Abstract
As a consequence of the Russian invasion of Ukraine, Europe is facing a shortage of chemical fertilizers for agriculture. Therefore, the use of byproducts of biomass anaerobic digestion, e.g., solid (SD) and liquid (LD) digestate, could be a key solution to cope with this problem. In this framework, the present study aimed to evaluate the effect of both SD and LD, derived from the same feedstock, on the biometric, physiological, and biochemical parameters of lettuce (Lactuca sativa L.) plants. Compared to the controls, the plants treated with 3% (w/w) SD showed a reduction in leaf fresh biomass, chlorophyll content, performance index, fractal dimension, and antiradical activity, while vitamin C increased by 18.8%. An opposite response was observed for the lettuce plants treated with 3% (v/w) LD, which showed an increase in all the above-mentioned parameters, except for vitamin C, which decreased by 39.8%. Interestingly, the content of malondialdehyde, which is correlated with cell membrane lipid peroxidation, increased in the SD-treated plants (+39.7%) and decreased (−42.1%) in the LD-treated plants. These results strongly support the use of LD in agriculture as a valuable product to improve the productivity and nutritional quality of crop plants.
1. Introduction
Europe is currently facing a shortage of chemical fertilizers for agriculture due to the Russian invasion of Ukraine. For this reason, an intensive search for bio-based solutions is now being conducted. On the other hand, the exponential growth of the human population, accompanied by increasing urbanization, have led to a significant increase in the amount of waste produced, especially organic waste. Anaerobic digestion (AD) is one of the most common sustainable processes for recycling and reusing organic waste such as animal manure, sewage sludge, organic fractions of municipal solid waste, and aquaculture, food and agricultural residues [1,2,3]. During this process, the organic fraction is microbially decomposed in the absence of oxygen to obtain mainly biogas, which consists of a mixture of gases, such as CH4, CO2, H2S, and NH3 [4]. Biogas is a source of green energy and is used mainly for the supply of heat and electricity [5]. Anaerobic digestion also produces digestate, which is a semi-solid product widely recognized in agriculture as a valuable plant fertilizer for its abundance of nutrients [6,7]. Furthermore, digestate has several molecules with phytohormonal action, e.g., auxin-like molecules and gibberellins [8,9,10,11], which promote seedling germination and plant growth and can enhance plant resistance to abiotic and biotic stresses [12]. However, due to its chemical compounds, when used as a fertilizer, digestate can also cause detrimental effects on crop plants, such as slow growth and early senescence [6]. For these reasons, the legislation (2019/1009/EU [13]) on this issue is continuously being updated, and limitations on N spreading (especially in the form of nitrates) on agricultural fields are regulated by Directive 91/676/EEC [14], which sets a maximum limit of 170 kg N ha−1 year−1.
The following two different fractions of the digestate can be obtained by means of a solid–liquid process: the solid fraction (solid digestate, SD) with a dry matter content > 15% and the liquid fraction (liquid digestate, LD) with a dry matter content < 15% [15]. Both fractions have specific physico-chemical properties that essentially depend on both the operating parameters and the feedstock used in the AD process [16,17]. After a stabilization process, through drying or composting, SD can be used as a biofertilizer [18]. The effects of SD are widely reported in the literature on various crops [19,20,21]. It has been shown that spreading SD on the soil surface can improve plant growth [22], but also modify soil characteristics by facilitating the volatilization of N forms, impacting crop growth [23]. On the other hand, it has also been demonstrated that the effects of long-term SD treatments on soil did not heavily influence soil fertility; therefore, the resulting environmental changes were not as pronounced [24]. Similar to SD, the LD is used as fertigation solution in horticulture [25] and in many other applications. For instance, LD can be recirculated within the AD reactor to limit both water and feedstock consumption [26]. However, LD effects on plant growth are still unclear. On the one hand, Odlare et al. [27] observed that short-term applications of LD did not negatively affect soil characteristics, such as pH. On the other hand, it has recently been shown that the long-term application of LD can lead to chemical soil contamination, due to the possible release of nutrients and ammonia, which can accumulate and contaminate the agroecosystem [16,28]. These variations in the soil properties may have both positive and negative effects on crops.
Lettuce (Lactuca sativa L.) is one of the most cultivated and consumed vegetables worldwide [29], and widely used as a model plant species to evaluate the possible effects of using natural products to limit contaminants and plant growth [30,31,32,33].
The present study aimed to evaluate the effect of the application of SD and LD on the growth of lettuce plants in terms of biometric (i.e., fresh weight and fractal analysis), photosynthetic (i.e., chlorophyll content and fluorescence), and biochemical (i.e., lipid peroxidation, total antioxidant power, and vitamin C content) parameters.
2. Materials and Methods
2.1. Digestate
The digestate was kindly provided by a biogas company located in southern Tuscany (Italy). It was derived from agricultural and zootechnical waste consisting of a mixture of maize, pomace, and turkey manure, which was anaerobically digested by the action of mesophilic microorganisms at a controlled temperature (20–45 °C). The solid part of the digestate (SD) was then mechanically separated from the liquid part (LD) using a helical compression machine. The microbiological analysis of the digestate did not evidence the presence of pathogenic microorganisms and the chemical characteristics for both fractions are provided by the biogas company, as reported in Table 1. For the SD, the pH and EC were determined according to Celletti et al. [34] with slight modifications. In brief, 2 g of air-dried and pulverized SD were diluted with distilled water in a ratio of 1:20 (w/v) and shaken for 2 h. After a centrifugation step (5 min at 4000 rpm), pH and EC were measured in the limpid supernatant using a pH meter (Hanna Instruments srl, Padova, Italy) and a conductivity meter (Delta Ohm, HD/8706, Padova, Italy), respectively. For the LD, the pH and EC were directly determined in the LD fraction using the above-mentioned instrumental devices.

Table 1.
Chemical and microbiological characteristics (mean ± standard error) of the solid (SD) and liquid digestate (LD) used in this study. pH and electrical conductivity (EC) were determined in a ratio of 1:20 (w/v) for the SD, while pH and EC were determined in the LD.
2.2. Plant Material and Growth Conditions
Lettuce (Lactuca sativa L.) was chosen as the model plant species, as one of the most economically important leafy vegetables. Thirty seedlings of lettuce (cv. “Cappuccio Biondo”) with a height of about 5 cm, placed in phytocells (5 × 5 × 4 cm), were purchased from a local nursery. Once in the laboratory, the seedlings were transplanted into black plastic pots (1 seedling/pot) (10 × 10 × 12 cm), previously filled with 400 g of air-dried soil sieved to 2 mm. Before transplanting, 3% (w/w) SD was added to the soil, forming the first batch of 10 plants, 3% (v/w) LD was added to the soil, forming the second batch, and no digestate was added to the soil, forming the remaining batch of 10 plants, which were used as a control (CTRL). Afterwards, the seedlings were placed in a greenhouse and grown for 5 weeks at 15 ± 2 °C, 400 μM m−2 s−1 PAR (day/night cycle of 10–14 h), 60 ± 1% RH. Every day, the pots were weighed with a scale to ensure the soil moisture remained constant (70% WHC) and rotated randomly to reduce any possible influence of microclimatic conditions inside the greenhouse. At harvest, the plants were carefully removed from each pot, and the leaves of each plant were weighed and stored at −20 °C; the soils of each plant were air-dried until they reached a constant weight and then sieved to 2 mm.
2.3. Soil Analyses
The chemical characteristics of the soil used for the experiment are reported in Table 2.

Table 2.
Chemical characteristics of the soil index used before transplanting plants.
Soil texture composition was determined by the Casagrande method [35]. Briefly, an amount of 40 g of soil, sieved to 2 mm, was added to 125 mL of sodium hexametaphosphate solution. The mixture was allowed to rest for 24 h before being stirred for 15 min. Subsequently, the dispersion was transferred to a beaker and filled to a volume of 1 L, marking the initiation of the sedimentation test. The moment of the onset of sedimentation was recorded in the densimeter, and the subsequent sedimentation times were recorded at intervals of 0.5, 1, 2, 4, 8, 15, and 30 min, as well as at 1, 2, 4, 8, and 24 h.
In the potting soils, the pH and electrical conductivity (EC) were determined according to the work of Celletti et al. [34] with slight modifications. In brief, 2 g of air-dried and pulverized potting soil was diluted with distilled water in a ratio of 1:20 (w/v) and shaken for 2 h. After a centrifugation step (5 min at 4000 rpm), the pH and EC were measured in the limpid supernatant using a pH meter (Hanna Instruments srl, Padova, Italy) and a conductivity meter (Delta Ohm, HD/8706, Padova, Italy), respectively.
For the mineral composition of the soil, the procedure proposed by Nelson et al. [36] was used, with minor modifications. A 250 mg sample of pulverized soil was weighed using an accurate scale and placed into a platinum crucible. Then, 0.1 g of lithium tetraborate and 0.4 g of lithium metaborate were added to the soil sample in the crucible. The soil sample and lithium salts were thoroughly mixed using a glass rod. The mixture was placed into a muffle furnace and heated to a temperature of 950 °C for a duration of 1 h and 30 min. Upon cooling, approximately 40 mL of 5% nitric acid (HNO3) was added to dissolve the melt. The resulting solution was filtered to remove any undissolved materials. Finally, the solution was made up to a final volume of 50 mL using ultrapure water. The total concentration of N, Ca, Fe, K, Mg, Mn, Na, P, and S in the soil samples was determined using inductively coupled plasma mass spectrometry (ICP-MS) with Perkin Elmer Elan 6100 and NexION 350 spectrometers. The analytical accuracy was evaluated by analyzing two standard reference materials, GBW 07411 (Chinese Soil), GBW 07311 e NIST 2709 (San Joaquin Soil).
The Walkley–Black procedure [37] was used to determine the organic C in the soil.
2.4. Plant Analyses
2.4.1. Main Photosynthetic Parameters
The total chlorophyll content and the performance index (PIABS), related to the chlorophyll a fluorescence, were non-destructively measured on the distal part of the three youngest and fully expanded leaves of each plant (10 measurements/leaf) using a chlorophyll content meter (CCM-300, Opti-Science, Hudson, NY, USA) and a plant efficiency analyzer (Handy PEA, Hansatech Ltd., Norfolk, UK), respectively.
2.4.2. Fractal Analysis
Fractal analysis is a universal mathematical method for characterizing most natural objects and processes [38]. A single methodological approach to calculating fractal dimensions allows a numerical description of the organization of natural structures of various origins, and hence a direct numerical comparison with each other [39]. The fractal dimension allows a single numerical value to be used as an indicator of plant biomass. Many structures have the fundamental property of geometric regularity, known as the invariance concerning scale, or “self-similarity”. If these objects are considered at a different scale, the same basic elements are always discovered. These repeating patterns determine the fractional, or fractal, dimension of the structure. Fractal geometry describes natural shapes in a way that cannot be described by Euclidean geometry [40].
To perform the fractal analysis on our samples (3 leaves/plant), scans of leaves of approx. the same length were executed using a professional scanner, so that we could evaluate the differences in the various treatments performed. We obtained high-resolution (1024 × 1024 pixels) PNG format images; an example of the fractal analysis is given in Figure S1.
Subsequently, the images obtained for the fractal analysis were transformed into black and white photos, i.e., binary images (Figure S2).
Finally, the binary images were handled with the MATLAB software (MathWorks Ltd., Natick, MA, USA) and the fractal dimensions of each plant were obtained with the box-counting method, which is commonly used to estimate the fractal dimension [38].
2.4.3. Non-Enzymatic Defense Response
The antiradical activity (ARA) was determined in 200 mg of leaves homogenized in 2 mL of 80% (v/v) ethanol. The homogenate was centrifuged at 15,000 rpm for 5 min and the supernatant (200 µL) was added to 1 mL of 2,2-diphenyl-1-picrylhydrazyl (DPPH) solution (prepared by dissolving 3.9 mg of DPPH in 100 mL of 80% (v/v) methanol). In addition, a blank and a control were prepared by adding 200 µL of 80% (v/v) ethanol in 1 mL of 80% (v/v) methanol and 0.2 mL of 80% (v/v) ethanol in 1 mL of DPPH solution, respectively. All the tubes were placed in the dark for 1 h and then the absorbance (Abs) was read at 517 nm with a UV–Vis spectrophotometer (Agilent 8453, Santa Clara, CA, USA). The results were expressed as the percentage of ARA, according to the following formula [41,42]:
The content of vitamin C (ascorbic acid) was determined according to Celletti et al. [43]. Briefly, 200 mg of leaves were homogenized in 0.8 mL of 10% (w/v) trichloroacetic acid (TCA). The homogenate was filtered on gauze, then kept in an ice bath for 5 min and finally centrifuged at 3000 rpm for 5 min. The supernatant (0.4 mL) was added to 1.6 mL of distilled water and 0.2 mL of 0.2 M Folin–Ciocalteu reagent (Carlo Erba, Cornaredo, MI, Italy). After 10 min of incubation in the dark, the samples were measured at 760 nm with a UV–Vis spectrophotometer (Agilent 8453, Santa Clara, CA, USA). The concentration of the samples was determined using a calibration curve prepared with 0.05–0.2 mL of a 100 µg mL−1 L-ascorbic acid (BioXtra, ≥99.0%, crystalline) stock solution.
2.4.4. Lipid Peroxidation
The level of lipid peroxidation was expressed as the content of malondialdehyde (MDA), which is one of the main metabolites that is reactive to 2-thiobarbituric acid (TBA), according to the procedure proposed by Silvestri et al. [44] with slight modifications. Briefly, 500 mg of leaves were homogenized in 5 mL of extraction solution, containing 0.25% (w/v) TBA dissolved in 10% (w/v) TCA. The homogenate was incubated at 95 °C for 30 min, and then quickly cooled on ice to stop the reaction. Subsequently, the completely cold samples were centrifuged at 5000 rpm for 20 min. The absorbance of the supernatant was measured at 532 nm and 600 nm with a UV–Vis spectrophotometer (Agilent 8453, Santa Clara, CA, USA). Non-specific turbidity correction was achieved by the subtraction of the absorbance value detected at 600 nm. The content of MDA was estimated using a molar extinction coefficient of the formed MDA–TBA complex of 155 mM−1 cm−1.
2.5. Statistical Analysis
Since the data demonstrated a normal distribution (Shapiro–Wilk test, p < 0.05), a one-way analysis of variance (ANOVA) was run, coupled with the least significant difference (LSD) test (p < 0.05) for post-hoc comparisons between the means. All calculations were run using the CoStat statistical software (Cohort, Berkeley, CA, USA).
3. Results
The addition of SD and LD significantly increased both the soil pH (+2.3% and +2.7%, respectively) and EC (+11.6% and 17.1%, respectively), compared to the relative controls (Figure 1A and B, respectively).
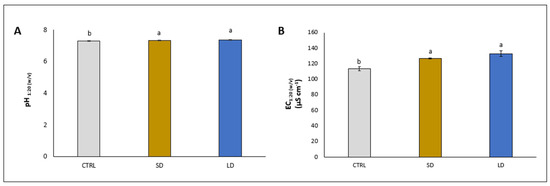
Figure 1.
pH (A) and electrical conductivity (EC) (B) in soils where lettuce plants were grown for 5 weeks without (CTRL) and with the application of digestate, either solid (SD) or liquid (LD). Parameters are expressed as mean ± standard error. Different letters indicate statistically significant differences (p < 0.05).
Among the biometric and photosynthetic parameters analyzed in the lettuce leaves, a similar trend was observed with the addition of SD and LD. Specifically, the SD significantly decreased the leaf fresh weight (−39.5%), chlorophyll content (−16.5%), PIABS (−25.1%), and fractal dimension (−2.3%), compared to their corresponding controls (Figure 2A, B, C, and D, respectively). On the contrary, LD significantly increased the leaf fresh weight (+22.9%), chlorophyll content (+6.2%), PIABS (+11.0%), and fractal dimension (+2.3%), compared to their corresponding controls (Figure 2A, B, C, and D, respectively).
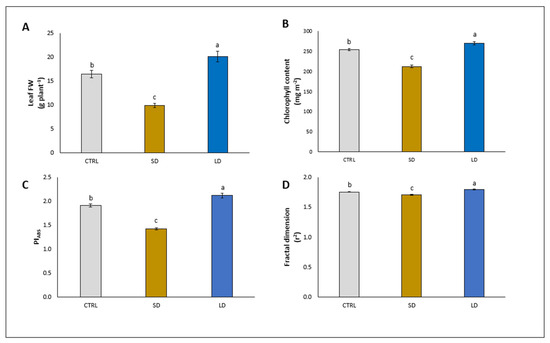
Figure 2.
Leaf fresh weight (FW) (A), chlorophyll content (B), performance index (PIABS) (C), and fractal dimension (D) in leaves of lettuce plants grown for 5 weeks without (CTRL) and with the application of digestate, either solid (SD) or liquid (LD). Parameters are expressed as mean ± standard error. Different letters indicate statistically significant differences (p < 0.05).
At the leaf level, it was evident that the antioxidant system was reduced (ARA: −29.3%) by the addition of SD, while it was increased (ARA: +28.6%) by the addition of LD, compared to the control (Figure 3A). Surprisingly, the determination of the content of vitamin C showed both a strong increase by +18.8% and a decrease by −39.8% with the addition of SD and LD, respectively, compared to the control (Figure 3B).
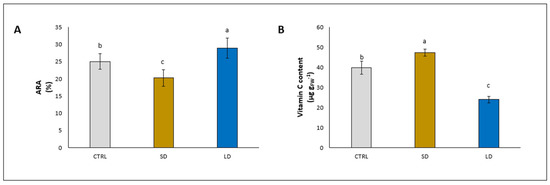
Figure 3.
Antiradical activity (ARA) (A) and vitamin C content (B) in leaves of lettuce plants grown for 5 weeks without (CTRL) and with the application of digestate, either solid (SD) or liquid (LD). Parameters are expressed as mean ± standard error. Different letters indicate statistically significant differences (p < 0.05).
In Figure 4, the content of MDA is shown, which increased (+39.7%) and decreased (−42.1%) in the leaves of SD- and LD-treated lettuce plants, respectively, in comparison with the control (Figure 4).
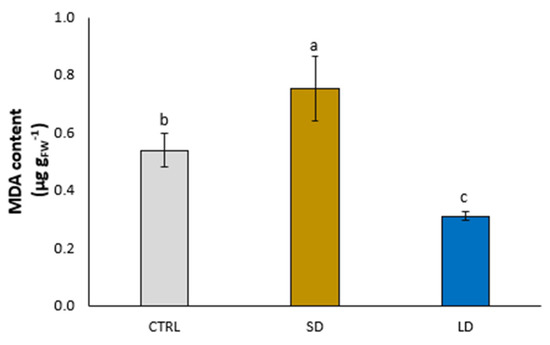
Figure 4.
Malondialdehyde (MDA) content in leaves of lettuce plants grown for 5 weeks without (CTRL) and with the application of digestate, either solid (SD) or liquid (LD). Parameters are expressed as mean ± standard error. Different letters indicate statistically significant differences (p < 0.05).
4. Discussion
Recently, in Italy, due to the war in Ukraine, the use of digestate has been authorized as a replacement for various synthetic chemical fertilizers to promote the use of eco-friendly practices in the production phase of biogas and to reduce the use of fertilizers according to the D.L. 21/2022 [45].
Plant biomass and chlorophyll content are parameters that are strictly related, since chlorophyll is a necessary pigment for plant growth, and an increase in chlorophyll content is usually correlated with an increase in plant biomass accumulation [30]. Due to the presence of irrelevant available amounts of nitrogen and phosphorus, the use of digestate can enhance photosynthesis, and thus increase the chlorophyll content, and plant biomass. Nevertheless, in some cases, the effects may be different [46]. In line with our results, Robles-Aguilar et al. [47] indicate that the application of SD decreased both the chlorophyll content and leaf fresh weight, whereas the application of LD increased both parameters. Mortola et al. [48] reported that a low SD application rate has no effect on the chlorophyll content and biomass of lettuce plants. In Chinese cabbage (Brassica pekinensis) plants, the addition of SD (obtained from a mixture of manure and food waste) impaired the early plant development stages and reduced the leaf chlorophyll content of the plants [49]. Treatments with SD performed on mustard green (Brassica juncea (L.) Czern.) plants showed different effects depending on the feedstock used to obtain the digestate; the food-waste digestate did not affect the biomass production of the plants, while the application of digestate from lignocellulosic biomass halted plant growth and root elongation [6].
Chlorophyll fluorescence is a very effective test for assessing the protective or stimulating effect of bioproducts on plants growth and physiology [30]. Indeed, in our study, the functionality of both photosystems I and II was noticeably higher in the LD-treated lettuce plants, as indicated by the increased PIABS value, compared with the control. Our results are consistent with several studies in the literature that show that LD addiction positively affects crop growth [50,51,52], with important biomass increases reported for wheat (Triticum aestivum L.) [53], giant Napier (Pennisetum purpureum Schumach.) [54], tomato (Solanum lycopersicum L.), and cucumber (Cucumis sativus L.) [55].
The antioxidant activity (ARA) is another indicator, strictly linked to general plant well-being, as it assesses the content of flavones, isoflavones, flavonoids, anthocyanin, coumarin lignans, catechins, and isocatechins [56]. Several epidemiological studies have shown that an increase in ARA in fruits and vegetables can have beneficial effects on human health, thus protecting against several cardiovascular diseases [57,58,59]. The application of the two fractions of digestate (SD and LD) produced completely opposed results in ARA determined in lettuce leaves, including a significant decrease when the plants were treated with SD and a significant increase when the plants were treated with LD. These latter results are consistent with those reported by Panuccio et al. [21,22], which showed that LD applications increased the ARA in tomato and cucumber fruits, respectively.
Vitamin C (ascorbic acid) is an essential micronutrient and a key element for the metabolism of all living organisms, especially for plants [60]. This compound plays an important role in the plant antioxidant system by offering protection against oxidative stress [61]. The content of vitamin C in our lettuce leaves showed a trend opposite to that of the general antioxidant defense system. In fact, it can be clearly noticed that while the SD treatment stimulated the production of vitamin C content, the LD treatment also decreased it. This different trend between vitamin C and ARA is explained by the fact that, as reported in the literature in lettuce leaves [62], the contribution by vitamin C to the total antioxidant power is below 1%. In addition, overall, plants can increase the vitamin C content within their organs/tissues to face stresses and not only when they are grown in optimal conditions [63]. Indeed, vitamin C is involved in several metabolic and biosynthetic pathways that regulate the process of plant growth and development [64]. Nevertheless, the level of vitamin C found in our samples, although significantly different among the treatments, falls within the range commonly reported for lettuce leaves [65,66].
Malondialdehyde is a natural by-product of cell membrane lipid peroxidation, usually analyzed to assess the damage caused by oxidative stress in plant tissues [67,68]. We observed an increase in MDA in SD-treated leaves and a decrease in LD-treated leaves. These results are consistent with those of ARA and vitamin C and with several other studies that show an increase in the content of MDA after different applications of digestate on sugar beet (Beta vulgaris L.) [69], rice (Orzya sativa L.) [53], and okra (Abelmoschus esculentus L.) [70]. The increase in MDA in our samples is also related to the reduction in leaf weight [71], further highlighting the damaging effects following the application of SD on lettuce plants. Moreover, MDA is related to vitamin C because both parameters are involved in oxidative stress, MDA allows us to assess the damage caused by oxidative stress in plant tissues and vitamin C plays an important role in the plant antioxidant system by offering protection against oxidative stress, so the increase in the similar trend observed in SD- and LD-treated plants reflects the overall status of the plants.
Fractal analysis in biology can be used to analyze the characteristics of objects, from molecules to ecosystems [72,73,74,75]. In this study, based on the value of the fractal dimension, binary images of scans of selected lettuce leaves were evaluated for changes in the physical characteristics of the leaf plates through changes in size and morphological organization by the properties of self-similarity and scale invariance in the geometric shape of the leaf plate. The characteristic of maximum space filling in the binary images of the lettuce leaf blade indicates an increase in the leaf blade area, which characterizes the increase in plant biomass [76,77]. In our study, an increase in plant biomass indicated the effectiveness of the LD as a fertilizer.
Overall, the results of this study show different effects of SD and LD on the growth, photosynthetic activity, and oxidative stress of lettuce plants, thus suggesting that only digestate in liquid form would seem to be able to enhance the growth, and thus the yield, of lettuce plants. The presence of a negative effect following applications with SD would seem to be attributable to the development of oxidative stress for the plant; however, the reason for this remains unknown. The negative effects of digestate on plants may depend on the inherent sensitivity of the species tested [49], thus suggesting how the pairing of two (or more) plants, one of which can be considered as more resistant while the other is less resistant, may help in the inter-prediction of the results obtained, especially when the results have to be interpreted from an environmental point of view [78].
5. Conclusions
This study investigated the effects of growing lettuce plants with a solid or liquid fraction of anaerobic digestate obtained from agricultural residues and animal manure. The results showed that these two fractions, although from the same raw material, affected the lettuce plants very differently. While the solid digestate showed negative effects overall, the application of liquid digestate resulted in the increased yield and improved nutraceutical quality of this crop that is consumed worldwide.
Although a larger field trial is needed to confirm the results of this study, our results suggest that the use of the liquid fraction of digestate in agriculture can be very effective in reducing dependence on the use of chemical fertilizers derived from fossil sources, thus representing an effective and sustainable approach to achieving the goal of a green and circular economy.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agronomy13030782/s1, Figure S1: Scanned leaves of lettuce plants grown for 5 weeks without (CTRL) (A) and with the application of digestate, either solid (SD) (B) or liquid (LD) (C); Figure S2: Binary images of leaves of lettuce plants grown for 5 weeks without (CTRL) (A) and with the application of digestate, either solid (SD) (B) or liquid (LD) (C).
Author Contributions
Conceptualization, S.L. and A.V.; methodology, R.F. and S.C.; formal analysis, R.F. and S.C.; investigation, R.F. and S.C.; resources, S.L.; data curation, R.F. and S.C.; writing—original draft preparation, R.F. and S.C.; writing—review and editing, R.F., S.C., S.L. and A.V.; supervision, S.C., A.V. and S.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data are available upon request by the corresponding author.
Acknowledgments
Marco Poltri (FUTURA ENERGIA a.r.l.) is acknowledged for kindly providing the digestate.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Schneider, N.; Gerber, M. Rheological Properties of Digestate from Agricultural Biogas Plants: An Overview of Measurement Techniques and Influencing Factors. Renew. Sustain. Energy Rev. 2020, 121, 109709. [Google Scholar] [CrossRef]
- Tampio, E.; Marttinen, S.; Rintala, J. Liquid Fertilizer Products from Anaerobic Digestion of Food Waste: Mass, Nutrient and Energy Balance of Four Digestate Liquid Treatment Systems. J. Clean. Prod. 2016, 125, 22–32. [Google Scholar] [CrossRef]
- Shi, L.; Simplicio, W.S.; Wu, G.; Hu, Z.; Hu, H.; Zhan, X. Nutrient Recovery from Digestate of Anaerobic Digestion of Livestock Manure: A Review. Curr. Pollut. Rep. 2018, 4, 74–83. [Google Scholar] [CrossRef]
- Amon, T.; Amon, B.; Kryvoruchko, V.; Zollitsch, W.; Mayer, K.; Gruber, L. Biogas Production from Maize and Dairy Cattle Manure-Influence of Biomass Composition on the Methane Yield. Bioresour. Technol. 2006, 100, 5777–5782. [Google Scholar] [CrossRef]
- Kougias, P.G.; Angelidaki, I. Biogas and Its Opportunities—A Review. Front. Environ. Sci. Eng. 2018, 12, 14. [Google Scholar] [CrossRef]
- Jamison, J.; Khanal, S.K.; Nguyen, N.H.; Deenik, J.L. Assessing the Effects of Digestates and Combinations of Digestates and Fertilizer on Yield and Nutrient Use of Brassica Juncea (Kai Choy). Agronomy 2021, 11, 509. [Google Scholar] [CrossRef]
- Celletti, S.; Lanz, M.; Bergamo, A.; Benedetti, V.; Basso, D.; Baratieri, M.; Cesco, S.; Mimmo, T. Evaluating the Aqueous Phase From Hydrothermal Carbonization of Cow Manure Digestate as Possible Fertilizer Solution for Plant Growth. Front. Plant Sci. 2021, 12, 1317. [Google Scholar] [CrossRef]
- Scaglia, B.; Pognani, M.; Adani, F. The Anaerobic Digestion Process Capability to Produce Biostimulant: The Case Study of the Dissolved Organic Matter (DOM) vs. Auxin-like Property. Sci. Total Environ. 2017, 589, 36–45. [Google Scholar] [CrossRef]
- Scaglia, B.; Pognani, M.; Adani, F. Evaluation of Hormone-like Activity of the Dissolved Organic Matter Fraction (DOM) of Compost and Digestate. Sci. Total Environ. 2015, 514, 314–321. [Google Scholar] [CrossRef]
- Yu, F.B.; Luo, X.P.; Song, C.F.; Shan, S.D. Concentrated Biogas Slurry Enhanced Soil Fertility and Tomato Quality. Acta Agric. Scand. 2009, 60, 262–268. [Google Scholar] [CrossRef]
- Liu, W.K.; Yang, Q.C.; Du, L. Soilless Cultivation for High-Quality Vegetables with Biogas Manure in China: Feasibility and Benefit Analysis. Renew. Agric. Food Syst. 2009, 24, 300–307. [Google Scholar] [CrossRef]
- Ronga, D.; Pane, C.; Zaccardelli, M.; Pecchioni, N. Use of Spent Coffee Ground Compost in Peat-Based Growing Media for the Production of Basil and Tomato Potting Plants. Commun. Soil Sci. Plant Anal. 2016, 47, 356–368. [Google Scholar] [CrossRef]
- Regulation (EU) 2019/1009 of the European Parliament and of the Council of 5 June 2019. Available online: https://eur-lex.europa.eu/eli/reg/2019/1009/oj (accessed on 5 March 2022).
- Council Directive of 12 December 1991. EUR-Lex-31991L0676-EN-EUR-Lex. Available online: europa.eu (accessed on 9 March 2022).
- Makádi, M.; Tomócsik, A.; Orosz, V. Digestate: A New Nutrient Source-Review Mineral Nutrition of Energy Crops View Project Long-Term Sewage Sludge Compost Utilization View Project Digestate: A New Nutrient Source-Review; Interopen Community Interest Company: London, UK, 2012. [Google Scholar] [CrossRef]
- Nkoa, R. Agricultural Benefits and Environmental Risks of Soil Fertilization with Anaerobic Digestates: A Review. Agron. Sustain. Dev. 2014, 34, 473–492. [Google Scholar] [CrossRef]
- Sheets, J.P.; Yang, L.; Ge, X.; Wang, Z.; Li, Y. Beyond Land Application: Emerging Technologies for the Treatment and Reuse of Anaerobically Digested Agricultural and Food Waste. Waste Manag. 2015, 44, 94–115. [Google Scholar] [CrossRef]
- Drosg, B.; Fuchs, W.; Al, T.; Madsen, S.M.; Linke, B. Nutrient Recovery by Biogas Digestate Processing; IEA: Paris, France, 2015. [Google Scholar]
- Fuchs, J.G.; Berner, A.; Mayer, J.; Smidt, E.; Schleiss, K. Influence of compost and digestates on plant growth and health: Potentials and limits. In Proceedings of the International Congress CODIS 2008, Solothurn, Switzerland, 27–29 February 2008; pp. 101–110. [Google Scholar]
- Möller, K.; Müller, T. Effects of anaerobic digestion on digestate nutrient availability and crop growth: A review. Eng. Life Sci. 2012, 12, 242–257. [Google Scholar] [CrossRef]
- Panuccio, M.R.; Papalia, T.; Attinà, E.; Giuffrè, A.; Muscolo, A. Use of Digestate as an Alternative to Mineral Fertilizer: Effects on Growth and Crop Quality. Arch. Agron. Soil Sci. 2019, 65, 700–711. [Google Scholar] [CrossRef]
- Panuccio, M.R.; Mallamaci, C.; Attinà, E.; Muscolo, A. Using Digestate as Fertilizer for a Sustainable Tomato Cultivation. Sustainability 2021, 13, 1574. [Google Scholar] [CrossRef]
- Chantigny, M.H.; Angers, D.A.; Bélanger, G.; Rochette, P.; Eriksen-Hamel, N.; Bittman, S.; Gasser, M.O. Yield and nutrient export of grain corn fertilized with raw and treated liquid swine manure. Agron. J. 2008, 100, 1303–1309. [Google Scholar] [CrossRef]
- Möller, K. Effects of anaerobic digestion on soil carbon and nitrogen turnover, N emissions, and soil biological activity. A review. Agron. Sustain. Dev. 2015, 35, 1021–1041. [Google Scholar] [CrossRef]
- Ronga, D.; Setti, L.; Salvarani, C.; de Leo, R.; Bedin, E.; Pulvirenti, A.; Milc, J.; Pecchioni, N.; Francia, E. Effects of Solid and Liquid Digestate for Hydroponic Baby Leaf Lettuce (Lactuca sativa L.) Cultivation. Sci. Hortic. 2019, 244, 172–181. [Google Scholar] [CrossRef]
- Estevez, M.M.; Sapci, Z.; Linjordet, R.; Schnürer, A.; Morken, J. Semi-Continuous Anaerobic Co-Digestion of Cow Manure and Steam-Exploded Salix with Recirculation of Liquid Digestate. J. Environ. Manag. 2014, 136, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Odlare, M.; Arthurson, V.; Pell, M.; Svensson, K.; Nehrenheim, E.; Abubaker, J. Land Application of Organic Waste–Effects on the Soil Ecosystem. Appl. Energy 2011, 88, 2210–2218. [Google Scholar] [CrossRef]
- Barłóg, P.; Hlisnikovský, L.; Kunzová, E. Effect of Digestate on Soil Organic Carbon and Plant-Available Nutrient Content Compared to Cattle Slurry and Mineral Fertilization. Agronomy 2020, 10, 379. [Google Scholar] [CrossRef]
- FAOSTAT. 2022. Available online: https://www.fao.org/faostat/en/ (accessed on 2 March 2022).
- Vannini, A.; Fedeli, R.; Guarnieri, M.; Loppi, S. Foliar Application of Wood Distillate Alleviates Ozone-Induced Damage in Lettuce (Lactuca Sativa L.). Toxics 2022, 10, 178. [Google Scholar] [CrossRef]
- Ntinas, G.K.; Bantis, F.; Koukounaras, A.; Kougias, P.G. Exploitation of Liquid Digestate as the Sole Nutrient Source for Floating Hydroponic Cultivation of Baby Lettuce (Lactuca sativa) in Greenhouses. Energies 2021, 14, 7199. [Google Scholar] [CrossRef]
- Montemurro, F.; Ferri, D.; Vitti, C.; Tittarelli, F.; Canali, S. Anaerobic Digestate and On-Farm Compost Application: Effects on Lettuce (Lactuca sativa L.) Crop Production and Soil Properties. Compost. Sci. Util. 2013, 18, 184–193. [Google Scholar] [CrossRef]
- Fedeli, R.; Vannini, A.; Guarnieri, M.; Monaci, F.; Loppi, S. Bio-Based Solutions for Agriculture: Foliar Application of Wood Distillate Alone and in Combination with Other Plant-Derived Corroborants Results in Different Effects on Lettuce (Lactuca sativa L.). Biology 2022, 11, 404. [Google Scholar] [CrossRef]
- Celletti, S.; Bergamo, A.; Benedetti, V.; Pecchi, M.; Patuzzi, F.; Basso, D.; Baratieri, M.; Cesco, S.; Mimmo, T. Phytotoxicity of Hydrochars Obtained by Hydrothermal Carbonization of Manure-Based Digestate. J. Environ. Manag. 2021, 280, 111635. [Google Scholar] [CrossRef]
- Casagrande, A. Die Ariiometer-Methode zur Bestimmung der Kornverteilung yon Boden und Anderen Materialen; Springer: Berlin/Heidelberg, Germany, 1934; p. 56S. [Google Scholar]
- Nelson, D.W.; Sommers, L.E.; Sparks, D.L. Methods of soil analysis. Part 3. Chemical methods. Soil Sci. Soc. Am. Book Ser. 1996, 5, 961–1010. [Google Scholar]
- Walkley, A.; Black, I.A. An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Hu, Y.; Shen, J.; Qi, Y.; Hu, Y.; Shen, J.; Qi, Y. Estimation of Rice Biomass at Different Growth Stages by Using Fractal Dimension in Image Processing. Appl. Sci. 2021, 11, 7151. [Google Scholar] [CrossRef]
- Kenkel, N.C.; Walker, D.J. Fractals and Ecology. Abstr. Bot. 1993, 17, 53–70. [Google Scholar]
- Lorimer, N.D. The Fractal Forest: Fractal Geometry and Applications in Forest Science; U.S. Department of Agriculture: Washington, DC, USA, 1994.
- Fedeli, R.; Vannini, A.; Celletti, S.; Maresca, V.; Munzi, S.; Cruz, C.; Alexandrov, D.; Guarnieri, M.; Loppi, S. Foliar Application of Wood Distillate Boosts Plant Yield and Nutritional Parameters of Chickpea. Ann. Appl. Biol. 2022. [Google Scholar] [CrossRef]
- Loppi, S.; Fedeli, R.; Canali, G.; Guarnieri, M.; Biagiotti, S.; Vannini, A. Comparison of the Mineral and Nutraceutical Profiles of Elephant Garlic (Allium ampeloprasum L.) Grown in Organic and Conventional Fields of Valdichiana, a Traditional Cultivation Area of Tuscany, Italy. Biology 2021, 10, 1058. [Google Scholar] [CrossRef]
- Celletti, S.; Fedeli, R.; Ghorbani, M.; Loppi, S. Impact of Starch-Based Bioplastic on Growth and Biochemical Parameters of Basil Plants. Sci. Total Environ. 2023, 159163. [Google Scholar] [CrossRef]
- Silvestri, C.; Celletti, S.; Cristofori, V.; Astolfi, S.; Ruggiero, B.; Rugini, E. Olive (Olea europaea L.) Plants Transgenic for Tobacco Osmotin Gene Are Less Sensitive to in Vitro-Induced Drought Stress. Acta Physiol. Plant. 2017, 39, 1–9. [Google Scholar] [CrossRef]
- Italian Minister Decree DECRETO-LEGGE 21 Marzo 2022, n. 21. Available online: https://www.gazzettaufficiale.it/eli/id/2022/03/21/22G00032/SG (accessed on 13 April 2022).
- Chen, J.; Cao, C.; Cai, M.; Yuan, B.; Zhai, J. Effect of Different Nitrogen Nutrition and Soil Water Potential on Physiological Parameters and Yield of Hybrid Rice. Field Crops Res. 2008, 14, 199–206. [Google Scholar]
- Robles-Aguilar, A.A.; Temperton, V.M.; Jablonowski, N.D. Maize Silage Digestate Application Affecting Germination and Early Growth of Maize Modulated by Soil Type. Agronomy 2019, 9, 473. [Google Scholar] [CrossRef]
- Mortola, N.; Rosa, V.; Cosentino, N.; Eiza, M. Potential Use of a Poultry Manure Digestate as a Biofertiliser: Evaluation of Soil Properties and Lactuca sativa Growth. Pedosphere 2019, 29, 60–69. [Google Scholar] [CrossRef]
- Maunuksela, L.; Herranen, M.; Torniainen, M. Quality Assessment of Biogas Plant End Products by Plant Bioassays. Int. J. Environ. 2012, 3, 305–310. [Google Scholar] [CrossRef]
- Zheng, X.; Fan, J.; Cui, J.; Wang, Y.; Zhou, J.; Ye, M.; Sun, M. Effects of Biogas Slurry Application on Peanut Yield, Soil Nutrients, Carbon Storage, and Microbial Activity in an Ultisol Soil in Southern China. J. Soils Sediments 2016, 16, 449–460. [Google Scholar] [CrossRef]
- Malav, L.C.; Khan, S.A.; Gupta, N. Impacts of Biogas Slurry Application on Soil Environment, Yield and Nutritional Quality of Baby Corn. Int. J. Plant Res. 2015, 28, 194–202. [Google Scholar] [CrossRef]
- Xu, C.; Tian, Y.; Sun, Y.; Dong, L. Effects of Biogas Slurry Irrigation on Growth, Photosynthesis, and Nutrient Status of Perilla Frutescens Seedlings. Commun. Soil Sci. Plant Anal. 2013, 44, 3381–3390. [Google Scholar] [CrossRef]
- Singh, R.P.; Singh, P.; Ibrahim, M.H.; Hashim, R. Land Application of Sewage Sludge: Physicochemical and Microbial Response. Rev. Environ. Contam. Toxicol. 2012, 214, 41–61. [Google Scholar] [CrossRef]
- Tan, F.; Zhu, Q.; Guo, X.; He, L. Effects of Digestate on Biomass of a Selected Energy Crop and Soil Properties. J. Sci. Food Agric. 2021, 101, 927–936. [Google Scholar] [CrossRef]
- Qi, X.; Zhang, S.; Wang, Y.; Wang, R. Advantages of the Integrated Pig-Biogas-Vegetable Greenhouse System in North China. Ecol. Eng. 2005, 24, 175–183. [Google Scholar] [CrossRef]
- Farrukh, A.; Iqbal, A.; Zafar, M. Antioxidant and Free Radical Scavenging Properties of Twelve Traditionally Used Indian Medicinal Plants. Turk. J. Biol. 2006, 30, 177–183. [Google Scholar]
- Mukherjee, P.K.; Nema, N.K.; Maity, N.; Sarkar, B.K. Phytochemical and Therapeutic Potential of Cucumber. Fitoterapia 2013, 84, 227–236. [Google Scholar] [CrossRef]
- Babajide, J.M.; Olaluwoye, A.A.; Taofik Shittu, T.A.; Adebisi, M.A. Physicochemical Properties and Phytochemical Components of Spiced Cucumber-Pineapple Fruit Drink. NIFOJ 2013, 31, 40–52. [Google Scholar] [CrossRef]
- DellaPenna, D. Carotenoid Synthesis and Function in Plants: Insights from Mutant Studies. In The Photochemistry of Carotenoids. Advances in Photosynthesis and Respiration; Springer: Berlin/Heidelberg, Germany, 1999; pp. 21–37. [Google Scholar] [CrossRef]
- Paciolla, C.; Fortunato, S.; Dipierro, N.; Paradiso, A.; de Leonardis, S.; Mastropasqua, L.; de Pinto, M.C. Vitamin C in Plants: From Functions to Biofortification. Antioxidants 2019, 8, 519. [Google Scholar] [CrossRef]
- Gupta, D.K.; Palma, J.M.; Corpas, F.J. Redox State as a Central Regulator of Plant-Cell Stress Responses. In Redox State as a Central Regulator of Plant-Cell Stress Responses; Springer International Publishing: Cham, Switzerland, 2016; pp. 1–386. [Google Scholar] [CrossRef]
- Szeto, Y.T.; Tomlinson, B.; Benzie, I.F.F. Total Antioxidant and Ascorbic Acid Content of Fresh Fruits and Vegetables: Implications for Dietary Planning and Food Preservation. Br. J. Nutr. 2002, 87, 55–59. [Google Scholar] [CrossRef]
- Locato, V.; Cimini, S.; de Gara, L. Strategies to Increase Vitamin C in Plants: From Plant Defense Perspective to Food Biofortification. Front. Plant Sci. 2013, 4, 152. [Google Scholar] [CrossRef]
- Foyer, C.H.; Noctor, G. Ascorbate and Glutathione: The Heart of the Redox Hub. Plant. Physiol. 2011, 155, 2–18. [Google Scholar] [CrossRef]
- Aćamović-Djoković, G.; Pavlović, R.; Mladenović, J.; Djurić, M. Vitamin C Content of Different Types of Lettuce Varieties 1. Acta Agric. Serb. 2011, XVI, 83–89. [Google Scholar]
- Llorach, R.; Martínez-Sánchez, A.; Tomás-Barberán, F.A.; Gil, M.I.; Ferreres, F. Characterisation of Polyphenols and Antioxidant Properties of Five Lettuce Varieties and Escarole. Food. Chem. 2008, 108, 1028–1038. [Google Scholar] [CrossRef]
- Ma, J.; Du, G.; Li, X.; Zhang, C.; Guo, J. A Major Locus Controlling Malondialdehyde Content under Water Stress Is Associated with Fusarium Crown Rot Resistance in Wheat. Mol. Genet. 2015, 290, 1955–1962. [Google Scholar] [CrossRef]
- Morales, M.; Munné-Bosch, S. Malondialdehyde: Facts and Artifacts. Plant Physiol. 2019, 180, 1246–1250. [Google Scholar] [CrossRef]
- Singh, R.P.; Agrawal, M. Effects of Sewage Sludge Amendment on Heavy Metal Accumulation and Consequent Responses of Beta Vulgaris Plants. Chemosphere 2007, 67, 2229–2240. [Google Scholar] [CrossRef]
- Srivastava, V.; Gupta, S.K.; Singh, P.; Sharma, B.; Singh, R.P. Biochemical, Physiological, and Yield Responses of Lady’s Finger (Abelmoschus esculentus L.) Grown on Varying Ratios of Municipal Solid Waste Vermicompost. Int. J. Recycl. Org. Waste Agric. 2018, 7, 241–250. [Google Scholar] [CrossRef]
- Jakhar, S.; Mukherjee, D. Chloroplast pigments, proteins, lipid peroxidation and activities of antioxidative enzymes during maturation and senescence of leaves and reproductive organs of Cajanus cajan L. Physiol. Mol. Biol. Plants 2014, 20, 171–180. [Google Scholar] [CrossRef]
- Song, C.; Gallos, L.K.; Havlin, S.; Makse, H.A. How to Calculate the Fractal Dimension of a Complex Network: The Box Covering. J. Stat. Mech. Theory Exp. 2007, 2007, P03006. [Google Scholar] [CrossRef]
- Nottale, L.; Chaline, J.; Grou, P. On the Fractal Structure of Evolutionary Trees. Fractals Biol. Med. 2002, 247–258. [Google Scholar] [CrossRef]
- Weibel, E.R. Design of Biological Organisms and Fractal Geometry. In Fractals in Biology and Medicine. Mathematics and Biosciences in Interaction; Nonnenmacher, T.F., Losa, G.A., Weibel, E.R., Eds.; Birkhäuser: Basel, Switzerland, 1994; pp. 68–85. [Google Scholar] [CrossRef]
- Su, J. A Spectrum Fractal Feature Classification Algorithm for Agriculture Crops with Hyper Spectrum Image. NASA 2011, 8002, 172–177. [Google Scholar] [CrossRef]
- Bayırlı, M.; Selvi, S.; Çakılcıoğlu, U. Determining Different Plant Leaves’ Fractal Dimensions: A New Approach to Taxonomical Study of Plants. Bangladesh J. Bot. 2014, 43, 267–275. [Google Scholar] [CrossRef]
- Ivanov, V.B.; Scherbakov, A.V. Assessment of the level of stress on plants of Western Siberian raised bogs by the method of fractal analysis. Sib. J. Life Sci. Agric. 2021, 13, 224–237. [Google Scholar] [CrossRef]
- Boluda, R.; Roca-Pérez, L.; Marimón, L. Soil plate bioassay: An effective method to determine ecotoxicological risks. Chemosphere 2011, 84, 1–8. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).