Metabolome and Transcriptome Analyses Provide Insights into Glucosinolate Accumulation in the Novel Vegetable Crop Cardamine violifolia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Total Glucosinolate Concentration
2.3. Detection of Glucosinolates via Widely Targeted Metabolome
2.4. Transcriptome Sequencing and Analysis
2.5. Conjoint Analysis of Transcriptome and Metabolome
2.6. Weighted Gene Correlation Network Analysis (WGCNA)
2.7. Validation of Transcriptome Data via Real-Time Quantitative PCR (RT-qPCR)
2.8. Statistical Analysis
3. Results
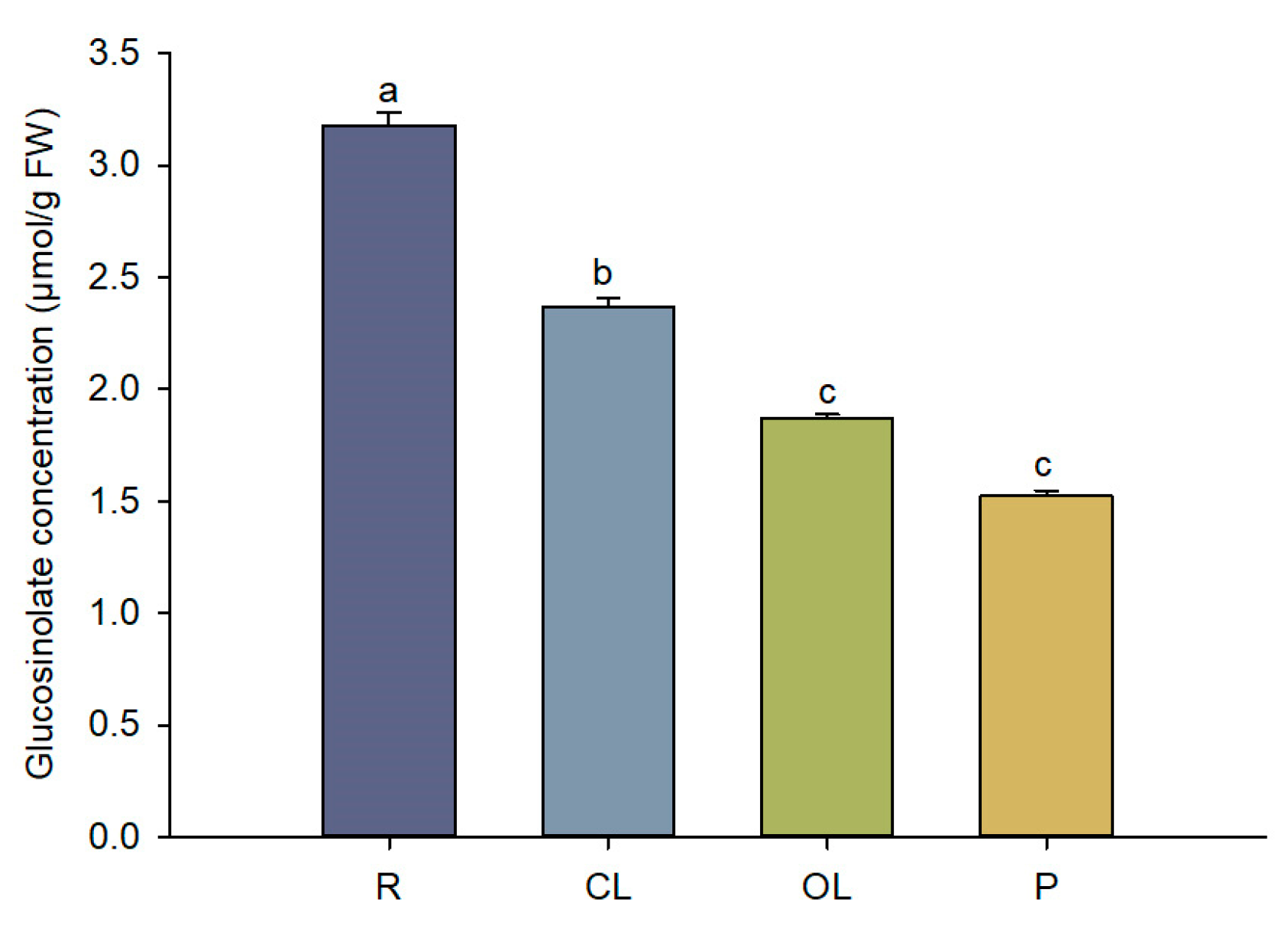
3.1. Total Glucosinolate Concentration in C. violifolia
3.2. Glucosinolate Profiles of C. violifolia
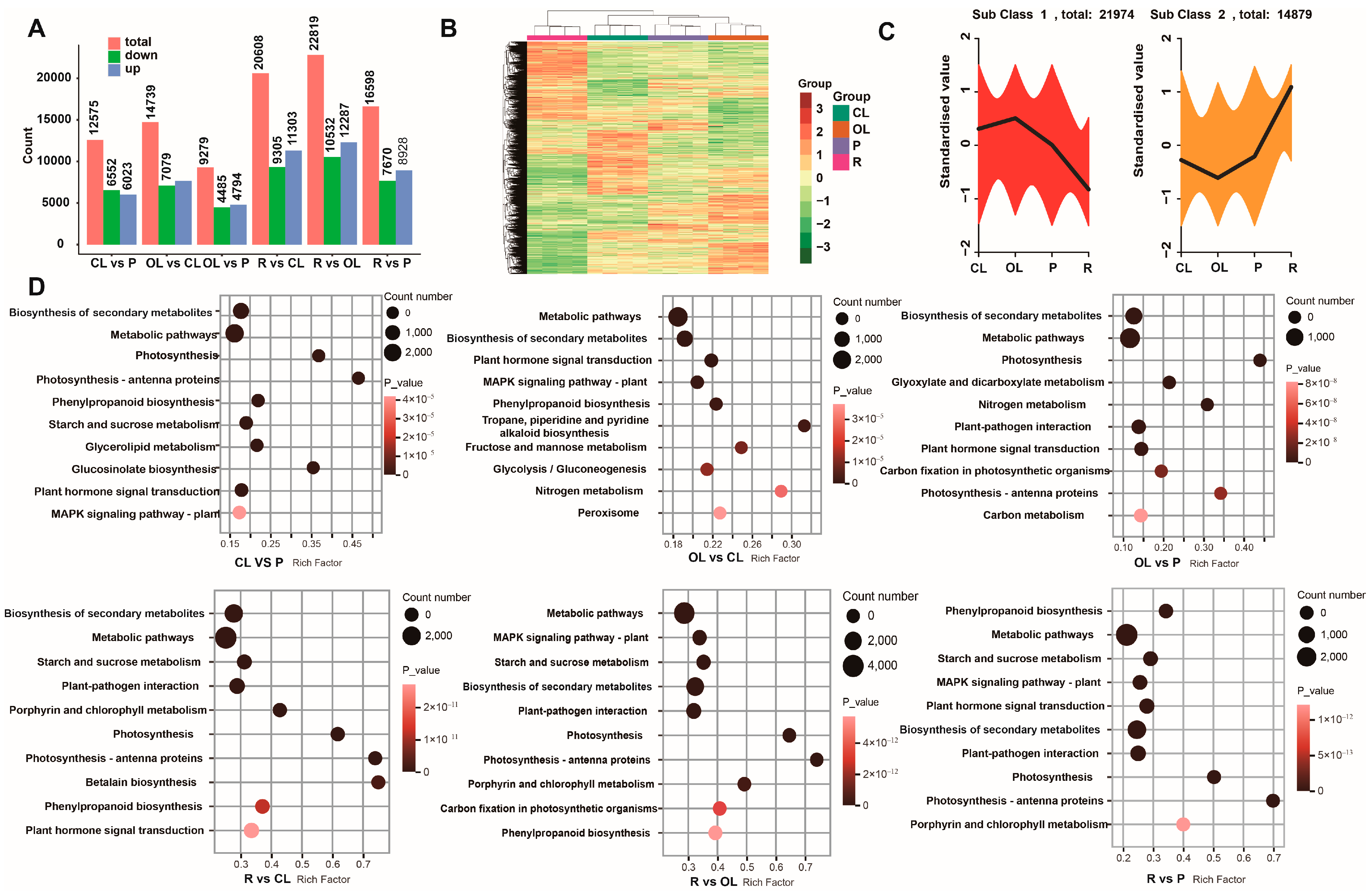
3.3. Transcriptome and DEG Analysis
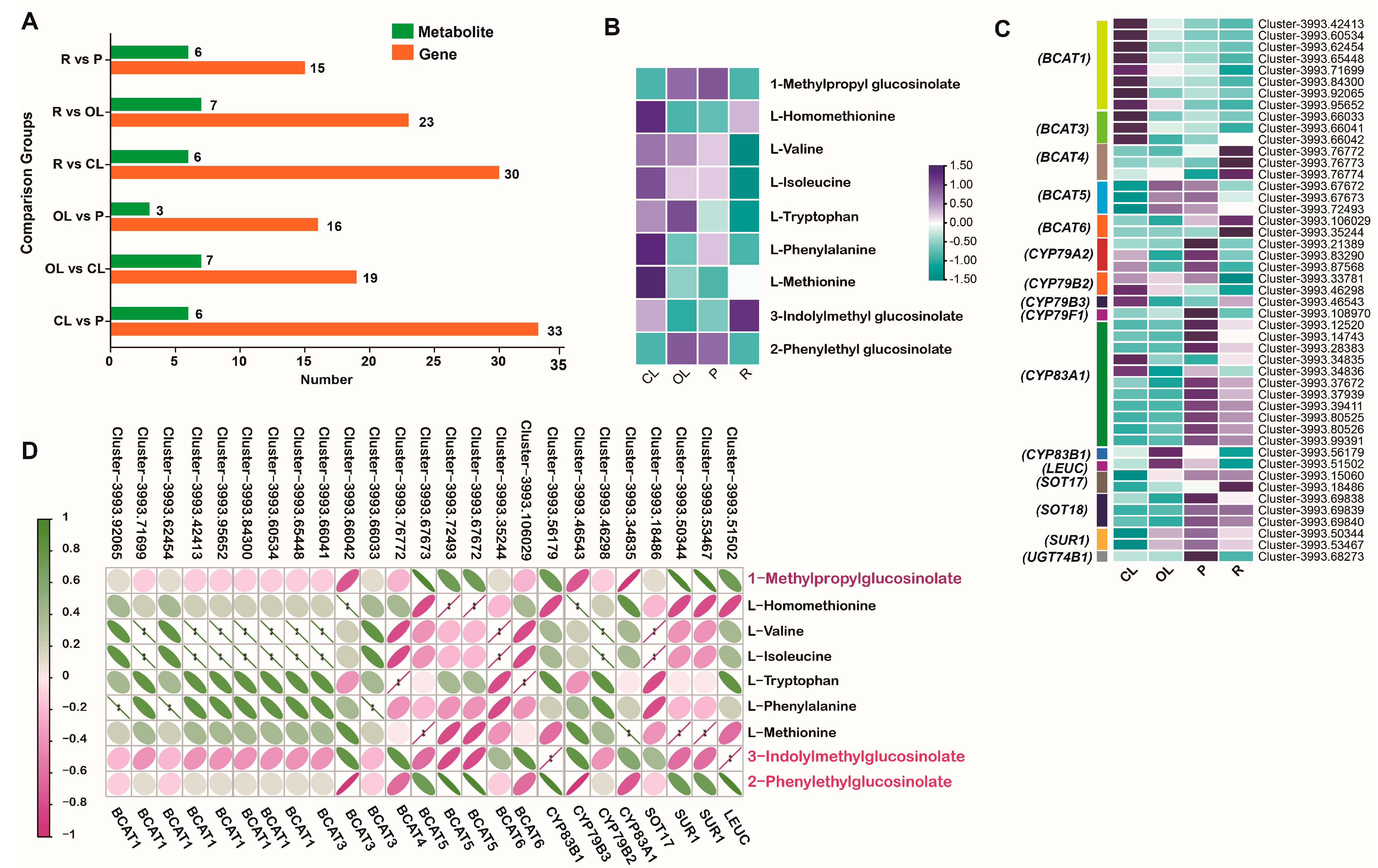
3.4. Correlation between the Metabolites and Genes in the Glucosinolate Biosynthesis Pathway
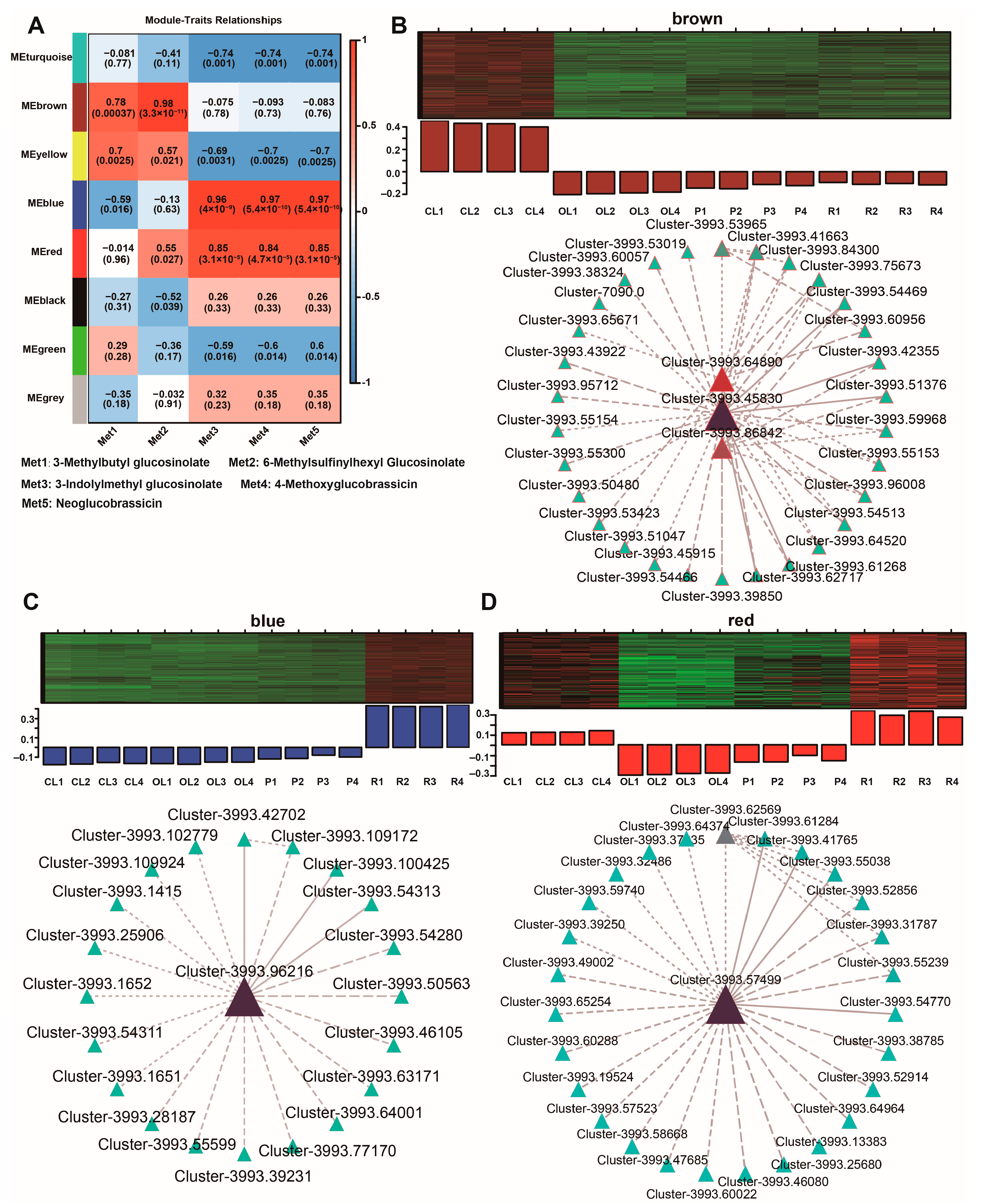
3.5. WGCNA Revealed Hub Genes Involved in the Accumulation of the Main Glucosinolates
3.6. Validation of the Transcriptome Data
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rao, S.; Yu, T.; Cong, X.; Lai, X.; Xiang, J.; Cao, J.; Liao, X.; Gou, Y.; Chao, W.; Xue, H.; et al. Transcriptome, proteome, and metabolome reveal the mechanism of tolerance to selenate toxicity in Cardamine violifolia. J. Hazard. Mater. 2021, 406, 124283. [Google Scholar] [CrossRef]
- Both, E.B.; Shao, S.; Xiang, J.; Jókai, Z.; Yin, H.; Liu, Y.; Magyar, A.; Dernovics, M. Selenolanthionine is the major water-soluble selenium compound in the selenium tolerant plant Cardamine violifolia. Biochim. Biophys. Acta (BBA) Gen. Subj. 2018, 1862, 2354–2362. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.; Cong, X.; Liu, H.; Hu, Y.; Yang, W.; Cheng, H.; Cheng, S.; Zhang, Y. Revealing the Phenolic Acids in Cardamine violifolia Leaves by Transcriptome and Metabolome Analyses. Metabolites 2022, 12, 1024. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Yin, J.; Wang, J.; Liu, X.; He, J.; Zhang, R.; Rao, S.; Cong, X.; Xiong, Y.; Wu, M. Selenium speciation and volatile flavor compound profiles in the edible flowers, stems, and leaves of selenium-hyperaccumulating vegetable Cardamine violifolia. Food Chem. 2023, 427, 136710. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.; Yu, T.; Cong, X.; Zhang, W.; Zhu, Z.; Liao, Y.; Ye, J.; Cheng, S.; Xu, F. Effects of selenate applied at two growth stages on the nutrient quality of Cardamine violifolia. Sci. Hortic. 2021, 288, 110352. [Google Scholar] [CrossRef]
- Prieto, M.A.; López, C.J.; Simal-Gandara, J. Glucosinolates: Molecular structure, breakdown, genetic, bioavailability, properties and healthy and adverse effects. Adv. Food Nutr. Res. 2019, 90, 305–350. [Google Scholar] [CrossRef] [PubMed]
- Blažević, I.; Montaut, S.; Burčul, F.; Olsen, C.E.; Burow, M.; Rollin, P.; Agerbirk, N. Glucosinolate structural diversity, identification, chemical synthesis and metabolism in plants. Phytochemistry 2020, 169, 112100. [Google Scholar] [CrossRef] [PubMed]
- Harun, S.; Abdullah-Zawawi, M.R.; Goh, H.H.; Mohamed-Hussein, Z.A. A Comprehensive Gene Inventory for Glucosinolate Biosynthetic Pathway in Arabidopsis thaliana. J. Agric. Food Chem. 2020, 68, 7281–7297. [Google Scholar] [CrossRef]
- Vanduchova, A.; Anzenbacher, P.; Anzenbacherova, E. Isothiocyanate from Broccoli, Sulforaphane, and Its Properties. J. Med. Food 2019, 22, 121–126. [Google Scholar] [CrossRef]
- Elkashty, O.A.; Tran, S.D. Sulforaphane as a Promising Natural Molecule for Cancer Prevention and Treatment. Curr. Med. Sci. 2021, 41, 250–269. [Google Scholar] [CrossRef]
- Kim, J.I.; Zhang, X.; Pascuzzi, P.E.; Liu, C.J.; Chapple, C. Glucosinolate and phenylpropanoid biosynthesis are linked by proteasome-dependent degradation of PAL. New Phytol. 2020, 225, 154–168. [Google Scholar] [CrossRef] [PubMed]
- Agneta, R.; Lelario, F.; De Maria, S.; Möllers, C.; Bufo, S.A.; Rivelli, A.R. Glucosinolate profile and distribution among plant tissues and phenological stages of field-grown horseradish. Phytochemistry 2014, 106, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Đulović, A.; Popović, M.; Burčul, F.; Čikeš Čulić, V.; Marijan, S.; Ruščić, M.; Anđelković, N.; Blažević, I. Glucosinolates of Sisymbrium officinale and S. orientale. Molecules 2022, 27, 8431. [Google Scholar] [CrossRef]
- Touw, A.J.; Verdecia Mogena, A.; Maedicke, A.; Sontowski, R.; van Dam, N.M.; Tsunoda, T. Both Biosynthesis and Transport Are Involved in Glucosinolate Accumulation During Root-Herbivory in Brassica rapa. Front. Plant Sci. 2020, 10, 1653. [Google Scholar] [CrossRef] [PubMed]
- Reichelt, M.; Brown, P.D.; Schneider, B.; Oldham, N.J.; Stauber, E.; Tokuhisa, J.; Kliebenstein, D.J.; Mitchell-Olds, T.; Gershenzon, J. Benzoic acid glucosinolate esters and other glucosinolates from Arabidopsis thaliana. Phytochemistry 2002, 59, 663–671. [Google Scholar] [CrossRef]
- Rao, S.; Gou, Y.; Yu, T.; Cong, X.; Gui, J.; Zhu, Z.; Zhang, W.; Liao, Y.; Ye, J.; Cheng, S.; et al. Effects of selenate on Se, flavonoid, and glucosinolate in broccoli florets by combined transcriptome and metabolome analyses. Food Res. Int. 2021, 146, 110463. [Google Scholar] [CrossRef]
- Montaut, S.; Grandbois, J.; Righetti, L.; Barillari, J.; Iori, R.; Rollin, P. Updated Glucosinolate Profile of Dithyrea wislizenii. J. Nat. Prod. 2009, 72, 889–893. [Google Scholar] [CrossRef]
- Cao, L.; Wang, Y.; Chen, X.; Deng, F.; Li, Z.; Wang, M.; Zhang, Y.; Su, R.; Kim, C.K. Discovery of novel glucosinolates inhibiting advanced glycation end products: Virtual screening and molecular dynamic simulation. Proteins 2023, 91, 1351–1360. [Google Scholar] [CrossRef]
- Agerbirk, N.; De Vos, M.; Kim, J.H.; Jander, G. Indole glucosinolate breakdown and its biological effects. Phytochem. Rev. 2009, 8, 101–120. [Google Scholar] [CrossRef]
- Renner, I.E.; Gardner, G.; Fritz, V.A. Manipulation of Continuous and End-of-Day Red/Far-Red Light Ratios Affects Glucobrassicin and Gluconasturtiin Accumulation in Cabbage (Brassica oleracea) and Watercress (Nasturtium officinale), Respectively. J. Agric. Food Chem. 2021, 69, 14126–14142. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Q.; Sun, H.; Zhang, Z.; Qian, H.; Zhao, X.; He, H.; Zhang, L. Glucosinolate Profiles in Different Organs of 111 Radish Accessions and Candidate Genes Involved in Converting Glucobrassicin to 4-Hydroxyglucobrassicin. J. Agric. Food Chem. 2022, 70, 488–497. [Google Scholar] [CrossRef]
- Li, Z.; Zheng, S.; Liu, Y.; Fang, Z.; Yang, L.; Zhuang, M.; Zhang, Y.; Lv, H.; Wang, Y.; Xu, D. Characterization of glucosinolates in 80 broccoli genotypes and different organs using UHPLC-Triple-TOF-MS method. Food Chem. 2021, 334, 127519. [Google Scholar] [CrossRef] [PubMed]
- Hornbacher, J.; Horst-Niessen, I.; Herrfurth, C.; Feussner, I.; Papenbrock, J. First experimental evidence suggests use of glucobrassicin as source of auxin in drought-stressed Arabidopsis thaliana. Front. Plant Sci. 2022, 13, 1025969. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.E. Indoles Derived from Glucobrassicin: Cancer Chemoprevention by Indole-3-Carbinol and 3,3′-Diindolylmethane. Front. Nutr. 2021, 8, 734334. [Google Scholar] [CrossRef] [PubMed]
- Wiesner, M.; Hanschen, F.; Schreiner, M.; Glatt, H.; Zrenner, R. Induced Production of 1-Methoxy-indol-3-ylmethyl Glucosinolate by Jasmonic Acid and Methyl Jasmonate in Sprouts and Leaves of Pak Choi (Brassica rapa ssp. chinensis). Int. J. Mol. Sci. 2013, 14, 14996–15016. [Google Scholar] [CrossRef] [PubMed]
- Kronbak, R.; Duus, F.; Vang, O. Effect of 4-Methoxyindole-3-carbinol on the Proliferation of Colon Cancer Cells In Vitro, When Treated Alone or in Combination with Indole-3-carbinol. J. Agric. Food Chem. 2010, 58, 8453–8459. [Google Scholar] [CrossRef]
- Glatt, H.; Engst, W.; Florian, S.; Schreiner, M.; Baasanjav-Gerber, C. Feeding Brassica vegetables to rats leads to the formation of characteristic DNA adducts (from 1-methoxy-3-indolylmethyl glucosinolate) in many tissues. Arch. Toxicol. 2022, 96, 933–944. [Google Scholar] [CrossRef]
- Badenes-Pérez, F.R.; Cartea, M.E. Glucosinolate Induction and Resistance to the Cabbage Moth, Mamestra brassicae, Differs among Kale Genotypes with High and Low Content of Sinigrin and Glucobrassicin. Plants 2021, 10, 1951. [Google Scholar] [CrossRef]
- Cong, C.; Yuan, X.; Hu, Y.; Chen, W.; Wang, Y.; Tao, L. Sinigrin attenuates angiotensin II-induced kidney injury by inactivating nuclear factor-κB and extracellular signal-regulated kinase signaling in vivo and in vitro. Int. J. Mol. Med. 2021, 48, 161. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, S. Antidiabetic Potential of Sinigrin Against Streptozotocin-Induced Diabetes via Modulating Inflammation and Oxidative Stress. Appl. Biochem. Biotechnol. 2022, 194, 4279–4291. [Google Scholar] [CrossRef]
- Kaiser, A.E.; Baniasadi, M.; Giansiracusa, D.; Giansiracusa, M.; Garcia, M.; Fryda, Z.; Wong, T.L.; Bishayee, A. Sulforaphane: A Broccoli Bioactive Phytocompound with Cancer Preventive Potential. Cancers 2021, 13, 4796. [Google Scholar] [CrossRef] [PubMed]
- Buffagni, V.; Vurro, F.; Janni, M.; Gullì, M.; Keller, A.A.; Marmiroli, N. Shaping Durum Wheat for the Future: Gene Expression Analyses and Metabolites Profiling Support the Contribution of BCAT Genes to Drought Stress Response. Front. Plant Sci. 2020, 11, 891. [Google Scholar] [CrossRef]
- Schuster, J.; Binder, S. The mitochondrial branched-chain aminotransferase (AtBCAT-1) is capable to initiate degradation of leucine, isoleucine and valine in almost all tissues in Arabidopsis thaliana. Plant Mol. Biol. 2005, 57, 241–254. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Dissing, M.M.; Agerbirk, N.; Crocoll, C.; Halkier, B.A. Characterization of Arabidopsis CYP79C1 and CYP79C2 by Glucosinolate Pathway Engineering in Nicotiana benthamiana Shows Substrate Specificity toward a Range of Aliphatic and Aromatic Amino Acids. Front. Plant Sci. 2020, 11, 57. [Google Scholar] [CrossRef] [PubMed]
- Essoh, A.P.; Monteiro, F.; Pena, A.R.; Pais, M.S.; Moura, M.; Romeiras, M.M. Exploring glucosinolates diversity in Brassicaceae: A genomic and chemical assessment for deciphering abiotic stress tolerance. Plant Physiol. Biochem. 2020, 150, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Nintemann, S.J.; Vik, D.; Svozil, J.; Bak, M.; Baerenfaller, K.; Burow, M.; Halkier, B.A. Unravelling Protein-Protein Interaction Networks Linked to Aliphatic and Indole Glucosinolate Biosynthetic Pathways in Arabidopsis. Front. Plant Sci. 2017, 8, 2028. [Google Scholar] [CrossRef] [PubMed]
- Ku, K.; Becker, T.M.; Juvik, J.A. Transcriptome and Metabolome Analyses of Glucosinolates in Two Broccoli Cultivars Following Jasmonate Treatment for the Induction of Glucosinolate Defense to Trichoplusia ni (Hübner). Int. J. Mol. Sci. 2016, 17, 1135. [Google Scholar] [CrossRef]
- Dadashipour, M.; Yamazaki, M.; Momonoi, K.; Tamura, K.; Fuhshuku, K.; Kanase, Y.; Uchimura, E.; Kaiyun, G.; Asano, Y. S-selective hydroxynitrile lyase from a plant Baliospermum montanum: Molecular characterization of recombinant enzyme. J. Biotechnol. 2011, 153, 100–110. [Google Scholar] [CrossRef]
- Dhara, A.; Raichaudhuri, A. ABCG transporter proteins with beneficial activity on plants. Phytochemistry 2021, 184, 112663. [Google Scholar] [CrossRef]
- Li, Z.; Ma, S.; Song, H.; Yang, Z.; Zhao, C.; Taylor, D.; Zhang, M. A 3-ketoacyl-CoA synthase 11 (KCS11) homolog from Malania oleifera synthesizes nervonic acid in plants rich in 11Z-eicosenoic acid. Tree Physiol. 2021, 41, 331–342. [Google Scholar] [CrossRef]
| Peak Area | ||||||||
|---|---|---|---|---|---|---|---|---|
| Number | Compounds | Q1/Q3 (Da) | Formula | CAS | R | OLs | NL | P |
| 1 | Sinigrin | 358.03/97.00 | C10H17NO9S2 | 3952-98-5 | 70,107 | 38,764 | 71,359 | 29,282 |
| 2 | 1-Methylpropyl glucosinolate | 374.06/96.96 | C11H21NO9S2 | 499-24-1 | -- | 1,391,350 | -- | 3,741,625 |
| 3 | 2-Hydroxy-3-butenyl glucosinolate | 388.04/96.96 | C11H18NO10S2 | 585-95-5 | 47,790 | 42,879 | 34,745 | 21,328 |
| 4 | 3-Methylbutyl glucosinolate | 388.07/96.96 | C12H23NO9S2 | 76265-22-0 | 442,681 | 849,380 | 2,954,000 | 1,790,425 |
| 5 | 4-Methylamyl Glucosinolate | 402.09/96.96 | C13H25NO9S2 | NR | 71,073 | 412,570 | 125,015 | 114,490 |
| 6 | 3-Methylsulfinylpropyl glucosinolate | 422.02/96.96 | C11H21NO10S3 | 554-88-1 | -- | 130,843 | 31,547 | 64,189 |
| 7 | 2-Phenylethyl glucosinolate | 422.06/96.96 | C15H21NO9S2 | 499-30-9 | -- | 119,572 | -- | 64,322 |
| 8 | Glucoraphanin | 436.04/96.96 | C12H23NO10S3 | 21414-41-5 | 19,852 | 18,207 | 23,126 | 19,318 |
| 9 | Glucocheirolin | 438.02/96.96 | C11H21NO11S3 | 15592-36-6 | 24,603 | 16,592 | 3254 | -- |
| 10 | m-Methoxybenzyl glucosinolate | 438.05/96.96 | C15H21NO10S2 | 111810-95-8 | -- | 607,625 | 157,487 | 252,432 |
| 11 | Indol-3-ylmethyl glucosinolate (glucobrassicin) | 447.05/96.96 | C16H20N2O9S2 | 4356-52-9 | 81,295,750 | 823,815 | 14,810,350 | 1,919,025 |
| 12 | 4-Methylsulfonyl-3-butenyl Glucosinolate | 450.02/96.96 | C12H20NO11S3- | NR | 30,813 | 13,232 | 22,992 | 7294 |
| 13 | 5-Methylsulfinylpentyl glucosinolate | 450.06/96.96 | C13H25NO10S3 | 499-37-6 | 1,019,337 | -- | 6292 | 14,157 |
| 14 | 6-Methylsulfinylhexyl Glucosinolate | 464.07/96.96 | C14H27NO10S3 | 33049-17-1 | 24,451,000 | 306,355 | 91,282,750 | 780,712 |
| 15 | Neoglucobrassicin | 477.06/96.96 | C17H22N2O10S2 | 5187-84-8 | 112,740,000 | 2,294,700 | 20,769,250 | 2,774,300 |
| 16 | 4-Methoxyglucobrassicin | 477.06/96.96 | C17H22N2O10S2 | 83327-21-3 | 115,092,500 | 2,199,975 | 19,990,250 | 2,528,200 |
| 17 | 6-(Methylsulfonyl)Hexyl Glucosinolate | 480.08/96.96 | C14H27NO11S3 | 74542-18-0 | 2,201,850 | 30,740 | 306,297 | 39,689 |
| 18 | 4-Benzoyloxybutyl glucosinolate | 494.08/96.96 | C18H24NO11S2 | NR | 1,734,900 | 7819 | -- | 34,278 |
| 19 | 1,4-Dimethoxyglucobrassicin | 507.08/476.06 | C18H24N2O11S2 | NR | 307,120 | 263 | 1150 | -- |
| Sum | 339,688,648 | 9,307,520 | 150,596,897 | 14,202,011 | ||||
| Database | Number of Genes | Percentage (%) |
|---|---|---|
| KEGG | 64,314 | 53.62 |
| Nr | 88,597 | 73.87 |
| SwissProt | 67,350 | 56.15 |
| TrEMBL | 88,360 | 73.67 |
| KOG | 53,817 | 44.87 |
| GO | 77,885 | 64.94 |
| Pfam | 60,211 | 50.2 |
| Annotated in at least one database | 90,233 | 75.23 |
| Total Unigenes | 119,939 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rao, S.; Gong, J.; Liu, H.; Liu, X.; Cheng, S.; Cheng, H.; Cong, X. Metabolome and Transcriptome Analyses Provide Insights into Glucosinolate Accumulation in the Novel Vegetable Crop Cardamine violifolia. Agronomy 2023, 13, 2760. https://doi.org/10.3390/agronomy13112760
Rao S, Gong J, Liu H, Liu X, Cheng S, Cheng H, Cong X. Metabolome and Transcriptome Analyses Provide Insights into Glucosinolate Accumulation in the Novel Vegetable Crop Cardamine violifolia. Agronomy. 2023; 13(11):2760. https://doi.org/10.3390/agronomy13112760
Chicago/Turabian StyleRao, Shen, Jue Gong, Haodong Liu, Xiaomeng Liu, Shuiyuan Cheng, Hua Cheng, and Xin Cong. 2023. "Metabolome and Transcriptome Analyses Provide Insights into Glucosinolate Accumulation in the Novel Vegetable Crop Cardamine violifolia" Agronomy 13, no. 11: 2760. https://doi.org/10.3390/agronomy13112760
APA StyleRao, S., Gong, J., Liu, H., Liu, X., Cheng, S., Cheng, H., & Cong, X. (2023). Metabolome and Transcriptome Analyses Provide Insights into Glucosinolate Accumulation in the Novel Vegetable Crop Cardamine violifolia. Agronomy, 13(11), 2760. https://doi.org/10.3390/agronomy13112760







