Abstract
The large outbreak of banana Fusarium wilt has become a bottleneck limiting the industry’s development, and crop rotation is a cost-effective and essential measure to overcome the obstacles of banana crop monoculture. The present work was carried out to explore the mechanisms of how changes in soil chemical properties and the reestablishment of soil microorganisms in high-incidence soils are affected by crop rotation and plant residue. In this study, pineapple–banana crop rotation and pineapple residue amendment were carried out to alleviate banana Fusarium wilt, and their effects on bacterial and fungal communities were analyzed using the MiSeq Illumina sequencing platform. Both pineapple–banana rotation and residue addition significantly reduced disease incidence. Moreover, pineapple rotation and residue amendment altered the bacterial and fungal community composition. The taxonomic and phylogenetic alpha diversity of bacteria and fungi significantly increased against disease suppression and nutrition competition. The relative abundances of the Burkholderia, Pseudomonas, Elaphocordyceps, Penicillium, and Talaromyces genera were higher, and the number of Fusarium was significantly lower in rotational soil than in banana monoculture soil. Finally, linear models (LM) showed that the Burkholderia and Talaromyces in crop rotation, and Aspergillus in residue amendment had a significantly negative relationship to disease incidence, which plays a key role in Fusarium reduction. To consider the economic benefits and protect the vitality of the soil, this study suggested that pineapple–banana rotation and pineapple residue amendment both could be considered for the sustainable management of banana wilt.
1. Introduction
Currently, soil-borne diseases are a serious threat to soil health and crop productivity [1,2], and have become one of the major problems for the sustainable development of intensive agriculture [3]. Banana (Musa spp.) is among the crops more severely affected by succession disorders caused by multiple abiotic (poor soil fertility, drought, high temperature, and salinity) and biotic (fungi, bacteria, viruses and pests) factors [4,5,6], particularly strains of the fungal pathogen Fusarium oxysporum f. sp. cubense tropical race 4 (TR4). This fungal pathogen can enter banana roots and cause Fusarium wilt disease of banana. Banana fusarium wilt is a serious soil-borne fungal disease which has become a bottleneck in the sustainable development of intensive agriculture and has now seriously threatened the world’s banana production [7,8,9,10,11,12]. At present, about 90% of banana varieties in China are threatened by TR4, so it is very important to ensure the sustainable and healthy development of banana industry [13].
For a long time, physical (e.g., exposure), chemical (e.g., soil fumigation), and biological (e.g., antagonistic microorganisms) methods have reduced the pathogens in the soil to some extent and have made important contributions to the effective prevention and control of soil-borne diseases, reducing the incidence of soil-borne fusarium wilt [14,15,16,17]. However, currently, the soil microbiota is severely imbalanced, and the short-term reduction in the pathogenic population does not fundamentally improve the balance of the soil microbiota in high-incidence banana soil [18]. Therefore, in order to gradually restore the healthy soil microbiome in the land with a high incidence of banana wilting, crop rotation may be an important measure to gradually reform the soil ecology. Several reviews show that soil pathogens can be reduced by using specific agricultural practices, including the incorporation of plant residues in the soil and crop rotation [19,20].
Crop rotation is an important measure for changing the soil ecology gradually [21]. Different crop rotation systems have different effects on the suppression of different diseases, which may be through mechanical mechanisms, such as blocking the circulation of pathogens, harmful chemical substances, and harmful microorganisms, improving soil fertility through root exudates and residues, and increasing soil microbial biomass and activity [22,23,24,25]. These strategies mostly rely on promoting the emergence of protective microbiomes or impacting the population of the pathogen directly, both of which account for effective disease control [8,26].
The soil microbiome plays an important role in promoting plant growth, nutrient uptake, disease resistance, and environmental stresses adaptation. Their richness, diversity, and composition largely determine the sustainability of agriculture. Recent studies have shown that modulation of the soil microbiome can reduce the number of pathogens in the soil, thereby suppressing crop disease. For instance, Wei et al. [27] reported that phages can “target” tomato cyanobacteria to disrupt their ability to survive while structuring the rhizosphere soil flora to restore community diversity and increasing the abundance of beneficial microbes. The abundance and activity of Pseudomonas, Sphingomonas, Penicillium, and Trichoderma spp. were found to be significantly higher than the Fusarium number in the crop rotation soil [28]. Improving soil microbial communities further improves soil fertility and crop yields, maintaining the persistence of plants and protecting soil health [29]. Additionally, there are many protozoans which can protect crop health by feeding on or producing beneficial antibacterial substances to defend against the invasion of other Fusarium pathogens [30]. More interestingly, the interactions between soil microbial communities are also closely related to the plant infection ability of Fusarium. Studies have shown that the rhizosphere microbial communities are more diverse and have a more complex microbial network [31]. In addition, rhizosphere microbial communities could produce more substances, inhibiting the growth of bacteria or fully occupy limited ecological niches, thus effectively suppressing pathogen invasion and reducing widespread outbreaks of soil-borne diseases [32].
Crop rotation and plant residue manipulation in the field are important strategies for soil-borne disease management [33,34]. Furthermore, studies have shown that plant root exudates and residues in soil can have a long-lasting effect on disease control as a result of crop rotation and relieve soil pressure by improving soil properties [35,36,37]. However, these strategies have received little attention, as efforts have mostly been given to investigate how such approaches relate to overall aspects of soil quality, nutrient cycle, and crop performance [38,39,40]. Therefore, the effect of these agricultural managements on crop rotation remains elusive [41]. This provides potential opportunities to explore beneficial results of agricultural management associated with soil-borne disease control [42].
In our previous work, banana–pineapple rotation was picked out for its high-efficiency in banana Fusarium wilt disease prevention [43]. In previous experiments, we found that different pineapple cultivars (Bali pineapple, Golden Pineapple and Tai nong 17 pineapple) planted with the soil of the infected banana garden showed significant differences in the reduction of Fusarium oxysporum number in the soil, as well as differences in the fungal and bacterial communities. Among them, Tai nong 17 pineapple had a better effect than others in inhibiting the number of Fusarium oxysporum [44]. A set of dynamic interactions with direct implications on plant performance between plant residue and soil-borne diseases have been confirmed [34]. Therefore, the effect of crop rotation consists of two parts: simple crop rotation and residue return. We hypothesize that the pineapple cultivar (Tai nong 17) with the best inhibition on the number of Fusarium oxysporum under crops rotation and residue amendment both have inhibitory effects on banana Fusarium wilt, while their work mechanism was still unknown. In this study, two pot experiments, crop rotation and residue amendment, were designed to investigate the improvement of soil physicochemical properties and soil microbiota, respectively. Further, we hypothesize that pineapple–banana crop rotation and pineapple residue addition better effect suppression on Fusarium wilt because improving soil physicochemical properties stimulates the growth of indigenous beneficial microorganisms, resulting in a significant reduction in disease incidence compared to monoculture. The aims of this study were (1) to evaluate the effect of the pineapple–banana crop rotation system on wilt disease in banana plantlets during the seedling period; (2) to estimate the changes in microbial community structure from the bulk soil to the rhizosphere soil zone; (3) to analyses the diversity of soil microorganisms; and (4) to study the relationship between the soil microbial community composition and environmental factors.
2. Materials and Methods
Effects of residue extract and root exudates on the growth of FOC4 (Fusarium oxysporum f.sp. cubense 4, provided by Nanjing Agricultural University). Preparation of banana and pineapple plant residues: Banana and pineapple residues were collected from a field located at Paigou village in Zhongyuan town, Qionghai City, Hainan Province, China (110°30′ E, 19°5′ N). Briefly, first, the parts of the plants were put into plastic packaging bags, placed in a dry ice blister box, and transported to the laboratory (<6 h). Second, these crop residues were carefully washed five times with sterile deionized water in the laboratory and divided into root, stem leaf, and fruit plant parts. Following that, these crop residues were chopped into tiny pieces and grounded down to powder. Each residue type was sieved through a 4-mm mesh, and the total nutrient content was measured. At last, fresh plant samples were dried, grounded and extracted at a ratio of 1:5 plant residue: deionized water for 48 h and aseptically filtered to obtain the extract master batch.
Preparation of banana and pineapple root exudates: The plants were removed from the soil, rinsed five times with tap water and five times with deionized water, and incubated in a plastic cup containing 300 mL of deionized water for 24 h. The solution containing the root exudate was combined and then slowly filtered (filter membrane pore size: 0.45 μm, size: Φ25 mm), and the filtrate was freeze-dried to dryness. All roots of pineapple plants involved in root exudate collection were then dried and weighed separately, the freeze-dried material was dissolved separately in an appropriate amount of deionized water, and the volume was fixed to one gram of dry weight root exudate per mL of root exudate master batch (i.e., 1 g of dry root exudate per mL) and stored at −20 °C.
The in vitro effects of residue extract and root exudates on the growth of FOC4 consisted of six treatments: CK: no residue, B_L: banana stem leaf residue, B_R: banana root residue, P_L: pineapple stem leaf residue, P_R: pineapple root residue, and P_F: pineapple fruit residue. Plates were poured according to different volume ratios 10%, 1% and 0.1% (1:10, 1:100, 1:1000) of residue extracts to water agar, respectively. Pathogen patches were inoculated into the center of the prepared plates with a 0.8-cm-diameter hole punch to obtain uniform growth of Fusarium oxysporum acuminatum (FOC4, Provided by Nanjing Agricultural University) of uniform media thickness. Percentage of mycelial growth inhibition, PIRG (%) = (diameter of control colonies − diameter of treated colonies)/(diameter of control colonies) × 100.
2.1. Pot Rotation Experimental Design
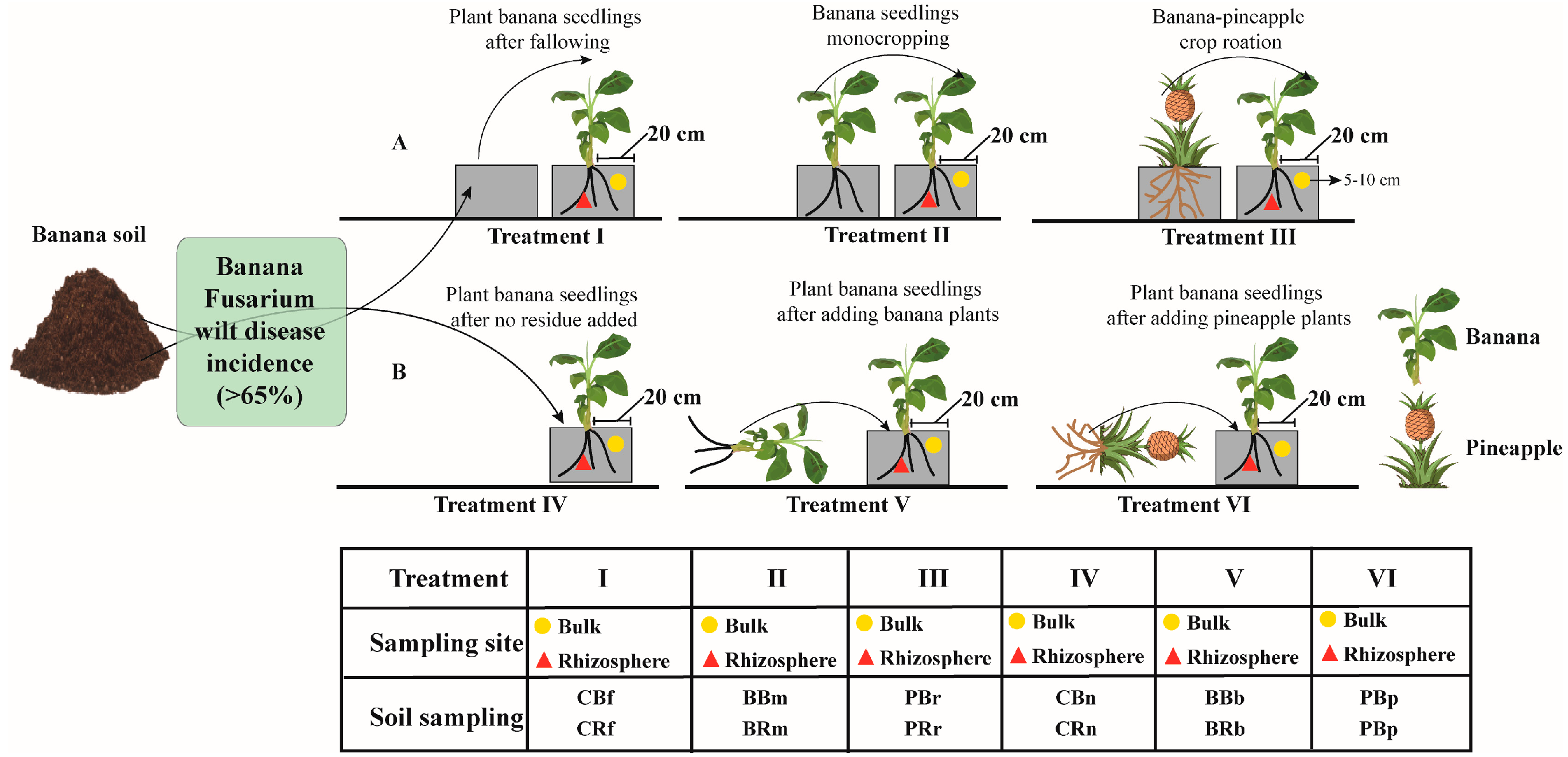
Soil with high banana Fusarium wilt disease incidence (>65%) was collected from the field trial site (Lingao Xinxing Farm, Hainan Province, China (109°77′ E, 19°77′ N), where bananas were continuously cropped for 11 years. The field trial site has a tropical monsoon climate with an average annual temperature of 23.8 °C, an average precipitation of 1786 mm, and an annual average of 2059 h of sunshine. The soil is an oxisol soil with a pH of 5.31, an organic matter content of 3.64 g·kg−1, an available phosphorus content of 15.64 mg·kg−1, an available potassium content of 411.67 mg·kg−1, and an available nitrogen content of 91.23 mg·kg−1. The excavated field soil was collected within the drip line next to the banana plant approximately 20 cm deep, thoroughly mixed, and immediately transferred to a greenhouse (average temperature of 36 °C and 37% humidity) at the College of Agriculture, Hainan University, Haikou, Hainan Province, China (110°34′ E, 20°06′ N).
A pot experiment was conducted from May 2018 to February 2019. It was completely randomized and included three treatments (i.e., Treatments I–III): fallow treatment (Treatment I), banana monoculture (Treatment II), and pineapple rotation (Treatment III). Each polypropylene pot (35 × 25 × 25 cm, length × width × height) had 10 kg of soil per pot, with six replicates of 10 pots per replicate. Before transplanting, commercial bio-organic fertilizer (a pure plant-derived bio-organic fertilizer containing 1.3% nitrogen (N), 1.1% phosphorus (P2O5), 1.0% potassium (K2O), organic matter ≥ 400 g·kg−1, and moisture ≤ 30%) was applied at a rate of 2% soil as a base fertilizer in the pots. After 5 days of transplanting, evenly grown pineapple and banana plantlets were transplanted into the pots (they were 2 months old before the pot experiment start). After 6 months planting, banana plantlets exhibited mild Fusarium wilt symptoms, such as yellow leaves and scabs at the base of the stem. Subsequently, the next crop of banana plantlets was continued by pulling out pineapple and banana plantlets and planting the banana seedlings. When the maximum incidence of treatment banana (B) was greater than 80% (4 months), the incidence of banana plantlets was counted for all treatments.
At this time, the whole banana plant was removed, and the bulk and rhizosphere soil were collected with sterile tweezers or brushes. The bulk soils of fallow, banana, and pineapple were defined as CBf, BBm, and PBr, respectively, while the rhizosphere soils of fallow, banana and pineapple were defined as CRf, BRm, and PRr, respectively (Figure 1A).
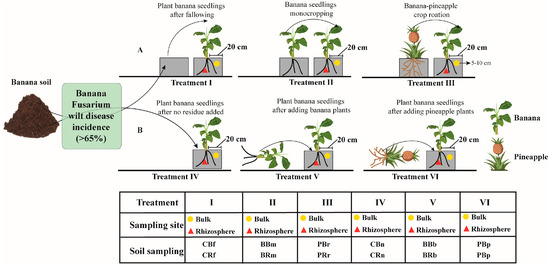
Figure 1.
Diagram of crop rotation, monocropping and residue addition sampling sites in the pot experiments. Experiment with different crop rotation (A); Experiment with different crop residues (B). CBf: Collect banana bulk soil after fallowing, CRf: Collect banana rhizosphere soil after fallowing; BBm: Collect banana bulk soil after monocropping, BRm: banana rhizosphere soil after monocropping; PBr: Collect banana bulk soil after pineapple–banana rotation, PRr: Collect banana rhizosphere soil after pineapple–banana rotation; CBn: Collect banana bulk soil after no residue added, CRn: Collect banana rhizosphere soil after no residue added; BBb: Collect banana bulk soil after adding banana residue, BRb: Collect banana rhizosphere soil after adding banana residue; PBp: Collect banana bulk soil after adding pineapple residue, PBp: Collect banana rhizosphere soil after adding pineapple residue.
2.2. Pineapple Residue Amendment Pot Experimental Design
From September 2018 to March 2019, an experiment was designed on the effect of crop residue addition on the incidence of banana seedlings. The banana and pineapple plants (Qionghai, Hainan) available in the field experiment were selected, chopped, and finely ground with an Oster blender, and then used in the pot experiment through a 2 mm mesh sieve. Crop residue was then thoroughly mixed with the pasteurized soil at a rate of 3.0% (v/v). There were three treatments (i.e., Treatments IV–VI) designed for soil collected from the field with the same potting specifications as above. Treatment IV was the control: no residue added, Treatment V was banana residue added, and Treatment VI was pineapple residue added. The experiment ended when the incidence of banana plantlets treated with added banana residues was over 60% (at six months). At this time, the whole banana plant was removed, and the bulk and rhizosphere soil were collected with sterile brushes. The bulk soils of fallow, banana, and pineapple were defined as CBn, BBb, and PBp, respectively, while the rhizosphere soils of fallow, banana, and pineapple were defined as CRn, BRb, and PRp, respectively (Figure 1B).
2.3. Disease Incidence Determination
Banana plants with typical wilt symptoms, such as yellow leaves, wilted leaves, and root rot, were counted as diseased plants, and the incidence rate was expressed as the percentage of the number of diseased banana plants to the total number of banana plants. In other words, the incidence rate (%) = diseased banana plants/total banana plants × 100% [28,43].
2.4. Soil Sampling Collection and DNA Extraction
When removing the plants, a soil auger was used to take 200 g soil samples from each plot at a depth of 5–10 cm and mixed into a composite sample for this treatment (Altitude: 10 m). Briefly, a total of 36 bulk soil samples (6 treatments × 6 mixed soil samples) were collected. Each of the 36 samples was made up of soil samples collected randomly from 10 pots (200 g per pot) out of the original 60 pots used for each treatment at a depth of 5–10 cm. The soil was mixed, homogenized, and sieved through a 2-mm nylon sieve to remove plant debris and was then divided equally into three subsamples. One subsample was air-dried for physicochemical analysis, and the other two were stored at 4 °C and −80 °C for microbiological analysis and DNA extraction, respectively. At this time, the whole plant was removed, and the bulk soil was removed by careful shaking. Soil still adhering to the roots was collected with sterile tweezers and defined as rhizosphere soil. Total soil DNA was extracted using a PowerSoil DNA Isolation Kit (MoBio Laboratories Inc., Carlsbad, CA, USA) following the manufacturer’s protocol. Bulk soil and rhizosphere soil samples of banana and pineapple collected from pot planting and residue addition tests were accurately weighed to 0.7000 g and 0.4000 g, respectively. A volume of 20 μL of DNA samples from each treatment was selected and sent to “Nanjing aomike Bio” for IlluminaMiSeq sequencing.
2.5. Quantitative PCR of Fusarium Oxysporum in Bulk and Rhizosphere Soil
The abundances of F. oxysporum in the bulk and rhizosphere soil were estimated by qPCR (Quantitative real-time PCR amplification) with primers FocSc-1 and FocSc-2, respectively (Table S1) [45,46]. The system for real-time fluorescence absolute quantification of pathogens at 50 μL was 2 μL of DNA template sample, 25 μL of premix reagent, 1 μL of primer 1 (FocSc-1) (10 pmol/L), 1 μL of primer 2 (FocSc-2) (10 pmol/L), and 21 μL of ddH2O. All soil DNA samples and the standard curve were analyzed using a 7500 Real-Time PCR System (Applied Biosystems™, Foster City, CA, USA) [28]. The melting curve and amplification efficiency were confirmed as standards for amplification. Six replicates of each sample were estimated, and the results were converted to log10 copy numbers (copy numbers g−1 soil) [47].
2.6. Soil Chemical Analysis and Culturable Microorganism Determination
Soil pH was assayed using a soil–water ratio of 1:5 (w/v) suspensions. The organic matter (OM) content was measured using the K2Cr2O7-H2SO4 oxidation method. Available potassium (AK) was extracted with ammonium acetate and analyzed by flame photometry. Available phosphorus (AP) was determined by molybdenum antimony colorimetry, and the available nitrogen (AN) content was determined following Liu et al. [48].
The total numbers of culturable Fusarium, bacteria, fungi, and actinomycetes were determined following Shen et al. [49]. The counts were as follows: the number of colonies forming units (CFU) formed on the plate was converted to the number of colonies formed per gram of dry soil and are expressed as CFU/g (dry soil) [50].
2.7. Illumina MiSeq Sequencing and Data Processing
The V4–V5 region of bacterial 16S rDNA and the fungal ITS region were amplified using the individual barcoded primers 520F/802R and ITS5F/ITS2R, respectively (Table S1) [51]. The PCR amplification procedures, including a 25-μL reaction volume containing 5 μL of 5 × reaction buffer, 5 μL of 5 × GC buffer, 1 μL of the 10 μM primer set, 2 μL of template DNA, 8.75 μL of ddH2O, and 5 μL of 100 mM dNTPs for 16S or 2 μL of 100 Mm dNTPs and 0.25 μL of DNA polymerase for ITS, were conducted according to Shen et al. [49]. The resulting PCR products were generated using the following PCR conditions: a temperature program of 2 min at 98 °C, followed by 28–30 cycles of 15 s at 98 °C, 55 °C for 30 s, and 30 s at 72 °C. The purified amplicons were pooled in equimolar amounts and analyzed with the Illumina MiSeq Aomike at Personal Biotechnology Co., Ltd. (Nanjing, China).
Raw sequences based on unique barcodes were assigned to soil samples following the QIIME software package (version 1.9.1) tutorials after elimination of the adaptors and primer sequences [52], and pairs of reads were merged using the FLASH software tool (version 1.2.7). The paired sequences were processed by the UPARSE pipeline to produce an operational taxonomic unit (OTU) table with USEARCH 11 and Perl scripts [53]. Briefly, sequences with a quality score < 0.5 or length < 200 bp were eliminated. The retained sequences were assembled to identify OTUs at 97% similarity, and chimaeras were removed based on the UCHIME method. Following that, high-quality OTUs were classified with the RDP Bacterial 16S database and the UNITE Fungal ITS database of the RDP classifier procedure for bacteria and fungi, respectively [54,55].
The raw sequence data were deposited in the NCBI Sequence Read Archive (SRA) database under accession numbers PRJNA745388 and PRJNA746047.
2.8. Statistical Analysis
Pot experiments were repeated over 2 seasons, and a very similar response trend was observed. Therefore, only sequencing data from the first pot experiment are presented herein. Microsoft Excel 2010 was used to process and graph the obtained data. Data were analyzed with SPSS 20.0 software, and one-way analysis of variance (ANOVA) was used to compare the data. The significance of differences between treatments was tested using the new complex polar difference method (Duncan’s test). R (3.6.0) language (vegan, ape, ggplot2, and corrplot packages) was used to perform principal coordinate analysis (PCoA) and Pearson and Spearman correlation analyses [46,56]. Permutational multivariate analysis of variance (PERMANOVA) and variance partitioning analysis (VPA) were based on the Bray-Curtis distance using the adonis and varpart functions within the R package vegan, respectively [57].
3. Results
3.1. Effects of Pineapple Rotation and Residue Amendment on Banana Fusarium Wilt Disease Incidence and Relative Abundance of Fusarium
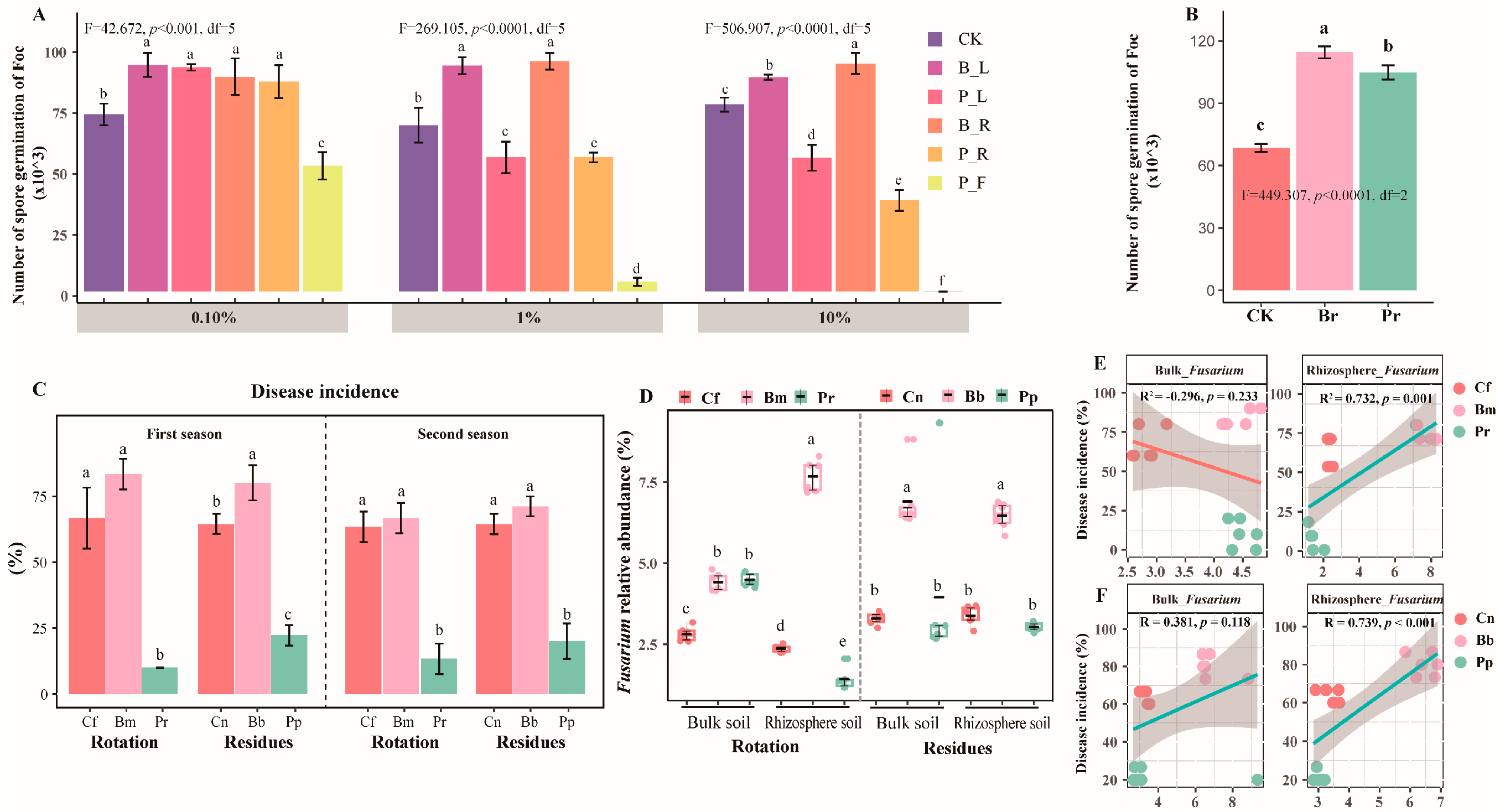
The in vitro effects of different pineapple and banana residues concentrations (0.1%, 1.0%, and 10.0%) on spore germination were evaluated (Figure 2A). The infusion of all parts of banana had a promoting effect on spore germination of the pathogen, while the infusion of all parts of pineapple had an inhibiting effect on spore germination, with the fruit infusion of pineapple having a highly significant effect on spore germination. In addition, compared to banana root exudates, pineapple root exudates were significantly less able to promote spore germination of the pathogen. The fungus significantly increased the number of pathogenic spores in adding banana and pineapple root exudates respectively compared to the control (Figure 2B). We observed an overall significantly reduced Fusarium wilt disease incidence in both the pineapple rotation and residue amendment systems in two seasons (Duncan’s t-test, p < 0.001) (Pr and Pp), which was significantly lower than the fallow and monoculture treatments (p < 0.001) (Figure 2C). The number of culturable bacteria was increased, while the number of culturable Fusarium was decreased in pineapple rotation and residue amendment treatments compared with the banana monoculture treatment (Table S2). Moreover, the abundance of rhizosphere Fusarium genera (rotation treatment) (p = 0.001), the residue treatment bulk Fusarium genera (p = 0.118), and the relative abundance of rhizosphere Fusarium genera (p < 0.001) were all positively correlated with disease incidence (Figure 2D), demonstrating the disease suppression ability of pineapple rotation and residue addition. In the rhizosphere soil, the relative abundance of Fusarium genera was significantly lower in the rotation than in the monoculture treatments (p < 0.05, Figure 2C). The qPCR results showed that significantly fewer Fusarium oxysporum were detected in the rotation rhizosphere (Duncan’s t-test, p < 0.05, Figure S1).
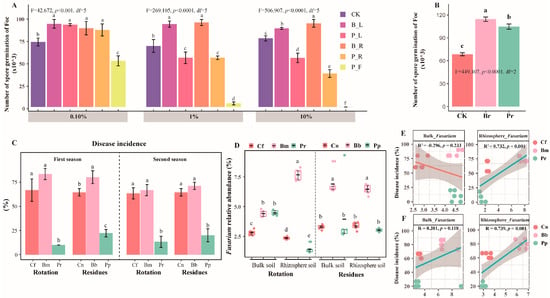
Figure 2.
Effects of root exudates from pineapple and banana on the spore germination of Foc (A). Effects of root exudates from pineapple and banana on the spore germination of Foc (B). Disease incidence of banana (%) (C) and the relative abundance of Fusarium spp. (D), and pearson correlations between DI and the relative abundance of Fusarium spp. in the bulk and rhizosphere with different crop rotation (E) and the relative abundance of Fusarium spp. in the bulk and rhizosphere with different crop residues (F) across all samples from six replicates. Different lowercase letters indicate statistically significant differences (p < 0.05) according to Duncan’s t-test. The treatment abbreviations are defined in Figure 1.
3.2. Effects of Pineapple Rotation and Residue Amendment on Microbial Community Structure
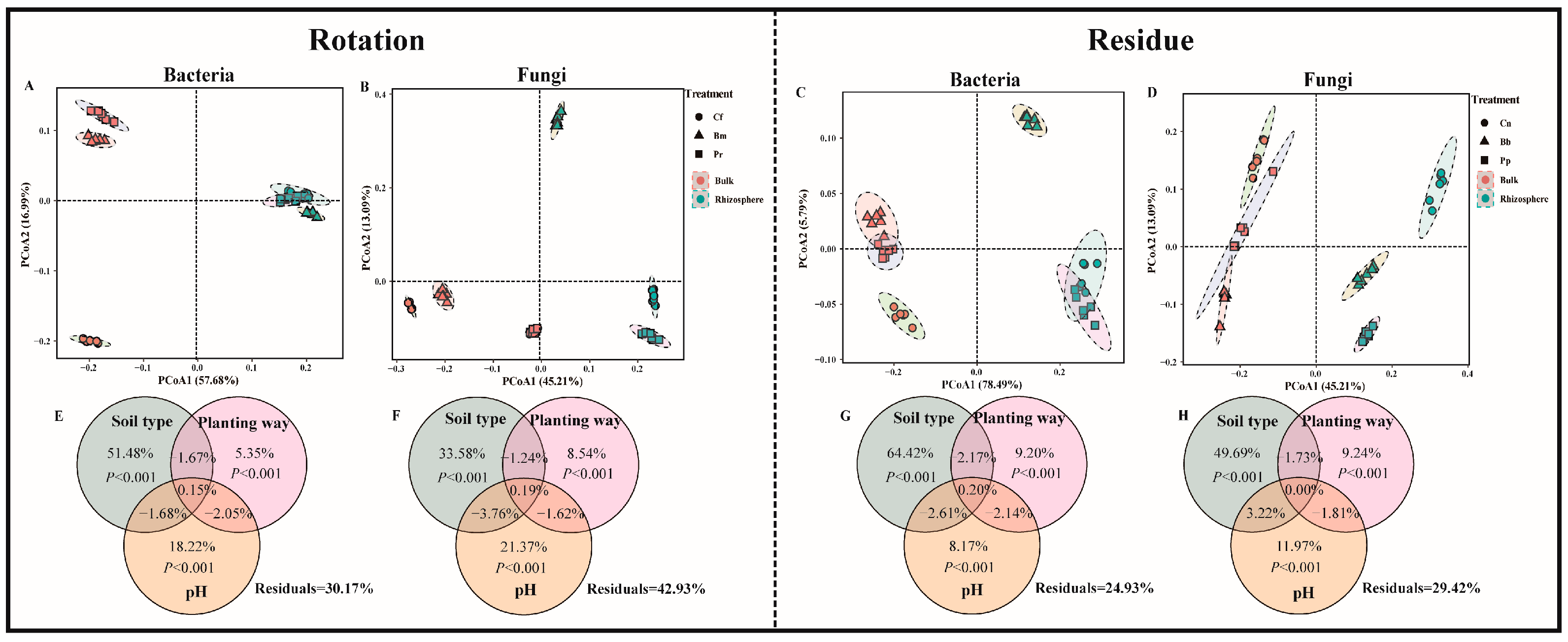
The PCoA plots (Figure 3A–D) showed significant differences in the bacterial and fungal community composition in the rotation and residue amendment systems (PERMANOVA, p < 0.001). In the rotation system, there was a significant difference in bacteria (p (bulk) < 0.01, p (rhizosphere) < 0.01). Moreover, the different rhizosphere soil microbial communities changed significantly from the corresponding initial soil microbial communities, and the microbial communities of different plants grown under the same soil type also differed significantly (p < 0.001) (Figure S2). The sequencing results were analyzed and detailed, and the microbial community richness and diversity sequencing results are shown in Supplementary Material (Table S4). PERMANOVA and VPA analyses revealed that soil rhizosphere microbial communities were significantly influenced by soil type (p < 0.001) and soil pH (p < 0.001). The relative importance of soil type and its soil pH on soil and rhizosphere microbial communities was greater than that of plant species (Figure 3E–H).
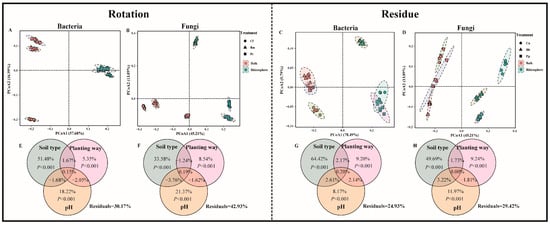
Figure 3.
PCoA based on Bray–Curtis distance of rotation (bacteria (A), fungi (B)) and residue (bacteria (C), fungi (D)) of soil community structure. Contributions of soil type, planting method, and soil chemicals (pH) to the assembly of soil bacterial (E,G) and fungal (F,H) communities were calculated based on variance partitioning analyses (VPAss) in rotation and residue, respectively, and the p value was determined by PERMANOVA. The treatment abbreviations are defined in Figure 1.
3.3. Effects of Pineapple Rotation and Residue Amendment on Taxonomic Composition
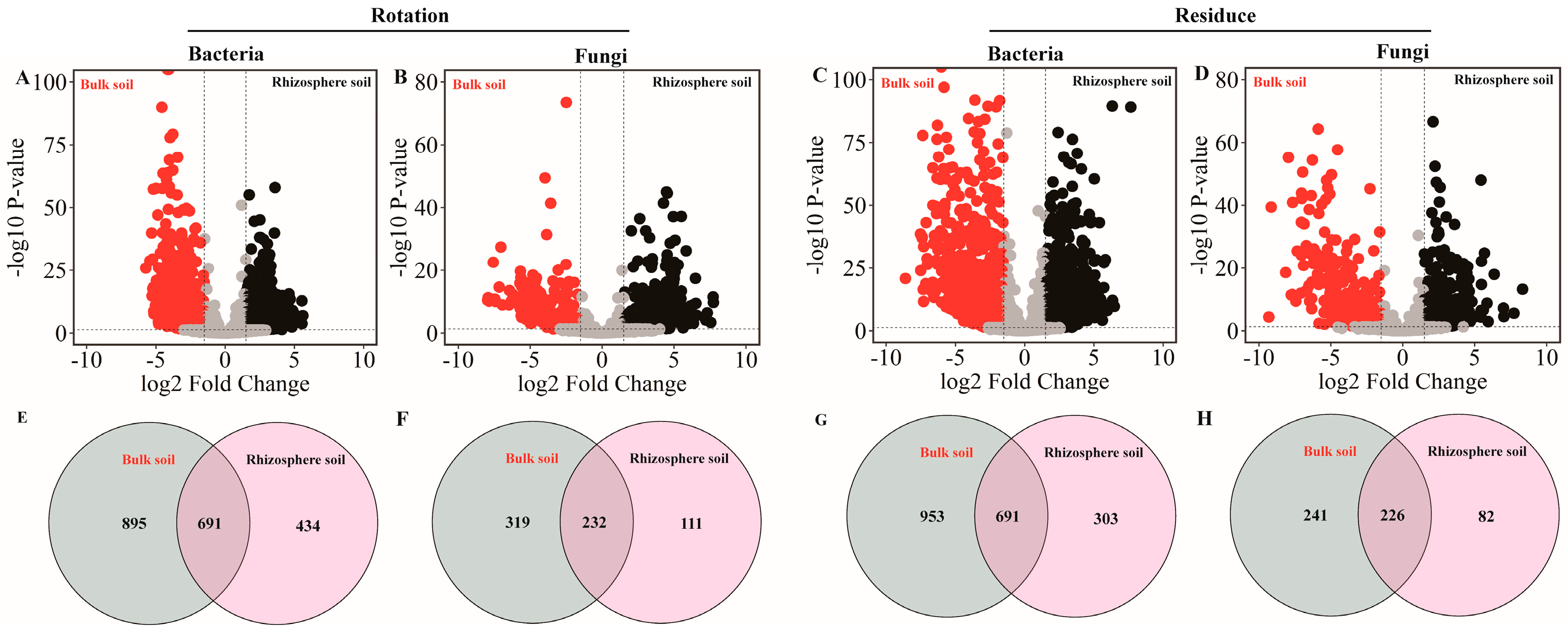
Volcano plot analysis of the sequence results revealed rotation and residue amendment bacterial and fungal community compositions with specific respective sets of OTUs (Figure 4A,B). With red indicating bulk soil and black indicating rhizosphere soil, we found that both the pineapple rotation and residue amendment treatment had a higher OTU number in the bulk soil. Selecting OTUs (relative abundance > 0.1%) for Venn analysis revealed that rotation and residue treatments had more bacteria, while fungi in the bulk soil had significantly higher OTU numbers than those in the rhizosphere soil (Figure 4C,D).
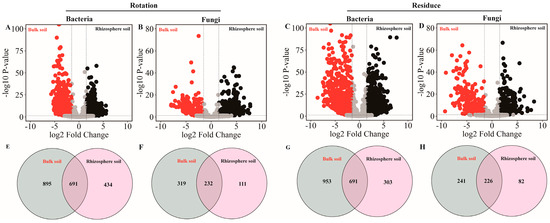
Figure 4.
The effects of soil type (bulk and rhizosphere soils) in the pineapple rotation and residue amendment on microbial compositions. The crop rotation (A,B) and crop residues (C,D) of bacterial and fungal,respectively. The bacterial and fungal OTUs with relative abundances that were significantly (log2-fold change > |1.5| and FDR adjusted p value < 0.05) different between the bulk (red) and rhizosphere (black) soils are colored in the volcano plot. Venn diagrams (E,F) represent the numbers of bacterial and fungal OTUs in bulk and rhizosphere soils originating from the crop rotation soil, respectively. Venn diagrams (G,H) represent the numbers of bacterial and fungal OTUs in bulk and rhizosphere soils originating from the crop residues soil, respectively.
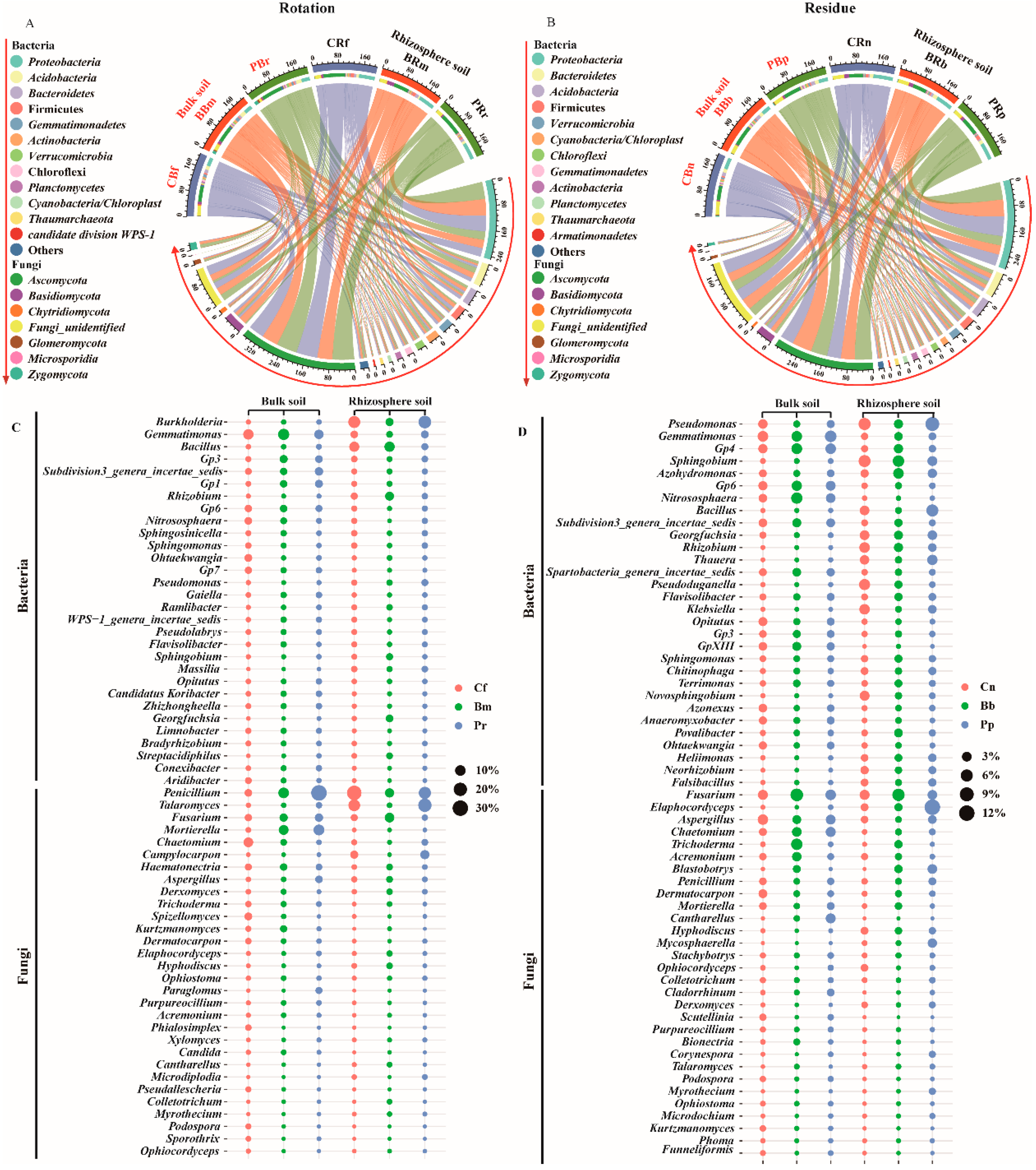
Pineapple rotations and residue amendment, which had the same phylum composition, consisting mainly of bacterial phyla Proteobacteria, Acidobacteria, Bacteroidetes, Firmicutes, Gemmatimonadetes, Actinobacteria, Verrucomicrobia, Chloroflexi, Planctomycetes, Cyanobacteria/Chloroplast, Thaumarchaeota and fungal phyla Ascomycota, and Basidiomycetes (Figure 5A,B). The Fusarium relative abundance was significantly negatively correlated with the bacterial phyla Thaumarchaeota (−0.482 **), Nitrospirae (−0.401 *), Firmicutes (−0.515 **), and Ascomycota (−0.608 **) (Table S6), which were generally consistent with previous findings on disease-suppressing soils [58].
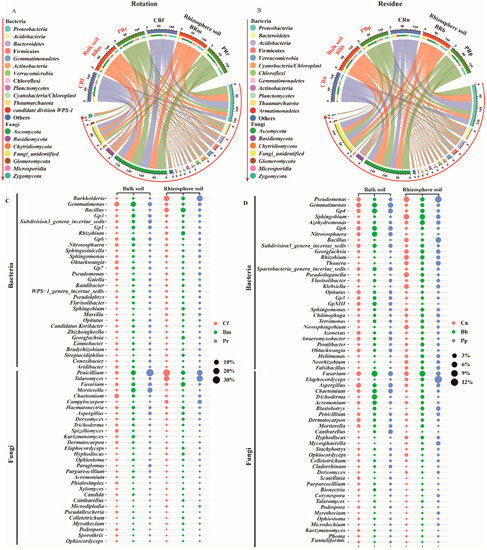
Figure 5.
Relative abundances (%) of the phyla and top 30 genera containing the rotation (A,C) and residue (B,D). Circle sizes in c and d represent the relative abundances of the genera. The treatment abbreviations are defined in Figure 1.
An analysis of top 30 genera of bacteria and fungi showed that the variability of genera significantly increased in the soil. In rotation, the Burkholderia, Bacillus, Rhizobium, Sphingosinicella, Pseudomonas, and Talaromyces genera were significantly increased in both rhizosphere and bulk soil, along with Gemmatimonas, Gp6, Nitrososphaera, Gp7, Penicillium, and Mortierella (Figure 5C). Among them, several genera were significantly negatively correlated with Fusarium, such as Burkholderia spp. (Pearson: −0.558 **, Spearman: −0.443 **), Pseudomonas spp. (Pearson: NS, Spearman: −0.363 *), and Talaromyces spp. (Pearson: −0.687 **, Spearman: −0.530 **) (Tables S7 and S9).
In residue rhizosphere, Pseudomonas, Sphingobium, Azohydromonas, Bacillus, Georgfuchsia, Rhizobium, Pseudoduganella, Klebsiella, Sphingomonas, Elaphocordyceps, and Penicillium genera were significantly increased, and in bulk soil, Gemmatimonas, Gp6, Nitrososphaera, Opitutus, Gp3, GpXIII, Aspergillus, and Chaetomium genera were significantly increased (Figure 5D). Among them, three genera were significantly negatively correlated with Fusarium spp.: Pseudomonas spp. (Pearson: −0.524 **, Spearman: −0.440 **), Aspergillus spp. (Pearson: −0.378 *, Spearman: −0.354 *), and Penicillium spp. (Pearson: −0.453 **, Spearman: −0.540 **) (Tables S8 and S9).
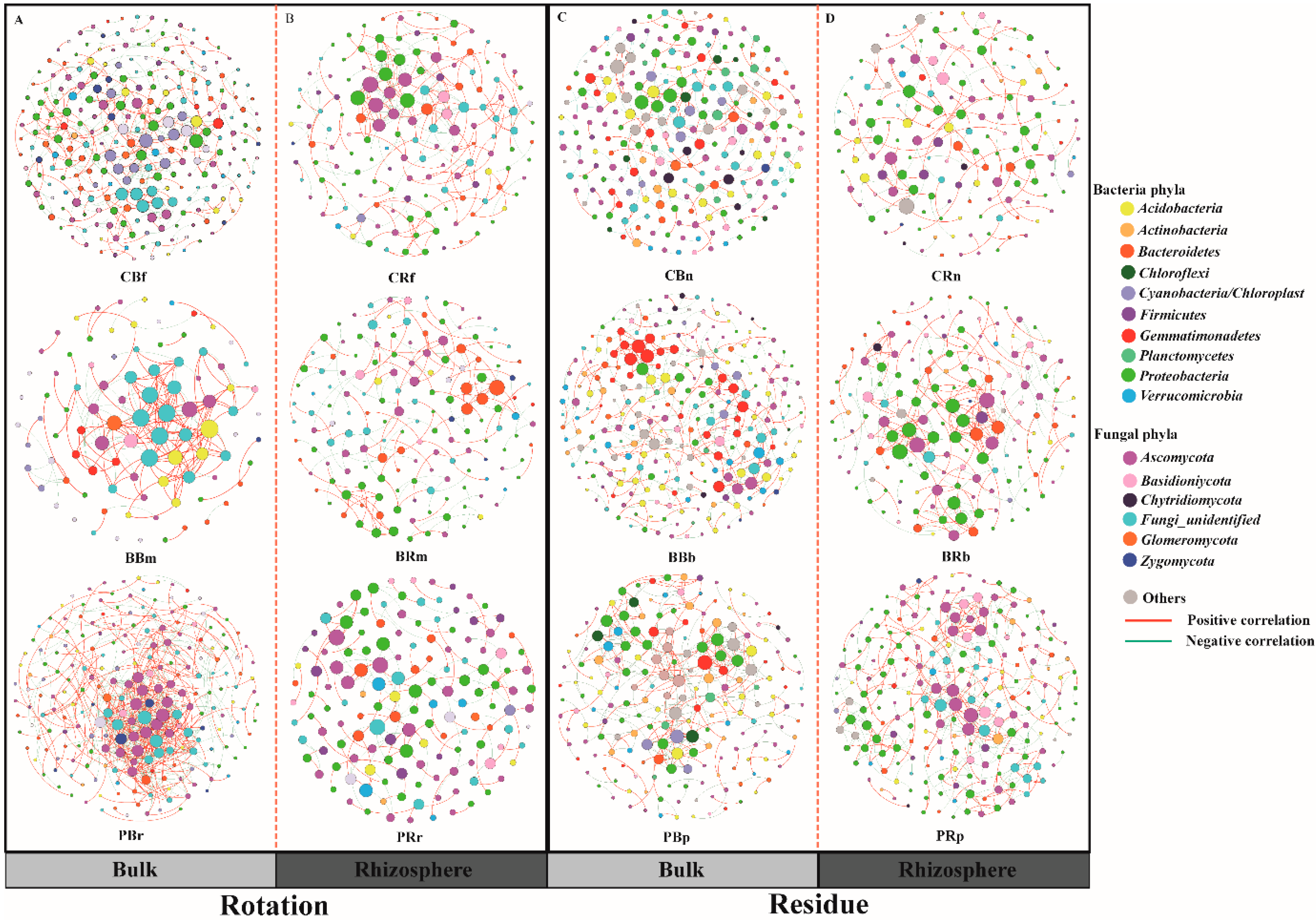
3.4. Bulk and Rhizosphere Network Construction through Effects on Specific Microbial Taxa
In this study, we constructed co-occurrence networks using random matrix theory (RMT) to determine the differences in bacterial and fungal assemblages (OTU relative abundance > 0.1%) in bulk and rhizosphere soils of the different treatments. All values of the calculated modularity index were larger than 0.4 (Table S10), indicating a typical modular structure [59]. Overall, pineapple rotation and residue amendment showed marked effects on the soil microbial network: the average path distance (GD), the average clustering coefficient (avgCC), and the modularity of the empirical networks were higher than those of the corresponding, identically sized random networks (Table S10). Here, we found that residue assemblages (in Figure 6C,D) formed more connected and more complex networks with fewer nodes but more connections (edges) between nodes compared with the bulk soil. Keystone taxa in network analysis can be computationally identified as hubs with a high within-module degree Zi (Zi ≥ 0.5 indicates that the nodes are “well connected” to other nodes in the module). The PBr and PRr treatments had some keystone taxa, such as Burkholderia and Pseudomonas, and no hub was found in the bulk or rhizosphere soil (Cf, Cn, Bm, and Bb) (Figure S3).
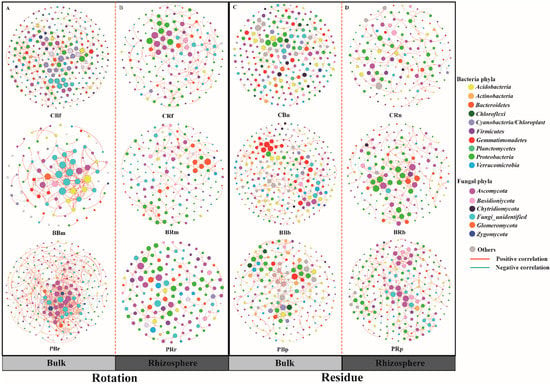
Figure 6.
Plant rhizosphere and the corresponding bulk soil networks in the rotation and residue addition systems. Bulk (A) and rhizosphere (B) soil microbial network under different crop rotation treatments; Bulk (C) and rhizosphere (D) soil microbial network under different crop residues treatments. Networks represent random matrix theory co-occurrence models derived from 6 biological replicates at each site, where nodes represent OTUs, and the edges between the nodes indicate significant correlations. A green edge indicates a negative covariation between two individual nodes, while a red edge indicates a positive covariation. The treatment abbreviations are defined in Figure 1.
3.5. Effects of The F. oxysporum, Microbial Communities, and Key Microorganism on Banana Fusarium Wilt Disease Incidence
To investigate the potentially relative important suppression predictors of banana Fusarium wilt disease incidence, linear models (LM) were used to identify the potential positive or negative effects of the number of Fusarium (including the number of F. oxysporum and the relative abundance of Fusarium genera), bacterial, and fungal communities (including bulk and rhizosphere communities), and key microorganism Burkholderia, Pseudomonas, Talaromyces, and Pseudomonas, Penicillium, and Aspergillus (bulk and rhizosphere soil) in the crop rotation and residue amendment treatment on banana Fusarium wilt disease incidence, respectively (Table 1).

Table 1.
Linear models (LM) for the relationships of microbial indicators with disease incidence and the relative importance of each indicator in the crop rotation and residue amendment system. p was results of ANOVAs.
In rotation treatment, importantly, Fungal-pcoa1 (rhizosphere) (F = 23.41, p = 0.001, Relative Importance = 10.89%), Fusarium genera relative abundance (rhizosphere) (F = 201.74, p < 0.001, Relative Importance = 12.70%), Burkholderia (rhizosphere) (F = 3.56, p = 0.092, Relative Importance = 13.79%), Talaromyces (bulk) (F = 64.34, p < 0.001, Relative Importance = 23.61%), and Talaromyces (rhizosphere) (F = 1.41, p = 0.265, Relative Importance = 19.09%) constrained disease incidence the most (with a relative importance more than 10%). Besides, based on linear regression analyses between disease incidence and selected microbial indicators, Fungal-pcoa1 (rhizosphere) (p = 0.001), Fusarium genera relative abundance (bulk) (p < 0.001), Fusarium relative abundance (rhizosphere) (p < 0.001), and Talaromyces (bulk) (p < 0.001) have a significant negative relationship to disease incidence (Table 1).
In residue amendment treatment, importantly, Fusarium genera relative abundance (rhizosphere) (F = 363.96, p < 0.001, Relative Importance = 25.49%) and Aspergillus (rhizosphere) (F = 16.15, p = 0.003, Relative Importance = 40.83%) constrained disease incidence the most (with a relative importance more than 10%). Besides, based on linear regression analyses between disease incidence and selected microbial indicators, Penicillium (bulk) (p = 0.051) and Aspergillus (rhizosphere) (p = 0.003) were significantly negative correlation with disease incidence (Table 1).
For physicochemical, soil organic matter (F = 8.94, p = 0.011, Relative Importance = 22.22%), the available potassium (F = 19.45, p = 0.001, Relative Importance = 36.45%) in rotation treatment, and the available phosphorus (F = 89.40, p < 0.001, Relative Importance = 16.37%), the available potassium (F = 223.59, p < 0.001, Relative Importance = 28.88%) and the available nitrogen (F = 7.69, p = 0.016, Relative Importance = 45.10%) in residue amendment treatment were top factors that significantly related to disease incidence with a relative importance more than 10% (Tables S3 and S11).
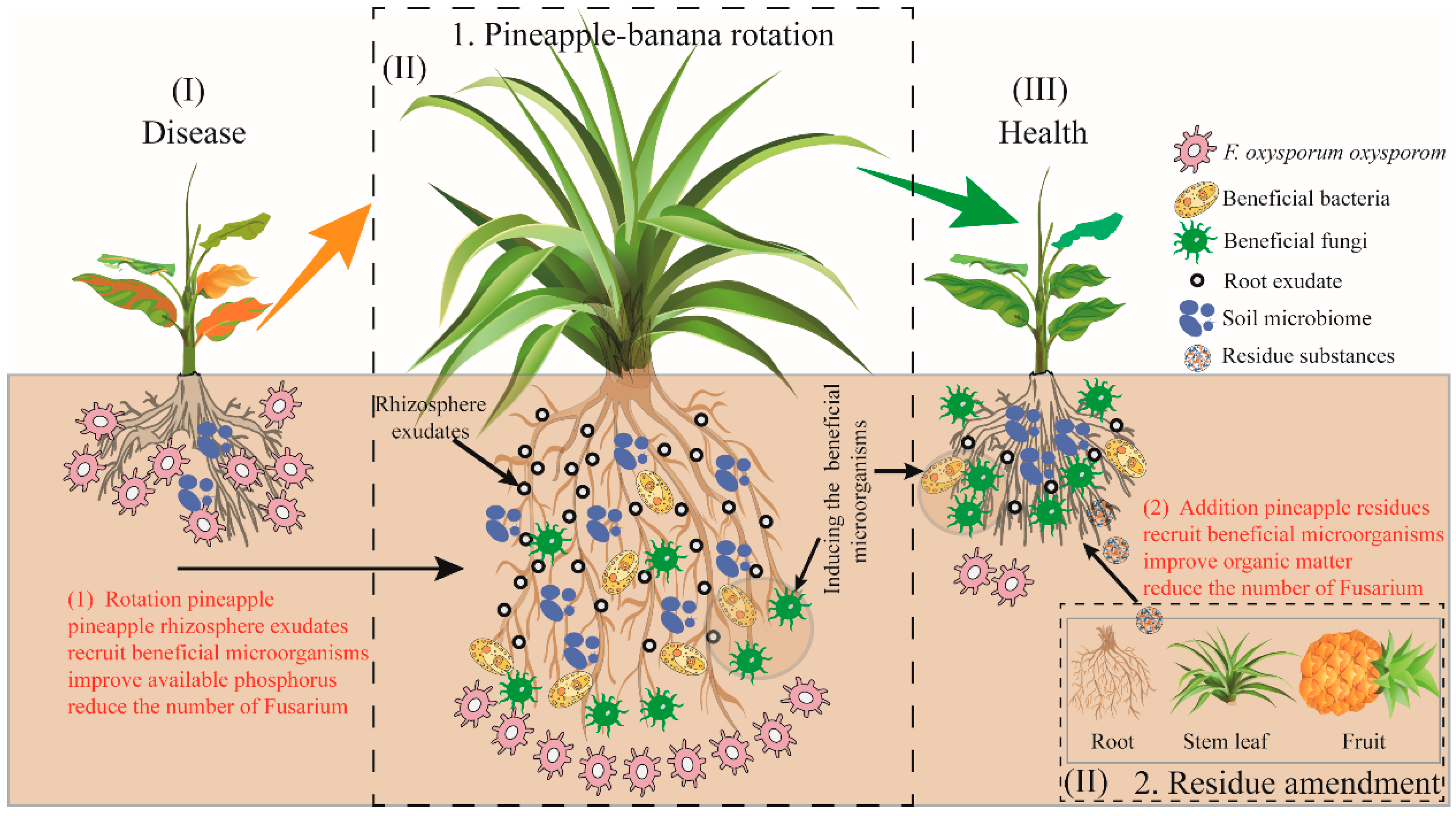
Based on the above results, a conceptual model illustrating potentially path was constructed with important suppression predictors (Figure 7). The conceptual mode indicated that soil characteristics and microbial community structure regulation were constitute the main two paths in disease suppression. Among all predictors, the key physicochemical factors AP (in rotation treatment) and OM (in residue treatment) were significantly affected by rotation and residue amendment, respectively. They were significant leading to the changes of fungal community and beneficial microorganism. Moreover, relative abundance of Fusarium was directly affected by the significant increases of Burkholderia, Talaromyces, and Aspergillus, thereby disease incidence was reduced (Figure 7).
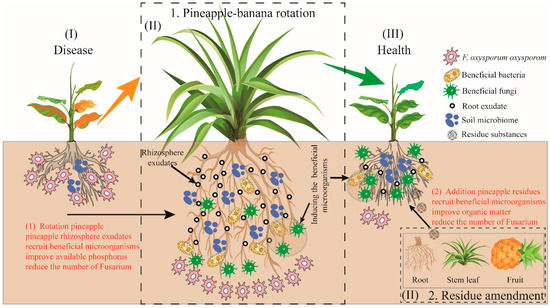
Figure 7.
The overview of the mechanism by which pineapple mediates microbiota to increase available nutrients in the banana–pineapple crop rotation and residue amendment system and reduce the incidence of banana.
4. Discussion
Different banana crop rotations can effectively reduce banana Fusarium wilt to varying degrees [28,60]. In this study, pot experiments revealed that pineapple–banana rotation and residue pineapple amendment could significantly enhance the suppression of Fusarium wilt disease compared with banana monocropping and residue addition. The results were a further extension of our previous report [43]. Notably, disease incidence reduction in crop rotation and residue amendment treatments could be attributed to a reduction in the number of Fusarium, as well as an increase in beneficial genera and changes in soil physicochemistry. Both the two steps in banana–pineapple rotation system, rotation, and residue amendment could inhibit fungal infections. Similar results have been shown for strawberry soil disease [61], likely due to the interruption of the host pathogen cycle in the root. Both high-throughput sequencing and qPCR showed that the Fusarium abundance was lower in both rotation and residue amendment treatments than in banana monoculture. Moreover, disease incidence has a significantly positive correlation with residue addition amount (Figure 2 and Figure S1). Pathogen density and disease incidence have been decreased in each pineapple residue treatment (P_L, P_R, and P_F). This finding aligns with our previous studies, indicating that using a pineapple–banana crop rotation system can effectively minimize the incidence of this pathogen [43]. Further, this study provides strong evidence that pineapple–banana rotation and residue amendment were both effective approaches for disease suppression.
Pineapple rotation may result in a significant decline in pathogen number via increasing some beneficial microorganisms. The disease suppression was potentially caused by a decrease in nutrient availability of pathogens which resulted from antagonistic and nutritional competition, parasitism, predation, induction of plant resistance, and interference of pathogenic signals. In addition, it seems possible that pineapple crops can stimulate beneficial bacterial microbiomes through root exudates to suppress pathogens during the crop rotation season [62].
The composition of the rhizosphere fungal community was clearly divided into different groups based on the PCoA results of the crop rotation and residue amendments, and the fungal communities were clearly clustered between the pineapple–banana rotation and residue pineapple addition treatments and the banana continuous crop and banana residue addition treatments (Figure 3). We hypothesize that soil fungi are less redundant than bacteria when exposed to environmental stresses of crop rotation and under the effective nutrients of residue addition. Additionally, VPA analysis showed that the variations in bacterial and fungal communities were best explained by the type of soil, followed by the pattern of cultivation. It has been previously reported that cultivated crops have a greater impact on microbial structure than cultivation patterns [59]. Future research elucidating the mechanisms of plant species-root exudate-soil microbial community interactions may be an effective way to study the suppression of pathogens [63]. Specifically, in our experiment, this suppressive emerged as a function of changes in the soil fungal communities rather than being mediated by changes in soil physicochemical properties. This was also further corroborated by linear models (LM), which traced the relationships among microbial communities, pathogen density, and banana disease incidence (Table S10).
Consistent with previous findings, the major bacterial phyla were Proteobacteria, Acidobacteria, Bacteroidetes, Firmicutes, Gemmatimonadetes, Actinobacteria, Verrucomicrobia, Chloroflexi, Planctomycetes, Cyanobacteria/Chloroplast, and Thaumarchaeota and the fungal phyla were Ascomycota and Basidiomycetes in this study (Figure 5A,B) [46,64]. The relative abundance of Firmicutes and Ascomycota increased after pineapple rotation and residue addition which also showed a strong negative correlation with F. oxysporum abundance (Table S6). This finding suggests that Firmicutes and Ascomycota may be involved in disease suppression through the representatives with biocontrol potential [65].
Pineapple–banana rotation significantly stimulated the relative abundance of the Burkholderia, Pseudomonas, and Talaromyces genera in bulk and rhizosphere soils (Figure 5C and Figure S2). These microorganisms showed significantly negative correlations with the abundance of F.oxysporum, and there is a large body of work demonstrating the stable suppression of disease by these beneficial microorganisms [66]. For example, Burkholderia spp. has been shown to be abundant in golden pineapple–banana rotations [43], and Pseudomonas spp. plays a key role in pathogen antagonism by stimulating the synthesis of a beneficial microbiome common to the native beneficial microorganisms, after the application of bioorganic fertilizers, to reduce the occurrence of Fusarium in banana [28]. Thus, they are widely reported as biological control agents. These microorganisms are capable of producing antifungal metabolites and can colonize the roots of plants, making them targets for biocontrol. Additionally, studies have shown that some fungal microorganisms pre-empt the microecological environment with pathogens, such as the effect of the mass production of Talaromyces spp. on pathogens populations [67]. This result is similar to Xiong et al. [68], who studied two treatments of vanilla wilt, where the crop rotation treatment significantly reduced the number of Fusarium oxysporum in the soil. In addition, both pineapple crop rotation and residue addition significantly reduced the incidence of banana seedling wilt, closely related to the decrease in the number of Fusarium oxysporum in the soil after pineapple cultivation.
According to Wang et al. [43], golden pineapple rotation can increase the organic matter and available phosphorus content in the soil with a decrease in the soil pH. The results of rotation treatment in this study were consistent with previous studies. PCoA, volcano plot, and VPA analyses revealed significant differences in microbial community structure after pineapple rotation, which is also consistent with previous studies [25,69,70]. Furthermore, we found that the two main components of banana–pineapple rotation, rotation only and residue addition, could both change soil microbial communities, and this may provide theoretical guidance for improving the operation of crop rotation.
Plant and soil microflora acted as important roles in soil-borne diseases suppression [28,71]. The species and number of beneficial microflorae in soil microorganisms are crucial for suppressing pathogens [72,73]. Numbers of Burkholderia, Pseudomonas, Penicillium, and Talaromyces were significantly increased in pineapple rotation treatment in the residue amendment, and Pseudomonas, Elaphocordyceps, and Aspergillus were significantly reduced in the residue amendment treatment. Wang et al. [43] and Steensels et al. [74] showed that the numbers of culturable bacteria and Burkholderia increased and the number of Fusarium oxysporum decreased significantly in pineapple rotation with bioorganic fertilizer. In herb and “maize–potato” rotations, Pseudomonas genus was abundant and significantly suppressed Fusarium oxysporum populations [16,47]. Moreover, studies have shown that Talaromyces and Aspergillus can have multiple plant protection functions and are corresponding antagonists against plant diseases [75,76,77]. Although Talaromyces and Aspergillus are fungal diseases in some crops, a significantly negative correlation was found between these two genera and Fusarium oxysporum in this experiment, which may be because the fungi Talaromyces and Aspergillus compete with Fusarium oxysporum for nutrients in the soil, resulting in a decrease in the number of Fusarium oxysporum [78].
The incidence of banana replanted after pineapple residue addition was significantly positively correlated with Fusarium oxysporum and culturable Fusarium oxysporum. In addition, it was significantly negatively correlated with soil pH, Pseudomonas, Aspergillus, and culturable bacteria. Penicillium and Burkholderia were abundantly present in the bulk and rhizosphere soils after pineapple residue addition, while Talaromyces and Aspergillus could pass through the pineapple rhizosphere to the soil and then survive in the next banana soil and Talaromyces could colonize the next banana soil. It was similar to the golden pineapple by Yuan et al., and the residual energy alleviated the occurrence of banana fusarium wilts, which verified that the abundance and colonization ability of Talaromyces and Aspergillus significantly increased after pineapple rotation [79,80]. Therefore, they are also potential key microorganisms in our case as Baileya and Lazarovitsb [81]. In summary, these results provide the basis for isolating and screening key beneficial microorganisms and the effect of their root exudates on these beneficial microorganisms. It provides some directions for the study of banana–pineapple rotation and residue amendment mechanisms.
5. Conclusions
This study further indicated that the addition of pineapple plant residues in the soil can also trigger soil suppression of a serious soil-borne pathogen (Fusarium spp.). Here, we described that this state of suppression is modulated by changes in the soil bacterial and fungal communities and highlighted one bacterial taxa (Burkholderia spp.) and two fungal taxa (Aspergillus spp. and Talaromyces spp.) that may be directly involved in pathogen suppression. Our next step is to isolate and screen these beneficial microorganisms and further explore the effects of root exudates on rhizosphere microorganisms in banana–pineapple rotation. In summary, our research may provide new avenues for exploring agricultural practices with a focus on beneficial outcomes that directly affect soil health and crop productivity in a viable and sustainable manner.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agronomy13020377/s1, Figure S1: The number of Fusarium oxysporum in rotation (A) and residue (B) treatment across all samples from six replicates; Figure S2: LDA scores (≥5) calculated for bacterial and fungal community composition differences at the top genus level between the difference treatments soils in the rotation (A and C) and the residue amendment (B and D), respectively; Figure S3: Zi-Pi value of the empirical molecular ecological networks of microbial communities in treatments; Table S1: Amplification primers used in this study; Table S2: Number of soil cultivable microorganism from the six treatments; Table S3: Soil properties in this study; Table S4: Bacterial and fungal community richness and diversity in the treatment and control; Table S5: Permutational multivariate analysis of variance (PERMANOVA) for principal coordinate analysis (PCoA); Table S6: Bacteria and fungal phylum level with Fusarium relative abundance correlation in the rotation and residue addition treatments; Table S7: Bacteria and fungi top 30 in the rotation; Table S8: Bacteria and fungi top 30 in the residue; Table S9: Pearson and spearman’s rank correlation coefficient between the abundant bacterial and fungal genus with the Fusarium abundance; Table S10: Topological properties of the empirical molecular ecological networks of microbial communities in treatments; Table S11: Linear models (LM) for the relationships of physicochemical indicators with disease incidence and the relative importance of each indicators in the crop rotation and residue amendment system.
Author Contributions
J.Y.: Conceptualization, Methodology, Software, Visualization, Writing—review & editing. Q.W.: Resources, Supervision. Y.W.: Resources, Supervision. X.C.: Resources, Supervision. W.G.: Resources, Supervision. Y.Z.: Resources, Supervision. B.W.: Investigation, Conceptualization, Methodology, Visualization, Writing—review & editing, Supervision, Funding acquisition. Y.R.: Resources, Supervision. All authors have read and agreed to the published version of the manuscript.
Funding
We are very grateful to Nanjing Personal Biotechnology Co., Ltd. for deep 16S rRNA and ITS barcod-edIllumina sequencing. In addition, we thank all staff members in the Hainan Key Laboratory for Sustainable Utilization of Tropical Bio-resources for their considerable help with banana and pineapple planting and management. This research was financially supported by The National Natural Science Foundation of China (41867006), the Hainan Provincial Fundation of China (320RC483) and the Key Research and Development Project of Hainan Province (ZDYF2021XDNY279).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Dita, M.A.; Waalwijk, C.; Buddenhagen, I.W.; Souza, M.T., Jr.; Kema, G.H.J. A molecular diagnostic for tropical race 4 of the banana fusarium wilt pathogen. Plant Pathol. 2010, 59, 348–357. [Google Scholar] [CrossRef]
- Mendes, R.; Garbeva, P.; Rainmakers, J.M. The rhizosphere microbiome: Significance of plant beneficial, plant pathogenic, and human pathogenic microorganisms. FEMS Microbiol. Rev. 2013, 37, 634–663. [Google Scholar] [CrossRef]
- Butler, D. Fungus threatens top banana. Nature 2013, 504, 195–196. [Google Scholar] [CrossRef] [PubMed]
- Mendes, L.W.; Kuramae, E.E.; Navarrete, A.A.; van Veen, J.A.; Tsai, S.M. Taxonomical and functional microbial community selection in soybean rhizosphere. ISME J. 2014, 8, 1577–1587. [Google Scholar] [CrossRef]
- Huang, Y.H.; Wang, R.C.; Li, C.H.; Zuo, C.W.; Wei, Y.R.; Zhang, L.; Yi, G.L. Control of Fusarium wilt in banana with Chinese leek. Eur. J. Plant Pathol. 2012, 134, 87–95. [Google Scholar] [CrossRef]
- Hwang, S.C.; Ko, W.H. Cavendish banana cultivars resistant to Fusarium wilt acquired through somaclonal variation in Taiwan. Plant Dis. 2004, 88, 580–588. [Google Scholar] [CrossRef]
- Abubakar, A.I.; Khairulmazmi, A.; Yasmeen, S.; Muhammad, A.A.W.; Abdulaziz, B.K.; Adamu, A.; Zobir, S.A.M.; Abdu, A.; Abdullah, S.N.A. Fusarium Wilt of Banana: Current Update and Sustainable Disease Control Using Classical and Essential Oils Approaches. Hortic. Plant J. 2022, in press. [Google Scholar] [CrossRef]
- Tao, C.Y.; Li, R.; Xiong, W.; Shen, Z.Z.; Liu, S.S.; Wang, B.B.; Ruan, Y.Z.; Geisen, S.; Shen, Q.R.; Kowalchuk, G.A. Bio-organic fertilizers stimulate indigenous soil Pseudomonas populations to enhance plant disease suppression. Microbiome 2020, 8, 137. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Cenci, A.; Rouard, M.; Zhang, D.; Wang, Y.Y.; Tang, W.H.; Zheng, S.J. Transcriptomic analysis of resistant and susceptible banana corms in response to infection by Fusarium oxysporum f. sp. cubense tropical race 4. Sci. Rep. 2019, 9, 8199. [Google Scholar] [CrossRef]
- Wang, B.B.; Yuan, J.; Zhang, J.; Shen, Z.Z.; Zhang, M.X.; Li, R.; Ruan, Y.Z.; Shen, Q.R. Effects of novel bioorganic fertilizer produced by Bacillus amyloliquefaciens W19 on antagonism of Fusarium wilt of banana. Biol. Fert. Soils 2013, 49, 435–446. [Google Scholar] [CrossRef]
- Sun, J.B.; Zou, L.P.; Li, W.B.; Yang, J.H.; Wang, Y.J.; Xia, Q.Y.; Peng, M. Rhizosphere soil properties and banana fusarium wilt suppression influenced by combined chemical and organic fertilizations. Agric. Ecosyst. Environ. 2018, 254, 60–68. [Google Scholar] [CrossRef]
- Ploetz, R.C.; Churchill, A.C.L. Fusarium wilt: The banana disease that refuses to go away. Acta Hortic. 2011, 897, 519–526. [Google Scholar] [CrossRef]
- Zhang, N.; He, X.; Zhang, J.; Raza, W.; Yang, X.M.; Ruan, Y.Z.; Shen, Q.R.; Huang, Q.W. Suppression of Fusarium wilt of banana with application of bio-organic fertilizers. Pedosphere 2014, 24, 613–624. [Google Scholar] [CrossRef]
- Lakshmanan, P.; Selvaraj, P.; Mohan, S. Efficacy of different methods for the control of Panama disease. Trop. Pest Manag. 2008, 33, 373–374. [Google Scholar] [CrossRef]
- Xue, C.; Shen, Z.Z.; Hao, Y.W.; Yu, S.T.; Li, Y.C.; Huang, W.J.; Chong, Y.; Ran, W.; Li, R.; Shen, Q.R. Fumigation coupled with bio-organic fertilizer for the suppression of watermelon Fusarium wilt disease re-shapes the soil microbiome. Appl. Soil Ecol. 2019, 140, 49–56. [Google Scholar] [CrossRef]
- Li, Y.; Guo, Q.; Wei, X.; Xue, Q.; Lai, H. Biocontrol effects of Penicillium griseofulvum against monkshood (Aconitum carmichaelii Debx.) root diseases caused by Sclerotium rolfsiii and Fusarium spp. J. Appl. Microbiol. 2019, 127, 1532–1545. [Google Scholar] [CrossRef]
- Su, L.X.; Shen, Z.Z.; Ou, Y.N.; Tao, C.Y.; Ruan, Y.Z.; Li, R.; Shen, Q.R. Novel soil fumigation strategy suppressed plant-parasitic nematodes associated with soil nematode community alterations in the field. Appl. Soil Ecol. 2017, 121, 135–142. [Google Scholar] [CrossRef]
- Ploetz, R.C. Management of Fusarium wilt of banana: A review with special reference to tropical race 4. Crop Prot. 2015, 73, 7–15. [Google Scholar] [CrossRef]
- Larkin, R.P. Soil health paradigms and implications for disease management. Annu. Rev. Phytopathol. 2015, 53, 199–221. [Google Scholar] [CrossRef] [PubMed]
- Bonanomi, G.; Lorito, M.; Vinale, F.; Woo, S.L. Organic amendments, beneficial microbes, and soil mcrobiota: Toward a unified framework for disease suppression. Annu. Rev. Phytopathol. 2018, 56, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Li, M.H.; Guo, J.J.; Ren, T.; Luo, G.W.; Shen, Q.R.; Lu, J.W.; Guo, S.W.; Ling, N. Crop rotation history constrains soil biodiversity and multifunctionality relationships. Agric. Ecosyst. Environ. 2021, 319, 107550. [Google Scholar] [CrossRef]
- Christen, O.; Sieling, K. Effect of different preceding crops and crop rotations on yield of Winter Oil-seed rape (Brassica napus L.). J. Agron. Crop Sci. 1995, 174, 265–271. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, J.; Shen, J.; Luo, Y.; Scheu, S.; Ke, X. Tillage, residue burning and crop rotation alter soil fungal community and water-stable aggregation in arable fields. Soil Tillage Res. 2010, 107, 71–79. [Google Scholar] [CrossRef]
- Larkin, R.P.; Halloran, J.M. Management effects of disease-suppressive rotation crops on potato yield and soil-borne disease and their economic implications in potato production. Am. J. Potato Res. 2014, 91, 429–439. [Google Scholar] [CrossRef]
- Wright, P.J.; Falloon, R.E.; Hedderley, D. Different vegetable crop rotations affect soil microbial communities and soil-borne diseases of potato and onion: Literature review and a long-term field evaluation. N. Z. J. Crop Hort. 2014, 43, 85–110. [Google Scholar] [CrossRef]
- Cunfer, B.M.; Buntin, G.D.; Phillips, D.V. Effect of crop rotation on take-all of wheat in double-cropping systems. Plant Dis. 2006, 90, 1161–1166. [Google Scholar] [CrossRef]
- Wei, Z.; Gu, Y.A.; Friman, V.P.; Kowalchuk, G.A.; Jousset, A. Initial soil microbiome composition and functioning predetermine future plant health. Sci. Adv. 2019, 5, eaaw0759. [Google Scholar] [CrossRef]
- Hong, S.; Jv, H.L.; Lu, M.; Wang, B.B.; Zhao, Y.; Ruan, Y.Z. Significant decline in banana Fusarium wilt disease is associated with soil microbiome reconstruction under chilli pepper-banana rotation. Eur. J. Soil Biol. 2020, 97, 103154. [Google Scholar] [CrossRef]
- Bender, S.F.; Wagg, C.; van der Heijden, M.G.A. An underground revolution: Biodiversity and soil ecological engineering for agricultural sustainability. Trends Ecol. Evol. 2016, 31, 440–452. [Google Scholar] [CrossRef]
- Guo, S.; Xiong, W.; Hang, X.N.; Gao, Z.L.; Jiao, Z.X.; Liu, H.J.; Mo, Y.N.; Zhang, N.; Kowalchuk, G.A.; Li, R.; et al. Protists as main indicators and determinants of plant performance. Microbiome 2021, 9, 64. [Google Scholar] [CrossRef]
- Ge, A.H.; Liang, Z.H.; Xiao, J.L.; Zhang, Y.; Zeng, Q.; Xiong, C.; Han, L.L.; Wang, J.T.; Zhang, L.M. Microbial assembly and association network in watermelon rhizosphere after soil fumigation for Fusarium wilt control. Agric. Ecosyst. Environ. 2021, 312, 107336. [Google Scholar] [CrossRef]
- Lambers, H.; Mougel, C.; Jailard, M.; Hinsinger, P. Plant-microbe-soil interactions in the rhizosphere: An evolutionary perspective. Plant Soil. 2009, 321, 83–115. [Google Scholar] [CrossRef]
- Mcdonald, G.K.; Peck, D. Effects of crop rotation, residue retention and sowing time on the incidence and survival of ascochyta blight and its effect on grain yield of field peas (pisum sativum l.). Field Crop Res. 2009, 111, 11–21. [Google Scholar] [CrossRef]
- Kerdraon, L.; Laval, V.; Suffert, F. Microbiomes and Pathogen Survival in Crop Residues, an Ecotone Between Plant and Soil. Phytobiomes J. 2019, 3, 246–255. [Google Scholar] [CrossRef]
- Hu, L.F.; Robert, C.A.M.; Cadot, S.; Zhang, X.; Ye, M.; Li, B.B.; Manzo, D.; Chervet, N.; Steinger, T.; van der Heijden, M.G.A.; et al. Root exudate metabolites drive plant-soil feedbacks on growth and defense by shaping the rhizosphere microbiota. Nat. Commun. 2018, 9, 2738. [Google Scholar] [CrossRef]
- Yuan, J.; Zhao, J.; Wen, T.; Zhao, M.; Li, R.; Goossens, P.; Huang, Q.W.; Bai, Y.; Vivanco, J.M.; Kowalchuk, G.A.; et al. Root exudates drive the soil-borne legacy of aboveground pathogen infection. Microbiome 2018, 6, 156. [Google Scholar] [CrossRef]
- Zhou, G.; Gao, S.; Lu, Y.; Liao, Y.; Nie, J.; Cao, W. Co-incorporation of green manure and rice straw improves rice production, soil chemical, biochemical and microbiological properties in a typical paddy field in southern China. Soil Tillage Res. 2020, 197, 104499. [Google Scholar] [CrossRef]
- Wang, M.; Pendall, E.; Fang, C.; Li, B.; Nie, M. A global perspective on agroecosystem nitrogen cycles after returning crop residue. Agric. Ecosyst. Environ. 2018, 266, 49–54. [Google Scholar] [CrossRef]
- Martinez-Feria, R.A.; Castellano, M.J.; Dietzel, R.N.; Helmers, M.J.; Liebman, M.; Huber, I.; Archontoulis, S.V. Linking crop- and soil-based approaches to evaluate system nitrogen-use efficiency and tradeoffs. Agric. Ecosyst. Environ. 2018, 256, 131–143. [Google Scholar] [CrossRef]
- Chen, J.; Heiling, M.; Resch, C.; Mbaye, M.; Gruber, R.; Dercon, G. Does maize and legume crop residue mulch matter in soil organic carbon sequestration? Agric. Ecosyst. Environ. 2018, 265, 123–131. [Google Scholar] [CrossRef]
- Afshan, S.; Ali, S.; Bharwana, S.A.; Rizwan, M.; Farid, M.; Abbas, F.; Ibrahim, M.; Mehmood, M.A.; Abbasi, G.H. Citric acid enhances the phytoextraction of chromium, plant growth, and photosynthesis by alleviating the oxidative damages in Brassica napus L. Environ. Sci. Pollut. Res. 2015, 22, 11679–11689. [Google Scholar] [CrossRef] [PubMed]
- De, C.U.; Patruno, L.; Avella, N.; Salimbeni, R.; Lacolla, G.; Cucci, G.; Crecchio, C. Soil management under tomato-wheat rotation increases the suppressive response against Fusarium wilt and tomato shoot growth by changing the microbial composition and chemical parameters. Appl. Soil Ecol. 2020, 154, 103601. [Google Scholar]
- Wang, B.B.; Li, R.; Ruan, Y.Z.; Ou, Y.N.; Zhao, Y.; Shen, Q.R. Pineapple-banana rotation reduced the amount of Fusarium oxysporum more than maize-banana rotation mainly through modulating fungal communities. Soil Biol. Biochem. 2015, 86, 77–86. [Google Scholar] [CrossRef]
- Yang, J.M.; Ren, X.Y.; Liu, M.Y.; Fan, P.S.; Ruan, Y.Z.; Zhao, Y.; Wang, B.B.; Li, R. Suppressing soil-borne Fusarium pathogens of bananas by planting different cultivars of pineapples, with comparisons of the resulting bacterial and fungal communities. Appl. Soil Ecol. 2022, 169, 104211. [Google Scholar] [CrossRef]
- Fierer, N.; Jackson, J.A.; Vilgalys, R.; Jackson, R.B. Assessment of soil microbial community structure by use of taxon-specific quantitative PCR assays. Appl. Environ. Microbiol. 2005, 71, 4117–4120. [Google Scholar] [CrossRef]
- Shen, Z.Z.; Xue, C.; Penton, C.R.; Thomashow, L.S.; Zhang, N.; Wang, B.B.; Ruan, Y.Z.; Li, R.; Shen, Q.R. Suppression of banana Panama disease induced by soil microbiome reconstruction through an integrated agricultural strategy. Soil Biol. Biochem. 2019, 128, 164–174. [Google Scholar] [CrossRef]
- Xiong, W.; Guo, S.; Jousset, A.; Zhao, Q.Y.; Wu, H.S.; Li, R.; Kowalchuk, G.A.; Shen, Q.R. Bio-fertilizer application induces soil suppressiveness against Fusarium wilt disease by reshaping the soil microbiome. Soil Biol. Biochem. 2017, 114, 238–247. [Google Scholar] [CrossRef]
- Liu, L.L.; Kong, J.J.; Cui, H.L.; Zhang, J.B.; Wang, F.H.; Cai, Z.C.; Huang, X.Q. Relationships of decomposability and C/N ratio in different types of organic matter with suppression of Fusarium oxysporum and microbial communities during reductive soil disinfestation. Biol. Control 2016, 101, 103–113. [Google Scholar] [CrossRef]
- Shen, Z.Z.; Ruan, Y.Z.; Wang, B.B.; Zhong, S.T.; Su, L.X.; Li, R.; Shen, Q.R. Effect of biofertilizer for suppressing Fusarium wilt disease of banana as well as enhancing microbial and chemical properties of soil under greenhouse trial. Appl. Soil Ecol. 2015, 93, 111–119. [Google Scholar] [CrossRef]
- Shen, Z.Z.; Ruan, Y.Z.; Xue, C.; Zhong, S.T.; Li, R.; Shen, Q.R. Soils naturally suppressive to banana Fusarium wilt disease harbor unique bacterial communities. Plant Soil. 2015, 393, 21–33. [Google Scholar] [CrossRef]
- Shen, Z.Z.; Xue, C.; Taylor, P.W.J.; Ou, Y.N.; Wang, B.B.; Zhao, Y.; Ruan, Y.Z.; Li, R.; Shen, Q.R. Soil pre-fumigation could effectively improve the disease suppressiveness of biofertilizer to banana Fusarium wilt disease by reshaping the soil microbiome. Biol. Fert. Soils 2018, 54, 793–806. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef]
- Edgar, R.C.; Haas, B.J.; Clemente, J.C.; Quince, C.; Knight, R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 2011, 27, 2194–2200. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef]
- Kõljalg, U.; Nilsson, R.H.; Abarenkov, K.; Tedersoo, L.; Taylor, A.F.S.; Bahram, M.; Bates, S.T.; Bruns, T.D.; Bengtsson-Palme, J.; Callaghan, T.M.; et al. Towards a unified paradigm for sequence-based identification of fungi. Mol. Ecol. 2013, 22, 5271–5277. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environmentfor Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019; Available online: https://www.R-project.org/ (accessed on 15 May 2021).
- Oksanen, J.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’Hara, R.B. Vegan: Community Ecology Package. R Package ed Version 2.4. 2012. Available online: http://cran.r-project.org/package=vegan (accessed on 20 May 2021).
- Shen, Z.Z.; Penton, C.R.; Lv, N.N.; Xue, C.; Yuan, X.F.; Ruan, Y.Z.; Li, R.; Shen, Q.R. Banana Fusarium wilt disease incidence is influenced by shifts of soil microbial communities under different monoculture spans. Microbiol. Ecol. 2018, 75, 739–750. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Bonkowshi, M.; Shen, Y.; Griffiths, B.S.; Jiang, Y.J.; Wang, X.Y.; Sun, B. Root ethylene mediates rhizosphere microbial community reconstruction when chemically detecting cyanide produced by neighbouring plants. Microbiome 2020, 8, 4. [Google Scholar] [CrossRef] [PubMed]
- Fan, P.S.; Lai, C.Y.; Yang, J.M.; Hong, S.; Yang, Y.; Wang, Q.; Wang, B.; Zhang, R.; Jia, Z.; Zhao, Y.; et al. Crop rotation suppresses soil-borne Fusarium wilt of banana and alters microbial communities. Arch. Agron. Soil Sci. 2022, 68, 447–459. [Google Scholar] [CrossRef]
- Fang, X.L.; You, M.P.; Barbetti, M.J. Reduced severity and impact of Fusarium wilt on strawberry by manipulation of soil pH, soil organic amendments and crop rotation. Eur. J. Plant Pathol. 2012, 134, 619–629. [Google Scholar] [CrossRef]
- Hao, W.Y.; Ren, L.X.; Ran, W.; Shen, Q.R. Allelopathic effects of root exudates from watermelon and rice plants on Fusarium oxysporum f. sp. niveum. Plant Soil. 2010, 336, 485–497. [Google Scholar] [CrossRef]
- Yin, C.T.; Casa Vargas, J.M.; Schlatter, D.C.; Hagerty, C.H.; Hulbert, S.H.; Paulitz, T.C. Rhizosphere community selection reveals bacteria associated with reduced root disease. Microbiome 2021, 9, 1–18. [Google Scholar] [CrossRef]
- Wang, B.B.; Sun, M.Z.; Yang, J.M.; Shen, Z.Z.; Ou, Y.N.; Fu, L.; Zhao, Y.; Li, R.; Ruan, Y.Z.; Shen, Q.R. Inducing banana Fusarium wilt disease suppression through soil microbiome reshaping by pineapple–banana rotation combined with biofertilizer application. Soil 2022, 8, 17–29. [Google Scholar] [CrossRef]
- Yang, J.M.; Duan, Y.J.; Liu, X.Y.; Sun, M.Z.; Wang, Y.M.; Liu, M.Y.; Zhu, Z.Q.; Shen, Z.Z.; Gao, W.; Wang, B.B.; et al. Reduction of banana fusarium wilt associated with soil microbiome reconstruction through green manure intercropping. Agric. Ecosyst. Environ. 2022, 337, 108065. [Google Scholar] [CrossRef]
- Hong, S.; Jv, H.L.; Yuan, X.F.; Geng, J.J.; Wang, B.B.; Zhao, Y.; Wang, Q.; Li, R.; Jia, Z.J.; Ruan, Y.Z. Soil Organic Nitrogen Indirectly Enhances Pepper-Residue-Mediated Soil Disease Suppression through Manipulation of Soil Microbiome. Agronomy 2022, 12, 2077. [Google Scholar] [CrossRef]
- Bahramian, D.; Naraghi, L.; Heydari, A. Effectiveness of the chemical stabilizers of talaromyces flavus in biological control of tomato and greenhouse cucumber vascular wilt disease. J. Plant Prot. Res. 2016, 56, 291–297. [Google Scholar] [CrossRef]
- Xiong, W.; Li, R.; Ren, Y.; Zhao, Q.Y.; Wu, H.S.; Jousset, A.; Shen, Q.R. Distinct roles for soil fungal and bacterial communities associated with the suppression of vanilla Fusarium wilt disease. Soil Biol. Biochem. 2017, 107, 198–207. [Google Scholar] [CrossRef]
- Zhalnina, K.; Louie, K.B.; Hao, Z.; Mansoori, N.; da Rocha, U.N.; Shi, S.J.; Cho, H.; Karaoz, U.; Loqué, D.; Bowen, B.P.; et al. Dynamic root exudate chemistry and microbial substrate preferences drive patterns in rhizosphere microbial community assembly. Nat. Microbiol. 2018, 3, 470–480. [Google Scholar] [CrossRef] [PubMed]
- Su, L.X.; Ruan, Y.Z.; Yang, X.J.; Wang, K.; Li, R.; Shen, Q.R. Suppression on plant-parasitic nematodes using a soil fumigation strategy based on ammonium bicarbonate and its effects on the nematode community. Sci. Rep.-UK 2015, 5, 17597. [Google Scholar] [CrossRef]
- Ling, N.; Wang, T.T.; Kuzyakov, Y. Rhizosphere bacteriome structure and functions. Nat. Commun. 2022, 13, 836. [Google Scholar] [CrossRef]
- Shen, Z.Z.; Zhong, S.T.; Wang, Y.G.; Wang, B.B.; Mei, X.L.; Li, R.; Ruan, Y.Z.; Shen, Q.R. Induced soil microbial suppression of banana fusarium wilt disease using compost and biofertilizers to improve yield and quality. Eur. J. Soil Biol. 2013, 57, 1–8. [Google Scholar] [CrossRef]
- Tian, B.N.; Xie, J.T.; Fu, Y.P.; Cheng, J.S.; Li, B.; Chen, T.; Zhao, Y.; Gao, Z.X.; Yang, P.Y.; Barbetti, M.J.; et al. A cosmopolitan fungal pathogen of dicots adopts an endophytic lifestyle on cereal crops and protects them from major fungal diseases. ISME J. 2020, 14, 3120–3135. [Google Scholar] [CrossRef] [PubMed]
- Steensels, J.; Gallone, B.; Voordeckers, K.; Verstrepen, K.J. Domestication of industrial microbes. Curr Biol. 2019, 29, R381–R393. [Google Scholar] [CrossRef]
- Zhai, M.M.; Niu, H.T.; Li, N.; Xiao, H.; Shi, Y.P.; Di, D.L.; Crews, P.; Wu, Q.X. Talaromycolides A–C, Novel Phenyl-Substituted Phthalides Isolated from the Green Chinese Onion-Derived Fungus Talaromyces pinophilus AF-02. J. Agric. Food Chem. 2015, 63, 9558–9564. [Google Scholar] [CrossRef]
- Naraghi, L.; Heydari, A.; Rezaee, S.; Razavi, M.; Khaledi, E.M. Biological control of tomato verticillium wilt disease by talaromyces flavus. J. Plant Prot. Res. 2010, 50, 360–365. [Google Scholar] [CrossRef]
- Moreno, A.B.; Penas, G.; Rufat, M.; Bravo, J.M.; Estopà, M.; Messeguer, J.; Segundo, B.S. Pathogen-induced production of the antifungal AFP protein from Aspergillus giganteus confers resistance to the blast fungus Magnaporthe grisea in transgenic rice. Mol. Plant-Microbe Interact. 2005, 18, 951–960. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.M.; Lin, M.H.; Rensing, C.; Qin, X.J.; Zhang, S.k.; Chen, J.; Wu, L.K.; Zhao, Y.L.; Lin, S.; Lin, W.X. Plant-mediated rhizospheric interactions in intraspecific intercropping alleviate the replanting disease of Radix pseudostellariae. Plant Soil 2020, 454, 411–430. [Google Scholar] [CrossRef]
- Yuan, X.F.; Hong, S.; Xiong, W.; Raza, W.; Shen, Z.Z.; Wang, B.B.; Li, R.; Ruan, Y.Z.; Shen, Q.R.; Dini-Andreote, F. Development of fungal-mediated soil suppressiveness against Fusarium wilt disease via plant residue manipulation. Microbiome 2021, 9, 200. [Google Scholar] [CrossRef]
- Yuan, X.F.; Wang, B.B.; Hong, S.; Xiong, W.; Shen, Z.Z.; Ruan, Y.Z.; Li, R.; Shen, Q.R.; Dini-Andreote, F. Promoting soil microbial-mediated suppressiveness against Fusarium wilt disease by the enrichment of specifc fungal taxa via crop rotation. Biol. Fert. Soils. 2021, 57, 1137–1153. [Google Scholar] [CrossRef]
- Baileya, K.L.; Lazarovitsb, G. Suppressing soil-borne diseases with residue management and organic amendments. Soil Tillage Res. 2003, 72, 169–180. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).