1. Introduction
Soil microorganisms play the most important role in the Earth’s ecosystems due to the high biogeochemical effect of their activity. In terms of global impact on the biosphere, the importance of soil microbiological processes can be compared only with photosynthesis [
1,
2,
3]. Soil microorganisms perform key functions in agriculture and ecosystems [
4,
5,
6].
The high activity and the huge scales of planetary transformations of substances made by microorganisms are caused by their huge number, widespread distribution, extraordinary growth rate and diversity of metabolic processes. Microorganisms have a high energy exchange and are the main agents of transformation of various chemical substances in the biosphere. Soil microbial communities directly influence soil functionality by participating in soil nutrient cycling and carbon storage [
7].
Soil microbiocenosis is a natural system, a complex set of microorganisms living in the soil. The way the system functions is the interrelationship of the soil and its microorganisms. They ensure the growth and development of plants in agrocenoses and contribute to the maintenance of biodiversity and functioning of ecosystems, provide nutrient cycles, fix nitrogen and participate in the formation of soil fertility.
Understanding the role and importance of soil microbiocenosis is important for the conservation of natural resources. Soil degradation caused by agriculture is a global and current problem associated with the loss of soil fertility. According to research by Alexandrovsky and Alexandrovskaya [
8] for approximately every 100 years 1 cm of humus horizon is formed, and recovery periods are also very long. Various types of agricultural activities (application of mineral fertilizers, chemicalization, irrigation, and soil cultivation systems) disturb the conditions necessary for the existence of microorganisms in the soil. The functioning of soil microbiota is disturbed under the influence of erosion, over-compaction, waterlogging, salinization and other degradation processes [
9]. The reaction of microorganisms depends on many factors: type and intensity of impact, type of microorganism, soil properties and soil regimes [
3]. The reaction of the microbial community to the use of soils in agriculture is expressed by a decrease in the number of microorganisms, a simplification of the structure of microbiocenosis, changes in species composition and dominant species, and indicators of their enzymatic activity. This indicates the possibility of deep disturbances of the initial state of soils and the formation of soils with properties atypical for the given landscape [
9]. Such transformation of microbiological properties is a dangerous phenomenon, since such changes will inevitably cause changes in the condition of plants and animals inhabiting the soil.
Soil enzymes are central to the natural environment because they catalyze innumerable biogeochemically important reactions in soils. Enzymatic activity is a multifactorial and multifunctional characteristic of soils [
10]. Enzymes in soils are products of the soil’s biocenosis metabolism, but the question of the participation of its various components in their accumulation is poorly studied.
Outperforming the functional links between the soil and the living organisms inhabiting it through mechanisms of substance–energy exchange, enzymes contribute to maintaining the integrity of biogeocenosis [
11,
12] and thus play an important role in soil cover and the characterization of its qualitative state [
13,
14,
15]. In general, the level of enzymatic activity characterizes the state of soil’s nutrition regime [
16].
Soil disturbance causes changes in the indicators of enzymatic activity. Enzymes are produced by all soil inhabitants, and especially actively by the soil microbial complex. Enzymes are a part of the lifetime excretions of all soil inhabitants and can also be transferred to the soil after their death. Preservation of enzymes in soil is facilitated by their adherence to soil particles. Enzymatic activity in soils, along with humus content and soil respiration indices, are considered to be the most informative diagnostic indicators.
The effect of the degradation of microbiological properties is different in different soils. The reaction of the soil microbial complex in response to agricultural use of soils goes through several stages or zones—homeostasis, stress, resistance and repression. The size of the homeostasis zone of a soil’s microbial mass can serve as a measure of soil resistance to a particular type of anthropogenic soil degradation. For example, in chernozems, the value of the homeostasis zone is an order of magnitude higher than in sod-podzolic soils [
1]. The state of the microbial complex is, on the one hand, an indicator of soil degradation and, on the other hand, an indicator of the soil’s recovery ability.
One of the determining factors of all soil biological processes in Transbaikalia is the sharp continental climate. Due to its remoteness from seas and oceans and location in the center of the Asian continent, Transbaikalia is the most continental region in our country. Low winter temperatures and air humidity lead to a loss of soil moisture by freezing and, thus, to changes in many soil processes. The coincidence of the periods of the highest moisture levels and the highest temperatures contributes to the activation of all biological processes in the soil within a short time.
There is evidence in the literature that microorganisms inhabiting extreme regions play an important role in regulating the climate and carbon balance of the Earth [
6]. The resistance of soil microbial communities to extreme climatic events has been studied, with special attention paid to drought. Reactions of soil microbial communities to extreme climatic events in space and time have been traced [
17]. The biomass, composition and activity of soil microbial communities are sensitive to climate impacts (reduced precipitation and warming) [
18]. Long-term drought stress can have a significant impact on the structure and activity of the soil microbial community. The significant impact of long-term drought stress affects soil moisture content, microbial community composition, and enzymatic activity [
19]. These stressful conditions reduce microbial populations, disrupt microbial structure, and reduce microbial activity, including enzyme production and nutrient cycling, resulting in decreased soil fertility. Despite the negative aspects of changes caused by drought, such harsh environmental conditions can cause adaptations in microbes and plants that allow them to survive and reproduce [
20,
21].
The spatial fragmentation of permafrost changes the hydrothermal regime and physicochemical properties of soils that control microbial activity responsible for organic matter decomposition. Due to a warming climate, there is a need to identify changes in microbial community and diversity to predict changes in soil function along the permafrost degradation gradient [
22].
Permafrost is melting at unprecedented rates, significantly altering landscapes and ecosystem trajectories by changing subsurface conditions, vegetation characteristics and soil properties. Hibernating microbes become active as temperatures rise and permafrost warms and thaws. Shotgun metagenome analysis showed how microbial communities and their potential functions vary with location and incubation temperature. This indicates different responses of permafrost microbes depending on their origin. These results have important implications for developing accurate predictions of microbial community accumulations during thawing at this location, which should be considered as a strong influencing factor [
23].
Soil microorganisms represent an important element in the response of climate change to agriculture through various cycles of nutrient substances and soil carbon sequestration [
24].
Thus, the extreme natural conditions of cryogenic ecosystems determine the peculiar composition and structure of microbiocenosis, increasing the vulnerability of microbiota, while promoting their adaptability to changes in conditions. Microbial biomass, its activity and the diversity of the microbial community are indicators of changes in the soil environment [
25]. Biological processes in them take place in a short growing season against the background of permafrost. The cryogenic character of soils imposes an imprint on the structure of microbial complexes, determines their dynamics and activity, thus determining the specificity of the process of substance transformation. Therefore, the analysis of soil microbiota of permafrost soils is relevant.
Publications on changes in the structure of microbiocenosis in the soils of Transbaikalia have studied these topics fragmentarily [
26,
27]. The aim of this study is to investigate the number of the main groups of microorganisms in microbial complexes, the carbon of microbial biomass and the enzymatic activity of permafrost soils under the influence of soil tillage. The obtained data can give an idea of microbiome stability in the soils of the region under agricultural impact.
The significance of the study lies in obtaining new data on the microbiocenosis of permafrost soils, which will make it possible to reveal the orientation and intensity of microbiological processes during their agricultural use.
2. Materials and Methods
2.1. Experimental Points
The objects of the study were virgin and arable grey forest non-podzolised soils (Greyic Phaeozems), permafrost meadow chernozemic soils (Turbic Chernozem Molliglossic), permafrost meadow soils (Gleyic Phaeozems) in the south of the Vitim Plateau and meal-carbonated chernozems (Haplic Chernozems Hypocalcic) in the Selenga mid-mountains of Western Transbaikalia (Russian Federation) (
Figure 1). The soils were classified according to Egorov and co-authors [
28], Shishov and co-authors [
29] and IUSS Working Group WRB [
30].
It is known that soil microorganisms are sensitive to their microenvironment. The structure and functions of their communities change depending on environmental conditions [
31], so we took soils with contrasting conditions of moisture and temperature as objects.
2.2. Soil Sampling
Samples were taken in different dynamics—May, July and September. Two variants of each soil type were selected for the study: virgin land and arable land. In grey forest non-podzolised soil, a variant of forest was additionally taken. On the studied arable soils, traditional tillage with a 20 cm deep moldboard was applied annually. Soil samples for analysis were taken from upper organogenic horizons: depth of 5–20 cm on virgin land and 0–20 cm on the arable variant (number of replicates n = 3). Soil samples were air-dried, pulverized and passed through a 2 mm sieve.
2.3. Analyses
Soil organic carbon (SOC, %) content was determined using an autoanalyzer SNC-100 (Scalar, Tokyo, Japan) [
32].
The total number of microorganisms was determined in situ using the luminescence microscopy method [
33]. When quantifying the cells of soil bacteria and mycelium of actinomycetes, preparations were stained with aqueous acridine orange solution, and calcofluor white was used to stain the mycelium of fungi. The number of cells (mycelium) per 1 g of soil was calculated using the formula:
N =
S1an/vS2c, where:
N is the number of cells (mycelium length, µm) in 1 g of soil;
S1 is the area of the preparation (µm
2);
a is the number of cells (mycelium length, µm) in one field of view (averaging was carried out over all preparations);
n is the dilution index of the soil suspension (mL);
v the volume of a drop applied to the glass (mL);
S2 is the microscope field of view area (μm
2);
c is the soil weight (g) [
34,
35].
Catalase was determined using the gasometric method modified by V.F. Kuprevich [
36]. The analysis procedure was as follows: a sample (1 g) of soil is placed into a 100 mL flask and 0.5 g of calcium carbonate is added. At the bottom of the flask, a small beaker with 5 mL of 3% hydrogen peroxide solution was placed carefully using tweezers. The flask was tightly closed with a rubber stopper with a glass tube, which was connected to the measuring burette with a thick-walled rubber hose through a tee. The two burettes were connected to the equalizing pear-shaped funnel with a rubber hose through a tee. The burettes and the pear were filled with water. The water level in the burettes and the pear was balanced by lowering or raising the pear, the latter was fixed at a certain height. Then, the tee was closed so as to eliminate the communication of the device with the external environment. It was necessary to keep the water level in the burettes at a certain level, which indicated the achievement of temperature equilibrium in the device. The experiment was carried out at a temperature of 18–20 °C. The beginning of the experiment was marked by a stopwatch at the moment when the vessel with hydrogen peroxide was tipped over and the contents of the flask were shaken. Shaking of the mixture was carried out during the whole experiment without touching the flask with hands. The released oxygen was displaced from the burettes into water after 3 min.
Invertase was determined using the method based on the copper reduction reaction modified by A.Sh. Galstyan [
36]. The analysis procedure was as follows: 5 g of soil was placed in a 50-mL flask, 0.5 mL of toluene, 10 mL of acetate buffer (pH = 4.7) and 10 mL of 5% sucrose solution were added. The flask was closed with a cork stopper, shaken and placed in a thermostat for 24 h at 30 °C. After incubation the contents of the flask were filtered into a 100 mL measuring flask and the volume was brought to the mark after two or three times washing the soil on the filter with water. In 20 mL of the filtrate reducing sugars were determined according to Bertrand. To do this, 10 mL of filtrate was transferred into a beaker with a capacity of 100 mL and immediately added 20 mL of Felling’s solution. The contents of the beaker were boiled (weakly) for 3 min, removed from the stove and left for 1 min at rest, leaving a red precipitate of copper oxide precipitates. The solution was then filtered into a Bunsen flask through an Allin tube with an asbestos filter, using a water-jet pump. The precipitate in the beaker and filter was washed by decanting with hot water until the reaction for sulfate ions was stopped. After washing, the filtrate was poured off and the Bunsen flask was rinsed thoroughly. The precipitate in the beaker and on the filter was dissolved iron-ammonium alum, acidified with sulfuric acid and passed through the filter. The beaker and filter were washed with hot water several times. The still hot solution was placed in a Bunsen flask titrated with 0.1 n. potassium permanganate to a faint pinking. To calculate the content of sugars in the volume of solution taken for analysis, the amount of permanganate was multiplied by 6.36 (1 mL of 0.1 n. KMnO
4 is equivalent to 6.36 mg Cu
2O). Invertase activity is calculated using the formula: X = Aν/Htν1, where A—amount of glucose (mg) taken for analysis; H—suspension (grams); ν—total volume of filtrate; t—incubation time (hour); ν1—volume of filtrate taken for analysis (ml). Controls were made with sterile soil (heated at 180 °C for one hour) and for the purity of the reagents. Invertase activity is expressed in milligrams of glucose per 1 g of soil for 24 h.
Determination of microbial biomass carbon (C-biomass) in soil was carried out using rehydration method according to T.G. Mirchink and N.S. Panikov [
37]. The analysis procedure was as follows: 5 g of soil was placed in a 50 mL conical flask and left in the thermostat at 65–70 °C for 24 h, then a soil extract of 0.5 M K
2S0
4 was prepared, with the ratio of soil:solution 1:5—for soils with organic matter content less than 3%, 1:2—for soils with organic matter content more than 3%. The suspension was shaken on a rotator for 30 min, centrifuged and in the supernatant the carbon content of organic matter was determined by bichromate oxidation method. For this purpose, 1.6 mL of transparent extract was mixed with 2.4 mL of chromium mixture in test tubes (6 g K
2Cr
20
7 was dissolved in 400 mL of distilled water, then in heat-resistant dishes 2 L of concentrated sulfuric acid was carefully added along the wall). The tubes were placed in a thermostat at 140 °C for 20 min, cooled and spectrophotometrically analyzed at 590 nm. Glucose or sucrose solutions in the concentration range of 100–500 mg C/L were used as standards. In parallel, a control determination was made with soil without drying. The amount of carbon of microbial biomass is calculated according to the formula: C mg/g soil = (C
1 − C
2)/0.3, where C
1 and C
2 are the amount of carbon in the soil extract after drying and in the fresh sample (mg of soil); 0.3 is a conversion factor equal to the proportion of cellular components solubilized after rehydration.
Soil temperature was determined using the loggers-TCR-0-U complex with a DS 1921 logger. Soil moisture was determined using the Decagon portable device with a 5tm sensor.
2.4. Calculations and Statistical Analysis
The Microsoft Excel and Statistica 12 programs were used for statistical processing of the data.
2.5. Conditions for the Formation of Microbiocenosis
Deep freezing of the soil profile and the long stay of soils in the frozen state, and rapid and significant drying of soils in the spring and summer seasons affect the soil biota of the studied soils. Permafrost in soil formation processes is a subfactor among the main factors of soil formation. It influences all factors of the soil formation process, changing soil properties and regimes.
Soil ecosystems consist of complex interactions between biological communities and physicochemical variables that contribute to the overall quality of soils [
4].
Table 1 below summarizes the main characteristics of grey forest non-podzolised soil (Greyic Phaeozems), permafrost meadow chernozemic (Turbic Chernozem Molliglossic), permafrost meadow soil (Gleyic Phaeozems) and chernozem meal-carbonated (Haplic Chernozem Hypocalcic).
One of the distinctive features of the ecological conditions of microbial cenosis development in Turbic Chernozem Molliglossic and Gleyic Phaeozems is low soil temperature. These soils are located at the southern boundary of permafrost distribution, which determines a spatially close combination of permafrost and non-permafrost areas. According to N.L. Melnichuk [
38], the thickness of permafrost rocks in the southern part of the Vitimsky Plateau reaches 100 m, the distribution is mostly continuous. The temperature of rocks at a depth of 12–14 m is −1.2–1.7 °C. The temperature of the lower part of the soil profile (290–300 cm) during the whole vegetation period is −0.1–0.2 °C. As the soil thaws from above, the temperature of its lower layers increases from −5.8 °C (early April) to −0.1 °C (August) [
39]. Multiyear permafrost in contact with the soil has a cooling effect not only on its lower part, but also on the entire profile. Therefore, during summer, the biologically active temperature drops along the soil profile only up to 60–80 cm from the surface, and the underlying soil layers at a depth of 1.5–2.5 m with a maximum thawing depth of 2.8–2.9 m remain permanently cold, due to which microbiological processes are inhibited in them.
The conditions of microbial cenosis functioning in grey forest non-podzolised soil (Greyic Phaeozems) and chernozem meal-carbonated (Haplic Chernozem Hypocalcic) are characterized by the same sharply continental soil climate, but unlike permafrost meadow chernozemic soils, there is no permanent permafrost in the soil profile affecting the soil thermal regime; these soils are long-seasonally permafrost. Despite the prolonged frozen state, these soils warm up faster and earlier, which increases possible periods of active microbiological activity. Due to frequent soil desiccation in meal-carbonated chernozems, moisture conditions are the limiting factor of microbiological processes. In grey forest non-podzolised soil, rather high soil skeletonization was observed, which leads to a decrease in the content of fine-grained soils, reduces the stock of nutrients and productive moisture.
In general, the conditions for microorganism development in these soils are favorable. The considered soils have rather high potential fertility, therefore the study of microbiocenosis of these soils is very important for agricultural industry.
Thus, the soils of Transbaikalia are a clear example of extreme conditions for microorganisms. The majority of bacteria in the studied soils are in a quiescent state, capable of withstanding long periods of starvation and drying. When favorable conditions associated with precipitation and an influx of plant residues into the soil occur, the cells apparently germinate and begin to actively multiply in order to ensure the fulfillment of their function in the ecosystem.
3. Results and Discussion
3.1. Hydrothermal Conditions of Soils
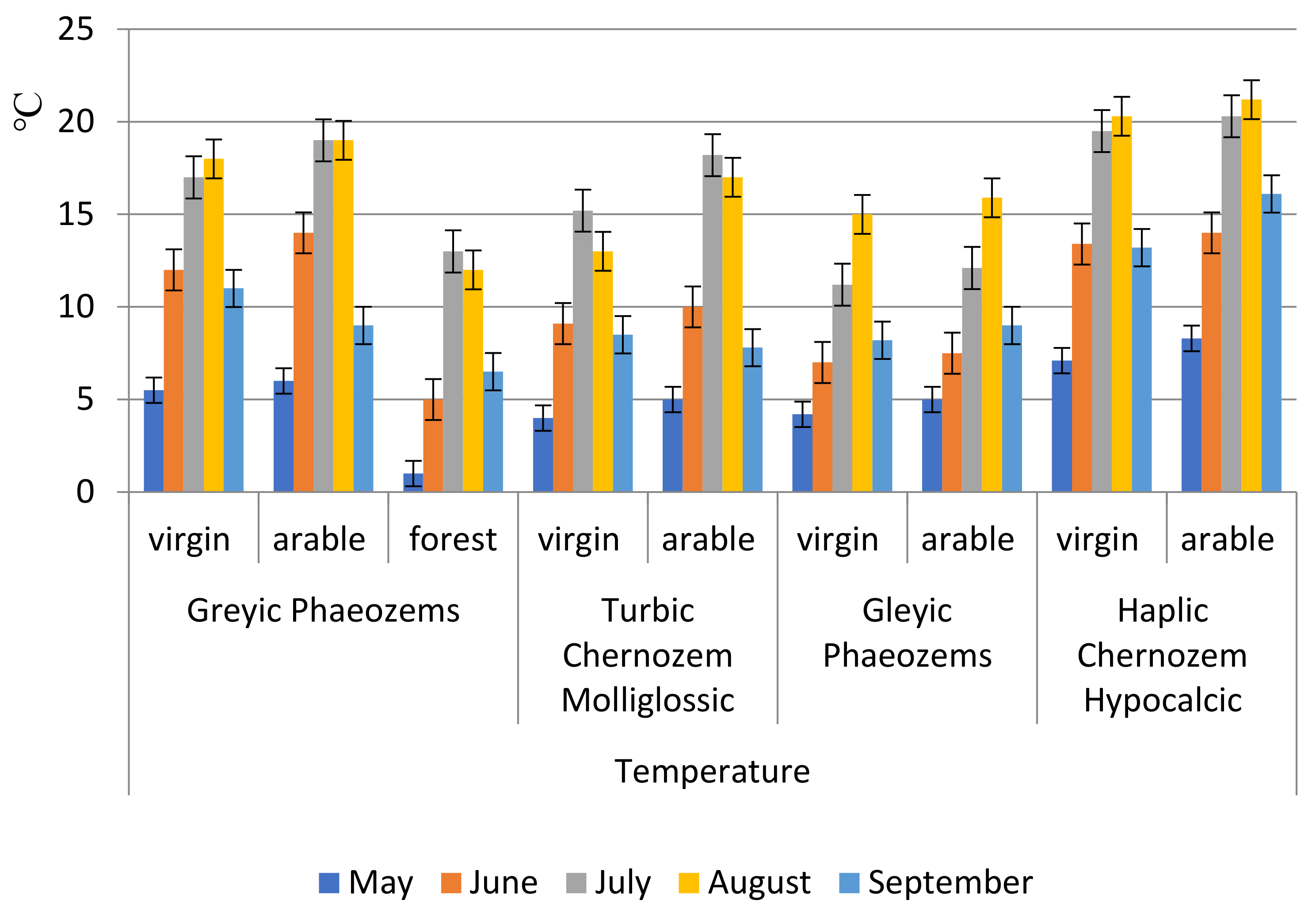
The hydrothermal conditions of permafrost meadow chernozemic soils and permafrost meadow soil as important components of the ecological conditions for the development of soil microorganisms are characterized by the following features. Maximum warming of the upper soil layers (0–20 cm) to 15–18 °C for a short time does not fully provide the optimal conditions for microorganism development (
Figure 2). Meal-carbonated chernozems warm up best of all. Arable variants warm up better than virgin ones.
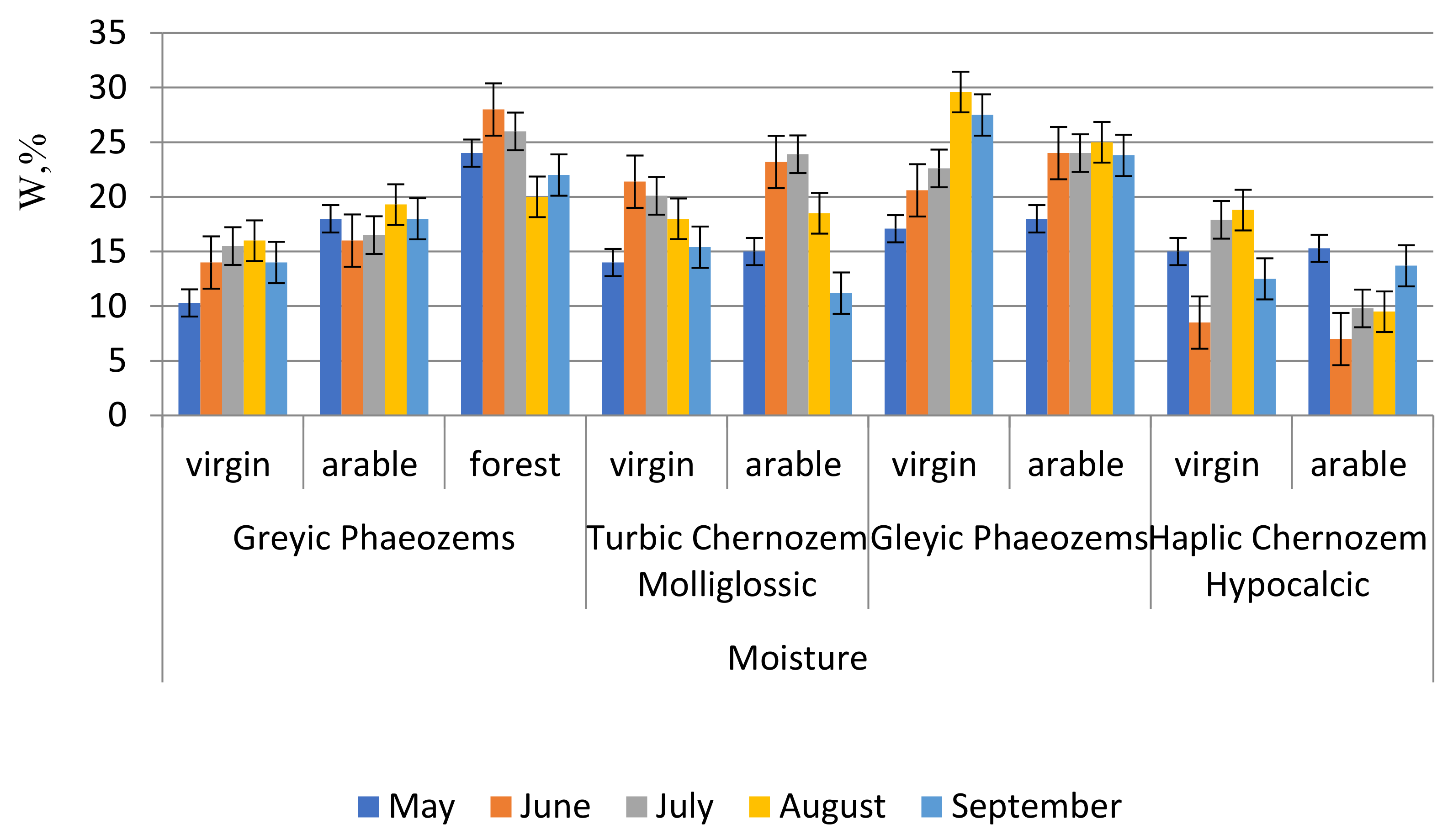
Soil moisture varies widely (
Figure 3). When large amounts of precipitation fall, anaerobic conditions are created. In the first half of the summer, the soil dries up despite the presence of permanent permafrost. In grey forest non-podzolised soils, moisture increases in the following order: virgin land–arable land–forest. Moisture conditions are low in meal-carbonated chernozems compared to the soils of the Yeravninskaya Basin. In all arable variants the soil moisture is higher than on virgin land, except for meal-carbonated chernozems. The latter is explained by the dry climate.
3.2. Microbial Biomass Carbon
Microbial biomass carbon is the most active and dynamic part of soil organic matter, which usually does not exceed 3–5% of the soil organic carbon content [
40,
41]. Microbial biomass carbon can be used as an indicator for assessing ecosystem productivity and as an early indicator of changes in soil organic matter [
41]. The C-biomass/SOC ratio serves as an indicator of soil organic carbon availability [
25,
42].
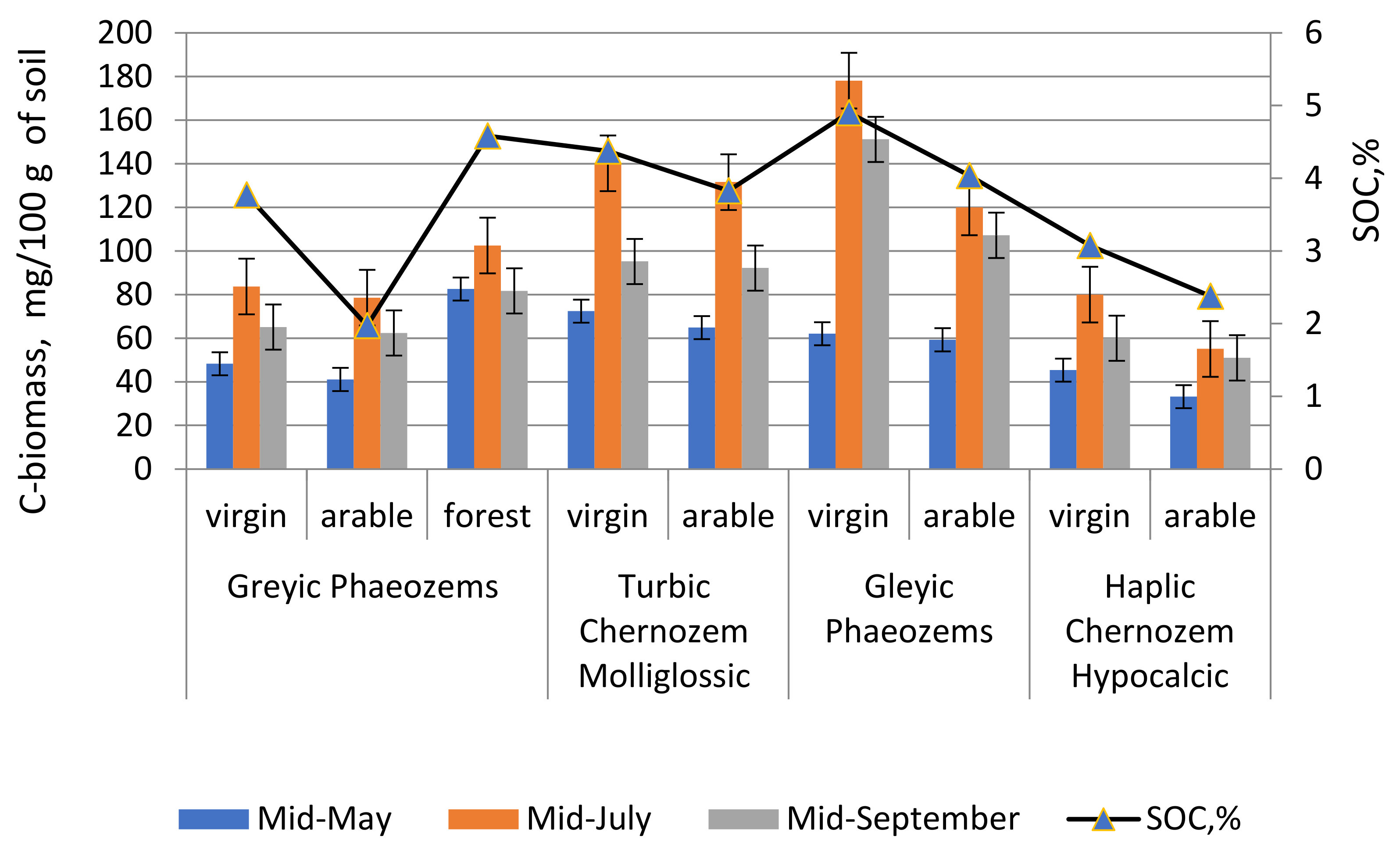
Figure 4 shows the data of microbial biomass carbon (on the main axis) and SOC values, % (on the auxiliary axis). From here we can see that, at the beginning of the growing season, the carbon index of microbial biomass at all experimental sites was low, which is due to deep soil freezing and slow spring warming.
With increasing soil temperature and after precipitation, the C-biomass index gradually increases at all experimental sites. The maximum accumulation of C-biomass was observed in semi-hydromorphic soils with sufficient moisture and humus resource, i.e., permafrost meadow chernozemic soils and permafrost meadow soils.
It is noted that in arable variants, C-biomasses are lower compared to virgin variants, which is explained by the humus resource.
Mealy carbonate black soil is located in more arid conditions than at other locations, here the lack of precipitation combined with a high air temperature led to a moisture deficit in soils, as a result of which low values of microbiological activity 55–80 mg/100 g are observed in the soil (
Figure 4).
During this period, the moisture content of the upper soil layers increases at the experimental sites, which caused a rise in C-biomass indicators.
In September, there is a decline in C-biomass indicators, which is associated with the fading of biological processes in the soil. The microbial biomass carbon gradually decreases at all experimental points.
The maximum amount of microbial biomass carbon was found in permafrost meadow soil—178.1 ± 32.30 mg/100 g of soil, which is explained by the abundance of rhizosphere microflora and a high SOC content. In permafrost meadow chernozemic soils, the average value of C-biomass in the upper soil layer was 140.2 ± 25.44 (V = 17.64%).
The accumulation of C-biomass in meal-carbonated chernozems is lower, despite better warming conditions, but here moisture is a limiting factor. C-biomass decreases insignificantly with agricultural use of soils.
Calculation of the coefficients of variation showed that the data set is homogeneous, i.e., significant. Microbial biomass carbon data have a strong correlation with the data for soil temperature and moisture (r = 0.75–0.99).
For comparison, in the chestnut soils of the region the indicators of C-biomass accumulation are low: on virgin land—32.2–37.6; on arable land—27.5–33.5 mg/100 g of soil [
43].
3.3. The Structure of Microbial Cenosis
3.3.1. Bacteria
Bacteria account for 37.8% of the microbial cenosis. The limiting factor of development is low soil moisture. The small specific weight of bacteria in the microflora is the result of the antagonistic effects of widespread actinomycetes. Intensive development of the latter is caused by their ability to adapt to low humidity and high physiological activity.
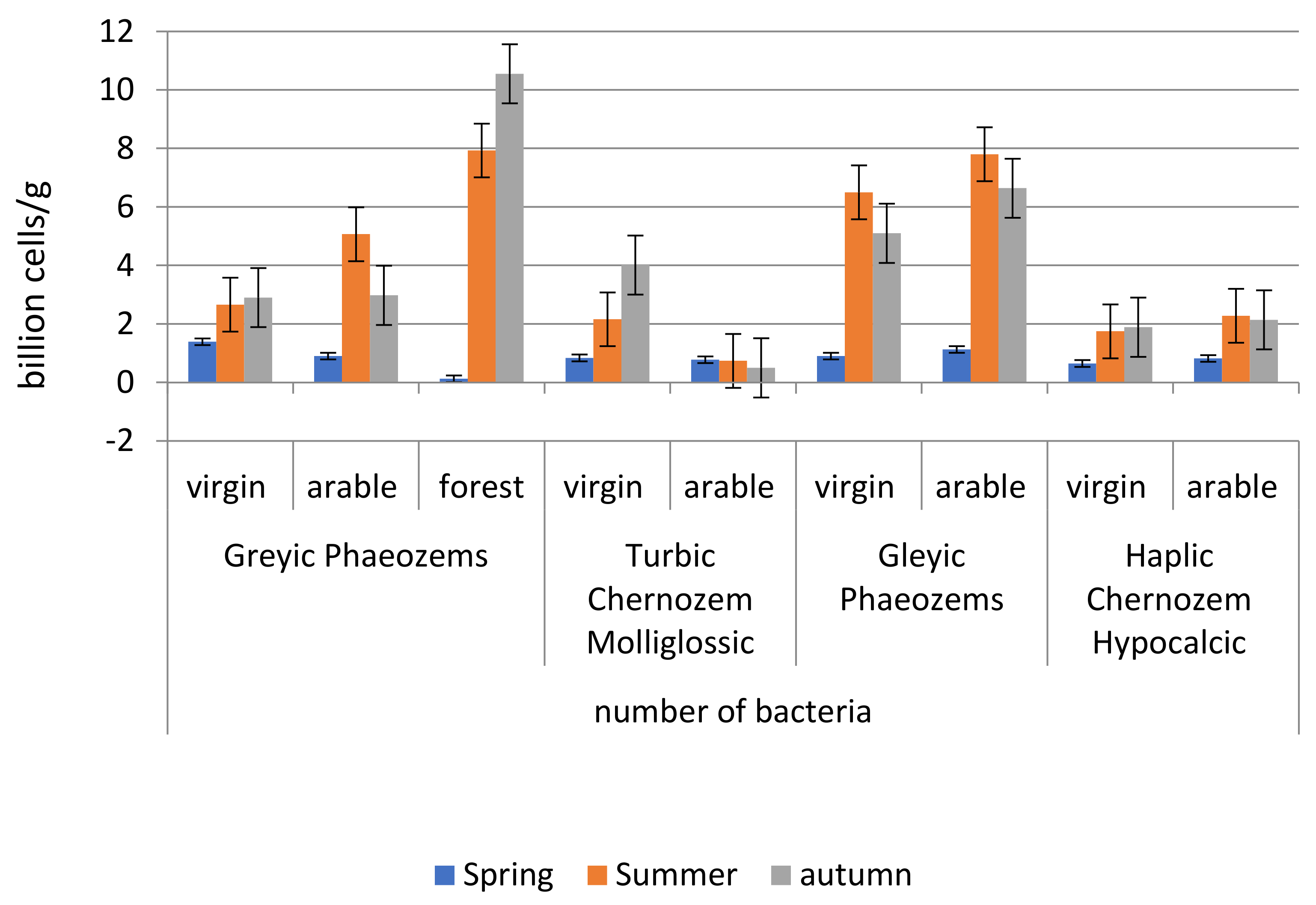
In spring, the number of bacteria is minimal compared to the summer and fall periods. The maximum number of bacteria in this period was found in virgin grey forest non-podzolised soils—1.4 billion cells/g (
Figure 5).
The summer period is characterized by an increase in bacterial abundance with the maximum reached in grey forest non-podzolised soils (arable land, forest) and on permafrost meadow soils (virgin land, arable land).
In the autumn period, the maximum number of bacteria was detected in grey forest non-podzolised soil, and the indicator decreases in the following order: forest—10.6 billion cells/g; arable land—5.1 billion cells/g; virgin lands—2.6 billion cells/g. The number of bacteria in permafrost meadow soils is also high and amounts to: on virgin land—5.1 billion cells/g; on arable land—6.6 billion cells/g. The lowest indicator of bacterial abundance (0.5 billion cells/g) was found in arable permafrost meadow chernozemic soils. Meal-carbonated chernozem was characterized by low bacterial content: on virgin land—1.9 billion cells/g; on arable land—2.1 billion cells/g.
The results of the study show that, in the structure of microbial cenosis, bacteria are predominant in grey forest non-podzolised soils and in permafrost meadow soils (
Figure 5). Observation of the dynamics of bacterial abundance showed that their maximum number was observed in the summer–autumn period.
During transformation of virgin soils into arable soils, the stocks and structure of microbiocenosis undergo change. This is due to the fact that, in addition to changes in physical conditions during tillage, improvement of the pore space structure, degree of watering and aeration in arable soil, the amplitude of daily, monthly and annual fluctuations of temperature and humidity expands.
The calculation of the correlation between the number of bacteria and temperature at all points is high (r = 0.89–0.99), the exception being permafrost meadow chernozemic soils (r = 0.19–0.30). The correlation of bacterial abundance with moisture and SOC of the studied soils is mainly high (r = 0.63–0.99).
The coefficient of variation of bacteria by seasons of the year in grey forest non-podzolised soils is uneven: virgin land—35%, arable land—69%, and forest—87%. In meal-carbonated chernozems the index is equal to: on virgin land—47% and on arable land—46%. In permafrost meadow chernozemic soils: on virgin land—68% and on arable land—22%, and in permafrost meadow soils: on virgin land—69% and on arable land—68%. According to the high percentage of variability, it can be concluded that this indicator is dynamic according to season.
3.3.2. Actinomycetes
Actinomycetes are filamentous chemoorganotrophic bacteria that form asexual spores. Actinomycetes are an integral part of the soil microbial complex, accounting for one quarter of the total number of bacteria [
44]. Actinomycete mycelium in soil, determined by luminescence microscopy, accounts for 5–15% of the total bacterial biomass [
45]. Most of them are capable of forming branching mycelium similar to fungal mycelium, but 5–7 times thinner. Actinomycetes are capable of being more successful, compared to other bacteria, in terms of their exploration of space and extending nutrient-deprived zones. Populations of these bacteria are usually larger in rhizosphere than in non-rhizosphere soil.
In each type of ecosystem, there are specific foci that are habitats of peculiar microorganisms that are practically undetectable in background substrates [
33].
The content of actinomycetes is dynamic. The low number of actinomycetes in arable soils is probably related to the reduction of organic residue inflow into the soil and the uniformity of biochemical composition of cultivated plants compared to virgin grasses. During the transformation of virgin soils into arable soils, the specific share of actinomycetes in the microbiocenosis decreases. In agrohumus hydrometamorphic permafrost soils, the share of actinomycetes is higher, which is probably associated with the inflow of plant residues into the soil, which is often accompanied by an increase in the number of actinomycetes. The maximum length of actinomycete mycelium was observed in grey forest non-podzolised soil (forest) in the autumn period—830 m/g (
Figure 6).
The wide distribution of actinomycetes in the studied soils is explained by the high adaptability of these microorganism species to the harsh soil climate. Despite the irregularity of hydrothermal indicators of the season, the spread of actinomycetes is constantly observed.
The high index of actinomycete mycelium length was observed in meal-carbonated chernozems, which reflects the evolution of these chernozems in xerophytic conditions and predetermines the specificity of organic matter mineralization towards deep mineralization. That is, to the final products, shifting the balance of mineralization—humification towards mineralization. The latter is partly explained by the low humus content of the steppe soils of the region with their usual biological productivity.
The correlation coefficients of actinomycetes are: (a) with temperature high r = 0.51–0.99, except for the arable variant and forest on grey forest non-podzolised soils; (b) with soil moisture high r = 0.73–0.95; and (c) with SOC content they are different: in grey forest non-podzolised soils the index is r = 0.61. In meal-carbonated chernozems the index is equal to r = 0.57. In permafrost meadow chernozemic soils r = 0.38 and in permafrost meadow soils r = 0.63.
The coefficient of variation of actinomycetes by season in grey forest non-podzolised soils is equal to: on virgin land—38%, on arable land—40%, and in forest—65%. In meal-carbonated chernozems the indicator is equal to: on virgin land—29% and on arable land—9%. In permafrost meadow chernozemic soils it equals: on virgin land—66% and on arable land—82%, and in permafrost meadow soils it equals: on virgin land—41% and on arable land—58%. Such a wide range of the coefficient of variation indicates the dynamics of the indicator according to the season.
3.3.3. Fungi
According to modern ideas among soil inhabitants, it is fungi that create the largest microbial biomass. They can account for 50–90% of the total biomass of microorganisms in soils. Fungi persist in soils in the form of a pool of spores, and under favorable conditions, spores germinate by growth tubes and there is an active development of mycelium. It is the mycelial type of structure, the development of fungal colonies in the form of repetitive mycelial modules, that allows fungi to actively spread in soil, colonizing various organic substrates [
46].
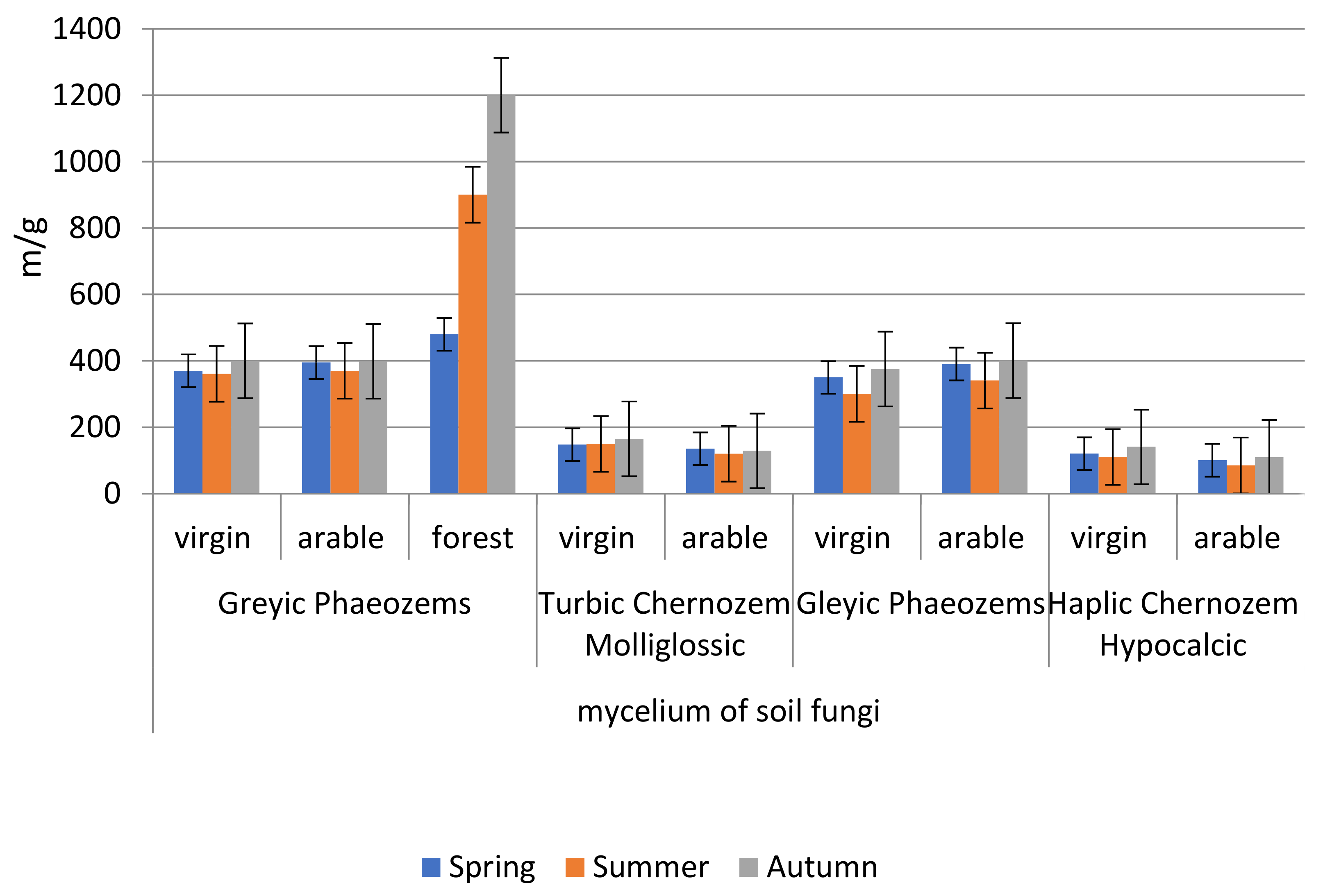
The maximum length of fungal mycelium was observed in grey forest non-podzolised (forest)—1200 m/g (
Figure 7). In arable variants, the length of fungal mycelium is high in grey forest non-podzolised soils and meadow permafrost soils. In chernozems and permafrost meadow chernozemic soils (virgin, arable) the indicator is much lower.
The correlation coefficients of fungal mycelium length: (a) with temperature on virgin soils are lower as r = 0.25–0.33 than on arable soils where r = 0.49–0.97; (b) with soil moisture they are high as r = 0.74–0.99 and (c) with SOC content they play no general regularity role as r = 0.10–0.99.
The coefficient of variation of mushroom mycelium length by season in all of the studied points is small, ranging from 4 to 12.8%; the exception is grey forest non-podzolised soil-forest, here the variability is 42%.
3.4. Enzymatic Activity in Soils
The catalase and invertase activities were studied in the soils we investigated. The choice of these enzymes is conditioned by two circumstances: their high sensitivity to external influences and the importance of organic matter transformation.
3.4.1. Catalase Activity in Soils
Among the redox enzymes, catalase, which destroys hydrogen peroxide poisonous to living organisms, is widespread in soils. Catalase is not only an intracellular enzyme, it is actively released by microorganisms into the environment, has high stability and can be accumulated and preserved in the soil for a long time. Some researchers recommend using catalase for preliminary assessment of soil activity and fertility.
The peculiarity of catalase activity is that it changes little down the profile, has an inverse dependence on soil moisture and a direct dependence on temperature [
47]. Catalase is produced by microorganisms in order to reduce the content of hydrogen peroxide, a product of microbial activity, in their environment; therefore, the distribution of catalase in soil layers characterizes either the distribution of microorganisms in the soil or the intensity of catalase transfer through the soil when microorganisms are located in the upper soil layer [
48].
Comparatively high activity of catalase was found by Nimaeva S.Sh. [
26], which smoothly decreases with depth. In virgin soils, the highest catalase activity is observed in the upper 0–20 cm layer—7.6–8.3 mL O
2 (1 g 3 min). In the arable layer, catalase activity varies within the 5.3–9.1 mL range, slightly decreasing in the transition horizon A/B and increasing again in the horizon C at a depth of 140–160 cm to values exceeding those of the arable layer. High catalase activity along the profile is partly connected with the chemical cleavage of hydrogen peroxide by iron and manganese compounds contained in the soil, because in the lower horizons of the studied soils the level of microorganisms and organic matter is insignificant. Soils are characterized by relatively high catalase activity, which indicates the increased intensity of the redox processes in them.
According to our studies, the maximum catalase activity, equal to 9.47 mL O2 (1 g/3 min), was found in grey forest non-podzolised soil (virgin soil). The lowest catalase activity (4.0 mL O2 1 g/3 min) was found in grey forest non-podzolised soil (forest). Approximately the same amount of catalase was detected in meal-carbonated chernozems and in permafrost meadow soil (4.37 and 4.27 mL, respectively). In grey forest non-podzolised soil (arable land) the activity was 5.73 mL. In permafrost meadow chernozemic soils, the catalase activity was also found to be low, equal to 6.60 mL. In general, catalase activity ranged from 4.0 to 9.47 mL O2 (1 g/3 min).
Thus, as a result of our studies, a relatively low activity of catalase was found, which indicates a low intensity of oxidation-reduction processes in these soils.
3.4.2. Invertase Activity in Soils
Invertase is a representative of a group of enzymes that catalyze the hydrolysis of disaccharides and polysaccharides by glucoside bonds in their molecules [
49]. Invertase catalyzes the hydrolysis of sucrose to monosaccharides. Thus, invertase activity is a good indicator of the intensity of organic matter decomposition in soil [
50].
The activity of this enzyme is closely related to genetic and ecological features of soils and is often used for diagnostic purposes [
51]. Invertase activity is directly correlated with the amount and availability of organic matter in the soil. A close correlation has been established between humus content and invertase activity [
50,
51,
52,
53,
54].
The activity of soil enzymes is on average higher in the upper soil layers, since the surface has a higher organic matter content and greater availability of air. The activity of invertase reaches its maximum in the surface layer of soil, while further down the profile its activity decreases [
50,
54,
55], which corresponds to a decrease in the humus content and the quantity of microflora [
54].
Maximum invertase activity was found in permafrost grey forest non-podzolised (virgin land)—51.5 mg glucose/1 g soil. Equal indices of enzymatic activity were found in other samples of grey forest non-podzolised: arable land—39.83 mg and forest—39.5 mg. The minimum of activity is characteristic of meal-carbonated chernozems. Here the activity is 24.33 mg. In contrast to the activity of other enzymes, relatively high activity of invertase is found in all variants.
The reason for the high activity of invertase in the above soils may be the high content of mobile carbohydrates in the soil. Since invertase activity is closely related to the content of water-soluble carbohydrates in the soil, the reason for its higher activity is probably the enrichment of the latter by roots.
The activity of the studied enzymes (catalase and invertase) is subject to significant fluctuations. They depend on a set of environmental factors (soil temperature and humidity and fresh plant residue inflow into the soil) affecting enzyme activity. In virgin soils, activity peaks were observed in the spring and autumn, in arable soils—they were in the summer and autumn.
4. Conclusions
The use of soils in agriculture changes soil microbiocenosis. The peculiarity of the structure of microbial cenosis of the studied soils of the region under consideration is its instability—a large qualitative and quantitative variability over time with a large proportion of actinomycetes.
The structure of microbial cenosis of virgin soils is more stable in comparison with arable soils. On virgin soils microbiocenosis is formed and functions within the established natural ecological conditions and is in a stable equilibrium state.
The low number of actinomycetes in arable soils is probably due to a decrease in the inflow of organic residues into the soil and the monotonous biochemical composition of cultivated plants compared to virgin grasses.
The maximum amount of C-biomass was recorded in permafrost meadow chernozemic soils and permafrost meadow soils, which are rich in humus and have good moisture content. During agricultural use of soils, C-biomass slightly decreases, which is associated with the reduction of the humus resource.
Assessment of catalase activity showed that soils are characterized by a low intensity of redox processes. The studied soils are characterized by high activity of invertase, which indicates a sufficiently high intensity of organic matter decomposition in the soil.
The main predictors of microbiocenosis development are SOC content, soil temperature and humidity, which is confirmed by statistical analysis of the obtained data.