Abstract
The extreme weather that humanity has been confronting in recent years is the result of climate change. All over the world, unknown plant species are disappearing daily, which humankind has not discovered and will never know. Since 1900, the angiosperms and gymnosperms have been disappearing at a frequency of three species per year, but it is worrying that this rate of disappearance is up to 500 times higher currently. These data, correlated with the information provided by the United Nations (the world population will reach 10 billion by the year 2050) and FAO (food insecurity and the decrease of feedstock) lead to a crucial need to conserve and study plant germplasm. Therefore, plant germplasm conserved, especially in gene banks, can represent an important source for the development of varieties with an increased resistance to abiotic stress factors. Considering the origin of the current species of Phaseolus vulgaris L. as being in two distinct centers with different gene pools (Andean and Mesoamerica), the aim of the article is to infer the ancestry of 27 landraces according to their sampling geographical origin and morphological and molecular traits based on DNA sequences of three genes associated with abiotic stress tolerance (drought and thermal stress): PvREB5A, PvDREB6B, and PvRPS4. Phaseolus vulgaris L. has two different centers of origin: the Mesoamerican and the Andean basins. In this research, 27 landraces were evaluated from different counties in Romania. Three genes, PvREB5A, PvDREB6B, and PvRPS4, were amplified by the PCR reaction, sequenced by the Sanger technique, and the data obtained were analyzed using MEGA XI software. For morphological data, the GraphPad Prism 9 software was used. According to PvDREB5A, 81.5% of all studied landraces belong to the Mesoamerican gene pool and 18.5% belong to the Andean. PvDREB6B revealed a high nucleotide and amino acid diversity between the Andean and Mesoamerican genotypes compared to the other evaluated genes. Also, the PvRPS4 gene from the chloroplast genome showed one SNP within its coding region, different for those two gene pools, which is directly involved in a nonsynonymous substitution. The morphological characteristics, such as weight for 100 seeds, length, height, width, weight, seed flatness, flatness index, seed elongation, and eccentricity index were determined. European landraces of Mesoamerican origin indicated a large seed size compared to Andean genotypes. This work can be a foundation for the identification of interesting traits that establish plant adaptation to abiotic stress and conserve landraces of common beans from genetic depletion.
1. Introduction
Common bean (Phaseolus vulgaris L.) belongs to the Fabaceae family, along with fava bean, lentil, soybean, or pea, and it is considered the most important legume for human consumption worldwide [1], representing an important source of high-quality proteins (20–30% from dry matter bass) with a significant lysine content [2,3]. Carbohydrates represent the main compound of common bean seeds, ensuring a significant energy supply to the consumer. Furthermore, other compounds, such as vitamins, minerals, antioxidants, and dietary fiber, can be found in these seeds, with potential benefits for human health (decreasing risk for a variety of diseases such as obesity, diabetes, cardiovascular diseases, or cancer) [4]. The common bean represents an important resource of food, especially for poor populations all over the world, and can be considered a potential functional food and staple crop for future food security [5,6,7]. This legume has the capability to create a symbiotic association with nitrogen-fixing bacteria, resulting in a very important relationship on an economic level because this fact can drastically reduce synthetic fertilizer usage [8]. According to the database of the Food and Agriculture Organisation (FAO), the harvested area, yield, and production of common beans between the years 2000–2021 gradually increased worldwide; on the other hand, in Romania, the values of these traits, gradually decreased between the same years (National Institute of Statistics of Romania—https://insse.ro/cms/ro/content/anuarele-statistice-ale-româniei (accessed on 23 January 2023) (Figure 1). In Romania, there are many local producers which cultivate common bean landraces adapted to the pedo-climatic conditions specific to different areas.
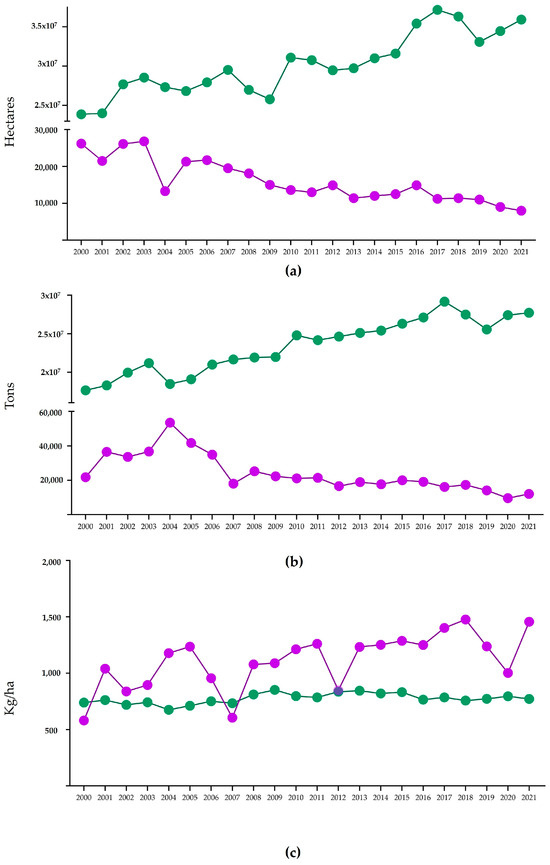
Figure 1.
Area (Ha), production (T), and yield (kg/ha) of common bean in Romania (purple) and worldwide (green) from 2000 to 2021: (a) Dry bean cultivated area in Romania and worldwide; (b) Dry bean production in Romania and worldwide; (c) Dry bean yield in Romania and worldwide.
Common beans originate from two different gene pools: Mesoamerica, now Central America (the region that extends from Mexico to Panama), and Andean, now South America (the area that extends from Colombia to Argentina) [9,10]. It is considered that common bean landraces from Mesoamerica and Andes were introduced to the European continent in 1492, after Columbus’ voyages, with poor evidence about their expansion [11]. Furthermore, the common bean was introduced into Europe at different times, using the same method, from Spain and Portugal. This theory is sustained by phaseolin protein structure [12,13,14]. Phaseolus vulgaris L. originated from the Mesoamerican gene pool and arrived in Europe in 1506, and again 22 years later, in 1528, the common bean from Andean was introduced to the European continent after the exploration of Peru by Pizarro [15]. China is considered the main producer of common beans worldwide, and it was demonstrated that Phaseolus vulgaris L. has the secondary center of diversity in China, where the Mesoamerican genotypes are more common than the Andean gene pools [16]. There is little information about the introduction and spread of the common bean in Romania. Some manuscripts relate that Phaseolus vulgaris L. was brought into Romanian agriculture technology at the end of the XVI century [17]. Until now, several studies have been carried out to show the gene pool diversity for samples from different areas of the world using various techniques: molecular markers such as RFPL [10], AFLP [18], RAPD [19], SSRs [20], cpSSRs [21], DNA (deoxyribonucleic acid) sequences [22], or other techniques that use seed proteins [23] and morphological traits [24]. Recently, Konzen et al. [25] demonstrated that the PvDREB5A gene, a sequence from the nuclear genome, can be used to prove the origin of common bean landraces. Phaseolus vulgaris L. from Mesoamerica has 483 bp the length of ORF, but for Andean landraces, the length is 474 bp (an INDEL of CGCAACAGCA). This represents a short IDEL of 9 bp sequence which codes three units of glutamine. Also, in the +33 position, one SNP is present, which affords the distinction of genotypes for the Mesoamerican (cytosine) and Andean (guanine) gene pools. Hence, this INDEL causes in Andean genotypes four units of glutamine in a row, while in Mesoamerican genotypes, there are seven units of this amino acid. Another sequence used as a molecular marker, in studies of the evolution and genetic diversity of the common bean, is phaseolin protein, which is the main seed storage protein: S-type phaseolin is possessed by Mesoamerican common beans, while T, C, or H are the phaseolin types, characteristic of Anden genotypes [26].
Despite the fact that data on morphological and agronomical traits cannot provide accurate information about belonging to a geographical area of common bean, they are still currently used along with the studies carried out at the molecular level [24]. For example, seeds from the Mesoamerica gene pool are smaller than those originated in the Andean area: the Mesoamerican beans’ hundred-seed weight (HSW) is smaller than 25 g per 100 seeds, while Andean beans are medium (HSW is between 25–40 g per 100 seeds) or large (HSW is bigger than 40 g per 100 seeds). However, in some regions, owing to their larger seed size, Andean beans are preferred by farmers and consumers [27,28].
The shape of common bean seeds can be another marker to evaluate the gene pool origin; an oval shape is characteristic of Andean beans and a cuboid or kidney shape for Mesoamerican genotypes [29]. However, several parameters, such as the flatness index, eccentricity index, seed flatness, or seed elongation can be used to assess seed shape. Cerda et al. indicate that a flatness index value of 1 shows a spherical or oval shape and a higher value is characteristic of flat seed shapes. The eccentricity index can vary from 1 (for sphere and ellipsoid shapes) to values greater than 2 (for spindle seed shapes). However, seed flatness and seed elongation parameters can be used to assess common bean seed shapes [30].
In this study, three genes (PvDREB5A, PvDREB6B, and PvRPS4) were selected to analyze the geographic origin of Phaseolus vulgaris L. local populations; two nuclear markers known to be involved in drought stress response in common bean (PvDREB5A, PvDREB6B). PvDREB5A, with a sequence length of 474 bp for Andean and 483 bp for Mesoamerican genotypes, also presents a specific SNP at +33 position guanine for Andean and cytosine for Mesoamerican genotypes. Konzen et al. [25] revealed 18 SNPs among the assessed common bean genotypes, according to the PvDREB6B gene, which can prove the geographical origin of Phaseolus vulgaris L. These genes have been poorly studied in the field of phylogeography, with most research focused on gene expression assessment under different abiotic stress conditions. Another analyzed gene is PvRPS4 from the chloroplast genome, which has not been studied until now in Phaseolus vulgaris L. as a marker able to discriminate between the two gene pools. The PvRPS4 gene is involved in drought stress response, and any SNP for Andean and Mesoamerican genotypes can be associated with a superior or inferior resistance to water scarcity. In the collection of the Romanian Vegetal Genetic Resources Bank, there are approximately 3200 different populations of the Phaseolus vulgaris L. species, representing landraces, were collected from all areas of the country. This germplasm is of particular relevance due to the traits it may contain, such as resistance to drought, high temperatures, or saline soil [31,32]. The necessity to collect, preserve, and study these samples is essential, especially since they are in danger of extinction. Old farmers who preserve these landraces on farms are fewer, and they gradually replace this germplasm with modern cultivars whose production yield is higher [33]. Assessments led in this field, of biogeography, can have extraordinary importance, especially for breeding programs [34] or to prevent genetic erosion of the common bean landraces [35]. These studies can be the starting point for determining traits of interest for breeders, which can lead to the development of varieties with superior characteristics in terms of the content of some compounds or those with increased resistance to different biotic or abiotic stress factors [36,37]. The aim of the article was to find out the geographic origin of Romanian common bean landraces from specific genetic pools: Andean or Mesoamerica. Phaseolus vulgaris L. from these pools differ by several biological traits. An assessment of the molecular and morphological characteristics of 27 Romanian landraces collected from different areas of the country and kept in the Romanian Gene Bank’s collection was undertaken. In this study, three genes (two from the nuclear and one from the chloroplast genomes) were sequenced and different levels of variability of their nucleotide’s patterns were shown. Simultaneously, seven morphological seed parameters were measured to reveal the diversity between populations from different geographic genetic pools (Andean/Mesoamerica).
2. Materials and Methods
2.1. Plant Material
The biological material used in the present study belongs to the Phaseolus vulgaris L. species, and it consists of 27 accessions from the Romanian Vegetal Genetic Resources Bank “Mihai Cristea” Suceava. The germplasm of common beans has Romanian origin, and it was collected from different geographical regions of the country between 1988–2008. The seeds of all these selected samples are white. These genotypes represent landraces, which are preserved at 4 °C temperatures and under medium-term storage in the gene bank collection. In Supplementary Table S1, “passport” information about exploited accession in this experiment, is accessible.
For each accession, twenty seeds were randomly selected and an average of these was used to measure the quantitative morphological traits per population. Eight seeds out of 20 were grown in laboratory conditions using a growth chamber with controlled temperature and humidity (Binder, KBWF 240, BINDER GmbH, Tuttlingen, Germany) at 25 °C and 80% relative humidity. The seeds were maintained in these conditions for fifteen days until the first leaves were developed and finally, these served as material for DNA isolation.
2.2. DNA Extraction
The total deoxyribonucleic acid was isolated from 40 mg of milled fresh leaves, using the Wizard® Genomic DNA Purification Kit (Promega, Madison, WI, USA) according to the manufacturer’s instructions. The DNA was resuspended in 100 µL of DNA Rehydration Solution. It was twice checked: qualitatively, using 1.5% agarose gel, and quantitatively, with NanoDrop™ One UV-VIS (Ultraviolet–Visible Spectroscopy) Spectrophotometer (Thermo Fisher Scientific GmbH, Dreieich, Germany). The DNA samples were stored at −20 °C for subsequent experiments. A total of 81 DNA samples, with 3 individuals per population, were isolated.
2.3. Polymerase Chain Reaction (PCR) Amplification, Purification, Validation, and Sequencing
The PCR reactions were performed in a 25 µL total volume using a premixed ready-to-use solution GoTaq® G2 Green Master Mix (Promega), containing: 12.5 µL Master Mix (GoTaq® G2 DNA Polymerase is provided in 2X Green GoTaq® G2 Reaction Buffer with pH 8.5, 400 µM dATP, 400 µM dGTP, 400 µM dCTP, 400 µM dTTP, and 3 mM MgCl2); 1.25 µL from each primer (0.5 µM final concentration); 2.5 µL genomic DNA (<250 ng final concentration) and nuclease-free water to 25 µL. In this experiment, three different regions, two from the nuclear genome (PvDREB5A and PvDREB6B) and one from the chloroplast genome (PvRPS4), were amplified (Table 1). All DNA products were checked by 2.5% agarose gel electrophoresis for unique fragments at the expected size. All PCR reactions were carried out with the Eppendorf Mastercycle (Eppendorf Group, Hamburg, Germany), and the cycling parameters were as follows: one cycle of 2 min at 95 °C, 30 cycles of 30 s at 94 °C, 30 s at 50–64 °C, 30 s at 72 °C, and a final extension, 5 min at 72 °C.

Table 1.
Nuclear DNA and cpDNA primers.
Amplicons were purified using the Wizard® SV Gel and PCR Clean-Up System (Promega), following the instructions of the manufacturer. After this, amplification products were quantified and prepared for the sequencing reactions with the same primer pairs from the original amplifications. PCR products were sequenced using 3730 and 3730xl DNA analyzers (Thermo Fisher Scientific GmbH, Dreieich, Germany ) and the Applied Biosystems Sequence Scanner program at CeMIA (CeMIA SA Company, University of Thessaly, Thessaly, Greece).
2.4. Morphological Analysis
The morphological characteristics, based on the list of descriptors for Phaseolus vulgaris L. (ECPGR—European Cooperative Program for Plant Genetic Resources), were registered for Romanian common bean landraces: the weight (g) for 100 seeds, the length (L; the longest distance across the seeds, parallel to the hilum was measured), the height (H; perpendicular to the length, the longest distance was measured), the width (W; the longest distance across the hilum of the seed was measured) [38], and the weight per seed (Figure 2). Furthermore, seed flatness (H/W), flatness index (L + H)/2 W), seed elongation (L/H), and eccentricity index (L/W) were calculated [24]. At the same time, the seed’s shape was recorded following the qualitative scale: cuboid, kidney, and oval.
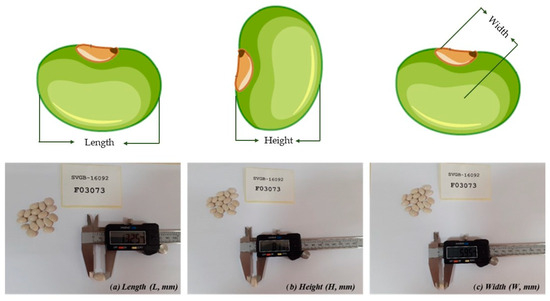
Figure 2.
Measurement protocol of seed length, height, and width for SVGB-16092 accession.
2.5. Data Analysis
Forward and reverse sequences were assembled with MEGA XI software. The alignments for the three regions were used to analyze the presence of single nucleotide polymorphisms (SNP) among the common bean panel genotypes. All these sequences were manually verified. At the same time, the evolutionary relationships of accessions were determined using the Neighbor-Joining method, with MEGA XI. DNA sequences were converted into proteins using the same software. Polymorphic information content (PIC) and heterozygosity values were estimated by exploiting Gene-Calc, an online free software (https://github.com/szymon6927/bio-gen-calc, accessed on 16 February 2023). The differences among the common beans for Andean and Mesoamerican gene pools were analyzed through GraphPAD Prism version 9 software. The calculated statistical variables are the following: mean, range (min, max), standard deviation, standard error of the mean, and coefficient of variation. To find the significant differences between the Andean and Mesoamerican gene pools, depending on several morphological traits, a two-way ANOVA was conducted using Tukey’s method. Using GraphPAD software 9.0.0, a principal component analysis (PCA) was conducted. At the same time, to demonstrate how genotypes for the common bean panels are distributed in different clusters, depending on morphological traits, dendrograms were constructed with IBM® SPSSR® Statistics version 20 software, using the Nearest Neighbor method.
3. Results
3.1. Origin of Romanian Common Bean Landraces—Genetic Perspective
The sequencing results showed that the Romanian common bean landraces can be classified into two groups according to geographical origin: Phaseolus vulgaris L. from the Mesoamerican, and Andean gene pools. PvDREB5A analysis of 27 accessions revealed that 5 of them (SVGB-1988, SVGB-18290, SVGB-11642, SVGB-14245, and SVGB-15078) have an ORF length of 474 bp, corresponding to an INDEL of 9 bp, and the presence of one SNP (G) in the +33 position of the gene. Therefore, 18.5% of the analyzed populations of common beans from the Romanian area belong to the Andean basin. Also, 22 common bean accessions (81.5%) belong to the Mesoamerican gene pool. This statement is grounded by the length of the gene, which is 483 bp, and in the +33 position is present in the C nucleotide (Figure 3). The distribution of these 5 populations originating from the Andes is concentrated in the northern half of Romanian territory (Figure 4). Regarding the cladistics structure, within a phylogenetic tree, through the Neighbor Joining method, the landrace populations form two general clades, depending on the geographical origin (Figure 5a). The evolutionary history was deductible using the Neighbor Joining method [39], and at the same time, the evolutionary distances were computed using the Maximum Composite Likelihood method [40] and can be found in the units of the number of base substitutions per site. Further, the percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (1000 replicates) are indicated next to the branches.
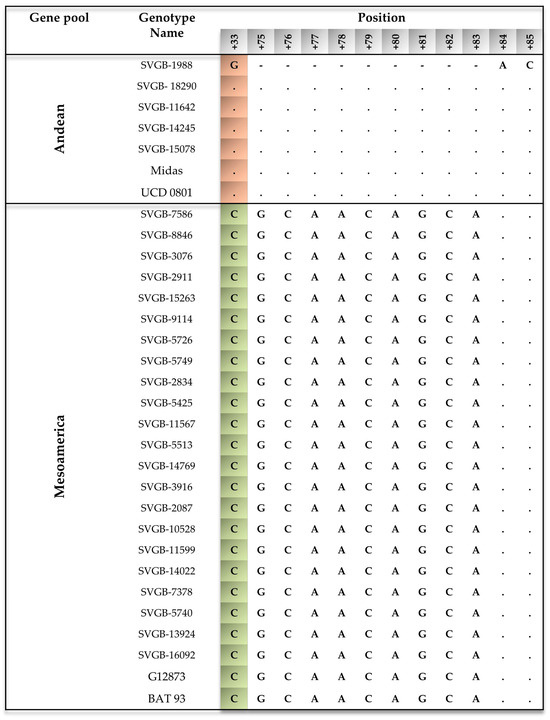
Figure 3.
Nucleotide variant patterns, obtained after Sanger sequencing, for the 27 common bean accessions assessed in this study, from the Romanian Gene Bank’s collection, according to the PvDREB5A gene. The genotypes Midas, UCD 0801 (Andean genotypes), G12873, and BAT 93 (Mesoamerican genotypes) were provided by Konzend et al. [25]. Andean SNPs were highlighted in orange and Mesoamerican SNPs in green.
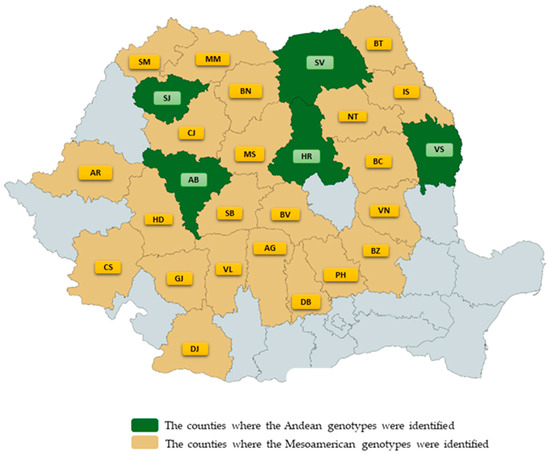
Figure 4.
The distribution of Phaseolus vulgaris L. species on Romanian territory, originating from the Andean and Mesoamerican genetic pools. The counties’ abbreviations can be found in Table S1 in Supplementary Materials.
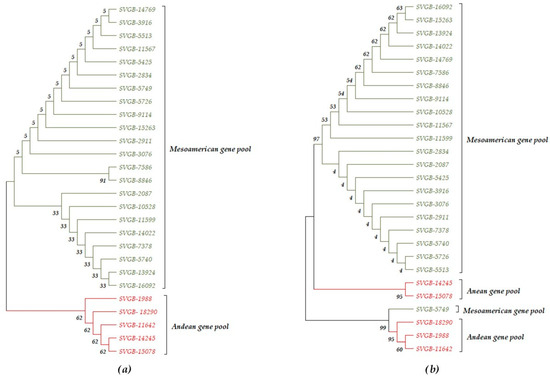
Figure 5.
The evolutionary history dendrogram was obtained using the Neighbor Joining method and bootstrap test: (a) PvDREB5A nuclear gene; (b) PvDREB6 nuclear gene. Andean genotypes were highlighted in red and Mesoamerican genotypes in green.
The PvDREB5A is a highly conserved coding sequence from the nuclear genome that presents one single nucleotide polymorphism (in the +33 position), and is devoid of any other nucleotide variability.
The polymorphisms identified in PvDREB6B represent the largest number of polymorphic sites detected. In total, 15 SNPs were identified within the common bean panel of genotypes. The SNPs were located at different positions of the ORF: +52, +288, +293, +307, +309, +478, +564, +669, +672, +694, +702, +723, +755, +781, and +791. For some of the common bean genotypes within the Andean gene pool, the nucleotide from a certain locus was different from the rest of the samples from the Andean basin, but the same with other accessions from the Mesoamerican basin (Figure 6). The point mutation from nine loci within PvDREB6B resulted in a change of some amino acid type, depending on geographic origin: for the Andean gene pool there were found isoleucine and glutamine in +18, +96, respectively, +98 locus. In the same site, for accession from the Mesoamerican gene pool, leucine, and histidine, respectively, leucine amino acids were identified. For the other six point mutations found in the protein sequence, there are differences in and between the geographic gene pool (+103, +232, +241, +252, +261, and +262). However, an interesting point of view is about two populations among the 27 samples assessed, SVGB-14245 and SVGB-15078. These landraces originate from the Andean geographic gene pool, according to PvDREB5A (due to the 9 bp INDEL and the SNP at position +33), but the results after PvDREB6B sequencing showed only three different SNPs from the Mesoamerican group. This fact can suggest the possible contribution of hybridization between groups of genes (Andean × Mesoamerica).
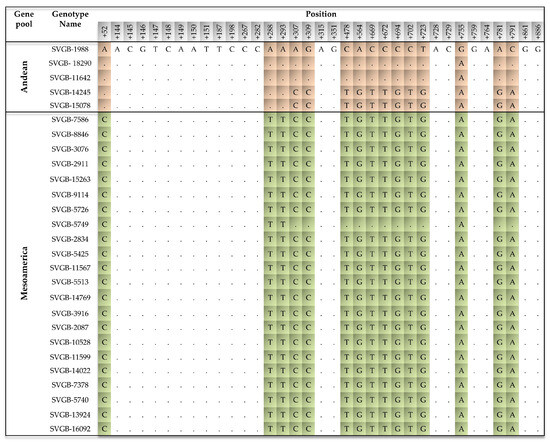
Figure 6.
The 15 SNPs identified in the target nuclear DNA, PvDREB6B, across all studied common bean (Phaseolus vulgaris L.) accessions. Andean SNPs were highlighted in orange and Mesoamerican SNPs in green.
The phylogenetic tree of the 27 populations of Phaseolus vulgaris L., based on the PvDREB6B sequence, led to the separation of the accessions differently compared to the PvDREB5A sequence. Thus, according to the Neighbor Joining method, two clades were formed. The first clade produces two subclades with populations from the Mesoamerican and Andean basins. In clade 2, there are three accessions with Andean origins and a single population from the Mesoamerican gene pool. Using the Bootstrap method as a Phylogeny Test with 1000 replications, the percentage of replicate trees in which the associated taxa clustered together are shown next to the branches (Figure 5b). Ribosomal protein S4 (PvRPS4) is a coding gene from the chloroplast genome, with a position in the large single copy (LSC). The ribosomal protein genes have been included in abiotic resistance studies [41], but it has not been included, until now, in the research about the phylogeography of the Phaseolus vulgaris L. species. In the common bean, the ribosomal protein S4 gene (Gene ID: 4961741) has a low molecular weight of 606 bp, encoding a protein sequence of 201 amino acids. In the context of this survey, only a part of the ORF1, 398 bp (ORF 1 bp = Codon Start—1 bp= and Codon Stop—606 bp; ORF2 = Codon Start—345 bp and Codon Stop—256 bp) was sequenced (Figure 7).
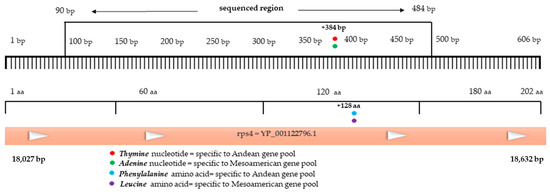
Figure 7.
Physical map of the PvRPS4 gene, with the delimitation of the sequenced ORF region and the nonsynonymous substitution.
The PvRPS4 gene sequences revealed a unique SNP in position +384, where populations from the Andean geographic basin present the thymine while those from the Mesoamerican gene pool present the adenine. The type of base pair from the +384 position also changes the amino acid type encoded, so that, in the case of populations originating from the Andean gene pool, the amino acid coded by the TTT codon is phenylalanine, while for the Mesoamerican accessions area, in the same locus, the amino acid is leucine (TTA codon) (Figure 8).
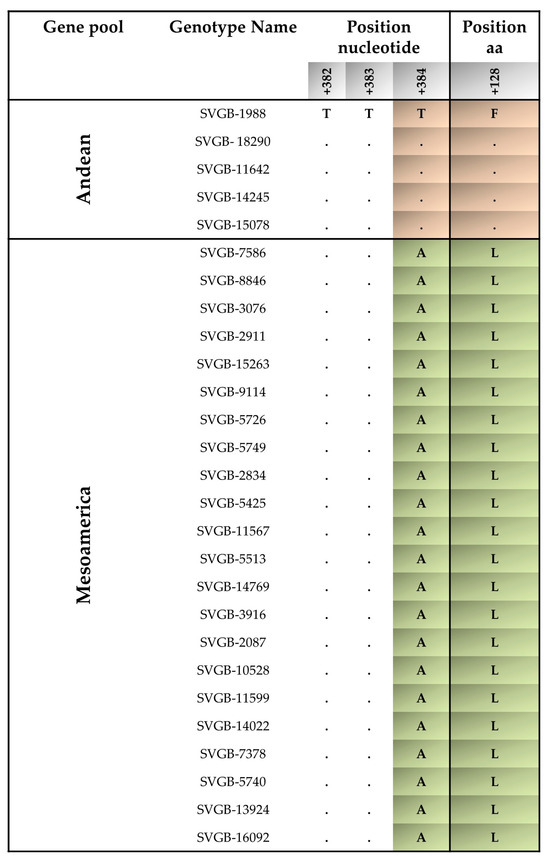
Figure 8.
An SNP present in position +384 of the PvRPS4 gene led to a nonsynonymous substitution. Andean genotypes were highlighted in orange and Mesoamerican genotypes in green.
The cladistic arrangement of the 27 accessions based on the PvRPS4 region within a phylogenetic tree, using the Neighbour Joining method led to the separation of populations according to their geographic origin. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (1000 replicates) can be followed next to the branches (Figure 9). Moreover, the PvRPS4 gene is highly conserved, with only one variable nucleotide, which was identified within the 398 bp site of the ORF.
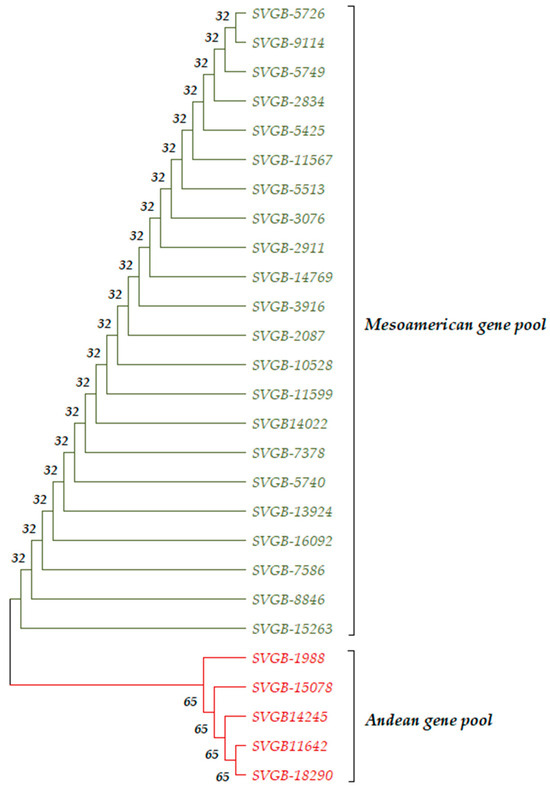
Figure 9.
The evolutionary history dendrogram was obtained using the Neighbor Joining method and bootstrap test for the PvRPS4 sequence. Andean genotypes were highlighted in red and Mesoamerican genotypes in green.
The cladistic arrangement of the 27 common bean landraces, inferred based on three evaluated genes (PvDRE5A, PvDREB6B, and PvRPS4), shows the following differences: the nucleotide patterns for the PvDREB5A and PvRPS4 genes generated two main distinct clades, according to the geographic gene pool, Mesoamerica/Andean (Figure 5a and Figure 9). Differences among the mentioned dendrograms refer to the percentage of replicate trees in which associated taxa clustered together in the bootstrap test (1000 replicates) and can be followed next to branches. The PvDREB6B gene generated a dendrogram with two main clades, for Andean and Mesoamerican basins (Figure 5b), but with a different topology, which can be considered a confirmation of a hybridization process between Andean and Mesoamerica populations for the SVGB-14245, SVGB-15078, and SVGB-5749 samples. The PvDREB6B gene shows the highest number of variable nucleotides, and PvDREB5A and PvRPS4 have the lowest number of variable nucleotides. Furthermore, the percentage of non-synonym mutations (0-fold degenerate site) varies between 64.5% for the ORF of PvDREB5A, regarding the Andean and Mesoamerican populations, and 67.08% for the PvRPS4 gene of the common bean panel originating from the Andean gene pool. Regarding the 2-fold degenerate sites, the highest value was recorded in the case of the PvRPS4 sequence, but the higher value was recorded for the populations from the Mesoamerican basin compared to the Andean ones (20.35% and 20.1%, respectively). Moreover, the highest percentage of synonymous mutations (4-fold degenerate site) was identified for the PvDREB6B sequence within the Mesoamerican accessions and the lowest value for the Mesoamerican and Andean genotypes, considering the PvRPS4 gene. More information about these values can be found in Table 2. The PIC value was 0.256 for the PvDREB5A and PvRPS4 regions, and the highest value was calculated for PvDREB6B (0.604). In addition to this, the values for heterozygosity were measured. The maximum value was 0.6502 for PvDREB6B, and the minimum was similar to the two other exploited markers from nuclear and chloroplast genomes, PvDREB5A and PvRPS4 (0.301).

Table 2.
The number of conserved and variable nucleotides, nucleotides parsimony info, nucleotides 0.2- and 4-fold degenerate sites, conserved and variable amino acids, amino acid parsimony info, and amino acid- singleton sites.
Another differentiation and similarity between the two populations, Andean and Mesoamerican, according to the PvDREB5A, PvDREB6B, and PvRPS4 genes, was obtained by the Bootstrap method, with the MEGA XI software. For instance, a few coefficients were calculated which can be seen in Table 3.

Table 3.
Summary values of four analyses that reveal within-group and between-group distances, coefficient of differentiation, and interpopulation diversity.
3.2. Origin of Romanian Common Bean Landraces—Morphological Perspective
The seeds of the Phaseolus vulgaris L. germplasm were analyzed following various morphological traits, such as weight, height, width, length, mass of 100 seeds, and the shape of the seeds. However, there was also determined the value of the flatness and eccentricity indices. Measurements of these parameters revealed a high diversity of common bean germplasm. In the below table (Table 4), are presented summary statistics variables for seven morphological traits for common bean’s seed from both gene pools.

Table 4.
Summary statistics for seven characteristics in 27 landraces of common bean (Phaseolus vulgaris L.).
For the Andean common bean, the mean for a 100-seed weight is 37.74 g, while for Mesoamerican seeds this value is higher. However, the maximum value belongs to an accession from the Mesoamerican gene pool, SVGB-9114. The highest coefficient of variation was calculated for the seed’s weight from the Mesoamerican and Andean gene pools (80%, respectively, 36.53%), and the lowest value was identified for the flatness index variable, both for the Andean and Mesoamerican basins (5.01%, respectively, 5.49%).
The main goal of PCA analysis is to reduce the number of linear combinations for seven variables. Depending on the eigenvalue, which is greater than 1, two components were obtained for common bean germplasm (27 accessions). The PCA biplot in Figure 10 showed Component 1 and Component 2, which add up to 72.53% of the total variance for the same seven morphological traits, but for an average of the values of all twenty-seven populations from both geographic pools genes. Component 1 accounted for 37.39%, and Component 2 accounted for 35.14% of the total variance. For this situation, it can be seen in the figure below that, in general, accessions from the Andean genetic background are placed in the same area as varieties from Mesoamerica, at the top of the chart. This fact may suggest some similarities between populations from different geographic genetic pools (Andean/Mesoamerica), depending on morphological traits. For PC1, the main contributor is the width, and for the second component (PC2), length, flatness index, eccentricity index, weight, height, and 100 seed weight are the most important contributors.
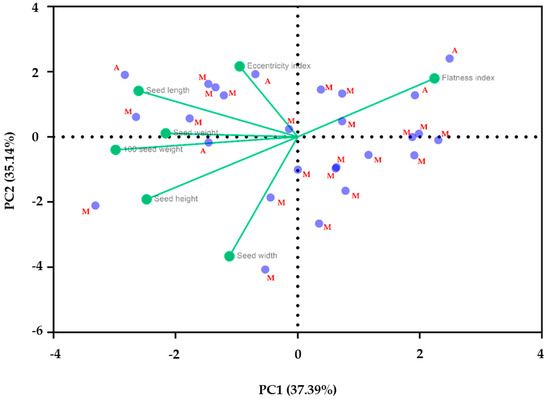
Figure 10.
A biplot of seven characteristics (seed width, seed height, seed weight, seed length, eccentricity, and flatness index) for 27 accessions of common bean germplasm.
The Tukey test revealed differences between groups that belong to both geographic gene pools, depending on morphological traits (Figure 11). In general, the large seeds belong to the Andean basin, while the smaller common bean seeds are specific to the Mesoamerican gene pool. The measurements of seed morphological parameters revealed some similarities between the populations of the Andean and Mesoamerican basins and even contradicted those stated above. A smaller difference, following the Tukey test, was reported between the Andean group and the Mesoamerican group, according to eccentricity (p ≤ 0.05) and flatness index (p ≤ 0.05), and no significant differences (p > 0.05) were indicated according to seed length, width, height, weight, and 100 seed weight. Last but not least, any conclusion about belonging to a certain geographical basin of common bean samples without molecular analysis is not conclusive.
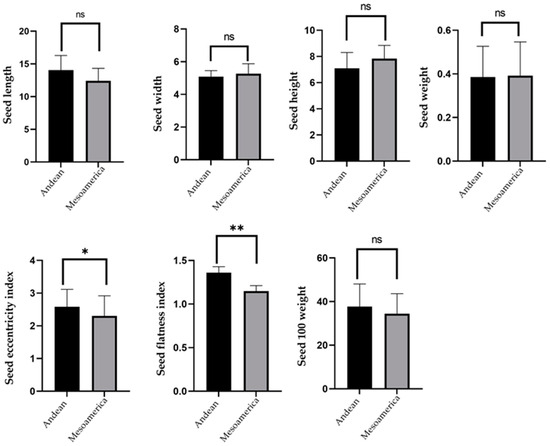
Figure 11.
Multiple comparisons between length, width, height, weight, flatness index, eccentricity index, and 100 seed weight between Andean and Mesoamerican gene pools were obtained with the two-way ANOVA method. Where ns, * and ** represent levels of nonsignificant (p > 0.05), significant (p ≤ 0.05), and very significant, respectively.
Nevertheless, information such as the 100 seed weight or the shape of the accession can provide knowledge about the geographical origin of Phaseolus vulgaris L. species. Thus, the following data were obtained: 29.64% of the studied accessions have large seeds (>40 g per 100 seeds), 62.96% are represented by medium seeds (25–40 g per 100 seeds), and only 7.4% of the analyzed common bean are characterized by small seeds (<25 g per 100 seeds). Just two of the analyzed genotypes, SVGB-18290 (M100 = 22.95 g) and SVGB-3076 (M100 = 24.21 g) would originate from Mesoamerica and the other accessions from the Andean gene pool, according to 100 seed weight. In order to highlight how varied the values are for the seven morphological traits of the common bean genotypes depending on geographic area, were created graphics that can be found in the Supplementary Materials (Supplementary Figure S1). Furthermore, in the Supplementary Materials (Supplementary Figures S2 and S3), the cladistics disposition for the 27 common bean genotypes, according to 100 seed weight and seed shape can be seen. Common bean germplasm was classified into two general clades, depending on the seed’s shape. Thus, Clade A includes all accessions whose seeds have an oval shape that fact can suggest an Andean origin. Instead, Clade B contains all genotypes that have cuboid and kidney shape and that fact can indicate a Mesoamerican place of origin. The studied landraces were classified into a dendrogram, forming three general clades according to 100 weights of seeds. Each of these three clades includes Andean germplasm.
4. Discussion
To find out the origin of the Romanian common bean local varieties from the specific geographic basins (Mesoamerica/Andean), an analysis of the molecular and morphological characteristics of several samples was undertaken. The 27 populations of common bean landraces evaluated in this study were collected from different areas of Romania and preserved in Gene Bank collection, in optimal conditions to maintain the viability of vegetal germoplasm. These local populations have not been assessed until now, considering their geographic origin or their resistance to abiotic and biotic stress factors. This research is the first that evaluated the phylogeography of the Romanian common bean landraces. In general, this type of study analyzes Phaseolus vulgaris L. from the European territory and less from Eastern Europe [13]. This biological material may be considered an extraordinary “raw material” that can be used in national and international breeding programs for creating varieties with increased resistance to different abiotic stress factors, especially in the current context of global warming.
The differences between the Andean and Mesoamerican gene pools, according to the PvDREB5A gene structure, are afforded by one SNP from the +33 position and an INDEL from 9 base pairs. The INDEL (deletion of 9 bp) codes for three units of glutamine amino acids and the SNP, from the +33 position, is a synonymous substitution and it can be called a silent mutation. After Sanger sequencing of the PvDREB5A marker, the results show that 18.5% of common bean genotypes analyzed are of Andean origin and 81.5% originated from the Mesoamerican geographic gene pool. These results are in contradiction with those reported by others. For instance, Logozzo et al. [27] reported that 75% of the cultivated common beans from the European territory have Andin origins. This statement is confirmed by others, as Phaseolus vulgaris L. from the Mesoamerican basin has a lower prevalence compared to common beans originating from the Andean [21,29,38,42]. According to Maras et al., Macedonia is an exception to this theory. In this country, the prevalence of Phaseolus vulgaris L. genotypes is bigger for Mesoamerican accessions than for the Andean varieties, especially in Eastern Macedonia. They explain that Phaseolus vulgaris L. species could have been introduced predominantly from the Mediterranean Basin in the western Balkans [43]. Another sequence analyzed is PvDREB6B, a gene that is involved in the water stress response of common beans. This sequence showed a high nucleotide and amino acid diversity within its ORF, compared to the PvDREB5A gene, which demonstrated only one SNP. Fifteen SNPs, within the PvDRE6B gene, were identified; some of them being involved in changing the encoded amino acid. Instead, Konzen et al. discovered 18 SNPs within PvDREB6B’s ORF [25]. However, an interesting fact was reported about the types of nucleotides and amino acids within the PvDREB6B gene of Andean and Mesoamerican genotypes. For instance, in the +755 position, just one Andean genotype was identified, guanine, as a nucleotide. Meanwhile, for the other Andean accessions and the Mesoamerican genotypes, adenine was reported as the base pair. This point mutation represents a non-synonymous substitution, thus revealing a different amino acid in the same position of the protein sequence. For SVGB-1988 accession, in the +252 position was found glycine (the GGG codon), and for the rest of the genotypes, from both gene pools was described glutamic acid (the GAG codon). At the same time, nine SNPs within PvDREB6B are nonsynonymous substitutions and six are silent mutations (synonymous substitutions). The Mesoamerican genotypes are characterized by a high value of nucleotide and amino acid diversity, compared to the Andean accessions, depending on the PvDREB6B gene. The higher level of diversity of the Mesoamerican varieties was also affirmed by others [44]. Based on the PvRPS4 gene, there is only one SNP difference between gene pools. This type of mutation is a nonsynonymous substitution. For the Andean group, the +384 locus was identified as thymine, but for Mesoamerican individuals in the same locus, was reported adenine nucleotide. This type of mutation reshaped the kind of amino acid. At the same time, there was no identified variability within gene pools, depending on the PvRPS4 gene. This type of SNP can be used in phylogeographical studies, to reveal the fact that the Phaseolus vulgaris L. species belongs to a certain basin. Moreover, all changes in amino acid types could model distinct functions within the Phaseolus vulgaris L. species, but obviously, in order to validate this statement, further investigation is necessary for all three sequences. To know how informative and polymorphic the markers used in this study are, polymorphism information content (PIC) was measured. SNP markers are co-dominant [45] and in case their PIC value is >0.5, this indicates a highly informative marker. Furthermore, markers can be informative if the PIC value ranges between 0.25–0.5 or quite informative when the PIC is <0.25 [46]. The PvDREB6B is a highly informative marker due to its PIC value of 0.6, and the other two markers (PvDREB5A and PvRPS4) are quite informative with a value of 0.25. Another measured trait was the heterozygosity of SNP markers. Heterozygosity can be defined as the probability of an individual being heterozygous at the markers locus. This fact is dependent on the number of alleles and their prevalence in the population. The heterozygosity can range from 0 value (without heterozygosis) to 1 (high number of alleles with equal frequency). The PvDREB6B gene indicated a high heterozygosis, due to its value of 0.65, while PvDREB5A and PvRPS4 showed a lower value, respectively, of 0.3 [47]. After Sanger reactions, the proportions of substitutions at the 4-fold degenerates sites, 2-fold degenerates sites, and 0-fold degenerates sites for the three genes were identified depending on the geographical gene pool. This way, the results showed that the highest value for substitutions at the 0-fold degenerates’ site was registered for the Andean genotypes, according to the PvRPS4 gene (67.08%), and the lowest percentage was registered for the Andean and Mesoamerican accessions, according to, this time, the PvDREB5A sequence (64.5%). The mutations that can happen at these sites are nonsynonymous, a fact that can show a high possibility to reshape the protein structure. These can be involved in diverse molecular mechanisms, associated with several abiotic stresses such as drought, heat, or cold tolerance in common beans. The substitutions at the 4-fold degenerate sites indicate that in these loci the changing of nucleotide will not change any amino acid in protein structure. This kind of substitution is useful for the evaluation of the rate of neutral evolution [48]. In this study, for 4-fold degenerated sites, Mesoamerican genotypes, depending on PvDREB6B, indicated the maximum value (15.67%) and the minimum value was demonstrated by the Andean and Mesoamerican landraces, described by PvRPS4 gene. Additionally, the number of parsimony-informative sites were determined. Within the Andean genotype, for PvDREB6B, was noticed the attendance of parsimony-informative sites (11/957). For the other studied sequences, the existence of parsimony-informative sites was not reported. The common bean accessions from the Andean gene pool presented a higher value of diversity than the Mesoamerican genotypes, due to the number of alleles within PvDREB6B gene. The combined phylogenetic tree according to the assessed three genes, PvDREB5A, PvDREB6B and PvRPS4, indicated similarities with the phylogenetic tree of the PvDREB6B gene, regarding cladistic arrangement. The evolutionary history was inferred using the Neighbor-Joining method. The percentage of replicate trees where associated taxa cluster together in the bootstrap test (1000 replicates) are noted next to branches. These values are different if we compare them with the phylogenetic tree of the PvDREB6B gene (Figure 5b). At the same time, evolutionary distances were calculated using the Maximum Composite Likelihood method (Figure 12).
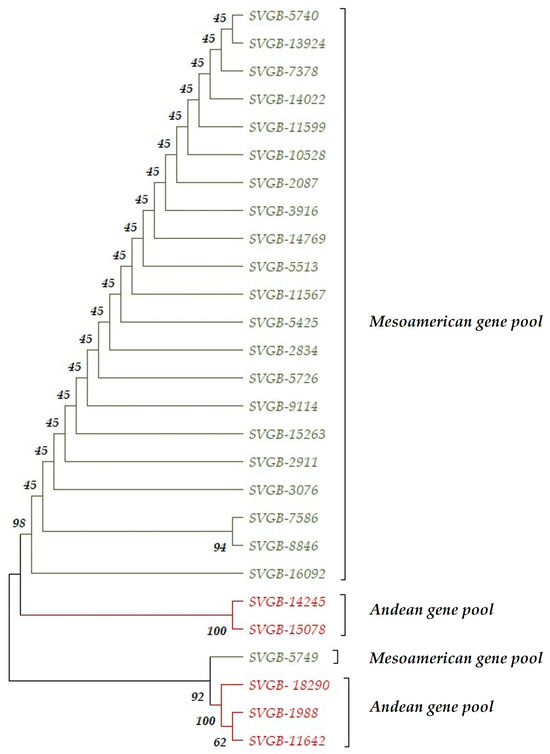
Figure 12.
The evolutionary history dendrogram was obtained using the Neighbor Joining method and bootstrap test for PvDDREB5A, PvDREB6B, and PvRPS4 sequences. Andean genotypes were highlighted in red and Mesoamerican genotypes in green.
Phaseolus vulgaris L. was classified into two major gene pools, based on seed size variances, those with large seeds belong to the Andean basin and small seeds belong to the Mesoamerican basin [49,50].
The results, after the measurement of 100 seed weight, showed that only two genotypes had Mesoamerican origin (SVGB-18290 and SVGB-3076) and the others, due to high value of weight, confirmed the Andean origin. This is in contradiction to the results according to molecular data analysis. This way, one of five genotypes that belongs to the Andean gene pool, depending on molecular data, has a 100-mass weight <25 g per 100 seeds (SVGB-18290 = 22.95 g) and 21 accessions from the Mesoamerican basin presented a higher value of 100 seed weight, excepting one landrace, which had the value of this trait, the <25 g per 100 seeds (SVGB-3076 = 24.21%). That showed an opposition to the general conclusion, that the Mesoamerican common bean type is represented by smaller seeds than the Andean center of origin. For instance, the highest value of 100 seed weight belongs to an accession from the Mesoamerican gene pool (SVGB-9114 = 61 g per 100 seeds). On the other hand, in previous studies, it was demonstrated that some European landraces of Mesoamerican origin are particularly large-seeded. This phenomenon was not mentioned for landraces from the Mesoamerican center of origin [27,51]. Another hypothesis of the larger seeds of the Mesoamerican basin is the results of hybridization between the Andean and Mesoamerican landraces which occurred after the insertion of Phaseolus vulgaris L. to Europe [52]. The cladistics categorization of the 27 studied common bean landraces, depending on 100 seed weight reveal heterogeneous samples, due to a high number of general clades and subclades. Regarding the shapes of the seeds, there were reported cuboid and kidney patterns for two accessions that belong to the Andean gene pool. That fact is contradictory with the theory that awards the oval seed’s shape in the Andean basin and the seed’s cuboid and kidney patterns in the Mesoamerican gene pool. Regarding the seed’s shape, there was a reported kidney pattern for two accessions that belong to the Andean gene pool (SVGB-18290 and SVGB-11642). This fact is in contradiction with the theory that awards the oval seed’s shape in the Andean basin and the seed’s cuboid and kidney patterns in the Mesoamerican gene pool [29]. The same results were obtained by Gepts et al. [9]. The morphological analysis of common beans’ germplasm showed the Andean origin of two studied landraces, but molecular assessment demonstrated the Mesoamerican center of origin [9]. This fact was also reported by others, larger seeds for Mesoamerican germplasm, compared to seeds from Andean populations [24]. Using principal component analysis, data about morphological traits were graphically represented. The position of common bean germplasm from both gene pools on the PCA Biplot indicates higher values of seed length, weight, flatness index, and eccentricity index for the Andean landraces, but for the Mesoamerican accessions there were registered high values of 100 seed weight, height, and width traits. Using ANOVA Tukey’s method, a significant difference between accessions from different gene pools and within each group, according to morphological traits was demonstrated. This was also confirmed by others [53,54].
Our data showed a higher prevalence of Mesoamerican genotypes compared to Andean genotypes on Romanian territory. However, on the European continent, the spreading of the two genotypes, Mesoamerican and Andean is different. Assessments show that common beans from both gene pools were dispersed on the European continent with variations among regions. For instance, it was demonstrated that the frequency of common bean landraces originating from Andean is predominant in Europe (76%) compared to Mesoamerican landraces (24%) [13]. In Europe, there are three macro-areas (Iberian Peninsula, Central-Northern Europe, and Italy), where the majority of seeds are the Andean type, and the Eastern and South-Eastern parts of the European continent are characterized by a higher frequency of Mesoamerican seeds [27,55,56]. In other areas of the world, the distribution of common bean germplasm is different. In the Central-East of the African continent, the prevalence of Andean type is higher than Mesoamerican (83%), but overall the frequency of these gene pools are approximately equal in Africa [57].
5. Conclusions
In this work, the geographic origin of 27 landraces of Phaseolus vulgaris L. from the Romania Gene Bank collection, using SNP markers and morphological traits were analyzed. However, the results obtained after the analysis at the molecular level are the ones that must be considered, because they show much greater credibility. Measurements of seven morphological parameters of seeds demonstrated a high diversity of common bean germplasm and some similarities between populations from different geographic genetic pools (Andean/Mesoamerica). Furthermore, results considering seed size came in contradiction with the well-known facts about the smaller size and Mesoamerican gene pool. It is not surprising that during domestication, similar sets of traits were selected over a wide range of plant species, including common beans (domestication syndrome), which shows numerous examples of convergent phenotypic evolution. Convergent evolution happened between the two gene pools of the common bean after the breaking of the spatial isolation of the Mesoamerican and Andean gene pools. First: this allowed spontaneous hybridization, thus increasing the possibility of novel genotypes and phenotypes. Second: the adaptation of the species to a novel agroecosystems which in Romania are not too extremely diverse and human needs increased the similarity of the traits of both gene pools. The percentage of the populations originating from the Mesoamerican basin, identified on the territory of Romania, is higher compared to that of the populations originating from the Andean basin. The number of the evaluated common bean landraces in this research is relatively small, compared to the Genebank collection of Phaseolus vulgaris L. Out of 27 common bean local populations evaluated, most of them have a Mesoamerican origin, but, to certainly state the geographical origin of the Phaseolus vulgaris L. species from the Romanian territory, further analysis is required. Also, the five Andean populations analyzed are spread in the northern half of the country. These results are different from those obtained until now, which show a greater spread of Andean populations on European territory, compared to those from the Mesoamerican basin. The morphological analysis of the seeds showed that common bean seeds originating from Mesoamerica are as large as or even larger than those originating from the Andean basin. On the other hand, the studies carried out until now have shown that the seeds originating from the Mesoamerican basin, present in the European area, are much larger compared to those from the center of origin. Also, the Mesoamerican germplasm spread on this territory may be the result of hybridization between the Mesoamerican and Andean populations, a process that took place at the time of the introduction of the Phaseolus vulgaris L. species in Europe. PvDREB6B gene showed the greatest nucleotide variability, with 15 SNPs being identified, instead PvDREB5A and PvRPS4 presented a single SNP each, depending on the center of origin. The different alleles can be studied further and associated with different resistance to abiotic stress factors, such as drought, high temperatures, or salt stress. Of course, in order to prove such a hypothesis, further studies are required. Our data confirms that molecular analyses are more accurate and relevant compared to morphological research for phylogeographic studies of common bean (Phaseolus vulgaris L.) germplasm.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agronomy13112820/s1, Table S1: The passport data of the analyzed common bean (Phaseolus vulgaris L.) landraces; Figure S1: Frequency distribution of 27 common bean (Phaseolus vulgaris L.) landraces, according to morphological traits, including Andean and Mesoamerican genotypes.; Figure S2: Dendrogram for 27 common bean accessions depending of 100 seed weight; Figure S3: Dendrogram for 27 common bean accessions depending on the shape of the seeds.
Author Contributions
Conceptualization, D.-L.G. and P.-M.G.; methodology, P.-M.G., L.-I.L. and D.-L.G.; writing—original draft preparation, P.-M.G., L.-I.L., S.S., D.-E.P., M.-M.C., A.-C.T., D.-M.S. and D.-L.G. writing—review and editing, D.-L.G., S.S. and M.-M.C.; supervision, D.-L.G. and S.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
This work has been supported by Romanian Gene Bank at the Vegetal Genetic Resources Bank “Mihai Cristea” Suceava.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Morales, F.J. Common Bean BT—Virus and Virus-Like Diseases of Major Crops in Developing Countries; Loebenstein, G., Thottappilly, G., Eds.; Springer: Dordrecht, The Netherlands, 2003; pp. 425–445. ISBN 978-94-007-0791-7. [Google Scholar]
- Los, F.G.B.; Zielinski, A.A.F.; Wojeicchowski, J.P.; Nogueira, A.; Demiate, I.M. Beans (Phaseolus vulgaris L.): Whole Seeds with Complex Chemical Composition. Curr. Opin. Food Sci. 2018, 19, 63–71. [Google Scholar] [CrossRef]
- Broughton, W.J.; Hernández, G.; Blair, M.; Beebe, S.; Gepts, P.; Vanderleyden, J. Beans (Phaseolus spp.)—Model Food Legumes. Plant Soil 2003, 252, 55–128. [Google Scholar] [CrossRef]
- Reynoso-Camacho, R.; Ramos-Gomez, M.; Loarca-Pina, G. Bioactive Components in Common Beans (Phaseolus vulgaris L.). Adv. Agric. Food Biotechnol. 2006, 661, 217–236. [Google Scholar]
- Nadeem, M.A.; Yeken, M.Z.; Shahid, M.Q.; Habyarimana, E.; Yılmaz, H.; Alsaleh, A.; Hatipoğlu, R.; Çilesiz, Y.; Khawar, K.M.; Ludidi, N.; et al. Common Bean as a Potential Crop for Future Food Security: An Overview of Past, Current and Future Contributions in Genomics, Transcriptomics, Transgenics and Proteomics. Biotechnol. Biotechnol. Equip. 2021, 35, 758–786. [Google Scholar] [CrossRef]
- Celmeli, T.; Sari, H.; Canci, H.; Sari, D.; Adak, A.; Eker, T.; Toker, C. The Nutritional Content of Common Bean (Phaseolus vulgaris L.) Landraces in Comparison to Modern Varieties. Agronomy 2018, 8, 166. [Google Scholar] [CrossRef]
- Catarino, S.; Brilhante, M.; Essoh, A.P.; Charrua, A.B.; Rangel, J.; Roxo, G.; Varela, E.; Moldão, M.; Ribeiro-Barros, A.; Bandeira, S.; et al. Exploring Physicochemical and Cytogenomic Diversity of African Cowpea and Common Bean. Sci. Rep. 2021, 11, 12838. [Google Scholar] [CrossRef]
- Vaclav, S. Nitrogen in Crop Production: An Account of Global Flows Adds. Glob. Biogeochem. Cycles 1999, 13, 647–662. [Google Scholar]
- Gepts, P.; Debouck, D. Origin, Domestication, and Evolution of the Common Bean (Phaseolus vulgaris L.). In Common Beans: Research for Crop Improvement; CAB International: Wallingford, UK, 1991; pp. 7–53. [Google Scholar]
- Chacón, S.M.I.; Pickersgill, B.; Debouck, D.G. Domestication Patterns in Common Bean (Phaseolus vulgaris L.) and the Origin of the Mesoamerican and Andean Cultivated Races. Theor. Appl. Genet. 2005, 110, 432–444. [Google Scholar] [CrossRef]
- Papa, R.; Nanni, L.; Sicard, D.; Rau, D.; Attene, G. Evolution of Genetic Diversity in Phaseolus vulgaris L. In Darwin’s Harvest: New Approaches to the Origins, Evolution, and Conservation of Crops; Motley, T.J., Zerega, N., Cross, H., Eds.; Columbia University Press: New York, NY, USA, 2006; pp. 121–142. [Google Scholar]
- Gepts, P. A Middle American and an Andean Common Bean Gene Pool BT—Genetic Resources of Phaseolus Beans: Their Maintenance, Domestication, Evolution and Utilization; Gepts, P., Ed.; Springer: Dordrecht, The Netherlands, 1988; pp. 375–390. ISBN 978-94-009-2786-5. [Google Scholar]
- Gepts, P.; Bliss, F.A. Dissemination Pathways of Common Bean (Phaseolus vulgaris, Fabaceae) Deduced from Phaseolin Electrophoretic Variability. II. Europe and Africa. Econ. Bot. 1988, 42, 86–104. [Google Scholar] [CrossRef]
- Zeven, A.C. The Introduction of the Common Bean (Phaseolus vulgaris L.) into Western Europe and the Phenotypic Variation of Dry Beans Collected in the Netherlands in 1946. Euphytica 1997, 94, 319–328. [Google Scholar] [CrossRef]
- Gioia, T.; Logozzo, G.; Attene, G.; Bellucci, E.; Benedettelli, S.; Negri, V.; Papa, R.; Spagnoletti Zeuli, P. Evidence for Introduction Bottleneck and Extensive Inter-Gene Pool (Mesoamerica × Andes) Hybridization in the European Common Bean (Phaseolus vulgaris L.) Germplasm. PLoS ONE 2013, 8, e75974. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Blair, M.W.; Wang, S. Genetic Diversity of Chinese Common Bean (Phaseolus vulgaris L.) Landraces Assessed with Simple Sequence Repeat Markers. Theor. Appl. Genet. 2008, 117, 629–640. [Google Scholar] [CrossRef] [PubMed]
- Olaru, C. Common Bean-Biology and Agriculture Technology; Romanian Writing: Craiova, Romania, 1982. [Google Scholar]
- Rossi, M.; Bitocchi, E.; Bellucci, E.; Nanni, L.; Rau, D.; Attene, G.; Papa, R. Linkage Disequilibrium and Population Structure in Wild and Domesticated Populations of Phaseolus vulgaris L. Evol. Appl. 2009, 2, 504–522. [Google Scholar] [CrossRef] [PubMed]
- Johns, M.A.; Skroch, P.W.; Nienhuis, J.; Hinrichsen, P.; Bascur, G.; Muñoz-Schick, C. Gene Pool Classification of Common Bean Landraces from Chile Based on RAPD and Morphological Data. Crop Sci. 1997, 37, 605–613. [Google Scholar] [CrossRef]
- Carović-Stanko, K.; Liber, Z.; Vidak, M.; Barešić, A.; Grdiša, M.; Lazarević, B.; Šatović, Z. Genetic Diversity of Croatian Common Bean Landraces. Front. Plant Sci. 2017, 8, 604. [Google Scholar] [CrossRef]
- Sicard, D.; Nanni, L.; Porfiri, O.; Bulfon, D.; Papa, R. Genetic Diversity of Phaseolus vulgaris L. and P. coccineus L. Landraces in Central Italy. Plant Breed. 2005, 124, 464–472. [Google Scholar] [CrossRef]
- Bitocchi, E.; Bellucci, E.; Giardini, A.; Rau, D.; Rodriguez, M.; Biagetti, E.; Santilocchi, R.; Zeuli, P.S.; Gioia, T.; Logozzo, G.; et al. Molecular Analysis of the Parallel Domestication of the Common Bean (Phaseolus vulgaris) in Mesoamerica and the Andes. New Phytol. 2013, 197, 300–313. [Google Scholar] [CrossRef]
- Gepts, P.; Osborn, T.C.; Rashka, K.; Bliss, F.A. Phaseolin-Protein Variability in Wild Forms and Landraces of the Common Bean (Phaseolus vulgaris): Evidence for Multiple Centers of Domestication. Econ. Bot. 1986, 40, 451–468. [Google Scholar] [CrossRef]
- Vidak, M.; Šatović, Z.; Liber, Z.; Grdiša, M.; Gunjača, J.; Kilian, A.; Carović-Stanko, K. Assessment of the Origin and Diversity of Croatian Common Bean Germplasm Using Phaseolin Type, SSR and SNP Markers and Morphological Traits. Plants 2021, 10, 665. [Google Scholar] [CrossRef]
- Konzen, E.R.; Recchia, G.H.; Cassieri, F.; Gomes Caldas, D.G.; Berny Mier, Y.; Teran, J.C.; Gepts, P.; Tsai, S.M. DREB Genes from Common Bean (Phaseolus vulgaris L.) Show Broad to Specific Abiotic Stress Responses and Distinct Levels of Nucleotide Diversity. Int. J. Genomics 2019, 2019, 9520642. [Google Scholar] [CrossRef]
- Gepts, P. Phaseolin as an Evolutionary Marker. In Current Plant Science and Biotechnology in Agriculture; Springer: Dordrecht, The Netherlands, 1988; pp. 215–241. [Google Scholar] [CrossRef]
- Logozzo, G.; Donnoli, R.; Macaluso, L.; Papa, R.; Knüpffer, H.; Zeuli, P.S. Analysis of the Contribution of Mesoamerican and Andean Gene Pools to European Common Bean (Phaseolus vulgaris L.) Germplasm and Strategies to Establish a Core Collection. Genet. Resour. Crop Evol. 2007, 54, 1763–1779. [Google Scholar] [CrossRef]
- Cichy, K.A.; Porch, T.G.; Beaver, J.S.; Cregan, P.; Fourie, D.; Glahn, R.P.; Grusak, M.A.; Kamfwa, K.; Katuuramu, D.N.; McClean, P.; et al. A Phaseolus vulgaris Diversity Panel for Andean Bean Improvement. Crop Sci. 2015, 55, 2149–2160. [Google Scholar] [CrossRef]
- Raggi, L.; Tiranti, B.; Negri, V. Italian Common Bean Landraces: Diversity and Population Structure. Genet. Resour. Crop Evol. 2013, 60, 1515–1530. [Google Scholar] [CrossRef]
- Cerdà, A.; García-Fayos, P. The Influence of Seed Size and Shape on Their Removal by Water Erosion. Catena 2002, 48, 293–301. [Google Scholar] [CrossRef]
- Dwivedi, S.L.; Ceccarelli, S.; Blair, M.W.; Upadhyaya, H.D.; Are, A.K.; Ortiz, R. Landrace Germplasm for Improving Yield and Abiotic Stress Adaptation. Trends Plant Sci. 2016, 21, 31–42. [Google Scholar] [CrossRef]
- Cortés, A.J.; Blair, M.W. Genotyping by Sequencing and Genome–Environment Associations in Wild Common Bean Predict Widespread Divergent Adaptation to Drought. Front. Plant Sci. 2018, 9, 128. [Google Scholar] [CrossRef]
- Negri, V. Landraces in Central Italy: Where and Why They Are Conserved and Perspectives for Their on-Farm Conservation. Genet. Resour. Crop Evol. 2003, 50, 871–885. [Google Scholar] [CrossRef]
- Shao, J.; Hao, Y.; Wang, L.; Xie, Y.; Zhang, H.; Bai, J.; Wu, J.; Fu, J. Development of a Model for Genomic Prediction of Multiple Traits in Common Bean Germplasm, Based on Population Structure. Plants 2022, 11, 1298. [Google Scholar] [CrossRef]
- Lioi, L.; Zuluaga, D.L.; Pavan, S.; Sonnante, G. Genotyping-by-Sequencing Reveals Molecular Genetic Diversity in Italian Common Bean Landraces. Diversity 2019, 11, 154. [Google Scholar] [CrossRef]
- Suárez, J.C.; Urban, M.O.; Contreras, A.T.; Grajales, M.Á.; Cajiao, C.; Beebe, S.E.; Rao, I.M. Adaptation of Interspecific Mesoamerican Common Bean Lines to Acid Soils and High Temperature in the Amazon Region of Colombia. Plants 2021, 10, 2412. [Google Scholar] [CrossRef]
- Beebe, S. Common Bean Breeding in the Tropics. Plant Breed. Rev. 2012, 36, 357–426. [Google Scholar]
- Leitão, S.T.; Dinis, M.; Veloso, M.M.; Šatović, Z.; Vaz Patto, M.C. Establishing the Bases for Introducing the Unexplored Portuguese Common Bean Germplasm into the Breeding World. Front. Plant Sci. 2017, 8, 1296. [Google Scholar] [CrossRef] [PubMed]
- Saitou, N.; Nei, M. The Neighbor-Joining Method: A New Method for Reconstructing Phylogenetic Trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Nei, M.; Kumar, S. Prospects for Inferring Very Large Phylogenies by Using the Neighbor-Joining Method. Proc. Natl. Acad. Sci. USA 2004, 101, 11030–11035. [Google Scholar] [CrossRef]
- Ruiz-Nieto, J.E.; Aguirre-Mancilla, C.L.; Acosta-Gallegos, J.A.; Raya-Pérez, J.C.; Piedra-Ibarra, E.; Vázquez-Medrano, J.; Montero-Tavera, V. Photosynthesis and Chloroplast Genes Are Involved in Water-Use Efficiency in Common Bean. Plant Physiol. Biochem. 2015, 86, 166–173. [Google Scholar] [CrossRef]
- Rodiño, A.P.; Santalla, M.; De Ron, A.M.; Singh, S.P. A Core Collection of Common Bean from the Iberian Peninsula. Euphytica 2003, 131, 165–175. [Google Scholar] [CrossRef]
- Maras, M.; Pipan, B.; Šuštar-Vozlič, J.; Todorović, V.; Ðurić, G.; Vasić, M.; Kratovalieva, S.; Ibusoska, A.; Agić, R.; Matotan, Z.; et al. Examination of Genetic Diversity of Common Bean from the Western Balkans. J. Am. Soc. Hortic. Sci. 2015, 140, 308–316. [Google Scholar] [CrossRef]
- Bitocchi, E.; Nanni, L.; Bellucci, E.; Rossi, M.; Giardini, A.; Zeuli, P.S.; Logozzo, G.; Stougaard, J.; McClean, P.; Attene, G.; et al. Mesoamerican Origin of the Common Bean (Phaseolus vulgaris L.) Is Revealed by Sequence Data. Proc. Natl. Acad. Sci. USA 2012, 109, E788–E796. [Google Scholar] [CrossRef]
- Liu, S.; Feuerstein, U.; Luesink, W.; Schulze, S.; Asp, T.; Studer, B.; Becker, H.C.; Dehmer, K.J. DArT, SNP, and SSR Analyses of Genetic Diversity in Lolium perenne L. Using Bulk Sampling. BMC Genet. 2018, 19, 10. [Google Scholar] [CrossRef]
- Botstein, D.; White, R.L.; Skolnick, M.; Davis, R.W. Construction of a Genetic Linkage Map in Man Using Restriction Fragment Length Polymorphisms. Am. J. Hum. Gen. 1980, 32, 314–331. [Google Scholar]
- Serrote, C.M.L.; Reiniger, L.R.S.; Silva, K.B.; dos Santos Rabaiolli, S.M.; Stefanel, C.M. Determining the Polymorphism Information Content of a Molecular Marker. Gene 2020, 726, 144175. [Google Scholar] [CrossRef] [PubMed]
- Nei, M.; Kumar, S. Molecular Evolution and Phylogenetics; Oxford University Press: New York, NY, USA, 2000; ISBN 0-19-513584-9. [Google Scholar]
- Blair, M.W.; Giraldo, M.C.; Buendía, H.F.; Tovar, E.; Duque, M.C.; Beebe, S.E. Microsatellite Marker Diversity in Common Bean (Phaseolus vulgaris L.). Theor. Appl. Genet. 2006, 113, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Polania, J.; Rao, I.M.; Cajiao, C.; Rivera, M.; Raatz, B.; Beebe, S. Physiological Traits Associated with Drought Resistance in Andean and Mesoamerican Genotypes of Common Bean (Phaseolus vulgaris L.). Euphytica 2016, 210, 17–29. [Google Scholar] [CrossRef]
- Zeven, A.C.; Waninge, J.; van Hintum, T.; Singh, S.P. Phenotypic Variation in a Core Collection of Common Bean (Phaseolus vulgaris L.) in the Netherlands. Euphytica 1999, 109, 93–106. [Google Scholar] [CrossRef]
- Rodiño, A.P.; Santalla, M.; González, A.M.; De Ron, A.M.; Singh, S.P. Novel Genetic Variation in Common Bean from the Iberian Peninsula. Crop Sci. 2006, 46, 2540–2546. [Google Scholar] [CrossRef]
- Gil, J.; Ron, A. De Variation in Phaseolus vulgaris in the Northwest of the Iberian Peninsula. Plant Breed. 1992, 109, 313–319. [Google Scholar] [CrossRef]
- Freitas, G.; Ganança, J.F.T.; Nóbrega, H.; Nunes, É.; Costa, G.; Slaski, J.J.; de Carvalho, M.Â.A.P. Morphological Evaluation of Common Bean Diversity on the Island of Madeira. Genet. Resour. Crop Evol. 2011, 58, 861–874. [Google Scholar] [CrossRef]
- Angioi, S.A.; Rau, D.; Nanni, L.; Bellucci, E.; Papa, R.; Attene, G. The Genetic Make-up of the European Landraces of the Common Bean. Plant Genet. Resour. 2011, 9, 197–201. [Google Scholar] [CrossRef]
- Bitocchi, E.; Rau, D.; Bellucci, E.; Rodriguez, M.; Murgia, M.L.; Gioia, T.; Santo, D.; Nanni, L.; Attene, G.; Papa, R. Beans (Phaseolus ssp.) as a Model for Understanding Crop Evolution. Front. Plant Sci. 2017, 8, 722. [Google Scholar] [CrossRef]
- Bellucci, E.; Bitocchi, E.; Rau, D.; Rodriguez, M.; Biagetti, E.; Giardini, A.; Attene, G.; Nanni, L.; Papa, R. Genomics of Origin, Domestication and Evolution of Phaseolus vulgaris. In Genomics of Plant Genetic Resources; Springer: Dordrecht, The Netherlands, 2014; pp. 483–507. ISBN 978-94-007-7571-8. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).