Chlorophyll Fluorescence Imaging Combined with Active Oxygen Metabolism for Classification of Similar Diseases in Cucumber Plants
Abstract
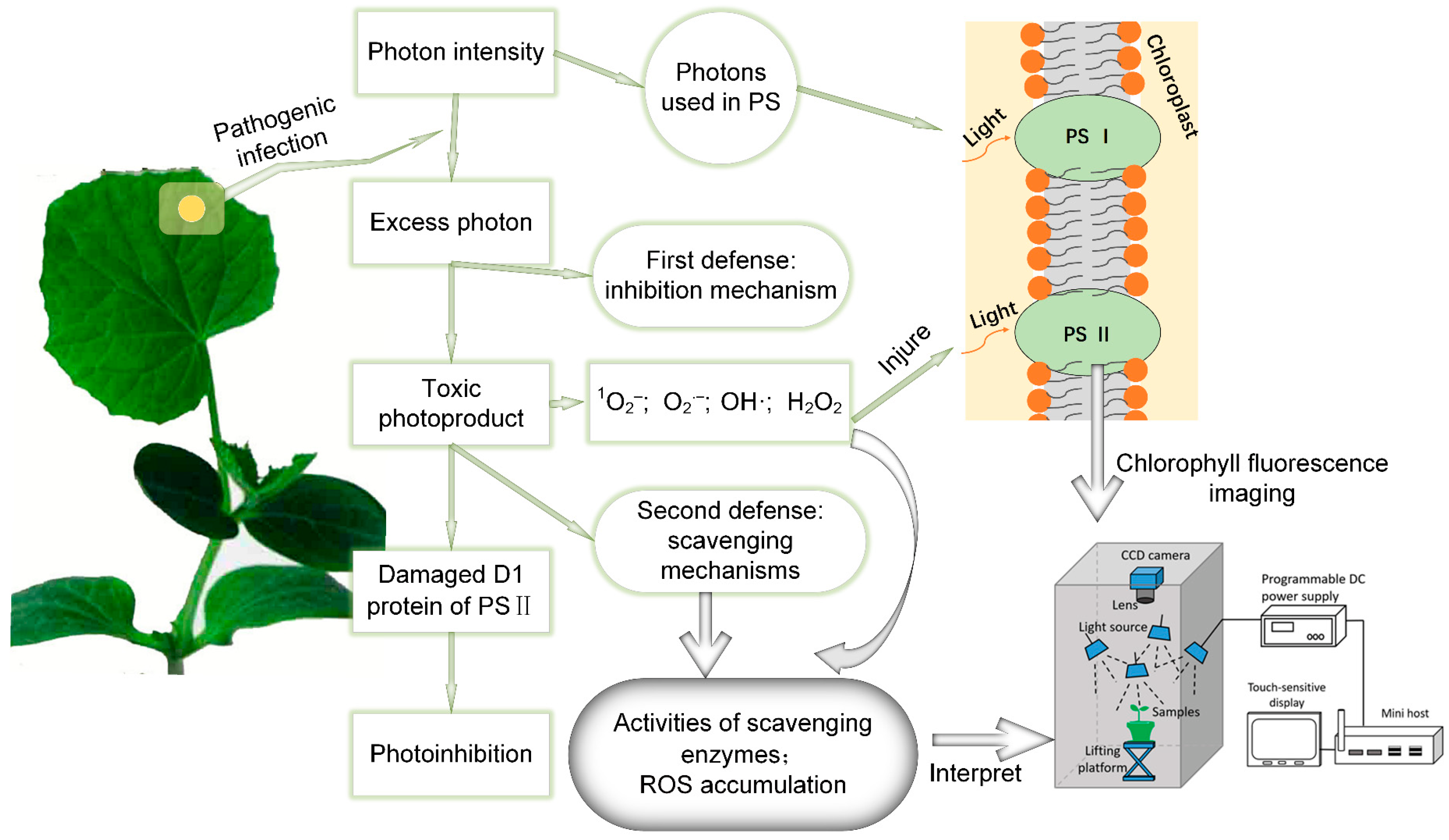
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
2.2. Pathogen Preparation and Plant Inoculation
2.3. Chlorophyll Fluorescence Imaging System and Data Acquisition
2.4. Determination of Nitrogen, Chlorophyll, MDA and H2O2 Content and Enzyme Activity in Cucumber Leaves
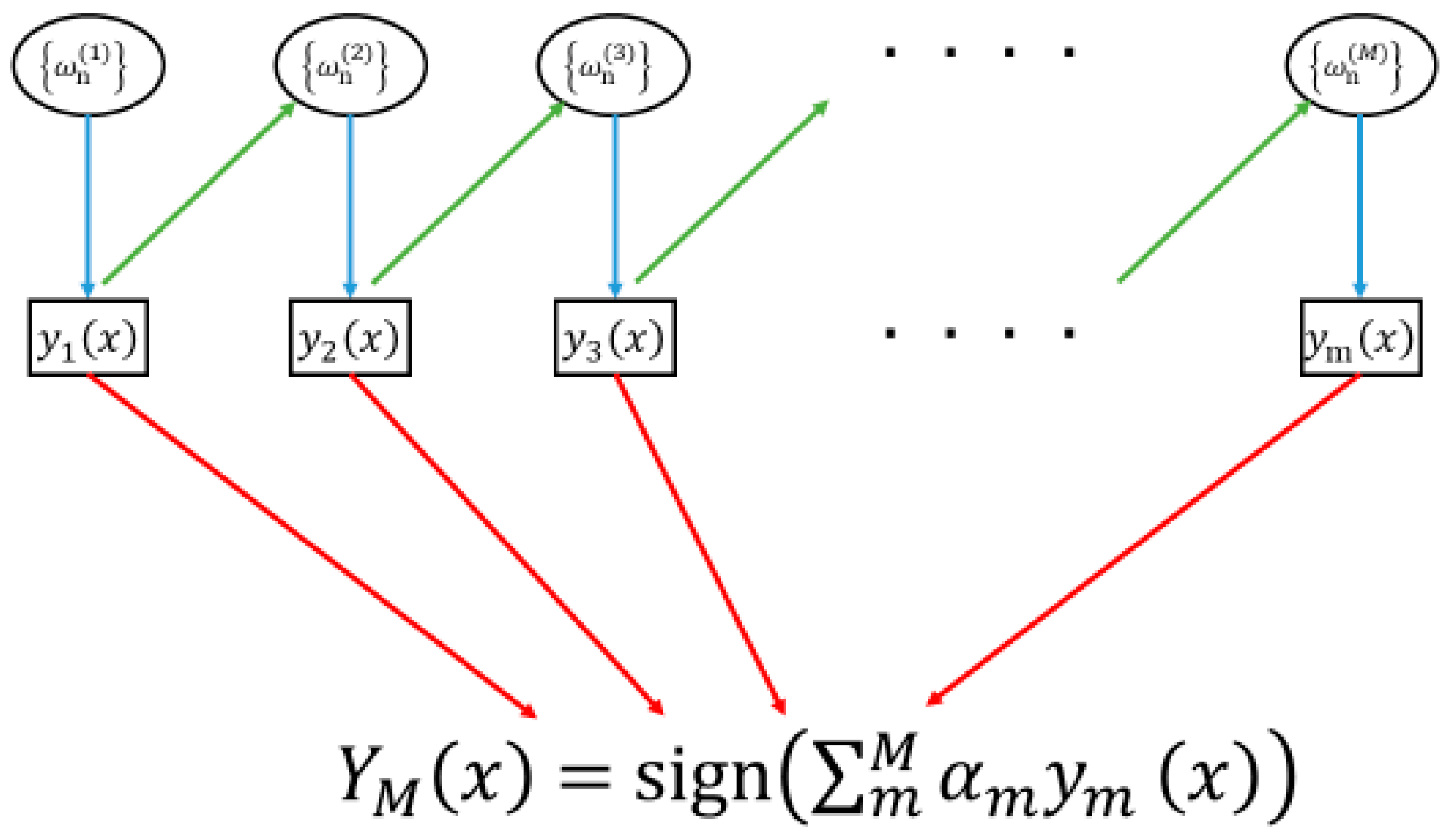
2.5. Data Processing and Analyzing
3. Results and Discussion
3.1. Effects of Fungal Infection on Chlorophyll, Nitrogen, H2O2 and MDA Content in Cucumber Leaves
3.2. Effects of Fungal Infection on Activity of Antioxidative Enzymes in Cucumber Leaves
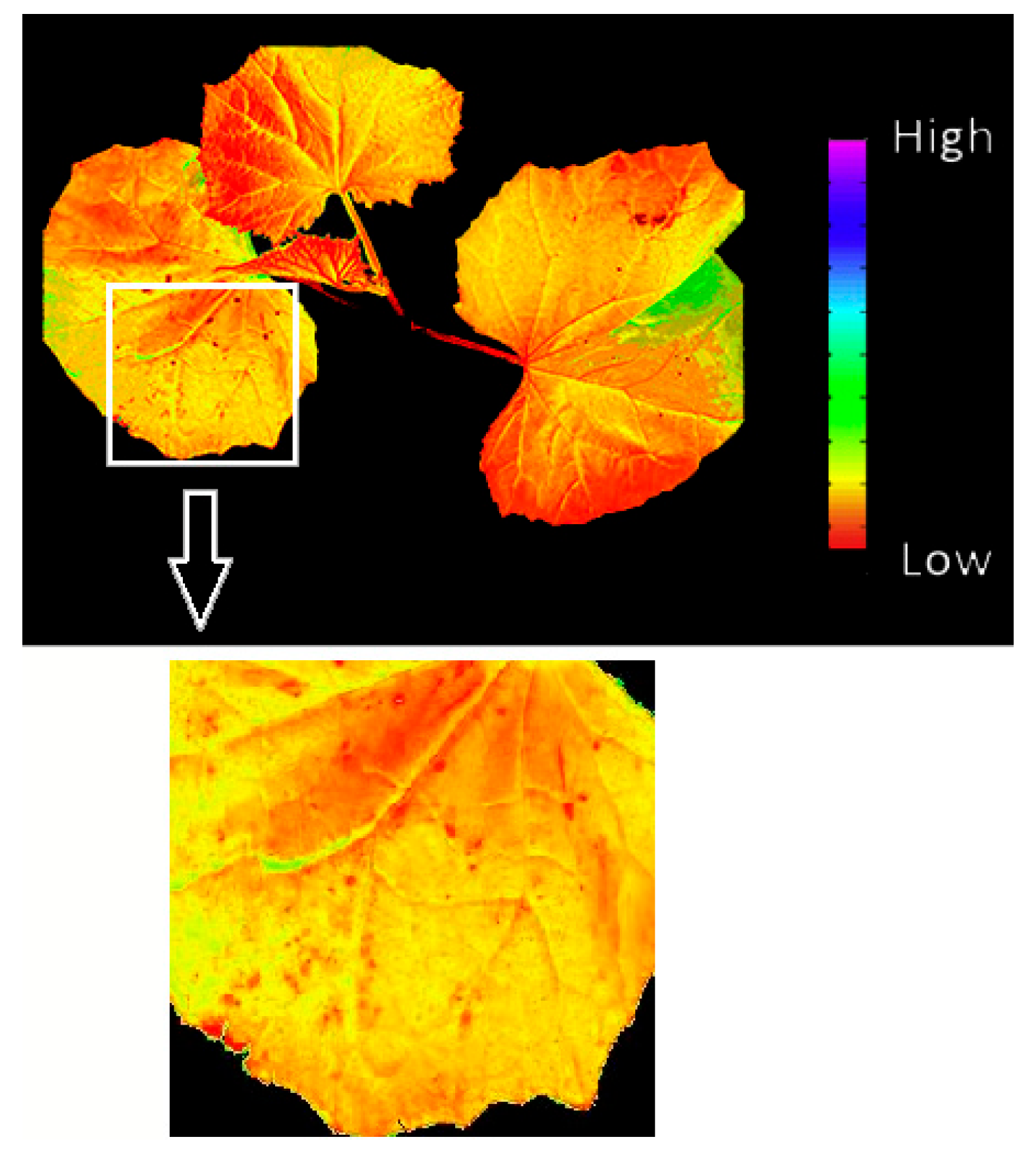
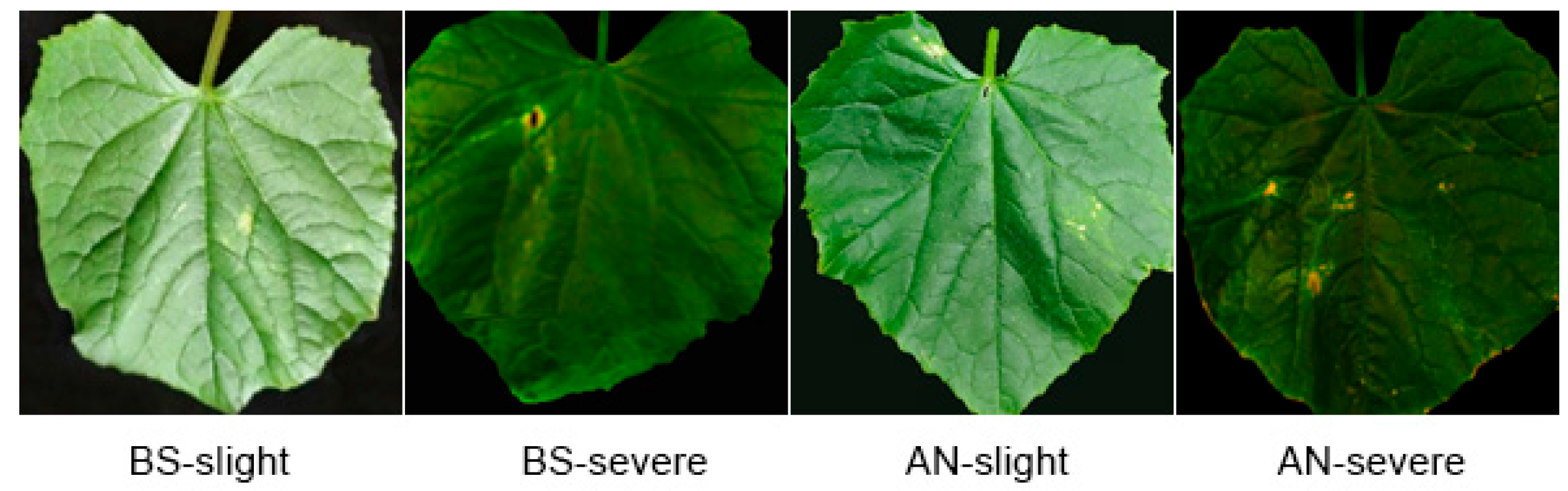
3.3. Effects of Fungal Infection in Chlorophyll Fluorescence Imagines of Diseased Cucumber Plants
3.4. Discriminant Results for Different Diseases in Cucumber Plants based on Multiple Chlorophyll Fluorescence Parameters
3.5. Discriminant Results for Early Diseased Cucumbers Based on Multiple Chlorophyll Fluorescence Parameters
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ma, T.; Yang, C.; Cai, F.; Cui, L.; Wang, Y. Optimizing fermentation of Bacillus amyloliquefaciens 3–5 and determining disease suppression and growth in cucumber (Cucumis sativus). Biol. Control 2022, 176, 105070. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, S.; Zhang, C.; Wang, X.; Shi, Y. Cucumber leaf disease identification with global pooling dilated convolutional neural network. Comput. Electron. Agric. 2019, 162, 422–430. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, J.; Huang, Y.; Tian, Y.; Yuan, L. Detection and discrimination of disease and insect stress of tea plants using hyperspectral imaging combined with wavelet analysis. Comput. Electron. Agric. 2022, 193, 106717. [Google Scholar] [CrossRef]
- Chouhan, S.S.; Singh, U.P.; Sharma, U.; Jain, S. Leaf disease segmentation and classification of Jatropha curcas L. and Pongamia PINNATA L. biofuel plants using computer vision based approaches. Measurement 2021, 171, 108796. [Google Scholar] [CrossRef]
- Ghanei Ghooshkhaneh, N.; Golzarian, M.R.; Mollazade, K. VIS-NIR spectroscopy for detection of citrus core rot caused by Alternaria alternata. Food Control 2023, 144, 109320. [Google Scholar] [CrossRef]
- Mastrodimos, N.; Lentzou, D.; Templalexis, C.; Tsitsigiannis, D.I.; Xanthopoulos, G. Development of thermography methodology for early diagnosis of fungal infection in table grapes: The case of Aspergillus carbonarius. Comput. Electron. Agric. 2019, 165, 104972. [Google Scholar] [CrossRef]
- Thakur, P.S.; Khanna, P.; Sheorey, T.; Ojha, A. Trends in vision-based machine learning techniques for plant disease identification: A systematic review. Expert Syst. Appl. 2022, 208, 118117. [Google Scholar] [CrossRef]
- Singh, V.; Sharma, N.; Singh, S. A review of imaging techniques for plant disease detection. Artif. Intell. Agric. 2020, 4, 229–242. [Google Scholar] [CrossRef]
- Feng, W.; He, L.; Zhang, H.; Guo, B.; Zhu, Y.; Wang, C.; Guo, T. Assessment of plant nitrogen status using chlorophyll fluorescence parameters of the upper leaves in winter wheat. Eur. J. Agron. 2015, 64, 78–87. [Google Scholar] [CrossRef]
- Zhou, C.; Le, J.; Hua, D.; He, T.; Mao, J. Imaging analysis of chlorophyll fluorescence induction for monitoring plant water and nitrogen treatments. Measurement 2019, 136, 478–486. [Google Scholar] [CrossRef]
- Ashrostaghi, T.; Aliniaeifard, S.; Shomali, A.; Azizinia, S.; Abbasi Koohpalekani, J.; Moosavi-Nezhad, M.; Gruda, N.S. Light intensity: The role player in cucumber response to cold stress. Agronomy 2022, 12, 201. [Google Scholar] [CrossRef]
- Schlie, T.; Dierend, W.; Köpcke, D.; Rath, T. Detecting low-oxygen stress of stored apples using chlorophyll fluorescence imaging and histogram division. Postharvest Biol. Technol. 2022, 189, 111901. [Google Scholar] [CrossRef]
- Chiu, Y.; Hsu, W.; Chang, Y. Detecting cabbage seedling diseases by using chlorophyll fluorescence. Eng. Agric. Environ. Food 2015, 8, 95–100. [Google Scholar] [CrossRef]
- Atta, B.M.; Saleem, M.; Ali, H.; Bilal, M.; Fayyaz, M. Application of fluorescence spectroscopy in wheat crop: Early disease detection and associated molecular changes. J. Fluoresc. 2020, 30, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Jushkov, A.N.; Borzykh, N.V.; Savelieva, N.N.; Zemisov, A.S. Chlorophyll fluorescence imaging in fruit plant breeding for resistance to dehydration and hyperthermia. J. Appl. Spectrosc. 2021, 87, 1087–1093. [Google Scholar] [CrossRef]
- Zhou, C.; Mao, J.; Zhao, H.; Rao, Z.; Zhang, B. Monitoring and predicting Fusarium wilt disease in cucumbers based on quantitative analysis of kinetic imaging of chlorophyll fluorescence. Appl. Opt. 2020, 59, 9118–9125. [Google Scholar] [CrossRef]
- Cen, H.; Weng, H.; Yao, J.; He, M.; Lv, J.; Hua, S.; Li, H.; He, Y. Chlorophyll fluorescence imaging uncovers photosynthetic fingerprint of citrus huanglongbing. Front. Plant Sci. 2017, 8, 1509. [Google Scholar] [CrossRef]
- Song, Y.; Liu, H.; Yang, Y.; He, J.; Yang, B.; Yang, L.; Zhou, X.; Liu, L.; Wang, P.; Yang, S. Novel 18β-glycyrrhetinic acid amide derivatives show dual-acting capabilities for control of plant bacterial diseases through ROS-mediated antibacterial efficiency and activation of plant defense responses. J. Integr. Agric. 2022. [Google Scholar] [CrossRef]
- Sun, J.; Lin, H.; Zhang, S.; Lin, Y.; Wang, H.; Lin, M.; Hung, Y.; Chen, Y. The roles of ROS production-scavenging system in Lasiodiplodia theobromae (Pat.) Griff. & Maubl.-induced pericarp browning and disease development of harvested longan fruit. Food Chem. 2018, 247, 16–22. [Google Scholar] [CrossRef]
- Segal, L.M.; Wilson, R.A. Reactive oxygen species metabolism and plant-fungal interactions. Fungal Genet. Biol. 2018, 110, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhou, Y.; Li, J.; Gu, X.; Zhao, L.; Li, B.; Wang, K.; Yang, Q.; Zhang, H. Pichia caribbica improves disease resistance of cherry tomatoes by regulating ROS metabolism. Biol. Control 2022, 169, 104870. [Google Scholar] [CrossRef]
- Zhao, L.; Xie, J.; Zhang, H.; Wang, Z.; Jiang, H.; Gao, S. Enzymatic activity and chlorophyll fluorescence imaging of maize seedlings (Zea mays L.) after exposure to low doses of chlorsulfuron and cadmium. J. Integr. Agric. 2018, 17, 826–836. [Google Scholar] [CrossRef]
- Tang, Q.; Zheng, X.; Guo, J.; Yu, T. Tomato SlPti5 plays a regulative role in the plant immune response against Botrytis cinerea through modulation of ROS system and hormone pathways. J. Integr. Agric. 2022, 21, 697–709. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, S.; Huang, Y.; Jones, J.B. Evaluation of a small molecule compound 3-indolylacetonitrile for control of bacterial spot on tomato. Crop Prot. 2019, 120, 7–12. [Google Scholar] [CrossRef]
- Bussotti, F.; Gerosa, G.; Digrado, A.; Pollastrini, M. Selection of chlorophyll fluorescence parameters as indicators of photosynthetic efficiency in large scale plant ecological studies. Ecol. Indic. 2020, 108, 105686. [Google Scholar] [CrossRef]
- Ye, M.; Zhu, L.; Li, X.; Ke, Y.; Huang, Y.; Chen, B.; Yu, H.; Li, H.; Feng, H. Estimation of the soil arsenic concentration using a geographically weighted XGBoost model based on hyperspectral data. Sci. Total Environ. 2023, 858, 159798. [Google Scholar] [CrossRef]
- Lin, Y.; Chen, M.; Lin, H.; Hung, Y.; Lin, Y.; Chen, Y.; Wang, H.; Shi, J. DNP and ATP induced alteration in disease development of Phomopsis longanae Chi-inoculated longan fruit by acting on energy status and reactive oxygen species production-scavenging system. Food Chem. 2017, 228, 497–505. [Google Scholar] [CrossRef]
- Lin, Y.; Lin, Y.; Lin, H.; Zhang, S.; Chen, Y.; Shi, J. Inhibitory effects of propyl gallate on browning and its relationship to active oxygen metabolism in pericarp of harvested longan fruit. LWT Food Sci. Technol. 2015, 60, 1122–1128. [Google Scholar] [CrossRef]
- Lanza, M.G.D.B.; Reis, A.R.D. Roles of selenium in mineral plant nutrition: ROS scavenging responses against abiotic stresses. Plant Physiol. Bioch. 2021, 164, 27–43. [Google Scholar] [CrossRef]
- Lin, Y.; Lin, H.; Zhang, S.; Chen, Y.; Chen, M.; Lin, Y. The role of active oxygen metabolism in hydrogen peroxide-induced pericarp browning of harvested longan fruit. Postharvest Biol. Technol. 2014, 96, 42–48. [Google Scholar] [CrossRef]
- Ghosh, R.; Barman, S.; Khatun, J.; Mandal, N.C. Biological control of Alternaria alternata causing leaf spot disease of Aloe vera using two strains of rhizobacteria. Biol. Control 2016, 97, 102–108. [Google Scholar] [CrossRef]
- Dong, Z.; Men, Y.; Li, Z.; Zou, Q.; Ji, J. Chlorophyll fluorescence imaging as a tool for analyzing the effects of chilling injury on tomato seedlings. Sci. Hortic. 2019, 246, 490–497. [Google Scholar] [CrossRef]
- Ma, J.; Du, K.; Zheng, F.; Zhang, L.; Sun, Z. Disease recognition system for greenhouse cucumbers based on deep convolutional neural network. Trans. Chin. Soc. Agric. Eng. 2018, 34, 186–192. [Google Scholar]


| Infection Time | Brown Spot Group | Anthracnose Group | Control Group | |||
|---|---|---|---|---|---|---|
| Nitrogen | Chlorophyll | Nitrogen | Chlorophyll | Nitrogen | Chlorophyll | |
| 0 h | 3.57 ± 0.06 a | 50.26 ± 2.86 a | 3.57 ± 0.15 a | 49.92 ± 2.97 a | 3.58 ± 0.13 ab | 49.72 ± 2.76 a |
| 24 h | 3.42 ± 0.23 ab | 45.24 ± 5.05 b | 3.50 ± 0.18 a | 48.38 ± 3.35 a | 3.56 ± 0.13 ab | 48.81 ± 3.72 a |
| 48 h | 3.31 ± 0.22 bc | 44.07 ± 4.36 bc | 3.48 ± 0.16 ab | 48.18 ± 4.06 a | 3.64 ± 0.16 a | 48.29 ± 2.92 a |
| 72 h | 3.25 ± 0.24 cd | 43.31 ± 4.95 bc | 3.40 ± 0.28 bc | 46.12 ± 2.98 b | 3.49 ± 0.14 b | 47.71 ± 3.73 a |
| Groups | Parameters | Infection Time | |||
|---|---|---|---|---|---|
| Healthy | 24 h | 48 h | 72 h | ||
| Brown spot | Fv/Fm | 0.840 ± 0.020 a | 0.828 ± 0.017 a | 0.825 ± 0.019 a | 0.819 ± 0.020 a |
| Y(II) | 0.214 ± 0.013 a | 0.200 ± 0.016 a | 0.211 ± 0.022 a | 0.217 ± 0.023 a | |
| qP | 0.366 ± 0.040 ab | 0.339 ± 0.030 a | 0.394 ± 0.050 b | 0.446 ± 0.039 c | |
| qN | 0.713 ± 0.047 a | 0.795 ± 0.015 b | 0.745 ± 0.017 a | 0.712 ± 0.020 a | |
| Y(NPQ) | 1.386 ± 0.140 a | 1.513 ± 0.060 a | 1.871 ± 0.091 b | 1.873 ± 0.163 b | |
| Anthracnose | Fv/Fm | 0.840 ± 0.020 a | 0.834 ± 0.024 a | 0.829 ± 0.025 a | 0.822 ± 0.026 a |
| Y(II) | 0.214 ± 0.013 a | 0.195 ± 0.017 b | 0.196 ± 0.016 ab | 0.207 ± 0.014 ab | |
| qP | 0.366 ± 0.040 ab | 0.345 ± 0.035 a | 0.396 ± 0.047 b | 0.438 ± 0.032 c | |
| qN | 0.713 ± 0.047 ab | 0.783 ± 0.023 c | 0.755 ± 0.037 bc | 0.708 ± 0.021 a | |
| Y(NPQ) | 1.386 ± 0.140 a | 1.506 ± 0.049 a | 1.772 ± 0.101 b | 1.801 ± 0.158 b | |
| Model | Class | Calibration (%) | Prediction | Class | Calibration (%) | Prediction | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Healthy | AN | Accuracy (%) | Healthy | AN | Accuracy (%) | |||||
| (A) SVM | Healthy | 98.5 | 31 | 3 | 91.2 | Healthy | 100.0 | 31 | 3 | 91.2 |
| BS | 95.0 | 3 | 37 | 92.5 | AN | 96.3 | 5 | 35 | 87.5 | |
| Overall | 96.6 | 34 | 40 | 91.9 | Overall | 97.9 | 36 | 38 | 89.2 | |
| (B) XG-Boost | Healthy | 100.0 | 31 | 3 | 91.2 | Healthy | 100.0 | 30 | 4 | 88.2 |
| BS | 98.7 | 1 | 39 | 97.5 | AN | 96.3 | 4 | 36 | 90.0 | |
| Overall | 99.3 | 32 | 42 | 94.6 | Overall | 97.9 | 34 | 40 | 89.2 | |
| Model | Class | Calibration (%) | Prediction | |||
|---|---|---|---|---|---|---|
| Healthy | AN | BS | Accuracy (%) | |||
| (A) SVM | Healthy | 90.1 | 26 | 4 | 4 | 76.4 |
| AN | 95.0 | 1 | 38 | 1 | 95.0 | |
| BS | 92.5 | 5 | 2 | 33 | 82.5 | |
| Overall | 92.9 | 32 | 44 | 38 | 85.1 | |
| (B) XGBoost | Healthy | 89.4 | 28 | 3 | 3 | 82.4 |
| AN | 98.8 | 1 | 39 | 0 | 97.5 | |
| BS | 90.0 | 6 | 0 | 34 | 85.0 | |
| Overall | 92.9 | 35 | 42 | 37 | 88.6 | |
| Model | Class | Calibration (%) | Prediction (%) | Class | Calibration (%) | Prediction (%) |
|---|---|---|---|---|---|---|
| (A) SVM | Healthy | 98.5 | 79.4 | Healthy | 97.5 | 85.3 |
| BS-slight | 85.0 | 85.0 | AN-slight | 90 | 75.0 | |
| BS-severe | 92.5 | 85.0 | AN-severe | 92.5 | 85.0 | |
| Overall | 93.2 | 82.4 | Overall | 93.3 | 82.4 | |
| (B) XGBoost | Healthy | 97.0 | 91.2 | Healthy | 93.9 | 82.4 |
| BS-slight | 92.5 | 90.0 | AN-slight | 87.5 | 80.0 | |
| BS-severe | 87.5 | 80.0 | AN-severe | 90.0 | 95.0 | |
| Overall | 93.2 | 87.8 | Overall | 90.4 | 85.1 |
| Model | Class | Calibration (%) | Prediction | |||||
|---|---|---|---|---|---|---|---|---|
| Healthy | BS-Slight | BS-Severe | AN-Slight | AN-Severe | Accuracy (%) | |||
| SVM | Healthy | 87.9 | 27 | 0 | 3 | 3 | 1 | 79.4 |
| BS-slight | 87.5 | 0 | 15 | 5 | 0 | 0 | 75.0 | |
| BS-severe | 85 | 1 | 4 | 15 | 0 | 0 | 75.0 | |
| AN-slight | 77.5 | 4 | 0 | 0 | 13 | 3 | 65.0 | |
| AN-severe | 82.5 | 2 | 2 | 0 | 1 | 15 | 75.0 | |
| Overall | 84.1 | 34 | 21 | 23 | 17 | 19 | 74.6 | |
| XGBoost | Healthy | 92.4 | 30 | 0 | 0 | 3 | 1 | 88.2 |
| BS-slight | 90.0 | 0 | 17 | 2 | 1 | 0 | 85.0 | |
| BS-severe | 82.5 | 2 | 3 | 15 | 0 | 0 | 75.0 | |
| AN-slight | 77.5 | 4 | 1 | 0 | 13 | 2 | 65.0 | |
| AN-severe | 85 | 2 | 0 | 1 | 2 | 15 | 75.0 | |
| Overall | 85.5 | 36 | 21 | 17 | 21 | 19 | 78.9 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.; Liu, T.; Wang, X.; Hu, Y. Chlorophyll Fluorescence Imaging Combined with Active Oxygen Metabolism for Classification of Similar Diseases in Cucumber Plants. Agronomy 2023, 13, 700. https://doi.org/10.3390/agronomy13030700
Sun Y, Liu T, Wang X, Hu Y. Chlorophyll Fluorescence Imaging Combined with Active Oxygen Metabolism for Classification of Similar Diseases in Cucumber Plants. Agronomy. 2023; 13(3):700. https://doi.org/10.3390/agronomy13030700
Chicago/Turabian StyleSun, Ye, Tan Liu, Xiaochan Wang, and Yonghong Hu. 2023. "Chlorophyll Fluorescence Imaging Combined with Active Oxygen Metabolism for Classification of Similar Diseases in Cucumber Plants" Agronomy 13, no. 3: 700. https://doi.org/10.3390/agronomy13030700
APA StyleSun, Y., Liu, T., Wang, X., & Hu, Y. (2023). Chlorophyll Fluorescence Imaging Combined with Active Oxygen Metabolism for Classification of Similar Diseases in Cucumber Plants. Agronomy, 13(3), 700. https://doi.org/10.3390/agronomy13030700






