Abstract
Saskatoon berry has become as important as a commercial fruit crop. One main goal is to release new plant cultivars well adapted to different climatic and soil conditions. Dormant seeds obtained from breeding are serious problems delaying the program. The seeds were directly extracted from fruits after harvest (unstored) or after storage at −18 °C for 6 months (stored) and subjected to modified stratification (3 °C) with KNO3, H2O2, NO, smoke-water (SW) or scarification using sandpaper or H2SO4 for 10, 20, 30, 40 min or treatments with pulsed radio frequency (PRF) or red light. The seeds were also subjected to warm–cool stratification (20/3 °C). Unstored seeds germinated in a higher percentage and with better uniformity (T75–T25) than stored seeds. Stored seeds positively affected the onset of seed germination (T1) and mean germination time (MGT). Dormancy breakage was promoted by stratification with KNO3, SW or scarification with sandpaper, H2SO4 or treatments with PRF. The recommended method for the breeding program of breaking seeds dormancy is when unstored seeds are subjected to stratification in KNO3 (0.2%) or SW (1:100). Depending on the applied methods, the percentage of seeds’ germination increased to 87% compared to untreated (64%) control seeds. The positive effects of the selected methods persisted during seedling development by stimulating their growth and enhancing the chlorophyll content index (SPAD) and effective quantum yield of PSII of chlorophyll in leaves (ΦPSII).
1. Introduction
The genus Amelanchier (family Rosaceae) includes about 25 species of shrubs or small deciduous trees. The most popular species are Saskatoon berry (A. alnifolia), Canadian serviceberry (A. canadensis), juneberry (A. lamarckii) and snowy mespilus (A. ovalis) [1]. Saskatoon berry (A. alnifolia Nutt.), a fruit-bearing shrub, is native to the Prairie Provinces of Canada and northern plains in the United States of America [2,3]. The most popular cultivars of this plant species, mainly grown in Canada and the USA, are ‘Smoky’, ‘Honeywood’, ‘Thiessen’, ‘Northline’ and ‘Martin’, which were brought to Poland in 2007. Recent research and observations showed that Saskatoon berry adapted well to Polish climatic and soil conditions and could be successfully cultivated in our conditions [4]. It can also grow in almost all soil types, except marshy sites and sands that are too dry and barren. Well-hardened shrubs withstand winter temperatures even down to −40 °C, and the buds and flowers are tolerant to spring frosts. Moreover, this crop is also interesting for growers because of its disease resistance, high fruit quality and yields, which are similar to the highbush blueberry [5].
The importance of this fruit crop has been increasing in such European countries as Finland, the Czech Republic, Lithuania, Latvia and Poland [2,6]. Depending on the genotype, the fruits of Saskatoon berry are particularly rich in bioactive compounds, such as: flavonols, phenolic acids, carotenoids, anthocyanins [7], pectins, vitamins (ascorbic acid, tocopherol, riboflavin, thiamin, pyridoxine) and other valuable antioxidants as well as minerals (potassium, calcium, manganese, magnesium, iron), sugars (glucose, fructose, sucrose, sorbitol) and organic acids [2,8]. The fruit of the Saskatoon berry can be used in the food industry in the production of beverages, jams, jellies, liqueurs or baked goods, or as an addition to ice cream or yogurts. The small scale of the Saskatoon berry’s cultivation, observed currently in our country, tends to increase the production of the fruit in the future [9].
In Poland, the first breeding program for developing new cultivars of the Saskatoon berry (A. alnifolia Nutt) was started in 2009 at the Institute of Pomology and Floriculture (currently the Institute of Horticulture—National Research Institute—InHort) in Skierniewice. The main breeding goals are to release new cultivars well adapted to the Polish climatic and soil conditions and high yielding with good quality of fruits. However, some problems are connected with the dormancy and low germinability of seeds, which prolong the breeding program of obtaining new cultivars [10].
Pulsed radio frequency (PRF), together with modified stratification, was applied in a previous study [11]. PRF had an additive role in breaking the dormancy of apple ‘Ligol’ by affecting biological changes in tissues due to the thermal effects or high intensity of electric fields, or as a result of both [12]. Electric fields can have a significant effect on cells due to the transmembrane potentials that they induce [12].
Smoke-water or smoke-saturated water (SW) is known to positively affect seeds with all types of seed dormancy [13]. This response concerned numerous horticultural and agricultural plants. Karrikin 1 (KAR1) in very low concentrations can stimulate not only the release of seed dormancy and the germination of many plant species but can also enhance seedling development. SW is derived from burning plant material and then bubbling the smoke through water [14].
However, published literature documenting the use of these methods as treatments for breaking the seed dormancy of the Saskatoon berry has still been lacking. Furthermore, the low percentage and dynamics of the germination of seeds obtained from the crossing programs are serious problems delaying the breeding work of this species. It causes the delayed emergence and reduced number of seedlings that constitute valuable breeding material for further evaluation and selection. It is expected that the development of methods of breaking seed dormancy in this species will increase their vigor and biological potential. As a result, this will accelerate and increase the emergence and growth of seedlings and will enable obtaining more seedlings from the same seed lot.
The objective of the current study was to accelerate the Saskatoon berry’s seed release from dormancy as well as juvenile plant growth and improve the chlorophyll content index (SPAD) and the effective quantum yield of PSII (ΦPSII) by the application of selected compounds and physical methods. In addition, this research is expected to determine the effectiveness of the tested methods in breaking seeds dormancy in order to accelerate the applied breeding programs of new Saskatoon berry cultivars.
2. Materials and Methods
2.1. Plant Material
The experiments were carried out at the Institute of Horticulture—National Research Institute (InHort) in Skierniewice on the Saskatoon berry (A. alnifolia Nutt.) seeds and seedlings of the Canadian cultivar ‘Martin’ (Figure 1). The seeds were collected at the beginning of July from the fully ripened fruit harvested from the 5-year-old bushes grown in the field of the Experimental Orchard in Dąbrowice (51.9163° N/20.1009° E), near Skierniewice, central Poland, belonging to the InHort. The seeds were directly extracted from fruits harvested in the summer of 2018 (unstored) and from fruits stored at −18 °C for 6 months. After storage, the seeds were placed at 5 °C and 30% relative humidity until the initiation of the experiments (2–5 months). The seeds were considered fully viable on the basis of the tetrazolium test. Tetrazolium tests were conducted before the start of experiments. The test was carried out according to the International Seed Testing Association (ISTA, 2003). The seed was considered viable when the entire embryo was evenly stained. Both seeds extracted from fruits directly after harvest or stored at −18 °C for 6 months were unable to germinate within 14 days at 3, 20 and 25 °C, indicating that they were dormant.

Figure 1.
Flowering shrubs (left), ripe fruits in clusters (center), and ripe seeds (right) of cv. ‘Martin’ of the Saskatoon berry.
2.2. Breaking of Seed Dormancy
The seeds were decontaminated in Chlorocid (1%) for 40 min and subjected to standard stratification; i.e., the seeds were sown onto two layers of filter paper moistened with distilled water and kept in darkness at a constant temperature of 3 ± 1 °C until they started germinating in this conditions; i.e., the radicle protrusion was observed with root lengths of 1–2 mm. The experimental unit was one Petri dish with fifty seeds. Experimental units were arranged as a factorial in a completely randomized design with three replications. The germinated seeds were transferred to 20 °C for further germination or sowing. The seeds stratified in the presence of distilled water served as a control. The seeds were also stratified with some modification, i.e., in the presence of potassium nitrate (KNO3; 0.2%), hydrogen peroxide (H2O2), nitric oxide (NO), and smoke-water (SW). NO was obtained by applying sodium nitroprusside (SNP) at the concentration of 5 mM for 3 h [15]. The seeds were also subjected to warm–cool stratification (20/3 °C), which was carried out at a moderate temperature (one day, 20 °C), and the next day at a low temperature (3 °C) for the first month, and then at a constant temperature of 3 °C for 12 weeks. All the treatments were performed at the same time. Saskatoon seeds germinate in low temperatures without moving them to 20 °C. Thus, the germination of the seeds was conducted at 3 °C. Saskatoon seeds belong to species that tolerate cool temperatures and germinate over a wide range of temperatures from 3 to 30 °C [16].
The other part of the seeds was subjected to physical treatments: mechanical scarification using sandpaper, chemical scarification (95% sulfuric acid, treatment time: 10, 20, 30, 40 min), exposure to red light (600–70 nm, Philips TLD 36W/5 lamps) or pulsed radio frequency (PRF). Mechanical scarification was carried out manually by rubbing the seeds against the rough surface of the sandpaper (100 grit) until the seed coat was damaged. The seeds were then transferred back to 3 °C. The seeds were then sown onto two layers of filter paper wetted with distilled water and transferred to darkness (constant temperature of 3 °C). The seeds subjected to red light were irradiated for 7 days followed by stratification at 3 °C in the presence of distilled water.
2.3. Pulsed Radio Frequency (PRF) Treatments
Pulsed radio frequency (PRF) was applied in three treatments for 20, 60 and 120 min at a voltage of 5, 25 or 50 V and a pulse repetition rate of 2, 4 or 8 Hz (pulses per second) and with a pulse time of 10 or 20 ms. PRF was used by applying an RFG 3C PLUS lesion generator (Radionics, Burlington, MA, USA). The generator, designed for medical applications, was a radio frequency power source equipped with pulse modulation and a provision for setting the exposure duration, pulse width, pulse repetition frequency, and the voltage supplied to parallel plate electrodes. The layers of moistened seeds were placed in a plexiglass container between two metal electrodes that adhered tightly to the seeds [11]. The experimental unit was one layer of moistened seeds transferred to one Petri dish. Each experimental run included three replications per treatment. The electrode PRF surface area was 13 × 17 cm, and the distance between the electrodes was 12 mm. The electrodes were connected to the RFG 3C PLUS lesion generator emitting the PRF with the mentioned parameters. After treatments with the PRF, the dynamics, the number of germinated seeds, the emergence of seedlings, the physiological activity and the growth of the plants were assessed [17]. PRF is commonly applied in medicine for treating various chronic pain syndromes, including neuropathic pain syndromes [18]. PRF treatment uses low-energy, short pulsations to modulate tissue characteristics [19].
2.4. Preparation of Smoke-Water (SW)
A smoke-water solution was obtained by igniting 5 kg of dry grass leaves (gathered from a grass field in Central Poland) in a 20 L specially designed furnace (length: 70 cm, breadth: 35 cm; height: 44 cm). Using compressed air, the smoke was bubbled through distilled water (500 mL) for 30 min (the water was yellow). The smoke extract was filtered through Whatman No. 1 filter paper and used as the stock solution. The stock smoke solution was diluted in the ratios 1:1, 1:10 and 1:100 with deionized water.
2.5. Percentage and Dynamics of Seed Germination and Mean Germination Time
The germinated seeds were counted every 5–7 days to estimate the percentage and dynamics of germination and the mean germination time (MGT). A seed was recorded as germinated when the radicle emerged from the pericarp. Seeds from each experimental variant were laid out in three replications of 50 seeds in each, in Petri dishes on distilled water (served as a control) or compound-moistened filter paper (Chmes) with a diameter of 85 mm, a pH of 7.0 and a weight of 250 g·m−2.
The germination rate, measured for seeds germinating after stratification, scarification and other treatments, was expressed as mean germination time (MGT), i.e., the average number of days required for the seeds to germinate. T1 was regarded as the beginning of seed germination, i.e., the time to reach one percent of germination [20,21]. T75–T25 was considered as germination uniformity, defined as the time from T75 to T25 (days), whereas T75 was the time to reach 75% of the final germination, and T25 was the time to reach 25% germination. The parameters T1 and T75–T25 were analyzed by means of the germinator package [20]. It is a simple, highly cost-efficient and flexible procedure for the high-throughput automatic scoring and evaluation of germination that can be implemented without the use of complex robotics. The full germinator package (for Windows operating systems) is freely available to the scientific community. It can be easily downloaded from the website (https://www.wur.nl/en/Research-Results/Chair-groups/Plant-Sciences/Laboratory-of-Plant-Physiology/Wageningen-Seed-Lab/Resources/Germinator.htm/ (accessed on 1 January 2010)). This website also contains a full manual and video demonstrations about the use of the various modules.
2.6. The Dynamics of Seedlings Growth (Initial Plant Growth)
After stratification, the germinating seeds were sown individually in the pots containing a medium consisting of peat and sand mixed in a proportion of 1:1 (v/v). The pots were transferred to greenhouse conditions. The seeds were sown only once at the same time. The seeds sowing into the pots took place at the same time. The plant height was measured twice (on 1st August and 1st September) from the soil surface to the top of the plant with a ruler. On both dates, 10 randomly chosen individuals in the middle rows were measured in three replicates.
2.7. Chlorophyll Content Index (CCI) and Quantum Efficiency of Photosystem II (ΦPSII)
The chlorophyll content index (CCI) in leaves was evaluated non-destructively using a portable Minolta SPAD-502 chlorophyll meter (Konica Minolta, Sensing, Inc, Japan). The results were expressed in SPAD units [22]. It is a handheld apparatus equipped with two light-emitting diodes and a silicon photodiode receptor that measures leaf transmittance in the red (650 nm—the measuring wavelength) and infrared (940 nm—a reference wavelength used to adjust for non-specific differences between samples) regions of the electromagnetic spectrum. The device uses transmittance values to derive a relative SPAD meter value (typically between 0.0 and 50.0) proportionally to the amount of chlorophyll in the sample [23]. Before measurement, the SPAD-502 m was calibrated using the reading checker supplied by the manufacturer [24,25]. The readings were measured from the different treatments at the midpoint on the topmost fully expanded leaves. Three readings, each from a different leaf, were taken per plant and then averaged as a single observation value for the plant. Ten plants were selected for one repetition. The experiment was performed in three repetitions.
Five readings were recorded between the base and the apex of each leaf blade. During measurements with the meter, the sensor head was shaded with the operator’s own body as recommended by the manufacturer to avoid direct sunlight from reaching the instrument.
The effective quantum yield of PSII photochemistry (ΦPSII) for a light-acclimated sample was calculated as (Fm’−Fs)/Fm’ according to the report [26] and was measured using a portable Pulse Amplitude Modulation (PAM) Chl fluorometer (FMS1, Hansatech Instruments Ltd., King’s Lynn, Norfolk, United Kingdom). ΦPSII is directly associated with the rate of electron transfer in PSII toward biochemical processes [27]. The measurements of ΦPSII were conducted on fully expanded leaves, and the measurements were taken at the same time of day. The fiber optic of the FMS-1 was positioned using the PPF/temperature leaf clip at a 60° angle from the upper surface of the leaf, and the distance between the leaf surface and the fiber optic was kept constant for all measurements.
2.8. Statistical Analyses
The experiments for the determination of the number of germinated seeds, mean germination time and seedling growth were performed in three replications. The PRF treatments, the chlorophyll content index (SPAD) and the effective quantum yield of PSII photochemistry (ΦPSII) were repeated four times. The least significant differences (LSD) were calculated at the level of p = 0.05 for all experimental data. The results were statistically analyzed by one-way analysis of variance in a random block design. Multiple comparisons of means for the combinations were performed with Tukey’s test at a significance level of α = 0.05 using STATISTICA v.13.1 software (StatSoft, Inc., Tulsa, OK, USA).
3. Results
3.1. Seed Germination of the Saskatoon berry after Stratification
Saskatoon berry seeds of cv. ‘Martin’ did not germinate at 20 °C for six months. The temporary failure of seeds to complete germination indicates that they were dormant. However, the seed dormancy was released after the seeds had been subjected to standard stratification (at 3 °C in the presence of H2O for 5 months). The breaking of the seeds’ dormancy significantly depended on the date of their extraction from the fruits, i.e., directly after harvest (unstored) or after storage at −18 °C for at least 6 months (until the end of seed germination).
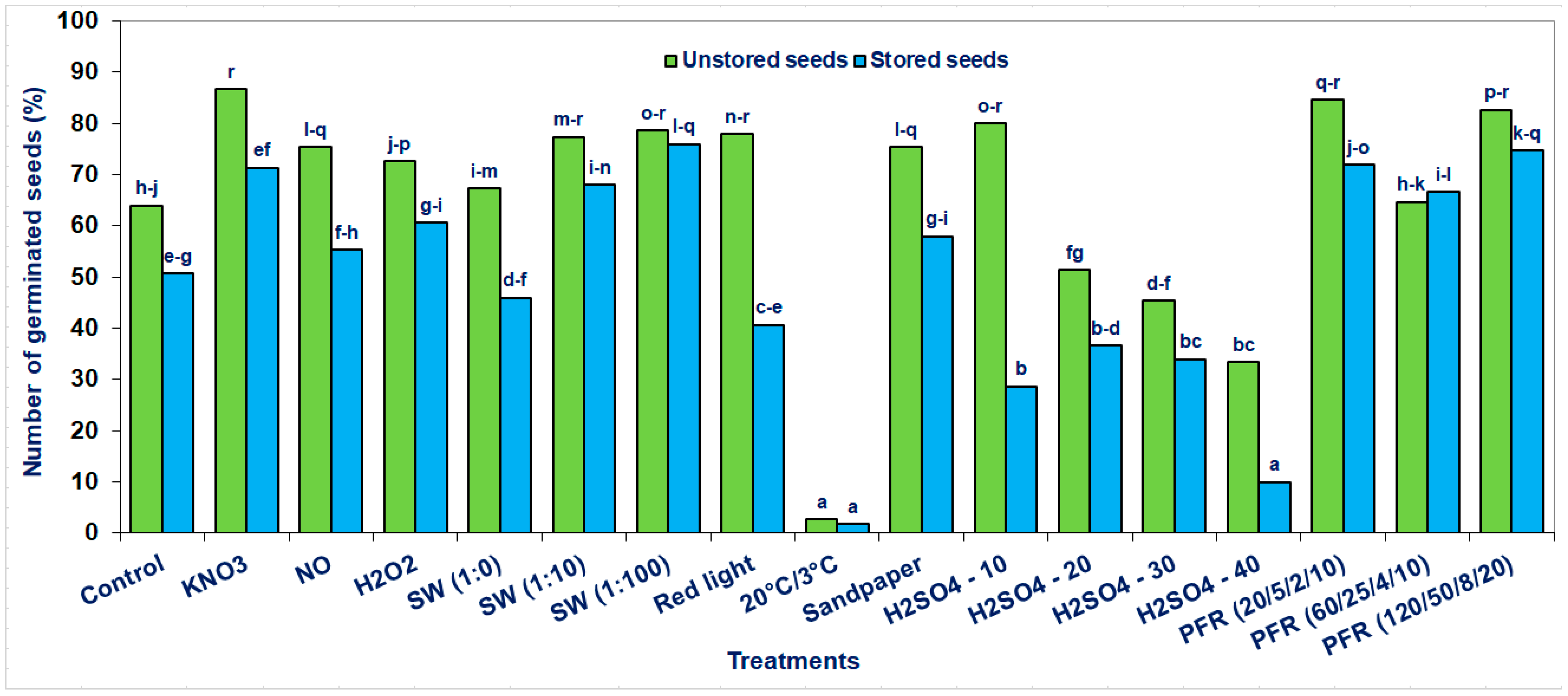
Unstored seeds germinated at a significantly higher percentage (13) in comparison with the stored ones when we take into consideration overall effects (Table 1). A similar difference was (13%) when comparing the unstored and then stratified in water (control) seeds with stored ones (Figure 2).

Table 1.
Overall effects of unstored seeds and seeds stored at −18 °C for 6 months on seed germination (%) and seedling height, Skierniewice, Poland, 2019.
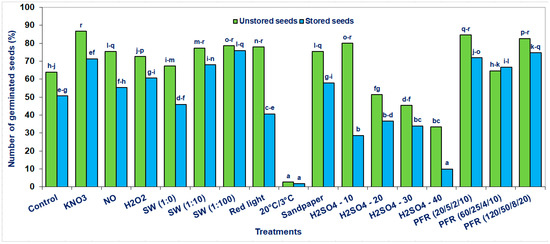
Figure 2.
Seed germination of the Saskatoon berry after stratification (3 °C) in the presence of KNO3, NO, H2O2, smoke-water (SW, in concentrations of 1:1, 1:10, 1:100), or scarification: sandpaper, sulfuric acid for 10, 20, 30 and 40 min or treatments with pulsed radio frequency (PRF, 20/5/2/10, 60/25/4/10, 120/50/8/20), red light and 20 °C/3 °C. The seeds were harvested directly from fruits (unstored) and stored at −18 °C for 6 months. The data within the graph and the color of the bar and with the same letter do not differ significantly according to Tukey’s test (5%). Values are the means of three replications of 50 seeds.
The most advantageous effects concerned seeds directly extracted from the fruit after harvest (unstored) and then subjected to stratification in the presence of KNO3 or treatments with PRF (20/5/2/10 and 120/50/8/20) or scarification with sandpaper, H2SO4 for 10 min or stratification with SW (1:100). Depending on the applied methods, the percentage of seed germination increased to 86.7% compared to untreated (64%) control seeds (Figure 2). In the case of stored seeds, the most pronounced methods to overcome the Saskatoon berry seed dormancy were observed after stratification with SW (1:100), PRF (120/50/8/20, 20/5/2/10 and 60/25/4/10) and KNO3. Depending on the method used, the most significant increase in the percentage of seed germination was 76%, compared to control seeds stratified in distilled water (51%).
3.2. Dynamics of Saskatoon berry Seed Germination
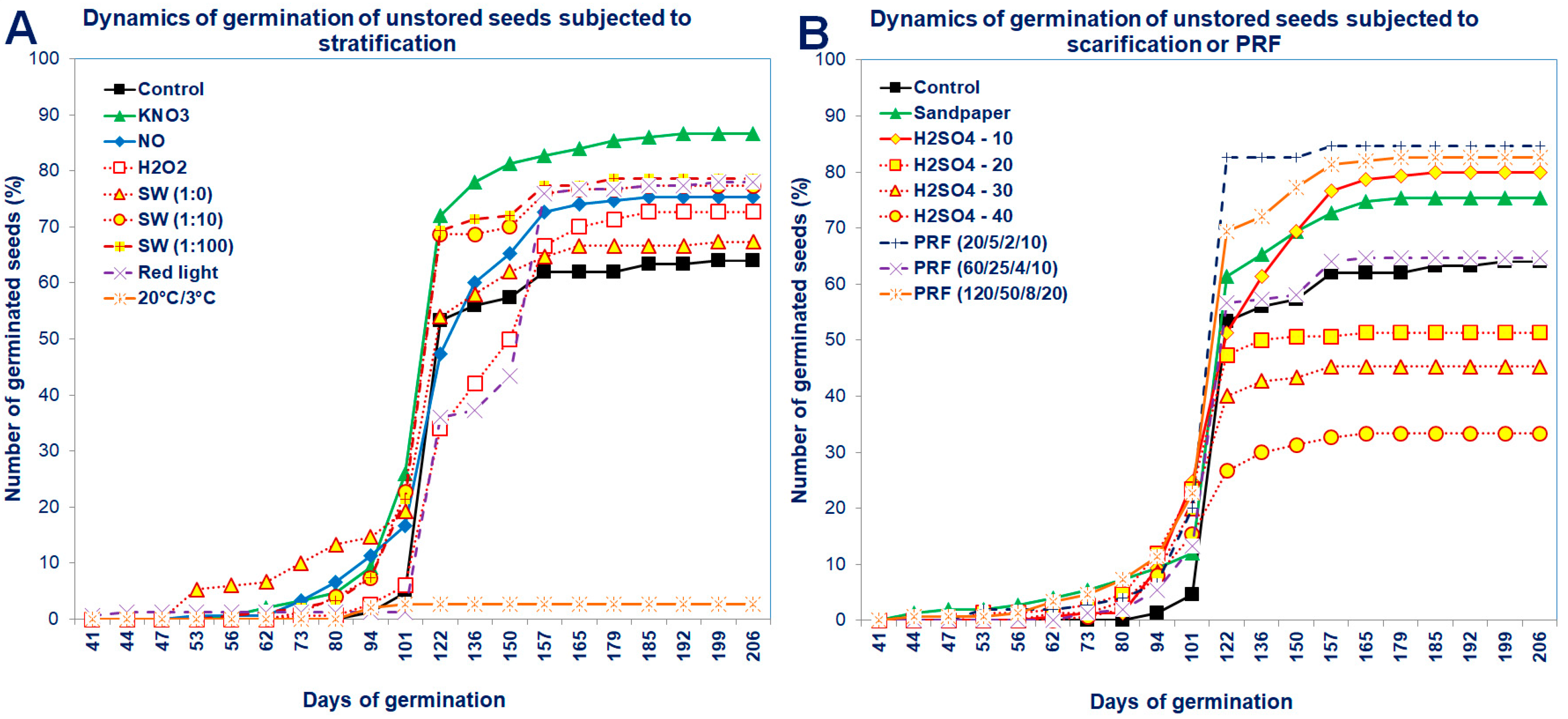
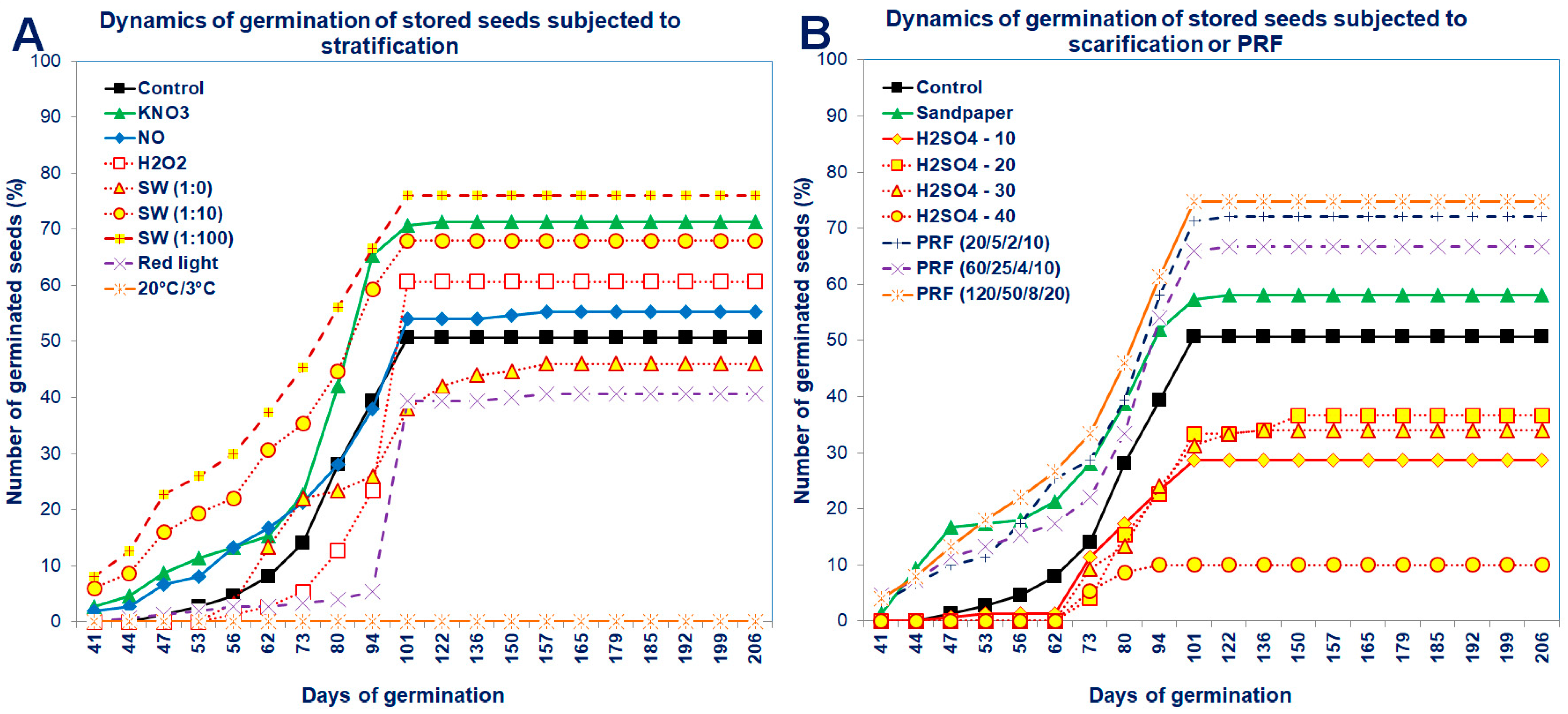
The dynamics of seed germination clearly showed that unstored seeds germinated in a higher percentage, although the beginning of their germination was delayed compared to stored seeds (Figure 3 and Figure 4). After standard stratification, the Saskatoon berry seeds germinated in a low percentage (at 63%), relatively late after sowing (98 days) and for a long time (approximately 40 days from the beginning of seed germination; Figure 3 and Figure 4).
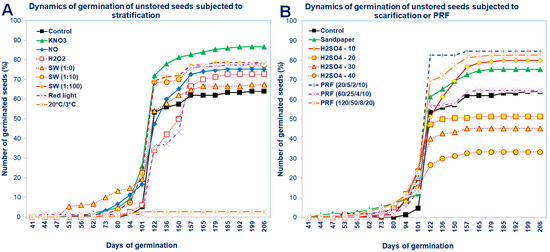
Figure 3.
Dynamics of germination of the unstored Saskatoon berry seeds subjected to (A) stratification (3 °C) in the presence of KNO3, NO, H2O2, smoke-water (SW, in concentrations of 1:1, 1:10, 1:100) and 20 °C/3 °C or (B) scarification: sandpaper, sulfuric acid for 10, 20, 30 and 40 min or treatments with red light or pulsed radio frequency (PRF; 20/5/2/10; 60/25/4/10; 120/50/8/20). Values are the means of three replications of 50 seeds.
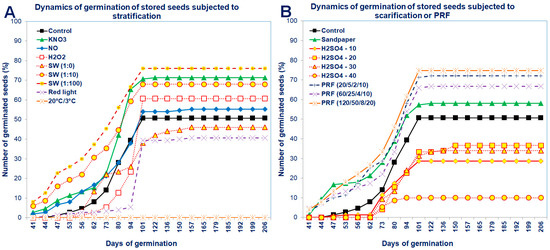
Figure 4.
Dynamics of germination of the stored Saskatoon berry seeds subjected to (A) stratification (3 °C) in the presence of KNO3, NO, H2O2, smoke-water (SW, in concentrations of 1:1, 1:10, 1:100), or 20 °C/3 °C or (B) scarification: sandpaper, sulfuric acid for 10, 20, 30 and 40 min or treatments with red light or with pulsed radio frequency (PRF, 20/5/2/10, 60/25/4/10, 120/50/8/20). Values are the means of three replications of 50 seeds.
The release of seed dormancy was clearly dependent on the method of seed stratification or scarification or the application of other physical treatments (red light or PRF). The applied methods predominantly contributed to a more efficient dormancy release.
3.3. The Onset of Seeds Germination
The storage of seeds at −18 °C for 6 months positively affected the onset of seed germination (T1, Table 1). Due to such treatments, the stored seeds started germination over 51 days earlier than the unstored ones, compared to stratified in water controls (Table 2). T1 was also significantly affected by the modified stratification, scarification, and other physical treatments (red light or PRF). The improvement of this parameter due to the application of these methods was more pronounced for unstored seeds than for stored seeds. The most favorable results for unstored seeds were obtained after seed scarification with sandpaper, stratification with SW (1:0) or treatments with PRF (120/50/8/20; Table 2, Figure 4). After these treatments, germination started 55, 50 and 47 days earlier, respectively, compared to unstored control seeds (T1 = 103 days from seeds sowing). For stored seeds, the most pronounced results were obtained after scarification in the presence of KNO3, SW (1:10 and 1:100), NO or scarification with sandpaper or treatments with PRF (20/5/2/10, 60/25/4/10 and 120/50/8/20). Due to such treatments, the seeds started germination 9 days earlier than stored control seeds.

Table 2.
T1, T75–T25 and MGT of unstored and stored Saskatoon seeds stratified in the presence of distilled water (control), KNO3, NO, H2O2, smoke-water or warm–cool stratified (20/3 °C) in distilled water or subjected to red light treatment or scarified (sandpaper, sulfuric acid for 10, 20, 30 and 40 min) or subjected to pulsed radio frequency (PRF, 20/5/2/10, 60/25/4/10, 120/50/8/20).
3.4. Uniformity (T75–T25) of Seeds Germination
The positive response of unstored seeds also concerned the uniformity of seed germination (T75–T25). Lower values of this parameter indicate better uniformity performance [20]. In the present study, the parameter for the unstored control seeds was lowered by 8.34 days compared to the stored one (Table 2). The storage of seeds in fruits at −18 °C for 6 months negatively affected the uniformity of germination (T75–T25; Table 1). In this respect, it was more advantageous and cheaper when Saskatoon berry seeds were directly extracted from fruits (unstored) and then subjected to the selected methods of breaking dormancy (Table 1). The best results were obtained when unstored seeds were treated with PRF (20/5/2/10). Due to the treatment, T75–T25 was reduced by 1 day compared to the control (untreated seeds). In the case of stored seeds, a positive effect was evident mainly when seeds were stratified in the presence of red light, H2O2, or when scarified in H2SO4 for 40 min. Due to these treatments, T75–T25 was improved by 17, 10 and 14 days, respectively.
3.5. Mean Germination Time
The overall data indicated that the seeds stored in fruits at −18 °C for 6 months beneficially affected mean germination time (MGT, Table 1). The storage conditions accelerated MGT by 37 days in comparison with the unstored seeds. MGT was also significantly affected by the modified stratification, scarification or treatments with PRF (Table 2). The most noticeable results were observed when stored seeds were stratified in the presence of SW (1:10, 1:100) or scarified by sandpaper (Table 2, Figure 3 and Figure 4). After such treatments, MGT was accelerated by 11, 14 and 10 days, respectively, compared to the control. In the case of unstored seeds, MGT was mainly improved provided they were subjected to stratification at 20 °C/3 °C or scarification with H2SO4 for 20 or 30 min. Due to these treatments, the seeds germinated on average 30, 16 and 14 days earlier, respectively, than the control seeds.
3.6. The Seedlings Height
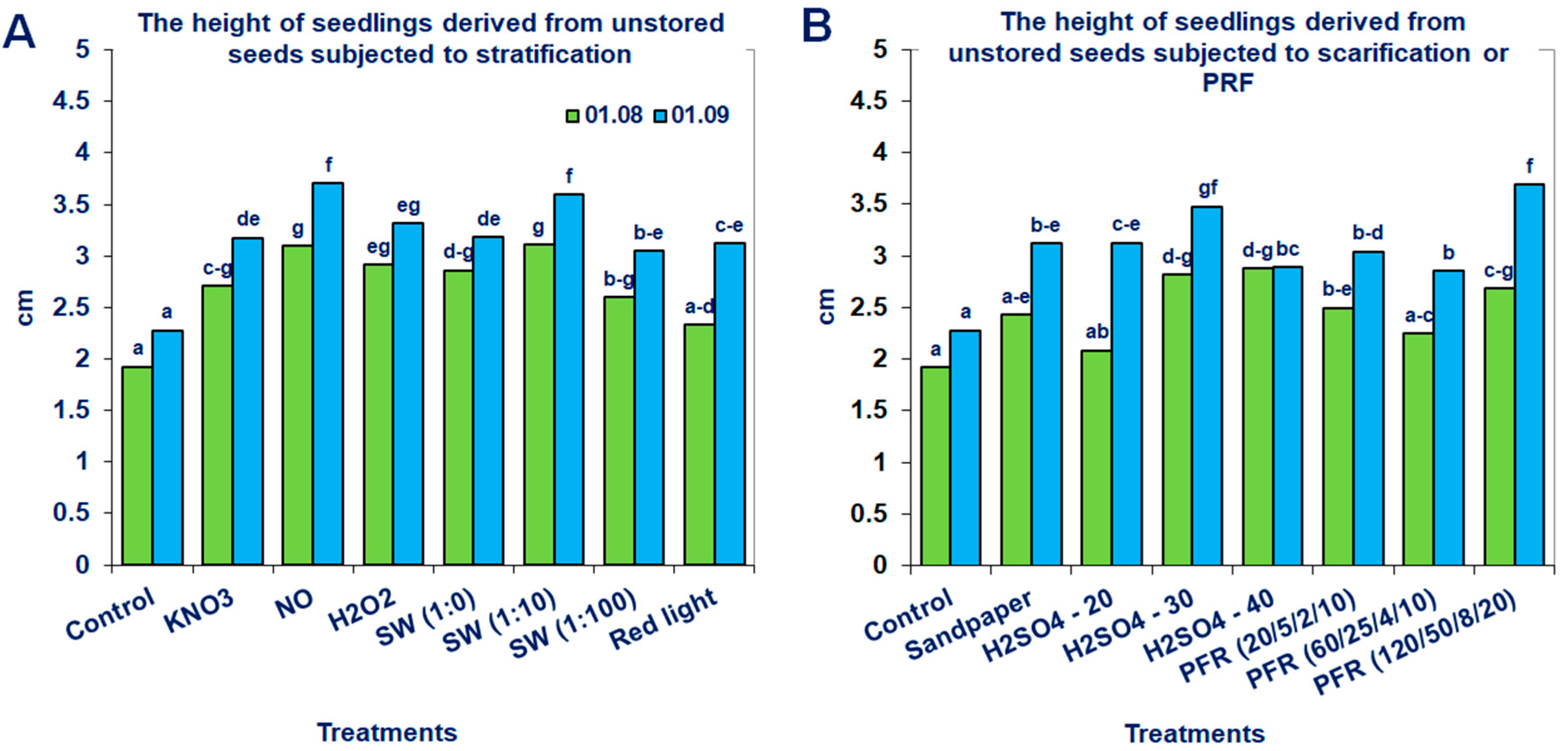
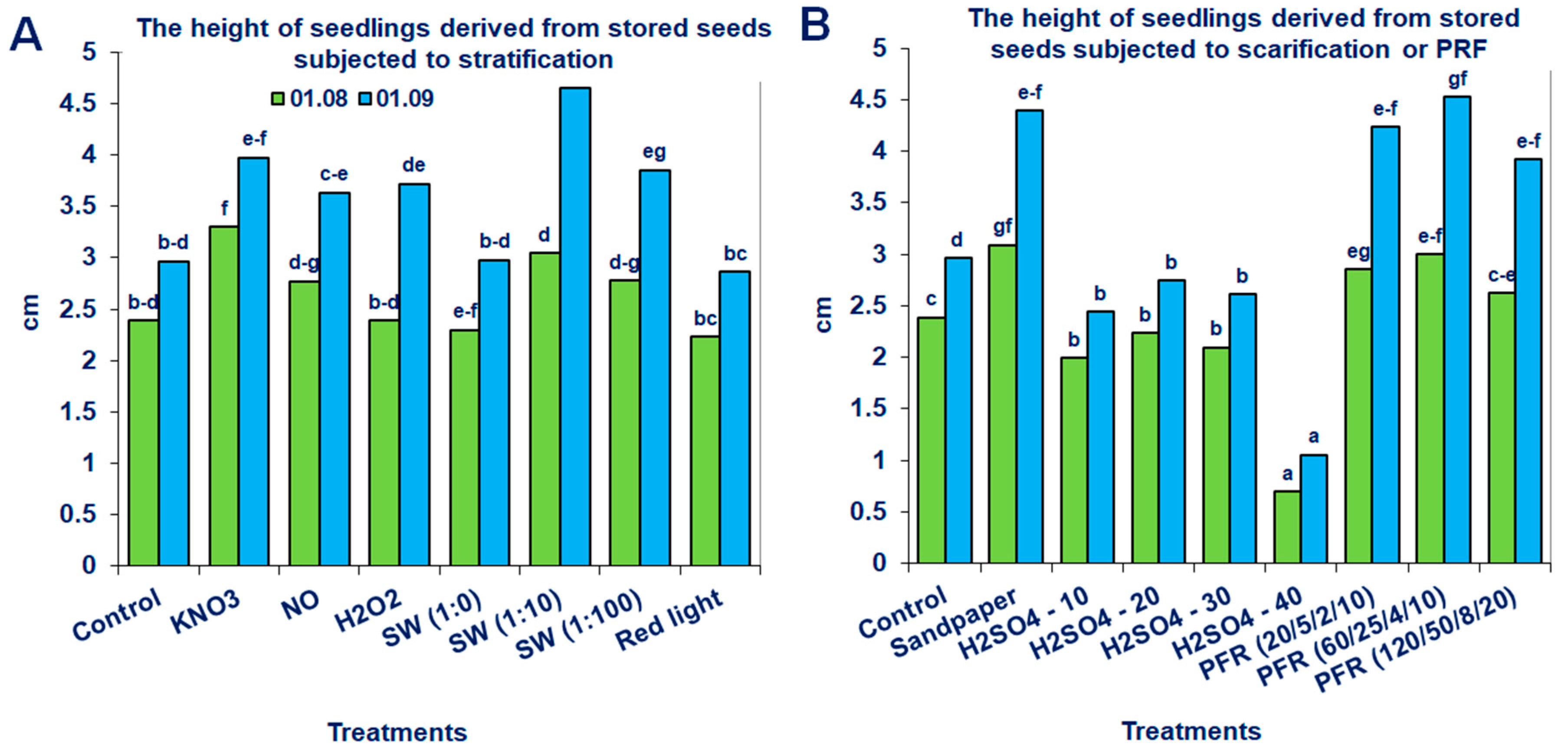
Neither the non-storage nor the storage of seeds in fruits at −18 °C for 6 months significantly promoted the height of the produced seedlings of the tested Saskatoon berry cultivar (Table 1). However, the application of modified stratification, scarification or treatments with PRF substantially affected this parameter (Figure 5 and Figure 6). The most beneficial effects were observed when the stored seeds were treated with PRF (20/5/2/10 or 60/25/4/10), scarified with sandpaper or stratified with SW (1:10; Figure 6). Due to these treatments, the height of the seedlings increased by 43, 52, 48 and 57%, respectively. The positive effects of the applied methods were also observed for unstored seeds. The most noticeable results were obtained when they were subjected to stratification in the presence of NO, SW (1:10), scarification with H2SO4 (30 min) or treatments with PRF (120/50/8/20; Figure 5). After these treatments, the growth of the evaluated seedlings measured on 1 September increased by 63, 58, 53 and 62%, respectively.
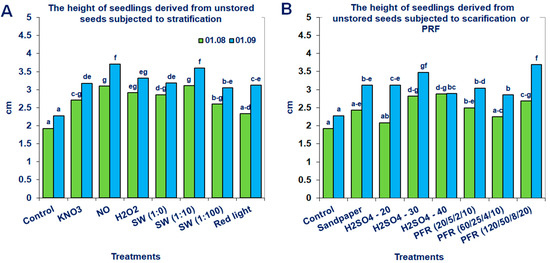
Figure 5.
Saskatoon berry seedling heights derived from unstored seeds subjected to (A) stratification (3 °C) in the presence of KNO3, NO, H2O2, smoke-water (SW, in concentrations of 1:1, 1:10, 1:100), red light and 20 °C/3 °C or (B) scarification: sandpaper, sulfuric acid for 10, 20, 30 and 40 min, or treatments with pulsed radio frequency (PRF, 20/5/2/10, 60/25/4/10, 120/50/8/20). The data within the graph (A or B) and the color of the bar and with the same letter do not differ significantly according to Tukey’s test (5%). Values are the means of three replications of 50 seeds.
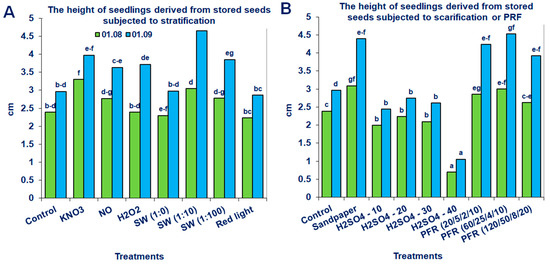
Figure 6.
Saskatoon berry seedling heights derived from stored seeds subjected to (A) stratification (3 °C) in the presence of KNO3, NO, H2O2, smoke-water (SW, in concentrations 1:1, 1:10, 1:100), red light or 20 °C/3 °C or (B) scarification: sandpaper, sulfuric acid for 10, 20, 30 and 40 min, or treatments with pulsed radio frequency (PRF, 20/5/2/10, 60/25/4/10, 120/50/8/20). The data within the graph (A or B) and the color of the bar and with the same letter do not differ significantly according to Tukey’s test (5%). Values are the means of three replications of 50 seeds.
3.7. Chlorophyll Content Index
The chlorophyll content index (SPAD) measured in the leaves of the Saskatoon berry seedlings was positively affected by modified stratification, scarification and seed treatments with PRF (Table 3). The effect was more prominent for seeds stored in fruits at −18 °C for 6 months than for the immediately harvested seeds (unstored). The most beneficial results were observed when stored seeds were scarified with sandpaper, treated with PRF (20/5/2/10) or stratified with SW (1:0). After these treatments, this parameter increased by 23.9, 19.1 and 18%, respectively. The same methods of treatment were also the most effective regarding unstored seeds.

Table 3.
Index of chlorophyll content (SPAD) and effective quantum yield of PSII (ΦPSII) of Saskatoon berry seedlings derived from unstored and stored seeds subjected to (A) stratification (3 °C) in the presence of KNO3, NO, H2O2, smoke-water (SW, in concentrations of 1:1, 1:10, 1:100), red light or 20 °C/3 °C or (B) scarification: sandpaper, sulfuric acid for 10, 20, 30 and 40 min, or treatments with pulsed radio frequency (PRF, 20/5/2/10, 60/25/4/10, 120/50/8/20).
3.8. The Effective Quantum Yield of PSII
The effective quantum yield of PSII (ΦPSII) was also significantly affected by the applied methods of breaking seed dormancy (Table 3). This concerned both unstored and stored seeds. The most beneficial results were observed when stored seeds were subjected to stratification with SW (1:10), H2O2 or scarification with H2SO4 (10 and 20 min), or treatments with PRF (20/5/2/10 and 120/50/8/20). In the case of seed stratification with SW (1:10), ΦPSII was increased by 25.4% compared to the control untreated seeds.
The obtained research indicated that Saskatoon berry seed dormancy release is accelerated by applying chosen methods of modified stratification, scarification, and pulsed radio frequency (PRF). Moreover, Saskatoon berry seed dormancy breakage is significantly dependent on the date of seed extraction from fruits.
4. Discussion
Seed germination is affected by external factors, including light, temperature, water and oxygen supply, nutrients, pathogens and toxins. It can also be affected by endogenous factors, including the stage of seed ripeness, hormone levels and seed dormancy [28]. The latter factor is common in many species of trees and shrubs.
Copeland and McDonald [29] stated that plants with a long history of domestication generally showed less seed dormancy than wild or more recently domesticated species. The Saskatoon berry seeds showed the phenomenon of dormancy [10,30], which allowed supposing that the process of the domestication of that species was relatively short. The occurrence of seed dormancy in the Saskatoon berry generates problems for seed producers, their customers, seed analysts and practical breeding programs, significantly prolonging this process. The latter problem also existed in breeding programs of apple trees. Previous research showed that seeds harvested from apples were in a state of dormancy, interpreted as a set of blocks imposed on a process or processes substantial for growth [11,31,32]. In order to overcome the mentioned blocks and accelerate the rate and improve germinability in the present study, some Saskatoon berry seeds were directly extracted from fruits after harvest (unstored seeds) and then subjected to stratification, scarification or treatments with other physical methods. Other seed lots were extracted from fruits after storage at −18 °C for 6 months and then subjected to the treatments mentioned above.
The presented data revealed that the date of seed extraction from the Saskatoon berry fruits determined their germinability (%), T75–T25, T1 and MGT. The storage of seeds at −18 °C for 6 months negatively affected germinability and T75–T25, and positively influenced T1 and MGT (Table 1 and Table 2). After the storage of seeds, dormancy release began 51.33 days earlier than in unstored seeds. As Saskatoon berry comes from the Prairie Provinces of Canada and northern plains in the USA, where matured fruits with seeds inside naturally experience exposure to low temperatures [2,3], seeds exposed to temperatures around and below 0 °C probably partly contributed to the dormancy release because of the origin of this species. This species also determines the tolerance of seeds with increased moisture content to freezing temperatures. Similarly, seeds of other species with up to 32% moisture content are resistant to freezing temperatures and can be stored in these conditions for several months [29]. However, after the storage of the Saskatoon berry seeds, they germinated at a level of 51%, and the end of this process occurred within 101 days from sowing. For the purpose of breeding programs, this is still not satisfactory.
Although the stored seeds showed improved T1 and MGT in comparison with unstored ones, the breeding program of new varieties needs to obtain as many healthy seedlings as possible. Moreover, the storage of seeds in the fruits at −18 °C for 6 months increases the cost of the entire procedure. However, these conditions are recommendable for the storage of Saskatoon berry fruits when needed. It is recommended to extract the seeds directly from fruits (unstored) because, after such treatments, the breeders will receive more seedlings. However, better T1 and MGT parameters of stored seeds in comparison to unstored seeds provoke further research in terms of increasing the percentage of germinated seeds and obtaining a larger number of seedlings.
In order to overcome dormancy more effectively, the seeds were subjected to modified stratification (exposure to low temperatures in the presence of compounds), mechanical and chemical scarification or treatments with red light or PRF or warm–cool stratification (20/3 °C). The present study showed that the most advantageous effects of dormancy breakage, with respect to germinability (%), were obtained when unstored seeds were stratified in the presence of KNO3 (0.2%), SW (1:10 and 1:100), NO or scarified with sandpaper or H2SO4 for 10 min or treated with red light or PRF (20/5/2/10 or 120/50/8/20) (Figure 2). Potassium nitrate (KNO3) is a widely used compound to promote germination and break seed dormancy. The concentration of 0.2% KNO3 is commonly recommended by the International Seed Testing Association (ISTA) and the Association of Official Seed Analysts (AOSA) for germination tests of many species [33]. The stimulatory effect of KNO3 application on dormancy breakage can be associated with improving cell wall permeability, thereby increasing the level of cellular metabolism and enzyme activity [34]. It can also be related to extending water absorption by inducing changes in water potential, promoting metabolic activity and auxin biosynthesis in the seeds [35] or changes in the levels of some phytohormones, thereby releasing physiological dormancy [34,36,37]. Grzesik et al. [11] reported that, due to stratification in the presence of KNO3 (0.2%), the dormancy of apple seeds considerably decreased, which was confirmed by the increased percentage and accelerated onset and the end of seed germination. Moreover, seed treatment with KNO3 during stratification significantly increased the physiological activity of seedling cells and tissues, which was expressed by the more significant index of chlorophyll content in the leaves, the activity of stomatal conductance, net photosynthesis, transpiration, and lower intercellular CO2 concentration [11]. Khan et al. [38] reported that this compound might contribute to auxin biosynthesis, ultimately triggering the growth of the embryo. Like in other studies, it can be concluded that KNO3 contributed to a more effective stratification and acted cooperatively or synergistically with stratification.
The present research indicated that stratification in the presence of hydrogen peroxide (H2O2) positively affected dormancy release. It was mainly expressed by improving the germination uniformity of stored seeds (T75–T25) (Table 2). Furthermore, this compound promoted further seedling growth (Figure 2). The findings are in agreement with another study, where exogenously applied H2O2 significantly increased early germination rates of sweet cherry seeds [39]. It has also been found that this compound promotes germination in other species both by oxidizing germination inhibitors and disintegrating hard seed coats. Hydrogen peroxide is also frequently used to sterilize the surface of seeds [30].
The present study showed that the stratification of unstored seeds in the presence of nitric oxide (NO) beneficially affected their germinability (%) and the further growth of seedlings. Moreover, it increased significantly also the onset of their germination (T1). A previous study with apple seeds indicated that NO was one of the most efficient compounds in dormancy breaking [11,17]. Similarly, Arc et al. [40] reported that NO was a potent dormancy-releasing agent in many species, including Arabidopsis. It was suggested to behave as an endogenous regulator of the physiological blockage of seed germination. Furthermore, NO efficiently counteracted the positive effect of abscisic acid (ABA) on seed dormancy maintenance by reducing ABA levels. Signorelli and Considine [41] demonstrated that NO interferes with the ABA signaling network at multiple steps, providing germination control.
Smoke-water (SW) also beneficially acted on the dormancy release of the Saskatoon berry seeds. Its application, together with stratification, positively affected the onset and percentage of seed germination, MGT, the further growth of seedlings, the index of chlorophyll content (SPAD) and the effective quantum yield of PSII (ΦPSII). Alahakoon et al. [42] reported that due to SW treatment, the germination percentage of many species was significantly increased, and the time taken for 50% germination was decreased. Cembrowska-Lech and Kępczyński [43] presented that karrikin 1 (KAR1), a key bioactive chemical present in smoke derived from plants, was involved in ABA degradation to phaseic acid and thereby induced almost the complete germination of dormant Avena fatua caryopses. Positive effects of SW diluted in the ratio 1:10 were maintained after seed sowings expressed by higher seedling height. This concerned both seedlings derived from unstored and stored seeds.
The presented study on the Saskatoon berry seeds clearly showed that PRF accelerated MGT and the onset of seed germination, increased the percentage of seed germination, further seedling growth, the index of chlorophyll content (SPAD) and the effective quantum yield of PSII (ΦPSII). Previous studies on apple seeds and some vegetable plant species also showed that pulsed radio frequency (PRF) positively contributed to dormancy alleviation [11,17]. Other studies revealed a reduction in the incidence of beetroot, lettuce and garden dill seed-borne fungi and improved their health status and germination capacity [17]. PRF produced by an RFG 3C PLUS lesion generator has been applied in medicine in the treatment of chronic pain. As this technique is employed to treat pain, movement, and mood disorders, it would probably have an advantageous influence on crucial blocked processes involved in the Saskatoon berry seeds’ dormancy removal. Its effectiveness depends on the chosen parameters: i.e., time of exposure, pulse repetition rate (pulses per second—Hz), pulse duration (ms) and voltage (V). Based on the former experiment, the seeds were subjected to exposure to PRF for 20, 60 and 120 min at a constant voltage of 5, 25 or 50 V, pulse repetition rates of 2, 4 or 4 Hz (pulses per second) with a pulse duration of 10 or 20 ms. It was found that biological changes in tissues during PRF exposure could appear as a result of the thermal effects or high intensity of electric fields or as a result of both [44]. However, the chosen parameters did not generate any heat in the present study. Thus, it should be assumed that PRF caused only the high intensity of electric fields. Electric fields could significantly affect cells because of the transmembrane potentials that they induce [12]. Although the mechanism of action of PRF was not fully understood, it was proved that it successfully contributed to the seed dormancy removal of Saskatoon berry.
From the present study, it is obvious that Saskatoon berry seeds exhibited physiological dormancy which was overcome by stratification in KNO3 (0.2%) or with SW (1:100) or by treatments with PRF (20/5/2/10 or 120/50/8/20). Rosner and Harrington [30] have also concluded that seeds of North American shrub species exhibit physiological embryo dormancy which can be overcome by stratification (moist prechill). However, the developed methods of breaking the dormancy of Saskatoon berry seeds can be nonrepeatable and connected with a genetic basis for variation as well as the significant population–year interaction effect [10]. According to Baskin and Baskin [45] the seeds of Saskatoon berry can be classified to a deep level of regular physiological dormancy, which can be broken by 12–24 weeks periods of cold stratification.
The obtained results indicated also that Saskatoon berry seed dormancy may also be partially associated with seed coat impermeability, which was released by scarification with sandpaper or H2SO4 for 10 min. These findings are in agreement with Hilton and Thomas’ [46] results. Physical dormancy could be associated with a greasy substance exuded from seeds during their imbibition. This sticky substance may partly contribute to limiting the access of oxygen and water to the seed embryo, as it forms a thick gelatinous barrier. Thereby, physiological dormancy is not the only dormancy mechanism, but also physical mechanisms are needed to break seed coat impermeability. The scarification of Saskatoon berry seeds in H2SO4 for longer than 10 min did not accelerate the seed dormancy release. Longer treatment with H2SO4 damaged not only the seed coat but also the endosperm layers of the seeds.
It is worth emphasizing that stratification with SW (1:0), scarification with sandpaper or treatments with PRF not only positively exerted dormancy release parameters but also the physiological properties of the seedlings obtained from the treated seeds. The positive changes concerned the index of chlorophyll content (SPAD) and the effective quantum yield of PSII (ΦPSII). Hawkins et al. [47] confirmed that SPAD-502 is an efficient tool for the non-destructive assessment of total foliar chlorophyll content and concentration across a range of plant ages, growing conditions and genotypes. The meter has been widely used in scientific research and agriculture, and many publications in the scientific literature describe its application [48]. The present research indicated that the positive effects of applying the selected methods on seed dormancy release were also maintained during further seedling growth and manifested by enhanced SPAD in leaves.
The chlorophyll fluorescence signal is susceptible to environmental influences and reflects the changes in photosynthesis [49]. The effective quantum yield of PSII (ΦPSII) was a real yield of active PSII reaction centers in converting absorbed light energy [50]. ΦPSII represented the fraction of the light energy used in photochemistry [26]. On the other hand, ΦPSII reflected the actual efficiency of photosynthesis [26]. From the present study, it was clearly evident that the ΦPSII in the leaves was significantly promoted by stratification with SW (1:0) or H2O2 or scarification by H2SO4 (for 10 and 20 min) or treatments with PRF (20/5/2/10 and 120/50/8/20). Due to these treatments, ΦPSII in chlorophyll was markedly enhanced.
The obtained results indicated also that the selected treatments not only accelerate and increase the germination of Saskatoon berry seeds but also positively affect the chlorophyll content and the functioning of the photosystem in leaves.
5. Conclusions
In recent years, there has been growing interest in developing new varieties of Saskatoon berries that are better adapted to different growing conditions and have improved fruit quality and yield. For practical application, it is recommended to break physiological dormancy by extracting the seeds directly from the fruits after harvest (unstored) and then subjecting them to stratification in the presence of KNO3 or SW (1:100). The seeds also exhibited physical dormancy. Further research is needed to elaborate if the combination of selected stratification methods with scarification with sandpaper or H2SO4 can additionally bring greater benefits for breeding programs.
The application of selected stratification, scarification and other treatments showed the improvement in the percentage, rate, onset and uniformity of Saskatoon berry seed germination, increasing the potential for obtaining new plants for breeding purposes. By using the selected methods of seed treatments, breeders can increase the efficiency of the production of seedlings for further the evaluation and selection of new valuable cultivars of Saskatoon berry.
Author Contributions
Conceptualization, K.G. and S.P.; methodology, K.G.; formal analysis, K.G.; investigation, E.D., S.G., B.S. and W.F.A.M.; data curation, K.G.; writing—original draft preparation, K.G.; writing—review and editing, S.P., Ł.S. and L.S.-P.; supervision, K.G. and L.S.-P.; funding acquisition, K.G. All authors have read and agreed to the published version of the manuscript.
Funding
The research was supported the Ministry of Agriculture and Rural Development as the statutory activity of the National Institute of Horticultural Research—No ZM/3/2019.
Data Availability Statement
Data are contained within this article.
Acknowledgments
We kindly thank Bert van Duin and Mieczysław Grzesik for using RFG 3C PLUS lesion generator (Radionics, Burlington, MA, USA).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Szpadzik, E.; Krupa, T. The yield, fruit quality and some of nutraceutical characteristics of saskatoon berries (Amelanchier alnifolia Nutt.) in the conditions of eastern Poland. Agriculture 2021, 11, 824. [Google Scholar] [CrossRef]
- Lachowicz, S.; Seliga, Ł.; Pluta, S. Distribution of phytochemicals and antioxidative potency in fruit peel, flesh, and seeds of Saskatoon berry. Food Chem. 2020, 305, 125430. [Google Scholar] [CrossRef]
- Mazza, G.; Cottrell, T. Carotenoids and cyanogenic glucosides in saskatoon berries (Amelanchier alnifolia Nutt.). J. Food Compos Anal. 2008, 21, 249–254. [Google Scholar] [CrossRef]
- Bieniek, A.; Markuszewski, B.; Kopytowski, J.; Pluta, S.; Markowski, J. Yielding and fruit quality of several cultivars and breeding clones of Amelanchier alnifolia grown in north-eastern Poland. Zemdirbyste-Agriculture 2019, 106, 351–358. [Google Scholar] [CrossRef]
- Hunková, J.; Szabóová, M.; Gajdošová, A. Protocols for Adventitious Regeneration of Amelanchier alnifolia var. cusickii and Lonicera kamtschatica ‘Jugana’. Plants 2021, 10, 1155. [Google Scholar] [CrossRef]
- Remphrey, W.R.; Pearn, L.P. A comparison of seed-propagated and micropropagated Amelanchier alnifolia (saskatoon). Yield and yield components in relation to crown architecture characteristics. Can. J. Plant Sci. 2006, 86, 499–510. [Google Scholar] [CrossRef]
- Jin, A.L.; Ozga, J.A.; Kennedy, J.A.; Koerner-Smith, J.L.; Botar, G.; Reinecke, D.M. Developmental profile of anthocyanin, flavonol, and proanthocyanidin type, content, and localization in saskatoon fruits (Amelanchier alnifolia Nutt.). J. Agric. Food Chem. 2015, 63, 1601–1614. [Google Scholar] [CrossRef]
- Eisele, T.A.; Drake, S.R. The partial compositional characteristics of apple juice from 175 apple varieties. J. Food Compos. Anal. 2005, 18, 213–221. [Google Scholar] [CrossRef]
- Migut, D.; Zaguła, G.; Gorzelany, J.; Pluta, S. Content of selected minerals in the fruit of Saskatoon berry (Amelanchier alnifolia Nutt.) genotypes grown in central Poland. J. Elem. 2019, 24, 1323–1333. [Google Scholar] [CrossRef]
- Acharya, S.N.; Chu, C.B.; Hermesh, R. Effects of population, environment and their interaction on saskatoon berry (Amelanchier alnifolia Nutt.) seed germination. Can. J. Plant Sci. 1989, 69, 277–284. [Google Scholar] [CrossRef]
- Grzesik, M.; Górnik, K.; Janas, R.; Lewandowki, M.; Romanowska-Duda, Z.; van Duijn, B. High efficiency stratification of apple cultivar Ligol seed dormancy by phytohormones, heat shock and pulsed radio frequency. J. Plant. Physiol. 2017, 219, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Chua, N.H.L.; Vissers, K.C.; Sluijter, M.E. Pulsed radiofrequency treatment in interventional pain management: Mechanisms and potential indications-a review. Acta Neurochir. 2011, 153, 763–771. [Google Scholar] [CrossRef]
- Kępczyński, J. Progress in utilizing plant-derived smoke water and smoke-derived KAR1 in plant tissue culture. Plant Cell Tiss. Organ Cult. 2020, 140, 271–278. [Google Scholar] [CrossRef]
- Chumpookam, J.; Lin, H.L.; Shiesh, C.C. Effect of smoke-water on seed germination and seedling growth of papaya (Carica papaya cv. Tainung No. 2). HortScience 2012, 47, 741–744. [Google Scholar] [CrossRef]
- Gniazdowska, A.; Dobrzyńska, U.; Babańczyk, T.; Bogatek, R. Breaking the apple embryo dormancy by nitric oxide involves the stimulation of ethylene production. Planta 2007, 225, 1051–1057. [Google Scholar] [CrossRef] [PubMed]
- Luna, T.; Wilkinson, K.M.; Dumroese, R.K. Seed germination and sowing options Chapter 9. In Tropical Nursery Manual: A Guide to Starting and Operating a Nursery for Native and Traditional Plants; Wilkinson, K.M., Landis, T.D., Haase, D.L., Daley, B.F., Dumroese, R.K., Eds.; Agriculture Handbook 732; U.S. Department of Agriculture, Forest Service: Washington, DC, USA, 2014; pp. 163–183. Available online: https://www.researchgate.net/publication/272790378 (accessed on 1 January 2014).
- Janas, R.; Górnik, K.; Grzesik, M.; Romanowska-Duda, Z.; van Duijn, B. Effectiveness of pulsed radio frequency in seed quality improvement of vegetable plant species. Int. Agrophys. 2019, 33, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Vanneste, T.; van Lantschoot, A.; van Boxem, K.; van Zundert, J. Pulsed radiofrequency in chronic pain: Wolters Kluwer. Curr. Opin. Anaesthesiol. 2017, 30, 577–582. [Google Scholar] [CrossRef]
- Jordan, S.; Catapano, M.; Sahni, S.; Ma, F.; Abd-Elsayed, A.; Visnjevac, O. Pulsed Radiofrequency in Interventional Pain Management: Cellular and Molecular Mechanisms of Action–An Update and Review. Pain Physician 2021, 24, 525. Available online: www.painphysicianjournal.com (accessed on 1 December 2021).
- Joosen, R.V.L.; Kodde, J.; Willems, L.A.J.; Ligterink, W.; van der Plas, L.H.W.; Hilhorst, H.W.M. Germinator: A software package for high-throughput scoring and curve fitting of Arabidopsis seed germination. Plant J. 2010, 62, 148–159. [Google Scholar] [CrossRef]
- Catusse, J.; Pestsova, E.; Bechetoille, C.; Job, C.; Meinhard, J.; Jagonak, A. Betaomics: A combined proteome and transcriptome profiling approach to characterize seed germination and vigour in sugarbeet seeds. In Proceedings of the 8th International Seeds Workshop (ISSS), Brisbane, Australia, 8–13 May 2005; Navie, S., Adkins, S., Ashmore, S., Eds.; CABI Publishing: Wallingford, Oxfordshire, 2007; pp. 169–178. [Google Scholar]
- Grzesik, M.; Romanowska-Duda, Z.B. Improvements in germination, growth, and metabolic activity of corn seedlings by grain conditioning and root application with Cyanobacteria and microalgae. Pol. J. Environ. Stud. 2014, 23, 1147–1153. Available online: https://www.researchgate.net/profile/Z-Romanowska-Duda/publication/267327911_Improvements_in_Germination_Growth_and_Metabolic_Activity_of_Corn_Seedlings_by_Grain_Conditioning_and_Root_Application_with_Cyanobacteria_and_Microalgae/links/562002c208aea35f267e135d/Improvements-in-Germination-Growth-and-Metabolic-Activity-of-Corn-Seedlings-by-Grain-Conditioning-and-Root-Application-with-Cyanobacteria-and-Microalgae.pdf (accessed on 1 January 2014).
- Uddling, J.; Gelang-Alfredsson, J.; Piikki, K.; Pleijel, H. Evaluating the relationship between leaf chlorophyll concentration and SPAD-502 chlorophyll meter readings. Photosynth. Res. 2007, 91, 37–46. [Google Scholar] [CrossRef] [PubMed]
- León, A.P.; Viña, S.Z.; Frezza, D.; Chaves, A.; Chiesa, A. Estimation of chlorophyll contents by correlations between SPAD-502 meter and chroma meter in butterhead lettuce. Commun. Soil Sci. Plant Anal. 2007, 38, 2877–2885. [Google Scholar] [CrossRef]
- Sas-Paszt, L.; Pruski, K.; Żurawicz, E.; Sumorok, B.; Derkowska, E.; Głuszek, S. The effect of organic mulches and mycorrhizal substrate on growth, yield and quality of Gold Milenium apples on M.9 rootstock. Can. J. Plant Sci. 2014, 94, 281–291. [Google Scholar] [CrossRef]
- Genty, B.; Briantais, J.M.; Baker, N.R. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim. Biophys. Acta (BBA) Gen. Subj. 1989, 990, 87–92. [Google Scholar] [CrossRef]
- Quero, G.; Bonnecarrère, V.; Simondi, S.; Santos, J.; Fernández, S.; Gutierrez, L. Genetic architecture of photosynthesis energy partitioning as revealed by a genome-wide association approach. Photosynth. Res. 2021, 150, 97–115. [Google Scholar] [CrossRef]
- Tiryaki, I.; Kaplan, S.A. Enhanced germination performance of dormant seeds of Eragrostis tef in the presence of light. Trop. Grassl. Forrajes. Trop. 2019, 7, 244–251. [Google Scholar] [CrossRef]
- Copeland, L.O.; McDonald, M.F. Principles of Seed Science and Technology; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Rosner, L.S.; Harrington, J.T. Effect of stratification in polyethylene glycol solutions on germination of three North American shrub species. Seed Sci. Technol. 2004, 32, 309–318. [Google Scholar] [CrossRef]
- Lewak, S. Metabolic control of embryonic dormancy in apple seed: Seven decades of research. Acta Physiol. Plant. 2011, 33, 1–24. [Google Scholar] [CrossRef]
- Chahtane, H.; Kim, W.; Lopez-Molina, L. Primary seed dormancy: A temporally multilayered riddle waiting to be unlocked. J. Exp. Bot. 2017, 68, 857–869. [Google Scholar] [CrossRef]
- Bareke, T. Biology of seed development and germination physiology. Adv. Plants Agric. Res. 2018, 8, 336–346. [Google Scholar] [CrossRef]
- Saffari, P.; Majd, A.; Jonoubi, P.; Najafi, F. Effect of treatments on seed dormancy breaking, seedling growth, and seedling antioxidant potential of Agrimonia eupatoria L. J. Appl. Res. Med. Aromat. Plants 2021, 20, 100282. [Google Scholar] [CrossRef]
- Kwon, H.J.; Shin, S.L.; Kim, Y.R.; Kim, S.Y. Effects of temperature, gibberellic acid, and KNO3 treatments on seed germination of the wild plant Maesa japonica. Seed Sci. Technol. 2020, 1, 65–72. [Google Scholar] [CrossRef]
- Mousavi, L.; Wan, I.; Wan, R.; Mousavi, M. Evaluation of Physicochemical Methods for Dormancy Breakage and Germination of Datura stramonium Seeds. J. Chem. Health Risks 2019, 9, 217–224. [Google Scholar] [CrossRef]
- Sohindji, F.S.; Sogbohossou, D.E.O.; Zohoungbogbo, H.P.F.; Houdegbe, C.A.; Achigan-Dako, E.G. Understanding Molecular Mechanisms of Seed Dormancy for Improved Germination in Traditional Leafy Vegetables: An Overview. Agronomy 2020, 10, 57. [Google Scholar] [CrossRef]
- Khan, J.; Rauf, M.A.; Ali, Z.; Khattack, M.S. Different stratification techniques on seed germination of pistachio cv. Wild. Pakistan J. Biol. Sci. 1999, 2, 1412–1414. [Google Scholar]
- Stein, M.; Serban, C.; McCord, P. Exogenous ethylene precursors and hydrogen peroxide aid in early seed dormancy release in sweet cherry. J. Am. Soc. Hortic. Sci. 2021, 146, 50–55. [Google Scholar] [CrossRef]
- Arc, E.; Galland, M.; Godin, B.; Cueff, G.; Rajjou, L. Nitric oxide implication in the control of seed dormancy and germination. Front. Plant Sci. 2013, 4, 346. [Google Scholar] [CrossRef] [PubMed]
- Signorelli, S.; Considine, M.J. Nitric Oxide Enables Germination by a Four-Pronged Attack on ABA-Induced Seed Dormancy. Front. Plant Sci. 2018, 9, 296. [Google Scholar] [CrossRef] [PubMed]
- Alahakoon, A.A.C.B.; Perera, G.A.D.; Merritt, D.J.; Turner, S.R.; Gama-Arachchige, N.S. Species-specific smoke effects on seed germination of plants from different habitats from Sri Lanka. Flora 2020, 263, 151530. [Google Scholar] [CrossRef]
- Cembrowska-Lech, D.; Kępczyński, J. Gibberellin-like effects of KAR 1 on dormancy release of Avena fatua caryopses include participation of non-enzymatic antioxidants and cell cycle activation in embryos. Planta 2016, 243, 531–548. [Google Scholar] [CrossRef]
- Nicholas, C.H.L.; Kris, V.C.P. Pulsed radiofrequency treatment in interventlonal pain management. QST Obs. Chronic Neck Pain 2011, 153, 107. [Google Scholar]
- Baskin, C.C.; Baskin, J.M. Mimicking the natural thermal environments experienced by seeds to break physiological dormancy to enhance seed testing and seedling production. Seed Sci. Technol. 2022, 50, 21–29. [Google Scholar] [CrossRef]
- Hilton, J.R.; Thomas, J.A. Regulation of pregerminative rates of respiration in seeds of various weed species by potassium nitrate. J. Exp. Bot. 1986, 37, 1516–1524. [Google Scholar] [CrossRef]
- Hawkins, T.S.; Gardiner, E.S.; Comer, G.S. Modeling the relationship between extractable chlorophyll and SPAD-502 readings for endangered plant species research. J. Nat. Conserv. 2009, 17, 123–127. [Google Scholar] [CrossRef]
- Ling, Q.; Huang, W.; Jarvis, P. Use of a SPAD-502 meter to measure leaf chlorophyll concentration in Arabidopsis thaliana. Photosynth. Res. 2011, 107, 209–214. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Schansker, G.; Ladle, R.J.; Goltsev, V.; Bosa, K.; Allakhverdiev, S.I. Frequently asked questions about in vivo chlorophyll fluorescence. Practical issues. Photosynth. Res. 2014, 122, 121–158. [Google Scholar] [CrossRef]
- Huang, B.; Mimouni, V.; Lukomska, E.; Morant-Manceau, A.; Bougaran, G. Carbon Partitioning and Lipid Remodeling During Phosphorus and Nitrogen Starvation in the Marine Microalga Diacronema lutheri (Haptophyta). J. Phycol. 2020, 56, 908–922. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).