Abstract
The Germplasm Bank of Stevia rebaudiana segregants of the University of Córdoba is a strategy for the use of genetic variability and efficient crop yield. There are genotypes with important characteristics such as: high tolerance to salt stress and climatic change (high CO2 in the Earth surface), late flowering, erect architecture, and high contents of steviol glycosides. However, there is a lack of in-depth studies of morphophysiological and biochemical indicators such as gas exchange, chlorophyll a fluorescence, chloroplast pigments, and antioxidant systems, which allow us to develop early selection tools for elite genotypes. The genotypes (L020, Morita II, and L102) were found to have elite characteristics such as high efficiency in water use, excellent biomass production, and a more robust antioxidant system than the genotypes (L057 and L082). The L020 genotype presented the highest content of stevioside and rebaudioside A, followed by the Morita II genotype. We found a close correlation between the electron transport rate and the mechanisms that increase photosystem complexes. In this sense, non-photochemical cooling modulated by the release of heat by the leaves is a fact that is confirmed by the greater activity of the xanthine pool to protect the photochemical complexes in S. rebaudiana.
1. Introduction
Stevia rebaudiana (Bert.) is a perennial herbaceous plant of the Asteraceae family. It was historically cultivated by aboriginal peoples [1], who believed that S. rebaudiana had the sweetest essence. The plant presents diterpenic glycosides 300 times sweeter than sucrose [2], being a powerful natural sweetener that is low in calories, has medicinal and therapeutic properties, and is beneficial to health. More than 144 genotypes are registered in the world, of which the Morita II genotype stands out as the most planted genotype in the world [3]. Yadav et al. [4] described a total of 183 randomly amplified polymorphic DNA (RAPD) markers in S. rebaudiana. Of the markers that showed amplification products, 35.5% detected polymorphic loci, and 62.5% of these marker loci segregated in a 1:1 ratio, indicating a high level of genetic diversity in S. rebaudiana. The high genetic variabilities of genotypes originating in Paraguay and Malaysia were described based on morphological, chemical, and molecular characteristics traits [5]. Studies such as these usually describe varieties or genotypes that are more adapted to certain conditions in a country or region [6].
Due to its industrial importance, Stevia is commercially cultivated in several parts of the world [7]. The Stevia industry is rising rapidly due to the increasing base of health-conscious consumers and preference for natural non-caloric sweeteners [8]. The global Stevia market size is estimated to be valued at US$ 647.7 million in 2022 [9] and is projected to grow at a compound annual growth rate (CAGR) of 8.0% to USD 1.14 billion in 2028 [10]. Stevia is also used extensively in the pharmaceutical and cosmetics industry due to its antimicrobial, antihypertensive, antioxidant, and skin hydration properties. [11]. According to a World Health Organization (WHO) survey [12], approximately 500 million people worldwide will be diabetic by 2030 due to the increased consumption of products sweetened with refined sugar, a market which can be satisfactorily replaced by natural sweeteners, with S. rebaudiana leading the consumer market. Today, China is the largest producer and exporter of Stevia, while Japan is the primary consumer [13].
To date, most of the research on the physiological responses in Stevia rebaudiana plants has concentrated on improving its crop production [14,15,16,17,18], salt content [19,20,21,22], and water tolerance [23] or retarding flowering to provide more time for leaf harvest [24,25,26]. Recently, Pompelli et al. [27] physiologically characterized two genotypes of S. rebaudiana cultivated under a CO2-enriched-atmosphere, proving that the photosynthetic efficiency of the species can be linked to a photoprotective efficiency and can overcome stomatic, mesophilic, and biochemical barriers that limit photosynthesis. Selection of the S. rebaudiana genotypes and the optimal environmental conditions that elicit higher stevioside production is the main goal. However, the performance of S. rebaudiana grown in land conditions has received meager attention. This research has as its main goal to analyze some morphological, physiological, biochemical, and enzymatic features in order to select a rapid and sensitive measurement tool based on these features to help test and select elite genotypes of S. rebaudiana. Within this assumption, the main goals of this study were the full characterization of a genotype with characteristics similar to the most cultivated genotype in the world (Morita II), presenting an efficiency of water use twice or three times higher than the Morita II genotype, with excellent characteristics of productivity and other genotypes that have leaf yield similar to the control cultivar, and incorporating the photoprotective efficiency of photosystems (PS), which sustains photosynthetic rates twice as high as Morita II genotype. However, the water use efficiency requires improvement.
2. Materials and Methods
2.1. Plant Material and Environmental Conditions
From the 115 segregants produced by self-crossing the Morita II, our research group (unpublished data) initially selected 86 genotypes, which were cultivated in a Biospace where 25 genotypes were selected with agromorphological characteristics of great interest, such as erect growth, large leaf volume [28], flowering delay [24], and high productivity [29]. These 25 genotypes were then cultivated in a type I biospace (1000 m2) [29] for 12 months, where 5 genotypes with very promising characteristics for plant genetic improvement were selected. This study, therefore, begins with these five genotypes with promising characteristics. Thus, 15-day-old cuttings of S. rebaudiana genotypes (L020, L057, L082, L102, and Morita) were transferred to land conditions with a pH of 6.08, organic matter of 1.64, Cation-Exchange Capacity (CEC) of 18.8, and clay loam—silty texture [29] at the experimental farm of the Faculty of Agricultural Sciences at the University of Cordoba, Montería, Colombia (8°47′37″ N; 75°51′51″ W, 15 m a.s.l.), experiencing a mean annual rainfall of 1346 mm, relative humidity of 84%, and mean annual temperature of 27.4 °C [30]. Montería is characterized as a tropical wet climate according to the Köppen climate classification and has a Martonne aridity index of 40.6 [30]. The cuttings were left to grow in this condition for 15 days; plantlets at 30 days were used in this physiological characterization. The plants were irrigated every other day using dripper tubes with a flow of 2.5-L h−1 and nominal diameter of 13 mm, with two drippers per plant, spaced 0.15 m apart to replace the evaporated water measured by the weather station.
2.2. Gas Exchange
The parameters of gas exchange were done on 30-day-old S. rebaudiana plantlets. The leaf gas exchange was determined on the 3rd attached fully expanded leaf from the apex, using a portable open-flow infrared gas analyzer (LI-6400XT; LI-COR Inc., Lincoln, NE, USA). The net photosynthesis (AN, μmol CO2 m−2 s−1), stomatal conductance (gs, μmol H2O m−2 s−1), transpiration (E, mmol H2O m−2 s−1), and internal CO2 concentration (Ci, μmol CO2 mol−1) were measured at approximately 09:00–11:00 h solar time, under a clear sky and under the leaf irradiance of 1300 μmol photons m−2 s−1 as 80% of light saturation point, as recently described [27], a fixed CO2 concentration (Ca) in 400 μmol mol−1, and an air flow of 400 μmol s−1 [31]. To evaluate if the decrease of AN in S. rebaudiana is limited by stomatic barriers, we applied the concept of a stomatal threshold limitation (SL), calculated according to the following formula: [32], where Ci is an internal CO2 concentration (in substomatal chamber) and Ca is a standard CO2, given by a CO2 cartridge (iSi pure CO2 cartridge, iSi inspiring solutions, Wien, Austria). From photosynthesis parameters, we calculated the intrinsic water use efficiency (WUEi) and vapor pressure deficit (VPD; kPa).
2.3. Chlorophyll a Fluorescence
In 30 min on dark adapted leaves, the full open oxidized fluorescence was measured at predawn (05:00 a.m.), midday (12:00 p.m.), and last afternoon (07:00 p.m.) using a pocket PEA chlorophyll fluorimeter (Hansatech Instruments, Norfolk, UK). Initially, initial fluorescence (F0) data were sampled at 10 µs intervals for the first 300 µseconds. Afterward, 3500 µmol m−2 s−1 intensity with a peak wavelength of 627 nm was applied to measure maximum fluorescence (Fm). These data were used to calculate the variable fluorescence as Fv (Fm-F0), Fv:Fm ratio, Fv:F0 ratio, and F0:Fm ratio. In addition, in light-adapted leaves, an actinic PAR (1200 μmol photons m−2 s−1) was applied for 300 s. Subsequently, a saturating white light pulse of 6000 μmol photons m−2 s−1 was used to achieve the light-adapted maximum fluorescence (Fm’), and finally a far-red illumination was applied to measure the light-adapted initial fluorescence (F0′). All other parameters were calculated in accordance with Pompelli et al. [33]. The fluorescence in light-adapted leaves was determined with a portable open-flow infrared gas analyzer (LI-6400XT; LI-COR Inc., Lincoln, NE, USA) integrated with fluorescence chamber heads (LI-6400-40; LI-COR Inc.).
2.4. Biochemical Analysis
2.4.1. Photosynthetic Pigments and Xantophyll Measurements
Chlorophyll a + b and total carotenoids were measured in the leaf samples at the same time the gas exchange measurements were performed. In addition, leaves were sampled at approximately predawn (05:00 a.m.) and midday (12:00 p.m.) to measure xanthophyll pool pigments. For this, the leaves were sampled and promptly immersed in liquid nitrogen (N2) to stop all biochemical reaction and then stored in darkness at −40 °C until use. Chlorophyll (Chl) was extracted with 100% methanol as previously described in Silva et al. [34] and quantified spectrophotometrically according to Lichtenthaler and Buschmann [35] equations, modified by Pompelli et al. [36] to adapt to microplate readings (ThermoScientific™ Multiskan™ GO, Missouri City, TX, USA). Violaxanthin (V), antheraxanthin (A), and zeaxanthin (Z) pigments were extracted with ice-cold 90% acetone, and the homogenate was collected in 2 mL microvials. All samples were then bubbled with gaseous nitrogen, after which they were kept in darkness for 30 min at 4 °C. Subsequently, the homogenate was then used to measure the xanthophyll pigments as fully previously detailed for S. rebaudiana leaves [27].
2.4.2. Protein Analysis
In the methanolic insoluble fraction, used to extract chlorophylls, soluble proteins were analyzed as per the previously tested protocol [37].
2.4.3. Antioxidant Enzyme Activity and Hydrogen Peroxide Analysis
Four attached fully expanded leaves were sampled and promptly immersed in N2 to stop all biochemical reaction and then stored in darkness at −40 °C until use. Catalase (CAT; EC 1.11.1.6) and ascorbate peroxidase (APX; EC 1.11.1.11) were extracted using extraction buffer containing 50 mM potassium phosphate buffer (pH 7.0), 2 mM EDTA (Sigma-Aldrich Chemical Co, Darmstadt, Germany, part number ED-500G), 20 mM ascorbic acid (Sigma-Aldrich, Darmstadt, Germany, part number A1300000), 10% Tween 20 (Sigma-Aldrich, Darmstadt, Germany, part number 11332465001), and 80% PVPP (Sigma-Aldrich, Darmstadt, Germany, part number 77627). The samples were centrifuged at 15,000× g for 10 min at 4 °C, and the supernatant transferred to new microtubes in an icebox. CAT activity was measured in 50 mM potassium phosphate buffer (pH 7.0) plus 0.1 mM H2O2. The reaction was started with the addition of a 30 μL sample, previously tested for its linearity. The absorbance was measured in 240 nm every 15 s, and delta (Δ) Abs was measured after 2 min. Activity measurement was estimated using the H2O2 extinction coefficient of 39.4 mM cm−1 and expressed on the basis of CAT units by soluble protein. One unit of CAT was defined as the amount of enzyme required to oxidize 1 μmol of H2O2 per minute. APX activity was measured in 50 mM potassium phosphate buffer (pH 7.0), 0.1 mM H2O2, and 0.5 mM ascorbic acid. The reaction was started with the addition of 40 μL sample, previously tested for its linearity. The absorbance was measured in 290 nm every 15 s, and ΔAbs measured after 2 min. Activity measurement was estimated using the ascorbic acid extinction coefficient of 2.8 mM cm−1 and expressed using APX units by soluble protein. One unit of APX was defined as the amount of enzyme required to oxidize 1 μmol of ascorbate per minute.
The hydrogen peroxide (H2O2) concentration was measured after extraction with 0.1% trichloroacetic acid (Sigma-Aldrich, Darmstadt, Germany, part number T6399). The samples were centrifuged at 15,000× g for 10 min at 4 °C, and the supernatant transferred to new microtubes in an icebox. The H2O2 concentration was estimated in 100 mM potassium phosphate buffer (pH 7.8) plus 1 M KI. The reaction was done with the addition of 40 μL sample, previously tested for its linearity. After 1 h in darkness, the absorbance was measured at 400 nm, and the results were expressed as dry weight (DW).
2.5. Steviol Glycoside Measurements
Steviol glycosides were measured in the leaf samples at the same time the gas exchange measurements were performed. Sixty-day-old leaves were used in this analysis. A protocol described in detail by Rivera-Avilez [24] was used.
2.6. Biomass Quantification
On 60-day S. rebaudiana plantlets, the dry weight of leaf and stalk was measured after 48 h at 70 °C. The DW was measured after dry cooling in an analytical balance (Sartorius Analytical Balance mod. ENTRIS224-1S, Bradford, MA, USA; accurate to 0.1 mg).
2.7. Experimental Design and Statistical Analyses
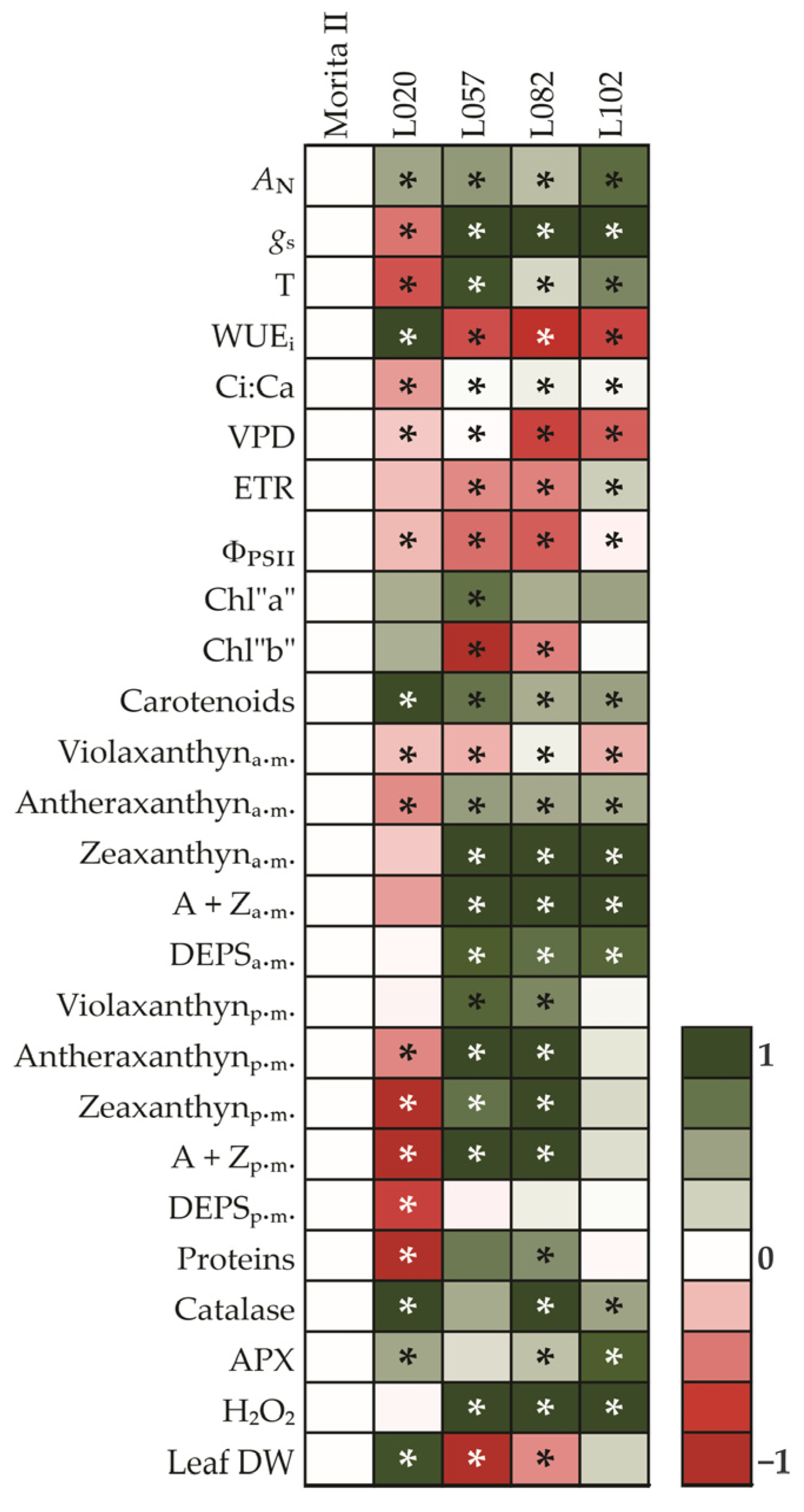
The experiments were conducted in a completely randomized block design composed of 5 genotypes (L020, L057, L082, L102, and Morita). The full open oxidized fluorescence was analyzed by two-way ANOVA, composed of 5 genotypes (L020, L057, L082, L102, and Morita) and 3 daily time (predawn, midday, and late afternoon) measurements. The VAZ was analyzed by two-way ANOVA, composed of 5 genotypes (L020, L057, L082, L102, and Morita) and 2 daily times (predawn and midday). All other features were analyzed by one-way ANOVA, composed of 5 genotypes (L020, L057, L082, L102, and Morita). All analyzed features were composed of 5 repetitions. The one-way ANOVA was performed to compare medians using an SNK test (p < 0.05) in SigmaPlot for Windows v. 14.0 (Systat Software, Inc., San Jose, CA, USA). The principal component analysis was estimated after multivariate analysis for all analyzed features in Minitab 18.1 (Minitab, Inc., Chicago, IL, USA). Heatmaps were used to compare the mean of each genotype, using the Morita genotype as a reference. After log2 transformation, the false color method was used, including a color scale. The heatmaps were constructed using Microsoft® Office 360 (Microsoft Corporation, Redmond, WA, USA) and CorelDRAW Graphics Suite X8 (Corel Corporation, Ottawa, ON, Canada).
3. Results
3.1. Gas Exchange
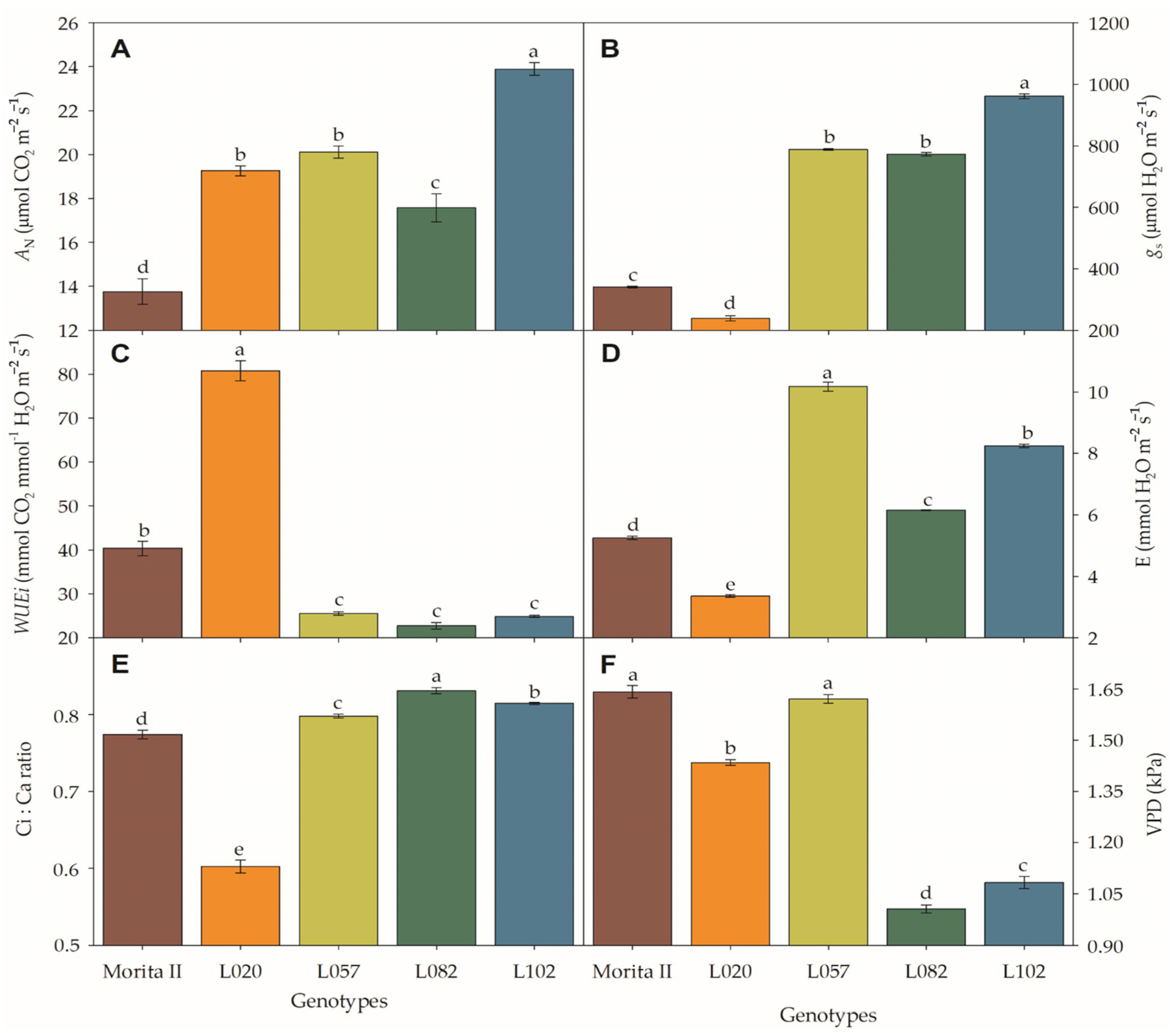
In respect to net photosynthesis (AN), all genotypes tested showed superiority to the model genotype (Morita II; AN = 13.8 μmol CO2 m−2 s−1) with AN 28% (17.6 μmol CO2 m−2 s−1 in L082), 40% (19.3 μmol CO2 m−2 s−1 in L020 genotype), 46% (20.1 μmol CO2 m−2 s−1 in L057 genotype), and 74% (23.9 μmol CO2 m−2 s−1 in L102 genotype) higher than the Morita II genotype (Figure 1A). The stomatal conductance (gs) (Figure 1B) showed a similar pattern to AN, with the following increases: Morita II (341 μmol H2O m−1 s−1), L082 (773.1 μmol H2O m−1 s−1), L057 (788.2 μmol H2O m−1 s−1), and L102 (961.5 mmol H2O m−1 s−1), which showed an AN 2.27, 2.31, and 2.82 times higher than the L020 genotype. Beyond these genotypes, the genotype L020 showed a gs of 239.4 μmol H2O m−1 s−1 or 30% less than that measured in the Morita II genotype. This pattern makes the L020 the best genotype in terms of the intrinsic water use efficiency (WUEi) (Figure 1D). The genotype L020 shows a WUEi twofold higher (80.8 mmol CO2 mmol−1 H2O m−2 s−1) than those measured in the Morita II genotype (40.3 mmol CO2 mmol−1 H2O m−2 s−1). L057, L102, and L082 showed a WUEi of 36.7% (25.5 mmol CO2 mmol−1 H2O m−2 s−1), 38.4% (24.9 mmol CO2 mmol−1 H2O m−2 s−1), and 43.6% (22.7 mmol CO2 mmol−1 H2O m−2 s−1) lower than the Morita II genotype (Figure 1D). The transpiration (E) showed an inverse pattern to those described in WUEi, where L082, L102, and L057 genotypes showed a 1.2-, 1,6-, and 1.9-fold higher E value than those in the Morita II genotype. Elsewhere, the L020 genotype showed an E 36% less than that measured in the Morita II genotype. This fall in E, by decreasing transpiration and carrying loss of latent heat from evaporation, could lead to an increase in leaf temperature; however, this did not happen with any of the treatments, with leaf temperatures not significantly different from Morita II genotype (values not shown).
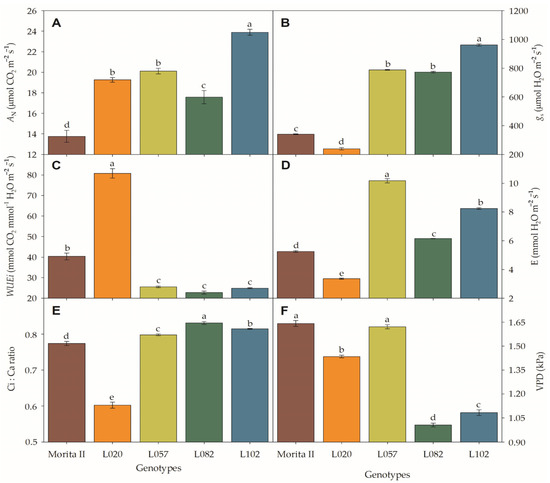
Figure 1.
Net photosynthesis (AN; (A)), stomatal conductance (gs; (B)), intrinsic water use efficiency (WUEi; (C)), transpiration (D,E), Ci:Ca ratio (E), and vapor pressure deficit (VPD; (F)), measured in genotypes of Stevia rebaudiana (Morita II, L020, L057, L082, and L102) under land conditions in the complete absence of any plant stress. In each feature, means followed by different letters denote statistical significance (p < 0.001). Means (±SD) (n = 5).
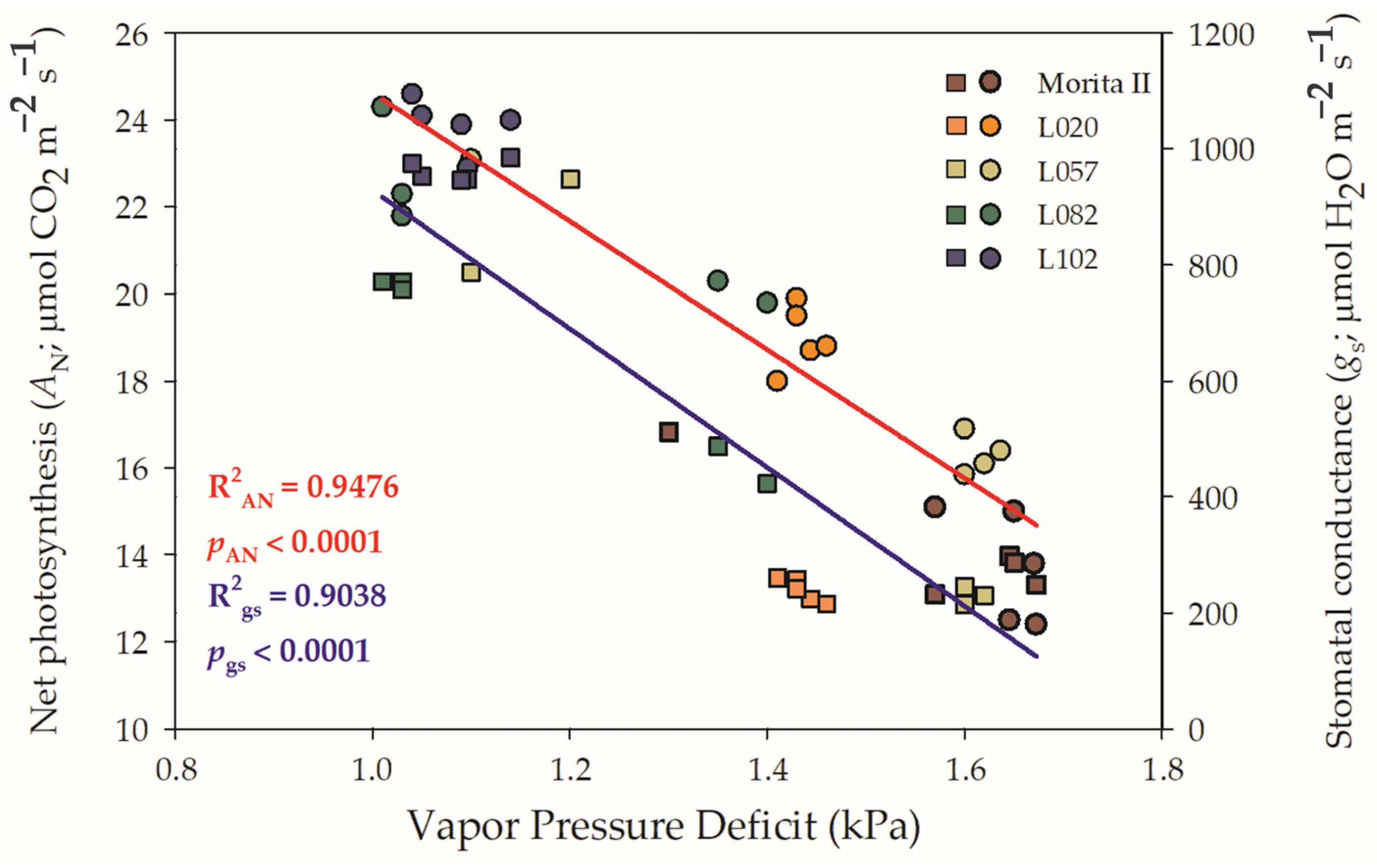
A probable decrease in gs was not the cause of the decrease in AN, especially considering that all other genotypes showed higher AN and gs than the control genotype (Morita II). In addition, the stomatal opening is not likely to be the cause of the decrease in AN because all genotypes presented a Ci:Ca higher than 0.5 or LS lesser than 0.4 (Figure 1E). However, genotypes L057, L120, and L082 had a respective VPD 1.2% (p = 0.394), 34%, and 39% higher than the Morita II genotype, while the genotype L020 showed a VPD 12.6% lower. In this sense, it should be noted that the increase in VPD consequently leads to a decrease in both AN and gs. However, the force that the VPD exerts on AN and gs is not the same as shown in Figure 2. The increase in VPD leads to a decrease in AN in an inversely proportional correlation of −0.764 (p = 2.94 10−5), while gs is decreased in −0.730 (p = 3.44 10−5), a correlation that is also evidenced by the higher determination factor for AN (0.95) than gs (0.90) (Supplementary Data File S1).
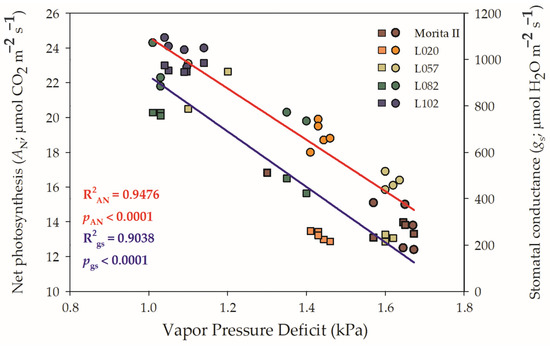
Figure 2.
Regression between vapor pressure deficit (VPD) and net photosynthesis (left y axis) and stomatal conductance (right y axis), measured in genotypes of Stevia rebaudiana (Morita II—brown; L020—orange; L057—dark yellow; L082—dark green; and L102—dark blue) under land conditions in the complete absence of any plant stress. Spheres denote the x–y between VPD and AN, while squares denote the x–y between VPD and gs. In both regressions, R2 and pvalue are shown.
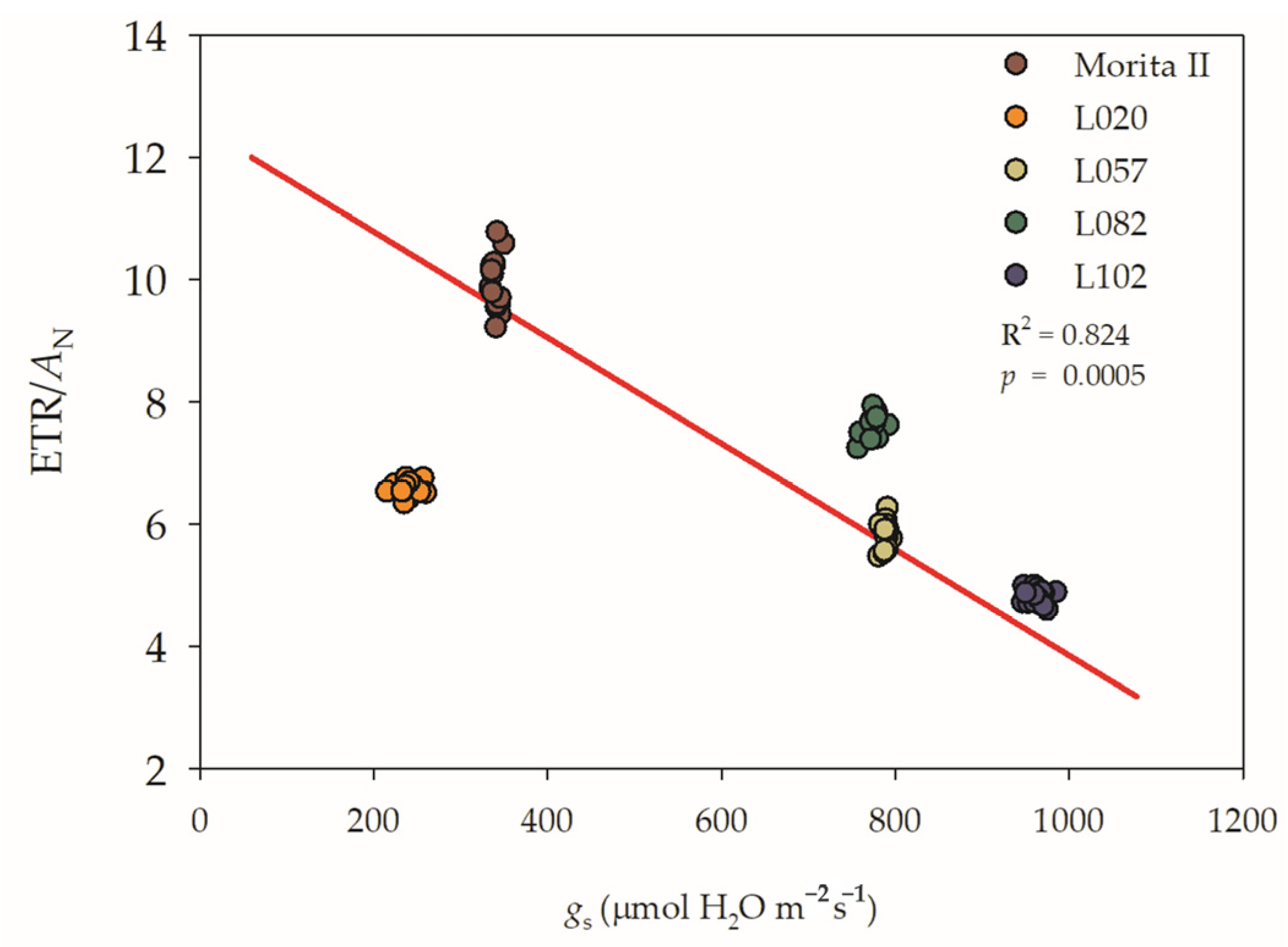
Regarding the strong negative interaction between AN and gs (−0.970; p = 1.40 10−15), one must understand that under normal conditions, the biggest loss for electrons is the photosynthesis that consumes CO2 (Figure 3). However, in stressed plants, most of the electrons coming from the electron transport rate (ETR) do not go to AN, because the stomata inhibit the CO2 absorption, and these electrons must be consumed in other ways than photosynthesis (for more details see Section 2.2). In this way, we showed that the L102 genotype has a greater use of ETR for PN; however, this pattern is a water-consuming process, because the higher use of electrons for photosynthesis is done with maximum gs. Contradicting this pattern, we observed that the Morita II genotype has the lowest efficiency for the use of electrons for PN; however, this pattern shows a lower water-consuming process. The genotype L020, in turn, showed a median efficiency of electrons used at the expense of the water-consuming process than the Morita II genotype. Both L057 and L082 have a median efficiency of electrons for PN, similar to genotype L020, but with a water-consuming process analogous to the L102 genotype (Figure 2).
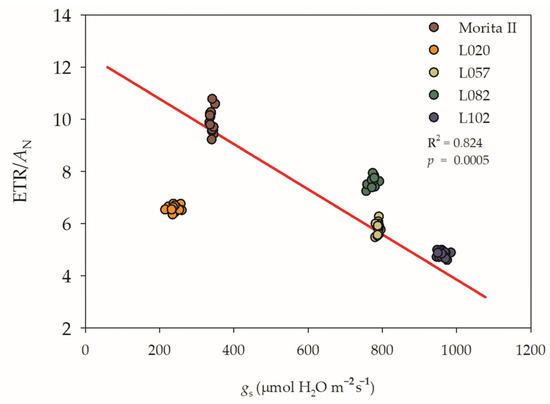
Figure 3.
Regression between the ratio of electron transport rate (ETR) and net photosynthesis (AN) versus stomatal conductance (gs) evaluated in five genotypes of Stevia rebaudiana (Morita II, L020, L057, L082, and L102) under land conditions in the complete absence of any plant stress. The regression R2 and pvalue are shown.
3.2. Chlorophyll a Fluorescence, Chlorophyll, and Carotenoid Concentration
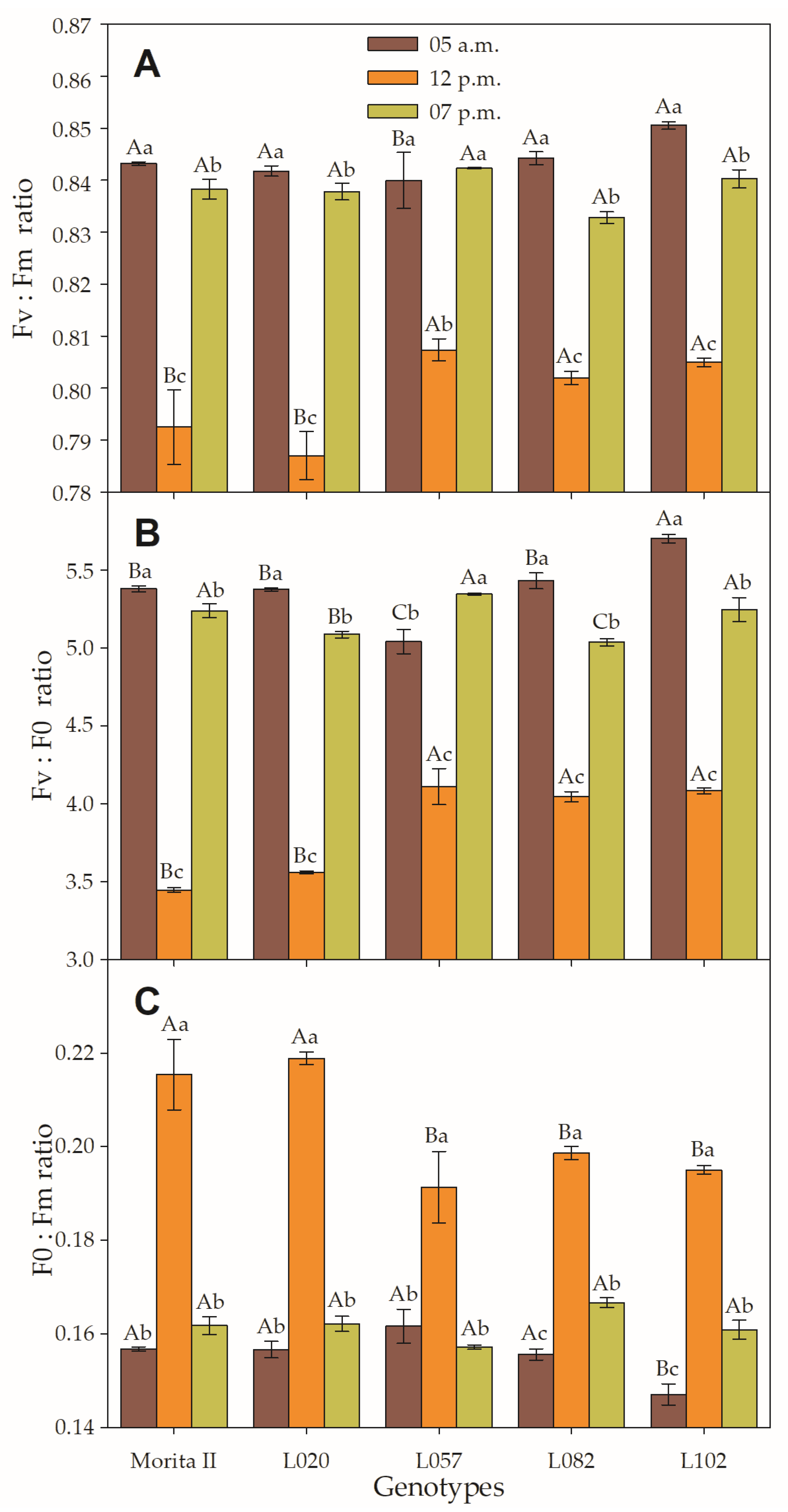
The Fv/Fm is a form of the dissipation of the reductor power in the PSII (Figure 4A) and therefore leads to a reduction of gs of the maximum efficiency of the PSII (ΦPSII; Figure 5C) and to the subsequent reduction of the β-Nicotinamide adenine dinucleotide 2′-phosphate (NADPH) formation, which results in decreased AN. In this sense, it could be stated that all plants, regardless of the evaluated genotype, are stressed by the supra-optimal amount of electron capture, since the Fv/Fm measured at midday was 3.9%, 5.0%, 5.4%, 6.0%, and 6.5% lower than that measured at predawn, respectively, in L057, L082, L102, Morita II, and L020 genotypes. However, it can be verified that, even if significantly lower, the values measured in the late afternoon were very similar to the values measured in the next predawn time; these values, although statistically different, demonstrate a physiologically insignificant difference. Two methods to analyze the integrity of PSII are the analysis of the Fv:F0 ratio and F0:Fm ratio (Figure 4B,C). At midday, the values of the Fv:F0 ratio ranged from 3.4 (in Morita II genotype) to 5.7 (in L102 genotype); these values had a strong and inverse correlation with the F0:Fm ratio (r = −0.876; p = 3.2 10−6; Supplementary Data File S1).
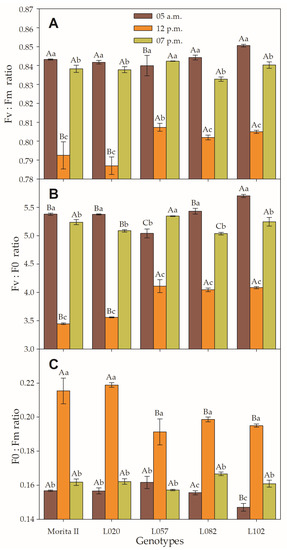
Figure 4.
(A) Variable-to-maximum (Fv:Fm ratio), (B) variable to minimum (Fv:F0 ratio), and (C) minimum to maximum (F0:Fm ratio) chlorophyll fluorescence evaluated in genotypes of Stevia rebaudiana (Morita II, L020, L057, L082, and L102) under land conditions in the complete absence of any plant stress. Means followed by different capital letters denote statistical significance between genotypes in the same evaluated time and different small letters denote statistical difference between evaluated times in the same genotype. Means ± SD (n = 5).
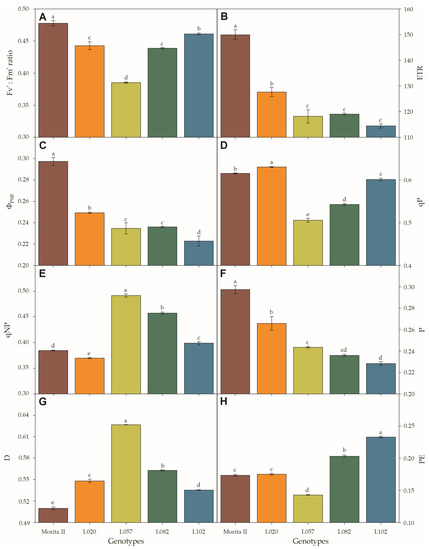
Figure 5.
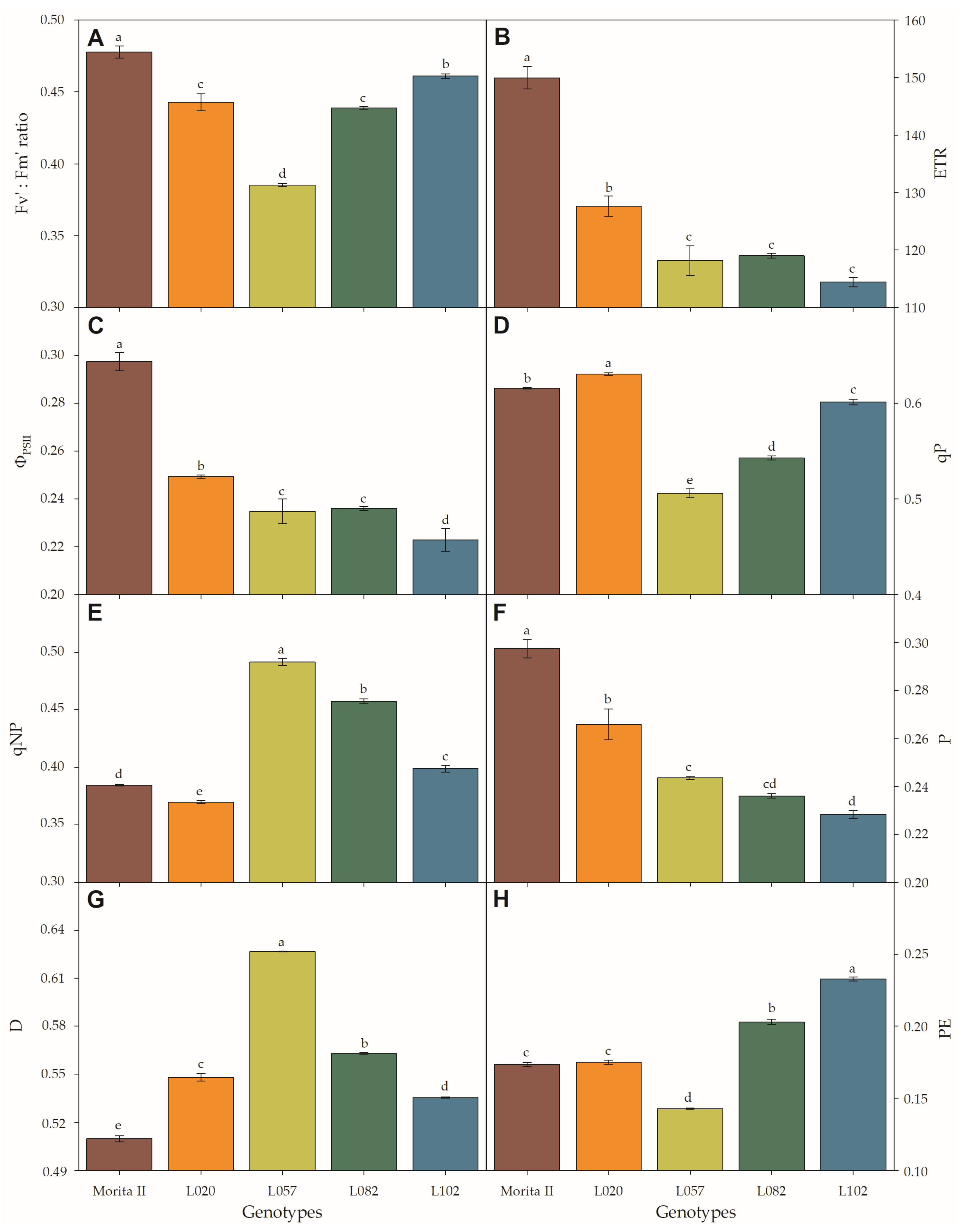
Light adapted variable-to-maximum (Fv’:Fm’ ratio) chlorophyll fluorescence (A), electron transport rate (ETR; (B)), quantum yield of photosystem II (ΦPSII; (C)), photochemical quenching (qP; (D)), non-photochemical quenching (qNP; (E)), fractions of absorbed photosynthetic active radiation used in photochemistry (P; (F)) or dissipated as heat (D; (G)), and the fraction neither used in photochemistry nor dissipated thermally (PE; (H)) evaluated in genotypes of Stevia rebaudiana (Morita II, L020, L057, L082, and L102) under land conditions in the complete absence of any plant stress. In each feature, means followed by different letters denote statistical significance (p < 0.001). Means ± SD (n = 5).
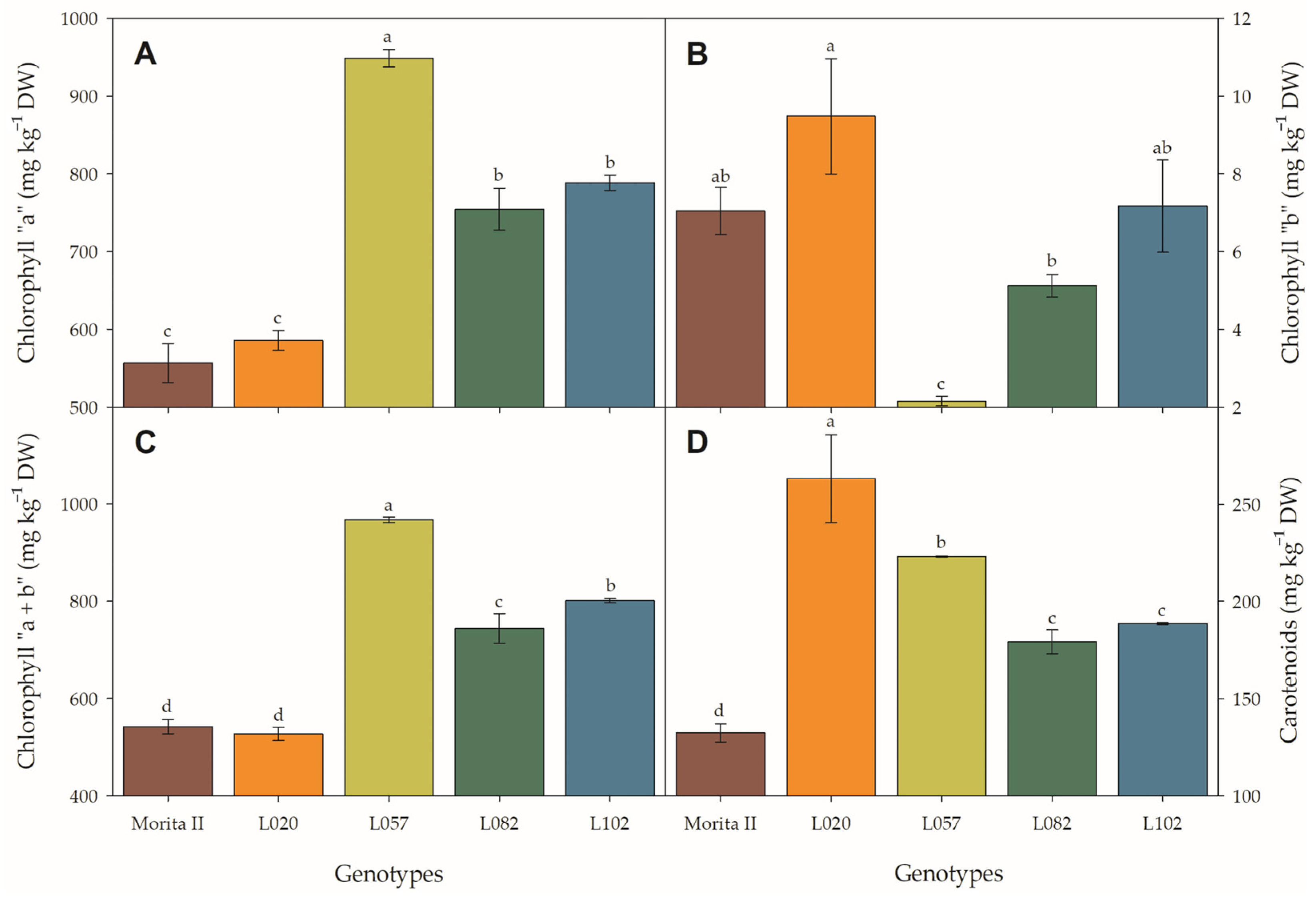
At first glance, the physiological characteristics evaluated in photosystem II (Figure 5) seem to resemble a photoinhibitory effect in all genotypes, with the exception of the Morita II genotype. This hypothesis is supported by the strong reduction of light-adapted variable-to-maximum chlorophyll fluorescence (Fv’:Fm’ ratio) and photochemical quenching (qP) (especially in genotype L057, with a reduction of 8.2% and 11.8%, respectively; Figure 5A,D) by the decrease in quantum yield of photosystem II (ΦPSII), ETR, and fractions of absorbed photosynthetic active radiation used in photochemistry (P) (Figure 5B,C,F) in all genotypes when compared with Morita II, and by the strong increase of non-photochemical quenching (qNP) and fractions of absorbed photosynthetic active radiation dissipated as heat (D) (especially in genotype L057, with an increase of 15.9% and 7.9%, respectively; Figure 5E,G). However, this hypothesis is not supported due to strong increases in chlorophyll concentration (Figure 6A–C) in genotypes L082 (1.4-fold higher), L102 (1.5-fold higher), and L057 (1.8-fold higher) compared to the Morita II genotype. The carotenoid pool (Figure 6D) corroborates the hypothesis of non-stressed plants, since the genotypes L082, L102, L057, and L020 showed an increase in carotenoid synthesis in an order of magnitude of 1.3-, 1.4-, 1.7-, and 2.0-fold higher than values measured in the Morita II genotype.
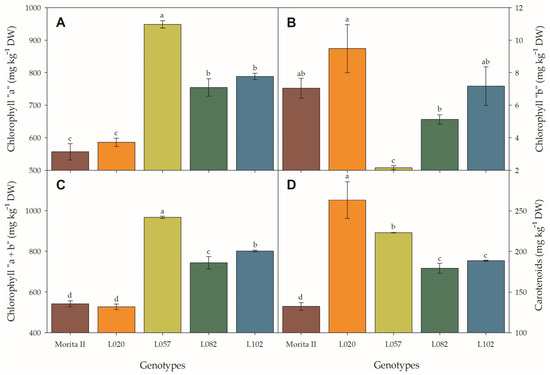
Figure 6.
Chlorophyll a (A), chlorophyll b (B), total chlorophyll (C), and total carotenoids (D) evaluated in genotypes of Stevia rebaudiana (Morita II, L020, L057, L082, and L102) under land conditions in the complete absence of any plant stress. In each feature, means followed by different letters denote statistical significance (p < 0.001). Means ± SD (n = 5).
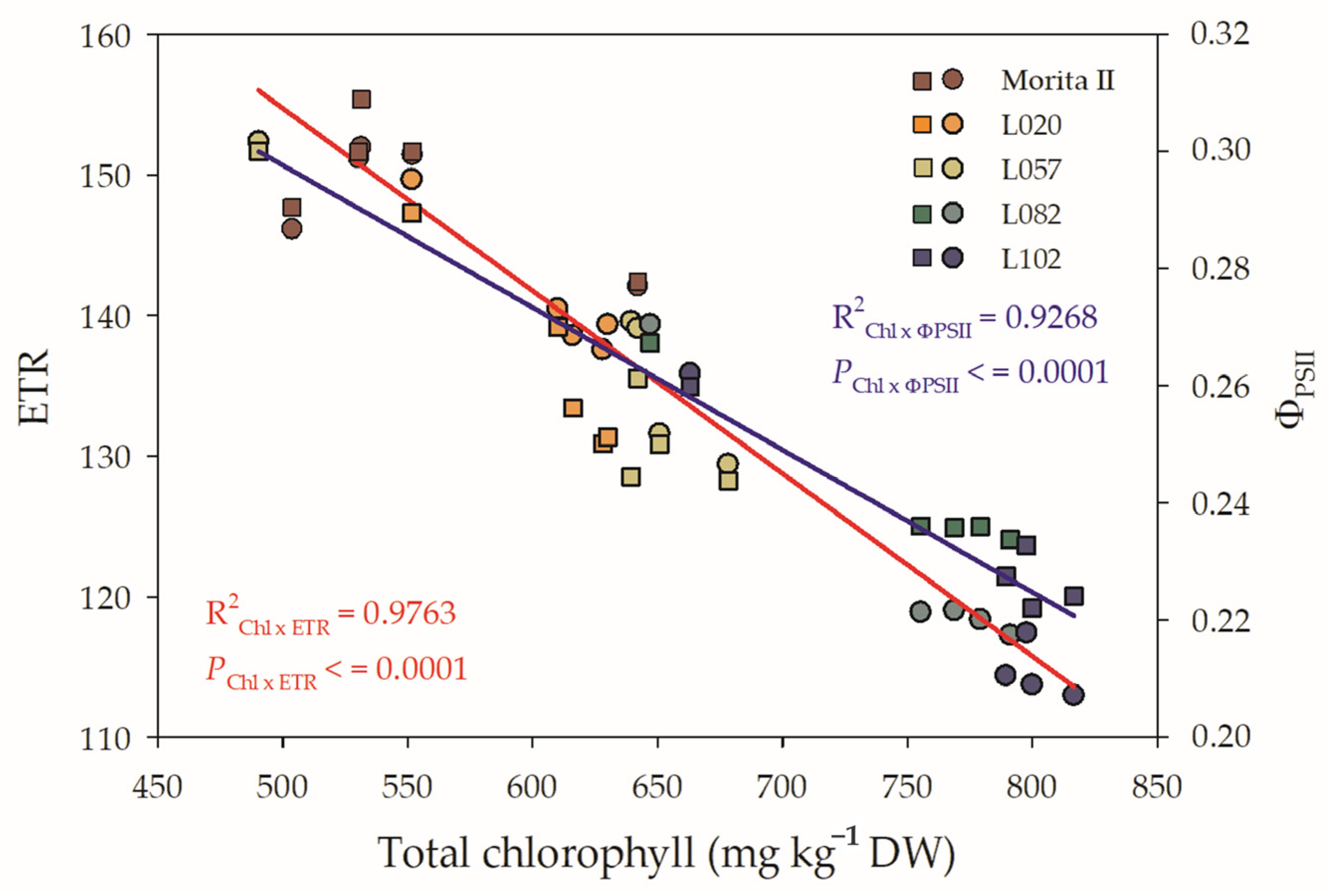
The photochemical reaction of photosynthesis is directly proportional to the capture of electrons through chlorophylls and the subsequent reduction of β-Nicotinamide adenine dinucleotide 2′-phosphate oxidized (NADP+), yielding NADPH, which is consumed in the Calvin-Benson cycle. However, both ETR and ΦPSII show a pattern that is influenced by the concentration of chlorophylls. Figure 7 shows that increasing the concentration of chlorophylls leads to a decrease in ETR and in the efficiency of using these electrons as a NADP+ photoreductor potential. However, the ETR seems to have a greater influence than ΦPSII, since the slope of the Chl x ETR curve presented an abrupt decrease, while the decrease related to the ΦPSII was less affected, although both characteristics have a strongly negative influence on the chlorophyll concentration. Figure 7 also shows that genotypes L082 and L102 show a low efficiency in the use of electrons captured by chlorophylls, potentially transported along ETR, and available to reduce NADP+. In the opposite way, the Morita II genotype is shown, due to its high efficiency in the use of electrons, to be an NADP+ reductor power. The L020 and L057 genotypes are of moderate efficiency.
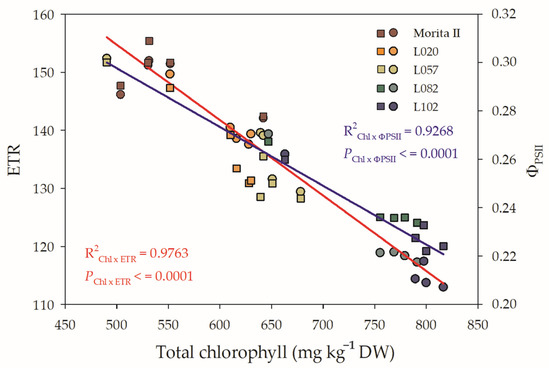
Figure 7.
Relationship between total chlorophyll (Chltotal) and electron transport rate (ETR; left y axis) and Chltotal and ΦPSII (ETR; right y axis) evaluated in genotypes of Stevia rebaudiana (Morita II, L020, L057, L082, and L102) under land conditions in the complete absence of any plant stress. The regression R2 and pvalue are shown.
3.3. Xantophyll Pigments
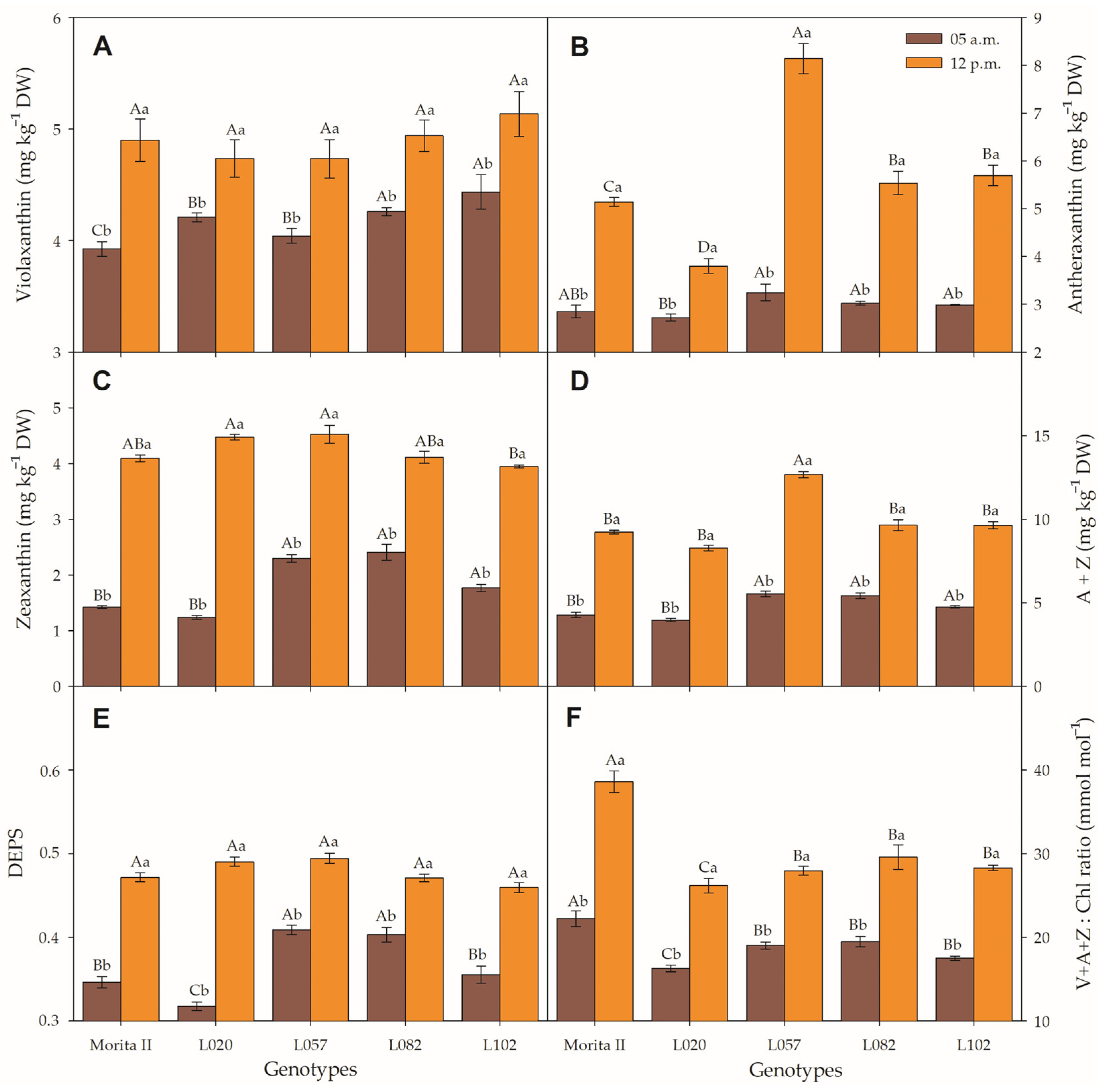
Before dawn, the concentration of V measured in genotypes L057, L020, L082, and L102 was respectively 3%, 7.2%, 8.5%, and 13% higher than measured in the Morita II genotype (Figure 8A). The V profile measured at midday was completely different, where all genotypes showed concentrations not statistically different between them. Antexanthin measured in the morning showed similar values for all genotypes, with a slight reduction (4.4%) found in genotype L020 in relation to the Morita II genotype (Figure 8B). However, at midday, all the genotypes showed statistically different values in comparison to Morita II genotype. The genotypes L082, L102, and L057 measured respectively 7.7%, 10.7%, and 58.3% more V than the Morita II genotype, at the same time that the L020 genotype showed a moderate reduction of 26.1%. With the exception of genotype L020, all genotypes showed an increase in Z concentration in relation to the Morita II genotype. The genotypes L102, L057, and L082 showed an increase in Z of 24.5%, 61.6%, and 69.6%, respectively. However, at midday, all genotypes showed similar values. Moreover, Z measured at midday was 70.8%, 97.1%, 123.3%, 188.3%, and 261.2% higher than the values measured at predawn, respectively, for genotypes L082, L057, L120, Morita II, and L020 (Figure 8C).
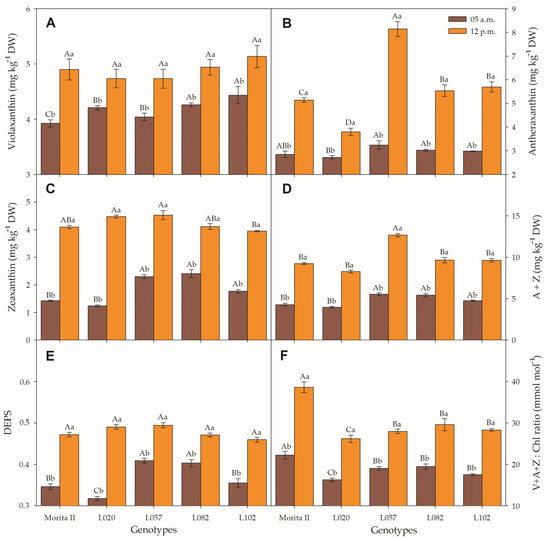
Figure 8.
Violaxanthin (V; (A)), Antheraxanthin (A; (B)), Zeaxanthin (Z; (C)), A + Z (D), DEPS (E), and VAZ Chl−1 (F) evaluated in genotypes of Stevia rebaudiana (Morita II, L020, L057, L082, and L102) under land conditions in the complete absence of any plant stress. Means followed by different capital letters denote statistical significance between genotypes in the same evaluated time and different small letters denote statistical difference between evaluate times in the same genotype. Means ± SD (n = 5).
A + Z increased in the same proportion that A and Z increased separately (Figure 8D), a fact confirmed by the strong correlation between A + Z and A (r = 0.915; p = 1.2 10−9) and Z (r = 0.991; p = 1.1 10−21). However, the correlation between these factors is stronger at predawn than midday (Supplementary Data File S1). DEPS expressed by the formula , evaluated in the predawn, showed that the genotypes L082 and L057 had a DEPS 16.4% and 18% higher, while the L102 genotype showed no difference, and the L020 genotype showed a reduction of 8.3% when compared to the Morita II genotype (Figure 8E). This is different from the pattern shown at midday, where all genotypes showed statistically non-significant values. V + A + Z (VAZ), when correctly expressed with chlorophyll molar weight, showed a pattern similar to DEPS; however, plants evaluated at midday showed a VAZ Chl−1 47%, 52%, 61%, 62%, and 73% higher in the plants when compared to plants evaluated at predawn (Figure 8F).
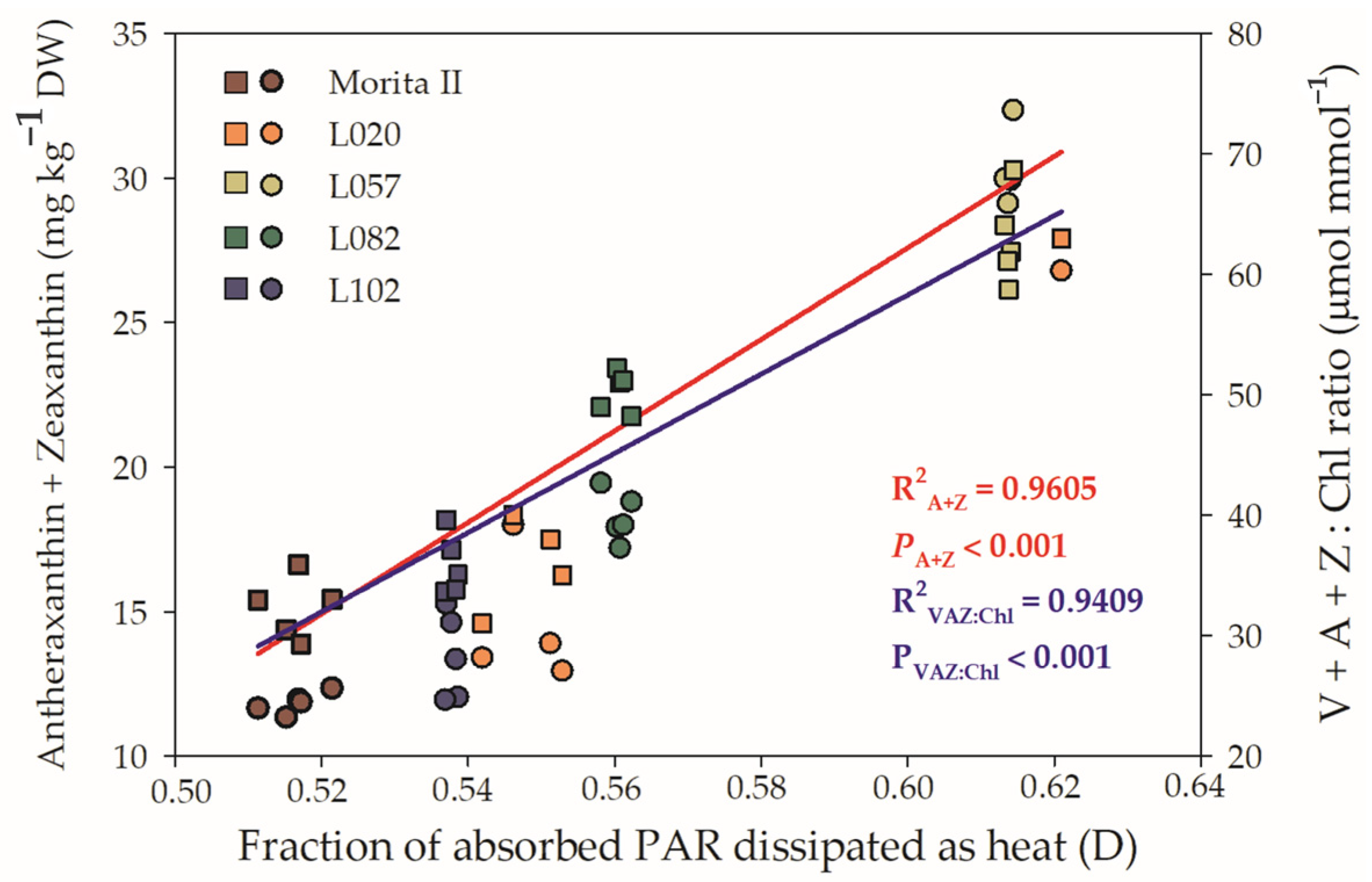
In accordance with Figure 5D, all genotypes showed a fraction of absorbed PAR dissipated as D as a way to extinguish the excess electrons that are transported through ETR. Xanthines, especially A and Z, have a great ability to safely dissipate heat and relieve photosystems. Thus, Figure 9 shows the relationship between D and A + Z, as well as D and VAZ Chl−1. The results show that both relationships have a strong correlation between them and D; however, A + Z has a correlation of 0.9605 with D, while VAZ Chl−1 has a correlation of 0.9409. These regressions are supported by Pearson’s correlations, which were, respectively, r = 0.554 (p = 0.0041) and r = 0.723 (p = 3 × 10−5) for D × A + Z and D × VAZ Ch−1, both measured in the morning. These r values are strongly increased when the correlation is made with the midday dataset: D × A + Z (r = 0.742; p = 2.17 × 10−5) and D × VAZ Ch−1 (r = 0.965; p = 3.6 × 10−12).
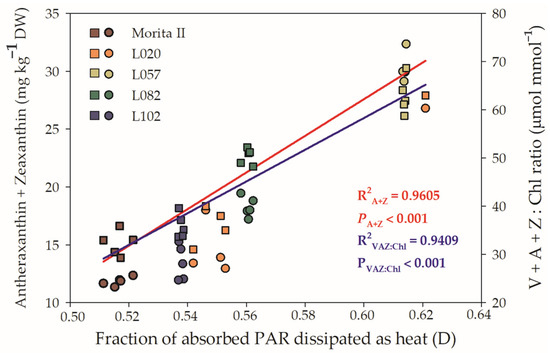
Figure 9.
Relationship between fraction of absorbed PAR dissipated as heat (D) and Antheraxanthin + Zeaxanthin (left y axis) and Violaxanthin + Antheraxanthin + Zeaxanthin Chl−1 (right y axis) evaluated in genotypes of Stevia rebaudiana (Morita II, L020, L057, L082, and L102) under land conditions in the complete absence of any plant stress. The regression R2 and pvalue are shown.
3.4. Proteins and Antioxidantive System
The concentration of soluble proteins was differentially modulated in the different genotypes. While genotype L102 showed no significant difference from the Morita II genotype, the genotypes L082 and L057 presented 1.5- and 1.6-fold higher protein than Morita II, while the genotype L020 presented 63% less protein than the Morita II genotype, or 76% and 78% less soluble protein, when compared to L082 and L057 genotypes, respectively (Table 1).

Table 1.
Soluble proteins, catalase and ascorbate peroxidase activity, and hydrogen peroxide concentration in genotypes of 60-day-old Stevia rebaudiana. All values denote the mean (±SD) of five repetitions. In each parameter, values followed by different letters are statistically significant at p ≤ 0.05.
Morita II, L057, and L102 genotypes showed similar catalase activity, while the L020 and L082 genotypes showed 2.3- and 5.7-fold higher protein than the Morita II genotype (Table 1). On the other hand, APX activity was increased in all genotypes when compared to the Morita II genotype. Thus, genotypes L057, L082, L020, and L120 showed 1.1-, 1.3-, 1.4-, and 1.8-fold higher APX activity than the Morita II genotype (Table 1).
3.5. Steviol Glycosides
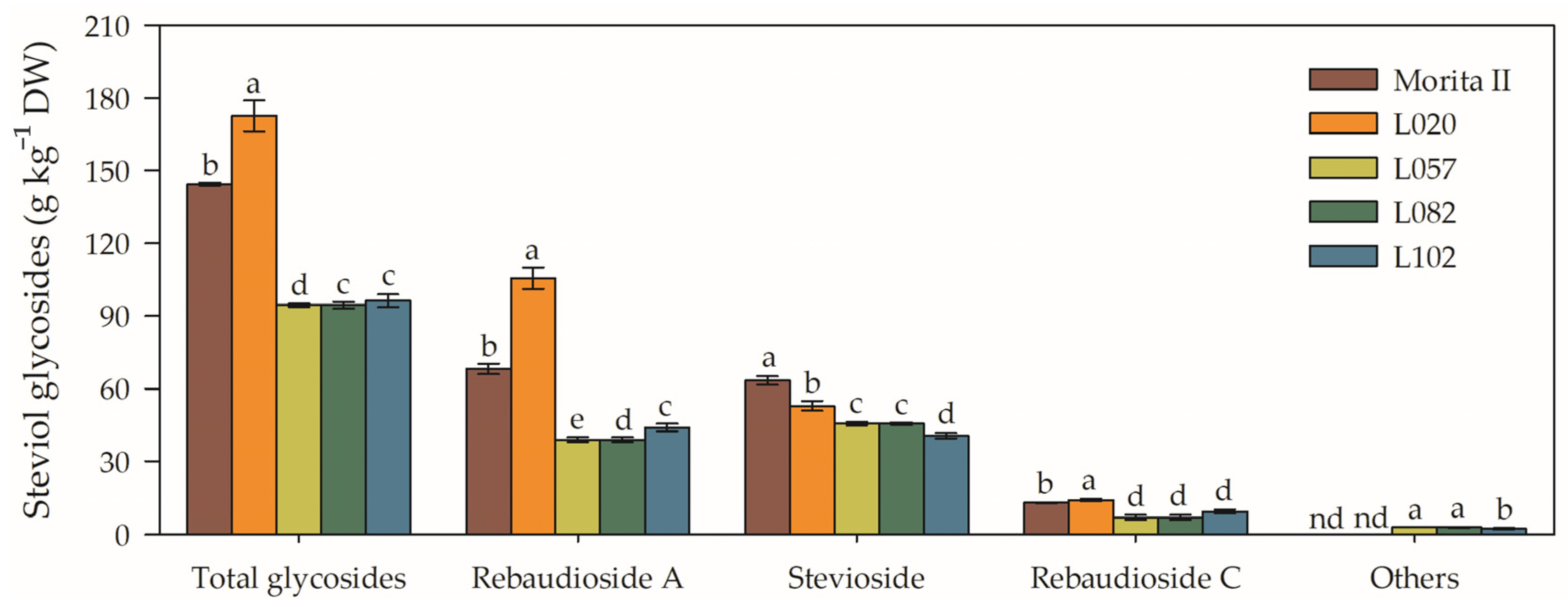
The genotype L020 showed the highest concentration of steviol glycosides (172.6 ± 6.4) among the evaluated genotypes, presenting 143.1%, 82.7%, 79.3%, and 19.4% more steviol glycosides, respectively, in relation to the genotypes L057, L082, L102, and Morita II (Figure 10). This higher concentration of steviol glycosides also translated into a higher concentration of rebaudioside A (105.7 ± 4.5), with the L020 genotype presenting 428.4%, 171%, 140.2%, and 55%, in the same order as described for steviol glycosides. On the other hand, Morita II was the genotype with the highest concentration of stevioside (63.4 ± 1.8), which was 56%, 40.8%, 39.1%, and 19.8% higher than the genotypes of L102, L057, L082, and L102, respectively (Figure 10).
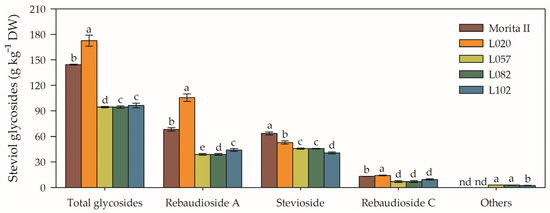
Figure 10.
Total glycosides, rebaudioside A, stevioside, rebaudioside C, and other compounds evaluated in genotypes of Stevia rebaudiana (Morita II, L020, L057, L082, and L102) under land conditions in the complete absence of any plant stress. Means followed by different letters denote statistical significance (p < 0.001). Means ± SD (n = 5). nd = not detected.
3.6. Biomass Produced after 60 Days
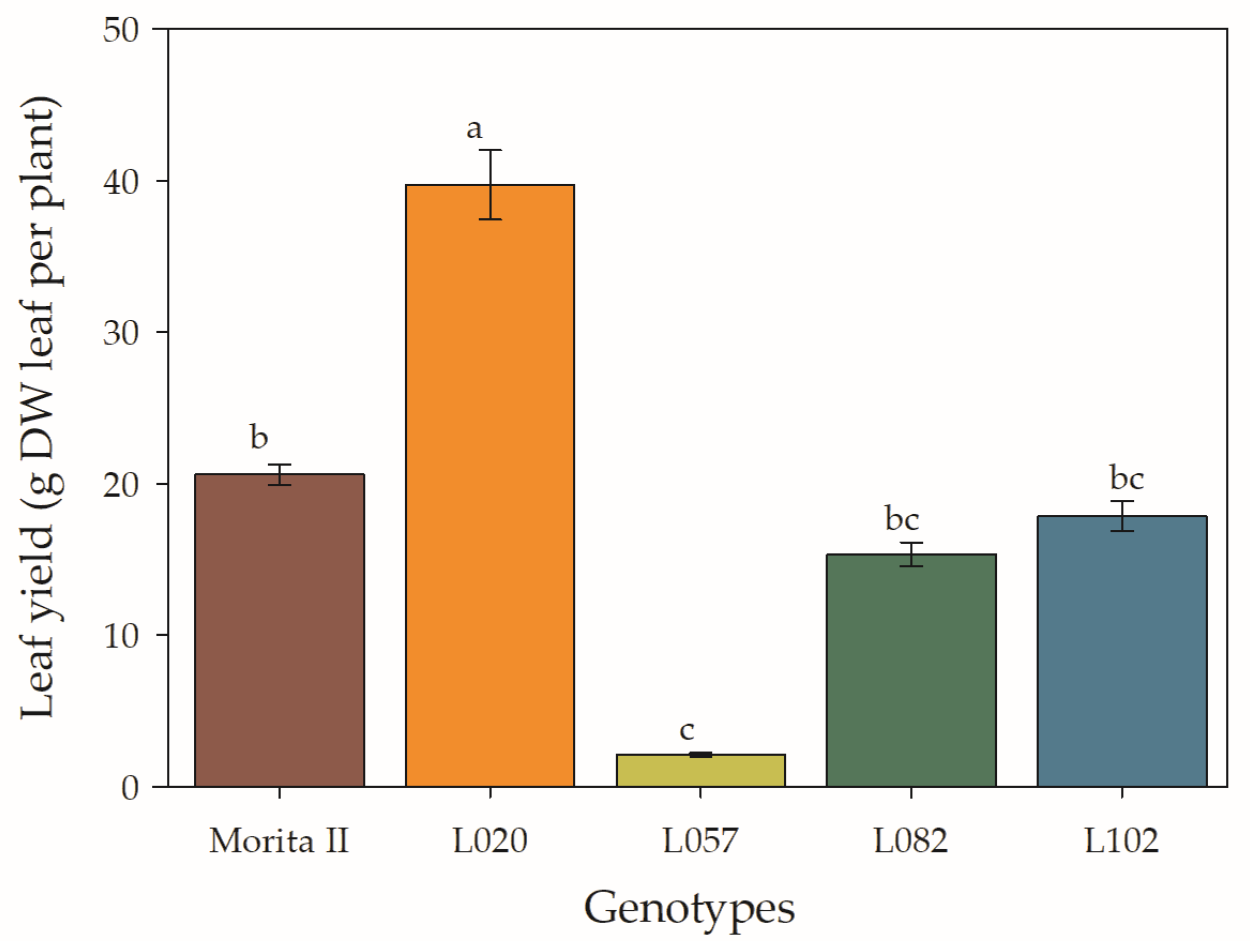
Biomass production is the final result of all the analyses that have been described thus far, as it expresses, in productivity, all the photosynthetic and photoprotective machinery in the production of more biomass. In this sense, it is verified that the L020 genotype accumulated 93% more biomass than the control genotype (Morita II), while the L102, L082, and 1057 genotypes accumulated, respectively, 13%, 26%, and 90% less biomass than the Morita II genotype (Figure 11).
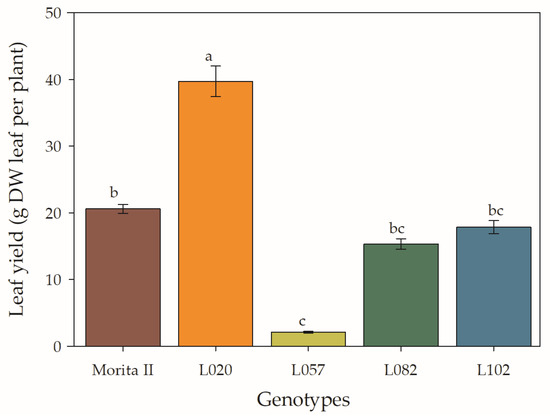
Figure 11.
Leaf yield measured in genotypes of Stevia rebaudiana (Morita II, L020, L057, L082, and L102) under land conditions in the complete absence of any plant stress. Means followed by different letters denote statistical significance (p < 0.001). Means ± SD (n = 5).
3.7. Multivariate Analysis
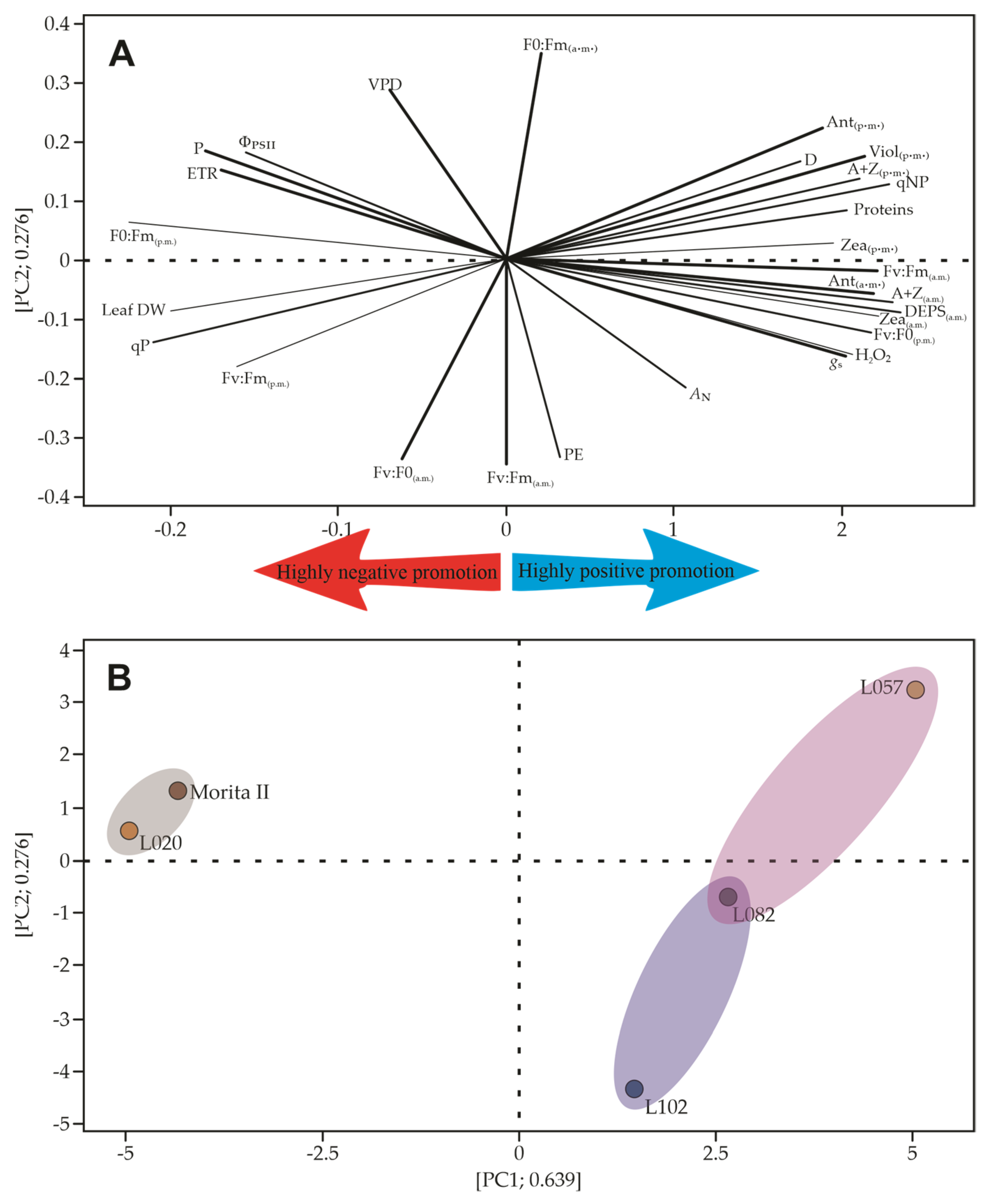
The multivariate analysis described by the principal component analysis (PCA) is a great strategy to search and rank similar patterns. The use of a wide variety of features makes PCA more robust; however, some factors tend to weigh less than others, and sometimes this excess of features can lead to a decrease in the sum of PC1 and PC2. Figure 11 shows that the analysis presented in this study explains 91.5% of the variations that may occur in the dataset. The lower axis of the PCA presents the data of gas exchange, chlorophyll a fluorescence, and predawn xanthine pool, while the upper axis of the PCA better explains the pattern of xanthines measured at midday, some chlorophyll a fluorescence data, as well as heat dissipation by means of D and VAZ (Figure 12A). Based on this pattern, it is possible to draw a dendrogram that clusters Morita II with L020 genotypes, as well as another cluster involving L057 and L082 genotypes. A third cluster is formed by the L120 genotype, which has some characteristics that can be shared with the L082 genotype (shading of the groups in Figure 12B).
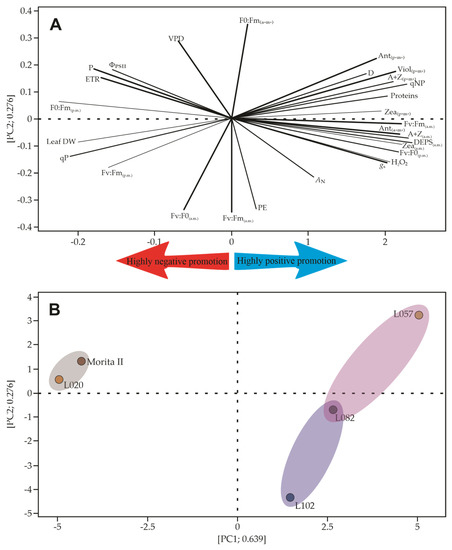
Figure 12.
Multivariate analysis to assess the morphophysiological characteristics of Stevia rebaudiana genotypes. (A) Spatial distribution of all analyzed features, showing the strength of each influencing the net photosynthesis (weak and strong lines). The first cluster has more influence on net photosynthesis, while the second influences chlorophyll a fluorescence and xanthines. In (B), all treatments are displayed in the PC1 and PC2 to show that Morita II and L020 genotypes are closely related. In addition, L102 shows a weak but significant relationship with the L082 genotype. All data were measured or estimated in five genotypes of Stevia rebaudiana plants. AN, net photosynthesis; Ant, antheraxanthin; Chl a, chlorophyll a; Chl b, chlorophyll b; Ci:Ca, internal-to-ambient CO2 concentration; D, fraction of absorbed photosynthetic active radiation dissipated as heat; DEPS, de-epoxidation state of the xanthophyll cycle; ETR, electron transport rate; FPSII, chlorophyll fluorescence and quantum yield of photosystem II; gs, stomatal conductance; Fv/F0, variable-to-initial chlorophyll fluorescence; Fv/Fm, variable-to-maximum chlorophyll fluorescence; NPQ, non-photochemical quenching; PC1-2, principal component axis 1 and axis 2; Vio, violaxanthin; Zea, zeaxanthin.
4. Discussion
Biomarkers are rapid physiological or biochemical assays that can detect a specific condition. In terms of our objectives, they permit us to detect the superiority of one or more characteristics that lead us to infer the agronomic and physiological superiority of these genotypes.
Some gas exchange features are able to be used for specific elite genotypes. Between them, the net photosynthesis (AN) and stomatal conductance are the most commonly used. Positive modulation of AN is expected of an elite genotype. S. rebaudiana is considered separately because the total AN is usually connected to produce more leaf area and thus more biological material in order to produce steviol glycosides. In this sense, studies showing the main objective of promoting leaf yield [38,39,40,41,42] or floral delay [3,24,43,44,45] are interesting in this species. Beyond this, the stomatal conductance flux is an important task, because the stomata opening elicits CO2 capture as well as water lost to atmosphere, which has an extremally negative water potential. So, in these contexts, the best genotype is the one that is most able to ensure photosynthesis with a low or very low stomatal opening, here described by intrinsic water use efficiency. In this sense, there is no doubt that genotype L020 is the best genotype among all studied genotypes, since its WUEi was 2.0-, 3.1-, 3.2-, and 3.6-fold more efficient in water use when compared with Morita II, L057, L102, and L082 genotypes, respectively. Additionally, the L020 genotype showed a moderate to high efficiency of electron use to drive photosynthesis in the same stomatal conductance base.
Plants absorb solar energy to drive their photosynthetic process. However, while beneficial, this process can be extremely dangerous for plants if the photons absorbed by the chlorophyll molecules are not quickly quenched [46,47,48,49,50]. There are essentially three ways to suppress the energy absorbed by chlorophyll molecules: (1) photons can be used to drive photosynthesis (direct and preferential pathway), and (2) thermal dissipation in the form of heat (D) or (3) re-emitted as light (fluorescence of chlorophyll). These three processes take place in competition, and any increase in the efficiency of one will result in a decrease in the efficiency of the other. A fourth process used by the plant to reduce the photon capture is to activate the photobleaching of chlorophyll [33,46,51,52,53,54,55], promoting the chlorophyllase gene expression [56,57,58]. Less Chl, leads to less captured energy and fewer dangerous molecules able to produce ROS. Thus, measurable physiological features, such as photosynthetic activity and chlorophyll fluorescence data, give us physiological parameters that guide photochemical and photoprotective events. Information about changes in photochemical efficiency and the use of photons can be obtained quickly and safely in order to allow an indirect evaluation of the yield of a crop species, thus serving as a biomarker in integrated genotype selection systems [55,59,60,61,62,63] as well as S. rebaudiana [27]. Fai et al. [62] described that a fluorescence assay could reliably predict the effects of nonphotosynthetic inhibitors on growth and select toxicity in a microalgal species Selenastrum capricornutum. This technique is widely reccomended as a rapid, inexpensive, and reliable method to select an interesting phenomemon [27,51,63] as a select elite genotype [27,51,63].
Another parameter widely used for the selection of elite genotypes is the ability of plants to activate their antioxidant system and make it active [63,64,65,66,67]. In addition, the presence of a more robust antioxidant system and a greater electron transport efficiency are characteristics that are sought as markers of the elite genotypes [27,59,63]. However, biomarkers have yet to be adopted in wide-scale and routine monitoring, despite the advantages that they convey [68]. S. rebaudiana is a fast-growing species with an excellent performance index [24,27], so both features are strictly connected with gas exchange and photochemical extinction events [20,24,27,69,70,71,72]. They could be markers, and as such, useful tools in the rapid selection of elite genotypes.
In the five genotypes studied, we found a significant variation in the content of stevioside and rebaudioside A. However, the L102 genotype presented content of stevioside and rebaudioside A similar to the L082 genotype; the L020 genotype presented the highest content of rebaudioside A. On the other hand, the Morita II genotype had the highest content of stevioside. Stevioside and rebaudioside A confer a higher sweetness than sucrose; however, the latter has a less bitter and metallic taste than the former [73,74]. Therefore, the selection of S. rebaudiana lines with a higher content of rebaudioside A is highly significant to improve sweetness; consequently, the L020 genotype stands out as an elite genotype, as it has a higher content of total glycosides, a higher amount of rebaudioside A, and a lower amount of stevioside, in addition to having the highest leaf yield and zero flowering throughout the study. In this study, we describe a lower concentration of stevioside and rebaudioside A when compared to other articles already published by our work team [24,27]. The reason for the lower concentrations of steviosides and rebaudioside A shown in this study likely comes from the fact that the plants used in this study were in the flowering process, when the vast majority of carbons produced in photosynthesis are used for the synthesis of reproductive organs [75,76] or gibberellin production [23,77]. The confirmation of this hypothesis could also be explained by the greater biomass production in the L020 genotype; the L057, L082, L102, and Morita II genotypes showed a 95%, 61%, 55%, and 48% lower leaf biomass than those of the L020 genotype. While the steviol glycoside levels shown in this work were lower than those published by our team, the steviol glycosides were in agreement with other published papers [26,42,78].
The photolysis of water (Hill reaction; [79]) yields electrons that are transported by PSII protein complexes to ferredoxin NADP reductase, which uses the proton gradient to direct the formation of NADPH, which is rapidly utilized in the Calvin-Benson cycle. However, stomatal closure prevents the uptake of atmospheric carbon, and thus the molecules that would be used to reduce CO2 to sugars are inhibited or drastically diminished. Without electron transport, the proton-motive force cannot be established; consequently, other components of PSI and PSII remain reduced and, with no other molecule for electron donation, they end up reducing molecular oxygen, giving rise to free radicals (ROS) [80,81]. However, plants have several alternative mechanisms, including cleaning mechanisms or avoidance mechanisms, for dissipating these electrons that reach the PSI. One of the main protection mechanisms is the reduction of the quantum yields of the PSII (herein called the Fv:Fm ratio). Thus, it can be concluded that the reduction of the Fv:Fm ratio is an indication of stress, which can be dynamic or photoinhibitory. It is dynamic when the plants are able to recover full functionality the next day, and photoinhibitory when the plants achieve a stress level that is so great that the defense system fails and proteins or large protein complexes such as PSII protein D1 are damaged [82]. In this study, we showed that in all genotypes a reversible photoinhibition occurred, as denoted by an initial decrease in the Fv:Fm ratio and Fv:F0 ratio and a strong increase of the F0:Fm ratio in plants evaluated at midday, parameters monitored in progression in late afternoon and the next predawn day. Thus, in our study, no photoinhibitory process was detected.
Recently, some scholars have described a characteristic of S. rebaudiana [27,59] where an increase in non-photochemical parameters serves as an efficient mechanism for dissipating excess light energy, thus minimizing ROS generation. Acosta-Motos et al. [59] recently reported that S. rebaudiana presenting high values of D is related to the inability of the plant to protect itself from excess light. However Acosta-Motos et al. [59] did not incorporate the VAZ cycle as a potent heat dissipator, mainly by lumen accidification as a result of photochemistry. This study and other recently published studies [27] consider the VAZ cycle as a potent heat dissipator and describe plants that present high values of A + Z as having an extraordinary capacity to dissipate energy as heat.
In accordance with Cantabella et al. [19], in the short term, it seems that S. rebaudiana can tightly control ROS generation by inducing different antioxidant mechanisms, such as ROS-scavenging enzymes (APX, POX, CAT, SOD). However Cantabella et al. [19] showed that non-stressed S. rebaudiana plants present a CAT activity of 2.1 mmol CAT min−1 mg−1 protein. In this study, we show a higher CAT activity, of 31.55 μmol CAT min−1 g−1 protein or 31.55 nmol CAT min−1 mg−1 protein; thus, our plants did not present any stress signals. The plants showed only 1.5% of the CAT activity described by Cantabella et al. [19] in non-strressed S. rebaudiana plants. A similar problem was identified in the antioxidant potential in S. rebaudiana callus in response to polyethylene glycol, paclobutrazol, and gibberellin treatments [83]. In this study, Hajihashemi et al. [83] described a non-stressed callus of S. rebaudiana CAT activity of ~12 mmol H2O2 min−1 mg−1 protein or 12 mol H2O2 min−1 g−1 protein, an activity 380-fold higher than values presented in this study. Hajihashemi et al. [84] described S. rebaudiana callus under paclobutrazol treatments with a CAT activity of ~4.5 mmol H2O2 min−1 mg−1 protein or ~4.5 mol H2O2 min−1 g−1 protein, an activity 143-fold higher than those results shown in our higher data. Saravi et al. [85] describe the effect of salt stress with a co-application of endophytic fungi in non-stressed plants as a CAT activity of 1.06 U CAT min−1 mg−1 protein. A superficial analysis of this value may confuse the reader because this author presented that one unit of CAT was defined by the change of absorbance in 60 s. Change of absorbance is an empirical form to express the enzyme activity, because the aborbance is directly proportional to protein in the reaction media and inversely proportional to the extinction coefficient of absorption (in this specific case, H2O2; i.e., 39.4 mmol−1 cm−1). Thus, without knowing the absorbance in each point, it is imposible to compare the values presented in this study with those presented by Saravi et al. [85].
Regarding ascorbate peroxidase, Saravi et al. [85] describes that non-stressed S. rebaudiana plants showed 2.29 U CAT min−1 mg−1 protein; however the manner in which the data is presented is very confusing, because without knowing the specific absorbance, it is imposible to compare our results with the results described in Saravi et al. [85]. Hajihashemi et al. [84] described the S. rebaudiana callus under paclobutrazol treatments as an APX activity of ~0.2 mmol ascorbate min−1 mg−1 protein or ~200 μmol H2O2 min−1 g−1 protein. These results are very similar to those presented in this study. A similar situation was described in the response of S. rebaudiana callus to polyethylene glycol, paclobutrazol, and gibberellin treatments [83]. In this study, Hajihashemi et al. [83] describe that the non-stressed callus showed an APX activity of ~0.9 mmol ascorbate min−1 mg−1 protein or 900 μmol ascorbate min−1 g−1 protein, an activity 6.5-fold higher than presented in this study.
Some scholars [59,86,87,88,89,90,91] describe that hydrogen peroxide (H2O2) can accumulate in response to a stressor or as an oxidative stress marker. In this study, we associate the H2O2 as a cell signaling strategy rather than the consequence of a stress process, as previously reported [92,93,94,95]. It has been established that it acts as a secondary messenger in signal transduction networks. Saravi et al. [85] describe a H2O2 concentration of 4.84 nmol H2O2 g FW−1 or 4.84 μmol H2O2 kg FW−1. At first glance, this value is much smaller than those described in the present study. However Saravi et al. [85] showed results in fresh weight (FW) base. To compare this value with the value presented in our study, the FW may be converted to dry weight (DW). Our research group, after a review of extensive published studies with many S. rebaudiana genotypes [20,24,27,28,29], conclude with a FW to DW conversion coefficient of 4.32 (i.e., 1 g of FW results in 248 mg DW). Then, applying this correction factor to the data of Saravi et al. [85], the H2O2 may be computed as 19.55 μmol H2O2 kg DW−1, a value similar to those presented in this study. However, the methodology used by Saravi et al. [85] is distinct from the methodology applied in this study, and so the results can not be compared. Similar results were presented by Hajihashemi et al. [83], who describe a H2O2 concentration of 1.8 μmol g−1 FW or 1.8 mmol kg−1 FW. Then, applying the conversion coefficient of 4.32, the final results are 7.27 mmol kg−1 DW, a result in growth media of 15% of the values presented in this study. Another example is presented in Hajihashemi et al. [84] where non-stressed S. rebaudiana plants showed a H2O2 concentration of 2.5 μmol g−1 FW or 2.5 mmol kg−1 FW, or 29.32 mmol kg−1 DW, after applying the conversion coefficient of 4.32. These last results are in accordance with our results measured in Morita II and L020 genotypes. In addition, the H2O2 concentration described in Oryza sativa [96] or Jatropha curcas [90] is lower than that presented in this study; however, the methodology used, the form of expression per unit, and fresh versus dry mass prevent the data presented by these authors from being compared with the data presented in this study. In accordance with Saxena et al. [92], under normal conditions, H2O2 emerges as an important factor during many biological processes.
Faced with strongly contrasting data, one might conclude that the data presented in this study are incorrect; however, as shown, these data are generally equivalent to others already published [33,55,97], including S. rebaudiana studies [83,84,98,99,100].
It is important to note that none of the regression analysis in this study resulted from collinear sampling (Supplementary Data File S2), which assumes collinearity to be a sampling artifact or a true reflection of population relationships [101]. According to these authors, collinearity must be considered when data are analyzed with regression analysis, as they can cause large fluctuations with insignificant changes in sampling. Data that result in collinear correlations can cause a misinterpretation, because the closer to collinearity, the more easily the x-axis values can predict the y-axis values, making the regression false and with a high determination index (R2) [102]. The absence of collinearity in all regressions presented in this study demonstrates the cohesion of the data and the ability of the data to estimate certain characteristics in an unbiased way, as reported by Næs and Mevik [102].
The heatmap is a technique widely used to compare profiles against a pattern (Figure 13). As this study aimed to characterize the genotypes and compare them with Morita II genotype, the heatmap technique provides the most interesting results. The direct analysis of the heatmap shows that the L020 genotype has a higher AN, a strong WUEi, a very extensive concentration of carotenoids, as well as a robust antioxidant system. These characteristics translate into a very impressive yield in dry mass, leading to a putative increase in total stevioside production per plant. On the other hand, the genotype L120 presents a higher AN in relation to both Morita II and L020 genotypes; however, this is accomplished by increases in both in gs and T, which make the genotype very inefficient in water use (WUEi). A significant increase in the predawn xanthine pool without a significant change in the midday level demonstrates that the genotype was not under any stress. In this sense, the accumulation of 2.5-fold H2O2 could be interpreted as a cell signaling strategy rather than the consequence of a stress process. The genotypes L057 and L082, in turn, showed an intermediate AN between the L020 and L120 genotypes, at the expense of a strong increase in gs and T and, consequently, a significant decrease in WUEi. The reduction in ETR and ΦPSII may indicate that these genotypes may be experiencing a stress event due to light excess. However, the expressive modulation of the xanthine pool evaluated both at predawn and midday shows that these varieties may provide a more robust system for excess energy dissipation, captured by chlorophylls. In this sense, the increase in CAT and APX activity, analyzed together with the H2O2 data, may indicate that these genotypes did not adapt well to the imposed conditions, a fact that translates into a strong reduction in leaf yield in these two genotypes. However, it should be noted that these analyses are preliminary and should not be considered as complete before an analysis of gene expression is conducted in the genotypes both in steady-state conditions, as presented in this study, and in stressful conditions, such as salinity, drought, and waterlogging, which are common in the Caribbean region where these genotypes are cultivated.
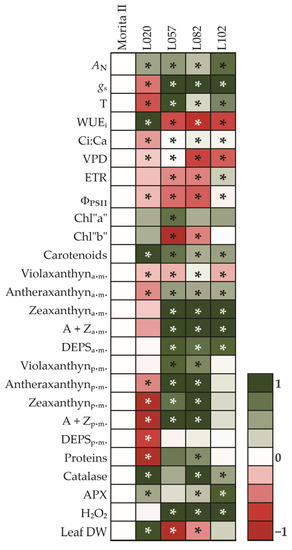
Figure 13.
Heat map representing all analyzed features in genotypes of Stevia rebaudiana. The color code of the heat map is given at the log(2) scale. Data are normalized with respect to the mean response calculated for control (Morita II genotype). To allow statistical assessment, individual plants from this set were normalized in the same way. Values represent means ± SE (SNK; p ≤ 0.05) of five biological replicates. An asterisk (*) indicates that the values from other samples are significantly different from controls.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agronomy13020403/s1, Supplementary Data File S1; Supplementary Data File S2.
Author Contributions
Conceptualization, L.A.R.-P., M.I.O.-O. and N.V.R.; methodology, L.A.R.-P. and M.F.P.; formal analysis, L.A.R.-P. and M.F.P.; investigation, L.A.R.-P., M.F.P., A.M.J.-R. and Y.Y.P.-R.; writing—original draft preparation, L.A.R.-P. and M.F.P.; writing—review and editing, L.A.R.-P., M.F.P., M.I.O.-O. and N.V.R.; supervision, M.I.O.-O. and N.V.R.; funding acquisition, L.A.R.-P., A.J.-O., J.d.D.J.-N., H.A.-T. and E.C.-C. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by Facultad de Ciencias Agrícolas, Universidad de Córdoba, CO, Colombia, through the program: Production systems and food security. Research line: Agri-food production systems and hydrobiological resources. Grants: FCA-04-19.
Data Availability Statement
Not applicable.
Acknowledgments
The authors thank Facultad de Ciencias Agrícolas, Universidad de Córdoba, CO, Colombia, and Instituto de Biotecnología de las Plantas, Universidad Central “Marta Abreu” de Las Villas, Cuba, for assistance with the conditions necessary for this study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hossain, M.F.; Islam, M.T.; Islam, M.A.; Akhtar, S. Cultivation and uses of stevia (Stevia rebaudiana Bertoni): A review. Afr. J. Food Agric. Nutr. Dev. 2017, 17, 12745–12757. [Google Scholar] [CrossRef]
- Naik, V.; Poyil, T. Application of stevia (Stevia rebaudiana Bertoni.) in food products. Pharma Innov. 2022, 11, 2056–2060. [Google Scholar]
- Hernández, K.V.; Moreno-Romero, J.; de la Torre, M.H.; Manríquez, C.P.; Leal, D.R.; Martínez-Garcia, J.F. Effect of light intensity on steviol glycosides production in leaves of Stevia rebaudiana plants. Phytochemistry 2022, 194, 113027. [Google Scholar] [CrossRef]
- Yadav, A.K.; Singh, S.; Dhyani, D.; Ahuja, P.S. A review on the improvement of stevia [Stevia rebaudiana (Bertoni)]. Can. J. Plant Sci. 2011, 91, 1–27. [Google Scholar] [CrossRef]
- Othman, H.S.; Osman, M.; Zainuddin, Z. Genetic variabilities of Stevia rebaudiana Bertoni cultivated in Malaysia as revealed by morphological, chemical and molecular characterisations. Agrivita 2018, 40, 267–283. [Google Scholar] [CrossRef]
- Francisco, F.; Pereira, G.P.; Machado, M.P.; Kanis, L.A.; Deschamps, C. Characterization of Stevia rebaudiana Bertoni A accessions cultived in southern Brazil. J. Agric. Sci. 2018, 10, 353–363. [Google Scholar] [CrossRef]
- Savita, S.M.; Sheela, K.; Sunanda, S.; Shankar, A.G.; Ramakrishna, P. Stevia rebaudiana—A functional component for food industry. J. Hum. Ecol. 2004, 15, 261–264. [Google Scholar] [CrossRef]
- Mahajan, M.; Anuradha, A.; Pal, P. Attaining higher biomass and steviol glycosides yields of Stevia rebaudiana through adjustment of plant population and nitrogen rate. Ind. Crop. Prod. 2021, 165, 113426. [Google Scholar] [CrossRef]
- Reports, V. Global Stevia Market Insights and Forecast to 2028. Stevia Market Place. 2022. Available online: https://www.linkedin.com/pulse/global-stevia-market-insights-forecast-2028-valuates-reports/?trk=pulse-article_more-articles_related-content-card (accessed on 26 October 2022).
- VMR. Global Rebaudioside A (Reb A) Market Market Size, Status and Forecast to 2028; Verified Market Research: New York, NY, USA, 2019; p. 94. [Google Scholar]
- Olazar, F.G.; Ferreira, E.R.; Valdovinos, V.; Kanasawa, S.; Stock, I.M.B.; Candia, N.B.; Ríos, D.F.; Arrua, A.A. Confusion or fraud? Labeling of Stevia sweeteners. South Fla. J. Develop. 2002, 3, 2264–2278. [Google Scholar] [CrossRef]
- News, U. Time to Get Off the Couch, WHO Warns, as 500 Million Risk Developing Chronic Illness. UN News—Global Perspective Human Stories. 2022. Available online: https://news.un.org/en/story/2022/10/1129662 (accessed on 26 October 2022).
- Gautam, R.D.; Kumar, R.; Kashyap, U.; Kumar, P.; Singh, S.; Singh, S.; Kumar, A. Genetic Improvement of Stevia: A Natural Non-Calorie Sweetener. In Plant Breeding—New Perspectives; Wang, P., Ed.; ACS Publications: Washington, DC, USA, 2022; pp. 1–23. [Google Scholar]
- Agronet. La Estevia en Colombia; Oferta y Cosumo. Available online: https://www.agronet.gov.co/Paginas/inicio.aspx (accessed on 26 October 2022).
- Basharat, S.; Huang, Z.; Gong, M.; Ahmed, A.; Hussain, I.; Li, J.; Du, G.L.L. A review on current conventional and biotechnical approaches to enhance biosynthesis of steviol glycosides in Stevia rebaudiana. Chin. J. Chem. Eng. 2021, 30, 92–104. [Google Scholar] [CrossRef]
- Benhmimou, A.; Ibriz, M.; Al Faïz, C.; Gaboun, F.; Douaik, A.; Amchra, F.Z.; Khiraoui, A.; Lage, M. Effects of planting density and harvesting time on productivity of natural sweetener plant (Stevia rebaudiana Bertoni.) in Larache Region, Morocco. Int. J. Plant Res. 2017, 7, 83–89. [Google Scholar] [CrossRef]
- Rodriguez-Paez, L.A.; Hernandez-Burgos, J.L.; Caballero, E.M.C.; Jarma-Orozco, A.; Santos, J.M.P. Rendimiento y calidad de hojas de Stevia rebaudiana Bert. bajo la oferta edafológica y dos niveles de radiación en cinco regiones de Colombia. Rev. UDCA Act. Div. Cient. 2016, 9, 77–85. [Google Scholar]
- Jarma-Orozco, A.; Combatt, E.M.; Cleves, J.A. Aspectos nutricionales y metabolismo de Stevia rebaudiana. Aspectos nutricionales y metabolismo de Stevia Rebaudiana (Bertoni). Agron. Colomb. 2010, 28, 199–208. [Google Scholar]
- Cantabella, D.; Piqueras, A.; Acosta-Motos, J.R.; Bernal-Vicente, A.; Hernández, J.A.; Díaz-Vivancos, P. Salt-tolerance mechanisms induced in Stevia rebaudiana Bertoni: Effects on mineral nutrition, antioxidative metabolism and steviol glycoside content. Plant Physiol. Bioch. 2017, 115, 484–496. [Google Scholar] [CrossRef] [PubMed]
- Castillejo-Morales, A.; Jarma-Orozco, A.; Pompelli, M.F. Physiological and morphological features denote that salt stress in Stevia rebaudiana is based on nonstomatic instead of stomatic limitation. Rev. Colomb. Cien Hortíc. 2021, 15, e12928. [Google Scholar] [CrossRef]
- Zeng, J.; Chen, A.; Li, D.; Wu, W. Effects of salt stress on the growth, physiological responses, and glycoside contents of Stevia rebaudiana Bertoni. J. Agric. Food Chem. 2013, 61, 5720–5726. [Google Scholar] [CrossRef]
- Hussin, S.; Geissler, N.; El-Far, M.M.M.; Koyro, H.W. Effects of salinity and short-term elevated atmospheric CO2 on the chemical equilibrium between CO2 fixation and photosynthetic electron transport of Stevia rebaudiana Bertoni. Plant Physiol. Bioch. 2017, 118, 178–186. [Google Scholar] [CrossRef]
- Hajihashemi, S.; Geuns, J.M.C. Gene transcription and steviol glycoside accumulation in Stevia rebaudiana under polyethylene glycol-induced drought stress in greenhouse cultivation. FEBS Open Bio 2016, 6, 937–944. [Google Scholar] [CrossRef]
- Rivera-Avilez, J.A.; Jarma-Orozco, A.; Pompelli, M.F. Stevia rebaudiana Bertoni: The interaction of night interruption on gas exchange, flowering delay, and steviol glycosides synthesis. Horticulturae 2021, 7, 543. [Google Scholar] [CrossRef]
- Yoneda, Y.; Shimizu, H.; Nakashima, H.; Miyasaka, J.; Ohdoi, K. Effects of light intensity and photoperiod on improving steviol glycosides content in Stevia rebaudiana (Bertoni) Bertoni while conserving light energy consumption. J. Appl. Res. Med. Aromat. Plants 2017, 7, 64–73. [Google Scholar] [CrossRef]
- Ceunen, S.; Werbrouck, S.; Geuns, J.M.C. Stimulation of steviol glycoside accumulation in Stevia rebaudiana by red LED light. J. Plant Physiol. 2012, 169, 749–752. [Google Scholar] [CrossRef]
- Pompelli, M.F.; Espitia-Romero, C.A.; Jaraba-Navas, J.D.; Rodriguez-Paez, L.A.; Jarma-Orozco, A. Stevia rebaudiana under a CO2 enrichment atmosphere: Can CO2 enrichment overcome stomatic, mesophilic and biochemical barriers that limit photosynthesis? Sustainability 2022, 14, 14269. [Google Scholar] [CrossRef]
- Hernández-Fernandéz, I.A.; Jarma-Orozco, A.; Pompelli, M.F. Allometric models for non-destructive leaf area measurement of stevia: An in depth and complete analysis. Hortic. Bras. 2021, 39, 207–217. [Google Scholar] [CrossRef]
- Jarma-Orozco, A.; Combatt-Baballero, E.; Jaraba-Navas, J. Growth and development of Stevia rebaudiana Bert., in high and low levels of radiation. Curr. Plant. Biol. 2020, 22, 100144. [Google Scholar] [CrossRef]
- Pompelli, M.F.; Mendes, K.R.; Ramos, M.V.; Santos, J.N.B.; Youssef, D.T.A.; Pereira, J.D.; Endres, L.; Jarma-Orozco, A.; Solano-Gomes, R.; Jarma-Arroyo, B.; et al. Mesophyll thickness and sclerophylly among Calotropis procera morphotypes reveal water-saved adaptation to environments. J. Arid. Land. 2019, 11, 795–810. [Google Scholar] [CrossRef]
- Corte-Real, N.; Miranda, P.V.V.C.; Endres, L.; Souza, E.R.; Pompelli, M.F. Tolerance to salinity in Jatropha curcas are genotype-dependent. Braz. J. Develop. 2019, 5, 22169–22199. [Google Scholar] [CrossRef]
- Wang, W.; Wang, C.; Pan, D.; Zhang, Y.; Luo, B.; Ji, J. Effects of drought stress on photosynthesis and chlorophyll fluorescence images of soybean (Glycine max) seedlings. Int. J. Agric. Biol. Eng. 2018, 11, 196–201. [Google Scholar] [CrossRef]
- Pompelli, M.F.; Martins, S.C.V.; Antunes, W.C.; Chaves, A.R.M.; DaMatta, F.M. Photosynthesis and photoprotection in coffee leaves is affected by nitrogen and light availabilities in winter conditions. J. Plant Physiol. 2010, 167, 1052–1060. [Google Scholar] [CrossRef]
- Silva, F.M.O.; Lichtenstein, G.; Alseekh, S.; Rosado-Souza, L.; Conte, M.; Suguiyama, V.F.; Lira, B.S.; Fanourakis, D.; Usadel, B.; Bhering, L.L.; et al. The genetic architecture of photosynthesis and plant growth-related traits in tomato. Plant Cell. Environ. 2017, 41, 327–341. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and carotenoids: Measurement and characterization by UV-VIS spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, 1, 1–8. [Google Scholar] [CrossRef]
- Pompelli, M.F.; França, S.C.S.; Tigre, R.C.; Oliveira, M.T.; Sacilot, M.; Pereira, E.C.G. Spectrophotometric determinations of chloroplastidic pigments in acetone, ethanol and dimethylsulphoxide. Braz. J. Biosc. 2013, 11, 52–58. [Google Scholar]
- Bradford, M. A rapid and quantitative method for quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Annu. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Ahmad, A.; Ali, H.; Khan, H.; Begam, A.; Khan, S.; Ali, S.S.; Ahmad, N.; Fazal, H.; Ali, M.; Hano, C.; et al. Effect of gibberellic acid on production of biomass, polyphenolics and steviol glycosides in adventitious root cultures of Stevia rebaudiana (Bert.). Plants 2020, 9, 420. [Google Scholar] [CrossRef] [PubMed]
- Akbari, F.; Arminian, A.; Kahriz, D.; Fazeli, A.; Ghaheri, M. Effect of nitrogen sources on gene expression of Stevia rebaudiana (Bertoni) under in vitro conditions. Cell Mol. Biol. 2018, 64, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Arradaza, C.C.; Cedo, M.L.O.; Zara, R.R.; Gonzaga, R.A. Gibberellin application influences on ex vitro growth, flowering and steviol glycoside accumulation of Stevia rebaudiana Bertoni. Int. J. Agric. Life Sci. 2019, 3, 75–83. [Google Scholar]
- Espitia, M.C.; Montoya, R.B.; Atgencio, L.S. Rendimiento de Stevia rebaudiana Bert. bajo tres arreglos poblacionales en el sinú medio. Rev. UDCA Actual. Divulg. Científica 2009, 12, 151–161. [Google Scholar]
- Gomes, E.N.; Moterle, D.; Biasi, L.A.; Koehler, H.S.; Kanis, L.A.; Deschamps, C. Plant densities and harvesting times on productive and physiological aspects of Stevia rebaudiana Bertoni grown in southern Brazil. An. Acad. Bras. Cienc. 2018, 90, 3249–3264. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Pal, P.; Masand, M.; Seth, R.; Kumar, A.; Singh, S.; Sharma, R.K. Comparative transcriptome analysis revealed gamma-irradiation mediated disruption of floral integrator gene(s) leading to prolonged vegetative phase in Stevia rebaudiana Bertoni. Plant Physiol. Biochem. 2020, 148, 90–102. [Google Scholar] [CrossRef]
- Truong, T.T.; Valíěek, P. Verification of growth and stevioside content of Stevia plants propagated by vegetative and generative method. Agric. Trop. Subtrop. 1999, 32, 79–84. [Google Scholar]
- Jarma-Orozco, A.; Ayala, C.C.; Herrera, C.F. Temperature and radiation effect on steviol glycosides production in Stevia rebaudiana in the Colombian humid Caribbean region. Rev. UDCA Actual. Divulg. Científica 2012, 15, 339–347. [Google Scholar]
- Terashima, I.; Fujita, T.; Inoue, T.; Chow, W.S.; Oguchi, R. Green light drives leaf photosynthesis more efficiently than red light in strong white light: Revisiting the enigmatic question of why leaves are green. Plant Cell Physiol. 2009, 50, 684–697. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.A.; Jifon, J.L.; Silva, J.A.G.; Sharma, V. Use of physiological parameters as fast tools to screen for drought tolerance in sugarcane. Braz. J. Plant Physiol. 2007, 19, 193–201. [Google Scholar] [CrossRef]
- Silva, J.; Santos, R. Can chlorophyll fluorescence be used to estimate photosynthetic production in the seagrass Zostera noltii? J. Exp. Mar. Biol. Ecol. 2004, 307, 207–216. [Google Scholar] [CrossRef]
- Rodriguez, E.; da Conceição Santos, M.; Azevedo, R.; Correia, C.; Moutinho-Pereira, J.; Oliveira, J.M.P.F.; Dias, M.C. Photosynthesis light-independent reactions are sensitive biomarkers to monitor lead phytotoxicity in a Pb-tolerant Pisum sativum cultivar. Environ. Sci. Pollut. Res. 2015, 22, 574–585. [Google Scholar] [CrossRef]
- Alho, L.O.G.; Souza, J.P.; Rocha, G.S.; Mansano, A.S.; Lombardi, A.T.; Sarmento, H.; Melão, M.G.G. Photosynthetic, morphological and biochemical biomarkers as tools to investigate copper oxide nanoparticle toxicity to a freshwater chlorophyceae. Environ. Pollut. 2020, 265, 114856. [Google Scholar] [CrossRef]
- Iqbal, W.; Afridi, M.Z.; Jamal, A.; Mihoub, A.; Saeed, M.F.; Székely, Á.; Zia, A.; Khan, M.A.; Jarma-Orozco, A.; Pompelli, M.F. Canola seed priming and its effect on gas exchange, chlorophyll photobleaching, and enzymatic activities in response to salt stress. Sustainabiity 2022, 14, 9377. [Google Scholar] [CrossRef]
- Pompelli, M.F.; Jarma-Orozco, A.; Rodrígues-Páez, L.A. Salinity in Jatropha curcas: A review of physiological, biochemical, and molecular factors involved. Agriculture 2022, 12, 594. [Google Scholar] [CrossRef]
- Tuba, Z.; Lichtenthaler, H.; Csintalan, Z.; Nagy, Z.; Szente, K. Loss of chlorophylls, cessation of photosynthesis CO2 assimilation and respiration in the poikilochlorophyllous plant Xerophyta scabrida during desiccation. Physiol. Plant. 1996, 96, 383–388. [Google Scholar] [CrossRef]
- Dos Santos, O.O.; Mendes, K.R.; Martins, S.V.C.; Batista-Silva, W.; dos Santos, M.A.; Figueirôa, J.M.; Souza, E.R.; Fernandes, D.; Araújo, W.L.; Pompelli, M.F. Physiological parameters and plasticity as key factors to understand pioneer and late successional species in the Atlantic Rainforest. Acta Physiol. Plant 2019, 41, 145. [Google Scholar] [CrossRef]
- Pompelli, M.F.; Arrieta, D.V.; Rodríguez, Y.Y.P.; Ramírez, A.M.J.; Bettin, A.M.V.; Avilez, M.A.Q.; Cárcamo, J.A.A.; Castaño, S.G.G.; González, L.M.M.; Cordero, E.D.F.; et al. Can Chlorophyll a fluorescence and photobleaching be a stress signal under abiotic stress in Vigna unguiculata L.? Sustainability 2022, 14, 15503. [Google Scholar] [CrossRef]
- Cha-Um, S.; Kirdmanee, C. Effect of salt stress on proline accumulation, photosynthetic ability and growth characters in two maize cultivars. Pak. J. Bot. 2009, 41, 87–98. [Google Scholar]
- Shin, Y.K.; Bhandari, S.R.; Jo, J.S.; Song, J.W.; Cho, M.C.; Yang, E.Y.; Lee, J.G. Response to salt stress in lettuce: Changes in chlorophyll fluorescence parameters, phytochemical contents, and antioxidant activities. Agronomy 2020, 10, 1627. [Google Scholar] [CrossRef]
- Holden, M. The breakdown of chlorophyll by chlorophyllase. Biochem. J. 1961, 78, 359–364. [Google Scholar] [CrossRef]
- Acosta-Motos, J.R.; Noguera-Vera, L.; Barba-Espín, G.; Piqueras, A.; Hernández, J.A. Antioxidant metabolism and chlorophyll fluorescence during the acclimatisation to ex vitro conditions of micropropagated Stevia rebaudiana Bertoni plants. Antioxidants 2019, 8, 615. [Google Scholar] [CrossRef] [PubMed]
- Dewir, Y.H.; El-Mahrouk, M.E.; Al-Shmgani, H.S.; Rihan, H.Z.; Teixeira da Silva, J.A.; Fuller, M.P. Photosynthetic and biochemical characterization of in vitro-derived African violet (Saintpaulia ionantha H.Wendl) plants to ex vitro conditions. J. Plant Interact. 2015, 10, 101–108. [Google Scholar] [CrossRef]
- Chaari-Rkhis, A.; Maalej, M.; Chelli-Chaabouni, A.; Fki, L.; Drira, N. Photosynthesis parameters during acclimatization of in vitro-grown olive plantlets. Photosynthetica 2015, 53, 613–616. [Google Scholar] [CrossRef]
- Fai, P.B.; Grant, A.; Reid, B. Chlorophyll a fluorescence as a biomarker for rapid toxicity assessment. Environ. Toxicol. Chem. 2007, 26, 1520–1531. [Google Scholar] [CrossRef]
- Pompelli, M.F.; Jarma-Orozco, A.; Rodriguéz-Paéz, L.A. Screening of Morphophysiological, Anatomical and Ultrastructural Traits to Improve the Elite Genotype Selection in Sugarcane (Saccharum officinarum L.). Horticulturae 2022, 11, 1069. [Google Scholar] [CrossRef]
- El-Mahrouk, M.E.; Dewir, Y.H.; Murthy, H.N.; Rihan, H.Z.; Al-Shmgani, H.S.; Fuller, M.P. Effect of photosynthetic photon flux density on growth, photosynthetic competence and antioxidant enzymes activity during ex vitro acclimatization of Dieenbachia cultivars. Plant Growth Regul. 2016, 79, 29–37. [Google Scholar] [CrossRef]
- Pompelli, M.F.; Barata-Luís, R.M.; Vitorino, H.S.; Gonçalves, E.R.; Rolim, E.V.; Santos, M.G.; Almeida-Cortez, J.S.; Endres, L. Photosynthesis, photoprotection and antioxidant activity of purging nut under drought deficit and recovery. Biomass Bioenerg. 2010, 34, 1207–1215. [Google Scholar] [CrossRef]
- Müller-Moulé, P.; Conklin, P.L.; Niyogi, K.K. Ascorbate deficiency can limit violaxanthin de-epoxidase activity in vivo. Plant Physiol. 2002, 128, 970–977. [Google Scholar] [CrossRef] [PubMed]
- Logan, B.A.; Grace, S.C.; Adams, W.W., III; Demmig-Adams, B. Seasonal differences in xanthophyll cycle characteristics and antioxidants in Mahonia repens growing in different light environments. Oecologia 1998, 116, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Galloway, T.S.; Brown, R.J.; Browne, M.A.; Dissanayake, A.; Lowe, D.; Jones, M.B.; Depledge, M.H. A multibiomarker approach to environmental assessment. Environ. Sci. Technol. 2004, 38, 1723–1731. [Google Scholar] [CrossRef] [PubMed]
- Debnath, M.; Ashwath, N.; Midmore, D.J. Physiological and morphological responses to abiotic stresses in two cultivars of Stevia rebaudiana (Bert.) Bertoni. S. Afr. J. Bot. 2019, 123, 124–132. [Google Scholar] [CrossRef]
- Wolwer-Rieck, U. The leaves of Stevia rebaudiana (Bertoni), their constituents and the analyses thereof: A review. J. Agric. Food Chem. 2012, 60, 886–895. [Google Scholar] [CrossRef] [PubMed]
- Tavakoli, H.; Tavakoli, N.; Moradi, F. The effect of the elicitors on the steviol glycosides biosynthesis pathway in Stevia rebaudiana. Funct. Plant. Biol. 2019, 46, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Serfaty, M.; Ibdah, M.; Fischer, R.; Chaimovitsh, D.; Saranga, Y.; Dudai, N. Dynamics of yield components and stevioside production in Stevia rebaudiana grown under different planting times, plant stands and harvesting regimes. Ind. Crop. Prod. 2013, 50, 731–736. [Google Scholar] [CrossRef]
- Prakash, I.; Dubois, G.E.; Clos, J.F.; Wilkens, K.L.; Fosdick, L.E. Development of rebiana, a natural, non-caloric sweetener. Food Chem. Toxicol. 2008, 46, S75–S82. [Google Scholar] [CrossRef]
- Bogado-Villalba, L.; Nakashima, H.N.; Britos, R.; Iehisa, J.C.m.; Giubi, M.E.F. Genotypic characterization and steviol glycoside quantification in a population of Stevia rebaudiana Bertoni from Paraguay. J. Crop. Sci. Biotech. 2021, 24, 145–152. [Google Scholar] [CrossRef]
- Saptari, R.T.; Esyanti, R.R.; Putranto, R.A. Growth and steviol glycoside content of Stevia rebaudiana Bertoni in the thin-layer liquid culture treated with late-stage gibberellin biosynthesis inhibitors. Sugar Tech 2020, 22, 179–190. [Google Scholar] [CrossRef]
- Yoneda, Y.; Shimizu, H.; Nakashima, H.; Miyasaka, J.; Ohdoi, K. Effect of treatment with gibberellin, gibberellin biosynthesis inhibitor, and auxin on steviol glycoside content in Stevia rebaudiana Bertoni. Sugar Tech 2018, 20, 482–491. [Google Scholar] [CrossRef]
- Hajihashemi, S.; Geuns, J.M.C. Steviol glycosides correlation to genes transcription revealed in gibberellin and paclobutrazol-treated Stevia rebaudiana. J. Plant Biochem. Biotechnol. 2017, 26, 387–394. [Google Scholar] [CrossRef]
- de Guzman, R.; Midmore, D.J.; Walsh, K.B. Do steviol glycosides act either as a carbon storage pool or in osmoregulation within leaves of Stevia rebaudiana? J. Nat. Prod. 2018, 81, 2357–2363. [Google Scholar] [CrossRef] [PubMed]
- Good, N.D. Carbon dioxide & the Hill reaction. Plant Phvsiol. 1963, 368, 298–304. [Google Scholar] [CrossRef]
- Bhattacharjee, S. The language of reactive oxygen species signaling in plants. J. Bot. 2012, 2012, 985298. [Google Scholar] [CrossRef]
- Buchanan, B.B.; Balmer, Y. Redox regulation: A broadening horizon. Annu. Rev. Plant Biol. 2005, 56, 187–220. [Google Scholar] [CrossRef]
- Kato, Y.; Sakamoto, W. Protein quality control in chloroplasts: A current model of D1 protein degradation in the photosystem II repair cycle. J. Biochem. 2009, 146, 463–469. [Google Scholar] [CrossRef]
- Hajihashemi, S.; Rajabpoor, S.; Djalovic, I. Antioxidant potential in Stevia rebaudiana callus in response to polyethylene glycol, paclobutrazol and gibberellin treatments. Physiol. Mol. Biol. Plants 2018, 24, 335–341. [Google Scholar] [CrossRef]
- Hajihashemi, S.; Ehsanpour, A.A. Antioxidant response of Stevia rebaudiana B. to polyethylene glycol and paclobutrazol treatments under in vitro culture. Appl. Biochem. Biotechnol. 2014, 172, 4038–4352. [Google Scholar] [CrossRef]
- Saravi, H.B.; Gholami, A.; Pirdashti, H.; Firouzabadi, M.B.; Asghari, A.; Yaghoubian, Y. Improvement of salt tolerance in Stevia rebaudiana by co-application of endophytic fungi and exogenous spermidine. Ind. Crop. Prod. 2022, 177, 114443. [Google Scholar] [CrossRef]
- Acosta-Motos, J.R.; Díaz-Vivancos, P.; Álvarez, S.; Fernández-García, N.; Sánchez-Blanco, M.J.; Hernández, J.A. NaCl-induced physiological and biochemical adaptative mechanisms in the ornamental Myrtus communis L. plants. J. Plant Physiol. 2015, 183, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Silva, E.N.; Ferreira-Silva, S.L.; Fontenele, A.V.; Ribeiro, R.V.; Viégas, R.A.; Silveira, J.A.G. Photosynthetic changes and protective mechanisms against oxidative damage subjected to isolated and combined drought and heat stresses in Jatropha curcas plants. J. Plant Physiol. 2010, 167, 1157–1164. [Google Scholar] [CrossRef]
- Liang, Y.; Chen, H.; Tang, M.-J.; Yang, P.-F.; Shen, S.-H. Responses of Jatropha curcas seedlings to cold stress: Photosynthesis-related proteins and chlorophyll fluorescence characteristics. Physiol. Plant 2007, 131, 508–517. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Anwar, A.; Saleemuddin, M. Immobilization and stabilization of invertase on Cajanus cajan lectin support. Bioresour. Technol. 2001, 79, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Cerqueira, J.V.A.; Silveira, J.A.G.; Carvalho, F.E.L.; Cunha, J.R.; Lima Neto, M.C. The regulation of P700 is an important photoprotective mechanism to NaCl-salinity in Jatropha curcas. Physiol. Plant 2019, 167, 404–417. [Google Scholar] [CrossRef]
- Carvalho, L.C.; Vilela, B.J.; Vidigal, P.; Mullineaux, P.M.; Amâncio, S. Activation of the ascorbate-glutathione cycle is an early response of micropropagated Vitis vinifera L. explants transferred to ex Vitro. Int. J. Plant Sci. 2006, 167, 759–770. [Google Scholar] [CrossRef]
- Saxena, I.; Srikanth, S.; Chen, Z. Cross talk between H2O2 and interacting signal molecules under plant stress response. Front. Plant Sci. 2016, 7, 570. [Google Scholar] [CrossRef]
- Pingbo, C.; Xia, L.; Kai, H.; Xiaodong, W.; Chuanchao, D.; Chuangen, L. Promotion of photosynthesis in transgenic rice over-expressing of maize C4 phosphoenolpyruvate carboxylase gene by nitric oxide donors. J. Plant. Physiol. 2014, 171, 458–466. [Google Scholar]
- Mazid, M.; Khan, T.A.; Mohammad, F. Role of nitric oxide in regulation of H2O2 mediating tolerance of plants to abiotic stress: A synergistic signalling approach. J. Stress Physiol. Biochem. 2011, 7, 34–74. [Google Scholar]
- Luna, C.M.; Pastori, G.M.; Driscoll, S.; Groten, K.; Bernard, S.; Foyer, C.H. Drought controls on H2O2 accumulation, catalase (CAT) activity and CAT gene expression in wheat. J. Exp. Bot. 2005, 56, 417–423. [Google Scholar] [CrossRef]
- Lee, D.H.; Kin, Y.S.; Lee, C.B. The inductive responses of the antioxidant enzymes by salt stress in the rice (Oryza sativa L.). J. Plant Physiol. 2001, 158, 737–745. [Google Scholar] [CrossRef]
- Pinheiro, H.A.; DaMatta, F.M.; Chaves, A.R.M.; Fontes, E.P.B.; Loureiro, M.E. Drought tolerance in relation to protection against oxidative stress in clones of Coffea canephora subjected to long-term drought. Plant Sci. 2004, 167, 1307–1314. [Google Scholar] [CrossRef]
- Dwivedi, S.; Alam, A.; Shekhawat, G.S.; Sharma, V.; Kumari, J. Enzymatic and non-enzymatic behaviour of Stevia rebaudiana (Bertoni) Bertoni against fluoride induced stress. Int. J. Sci. Res. Knowl. 2016, 4, 69–76. [Google Scholar] [CrossRef]
- Peynevandi, K.M.; Razavi, M.M.; Zahri, Z. The ameliorating effects of polyamine supplement on physiological and biochemical parameters of Stevia rebaudiana Bertoni under cold stress. Plant Prod. Sci. 2018, 21, 123–131. [Google Scholar] [CrossRef]
- Dwivedi, S.; Alam, A.; Shekhawat, G.S. Antioxidant response of Stevia rebaudiana (Bertoni) Bertoni (Angiosperms; Asteraceae) during developing phase of suspension cell culture. Plant Sci. Today 2016, 3, 115–123. [Google Scholar] [CrossRef]
- Mason, C.H.; Perreault, W.D. Collinearity, power, and interpretation of multiple regression analysis. J. Mark. Res. 1991, 28, 268–280. [Google Scholar] [CrossRef]
- Næs, T.; Mevik, B.-H. Understanding the collinearity problem in regression and discriminant analysis. J. Chemom. 2001, 15, 413–426. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).