Abstract
The formation of bulbils, which are storage organs, is an important agronomic trait and a unique morphological feature in the vegetative reproduction of yam. We found a landrace of water yam (Dioscorea alata L.), which rarely forms bulbils, that produces bulbils during periods of high rainfall. We investigated the physiological mechanism of bulbil formation in response to over-moist soil and relevant factors at the single plant level. Waterlogging (WL) treatment markedly increased the number of bulbils initiated, predominantly toward the upper nodes. This formed-bulbil was an accessory bud developed as a storage organ in leaf axils. Photosynthetic capacity decreased under WL, attributed to stress-induced stomatal closure. WL stress also reduced dry matter partitioning to the belowground organs. During tuber enlargement in WL plants, photosynthetic products accumulated in the aboveground organs and were transported to the bulbils as a result of reduced translocation to belowground organs. We investigated the effect of abscisic acid (ABA) on bulbil formation on the basis of changes in the sink–source balance in response to WL stress. ABA treatment of leaf axils enhanced bulbil formation in unstressed plants, suggesting that increased ABA is one of the factors that initiate bulbils. Our study shows that bulbil initiation occurs as a result of changes in physiological conditions in response to WL stress. This finding may provide fundamental information for the control of bulbil production. This response of bulbil formation, as an environmentally adaptive trait of the tropical water yam, may underlie the survival strategy of vegetatively propagated plants.
1. Introduction
Yams, Dioscorea spp., are a major tuber crop and are widely grown as an important security food source for millions of people in the tropics and subtropics [1]. The genus has over 600 species, of which about 10 are cultivated and have economic importance [2]. Dioscorea species are genetically diverse [3,4]. One of yams’ distinctive ecological traits is the formation of aerial tubers known as bulbils. A bulbil is a small tuber that develops by plastic development of meristematic tissue in leaf axils and in aboveground storage organs [5,6]. Bulbil formation is an uncommon vegetative propagation trait seen in several plant species, also including garlic, daylily, and rudbeckia. Bulbiliferous plants develop a unique bulbil corresponding to their ecological or evolutionary niche in their growth environments [5,7,8,9]. Yam bulbils are widely produced for food and for pharmaceutical uses [10]. In general, yams are not often reproduced by seed owing to their low seed fertility, and are reproduced mainly by vegetative propagation using belowground tubers or bulbils. After detaching from the mother plant, mature bulbils can grow as independent plants in the next growing season [11,12]. Bulbils thus provide a significant advantage for proliferation through asexual reproduction. Thus, clarifying the mechanism of bulbil formation is interesting not only for agricultural production, but also for plant ecology, as it could lead to an understanding of reproductive strategies.
Three main species of yams are grown in Japan: Chinese yam (Dioscorea opposita Thunb.), Japanese yam (D. japonica Thunb.), and water yam (D. alata L.). It is well known that Chinese yam and Japanese yam grown in Japan produce large numbers of bulbils during the tuber enlargement phase [13,14,15]. Previous studies have demonstrated that Exogenous gibberellin (GA) suppresses bulbil formation and that treatment with a gibberellin (GA) synthesis inhibitor increases bulbil formation in both species [13,16,17]. In contrast, few cultivars of water yam produce bulbils in Japan [18]. Overall, little is known about bulbil formation in water yam or its growth regulation mechanism.
Although most water yam cultivars have been assumed to not form bulbils in Japan, we have discovered a landrace, ‘Nagaimo (marukei)’, that forms a lot of bulbils in previous field experiments [19]. Its bulbil formation is accelerated during autumn (tuber-enlargement phase), especially under heavy rainfall (Figure S1a,b). Thus, we hypothesized that high soil moisture promotes bulbil formation. However, there have been no reports on the relationship between bulbil formation and soil moisture condition in yams. Water yam originated in the tropics and thus may be adapted to growth in wet humid environments. The characteristic of bulbil formation in response to excess soil water stress is unique and will provide fundamental information in unraveling the environmental adaptation of vegetatively propagated plants. Moreover, as bulbils are used not only for food but also for establishing the next crop, the ability to promote their formation in the field would contribute to the establishment of a stable technique for seed tuber propagation.
Here, to clarify the mechanisms of bulbil initiation in response to over-wet soil and related factors, we evaluated spatiotemporal bulbil formation and changes in the source–sink balance under waterlogging stress at the plant level. Focusing on phytohormones expected to be associated with bulbil initiation, we investigated the effects of abscisic acid (ABA) and GA treatments on bulbil formation.
2. Materials and Methods
2.1. Plant Material and Growth Conditions
Pot experiments were carried out from July to November in 2019 and 2020 at the Ito Plant Experiment Fields and Facilities (33°590′ N, 130°20′ E), Faculty of Agriculture, Kyushu University, Fukuoka, Japan. We grew the D. alata Japanese landrace ‘Nagaimo (marukei)’ (JP173293), from the National Institute of Agrobiological Sciences Genebank, which we had previously noted forms bulbils.
Seed setts from mature tubers grown in an experimental upland field at Kyushu University in 2018 or 2019 were cut into pieces of 4.5–6.0 g, treated with fungicide (benomyl), and incubated in a plastic bag with vermiculite at 30 °C in the dark for 1 week. The moisture content of the vermiculite was then adjusted to 50% (w/w) to induce sprouting. After 3 weeks, sprouted setts was selected and transplanted into long plastic pots (200 cm2, 40 cm height) containing 8 L sandy loam soil.
As basal dressing, 1.6 g N/P/K and 0.2 g Mg per pot were applied as compound fertilizer. In addition, topdressed compound fertilizer was applied at 45 days after transplanting at 0.64 g N/P/K and 0.08 g Mg per pot. Afterwards, plants were grown outdoors with sufficient water, and their growth was periodically surveyed. To adjust the onset of tuber enlargement, we set the daylength to 10 h light/14 h dark from 50 days after transplanting in a photoperiod-controlling apparatus set outdoors.
2.2. Waterlogging Treatment
For waterlogging treatment, pots were placed into a plastic container (92 cm × 61 cm, 20 cm high) with tap water (six pots in each container) and were imposed by keeping water of 20 cm below soil surface (bottom half of soil was waterlogged, WL); for control plants (no waterlogging, Ctrl), soil humidity was kept well drained by daily watering. The waterlogging treatment started at 68 days after transplanting (tuber-enlargement phase) and maintained that water level until sampling. During the treatment period, mean soil moisture content in the pots was 20.1 ± 0.5% for the waterlogging condition and 11.8 ± 1.5% for the control condition.
2.3. Evaluation of Bulbil Formation
An initiated bulbil was defined as one that developed in a leaf axil and reached 1 mm in diameter (Figure S1c,d). The number of bulbils per axil was defined as the bulbil formation rate (%). We investigated changes in this rate per site on the vine. First, the vine was divided into a main vine (M) and branch vine (B). On the basis of total node number, the main vine was divided into five positions by leaf axil, defined as M1 (top) to M5 (bottom). Branch vines were classified into upper, middle, and lower nodes according to the site of connection with the main vine. The position of bulbil formation on each vine was also classified into upper (1), middle (2), and lower (3). The number of bulbils per vine was determined every 7 days from the start of treatment, and the position of formation was marked on the vine. We investigated the site and time of bulbil formation, the number of leaf axils, and the formation rate (%) relative to the number of leaf axils on the vines sampled at harvest. From these data, spatiotemporal bulbil formation was represented in a heat map.
2.4. Microscopic Observation of Leaf Axils
Leaf axils were sampled separately for bulbils formed and non-formed at the harvesting stage. Samples were fixed in formalin-acetic acid-alcohol (FAA) solution and were then hand-sectioned using a razor blade. The longitudinal sections were observed under a light microscope at 40× magnification.
2.5. Measurement of Dry Matter Production
Plants were sampled at 0, 20, and 50 days of treatment and divided into leaves, stems (vines), bulbils, tuber, and roots. Subsequently, the samples were oven-dried at 80 °C for 3 days to determine their dry weight. From the dry weight of the aboveground and belowground parts, top/root ratio (T/R ratio) was calculated [20].
2.6. Measurement of Photosynthetic Gas Exchange
Photosynthetic gas exchange of the most recently expanded leaf of three to five plants from each treatment was measured at 0, 20, and 50 days of treatment. The net photosynthetic rate, stomatal conductance, and intercellular CO2 concentration were measured between 09:00 and 12:30 with a portable photosynthesis measurement system (LI-6400; Li-Cor, Inc., Lincoln, NE, USA). The photosynthetic photon flux density inside the chamber was set at 1500 mol m−2 s−1 by means of an internal light source. The CO2 concentration in the air entering the leaf chamber was adjusted to 400 mol mol−1. The air temperature in the chamber was adjusted to 25 °C and the relative humidity was controlled to ~60%.
2.7. Evaluation of 13C-Photoassimilate Translocation
In this experiment, the translocation traits were evaluated for the following three periods: 30 days (0–30 DAT) or 50 days (0–50 DAT) from the date of 13C feed at the start of WL treatment, and 30 days (20–50 DAT) after the 13C feed at 20 days after the WL treatment.
13CO2 was fed to both Ctrl and WL plants at 09:30–10:30 on 0 DAT or 20 DAT. A centrifuge tube containing 1.0 g of stable-isotope-labeled barium carbonate (Ba13CO3, 99 atom% 13C) was placed in a polyethylene bag. Ten milliliters of 30% lactic acid was added to the Ba13CO3, 13CO2 was supplied to whole shoot of two plants, and the polyethylene bag was opened 6 h after the start of the feeding. Thirty or 50 days after the 13CO2 feeding, three plants for each treatment were separated into bulbils, leaves, vines, tubers, and roots. The plant samples were dried in an open air oven at 80 °C for more than 3 days, weighed, and ground to powder with a sample mill.
13C abundance in plant material was determined with a mass spectrometer (Flash2000-DELTAplus Advantage ConFloⅢ System (ThermoFisher Scientific), Waltham, MA, USA). The 13C atom percentage excess in the plant sample was calculated as the difference in 13C atom percentage between the sample and a standard chemical of glycine. From the 13C labelled carbon amount of each part, the 13C-photosyntate allocation rate was calculated using the following equation:
Photoassimilate allocation rate = (13C amount recovered from each part/13C amount recovered from whole plant) × 100
2.8. ABA and GA Treatments
To investigate the effect of ABA and GA on bulbil formation, 100 µM ABA (Sigma-Aldrich, St. Louis, MO, USA) and 80 µM GA3 (Sigma-Aldrich, St. Louis, MO, USA) containing 0.05% Triton X-100 were applied to all leaf axils of Ctrl and WL plants, respectively. These treatments were performed every 3 days using a brush. To prevent degradation of phytohormone, both treatments were conducted after sunset between 20:00 and 22:00. The number of bulbils was then measured 15 days after the start of phytohormone treatment.
2.9. Statistical Analysis
Student’s t-test was applied to test the significance of differences between the data from control and waterlogged soil conditions. Data on 13C allocation in each organ were statistically evaluated by analysis of variance (ANOVA), and Tukey’s test at p < 0.05 was used for the comparison of means.
3. Results and Discussion
3.1. Effects of Waterlogging Stress on Bulbil Formation
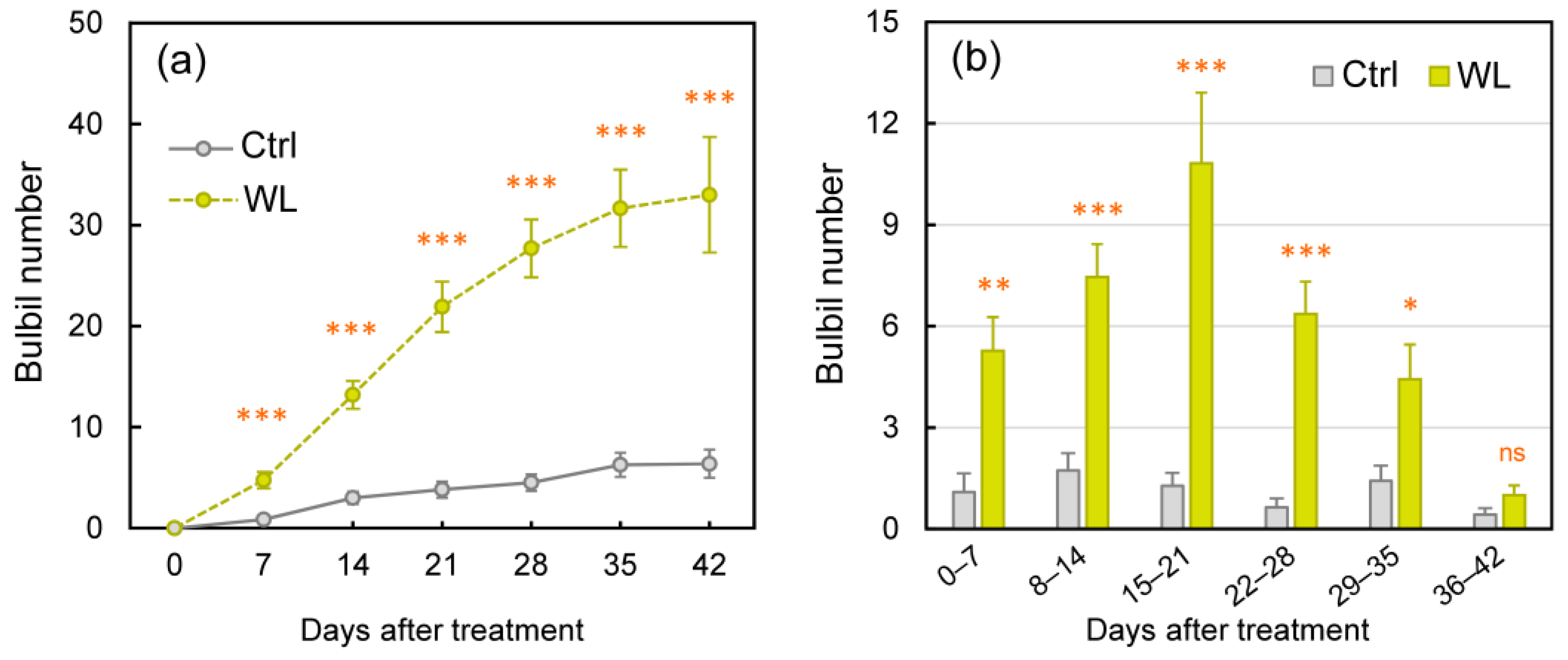
We assessed bulbil formation every 7 days (Figure 1). In control (Ctrl) plants, a few bulbils had formed by 7 days after the onset of treatment (DAT), and the numbers increased slowly. In waterlogged (WL) plants, in contrast, significantly more bulbils were formed from 7 to 42 DAT than in Ctrl (p < 0.001; Figure 1a). Significantly more bulbils were formed per week in WL than in Ctrl until week 5, peaking at 2 weeks at 1.7 in Ctrl and at 3 weeks at 10.8 in WL (p < 0.001; Figure 1b). By 42 DAT, bulbil numbers averaged 6.4 in Ctrl and 33.0 in WL (a ratio of 5.2×). In addition, the bulbil formation rate relative to total leaf axils was also significantly higher: 44 ± 6% in WL versus 10 ± 3% in Ctrl (p < 0.001) at 42 DAT.
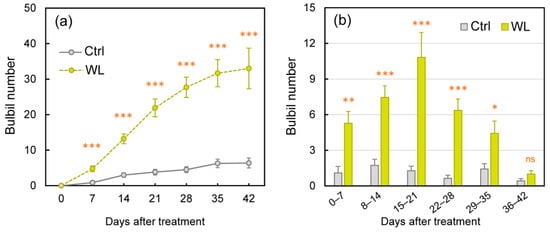
Figure 1.
Changes in formed bulbil number under control (Ctrl) and waterlogged (WL) soil conditions. (a) Total bulbil number and (b) number of formed bulbils every seven days. Values are the means ± S.E. of 7–11 replications derived from different plant. * p < 0.05, ** p < 0.01, *** p < 0.001; ns, not significantly different (Student’s t-test).
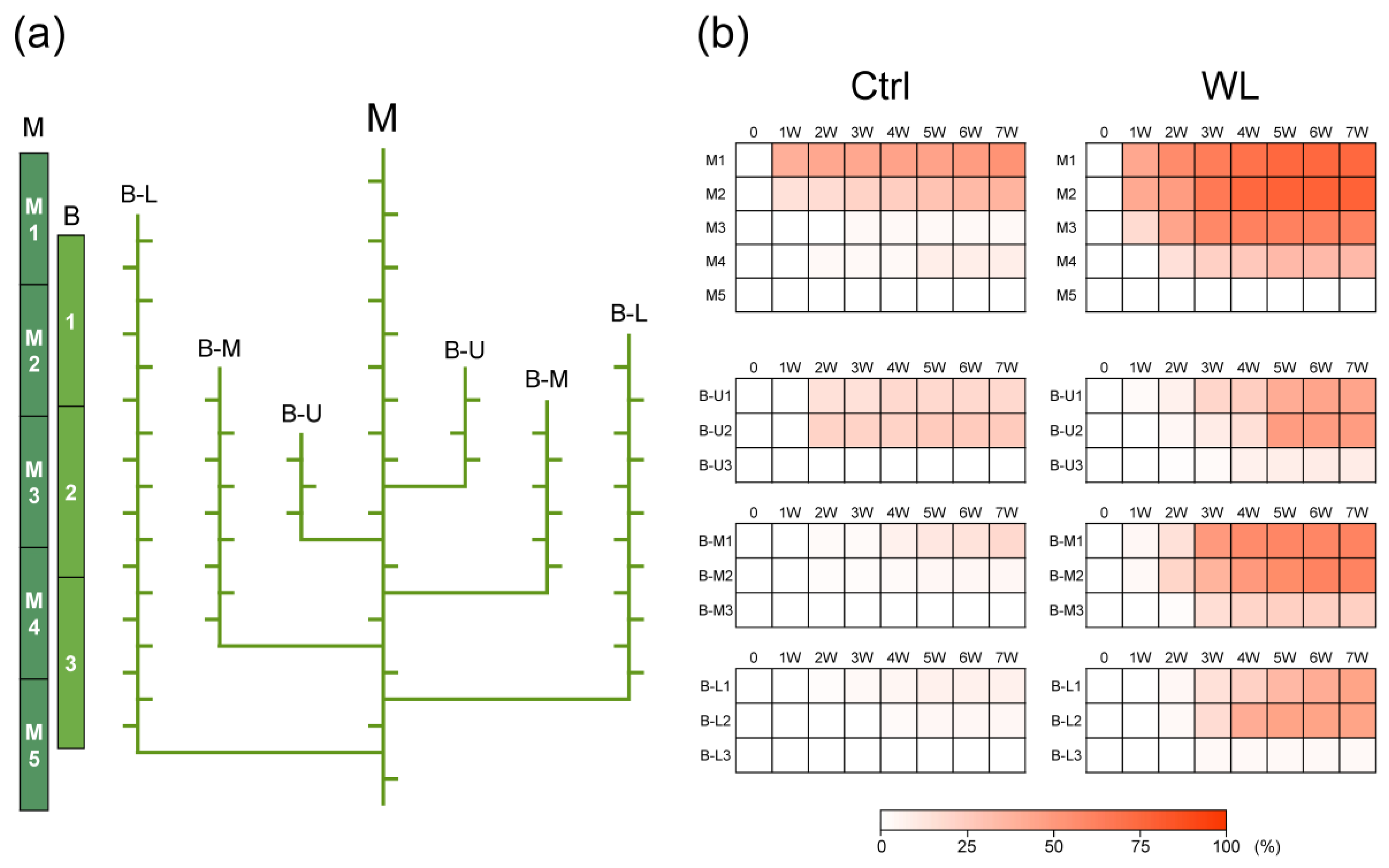
We examined the change in the bulbil formation rate by position of the leaf axils on vines. In both Ctrl and WL plants, bulbils tended to form earlier in the higher (younger) axils of main and branch vines (Figure 2). At 42 DAT, the bulbil formation rate on the main vine in Ctrl was 53% at M1, 37% at M2, 3% at M3, 9% at M4, and 0% at M5; and that in WL was 74% at M1, 77% at M2, 62% at M3, 33% at M4, and 0% at M5. In WL, it was particularly high on the upper nodes of the main vine, and it tended to be higher in the upper and middle nodes on the branch vines: 19% in the upper nodes (U1) and 25% in the middle nodes (U2) of B-U at 2 weeks and 7–19% in the upper nodes (M1 and L1) of B-M and B-L; and it was higher in B-M and B-L, which had more leaf axils, than in Ctrl, and increased from 1 week in B-M1 and B-M2 and from 2 weeks in B-L1 and B-L2, becoming remarkably higher at 7 weeks (61% and 46%, respectively). Moreover, WL plants, but not Ctrl plants, formed bulbils in B-U3, B-M3, and B-L3. These observations indicate that water yam begins early bulbil formation in response to waterlogging stress. Water yam may begin bulbil formation as an adaptive response to wet soil. Furthermore, WL stress also markedly increased the bulbil formation rate in the upper nodes, close to the growing point, forming clones at positions distant from the underground roots and tubers, which are directly exposed to the stress. Thus, with low seed fertility, water yam may have ecological characteristics that promote asexual reproduction at a greater distance from the mother plant when encountering waterlogging stress.
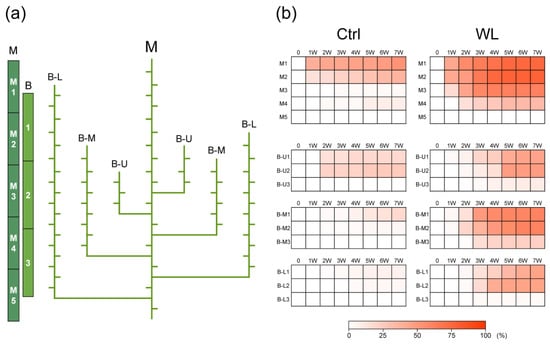
Figure 2.
Comparison of spatiotemporal bulbil formation between control (Ctrl) and waterlogged (WL) soil conditions. (a) Classification of leaf axil positions. The main stem (M) was divided into five parts (M1 to M5). The branch stems (B) were separated into three positions (upper: U; middle: M; lower: L) and divided into three parts (1 to 3). (b) The heat map represents weekly changes in percentage of formed bulbils in all leaf axils at each part. The data are the means of seven replications derived from different plants.
3.2. Morphological Characteristics of Bulbil
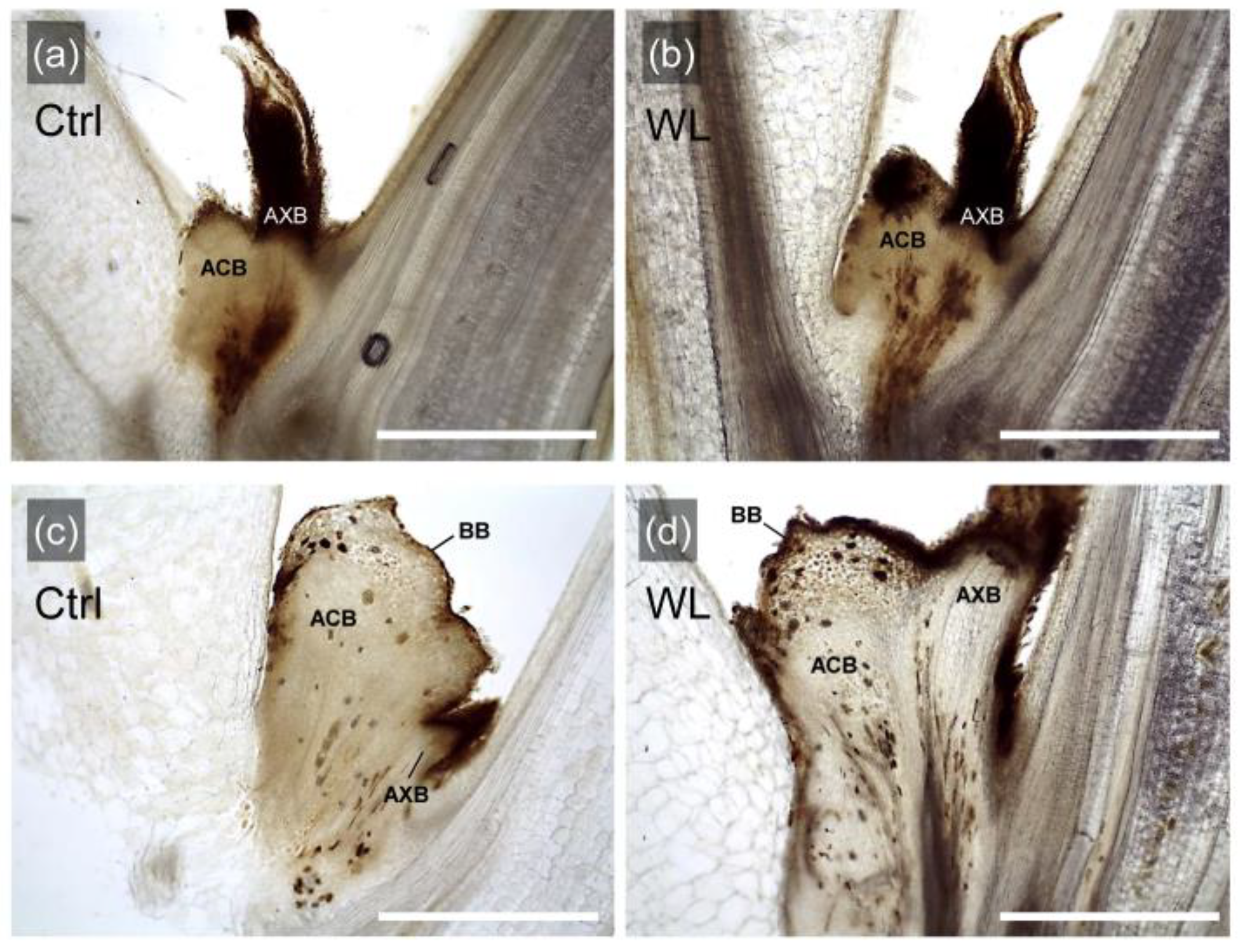
In order to characterize the morphology of bulbils formed by WL stress, the morphological traits of formed bulbils or unformed leaf axils were compared between treatments (Figure 3). In the leaf axils, axillary bud and accessory bud were adjacent to each other in both Ctrl and WL plants, regardless of the presence or absence of bulbil formation. In addition, these axillary and accessory buds were joined at the base of the leaf axil and their vascular bundles were connected. The upper part of the accessory bud tissue (globular tissue) developed into a storage organ, which was identified as a bulbil [21], and was common in both treatments (Figure 3c,d). These results suggested that WL stress does not cause a specific change in leaf axillary morphology to form bulbils, but that promotes the development of accessory buds, which significantly increases the bulbil formation rate. In pea, development of accessory bud in the leaf axils involves apical dominance and is subject to correlative inhibition by axillary bud growth, thus removal of the axillary bud promotes outgrowth of the accessory bud [22]. Indeed, no axillary bud outgrowth was observed in the leaf axils where bulbils were formed in our study. Therefore, it was indicated that water yam has a unique trait of promoting accessory bud development and differentiation into the assimilate storage organ (bulbil), probably when axillary bud outgrowth is suppressed.
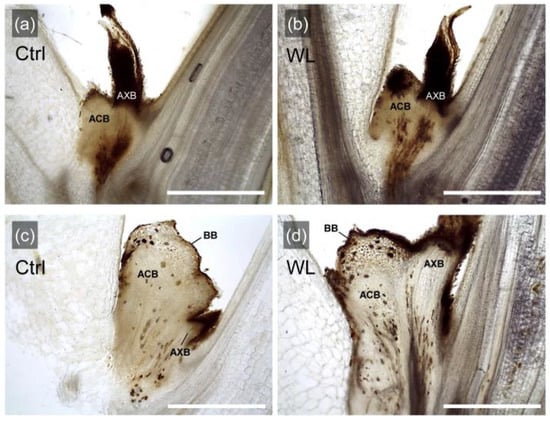
Figure 3.
Longitudinal sections of leaf axils grown under control (Ctrl) and waterlogged (WL) soil conditions. Leaf axils without bulbil formation in Ctrl (a) and WL (b); leaf axils with formed bulbils in Ctrl (c) and WL (d). AXB, axillary bud; ACB, accessory bud; BB, bulbil. Scale bars, 1 mm.
3.3. Dry Matter Production and Tuber Yield under Waterlogging Stress
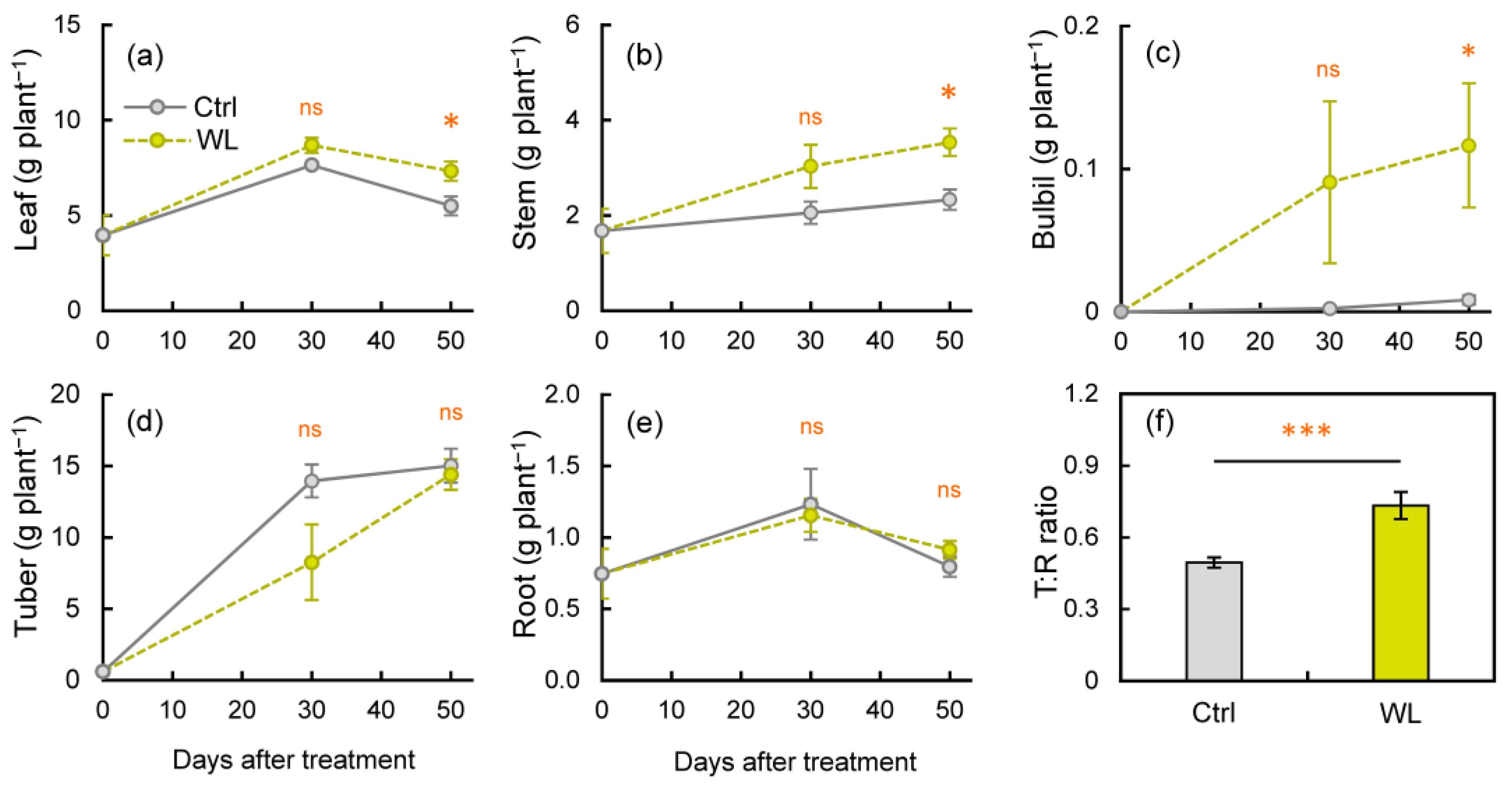
As the bulbil is a sink organ, its formation may involve changes in biomass partitioning in response to stress. We investigated the effects of WL stress on organ-specific dry matter (DM) weights. The DM weights of leaves and stems were significantly higher (p < 0.05) in WL than in Ctrl at 50 DAT (Figure 4a,b). Bulbil weights were notably higher in WL than in Ctrl, significantly so at 50 DAT (p < 0.05, Figure 4c). By contrast, there was little difference in root weight, but the degree of increase in tuber weight tended to be smaller in WL (Figure 4d,e). As a result, the T/R ratio at 50 DAT was significantly higher in WL than in Ctrl (p < 0.001; Figure 4f). These results suggest that WL lowers DM partitioning to belowground tubers in response to excess soil moisture and partially suppresses assimilate transport from aboveground to belowground organs. In addition, there was no significant difference in tuber yield (Figure S2), but under WL conditions, the tuber became malformed and the appearance quality decreased (Figure S3). It is known that normal tuber growth is inhibited by excessive soil moisture in other tuber crops [23], which appears to be the case in water yam. To improve the WL tolerance of water yam in fields, it will be necessary to consider the enlargement of tubers with a normal shape.
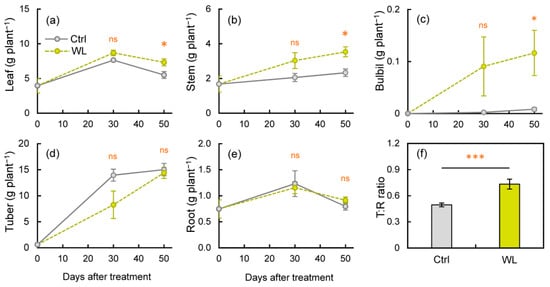
Figure 4.
Changes in dry matter production at each organ: (a) leaf, (b) stem, (c) bulbil, (d) tuber, and (e) root, under control (Ctrl) and waterlogged (WL) soil conditions, as well as (f) top/root ratio (T:R ratio) at 50 days after treatment. Values are the means ± S.E. of three to six replications derived from different plants. * p < 0.05, *** p < 0.001; ns, not significantly different (Student’s t-test).
Although total bulbil weight was increased by WL through the promotion of bulbil formation, only 9% of bulbils tended to enlarge to >1 cm in diameter under WL. Therefore, we infer that excess soil moisture stress was mainly responsible for increasing the number of bulbils formed, and that other factors controlled the subsequent promotion of enlargement growth. Factors might involve the physiological state of the meristematic tissue in the leaf axils, such as sucrose concentration.
3.4. Effect of Waterlogging Stress on Photosynthetic Traits and Photoassimilate Allocation
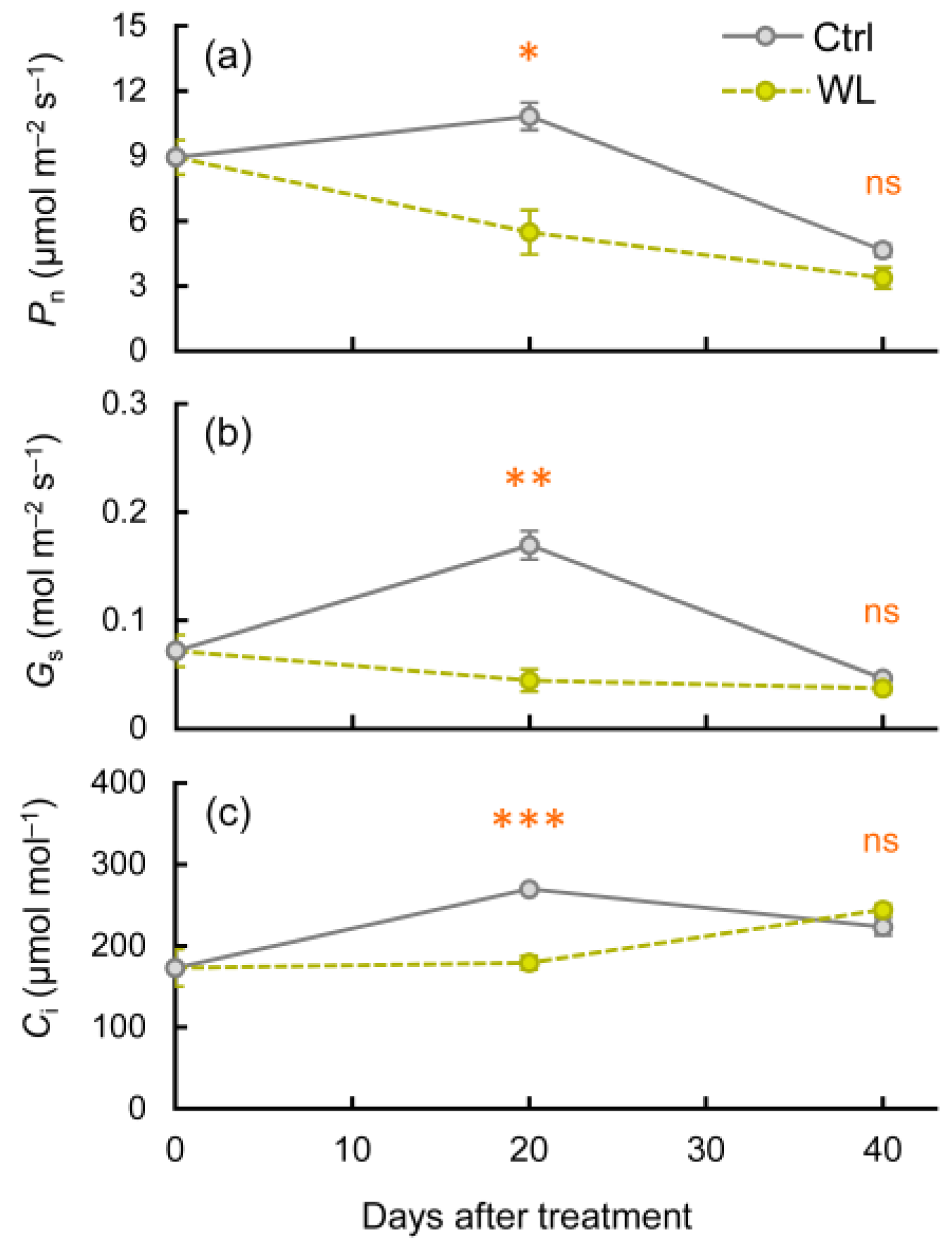
We investigated the photosynthetic and translocation traits to determine the physiological characteristics involved in bulbil formation. We compared the photosynthetic ability of single leaves between treatments. The net photosynthetic rate (Pn), stomatal conductance (Gs), and intercellular CO2 concentration (Ci) were significantly lower in WL than in Ctrl at 20 DAT (Figure 5a–c). Thereafter, Pn and Gs decreased in both treatments, with a faster decrease in Ctrl (Figure 5a,b), and there was no significant difference in Pn, Gs, or Ci between treatments at 40 DAT (Figure 5a–c). As this study was conducted during the tuber-enlargement harvest period, the reduction in photosynthetic capacity in the Ctrl after treatment could be caused by leaf senescence. Hence, although the photosynthetic capacity of individual leaves declined with age, WL stress reduced the carbonic acid fixation capacity and stomatal aperture. Photosynthetic characteristics under waterlogging have been studied in other crop species, in which reduced photosynthesis due to accelerated stomatal closure has been reported [24,25,26,27]. Our photosynthetic characteristics of water yam are generally consistent with the results of previous reports. In over-humidified soil, diffused oxygen concentrations drop sharply and direct oxygen supply from the atmosphere to the roots is inhibited, suppressing the aerobic respiration of roots and plant growth [28]. In the context of source capacity in flooded plants, stomatal closure is induced in part by root-to-leaf signaling, possibly via reduced cytokinin synthesis along with increased transport of ABA and ethylene [29,30]. In water yam, WL treatment reduced the photosynthetic capacity, suggesting that roots sensed waterlogging stress and promoted stomatal closure as a result of changes in the balance of plant hormones such as ABA and cytokinin.
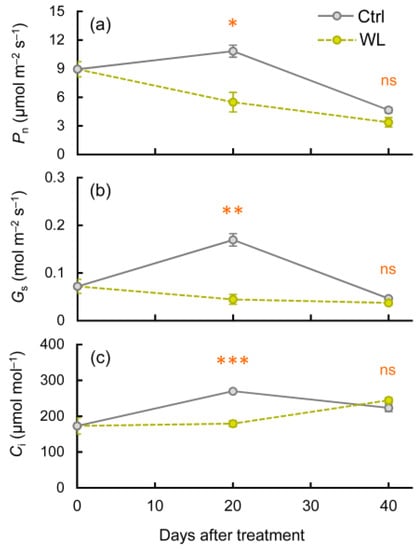
Figure 5.
Changes in photosynthetic traits under control (Ctrl) and waterlogged (WL) soil conditions. (a) Net photosynthetic rate (Pn), (b) stomatal conductance (Gs), and (c) intercellular CO2 concentration (Ci). Values are the means ± S.E. of three to five replications derived from different plants. * p < 0.05, ** p < 0.01, *** p < 0.001; ns, not significantly different (Student’s t-test).
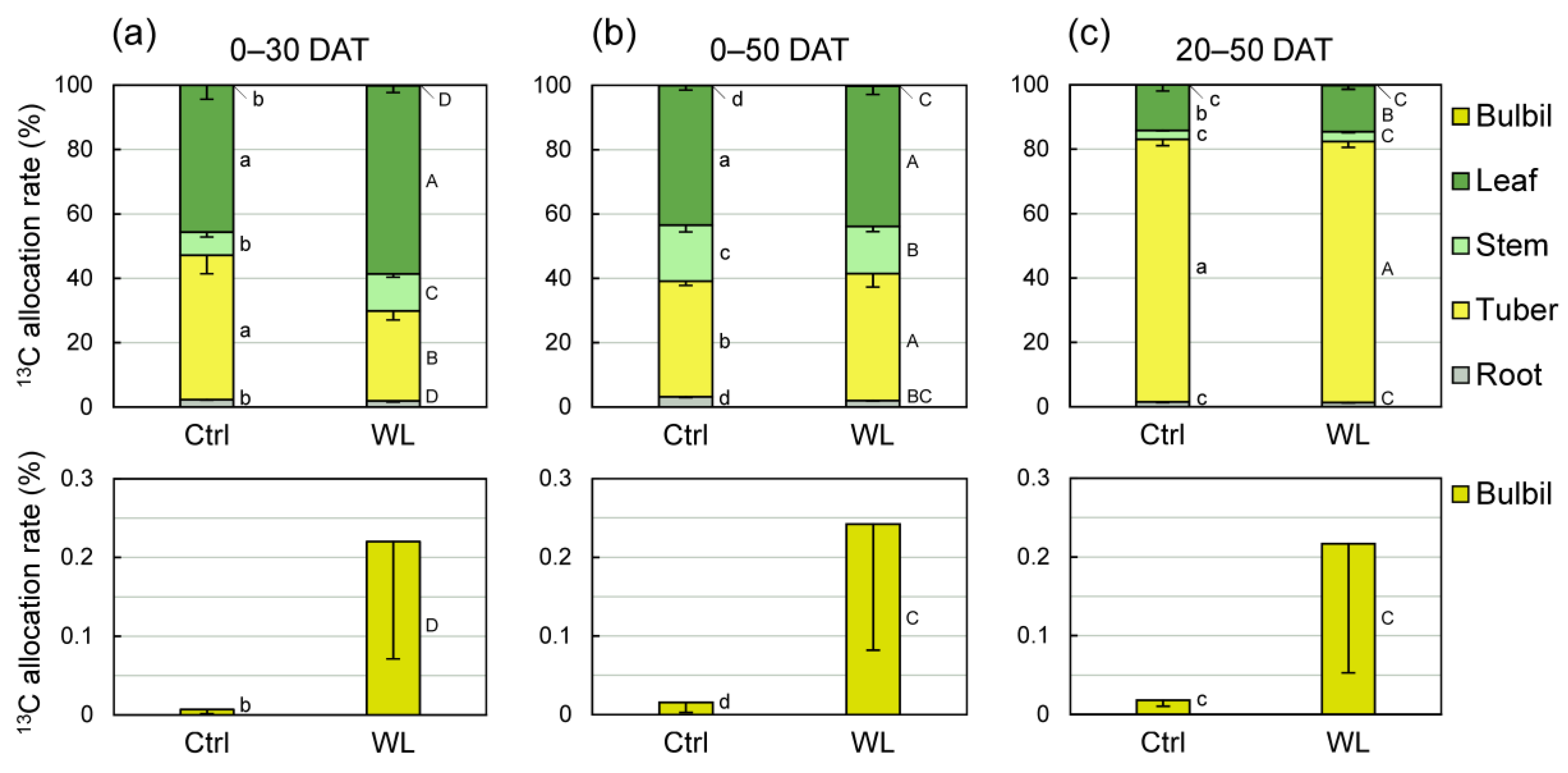
Given that WL stress altered the source capacity, we conducted a 13C tracer experiment to investigate translocation characteristics under WL. At 30 days after feeding of 13CO2 (0–30 DAT), plants distributed less 13C photoassimilate (28%) to tubers in WL than in Ctrl (45%; Figure 6a). At 30 days after feeding of 13CO2 (0–30 DAT), plants distributed less 13C photoassimilate to tubers in WL (28%) than in Ctrl (45%), and the 13C allocation rate of tuber was significantly lower than that of leaves in WL plants (Figure 6a). Leaves and stems distributed more 13C to aboveground parts in WL than in Ctrl. The distribution rate to bulbils in WL (0.220%) was ~30× that in Ctrl (0.007%). At 50 days after feeding of 13CO2 (0–50 DAT), in contrast, plants showed no difference in 13C partitioning by organ between treatments, and the distribution rate to tubers was similar, at 36% to 39% (Figure 6b). The 13C distribution rate to bulbils was almost same as that at 0–30 DAT, suggesting that photoassimilate fed at the start of WL treatment (0 DAT) was transported to bulbils by 20 DAT and accumulated in them without being retranslocated over the next 30 days (20–50 DAT). On the other hand, in plants fed 13CO2 at 20 DAT, ~81% of photoassimilate was transported to the tubers, with no clear difference in 13C partitioning between treatments (Figure 6c). 13C partitioning to bulbils at 20–50 DAT was 0.217% in WL, almost the same as at 0–30 DAT. These results reveal that the source–sink balance is altered in response to WL stress. Particularly, at 0–30 DAT, when bulbil formation is accelerated, the rate of photoassimilate partitioning to aboveground organs is increased owing to the suppression of assimilate transport from leaves to tubers. Increased concentrations of sugars may be involved in bulbil formation when leaves and vines become temporary sinks.
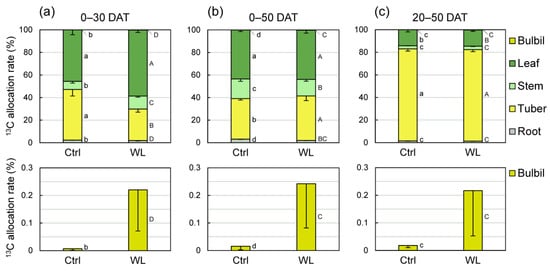
Figure 6.
Comparison of 13C-photoassimilate allocation rates between control (Ctrl) and waterlogged (WL) soil conditions at (a) 30 and (b,c) 50 days after treatment (DAT). 13CO2 was applied to whole shoot at (a,b) 0 and (c) 20 DAT. Values are the means ± S.E. of three replications derived from different plants. Bars followed by the same letter represent no significant difference in each organ by Tukey’s test at the 5% level.
3.5. Effects of ABA and GA Treatment on Bulbil Formation
Under WL conditions, leaf stomatal closure was accelerated (Figure 5b), suggesting that ABA synthesized in root sensing stress is transported to the shoot, increasing the ABA concentration in the above-ground organs (including leaf axils). Moreover, previous studies using other yam species have indicated that GA is involved in the inhibition of bulbil formation [13,16,17]. Therefore, we focused on both phytohormones and investigated their relationship with bulbil formation. We applied the phytohormones ABA and GA to leaf axils of Ctrl and WL plants, respectively.
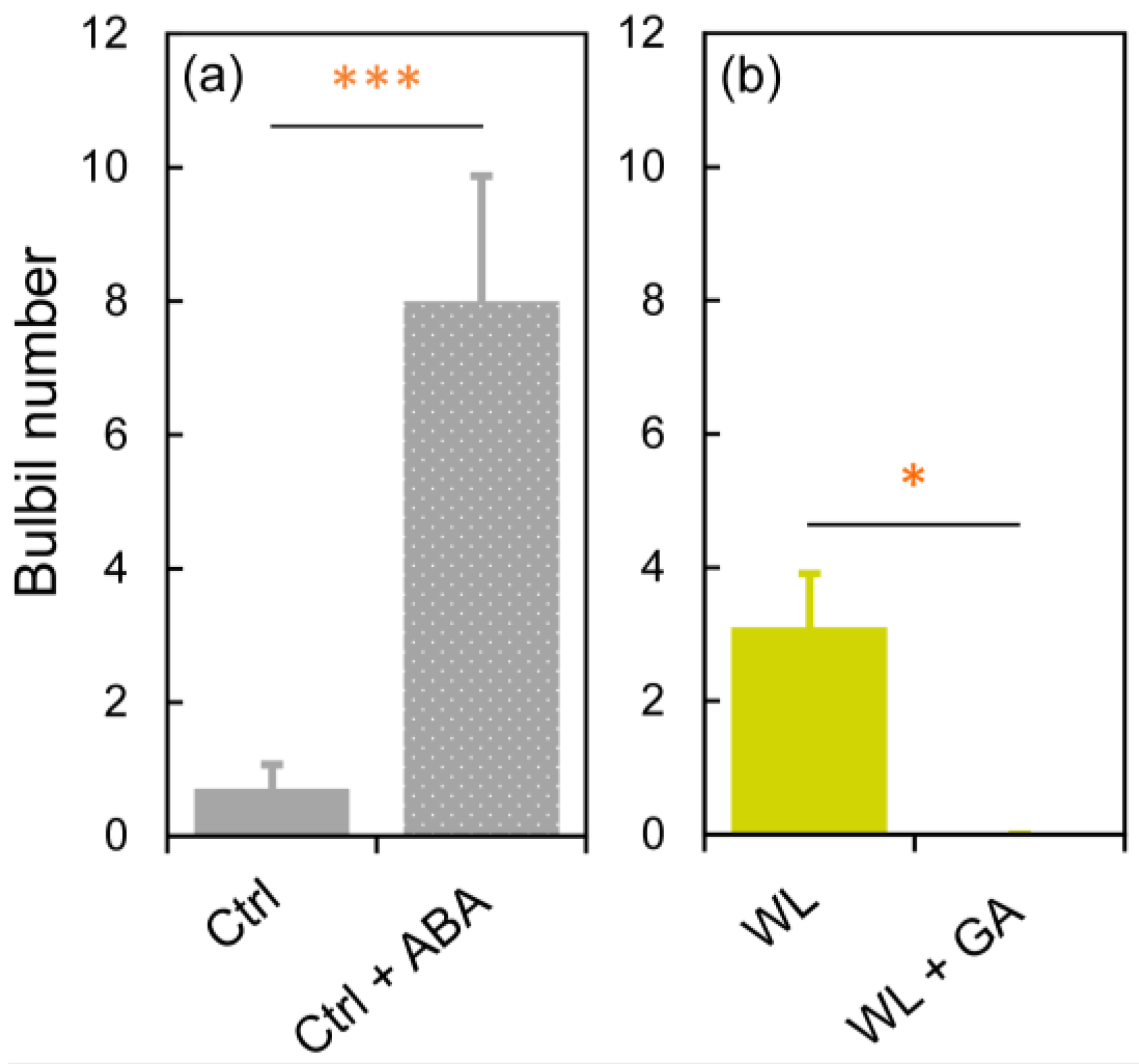
In Ctrl, significantly more bulbils (p < 0.001) were formed at 15 DAT in ‘Ctrl + ABA’ than in ‘Ctrl’, indicating that ABA treatment promoted bulbil formation (Figure 7a). In WL, no bulbils were formed in ‘WL + GA’, significantly different (p < 0.05) from that in ‘WL’, indicating that GA treatment suppressed bulbil formation under WL (Figure 7b). These results suggest that increased ABA concentrations in leaf axils are associated with bulbil formation, and that bulbil formation in response to WL stress is suppressed by high GA concentrations in water yam. A comprehensive gene expression analysis in water yam bulbils from early formation to maturity was recently performed [31]. From when bulbil formation is confirmed, the expression of auxin-, cytokinin-, and sucrose-related genes changes greatly, suggesting that these are key factors in the development of bulbils. In particular, localized auxin in the bulbil is transiently produced by stimulation of auxin synthesis, which seems to be necessary for the activation of the bulbil initiation [31]. Thus, leaf axillary bulbil formation may involve apical dominance via auxin–cytokinin crosstalk, and it is presumed that these regulate the antagonistic development between axillary and accessory buds. Indeed, bulbil formation rates in both Ctrl and WL plants were higher in the upper nodes, close to the growing point (Figure 2b), supporting involvement with apical dominance. Based on this predicted bulbil formation mechanism in water yam, the effects of GA and ABA on bulbil initiation under different soil moisture conditions are discussed below.
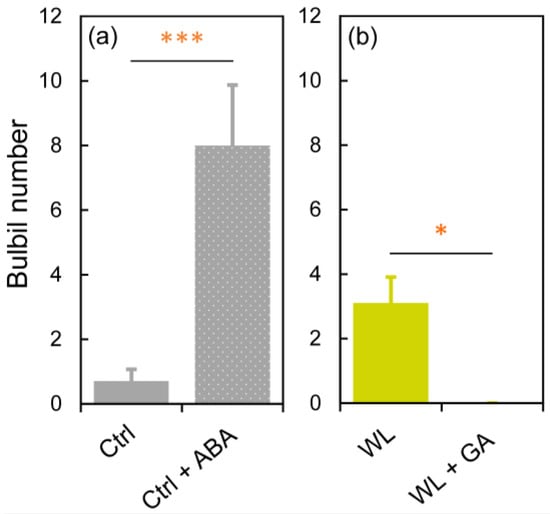
Figure 7.
The effects of (a) abscisic acid (ABA) and (b) gibberelline (GA) on bulbil formation at 15 days after treatment in water yam grown under (a) control (Ctrl) and (b) waterlogged (WL) soil conditions. All leaf axils were treated every four days with no hormone (Ctrl, WL), 100 µM ABA (Ctrl + ABA), or 80 µM GA (WL + GA). Values are the means ± S.E. of 4–10 replications derived from different plants. * p < 0.05, *** p < 0.001 (Student’s t-test).
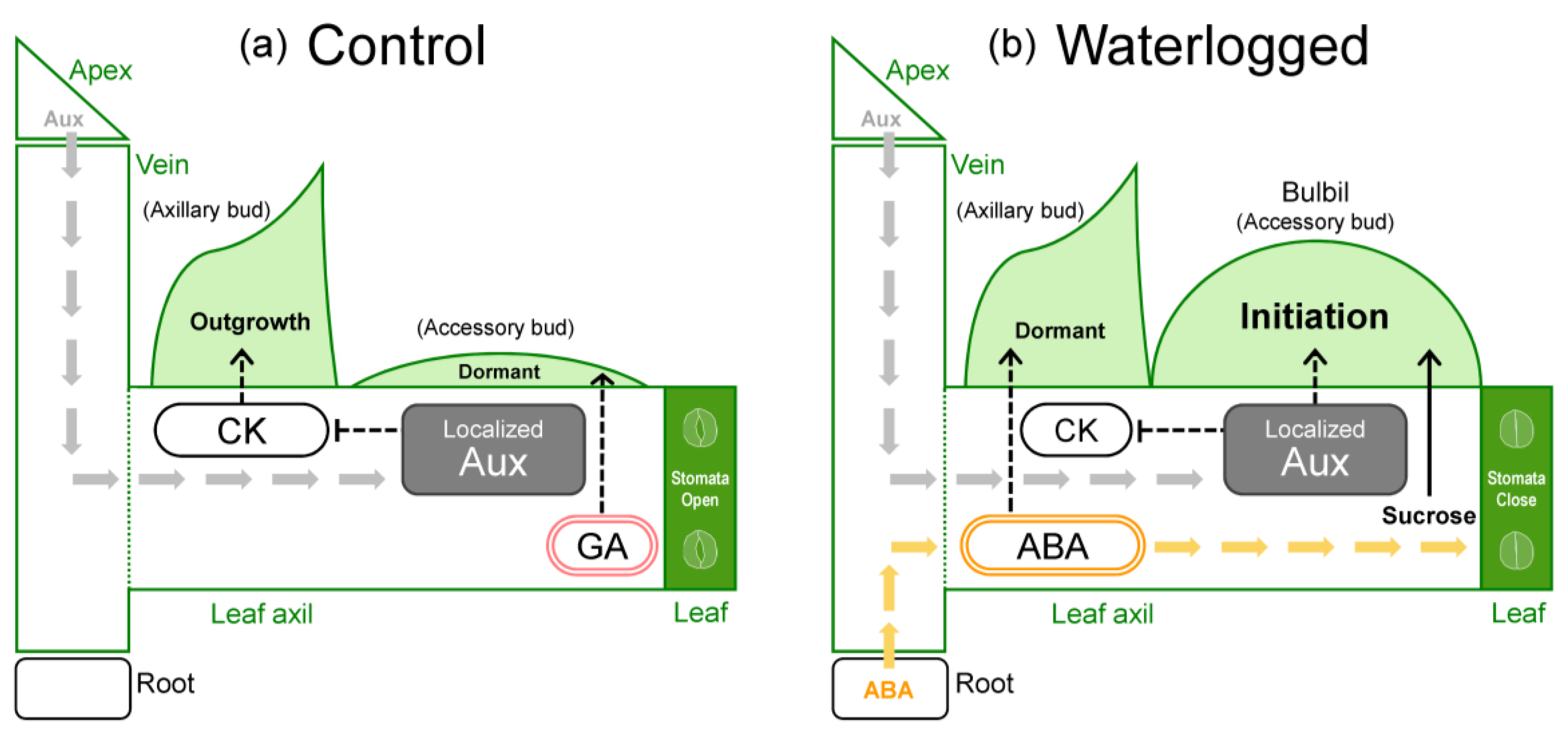
First, the suppression of bulbil formation by GA is discussed. GA treatment reduces the number of bulbils formed in Chinese yam and Japanese yam [13,14]. We confirmed that bulbils were not formed under GA treatment in WL conditions (Figure 7b), which induce bulbil formation in water yam. Therefore, we consider that the suppressive effect of GA on bulbil formation is common in Dioscorea. Indeed, it is also known that tuberization is suppressed by high GA levels in another tuber crop, potato [32]. In yam species, GA has been reported to induce tuber dormancy in both underground tubers and bulbs [33,34]. This GA response is one of the unique characteristics of yam. Based on this, it is possible that increased GA levels in leaf axils induced dormancy of accessory bud that differentiates into the storage organ (Figure 8a), resulting in the suppression of bulbil formation in WL plants as well.
Next, the induction of bulbil formation by ABA (Figure 7a) is discussed. The induction of bulbil formation by exogenous ABA has not been reported in any yam species. Our results suggested that ABA transported from roots to leaf axils is probably involved as a signal for bulbil formation in response to WL stress. In fact, no changes in the expression of ABA synthesis-related genes were observed in the formed bulbil at the initiation stage in a previous study [31]. Recently, BLANCHED 1b (StBRC1b), a gene that suppresses aerial tuber formation in potato, was isolated and its molecular function was analyzed [35]. tBRC1b protein synthesized in leaves is transported to the leaf axils and induces axillary bud dormancy, either directly or via enhancement of ABA synthesis. This indicates that increased ABA concentration via StBRC1b inhibits the formation of aerial tubers through the promotion of axillary bud dormancy. However, those results differ from ours. This difference may be due to the fact that the aerial tuber of potato is a differentiated organ of axillary bud, whereas the bulbil of water yam is a differentiated organ of accessory bud (Figure 3), resulting in different anatomical characteristics. In previous studies on axillary branching, it has been reported that ABA acts on dormancy or growth inhibition of axillary bud [22,36,37,38]. Moreover, axillary and accessory buds exhibit antagonistic growth in leaf axils [22]. Based on these findings, ABA probably does not directly induce bulbil differentiation, but indirectly induces accessory bud differentiation by localized auxin, perhaps by acting to promote dormancy in the axillary bud and by affecting apical dominance in the leaf axils through cross-talk between auxin and cytokinin (Figure 8b). In addition, bulbil initiation may also involve a decrease in cytokinin level in leaf axils via a reduction in root cytokinin synthesis due to WL stress.
4. Conclusions
The physiological responses of water yam at the plant level to waterlogging stress can be summarized as follows (Figure 8b). Underground organs (roots or tubers) sense excessive soil moisture and probably signal it by an increase in ABA levels in the aboveground parts. As a result, source activity is reduced with accelerated stomatal closure in leaves and translocation to the belowground organs is inhibited, allowing assimilates to accumulate in the aboveground organs (promoting sugar transport to leaf axil). Presumably, increased ABA concentration in leaf axil indirectly promotes the auxin-induced start of bulbil initiation in accessory buds via the induction of axillary bud dormancy. In addition, bulbil formation has an ontogenetic order, with priority given to the upper nodes.
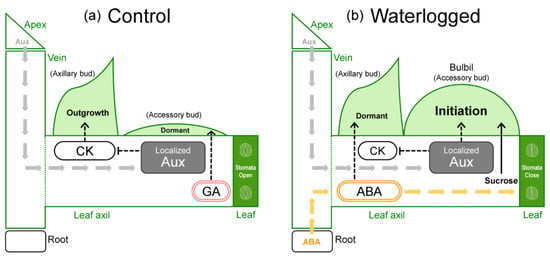
Figure 8.
Schematic model of the physiological regulation of bulbil formation by plant hormones and sucrose under (a) control and (b) waterlogged soil conditions. CK, cytokinin; Aux, auxin; ABA, abscisic acid; GA, gibberellin.
Figure 8.
Schematic model of the physiological regulation of bulbil formation by plant hormones and sucrose under (a) control and (b) waterlogged soil conditions. CK, cytokinin; Aux, auxin; ABA, abscisic acid; GA, gibberellin.
Our results show a preliminarily but reasonable stress response in plants. With a tropical origin, water yam is considered to be adapted to high rainfall. For the vegetative propagation of water yam, bulbils, as well as underground tubers, are essential for clonal reproduction, and their ability to form is an important factor in the yam’s survival and reproductive strategies. It would be interesting to understand which of the reproductive organs, below-ground or above-ground, are preferentially developed in response to environmental stresses. However, as this study was conducted using a single cultivar of D. alata, the identification of key regulators and an evaluation system capable of large-scale screening are required to reveal bulbil formation at the species or genus level. We plan to elucidate the molecular basis of bulbil initiation in water yam in order to understand the mechanism of bulbil formation via interactions between ABA and other phytohormones.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/agronomy13020484/s1, Figure S1: Gross morphology of bulbils in Dioscorea alata (c.v. Nagaimo (marukei)). Bulbils formed and matured under field condition (a,b). Bulbils formed in leaf axils in pot experiments (tuber wide > 1mm) (c,d). Figure S2: Comparison of fresh tuber yield at harvest (50 days after treatment) between control (Ctrl) and waterlogged (WL) soil conditions. Values are the means ± S.E. of seven replications derived from different plants. ns, not significantly different (Student’s t-test). Figure S3: Tuber shape at harvest (50 days after treatment) grown under control (Ctrl) and waterlogged (WL) soil conditions. Scale bars, 10 cm.
Author Contributions
Conceptualization, N.H. and Y.I.; methodology, N.H.; investigation, N.H. and T.M.; writing—original draft preparation, N.H.; review and editing, Y.I., C.S. and T.T. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by JSPS KAKENHI (Grant Nos. JP19K15824 and JP21K05542) to N.H.
Data Availability Statement
Not applicable.
Acknowledgments
The authors thank the National Institute of Agrobiological Sciences Genebank for providing the water yam germplasm. We would like to thank Kenji Hirao (Crop Husbandry Laboratory, Faculty of Education, University of Teacher Education Fukuoka, Japan) for use of the portable photosynthesis measurement system. We are grateful to Tomomi Abiko (Faculty of Agriculture, Experimental Farm, Kyushu University, Japan) for fruitful comments on waterlogging stress in tuber crop. We also thank Hue Thi Nong (Faculty of Biotechnology, Vietnam National University of Agriculture, Vietnam) for technical assistance.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ayensu, E.S.; Coursey, D.G. Guinea yams: The botany, ethnobotany, use and possible future of yams in West Africa. Econ. Bot. 1972, 26, 301–318. [Google Scholar] [CrossRef]
- Sartie, A.; Asiedu, R.; Franco, J. Genetic and phenotypic diversity in a germplasm working collection of cultivated tropical yams (Dioscorea spp.). Genet. Resour. Crop. Evol. 2012, 59, 1753–1765. [Google Scholar] [CrossRef]
- Lebot, V. Section III. In Yams in Tropical Root and Tuber Crops: Cassava, Sweet Potato, Yams and Aroids 181–275; CABI: Wallingford, UK, 2008. [Google Scholar] [CrossRef]
- Girma, G.; Hyma, K.E.; Asiedu, R.; Mitchell, S.E.; Gedil, M.; Spillane, C. Next generation sequencing based genotyping, cytometry and phenotyping for understanding diversity and evolution of guinea yams. Theor. Appl. Genet. 2014, 127, 1783–1794. [Google Scholar] [CrossRef] [PubMed]
- Wickham, L.D.; Wilson, L.A.; Passam, H.C. The origin, development and germination of bulbils in two Dioscorea species. Ann. Bot. 1982, 50, 621–627. [Google Scholar] [CrossRef]
- Murty, Y.S.; Purnima. Morphology, anatomy and development of bulbil in some Dioscoreas. Proc. Indian Acad. Sci. (Plant Sci.) 1983, 92, 443–449. [Google Scholar] [CrossRef]
- Szarek, S.R.; Driscoll, B.; Shohet, C.; Priebe, S. Bulbil production in Agave (Agavaceae) and related genera. Southwest. Nat. 1996, 41, 465–469. [Google Scholar]
- Ronsheim, M.L.; Bever, J.D. Genetic variation and evolutionary tradeoffs for sexual and asexual reproductive modes in Allium vineale (Liliaceae). Am. J. Bot. 2000, 87, 1769–1777. [Google Scholar] [CrossRef]
- Wang, C.N.; Moller, M.; Cronk, Q.C. Altered expression of GFLO, the Gesneriaceae homologue of FLORICAULA/LEAFY, is associated with the transition to bulbil formation in Titanotrichum oldhamii. Dev. Genes Evol. 2004, 214, 122–127. [Google Scholar] [CrossRef]
- Asiedu, R.; Sartie, A. Crops that feed the world 1. Yams. Food Secur. 2010, 2, 305–315. [Google Scholar] [CrossRef]
- Walck, J.L.; Cofer, M.S.; Hidayati, S.N. Understanding the germination of bulbils from an ecological perspective: A case study on Chinese yam (Dioscorea polystachya). Ann. Bot. 2010, 106, 945–955. [Google Scholar] [CrossRef]
- Zona, S.; Howard, C.C. Aerial vegetative diaspores of angiosperms: Terminology, organography, and dispersal. Flora 2021, 287, 151989. [Google Scholar] [CrossRef]
- Yoshida, Y.; Takahashi, H.; Kanda, H.; Kanahama, K. Interactive effects of photoperiods and plant growth regulators on the development of tubers and flowering spikes in Chinese Yam (Dioscorea opposita) cv. Nagaimo. J. Jpn. Soc. Hort. Sci. 2002, 71, 752–757. [Google Scholar] [CrossRef]
- Yoshida, Y.; Takahashi, H.; Kanda, H.; Kanahama, K. Effect of seed tuber weights on the development of tubers and flowering spikes in Japanese yams (Dioscorea japonica) grown under different photoperiods and with plant growth regulators. J. Japan. Soc. Hortic. Sci. 2007, 76, 230–236. [Google Scholar] [CrossRef]
- Epping, J.; Laibach, N. An underutilized orphan tuber crop—Chinese yam: A review. Planta 2020, 252, 58. [Google Scholar] [CrossRef]
- Kim, S.K.; Lee, S.C.; Lee, B.H.; Choi, H.J.; Kim, K.U.; Lee, I.J. Bulbil formation and yield responses of Chinese yam to application of gibberellic acid, mepiquat chloride and trinexapac-ethyl. J. Agron. Crop. Sci. 2003, 189, 255–260. [Google Scholar] [CrossRef]
- Kim, S.K.; Lee, S.C.; Shin, D.H.; Jang, S.W.; Nam, J.W.; Park , T.S.; Lee, I.J. Quantification of endogenous gibberellins in leaves and tubers of Chinese yam, Dioscorea opposita Thunb. cv. Tsukune during tuber enlargement. Plant Growth Regul. 2003, 39, 125–130. [Google Scholar] [CrossRef]
- Shiwachi, H.; Chang, K.J.; Hayashi, M. Ecological and morphological characterization and general evaluation of the introduced yams (Dioscorea alata L.). Bull. Fac. Agric. Kagoshima Univ. 1995, 45, 1–17. [Google Scholar]
- Hamaoka, N.; Moriyama, T.; Taniguchi, T.; Suriyasak, C.; Ishibashi, Y. Identification of phenotypic traits associated with tuber yield performance in non-staking cultivation of water yam (Dioscorea alata L.). Agronomy 2022, 12, 2323. [Google Scholar] [CrossRef]
- Hamaoka, N.; Uchida, Y.; Tomita, M.; Kumagai, E.; Araki, T.; Ueno, O. Genetic variations in dry matter production, nitrogen uptake, and nitrogen use efficiency in the AA genome Oryza species grown under different nitrogen conditions. Plant Prod. Sci. 2013, 16, 107–116. [Google Scholar] [CrossRef]
- Sanada, A.; Cheng, C.; Kikuno, H.; Siwachi, H. Bulbil dormancy and formation in water yam (Dioscorea alata L.). Trop. Agr. Develop. 2018, 62, 109–114. [Google Scholar]
- Shimizu-Sato, S.; Mori, H. Control of outgrowth and dormancy in axillary buds. Plant Physiol. 2001, 127, 1405–1413. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Jayapal, A.; Padmanabhan, V.B. Tropical tuber crops. In Abiotic Stress Physiology of Horticultural Crops; Springer: New Delhi, India, 2016; pp. 358–359. [Google Scholar]
- Else, M.A.; Tiekstra, A.E.; Croker, S.J.; Davies, W.J.; Jackson, M.B. Stomatal closure in flooded tomato plants involves abscisic acid and a chemically unidentified anti-transpirant in xylem sap. Plant Physiol. 1996, 112, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Nawata, E.; Hosokawa, M.; Domae, Y.; Sakuratani, T. Alterations in photosynthesis and some antioxidant enzymatic activities of mungbean subjected to waterlogging. Plant Sci. 2002, 163, 117–123. [Google Scholar] [CrossRef]
- Araki, T.; Nguyen, M.T.P.; Kubota, F. Specific feature in photosynthetic response of kenaf (Hibiscus cannabinus L.) to flooding stress. Environ. Control Biol. 2012, 50, 127–134. [Google Scholar] [CrossRef]
- Ploschuk, R.A.; Miralles, D.J.; Colmer, T.D.; Ploschuk, E.L.; Striker, G.G. Waterlogging of winter crops at early and late stages: Impacts on leaf physiology, growth and yield. Front. Plant Sci. 2018, 9, 1863. [Google Scholar] [CrossRef]
- Pan, J.; Sharif, R.; Xu, X.; Chen, X. Mechanisms of waterlogging tolerance in plants: Research progress and prospects. Front. Plant Sci. 2021, 11, 627331. [Google Scholar] [CrossRef]
- Nilsen, E.T.; Orcutt, D.M. Physiology of Plants under Stress; John Wiley & Sons, Inc.: New York, NY, USA, 1996. [Google Scholar]
- Herzog, M.; Striker, G.G.; Colmer, T.D.; Pedersen, O. Mechanisms of waterlogging tolerance in wheat–a review of root and shoot physiology. Plant Cell Environ. 2016, 39, 1068–1086. [Google Scholar] [CrossRef]
- Wu, Z.G.; Jiang, W.; Tao, Z.M.; Pan, X.J.; Yu, W.H.; Huang, H.L. Morphological and stage-specific transcriptome analyses reveal distinct regulatory programs underlying yam (Dioscorea alata L.) bulbil growth. J. Exp. Bot. 2020, 71, 1899–1914. [Google Scholar] [CrossRef]
- Jackson, S.D.; Prat, S. Control of tuberization in potato by gibberellins and phytochrome B. Physiol. Plant 1996, 98, 407–412. [Google Scholar] [CrossRef]
- Okagami, N.; Nagao, M. Gibberellin-induced dormancy in bulbils of Dioscorea. Planta 1971, 101, 91–94. [Google Scholar] [CrossRef]
- Tanno, N.; Yokota, T.; Abe, M.; Okagami, N. Identification of endogenous gibberellins in dormant bulbils of Chinese yam, Dioscorea opposita. Plant Physiol. 1992, 100, 1823–1826. [Google Scholar] [CrossRef] [PubMed]
- Nicolas, M.; Torres-Pérez, R.; Wahl, V.; Cruz-Oró, E.; Rodríguez-Buey, M.L.; Zamarreño, A.M.; Martín-Jouve, B.; García-Mina, J.M.; Oliveros, J.C.; Prat, S.; et al. Spatial control of potato tuberization by the TCP transcription factor BRANCHED1b. Nat. Plants 2022, 8, 281–294. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.; Finlayson, S.A. Abscisic acid is a general negative regulator of Arabidopsis axillary bud growth. Plant Physiol. 2015, 169, 611–626. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Grandio, E.; Pajoro, A.; Franco-Zorrilla, J.M.; Tarancon, C.; Immink, R.G.H.; Cubas, P. Abscisic acid signaling is controlled by a BRANCHED1/HD-ZIP I cascade in Arabidopsis axillary buds. Proc. Natl. Acad. Sci. USA 2017, 114, E245–E254. [Google Scholar] [CrossRef] [PubMed]
- Noriega, X.; Pérez, F.J. ABA biosynthesis genes are down-regulated while auxin and cytokinin biosynthesis genes are up-regulated during the release of grapevine buds from endodormancy. J. Plant Growth Regul. 2017, 36, 814–823. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).