Abstract
This work investigates the effects of an organic fertilizer enriched in Ca and Mg and two bacterial inoculants, applied alone and in combination, on soil fertility, plant growth, nutrition, and production of secondary metabolites, namely, acemannan and total phenolic compounds (TPCs), by Aloe vera (Aloe barbadensis Miller), under field cultivation. The first inoculum consisted of five native bacterial strains (Pseudomonas sp., Enterobacter sp., and three strains of Pantoea sp.), characterized in vitro as putative plant growth promoters, isolated from local organic farming fields of Aloe vera. The second inoculant was a commercial product (BACTILIS-S and HUMOFERT) and consisted of three Bacillus species: B. pumilus, B. amyloliquefaciens, and B. subtilis. The organic fertilizer (HUMO-CAL M-8O) was a mixture of humic and fulvic acids, with an additional CaCO3 (40% w/w) and MgO (4% w/w). The most significant increase in the content of acemannan and TPCs was detected under single application of the organic fertilizer, which was linked to enhanced concentration of Mg and Ca in the leaf gel. The concentration of acemannan tended to be increased with the combined application of the organic fertilizer and microbial inoculants. TPCs were significantly increased in both single and combined treatments, seemingly related to Fe concentration in the leaf rinds.
1. Introduction
Aloe vera (Aloe barbadensis Miller) constitutes one of the most valuable medicinal plants with many uses reported over thousands of years [1]. To date, it is used worldwide as an ingredient in nutraceuticals, pharmaceuticals, and cosmetics [2,3,4]. Aloe vera is a xerophytic succulent plant, which belongs to the Asphodelaceae family, originating from Africa and the Arabian Peninsula [5,6]. These plants have the capability to produce lateral shoots (also known as offshoots, suckers, etc.), which may be used as propagation material. Planting of offshoots constitutes the main propagation technique of Aloe vera [7]. Its succulent leaves consist of two parts, the outer photosynthetically activate green rind and the inner leaf pulp (also called gel), which serves primarily for water and energy storage. Crassulacean acid metabolism (CAM) supports the plant’s tolerance to xerophytic regions, as it improves water use efficiency [5].
Several secondary metabolites present in the leaf gel of Aloe vera are thought to be directly related to the plant’s beneficial characteristics. The leaf gel consists of 98–99% water, while the remaining 1–2% constitutes several biodrastic molecules, such as vitamins, proteins, phenol compounds, and carbohydrates [8,9,10,11]. Beta-(1→4) acetylated glucomannan, also, known as acemannan, is one of the most remarkable polysaccharides in Aloe vera leaf gel [12]. Anticancer, anti-inflammatory, antidiabetic, and wound-healing properties have been attributed to acemannan [3,13,14,15]. In addition to being components of the cell wall, glucomannans may play the role of storage polysaccharides in membrane-bound granules in specialized tissues such as Dendrobium orchid stems [16,17]. In Aloe vera plants, acemannan acts as a solute, contributing to the plant’s tolerance to water deficit [5,18].
Plant secondary metabolites, in addition to acting as functional molecules for crop defense against stressors, are of great interest due to their beneficial effects on human health and their potential pharmaceutical, cosmetic, and industrial use. However, they are found in very low concentrations in the plant tissues; therefore, there is increased interest in increasing the concentration of these molecules [19]. Strategies for achieving this goal include breeding approaches [20], engineering plant cell cultures [21], and heterologous gene expression [22]. Among them, there is growing interest in understanding the environmental factors (biotic and abiotic, acting as elicitors) that trigger and enhance the metabolite production in planta [19,23,24]. Organic compounds, such as humic and fulvic acids, have been found to act as elicitors, increasing the accumulation of secondary metabolites in plants [25,26]. Organic fertilization, in addition to its several beneficial effects for plants, contributes to the maintenance of soil fertility [27]. The uncontrolled use of chemical fertilizers to promote plant growth that started with the “green revolution” led to serious side-effects related to environmental damage [8,28,29,30]. Recently, in the strategy framework 2022–2023 of the FAO [31], the need for more sustainable agricultural practices, such as organic farming, is noted.
The application of plant growth-promoting rhizobacteria (PGPR) is also an innovative efficient tool for improving plant growth and metabolism [32,33,34]. These microbes may exist in plants roots or in the rhizospheric soil, and they can positively affect plants, either directly or indirectly [35]. Moreover, PGPR can act as bio-elicitors, inducing the synthesis of secondary metabolites in plants [36,37,38,39]. Nevertheless, the interactions between plants and microbes are dynamic; they may depend on environmental conditions and/or be species specific [40]. Investigations focusing on the potential effects of combined applications of PGPR with organic fertilization on the secondary metabolism of medicinal plants are still limited.
In the current study, we examined the combined effect of organic fertilization and PGPR inoculants on soil fertility, plant growth, nutrient content, and secondary metabolite production (acemmanan and total phenolic compounds (TPCs)) of Aloe vera plants. We used as microbial inoculants a consortium of the PGPR species Pseudomonas sp., Enterobacter sp., and three strains of Pantoea sp., which were previously isolated from Aloe vera roots, as well as a commercial biofertilizer (BACTILIS-S, HUMOFERT) containing Bacillus sp. strains. The organic fertilizer (HUMO-CAL M-8O) was also a commercial product, composed of a mixture of humic and fulvic acids with 40% w/w CaCO3 and 4% w/w MgO. It was hypothesized that the combined application of organic fertilizer with each microbial inoculant would lead to an improved nutritional status of Aloe vera plants, which would also influence their bioactive compound composition and crop productivity.
2. Materials and Methods
2.1. Experimental Design and Applications
The study was conducted in Neapoli (Laconia, Greece), from June to November 2022. The region is characterized by a typical Mediterranean climate with a xeric soil moisture and thermic soil temperature regime (latitude: 36.54039, longitude: 23.02571). The soil texture was determined as sandy [41]. One year old offshoots were used as plant material with 70 × 80 cm planting distance and a plantation framework of 10,000 plants/ha. The field experiment treatments included the “no application” control (C1), three single treatments ((i) “application of organic fertilizer” (C2), (ii) “inoculation with BACTILLIS- S” (C3), and (iii) “inoculation with the five native isolated PGPR strains” (C4)) and two combined treatments ((i) “application of the organic fertilizer in combination with BACTILLIS-S” (C5) and (ii) “application of the organic fertilizer and the five PGPR isolates” (C6)). A total of 36 plants were used per treatment, arranged according to a complete block design.
The commercial organic fertilizer treatment consisted of an organic mixture containing biologically processed leonardite rich in humic and fulvic acids (10–12% w/w in total), with extra content of CaCO3 (40% w/w) and MgO (4% w/w). The mixture was poor in macronutrients N, P2O5, and K2O (<1% w/w). Before plantation, 50 g of organic fertilizer was added to every planting pit. The consortium inoculant of the bacterial isolates included five native strains previously isolated from Aloe vera roots, derived from cultivations in Neapoli (Laconia, Greece), which showed positive results for in vitro plant growth-promoting tests, i.e., solubilization of phosphorus, production of indole-3-acetic acid (IAA), and siderophore production. BACTILLIS-S, the commercial biofertilizer, contained lyophilized bacterial strains of three Bacillus species: B. pumilus, B. amyloliquefaciens, and B. subtilis. The five PGPR isolates were grown separately in UM broth at 30 °C for 24 h in an orbital shaker; prior to inoculation, a consortium was prepared by mixing equal volumes of all individual cultures. Both the consortium of five isolates and the BACTILLIS-S inoculum were diluted to 108 cfu/mL, and 100 mL of inoculum was applied per plant. Inoculation was repeated after 20 days.
2.2. Soil Properties Analysis
Soil sampling was performed twice, at 20 days after the application of organic fertilizer and bacterial inocula and at harvest (6 months after the applications). At each sampling time, three soil samples (0–10 cm) per treatment were collected. All soil samples were air-dried and sieved to <2 mm prior to analysis.
The pH was measured using a standard glass/calomel electrode in 1:2.5 w/v soil–CaCl2 (0.01M) ratio suspensions [42]. Electrical conductivity (EC) measurement was conducted in a solution of 10 g of soil with 25 mL of dH2O [42]. Soil total organic carbon was estimated according to Walkley and Black’s wet digestion method [43], and total N was estimated by titration after distillation of NH3, via Kjeldahl digestion [44]. Exchangeable cations and extractable Zn, Fe, Mn, and Cu were determined using the ammonium acetate and diethylenetriaminepentaacetic acid (DTPA) extraction methods [45,46], respectively. Concentrations of Mg, Fe, Zn, Mn, and Cu were measured by flame atomic absorption spectrophotometry (Varian, A–300; Varian Techtron Pty. Limited, Australia), using an air–acetylene flame, while Ca concentration, using an acetylene–N2O flame. K and Na were measured by flame photometry (PG 2000 Instruments). The available P in soil samples was estimated by extraction with sodium bicarbonate [47], followed by Murphy and Riley’s color reaction method with a T60 UV/Vis spectrophotometer (PG instruments, United Kingdom), at 880 nm wavelength.
2.3. Morpho-Anatomical Measurments
The analysis took place at harvest time. Plant growth was determined by measuring height, width, total number of leaves (NOL), and total number of offshoots (NOO) per plant. Twelve replicates (plants) were used per treatment.
2.4. Leaf and Gel Analysis for Minerals
One leaf per plant was collected for the determination of mineral content in the outer leaf rind and of concentration of minerals, acemannan, and TPCs in the inner leaf gel, with six replicates for each treatment. Leaf samples were collected in the morning and were separated immediately to the outer leaf rind and inner leaf gel parts. Afterward, rind samples were dried for further analysis, and gel samples were homogenized and kept at −20 °C, until lyophilization.
For mineral composition analysis, samples of the dried rinds of all plants were finely ground in a stainless-steel Wiley mill. A subsample of 0.5 g was heated to ash at 550 °C. The rind extract was digested with 5 mL of 65% HNO3, diluted to 25 mL with dH2O, and filtered. For the inner leaf gel, a subsample of 50 mg lyophilized gel of each sample was heated to ash at 550 °C. The extract was digested with 1 mL of 65% HNO3, diluted to 10 mL with dH2O, and filtered. Total concentration of P in the rind and lyophilized gel samples was determined following the Murphy and Riley color reaction method, with a PG T60 UV/Vis spectrophotometer, at 880 nm wavelength [48]. For rind samples, concentration of Mg, Fe, Zn, Mn, and Cu were determined by flame atomic absorption spectrophotometry (Varian, A–300; Varian Techtron Pty. Limited, Mulgrave, Australia), using an air–acetylene flame, while Ca concentration was determined using an acetylene–N2O flame. K and Na were measured by flame photometry (PG 2000 Instruments), and N was measured using the Kjeldahl method [49]. For the lyophilized gel samples, the concentration of the macro-elements Ca, Mg, K, and Na was determined as for the rind samples.
2.5. Acemannan Quantification
Acemannan was quantified spectrophotometrically at 540 nm, using the Congo Red method, according to Eberendu et al. [50] and Candarelli et al. [51], with some modifications. Briefly, 10 mg of lyophilized gel of each sample was diluted in approximately 35 mL of distilled water and, after overnight shaking at 28 °C, placed in an ultrasonic bath for 30 min. Afterward, the extract was diluted to 50 mL and passed through a 0.45μm filter, before the color reaction. Konjac glucomannan (Megazyme) was used as the standard [12].
2.6. Quantification of Total Phenolic Content
Τhe total phenolic content of each lyophilized gel sample was estimated using the Folin–Ciocâlteu method [52]. For the extraction, 25 mg of lyophilized gel was diluted in 2 mL of 80% MeOH and centrifuged at 12,000 rpm for 15 min. This step was repeated twice. Then, 50 μL of the methanol extract was combined with 3.95 mL of distilled water and 250 μL of Folin–Ciocâlteu reagent. After 1 min, 270 μL of 20% Na2CO3 was added to the mixture. The absorbance was measured after 2 h of incubation in the dark with a PG T60 UV/Vis spectrophotometer, at 760 nm wavelength. A solution (1 mg/mL) of gallic acid was used to construct the standard calibration curve.
2.7. Data Analysis
All data analyses were conducted in R v4.2.2 (R Core Team, Vienna, Austria, 2022). We tested for main effects of treatments using one-way analysis of variance (ANOVA). For the comparisons between means, Duncan’s multiple range test (p < 0.05) was employed, using the R package agricolae [53]. Pearson’s correlation was used for pairwise comparisons of acemannan with (i) mineral and TOC concentration in the inner leaf gel, and (ii) nutrient concentration in the outer leaf rind. A p-value ≤ 0.05 was considered to indicate statistical significance. All plots were designed with the ggplot2 package [54].
3. Results and Discussion
3.1. Soil Properties
In order to determinate whether the treatments improved soil fertility, soil parameters such as pH, electrical conductivity (EC), and contents of total organic carbon (TOC), total N, exchangeable Ca, Mg, K, and Na, and available micronutrients Fe, Mn, Zn, and Cu were measured at two different periods (Table 1). At the first soil sampling, pH increased in C3, C5, and C2 treatments, compared to C1 (Table 1). However, the pH increase remained significant until the second sampling (6 months after the application) for the C3 treatment only (Table 1). On the contrary, no significant differences were recorded in the first or second samplings regarding the EC among all treatments (Table 1). Regarding TOC, organic fertilizer seemingly induced a positive effect in all treatments, although significant differences were observed only during the first sampling (Table 1). Despite the fact that application with organic fertilizer induced a positive effect on ΤN content, the C/N ratio showed a clear increase of 47% compared to C1, at the first sampling (Table 1). Both combined treatments of organic fertilizer with microbial inoculants led to a reduction in C/N ratio at the first sampling (Table 1).

Table 1.
The effects of fertilizer and microbial inoculants on soil properties.
In terms of the other macronutrients, only Na was not significantly affected by the treatments (Table 1). Soil Mg concentrations significantly increased in C3 and C5 treatments at the first sampling, while, for C2 treatment, the observed increase was not significant (Table 1). Nonetheless, the Ca increase was more pronounced for the C2 treatment, with a 29% increment compared to C1 (Table 1). Furthermore, most treatments increased K concentrations in relation to C1: 126.3% for C5, 92.6% for C3, 79.8% for C6, and 42.3% for C2 (Table 1). Regarding P concentrations, individual treatments of BACTILLIS S and organic fertilizer had the most positive effect, while combined treatments were not found to promote an additive effect compared to C1 (Table 1). Moreover, significant differences were noted in the first but not the second sampling.
Treatments resulted in significant increases with respect to the concentrations of micronutrients compared to C1, except for Mn (Table 2). However, these changes were noticeable only in the first sampling. Regarding Fe, Zn, and Cu, the most significant increase was recorded in the C2 treatment compared to control. Moreover, significant differences in Cu concentrations were also detected for C3 and C5 treatments, showing 46.7% and 40% increases, respectively (Table 2).

Table 2.
The effects of fertilizer and microbial inoculants on soil concentration of micro-elements.
The benefits of humic and fulvic acids in soil fertility have been widely documented [55]. According to our results, the mixture of humic and fulvic acids with CaCO3 and MgO led to a significant increase in the concentration of TOC and available soil Ca, Mg, K, P, and Fe, which was linked to the significant increase in soil pH. BACTILLIS S treatment had a positive effect on the concentration of K, P, and Cu. Bacillus spp. have been found to solubilize K, mineralize organic material, and solubilize unavailable forms of P [56,57]. Moreover, Bacillus spp., such as B. subtillis are known to produce organic and inorganic acids and chelating compounds, providing plants with available forms of micronutrients [56,58].
3.2. Plant Growth
To examine the effects of treatments on Aloe vera plant growth, the height, width, number of leaves (LOL), and number offshoots (NOO) were determined (Table 3). No differences were detected, despite the observed changes in soil fertility. Many studies have reported the benefits of organic fertilization and inoculation with PGPR for plant growth, but this effect depends on the environment and host genotype [59,60]. Our findings are in line with Khajeeyan et al. [61], who recorded no significant effect on Aloe vera growth after the application of PGPR Pseudomonas and Pantoea sp., in a field experiment over 2 years.

Table 3.
The effects of fertilizer and microbial inoculants on Aloe vera growth.
3.3. Leaf Rind Mineral Concentrations
The macronutrient content of leaf rind samples was affected by treatment application, except K, which did not differ among treatments (Table 4), despite showing a significant increase in the soil (Table 1). On the contrary, all treatments had a positive impact on N content, whereas the most pronounced effects were for C3 and C6, which exhibited 36.9% and 33.4% increases, respectively. The sole application of organic fertilizer (C2 treatment) significantly increased Ca and Mg content. Regarding P, the combined application of organic fertilizer and BACTILLIS S (C5 treatment) significant increased P concentration by 128% compared to C1. On the contrary, all treatments led to a reduction in the Na content compared to C1, especially the two treatments which contained BACTILLIS-S (C3 and C5).

Table 4.
The effects of fertilizer and microbial inoculants on mineral composition in the Aloe vera outer green rind of the leaves.
Fe concentrations showed significant increases of 77.6%, 71.6%, 68%, 56.2%, and 53.9% for C2, C6, C4, C5, and C3, respectively. Moreover, Mn concentrations were positively affected, for the most part, by PGPR isolates and the organic fertilizer treatment. Cu and Zn concentrations in rinds showed no significant differences among treatments.
The significant increase in N content caused by C3 and C6 indicated the beneficial effect of BACTILLIS S on N uptake from Aloe vera plants, linked to the reduced C/N ratio in soil for the C6 treatment compared to C2. A positive result in terms of N content was also reported after the application of Bacillus pumilus in the CAM plant Mammillaria fraileana in a pot experiment [62]. Diverse species of Bacillus are known to act as diazotrophs, providing the plants with available forms of nitrogen [56,63]. Additionally, although the single application of BACTILLIS S led to the most significant increase in the soil, the concentration of P in the rind was more pronounced in the combined application of BACTILLIS S with the organic fertilizer, indicating the synergistic effect of the latter on P accumulation in the rind. Furthermore, the increase in Fe content was attributed to the siderophore production capability, as previously mentioned for Bacillus spp., Pseudomonas spp., Enterobacter spp., and Pantoae spp. [10,62,64,65,66]; this was confirmed in vitro for the strains used in this experiment.
3.4. Gel Macronutrient Concentration
Treatments caused significant changes in the accumulation of all examined macronutrients. The application of the organic fertilizer, enriched in Ca and Mg, had the most positive effect on the Ca content (significant increase of 19.2% compared to C1). Nevertheless, the combined treatment of organic fertilizer and PGPR isolates (C6) led to a surprisingly significant reduction in Ca content by 22.1% compared to C1. In addition, C3 showed a decrease in Ca concentration by 31.9%. Similarly, the sole application of organic fertilizer and PGPR isolates resulted in significant increases in Mg concentration of 27.6% and 15.5%, respectively, compared to C1; however, the combined treatment (C6) was not found to have an additive effect. This consistent determination of a positive effect of the organic fertilizer on Ca and Mg accumulation in the leaves by the application of PGPR isolates indicates a potential role of PGPR in the leaf transpiration of Aloe vera. Ca and Mg reside in plant leaves as a result of increased transpiration, which leads leaves to accumulate increased amounts of these elements [67]. However, PGPR have been shown to improve water management and to control leaf transpiration, mainly by interfering with the ABA signaling pathway [68]. We, therefore, suggest that the observed control of Ca and Mg accumulation in the leaves of the Aloe vera plants by the application of the PGPR is probably related to reduced transpiration and improved water status management indued by the application of endophytic bacterial isolates. Regarding K, all treatments with organic fertilizer or PGPR isolates showed a negative effect. K content was found to be increased only by the single application of BACTILLIS S (C3). BACTILLIS S also resulted in a significant increase in the concentration of P compared to C1, not only in the combined application with the organic fertilizer, such as rind samples, but also when applied alone. Although not a nutrient, we also measured Na content in the leaves, since it interferes with plant physiology, especially under drought/salinity conditions. Na content was significantly lower by 33.9% for C5 in comparison to C1.
Little information is available on the impact of soil fertility in the nutrient concentration in Aloe vera gel because, in most studies, the whole leaf was used for nutrient analysis. Chowdhury et al. [69] observed an increase in the concentration of P in the gel of Aloe vera plants, treated with poultry manure combined with inorganic fertilization in all doses applied, compared to control. According to our results, organic fertilization caused a significant augmentation in the concentration of Ca and Mg in rind and gel samples (Table 5). However, the concentration of K, was not found to be changed in the rind (Table 4), whereas, in gel samples (Table 5), it was significantly higher compared to C1 when the plants were inoculated with BACTILLIS S. This observation is related to the increased concentration of K in soil samples of BACTILLIS S treatment, which may be the mechanism via which BACTILLIS S conveys enhanced plant tolerance to drought, since K is a major plant cell osmoticum [70]. Similarly, the positive effect of the individual treatment of BACTILLIS S on P concentration was found to be significant in the gel and soil, but not in the rind samples. Nonetheless, the combined application of BACTILLIS S with organic fertilization led to a significant accumulation of P in the rind and gel samples. An opposite trend was observed for Na concentration, which decreased in gel samples under the combined treatment of BACTILLIS S and organic fertilizer.

Table 5.
The effects of fertilizer and microbial inoculants on mineral composition in the Aloe vera inner leaf gel.
3.5. Acemannan and TPC Concentrations
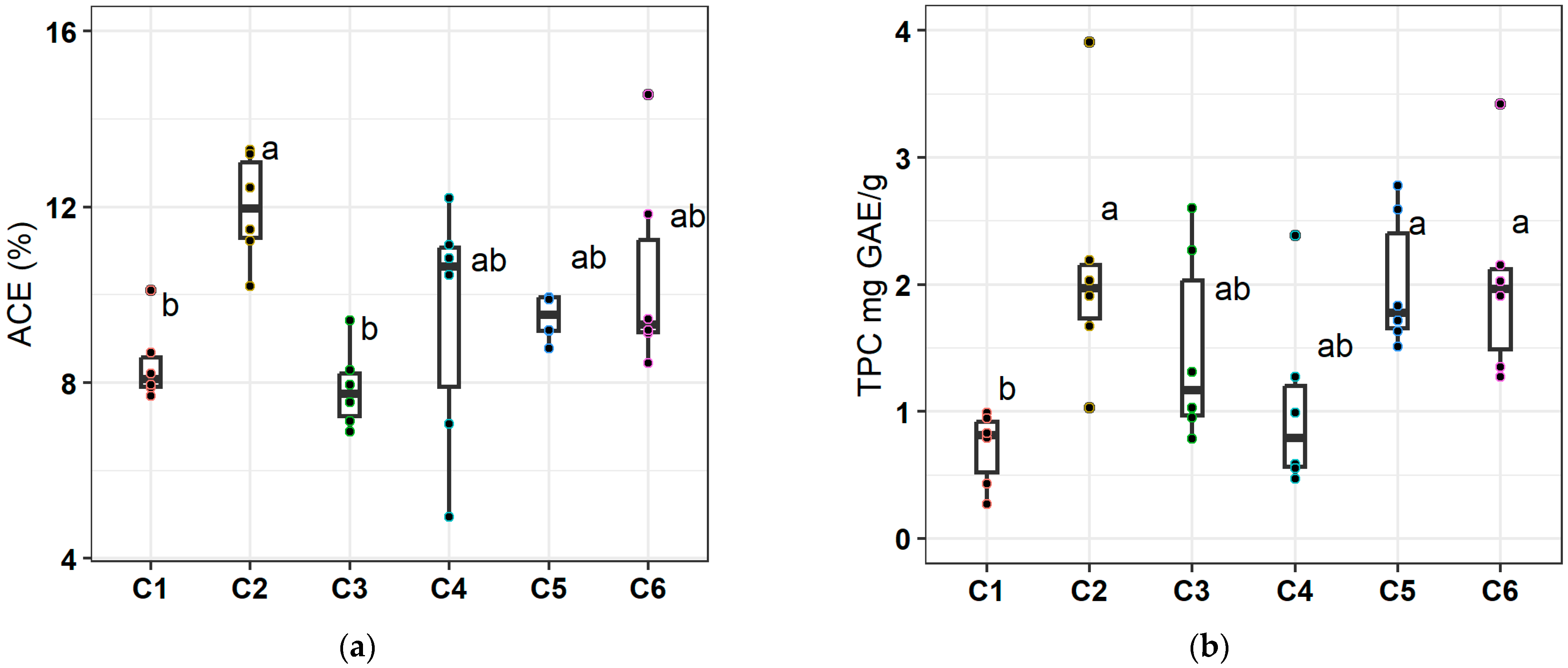
To examine whether changes in nutrient status were related to modifications in the accumulation of specific secondary metabolites in the inner leaf gel of Aloe vera plants, we measured the concentrations of acemannan (Figure 1a) and TPC (Figure 1b) in the lyophilized gel samples. The accumulation of both acemannan and TPC was positively affected by the application of the organic fertilizer. PGPR inoculation induced an increase in acemannan, while BACTILLIS S induced an increase in TPC. Specifically, the highest acemannan concentrations were recorded in the single treatment of organic fertilizer (42.1% compared to value of C1 samples), while, in combined treatments, no significant increase was observed. The positive effect of organic fertilizer was more pronounced for TPC with respect to acemannan, while C2, C6, and C5 treatments led to significant average increases compared to C1.
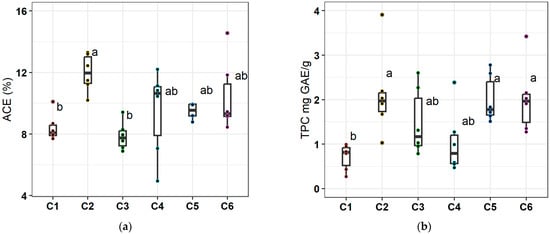
Figure 1.
Variation in the content of (a) acemannan (% d.w.) in the inner leaf gel, and (b) total phenolic content (TPC; mg GAE/g) in the inner leaf gel, across treatments. C1 = control, C2 = organic fertilizer, C3 = BACTILLIS S, C4 = PGPR isolates, C5 = organic fertilizer plus BACTILLIS S, C6 = organic fertilizer plus PGPR isolates. The upper and lower box boundaries indicate the 75th and the 25th percentiles, respectively; the midline indicates the median, and the whiskers above and below indicate the 90th and 10th percentiles, respectively; the dots indicate outliers (n = 6). Boxes with different letter are significantly different according to Duncan’s multiple range test (p < 0.05).
3.6. Correlation Analysis
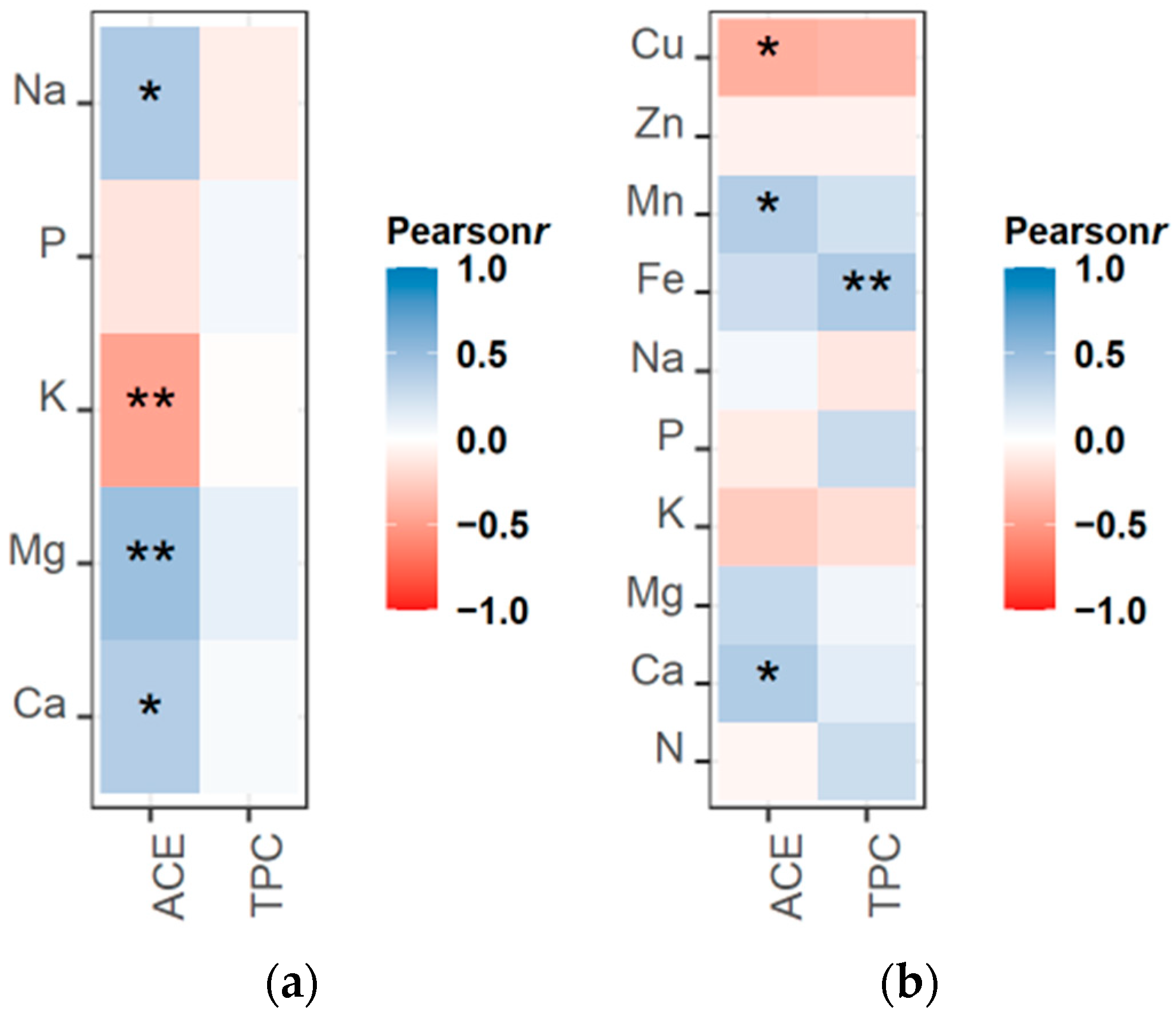
Next, we examined if there was a link between nutrient content and the concentrations of acemannan and TPC in the leaf gel (Figure 2a) and in the rind (Figure 2b). The results showed a strong positive correlation between Mg content in the gel and acemannan concentration. Interestingly, Ca content was not as strongly correlated as Mg content with acemannan concentration, but a significant correlation was still observed for gel and rind samples. A positive association with acemannan concentration was also observed with Na in gel samples and Mn in rind samples. An opposite pattern was observed for K gel content and Cu content in rind samples, which were correlated negatively with acemannan accumulation. Regarding TPC, their concentration was found to only correlate with the concentration of Fe in the rind samples.
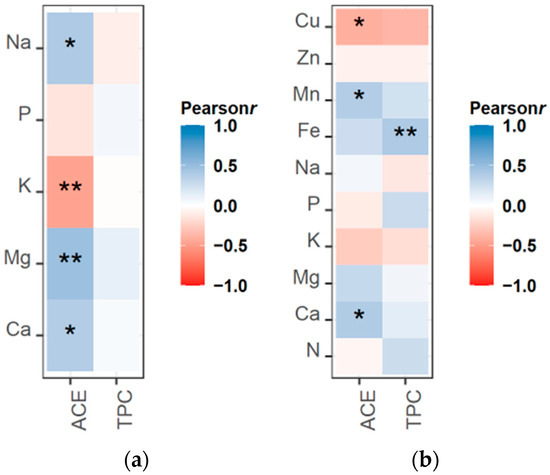
Figure 2.
Correlogram representing Pearson’s correlation coefficient ranking of ACE and TPC with (a) the concentration of macronutrients in the inner leaf gel, and (b) the concentration of macronutrients and micronutrients in the outer leaf rind. Asterisks indicate the significance level of the correlation: * p < 0.05, ** p < 0.01.
Acemannan accumulation was positive affected, for the most part, by all applications of organic fertilizer, as well as by inoculation with the PGPR isolates. For both the organic fertilizer and the PGPR isolates, gel and rind concentrations of Mg and Ca were higher than in the other treatments. Furthermore, in all treatments which contained the organic fertilizer (which was enriched in Ca and Mg), the concentration of available Ca and Mg in the soil was increased. In contrast, single applications of organic fertilizer and PGPR resulted in a reduction in K concentration in the gel and the rind, despite a small increase in K availability recorded in the soil. Apparently, the excess availability of Ca and Mg led to competition between K and Ca/Mg uptake, resulting in reduced uptake of K. Zhang et al. [71] also observed a significant increase in the total soluble sugars of banana plants after the application of a mixture of a calcium magnesium phosphate fertilizer and an organic fertilizer, which was linked to the improved Mg content in the leaves. In addition, the beneficial impact of PGPR isolates on the acemannan concentration is in line with previous investigations, including inoculations with Pseudomonas spp. [40,72] or Enterobacter spp. [73] and sugar elevation in plant tissues. Nevertheless, investigations focusing on the impact of organic fertilization and biostimulant on acemannan production are still limited. A significant increase in the concentration of acemannan (referred to as β-polysaccharides) in the gel of Aloe vera plants, was reported by Cardarelli et al. [51], after the application of a mixed inoculum, consisting of the arbuscular mycorrhiza fungi Glomus intraradices and Glomus mosseae, in a pot experiment.
Regarding TPC, all organic fertilizer applications resulted in increased concentrations, but a correlation was only observed with rind Fe concentration. These results are in agreement with the reported increase in TPC in Aloe vera plants following the application of poultry manure in a field experiment [69]. TPC concentration was also enhanced by all applications of BACTILLIS S. This is in line with previous findings, such as the increase in phenolic compounds of Coriandrum sativum L. and Cichorium endivia L., after the application of a Bacillus halotolerans biofertilizer [74,75]. Moreover, Bacillus subtillis inoculation led to increased TPC levels in tomato plants [76]. Li and Jiang [77] also observed that inoculation of maize with Bacillus aquimaris caused an increase in TPC, under both normal and salt stress conditions.
4. Conclusions
The results of this study showed that bioactive compounds in Aloe vera such as acemannan and total phenolic content were positively affected by organic fertilization rich in Ca and Mg, as well as by microbial biostimulant applications, despite the absence of noticeable changes in plant growth. Although the increase induced by the single application of organic fertilizer was more pronounced, the inoculation with the consortium of PGPR isolates and BACTILLIS-S seemed to also enhance the accumulation of acemannan and TPC. Moreover, the organic fertilizer and the microbial biostimulants of this study improved soil fertility and led to significant differences in the nutrient content of the leaf gel and rind. Noteworthily, the strong correlation between the nutrient content of the leaf gel and rind with the bioactive compounds of Aloe vera plants, particularly between acemmanan and Mg in the gel, and between TPC with Fe in the rind, supports the hypothesis that nutrient acquisition plays a significant role in the secondary metabolism of these plants. This study presents a basis for further investigation of sustainable agricultural practices which promote the production of valuable secondary metabolites, contributing to the resourceful cultivation of Aloe vera plants, as well as to human health.
Author Contributions
Conceptualization, C.N.N., C.E. and D.G.; methodology, C.N.N., A.C., M.T. and A.G.K.; validation, C.N.N., C.E. and D.G.; formal analysis, C.N.N.; investigation, C.N.N.; resources, A.C.; data curation, C.N.N. and D.G.; writing—original draft preparation, C.N.N.; writing—review and editing, C.N.N., M.T. and D.G.; supervision, C.E. and D.G.; project administration, D.G.; funding acquisition, D.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Acknowledgments
The authors would like to thank Voion Aloe vera S.A., especially Georgia Mitropoulou, and Aloe vera producers for their guidance and help. The authors also thank Filio Demirtzoglou and HUMOFERT S.A. for providing BACTILLIS S. Moreover, the authors thank Vasiliki Skiada and Ifigeneia Tsopi for valuable help and discussion.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Añibarro-Ortega, M.; Pinela, J.; Barros, L.; Ćirić, A.; Silva, S.P.; Coelho, E.; Mocan, A.; Calhelha, R.C.; Soković, M.; Coimbra, M.A.; et al. Compositional Features and Bioactive Properties of Aloe vera Leaf (Fillet, Mucilage, and Rind) and Flower. Antioxidants 2019, 8, 444. [Google Scholar] [CrossRef]
- Kahramanoğlu, İ.; Chen, C.; Chen, J.; Wan, C. Chemical Constituents, Antimicrobial Activity, and Food Preservative Characteristics of Aloe vera Gel. Agronomy 2019, 9, 831. [Google Scholar] [CrossRef]
- Sánchez-Machado, D.I.; López-Cervantes, J.; Sendón, R.; Sanches-Silva, A. Aloe vera: Ancient Knowledge with New Frontiers. Trends Food Sci. Technol. 2017, 61, 94–102. [Google Scholar] [CrossRef]
- Eshun, K.; He, Q. Aloe vera: A Valuable Ingredient for the Food, Pharmaceutical and Cosmetic Industries—A Review. Crit. Rev. Food Sci. Nutr. 2004, 44, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Salinas, P.; Salinas, C.; Contreras, R.A.; Zuñiga, G.E.; Dupree, P.; Cardemil, L. Water Deficit and Abscisic Acid Treatments Increase the Expression of a Glucomannan Mannosyltransferase Gene (GMMT) in Aloe vera Burm. F. Phytochemistry 2019, 159, 90–101. [Google Scholar] [CrossRef] [PubMed]
- Kaparakou, E.H.; Kanakis, C.D.; Gerogianni, M.; Maniati, M.; Vekrellis, K.; Skotti, E.; Tarantilis, P.A. Quantitative Determination of Aloin, Antioxidant Activity, and Toxicity of Aloe vera Leaf Gel Products from Greece. J. Sci. Food Agric. 2021, 101, 414–423. [Google Scholar] [CrossRef] [PubMed]
- Cristiano, G.; Murillo-Amador, B.; De Lucia, B. Propagation Techniques and Agronomic Requirements for the Cultivation of Barbados Aloe (Aloe vera (L.) Burm. F.)—A Review. Front. Plant Sci. 2016, 7, 1410. [Google Scholar] [CrossRef]
- Ning, C.; Gao, P.; Wang, B.; Lin, W.; Jiang, N.; Cai, K. Impacts of Chemical Fertilizer Reduction and Organic Amendments Supplementation on Soil Nutrient, Enzyme Activity and Heavy Metal Content. J. Integr. Agric. 2017, 16, 1819–1831. [Google Scholar] [CrossRef]
- Vega-Gálvez, A.; Miranda, M.; Aranda, M.; Henriquez, K.; Vergara, J.; Tabilo-Munizaga, G.; Pérez-Won, M. Effect of High Hydrostatic Pressure on Functional Properties and Quality Characteristics of Aloe vera Gel (Aloe barbadensis Miller). Food Chem. 2011, 129, 1060–1065. [Google Scholar] [CrossRef]
- Kumar, S.; Yadav, A.; Yadav, M.; Yadav, J.P. Effect of Climate Change on Phytochemical Diversity, Total Phenolic Content and in Vitro Antioxidant Activity of Aloe vera (L.) Burm.f. BMC Res. Notes 2017, 10, 60. [Google Scholar] [CrossRef]
- Canche-Escamilla, G.; Colli-Acevedo, P.; Borges-Argaez, R.; Quintana-Owen, P.; May-Crespo, J.F.; Cáceres-Farfan, M.; Yam Puc, J.A.; Sansores-Peraza, P.; Vera-Ku, B.M. Extraction of Phenolic Components from an Aloe vera (Aloe barbadensis Miller) Crop and Their Potential as Antimicrobials and Textile Dyes. Sustain. Chem. Pharm. 2019, 14, 100168. [Google Scholar] [CrossRef]
- Quezada, M.P.; Salinas, C.; Gotteland, M.; Cardemil, L. Acemannan and Fructans from Aloe vera (Aloe barbadensis Miller) Plants as Novel Prebiotics. J. Agric. Food Chem. 2017, 65, 10029–10039. [Google Scholar] [CrossRef]
- Minjares-Fuentes, J.R.; Femenia, A. Effect of Processing on the Bioactive Polysaccharides and Phenolic Compounds from Aloe vera (Aloe barbadensis Miller). In Dietary Fiber Functionality in Food and Nutraceuticals; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2017; pp. 263–287. ISBN 978-1-119-13810-5. [Google Scholar]
- Hamman, J.H. Composition and Applications of Aloe vera Leaf Gel. Molecules 2008, 13, 1599–1616. [Google Scholar] [CrossRef] [PubMed]
- Sadgrove, N.J.; Simmonds, M.S.J. Pharmacodynamics of Aloe vera and Acemannan in Therapeutic Applications for Skin, Digestion, and Immunomodulation. Phytother. Res. 2021, 35, 6572–6584. [Google Scholar] [CrossRef] [PubMed]
- Voiniciuc, C. Modern Mannan: A Hemicellulose’s Journey. New Phytol. 2022, 234, 1175–1184. [Google Scholar] [CrossRef]
- He, C.; Wu, K.; Zhang, J.; Liu, X.; Zeng, S.; Yu, Z.; Zhang, X.; Teixeira da Silva, J.A.; Deng, R.; Tan, J.; et al. Cytochemical Localization of Polysaccharides in Dendrobium Officinale and the Involvement of DoCSLA6 in the Synthesis of Mannan Polysaccharides. Front. Plant Sci. 2017, 8, 173. [Google Scholar] [CrossRef]
- Femenia, A.; Sánchez, E.S.; Simal, S.; Rosselló, C. Compositional Features of Polysaccharides from Aloe vera (Aloe barbadensis Miller) Plant Tissues. Carbohydr. Polym. 1999, 39, 109–117. [Google Scholar] [CrossRef]
- Yang, L.; Wen, K.-S.; Ruan, X.; Zhao, Y.-X.; Wei, F.; Wang, Q. Response of Plant Secondary Metabolites to Environmental Factors. Molecules 2018, 23, 762. [Google Scholar] [CrossRef]
- Douglas, A.E. Strategies for Enhanced Crop Resistance to Insect Pests. Annu. Rev. Plant Biol. 2018, 69, 637–660. [Google Scholar] [CrossRef]
- Murthy, H.N.; Lee, E.-J.; Paek, K.-Y. Production of Secondary Metabolites from Cell and Organ Cultures: Strategies and Approaches for Biomass Improvement and Metabolite Accumulation. Plant Cell Tiss. Organ Cult. 2014, 118, 1–16. [Google Scholar] [CrossRef]
- Arsenault, P.R.; Wobbe, K.K.; Weathers, P.J. Recent Advances in Artemisinin Production through Heterologous Expression. Curr. Med. Chem. 2008, 15, 2886–2896. [Google Scholar] [CrossRef]
- Sampaio, B.L.; Edrada-Ebel, R.; Da Costa, F.B. Effect of the Environment on the Secondary Metabolic Profile of Tithonia Diversifolia: A Model for Environmental Metabolomics of Plants. Sci. Rep. 2016, 6, 29265. [Google Scholar] [CrossRef]
- Pant, P.; Pandey, S.; Dall’Acqua, S. The Influence of Environmental Conditions on Secondary Metabolites in Medicinal Plants: A Literature Review. Chem. Biodivers. 2021, 18, e2100345. [Google Scholar] [CrossRef]
- Qiu, C.; Sun, J.; Shen, J.; Zhang, S.; Ding, Y.; Gai, Z.; Fan, K.; Song, L.; Chen, B.; Ding, Z.; et al. Fulvic Acid Enhances Drought Resistance in Tea Plants by Regulating the Starch and Sucrose Metabolism and Certain Secondary Metabolism. J. Proteom. 2021, 247, 104337. [Google Scholar] [CrossRef]
- Pereira, M.M.A.; Morais, L.C.; Marques, E.A.; Martins, A.D.; Cavalcanti, V.P.; Rodrigues, F.A.; Gonçalves, W.M.; Blank, A.F.; Pasqual, M.; Dória, J. Humic Substances and Efficient Microorganisms: Elicitation of Medicinal Plants—A Review. JAS 2019, 11, 268. [Google Scholar] [CrossRef]
- Wei, X.; Chen, J.; Gao, B.; Wang, Z. Chapter 39—Role of Controlled and Slow Release Fertilizers in Fruit Crop Nutrition. In Fruit Crops; Srivastava, A.K., Hu, C., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 555–566. ISBN 978-0-12-818732-6. [Google Scholar]
- Soil Atlas 2015|Global Soil Week. Available online: https://gsf.globalsoilweek.org/soilatlas-2015 (accessed on 14 December 2022).
- Riahi, L.; Cherif, H.; Miladi, S.; Neifar, M.; Bejaoui, B.; Chouchane, H.; Masmoudi, A.S.; Cherif, A. Use of Plant Growth Promoting Bacteria as an Efficient Biotechnological Tool to Enhance the Biomass and Secondary Metabolites Production of the Industrial Crop Pelargonium Graveolens L’Hér. under Semi-Controlled Conditions. Ind. Crops Prod. 2020, 154, 112721. [Google Scholar] [CrossRef]
- Tennakoon, P.L.K.; Rajapaksha, R.M.C.P.; Hettiarachchi, L.S.K. Tea Yield Maintained in PGPR Inoculated Field Plants despite Significant Reduction in Fertilizer Application. Rhizosphere 2019, 10, 100146. [Google Scholar] [CrossRef]
- Building a Sustainable and Food-Secure World Starts with the Right to Food. Available online: http://www.fao.org/publications/highlights-detail/en/c/1458917/ (accessed on 6 February 2023).
- Das, P.P.; Singh, K.R.; Nagpure, G.; Mansoori, A.; Singh, R.P.; Ghazi, I.A.; Kumar, A.; Singh, J. Plant-Soil-Microbes: A Tripartite Interaction for Nutrient Acquisition and Better Plant Growth for Sustainable Agricultural Practices. Environ. Res. 2022, 214, 113821. [Google Scholar] [CrossRef] [PubMed]
- Egamberdieva, D.; Shrivastava, S.; Varma, A. (Eds.) Plant-Growth-Promoting Rhizobacteria (PGPR) and Medicinal Plants; Soil Biology; Springer International Publishing: Cham, Switzerland, 2015; Volume 42, ISBN 978-3-319-13400-0. [Google Scholar]
- Ibort, P.; Imai, H.; Uemura, M.; Aroca, R. Proteomic Analysis Reveals That Tomato Interaction with Plant Growth Promoting Bacteria Is Highly Determined by Ethylene Perception. J. Plant Physiol. 2018, 220, 43–59. [Google Scholar] [CrossRef]
- Lugtenberg, B.; Kamilova, F. Plant-Growth-Promoting Rhizobacteria. Annu. Rev. Microbiol. 2009, 63, 541–556. [Google Scholar] [CrossRef]
- Cai, Z.; Kastell, A.; Speiser, C.; Smetanska, I. Enhanced Resveratrol Production in Vitis Vinifera Cell Suspension Cultures by Heavy Metals without Loss of Cell Viability. Appl. Biochem. Biotechnol. 2013, 171, 330–340. [Google Scholar] [CrossRef] [PubMed]
- Cappellari, L.D.R.; Santoro, M.V.; Schmidt, A.; Gershenzon, J.; Banchio, E. Induction of Essential Oil Production in Mentha x Piperita by Plant Growth Promoting Bacteria Was Correlated with an Increase in Jasmonate and Salicylate Levels and a Higher Density of Glandular Trichomes. Plant Physiol. Biochem. 2019, 141, 142–153. [Google Scholar] [CrossRef] [PubMed]
- Walker, V.; Bertrand, C.; Bellvert, F.; Moënne-Loccoz, Y.; Bally, R.; Comte, G. Host Plant Secondary Metabolite Profiling Shows a Complex, Strain-Dependent Response of Maize to Plant Growth-Promoting Rhizobacteria of the Genus Azospirillum. New Phytol. 2011, 189, 494–506. [Google Scholar] [CrossRef] [PubMed]
- Miliordos, D.-E.; Tsiknia, M.; Kontoudakis, N.; Dimopoulou, M.; Bouyioukos, C.; Kotseridis, Y. Impact of Application of Abscisic Acid, Benzothiadiazole and Chitosan on Berry Quality Characteristics and Plant Associated Microbial Communities of Vitis Vinifera L Var. Mouhtaro Plants. Sustainability 2021, 13, 5802. [Google Scholar] [CrossRef]
- Desrut, A.; Moumen, B.; Thibault, F.; Le Hir, R.; Coutos-Thévenot, P.; Vriet, C. Beneficial Rhizobacteria Pseudomonas simiae WCS417 Induce Major Transcriptional Changes in Plant Sugar Transport. J. Exp. Bot. 2020, 71, 7301–7315. [Google Scholar] [CrossRef]
- Bouyoucos, G.J. A Recalibration of the Hydrometer Method for Making Mechanical Analysis of Soils1. Agron. J. 1951, 43, 434–438. [Google Scholar] [CrossRef]
- Carter, M.R. Soil Sampling and Methods of Analysis; CRC Press: Boca Raton, FL, USA, 1993; ISBN 978-0-87371-861-5. [Google Scholar]
- Nelson, D.W.; Sommers, L.E. Total Carbon, Organic Carbon, and Organic Matter. In Methods of Soil Analysis; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 1983; pp. 539–579. ISBN 978-0-89118-977-0. [Google Scholar]
- Bremner, J.M.; Mulvaney, C.S. Nitrogen—Total. In Methods of Soil Analysis; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 1983; pp. 595–624. ISBN 978-0-89118-977-0. [Google Scholar]
- Lindsay, W.L.; Norvell, W.A. Development of a DTPA Soil Test for Zinc, Iron, Manganese, and Copper. Soil Sci. Soc. Am. J. 1978, 42, 421–428. [Google Scholar] [CrossRef]
- Massas, I.; Kalivas, D.; Ehaliotis, C.; Gasparatos, D. Total and Available Heavy Metal Concentrations in Soils of the Thriassio Plain (Greece) and Assessment of Soil Pollution Indexes. Environ. Monit. Assess. 2013, 185, 6751–6766. [Google Scholar] [CrossRef]
- Olsen, S.R. Estimation of Available Phosphorus in Soils by Extraction with Sodium Bicarbonate; U.S. Department of Agriculture: Washington, DC, USA, 1954. [Google Scholar]
- Dick, W.A.; Tabatabai, M.A. Determination of Orthophosphate in Aqueous Solutions Containing Labile Organic and Inorganic Phosphorus Compounds. J. Environ. Qual. 1977, 6, 82–85. [Google Scholar] [CrossRef]
- Bremner, J.M. Total Nitrogen. In Methods of Soil Analysis; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 1965; pp. 1149–1178. ISBN 978-0-89118-204-7. [Google Scholar]
- Eberendu, A.R.; Luta, G.; Edwards, J.A.; McAnalley, B.H.; Davis, B.; Rodriguez, S.; Henry, C.R. Quantitative Colorimetric Analysis of Aloe Polysaccharides as a Measure of Aloe vera Quality in Commercial Products. J. AOAC Int. 2005, 88, 684–691. [Google Scholar] [CrossRef]
- Cardarelli, M.; Rouphael, Y.; Rea, E.; Lucini, L.; Pellizzoni, M.; Colla, G. Effects of Fertilization, Arbuscular Mycorrhiza, and Salinity on Growth, Yield, and Bioactive Compounds of Two Aloe Species. Horts 2013, 48, 568–575. [Google Scholar] [CrossRef]
- Folin, O.; Ciocalteu, V. On Tyrosine and Tryptophane Determinations in Proteins. J. Biol. Chem. 1927, 73, 627–650. [Google Scholar] [CrossRef]
- Mendiburu, F.D.; Simon, R. Agricolae—Ten Years of an Open Source Statistical Tool for Experiments in Breeding, Agriculture and Biology; PeerJ Inc.: California, CA, USA, 2015. [Google Scholar]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016; ISBN 978-3-319-24277-4. [Google Scholar]
- Olk, D.C.; Dinnes, D.L.; Rene Scoresby, J.; Callaway, C.R.; Darlington, J.W. Humic Products in Agriculture: Potential Benefits and Research Challenges—A Review. J. Soils Sediments 2018, 18, 2881–2891. [Google Scholar] [CrossRef]
- Saxena, A.K.; Kumar, M.; Chakdar, H.; Anuroopa, N.; Bagyaraj, D.J. Bacillus Species in Soil as a Natural Resource for Plant Health and Nutrition. J. Appl. Microbiol. 2020, 128, 1583–1594. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Nadeem, S.M.; Naveed, M.; Zahir, Z.A. Potassium-Solubilizing Bacteria and Their Application in Agriculture. In Potassium Solubilizing Microorganisms for Sustainable Agriculture; Meena, V.S., Maurya, B.R., Verma, J.P., Meena, R.S., Eds.; Springer India: New Delhi, India, 2016; pp. 293–313. ISBN 978-81-322-2776-2. [Google Scholar]
- Meena: Agriculturally Important Microbes for Sustainable. Available online: https://scholar.google.com/scholar_lookup?hl=en&publication_year=2017&pages=25-61&author=N.+Kumawat&author=R.+Kumar&author=S.+Kumar&author=V.S.+Meena&isbn=%00null%00&title=Applications+in+Crop+Production+and+Protection (accessed on 29 December 2022).
- Bhaskar, R.; Xavier, L.S.E.; Udayakumaran, G.; Kumar, D.S.; Venkatesh, R.; Nagella, P. Biotic Elicitors: A Boon for the in-Vitro Production of Plant Secondary Metabolites. Plant Cell Tiss. Organ Cult. 2022, 149, 7–24. [Google Scholar] [CrossRef]
- Mahapatra, S.; Yadav, R.; Ramakrishna, W. Bacillus subtilis Impact on Plant Growth, Soil Health and Environment: Dr. Jekyll and Mr. Hyde. J. Appl. Microbiol. 2022, 132, 3543–3562. [Google Scholar] [CrossRef] [PubMed]
- Khajeeyan, R.; Salehi, A.; Movahhedi Dehnavi, M.; Farajee, H.; Kohanmoo, M.A. Growth Parameters, Water Productivity and Aloin Content of Aloe vera Affected by Mycorrhiza and PGPR Application under Different Irrigation Regimes. South Afr. J. Bot. 2022, 147, 1188–1198. [Google Scholar] [CrossRef]
- Lopez, B.R.; Tinoco-Ojanguren, C.; Bacilio, M.; Mendoza, A.; Bashan, Y. Endophytic Bacteria of the Rock-Dwelling Cactus Mammillaria Fraileana Affect Plant Growth and Mobilization of Elements from Rocks. Environ. Exp. Bot. 2012, 81, 26–36. [Google Scholar] [CrossRef]
- Di, Y.; Kui, L.; Singh, P.; Liu, L.; Xie, L.; He, L.; Li, F. Identification and Characterization of Bacillus subtilis B9: A Diazotrophic Plant Growth-Promoting Endophytic Bacterium Isolated from Sugarcane Root. J Plant Growth Regul. 2022, 41, 1–18. [Google Scholar] [CrossRef]
- Kalinowski, B.E.; Liermann, L.J.; Givens, S.; Brantley, S.L. Rates of Bacteria-Promoted Solubilization of Fe from Minerals: A Review of Problems and Approaches. Chem. Geol. 2000, 169, 357–370. [Google Scholar] [CrossRef]
- Sayyed, R.Z.; Chincholkar, S.B.; Reddy, M.S.; Gangurde, N.S.; Patel, P.R. Siderophore Producing PGPR for Crop Nutrition and Phytopathogen Suppression. In Bacteria in Agrobiology: Disease Management; Maheshwari, D.K., Ed.; Springer: Berlin, Heidelberg, 2013; pp. 449–471. ISBN 978-3-642-33639-3. [Google Scholar]
- Lv, L.; Luo, J.; Ahmed, T.; Zaki, H.E.M.; Tian, Y.; Shahid, M.S.; Chen, J.; Li, B. Beneficial Effect and Potential Risk of Pantoea on Rice Production. Plants 2022, 11, 2608. [Google Scholar] [CrossRef]
- Gilliham, M.; Dayod, M.; Hocking, B.J.; Xu, B.; Conn, S.J.; Kaiser, B.N.; Leigh, R.A.; Tyerman, S.D. Calcium Delivery and Storage in Plant Leaves: Exploring the Link with Water Flow. J. Exp. Bot. 2011, 62, 2233–2250. [Google Scholar] [CrossRef]
- Kim, Y.-C.; Glick, B.R.; Bashan, Y.; Ryu, C.-M. Enhancement of Plant Drought Tolerance by Microbes. In Plant Responses to Drought Stress: From Morphological to Molecular Features; Aroca, R., Ed.; Springer: Berlin, Heidelberg, 2012; pp. 383–413. ISBN 978-3-642-32653-0. [Google Scholar]
- Chowdhury, T.; Chowdhury, M.A.H.; Qingyue, W.; Enyoh, C.E.; Wang, W.; Khan, M.S.I. Nutrient Uptake and Pharmaceutical Compounds of Aloe vera as Influenced by Integration of Inorganic Fertilizer and Poultry Manure in Soil. Heliyon 2021, 7, e07464. [Google Scholar] [CrossRef]
- Ragel, P.; Raddatz, N.; Leidi, E.O.; Quintero, F.J.; Pardo, J.M. Regulation of K+ Nutrition in Plants. Front. Plant Sci. 2019, 10, 281. [Google Scholar] [CrossRef]
- Zhang, C.; Zhou, T.; Zhu, L.; Du, Z.; Li, B.; Wang, J.; Wang, J.; Sun, Y. Using Enzyme Activities and Soil Microbial Diversity to Understand the Effects of Fluoxastrobin on Microorganisms in Fluvo-Aquic Soil. Sci. Total Environ. 2019, 666, 89–93. [Google Scholar] [CrossRef]
- Sandhya, V.; Ali, S.Z.; Grover, M.; Reddy, G.; Venkateswarlu, B. Effect of Plant Growth Promoting Pseudomonas spp. on Compatible Solutes, Antioxidant Status and Plant Growth of Maize under Drought Stress. Plant Growth Regul. 2010, 62, 21–30. [Google Scholar] [CrossRef]
- Singh, R.P.; Jha, P.; Jha, P.N. Bio-Inoculation of Plant Growth-Promoting Rhizobacterium Enterobacter cloacae ZNP-3 Increased Resistance Against Salt and Temperature Stresses in Wheat Plant (Triticum aestivum L.). J. Plant Growth Regul. 2017, 36, 783–798. [Google Scholar] [CrossRef]
- Jiménez-Gómez, A.; García-Estévez, I.; García-Fraile, P.; Escribano-Bailón, M.T.; Rivas, R. Increase in Phenolic Compounds of Coriandrum sativum L. after the Application of a Bacillus halotolerans Biofertilizer. J. Sci. Food Agric. 2020, 100, 2742–2749. [Google Scholar] [CrossRef]
- Jiménez-Gómez, A.; García-Estévez, I.; Escribano-Bailón, M.T.; García-Fraile, P.; Rivas, R. Bacterial Fertilizers Based on Rhizobium laguerreae and Bacillus halotolerans Enhance Cichorium endivia L. Phenolic Compound and Mineral Contents and Plant Development. Foods 2021, 10, 424. [Google Scholar] [CrossRef]
- Chandrasekaran, M.; Chun, S.C.; Oh, J.W.; Paramasivan, M.; Saini, R.K.; Sahayarayan, J.J. Bacillus subtilis CBR05 for Tomato (Solanum lycopersicum) Fruits in South Korea as a Novel Plant Probiotic Bacterium (PPB): Implications from Total Phenolics, Flavonoids, and Carotenoids Content for Fruit Quality. Agronomy 2019, 9, 838. [Google Scholar] [CrossRef]
- Li, H.Q.; Jiang, X.W. Inoculation with Plant Growth-Promoting Bacteria (PGPB) Improves Salt Tolerance of Maize Seedling. Russ. J. Plant Physiol. 2017, 64, 235–241. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).