Dynamic of Phenolic Compounds in Guadua angustifolia Kunth under Chemical, Organic, and Biological Fertilization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Location of the Experiment and Biological Material
2.2. Experimental Setup and Sampling
2.3. Extraction and Identification of Phenolic Compounds
2.4. Extraction and Amplification of Root Bacterial DNA
2.5. Statistical Analyses and Data Handling
3. Results
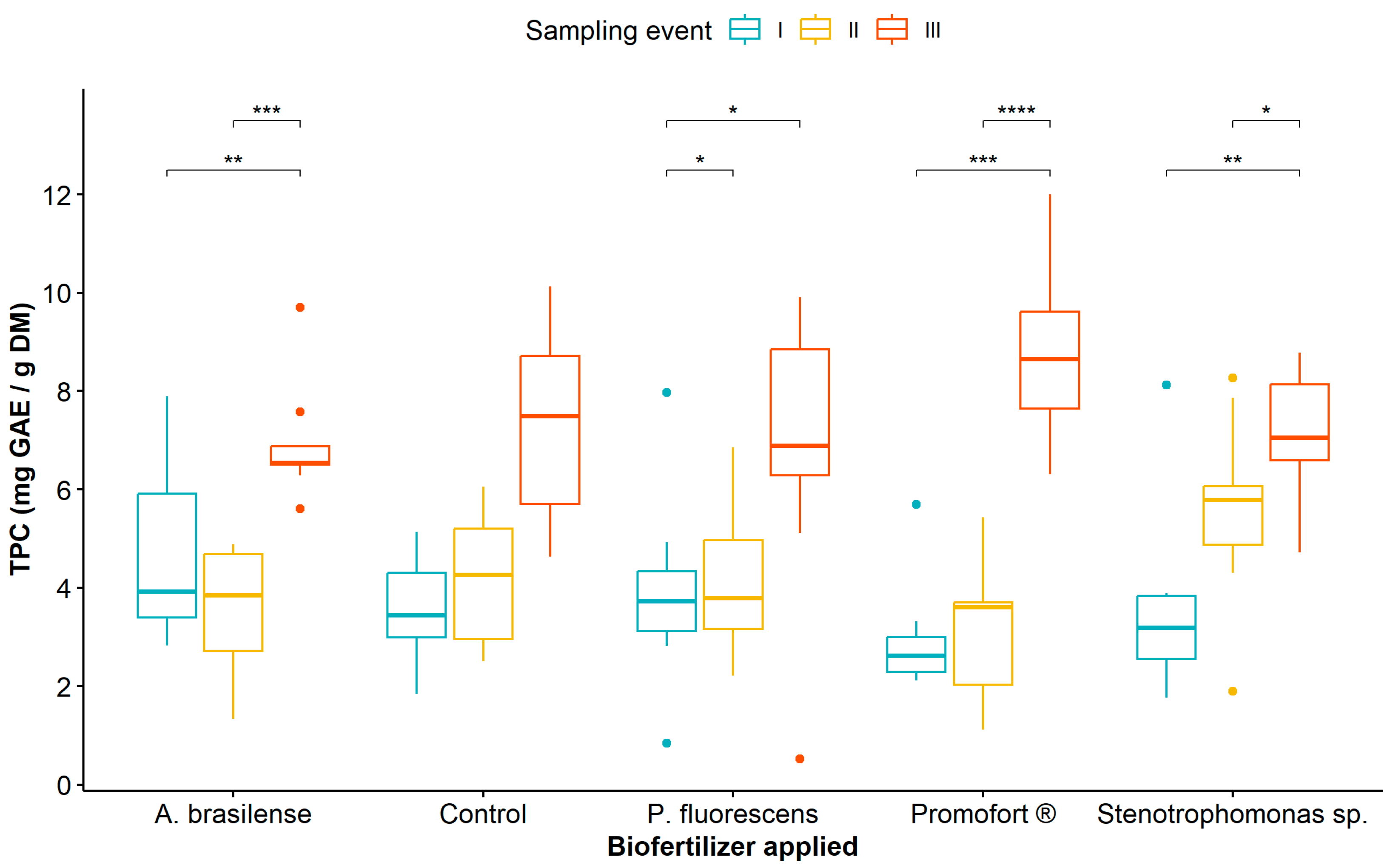
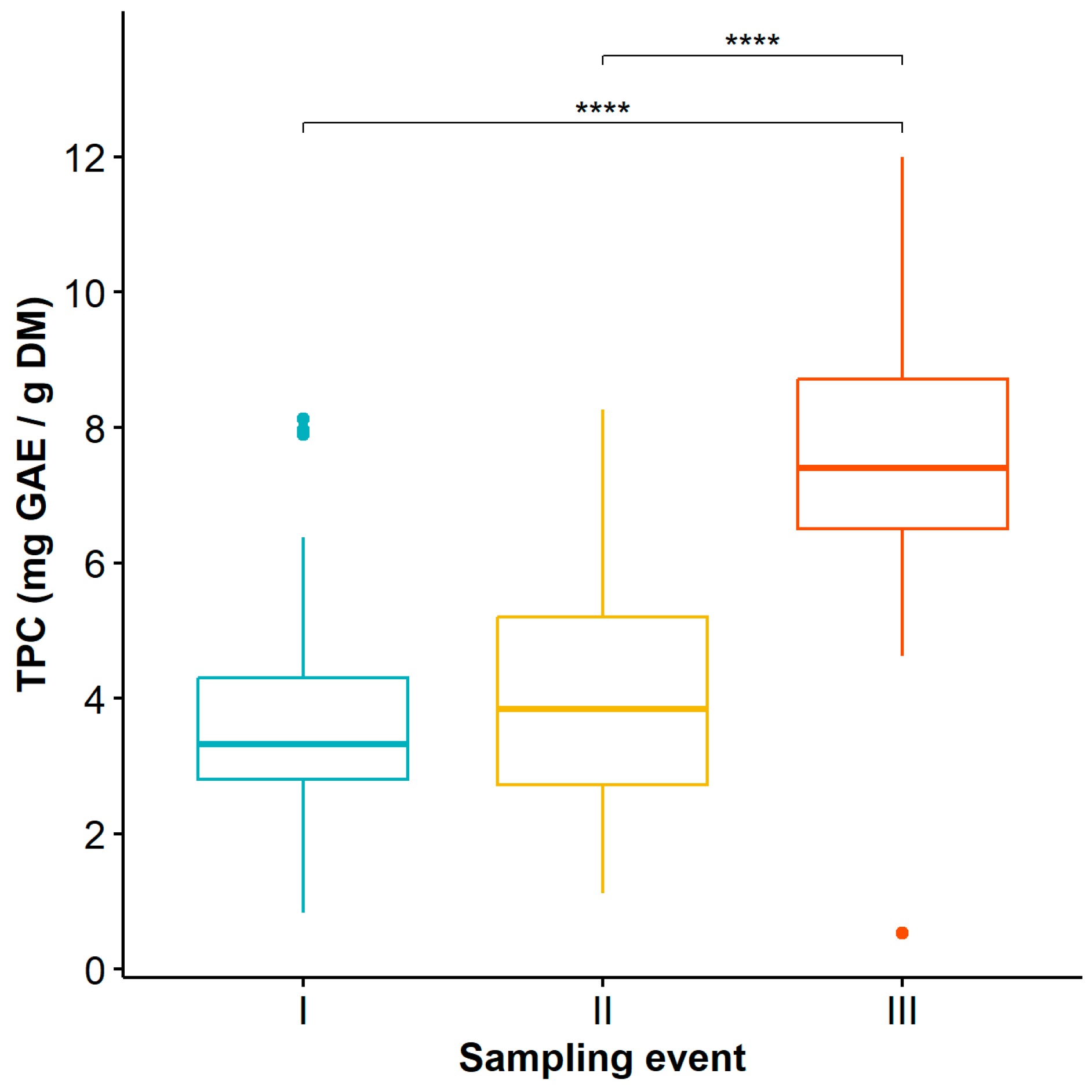
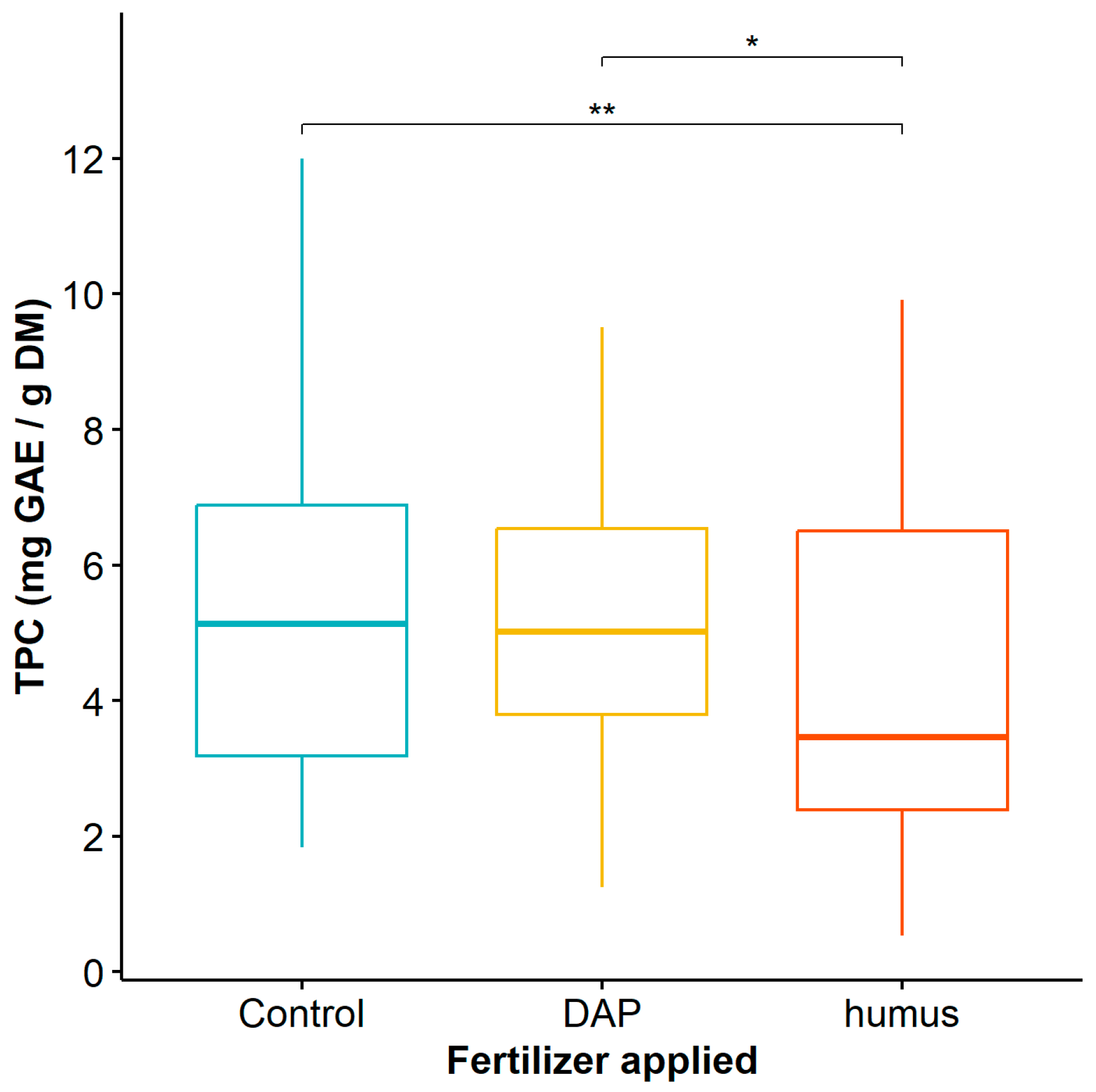
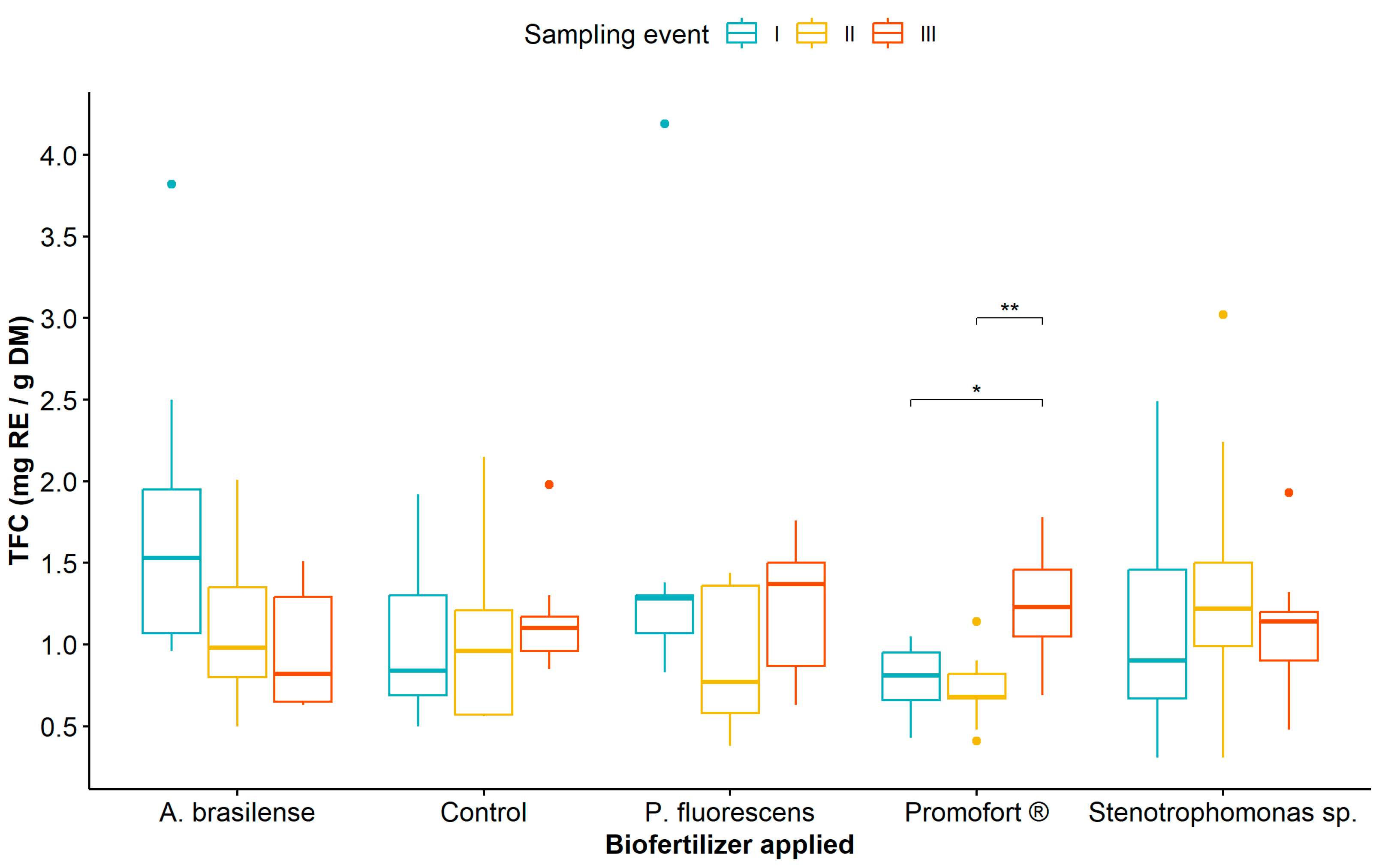
3.1. Analysis of Phenolic Compound Content
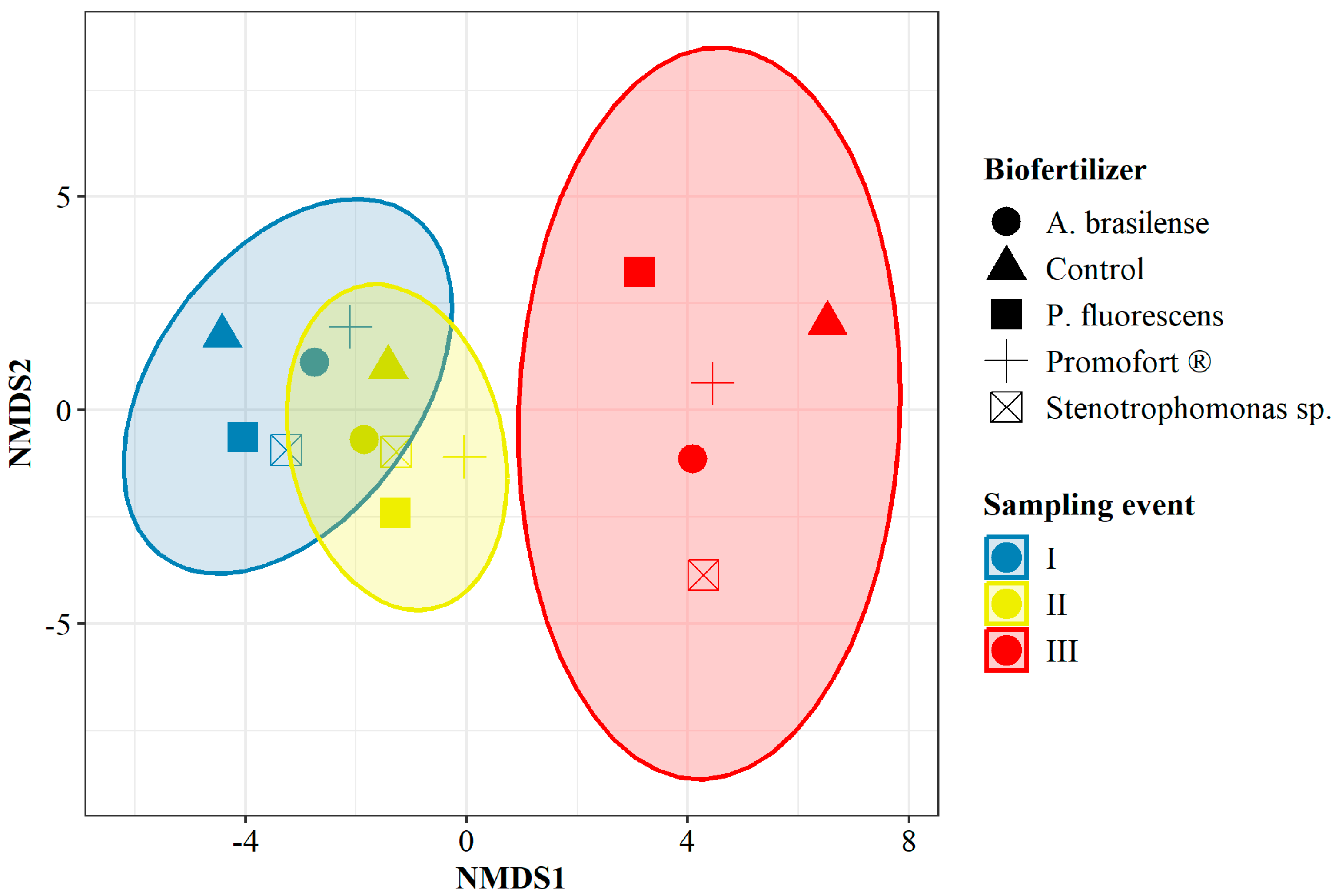
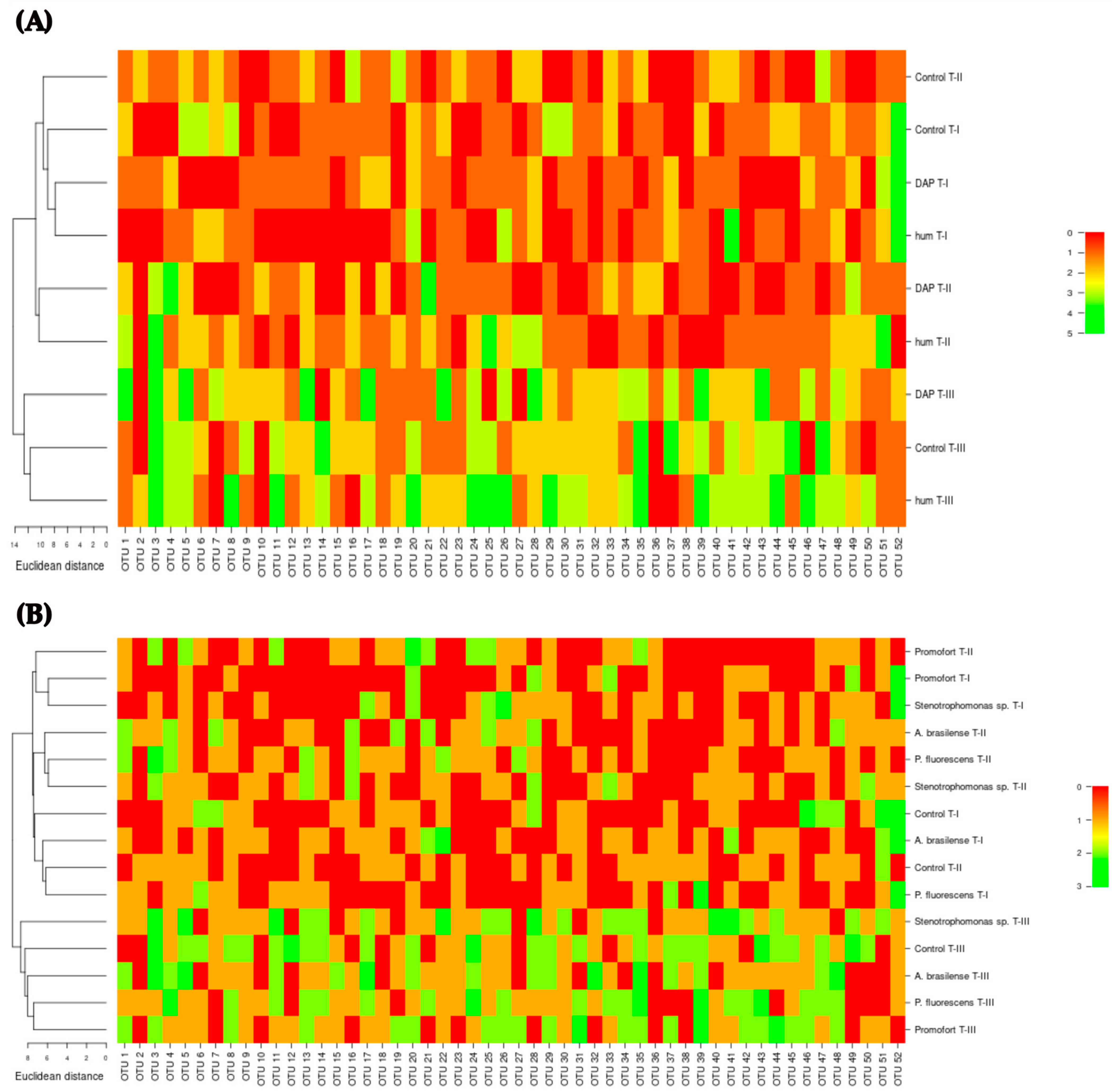
3.2. Analysis of the Bacterial Profile in G. angustifolia Roots
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Clark, L.G.; Londoño, X.; Ruiz-Sanchez, E. Bamboo Taxonomy and Habitat. In Bamboo; Liese, W., Köhl, M., Eds.; Springer International Publishing: Cham, Switzerland, 2015; Volume 10, pp. 1–30. [Google Scholar] [CrossRef]
- Judziewicz, E.J.; Clark, L.G.; Londoño, X. American Bamboos; Smithsonian Institution Press: Washington, DC, USA, 1999. [Google Scholar]
- Londoño, X. El bambú en Colombia. Biotecnol. Veg. 2011, 11, 143–154. [Google Scholar]
- Long, T.T.; Yanxia, L.; Jayaraman, D. Global Priority Species of Economically Important Bamboo; INBAR Technical Report no. 44; International Bamboo and Rattan Organization (INBAR): Beijing, China, 2022; Available online: https://www.inbar.int/resources/inbar_publications/global-priority-species-economically-important-bamboo (accessed on 15 September 2023).
- Reyes-Agüero, J.A.; Cruz-Armendáriz, N.M.; Ruiz-Sanchez, E. Servicios ecosistémicos de las especies nativas e introducidas de bambú en la Huasteca Potosina, México: Usos del bambú. Acta Bot. Mex. 2023, 130, e2132. [Google Scholar] [CrossRef]
- Akinlabi, E.T.; Anane-Fenin, K.; Akwada, D.R. Bamboo; Springer International Publishing: Cham, Switzerland, 2017. [Google Scholar] [CrossRef]
- Durango, C.; Gallardo, A.; Contreras, A. Estudios para el aprovechamiento potencial de hojas de Guadua angustifolia Kunth (Poaceae), para el sector cosmético. Rev. Cuba. Farm. 2015, 49, 535–542. [Google Scholar]
- Chitiva, L.C.; Lozano-Puentes, H.S.; Londoño, X.; Leão, T.F.; Cala, M.P.; Ruiz-Sanchez, E.; Díaz-Ariza, L.A.; Prieto-Rodríguez, J.A.; Castro-Gamboa, I.; Costa, G.M. Untargeted metabolomics approach and molecular networking analysis reveal changes in chemical composition under the influence of altitudinal variation in bamboo species. Front. Mol. Biosci. 2023, 10, 1192088. [Google Scholar] [CrossRef] [PubMed]
- Pang, Z.; Chen, J.; Wang, T.; Gao, C.; Li, Z.; Guo, L.; Xu, J.; Cheng, Y. Linking Plant Secondary Metabolites and Plant Microbiomes: A Review. Front. Plant Sci. 2021, 12, 621276. [Google Scholar] [CrossRef]
- Narayanan, Z.; Glick, B.R. Secondary Metabolites Produced by Plant Growth-Promoting Bacterial Endophytes. Microorganisms 2022, 10, 2008. [Google Scholar] [CrossRef]
- Ghitti, E.; Rolli, E.; Crotti, E.; Borin, S. Flavonoids Are Intra- and Inter-Kingdom Modulator Signals. Microorganisms 2022, 10, 2479. [Google Scholar] [CrossRef]
- Naguib, A.E.-M.M.; El-Baz, F.K.; Salama, Z.A.; Hanaa, H.A.E.B.; Ali, H.F.; Gaafar, A.A. Enhancement of phenolics, flavonoids and glucosinolates of Broccoli (Brassica oleracea var. Italica) as antioxidants in response to organic and bio-organic fertilizers. J. Saudi Soc. Agric. Sci. 2012, 11, 135–142. [Google Scholar] [CrossRef]
- Verma, N.; Shukla, S. Impact of various factors responsible for fluctuation in plant secondary metabolites. J. Appl. Res. Med. Aromat. Plants 2015, 2, 105–113. [Google Scholar] [CrossRef]
- Barroso, M.R.; Martins, N.; Barros, L.; Antonio, A.L.; Rodrigues, M.; Sousa, M.J.; Santos-Buelga, C.; Ferreira, I.C. Assessment of the nitrogen fertilization effect on bioactive compounds of frozen fresh and dried samples of Stevia rebaudiana Bertoni. Food Chem. 2018, 243, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Amarowicz, R.; Cwalina-Ambroziak, B.; Janiak, M.A.; Bogucka, B. Effect of N Fertilization on the Content of Phenolic Compounds in Jerusalem Artichoke (Helianthus tuberosus L.) Tubers and Their Antioxidant Capacity. Agronomy 2020, 10, 1215. [Google Scholar] [CrossRef]
- Wei, X.; Chen, J.; Gao, B.; Wang, Z. Role of controlled and slow release fertilizers in fruit crop nutrition. In Fruit Crops; Srivastava, A.K., Hu, C., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 555–566. [Google Scholar] [CrossRef]
- Ramaiya, S.D.; Lee, H.H.; Xiao, Y.J.; Shahbani, N.S.; Zakaria, M.H.; Bujang, J.S.; Sarker, U. Organic cultivation practices enhanced antioxidant activities and secondary metabolites in giant granadilla (Passiflora quadrangularis L.). PLoS ONE 2021, 16, e0255059. [Google Scholar] [CrossRef] [PubMed]
- Sałata, A.; Nurzyńska-Wierdak, R.; Kalisz, A.; Kunicki, E.; Ibáñez-Asensio, S.; Moreno-Ramón, H. Effects of Organic Cropping on Phenolic Compounds and Antioxidant Capacity of Globe Artichoke Herbs. Agronomy 2022, 12, 192. [Google Scholar] [CrossRef]
- Colipano, J.M.; Cagasan, U.A. A review on the impact of organic, conventional and nano fertilizer application in crop production. Eurasian J. Agric. Res. 2022, 6, 101–109. [Google Scholar]
- Alizadeh, A.; Khoshkhui, M.; Javidnia, K.; Firuzi, O.R.; Tafazoli, E.; Khalighi, A. Effects of fertilizer on yield, essential oil composition, total phenolic content and antioxidant activity in Satureja hortensis L. (Lamiaceae) cultivated in Iran. J. Med. Plants Res. 2010, 4, 33–40. [Google Scholar] [CrossRef]
- Moradzadeh, S.; Moghaddam, S.S.; Rahimi, A.; Pourakbar, L.; Sayyed, R.Z. Combined bio-chemical fertilizers ameliorate agro-biochemical attributes of black cumin (Nigella sativa L.). Sci. Rep. 2021, 11, 11399. [Google Scholar] [CrossRef]
- Cevheri, C.I.; Sakin, E.; Ramazanoglu, E. Effects of different fertilizers on some soil enzymes activity and chlorophyll contents of two cotton (G. hirsutum L.) varieties grown in a saline and non-saline soil. J. Plant Nutr. 2022, 45, 95–106. [Google Scholar] [CrossRef]
- Chuma, G.B.; Mulalisi, B.; Mondo, J.M.; Ndeko, A.B.; Bora, F.S.; Bagula, E.M.; Mushagalusa, G.N.; Civava, R. Di-ammonium phosphate (DAP) and plant density improve grain yield, nodulation capacity, and profitability of peas (Pisum sativum L.) on ferralsols in eastern D.R. Congo. CABI Agric. Biosci. 2022, 3, 65. [Google Scholar] [CrossRef]
- Zhang, J.; Bei, S.; Li, B.; Zhang, J.; Christie, P.; Li, X. Organic fertilizer, but not heavy liming, enhances banana biomass, increases soil organic carbon and modifies soil microbiota. Appl. Soil Ecol. 2018, 136, 67–79. [Google Scholar] [CrossRef]
- Zhaoxiang, W.; Huihu, L.; Qiaoli, L.; Changyan, Y.; Faxin, Y. Application of bio-organic fertilizer, not biochar, in degraded red soil improves soil nutrients and plant growth. Rhizosphere 2020, 16, 100264. [Google Scholar] [CrossRef]
- Kumari, M.; Swarupa, P.; Kesari, K.K.; Kumar, A. Microbial Inoculants as Plant Biostimulants: A Review on Risk Status. Life 2023, 13, 12. [Google Scholar] [CrossRef] [PubMed]
- O’Callaghan, M.; Ballard, R.A.; Wright, D. Soil microbial inoculants for sustainable agriculture: Limitations and opportunities. Soil Use Manag. 2022, 38, 1340–1369. [Google Scholar] [CrossRef]
- Li, Y.; Li, Q.; Guan, G.; Chen, S. Phosphate solubilizing bacteria stimulate wheat rhizosphere and endosphere biological nitrogen fixation by improving phosphorus content. PeerJ 2020, 8, e9062. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yan, X.; Su, M.; Li, J.; Man, T.; Wang, S.; Li, C.; Gao, S.; Zhang, R.; Zhang, M.; et al. Isolation of potassium solubilizing bacteria in soil and preparation of liquid bacteria fertilizer from food wastewater. Biochem. Eng. J. 2022, 181, 108378. [Google Scholar] [CrossRef]
- Ghaderimokri, L.; Rezaei-Chiyaneh, E.; Ghiyasi, M.; Gheshlaghi, M.; Battaglia, M.L.; Siddique, K.H.M. Application of humic acid and biofertilizers changes oil and phenolic compounds of fennel and fenugreek in intercropping systems. Sci. Rep. 2022, 12, 1–14. [Google Scholar] [CrossRef]
- Khalid, M.; Hassani, D.; Bilal, M.; Asad, F.; Huang, D. Influence of bio-fertilizer containing beneficial fungi and rhizospheric bacteria on health promoting compounds and antioxidant activity of Spinacia oleracea L. Bot. Stud. 2017, 58, 35. [Google Scholar] [CrossRef]
- Dasgan, H.Y.; Aldiyab, A.; Elgudayem, F.; Ikiz, B.; Gruda, N.S. Effect of biofertilizers on leaf yield, nitrate amount, mineral content and antioxidants of basil (Ocimum basilicum L.) in a floating culture. Sci. Rep. 2022, 12, 20917. [Google Scholar] [CrossRef]
- Villamarin-Raad, D.A.; Lozano-Puentes, H.S.; Chitiva, L.C.; Costa, G.M.; Díaz-Gallo, S.A.; Díaz-Ariza, L.A. Changes in Phenolic Profile and Total Phenol and Total Flavonoid Contents of Guadua angustifolia Kunth Plants under Organic and Conventional Fertilization. ACS Omega 2023, 8, 41223–41231. [Google Scholar] [CrossRef]
- Bouderias, S.; Teszlák, P.; Jakab, G.; Kőrösi, L. Age- and season-dependent pattern of flavonol glycosides in Cabernet Sauvignon grapevine leaves. Sci. Rep. 2020, 10, 14241. [Google Scholar] [CrossRef]
- Saffaryazdi, A.; Ganjeali, A.; Farhoosh, R.; Cheniany, M. Variation in phenolic compounds, α-linolenic acid and linoleic acid contents and antioxidant activity of purslane (Portulaca oleracea L.) during phenological growth stages. Physiol. Mol. Biol. Plants 2020, 26, 1519–1529. [Google Scholar] [CrossRef]
- Lavola, A.; Maukonen, M.; Julkunen-Tiitto, R. Variability in the composition of phenolic compounds in winter-dormant Salix pyrolifolia in relation to plant part and age. Phytochemistry 2018, 153, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Kleuter, M.; Dinani, S.T.; Trindade, L.M.; van der Goot, A.J. The role of plant age and leaf position on protein extraction and phenolic compounds removal from tomato (Solanum lycopersicum) leaves using food-grade solvents. Food Chem. 2023, 406, 135072. [Google Scholar] [CrossRef] [PubMed]
- Qudsieh, H.Y.M.; Yusof, S.; Osman, A.; Rahman, R.A. Effect of Maturity on Chlorophyll, Tannin, Color, and Polyphenol Oxidase (PPO) Activity of Sugarcane Juice (Saccharum officinarum Var. Yellow Cane). J. Agric. Food Chem. 2002, 50, 1615–1618. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Bals, O.; Grimi, N.; Vorobiev, E. A new way for the oil plant biomass valorization: Polyphenols and proteins extraction from rapeseed stems and leaves assisted by pulsed electric fields. Ind. Crop. Prod. 2015, 74, 309–318. [Google Scholar] [CrossRef]
- O’brien, F.J.M.; Dumont, M.G.; Webb, J.S.; Poppy, G.M. Rhizosphere Bacterial Communities Differ According to Fertilizer Regimes and Cabbage (Brassica oleracea var. capitata L.) Harvest Time, but Not Aphid Herbivory. Front. Microbiol. 2018, 9, 1620. [Google Scholar] [CrossRef] [PubMed]
- Dang, P.; Li, C.; Lu, C.; Zhang, M.; Huang, T.; Wan, C.; Wang, H.; Chen, Y.; Qin, X.; Liao, Y.; et al. Effect of fertilizer management on the soil bacterial community in agroecosystems across the globe. Agric. Ecosyst. Environ. 2022, 326, 107795. [Google Scholar] [CrossRef]
- Hartmann, M.; Frey, B.; Mayer, J.; Mäder, P.; Widmer, F. Distinct soil microbial diversity under long-term organic and conventional farming. ISME J. 2014, 9, 1177–1194. [Google Scholar] [CrossRef]
- Xu, F.; Sun, G.; Du, W.; Ai, F.; Yin, Y.; Guo, H. Impacts of Chemical and Organic Fertilizers on the Bacterial Communities, Sulfonamides and Sulfonamide Resistance Genes in Paddy Soil Under Rice-Wheat Rotation. Bull. Environ. Contam. Toxicol. 2022, 110, 20. [Google Scholar] [CrossRef] [PubMed]
- Pershina, E.; Valkonen, J.; Kurki, P.; Ivanova, E.; Chirak, E.; Korvigo, I.; Provorov, N.; Andronov, E. Comparative Analysis of Prokaryotic Communities Associated with Organic and Conventional Farming Systems. PLoS ONE 2015, 10, e0145072. [Google Scholar] [CrossRef]
- Herzog, S.; Wemheuer, F.; Wemheuer, B.; Daniel, R. Effects of Fertilization and Sampling Time on Composition and Diversity of Entire and Active Bacterial Communities in German Grassland Soils. PLoS ONE 2015, 10, e0145575. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Ni, T.; Li, Y.; Xiong, W.; Ran, W.; Shen, B.; Shen, Q.; Zhang, R. Responses of Bacterial Communities in Arable Soils in a Rice-Wheat Cropping System to Different Fertilizer Regimes and Sampling Times. PLoS ONE 2014, 9, e85301. [Google Scholar] [CrossRef]
- Zhong, W.; Gu, T.; Wang, W.; Zhang, B.; Lin, X.; Huang, Q.; Shen, W. The effects of mineral fertilizer and organic manure on soil microbial community and diversity. Plant Soil 2009, 326, 511–522. [Google Scholar] [CrossRef]
- Díaz Ariza, L.A. Bioinoculant Composition. U.S. Patent 11,001,536 B2, 11 May 2021. [Google Scholar]
- Lozano-Puentes, H.S.; Sánchez-Matiz, J.J.; Ruiz-Sanchez, E.; Costa, G.M.; Díaz, L.A. Guadua angustifolia Kunth Leaves as a Source for Bioactive Phenolic Compounds: Optimization of Ultrasound-Assisted Extraction Using Response Surface Methodology and Antioxidant Activities. SSRN 2023. submitted. [Google Scholar]
- Lamuela-Raventós, R.M. Folin-Ciocalteu method for the measurement of total phenolic content and antioxidant capacity. In Measurement of Antioxidant Activity & Capacity: Recent Trends and Applications; John Wiley & Sons, Ltd.: Chichester, UK, 2017; pp. 107–115. [Google Scholar] [CrossRef]
- Chandra, S.; Khan, S.; Avula, B.; Lata, H.; Yang, M.H.; ElSohly, M.A.; Khan, I.A. Assessment of Total Phenolic and Flavonoid Content, Antioxidant Properties, and Yield of Aeroponically and Conventionally Grown Leafy Vegetables and Fruit Crops: A Comparative Study. Evid.-Based Complement. Altern. Med. 2014, 2014, 253875. [Google Scholar] [CrossRef]
- Doyle, J.J.; Doyle, J.L. Isolation of plant DNA from fresh tissue. Focus 1990, 12, 13–15. [Google Scholar]
- Heuer, H.; Krsek, M.; Baker, P.; Smalla, K.; Wellington, E.M. Analysis of actinomycete communities by specific amplification of genes encoding 16S rRNA and gel-electrophoretic separation in denaturing gradients. Appl. Environ. Microbiol. 1997, 63, 3233–3241. [Google Scholar] [CrossRef] [PubMed]
- Valášková, V.; Baldrian, P. Denaturing gradient gel electrophoresis as a fingerprinting method for the analysis of soil microbial communities. Plant Soil Environ. 2009, 55, 413–423. [Google Scholar] [CrossRef]
- Myers, R.M.; Lumelsky, N.; Lerman, L.S.; Maniatis, T. Detection of single base substitutions in total genomic DNA. Nature 1985, 313, 495–498. [Google Scholar] [CrossRef]
- Tukey, J.W. Comparing Individual Means in the Analysis of Variance. Biometrics 1949, 5, 99–114. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, S.S.; Wilk, M.B.; Chen, H.J. A Comparative Study of Various Tests for Normality. J. Am. Stat. Assoc. 1968, 63, 1343. [Google Scholar] [CrossRef]
- Conover, W.J.; Iman, R.L. Rank Transformations as a Bridge Between Parametric and Nonparametric Statistics. Am. Stat. 1981, 35, 124. [Google Scholar] [CrossRef]
- Greenhouse, S.W.; Geisser, S. On methods in the analysis of profile data. Psychometrika 1959, 24, 95–112. [Google Scholar] [CrossRef]
- Huynh, H.; Feldt, L.S. Estimation of the Box Correction for Degrees of Freedom from Sample Data in Randomized Block and Split-Plot Designs. J. Educ. Stat. 1976, 1, 69. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023; Available online: https://www.R-project.org (accessed on 9 August 2023).
- Lawrence, M.A. Ez: Easy Analysis and Visualization of Factorial Experiments; R package v4.4-0; CRAN: Vienna, Austria, 2016; Available online: https://CRAN.R-project.org/package=ez (accessed on 9 August 2023).
- Kassambara, A. Rstatix: Pipe-Friendly Framework for Basic Statistical Tests; R package v0.7.2; CRAN: Vienna, Austria, 2023; Available online: https://CRAN.R-project.org/package=rstatix (accessed on 9 August 2023).
- Kassambara, A. Ggpubr: ‘ggplot2’ Based Publication Ready Plots; R package v0.6.0; CRAN: Vienna, Austria, 2023; Available online: https://CRAN.R-project.org/package=ggpubr (accessed on 9 August 2023).
- Oksanen, J.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; Stevens, M.H.H.; Wagner, H. Package ‘vegan’. In Community Ecology Package, R package version 2.6-4; CRAN: Vienna, Austria, 2022; Available online: https://CRAN.R-project.org/package=vegan (accessed on 9 August 2023).
- Buttigieg, P.L.; Ramette, A. A guide to statistical analysis in microbial ecology: A community-focused, living review of multivariate data analyses. FEMS Microbiol. Ecol. 2014, 90, 543–550. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, J.N.; Myers, T.G.; O’Connor, P.M.; Friend, S.H., Jr.; Fornace, A.J.; Kohn, K.W.; Fojo, T.; Bates, S.E.; Rubinstein, L.V.; Anderson, N.L.; et al. An Information-Intensive Approach to the Molecular Pharmacology of Cancer. Science 1997, 275, 343–349. [Google Scholar] [CrossRef]
- Gupta, R.; Singh, A.; Gupta, M.M.; Pandey, R. Cumulative role of bioinoculants on growth, antioxidant potential and artemisinin content in Artemisia annua L. under organic field conditions. World J. Microbiol. Biotechnol. 2016, 32, 167. [Google Scholar] [CrossRef] [PubMed]
- Helaly, A.A.; Mady, E.; Salem, E.A.; Randhir, T.O. Stimulatory effects of growth-promoting bacteria on growth, nutritional composition, and yield of kale plants. J. Plant Nutr. 2022, 45, 2465–2477. [Google Scholar] [CrossRef]
- Chiappero, J.; Cappellari, L.d.R.; Alderete, L.G.S.; Palermo, T.B.; Banchio, E. Plant growth promoting rhizobacteria improve the antioxidant status in Mentha piperita grown under drought stress leading to an enhancement of plant growth and total phenolic content. Ind. Crop. Prod. 2019, 139, 111553. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, Z.; Chen, Z.; Kowalchuk, G.A.; Fu, X.; Kuramae, E.E. Microbial inoculants modulate growth traits, nutrients acquisition and bioactive compounds accumulation of Cyclocarya paliurus (Batal.) Iljinskaja under degraded field condition. For. Ecol. Manag. 2021, 482, 118897. [Google Scholar] [CrossRef]
- Lewis, D.R.; Ramirez, M.V.; Miller, N.D.; Vallabhaneni, P.; Ray, W.K.; Helm, R.F.; Winkel, B.S.; Muday, G.K. Auxin and Ethylene Induce Flavonol Accumulation through Distinct Transcriptional Networks. Plant Physiol. 2011, 156, 144–164. [Google Scholar] [CrossRef]
- Luo, J.; Zhou, J.-J.; Zhang, J.-Z. Aux/IAA Gene Family in Plants: Molecular Structure, Regulation, and Function. Int. J. Mol. Sci. 2018, 19, 259. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Duan, G.; Li, C.; Liu, L.; Han, G.; Zhang, Y.; Wang, C. The Crosstalks Between Jasmonic Acid and Other Plant Hormone Signaling Highlight the Involvement of Jasmonic Acid as a Core Component in Plant Response to Biotic and Abiotic Stresses. Front. Plant Sci. 2019, 10, 1349. [Google Scholar] [CrossRef] [PubMed]
- Sabbagh, E.; Sabbagh, S.K.; Panjehkeh, N.; Bolok-Yazdi, H.R. Jasmonic Acid Induced Systemic Resistance in Infected Cucumber by Pythium aphanidermatum. J. Agric. Sci. 2018, 24, 143–152. [Google Scholar] [CrossRef]
- Ibrahim, M.H.; Jaafar, H.Z.E.; Karimi, E.; Ghasemzadeh, A. Impact of Organic and Inorganic Fertilizers Application on the Phytochemical and Antioxidant Activity of Kacip Fatimah (Labisia pumila Benth). Molecules 2013, 18, 10973–10988. [Google Scholar] [CrossRef] [PubMed]
- Bayat, H.; Shafie, F.; Aminifard, M.H.; Daghighi, S. Comparative effects of humic and fulvic acids as biostimulants on growth, antioxidant activity and nutrient content of yarrow (Achillea millefolium L.). Sci. Hortic. 2021, 279, 109912. [Google Scholar] [CrossRef]
- Kaur, A.; Pati, P.K.; Ohri, P.; Kaur, A. Effects of Vermicompost and Vermicompost Leachate on the Biochemical and Physiological Response of Withania somnifera (L.) Dunal. J. Soil Sci. Plant Nutr. 2022, 22, 3228–3242. [Google Scholar] [CrossRef]
- Marlin, M.; Simarmata, M.; Salamah, U.; Nurcholis, W. Effect of nitrogen and potassium application on growth, total phenolic, flavonoid contents, and antioxidant activity of Eleutherine palmifolia. AIMS Agric. Food 2022, 7, 580–593. [Google Scholar] [CrossRef]
- Barzegar, T.; Mohammadi, S.; Ghahremani, Z. Effect of nitrogen and potassium fertilizer on growth, yield and chemical composition of sweet fennel. J. Plant Nutr. 2020, 43, 1189–1204. [Google Scholar] [CrossRef]
- Lillo, C.; Lea, U.S.; Ruoff, P. Nutrient depletion as a key factor for manipulating gene expression and product formation in different branches of the flavonoid pathway. Plant Cell Environ. 2008, 31, 587–601. [Google Scholar] [CrossRef]
- Deng, B.; Li, Y.; Lei, G.; Liu, G. Effects of nitrogen availability on mineral nutrient balance and flavonoid accumulation in Cyclocarya paliurus. Plant Physiol. Biochem. 2019, 135, 111–118. [Google Scholar] [CrossRef]
- Heimler, D.; Romani, A.; Ieri, F. Plant polyphenol content, soil fertilization and agricultural management: A review. Eur. Food Res. Technol. 2017, 243, 1107–1115. [Google Scholar] [CrossRef]
- Wu, Q.; Zhang, J.; Liu, X.; Chang, T.; Wang, Q.; Shaghaleh, H.; Hamoud, Y.A. Effects of biochar and vermicompost on microorganisms and enzymatic activities in greenhouse soil. Front. Environ. Sci. 2023, 10, 1060277. [Google Scholar] [CrossRef]
- Liu, M.; Wang, C.; Liu, X.; Lu, Y.; Wang, Y. Saline-alkali soil applied with vermicompost and humic acid fertilizer improved macroaggregate microstructure to enhance salt leaching and inhibit nitrogen losses. Appl. Soil Ecol. 2020, 156, 103705. [Google Scholar] [CrossRef]
- Wang, R.; Hou, T.; Sun, Q.; Ji, L.; Lei, J.; Zhang, J. Organic Fertilizers and Soil Conditioner Recover Chemical Fertilizer-Induced Changes in Soil Bacterial Community Diversity in Wine Grape Rhizosphere Soil. Pol. J. Environ. Stud. 2021, 30, 1853–1863. [Google Scholar] [CrossRef]
- Zainuddin, N.; Keni, M.F.; Ibrahim, S.A.S.; Masri, M.M.M. Effect of integrated biofertilizers with chemical fertilizers on the oil palm growth and soil microbial diversity. Biocatal. Agric. Biotechnol. 2022, 39, 102237. [Google Scholar] [CrossRef]
- Cornell, C.; Kokkoris, V.; Richards, A.; Horst, C.; Rosa, D.; Bennett, J.A.; Hart, M.M. Do Bioinoculants Affect Resident Microbial Communities? A Meta-Analysis. Front. Agron. 2021, 3, 753474. [Google Scholar] [CrossRef]
- Thiergart, T.; Durán, P.; Ellis, T.; Vannier, N.; Garrido-Oter, R.; Kemen, E.; Roux, F.; Alonso-Blanco, C.; Ågren, J.; Schulze-Lefert, P.; et al. Root microbiota assembly and adaptive differentiation among European Arabidopsis populations. Nat. Ecol. Evol. 2019, 4, 122–131. [Google Scholar] [CrossRef]
- Emmett, B.D.; Buckley, D.H.; Drinkwater, L.E. Plant growth rate and nitrogen uptake shape rhizosphere bacterial community composition and activity in an agricultural field. New Phytol. 2019, 225, 960–973. [Google Scholar] [CrossRef]
- Zhalnina, K.; Louie, K.B.; Hao, Z.; Mansoori, N.; da Rocha, U.N.; Shi, S.; Cho, H.; Karaoz, U.; Loqué, D.; Bowen, B.P.; et al. Dynamic root exudate chemistry and microbial substrate preferences drive patterns in rhizosphere microbial community assembly. Nat. Microbiol. 2018, 3, 470–480. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Matiz, J.J.; Lozano-Puentes, H.S.; Villamarín-Raad, D.A.; Díaz-Gallo, S.A.; Díaz-Ariza, L.A. Dynamic of Phenolic Compounds in Guadua angustifolia Kunth under Chemical, Organic, and Biological Fertilization. Agronomy 2023, 13, 2782. https://doi.org/10.3390/agronomy13112782
Sánchez-Matiz JJ, Lozano-Puentes HS, Villamarín-Raad DA, Díaz-Gallo SA, Díaz-Ariza LA. Dynamic of Phenolic Compounds in Guadua angustifolia Kunth under Chemical, Organic, and Biological Fertilization. Agronomy. 2023; 13(11):2782. https://doi.org/10.3390/agronomy13112782
Chicago/Turabian StyleSánchez-Matiz, Juan José, Hair Santiago Lozano-Puentes, David A. Villamarín-Raad, Sergio A. Díaz-Gallo, and Lucía Ana Díaz-Ariza. 2023. "Dynamic of Phenolic Compounds in Guadua angustifolia Kunth under Chemical, Organic, and Biological Fertilization" Agronomy 13, no. 11: 2782. https://doi.org/10.3390/agronomy13112782
APA StyleSánchez-Matiz, J. J., Lozano-Puentes, H. S., Villamarín-Raad, D. A., Díaz-Gallo, S. A., & Díaz-Ariza, L. A. (2023). Dynamic of Phenolic Compounds in Guadua angustifolia Kunth under Chemical, Organic, and Biological Fertilization. Agronomy, 13(11), 2782. https://doi.org/10.3390/agronomy13112782





