Preliminary Study on the Diversity of Soil Oribatid Mite (Acari: Oribatida) Community Reveals Both Longitudinal and Latitudinal Patterns in Paddy Fields along the Middle and Lower Reaches of Yangtze River, China
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Study Area and Sample Collection
2.3. Identification
2.4. Environmental Variable
2.5. Data Analysis
3. Results
3.1. Composition and Diversity of the Soil Oribatid Mite Community along MLYR
3.2. Correlation between Diversity and Geographical Variables
4. Discussion
4.1. Community Composition and Diversity along MLYR
4.2. Spatial Patterns of the Soil Oribatid Mite Community along MLYR
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Huang, C.; Xu, Y.; Zang, R. Variations in Functional Richness and Assembly Mechanisms of the Subtropical Evergreen Broadleaved Forest Communities along Geographical and Environmental Gradients. Forests 2022, 13, 1206. [Google Scholar] [CrossRef]
- Pyle, P.; Saracco, J.F.; DeSante, D.F. Evidence of widespread movements from breeding to molting grounds by North American landbirds. Am. Ornithol. Soc. 2018, 135, 506–520. [Google Scholar] [CrossRef]
- Maestri, R.; Patterson, B.D. Patterns of Species Richness and Turnover for the South American Rodent Fauna. PLoS ONE 2018, 11, e0151895. [Google Scholar] [CrossRef] [PubMed]
- Jego, L.; Li, R.; Roudine, S.; Ma, C.S.; Lann, C.L.; Ma, G.; Baaren, J.V. Parasitoid ecology along geographic gradients: Lessons for climate change studies. Insect Sci. 2023, 57, 101036. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Song, G.; Zhang, D.; Jia, C.; Fan, P.; Hao, Y.; Ji, Y.; Lei, F. Distribution pattern and driving factors of genetic diversity of passerine birds in the Mountains of Southwest China. Avian Res. 2022, 13, 100043. [Google Scholar] [CrossRef]
- Bose, A.K.; Scherrer, D.; Camarero, J.J.; Ziche, D.; Babst, F.; Bigler, C.; Bolte, A.; Dorado-Liñán, I.; Etzold, S.; Fonti, P.; et al. Climate sensitivity and drought seasonality determine post-drought growth recovery of Quercus petraea and Quercus robur in Europe. Sci. Total Environ. 2021, 784, 147222. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Shi, X.; Fan, P.; Zhou, X.; Sheng, J.; Zhang, Y. Relationship of species diversity between overstory trees and understory herbs along the environmental gradients in the Tianshan Wild Fruit Forests, Northwest China. J. Arid Land 2020, 12, 618–629. [Google Scholar] [CrossRef]
- Zhang, L.; Sun, P.; Huettmann, F.; Liu, S. Where should China practice forestry in a warming world? Glob. Change Biol. 2022, 28, 2461–2475. [Google Scholar] [CrossRef]
- Mumladze, L.; Murvanidze, M.; Maraun, M.; Salakaia, M. Oribatid mite communities along an elevational gradient in Sairme gorge (Caucasus). Exp. Appl. Acarol. 2015, 66, 41–51. [Google Scholar] [CrossRef]
- Raymond-Léonard, L.J.; Bouchard, M.; Handa, I.T. Dead wood provides habitat for springtails across a latitudinal gradient of forests in Quebec, Canada. For. Ecol. Manag. 2020, 472, 118237. [Google Scholar] [CrossRef]
- Lehmitz, R.; Russell, D.; Hohberg, K.; Christian, A.; Xylander, W.E.R. Active dispersal of oribatid mites into young soils. Appl. Soil Ecol. 2012, 55, 10–19. [Google Scholar] [CrossRef]
- Caruso, T.; Schaefer, I.; Monson, F.; Keith, A.M. Oribatid mites show how climate and latitudinal gradients in organic matter can drive large-scale biodiversity patterns of soil communities. J. Biogeogr. 2019, 46, 611–620. [Google Scholar] [CrossRef]
- Mumladze, L.; Murvanidze, M.; Behan-Pelletier, V. Compositional patterns in Holarctic peat bog inhabiting oribatid mite (Acari: Oribatida) communities. Pedobiologia 2013, 56, 41–48. [Google Scholar] [CrossRef]
- Dirilgen, T.; Arroyo, J.; Dimmers, W.; Faber, J.; Stone, D.; da Silva, P.M.; Carvalho, F.; Schmelz, R.; Griffiths, B.; Francisco, R. Mite community composition across a European transect and its relationships to variation in other components of soil biodiversity. Appl. Soil Ecol. 2016, 97, 86–97. [Google Scholar] [CrossRef]
- Wilschut, R.A.; Geisen, S.; Martens, H.; Kostenko, O.; de Hollander, M.; Ten Hooven, F.C.; Weser, C.; Snoek, L.B.; Bloem, J.; Caković, D. Latitudinal variation in soil nematode communities under climate warming-related range-expanding and native plants. Glob. Change Biol. 2019, 25, 2714–2726. [Google Scholar] [CrossRef] [PubMed]
- Slatyer, R.A.; Schoville, S.D. Physiological limits along an elevational gradient in a radiation of montane ground beetles. PLoS ONE 2016, 11, e0151959. [Google Scholar] [CrossRef] [PubMed]
- Baselga, A.; Lobo, J.M.; Svenning, J.C.; Aragón, P.; Araújo, M.B. Dispersal ability modulates the strength of the latitudinal richness gradient in European beetles. Glob. Ecol. Biogeogr. 2012, 21, 1106–1113. [Google Scholar] [CrossRef]
- González, G.; Garcia, E.; Cruz, V.; Borges, S.; Zalamea, M.; Rivera, M.M. Earthworm communities along an elevation gradient in Northeastern Puerto Rico. Eur. J. Soil Biol. 2007, 43, 24–32. [Google Scholar] [CrossRef]
- Gabriac, Q.; Ganault, P.; Barois, I.; Aranda-Delgado, E.; Cimetière, E.; Cortet, J.; Gautier, M.; Hedde, M.; Marchán, D.F.; Reyes, J.C.P. Environmental drivers of earthworm communities along an elevational gradient in the French Alps. Eur. J. Soil Biol. 2023, 116, 103477. [Google Scholar] [CrossRef]
- Dinca, L.; Onet, A.; Samuel, A.D.; Tognetti, R.; Uhl, E.; Bosela, M.; Gömöryová, E.; Bielak, K.; Skrzyszewski, J.; Hukic, E.; et al. Microbial soil biodiversity in beech forests of European mountains. Can. J. For. Res. 2021, 51, 1833–1845. [Google Scholar] [CrossRef]
- Fiera, C.; Ulrich, W. Spatial patterns in the distribution of European springtails (Hexapoda: Collembola). Biol. J. Linn. Soc. 2012, 105, 498–506. [Google Scholar] [CrossRef]
- Zaitsev, A.S.; Wolters, V. Geographic determinants of oribatid mite communities structure and diversity across Europe: A longitudinal perspective. Eur. J. Soil Biol. 2006, 42, 358–361. [Google Scholar] [CrossRef]
- Ulrich, W.; Fattorini, S. Longitudinal gradients in the phylogenetic community structure of European Tenebrionidae (Coleoptera) do not coincide with the major routes of postglacial colonization. Ecography 2013, 36, 1106–1116. [Google Scholar] [CrossRef]
- Vázquez, D.P.; Stevens, R.D. The latitudinal gradient in niche breadth: Concepts and evidence. Am. Nat. 2004, 164, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Callisto, M.; Goulart, M. Invertebrate drift along a longitudinal gradient in a Neotropical stream in Serra do Cipo’ National Park, Brazil. Hydrobiologia 2005, 539, 47–56. [Google Scholar] [CrossRef]
- Carroll, C.; Noss, R.F. Rewilding in the face of climate change. Conserv. Biol. 2020, 35, 155–167. [Google Scholar] [CrossRef] [PubMed]
- Procheş, Ş.; Watkeys, M.K.; Ramsay, L.F.; Cowling, R.M. Why we should be looking for longitudinal patterns in biodiversity. Front. Ecol. Evol. 2023, 11, 1032827. [Google Scholar] [CrossRef]
- Schatz, H.; Behan-Pelletier, V. Global diversity of oribatids (Oribatida: Acari: Arachnida). Hydrobiologia 2008, 595, 323–328. [Google Scholar] [CrossRef]
- Luptácik, P.; Miklisová, D.; Kovác, L. Diversity and community structure of soil Oribatida (Acari) in an arable field with alluvial soils. Eur. J. Soil Biol. 2012, 50, 97–105. [Google Scholar] [CrossRef]
- Gao, M.; Guo, Y.; Liu, J.; Liu, J.; Adl, S.; Wu, D.; Lu, T. Contrasting beta diversity of spiders, carabids, and ants at local and regional scales in a black soil region, northeast China. Soil Ecol. Lett. 2021, 3, 103–114. [Google Scholar] [CrossRef]
- Elyamine, A.M.; Moussa, M.G.; Ismael, M.A.; Wei, J.; Zhao, Y.; Wu, Y.; Hu, C. Earthworms, Rice Straw, and Plant Interactions Change the Organic Connections in Soil and Promote the Decontamination of Cadmium in Soil. Int. J. Environ. Res. Public Health 2018, 15, 2398. [Google Scholar] [CrossRef] [PubMed]
- Kishimoto-Yamada, K.; Minamiya, Y. Earthworm species and density in semi-natural grasslands on rice paddy levees in Japanese satoyama. Biodivers. Data J. 2020, 8, e56531. [Google Scholar] [CrossRef] [PubMed]
- Folgarait, P.J.; Thomas, F.; Desjardins, T.; Grimaldi, M.; Tayasu, I.; Curmi, P.; Lavelle, P.M. Soil properties and the macrofauna community in abandoned irrigated rice fields of northeastern Argentina. Biol. Fertil. Soils 2003, 38, 349–357. [Google Scholar] [CrossRef]
- Folgarait, P.J.; Gorosito, N.; Pizzio, R.; Rossi, J.P.; Fernández, J. Camponotus punctulatus ant’s demography: A temporal study across land-use types and spatial scales. Insectes Sociaux 2007, 54, 42–52. [Google Scholar] [CrossRef]
- Chang, L.; Wu, H.; Wu, D.; Sun, X. Effect of tillage and farming management on Collembola in marsh soils. Appl. Soil Ecol. 2013, 64, 112–117. [Google Scholar] [CrossRef]
- Bunyangha, J.; Muthumbi, A.W.; Gichuki, N.N.; Majaliwa, M.J.G.; Egeru, A. Soil Macroinvertebrate Response to Paddy Rice Farming Pathways in Mpologoma Catchment, Uganda. Agronomy 2022, 12, 312. [Google Scholar] [CrossRef]
- Wang, J.; Yang, H.; Gourley, J.; Adhikari, P.; Li, L.; Su, F. Quantitative assessment of climate change and human impacts on long-term hydrologic response: A case study in a sub-basin of the Yellow River, China. Int. J. Climatol. 2021, 30, 2130–2137. [Google Scholar] [CrossRef]
- Dagg, M.; Benner, R.; Lohrenz, S.; Lawrence, D. Transformation of dissolved and particulate materials on continental shelves influenced by large rivers: Plume processes. Cont. Shelf Res. 2004, 24, 833–858. [Google Scholar] [CrossRef]
- Xu, Z.; Hu, C.; Wang, X.; Wang, L.; Xing, J.; He, X.; Wang, Z.; Zhao, P. Distribution characteristics of plastic film residue in long-term mulched farmland soil. Soil Ecol. Lett. 2023, 5, 220144. [Google Scholar] [CrossRef]
- Wang, Z.; Shao, D.; Westerhoff, P. Wastewater discharge impact on drinking water sources along the Yangtze River (China). Sci. Total Environ. 2017, 599, 1399–1407. [Google Scholar] [CrossRef]
- Fan, K.; Wang, H.; Choi, Y.J. A physically-based statistical forecast model for the middle-lower reaches of the Yangtze River Valley summer rainfall. Chin. Sci. Bull. 2008, 53, 602–609. [Google Scholar] [CrossRef]
- Shen, S.; Yang, S.; Zhao, Y.; Xu, Y.; Zhao, X.; Wang, Z.; Liu, J.; Zhang, W. Simulating the rice yield change in the middle and lower reaches of the Yangtze River under SRES B2 scenario. Acta Ecol. Sin. 2011, 31, 40–48. [Google Scholar] [CrossRef]
- National Bureau of Statistic of China. China Statistical Yearbook; China Statistical Press: Beijing, China, 2022. [Google Scholar]
- Ding, Y.; Wang, W.; Song, R.; Shao, Q.; Jiao, X.; Xing, W. Modeling spatial and temporal variability of the impact of climate change on rice irrigation water requirements in the middle and lower reaches of the Yangtze River, China. Agric. Water Manag. 2017, 193, 89–101. [Google Scholar] [CrossRef]
- Zhan, L.; Li, X.; Lu, J.; Liao, Z.; Ren, T.; Cong, R. Potassium fixation and release characteristics of several normal and K-exhausted soils in the middle and lower reaches of the Yangtse River, China. Commun. Soil Sci. Plant Anal. 2014, 45, 2921–2931. [Google Scholar] [CrossRef]
- Xiong, X.; Wu, C.; Elser, J.J.; Mei, Z.; Hao, Y. Occurrence and fate of microplastic debris in middle and lower reaches of the Yangtze River–from inland to the sea. Sci. Total Environ. 2019, 659, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Yin, W.; Hu, S.; Shen, Y.; Ning, Y.; Sun, X.; Wu, J.; Zhu, G.; Zhang, Y.; Wang, M.; Chen, J.; et al. Pictorical Keys to Soil Animals of China; Science Press: Beijing, China, 1998. [Google Scholar]
- Raunkiaer, C. Life Forms of Plants and Statistical Plant Geography; The Clarendon Press: Oxford, UK, 1934. [Google Scholar]
- Gao, M.; Liu, D.; Lin, L.; Wu, D. The small-scale structure of a soil mite metacommunity. Eur. J. Soil Biol. 2016, 74, 69–75. [Google Scholar] [CrossRef]
- Condit, R.; Ashton, P.S.; Baker, P.; Bunyavejchewin, S.; Gunatilleke, S.; Gunatilleke, N.; Hubbell, S.P.; Foster, R.B.; Itoh, A.; LaFrankie, J.V.; et al. Spatial patterns in the distribution of tropical tree species. Science 2000, 288, 1414–1418. [Google Scholar] [CrossRef] [PubMed]
- Gergócs, V.; Hufnagel, L. Application of oribatid mites as indicators. Appl. Ecol. Environ. Res. 2009, 7, 79–98. [Google Scholar] [CrossRef]
- Aoki, J. Analysis of Oribatid Communities by Relative Abundance in the Species and Individual Numbers of the Three Major Groups (MGP-Analysis). Bull. Inst. Environ. Sci. Technol. Yokohama Natl. Univ. 1983, 10, 171–176. [Google Scholar]
- Shannon, C.E. A mathematical theory of communication. Bell Syst. Tech. J. 1948, 27, 379–423. [Google Scholar] [CrossRef]
- Simpson, E.H. Measurement of diversity. Nature 1949, 163, 688. [Google Scholar] [CrossRef]
- Pielou, E.C. The measurement of diversity in different types of biological collections. J. Theor. Biol. 1966, 13, 131–144. [Google Scholar] [CrossRef]
- Si, X.; Pimm, S.L.; Russell, G.J.; Ding, P. Turnover of breeding bird communities on islands in an inundated lake. J. Biogeogr. 2015, 41, 2283–2292. [Google Scholar] [CrossRef]
- Adler, D.; Kelly, S.T. Package ‘Vioplot’, R Package Version 0.4.0. 2005. Available online: https://cran.r-project.org/web/packages/vioplot/index.html (accessed on 30 September 2023).
- Oksanen, J.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’hara, R.; Simpson, G.L.; Solymos, P.; Stevens, M.H.H.; Wagner, H. Package ‘Vegan’, R Package Version 2.6-4. 2013. Available online: https://search.r-project.org/CRAN/refmans/vegan/html/vegan-deprecated.html (accessed on 30 September 2023).
- Paradis, E.; Blomberg, S.; Bolker, B.; Brown, J.; Claude, J.; Cuong, H.S. Desper, Package ‘Ape’, R Package Version 5.7. 2019. Available online: https://search.r-project.org/CRAN/refmans/aphylo/html/ape-methods.html (accessed on 30 September 2023).
- Fox, J.; Weisberg, S.; Adler, D.; Bates, D.; Baud-Bovy, G.; Ellison, S.; Firth, D.; Friendly, M.; Gorjanc, G.; Graves, S. Package ‘Car’, R Package Version 3.1-1. 2012. Available online: https://search.r-project.org/CRAN/refmans/car/html/car-deprecated.html (accessed on 30 September 2023).
- Pedersen, T.L. Package ‘Patchwork’, R package version 1.1.2. 2019. Available online: https://search.r-project.org/CRAN/refmans/patchwork/html/patchwork-package.html (accessed on 30 September 2023).
- Lai, J.; Zou, Y.; Zhang, S.; Zhang, X.; Mao, L. glmm. hp: An R package for computing individual effect of predictors in generalized linear mixed models. J. Plant Ecol. 2022, 15, 1302–1307. [Google Scholar] [CrossRef]
- Menta, C. Soil fauna diversity-function, soil degradation, biological indices, soil restoration. Biodivers. Conserv. Util. A Divers. World 2012, 59–94. [Google Scholar] [CrossRef]
- Knoepp, J.D.; Coleman, D.C.; Crossley, D.A., Jr.; Clark, J.S. Biological indices of soil quality: An ecosystem case study of their use. For. Ecol. Manag. 2000, 138, 357–368. [Google Scholar] [CrossRef]
- Roy, S.; Roy, M.M.; Jaiswal, A.K.; Baitha, A. Soil arthropods in maintaining soil health: Thrust areas for sugarcane production systems. Sugar Tech 2018, 20, 376–391. [Google Scholar] [CrossRef]
- Lavelle, P. Diversity of soil fauna and ecosystem function. Biol. Int. 1996, 33, 3–16. [Google Scholar]
- Cardoso, E.J.B.N.; Vasconcellos, R.L.F.; Bini, D.; Miyauchi, M.Y.H.; Santos, C.A.d.; Alves, P.R.L.; Paula, A.M.d.; Nakatani, A.S.; Pereira, J.d.M.; Nogueira, M.A. Soil health: Looking for suitable indicators. What should be considered to assess the effects of use and management on soil health? Sci. Agric. 2013, 70, 274–289. [Google Scholar] [CrossRef]
- Decaëns, T.; Jiménez, J.J.; Gioia, C.; Measey, G.J.; Lavelle, P. The values of soil animals for conservation biology. Eur. J. Soil Biol. 2006, 42, 23–38. [Google Scholar] [CrossRef]
- Singh, L.A.; Ray, D.C. Effect of no-tillage and tillage on the ecology of mite, Acarina (Oribatida) in two different farming systems of paddy field in Cachar district of Assam. J. Enviornmental Biol. 2015, 36, 319–323. [Google Scholar]
- Behan-Pelletier, V.M. Oribatid mite biodiversity in agroecosystems: Role for bioindication. Agric. Ecosyst. Environ. 1999, 74, 411–423. [Google Scholar] [CrossRef]
- Qin, Y.; Payne, R.; Yang, X.; Ya, M.; Xue, J.; Gu, Y.; Xie, S. Testate amoebae as indicators of water quality and contamination in shallow lakes of the Middle and Lower Yangtze Plain. Environ. Earth Sci. 2016, 75, 627. [Google Scholar] [CrossRef]
- Otilia, I. Density, diversity and distribution of the oribatid mites (Acari, Oribatida) in some cultivated soils from North-Eastern Romania. Lucr. Ştiinţifice 2008, 51, 1–6. [Google Scholar]
- Ivan, O. Diversity and distribution of the oribatid mites (Acari, Oribatida) in some grassland ecosystems from the lower section of the Prut meadow (Romania). Agronomie 2009, 52, 359–364. [Google Scholar]
- Zaitsev, A.S.; van Straalen, N.M. Species diversity and metal accumulation in oribatid mites (Acari, Oribatida) of forests affected by a metallurgical plant. Pedobiologia 2001, 45, 467–479. [Google Scholar] [CrossRef]
- Wei, Q.; Zhou, Y.; Xiao, N.; Huang, J.; Xiao, H.; Chen, H. Effects of three typical grass cultivation patterns on the community structure of soil mites in rocky desertification control area, Guizhou, China. Environ. Res. Commun. 2020, 4, 045008. [Google Scholar] [CrossRef]
- Ding, C.; Dai, Z.; Xue, X.; Li, H.; Liu, M.; Chen, X.; Zhou, J.; Zhang, B.; Hu, F. Effects of vegetations restoration on soil mite community in degraded red soil, Subtropical China. Acta Ecol. Sin. 2008, 28, 4772–4781. [Google Scholar] [CrossRef]
- Yin, X.; Wang, H.; Zhou, D. Characteristics of soil animals’ communities in different agricultural ecosystem in the Songnen grassland of China. Acta Ecol. Sin. 2003, 23, 1071–1078. [Google Scholar]
- John, K.; Zaitsev, A.S.; Wolters, V. Soil fauna groups respond differentially to changes in crop rotation cycles in rice production systems. Pedobiologia 2021, 84, 150703. [Google Scholar] [CrossRef]
- Shen, W.; Lin, X.; Gao, N.; Zhang, H.; Yin, R.; Shi, W.; Duan, Z. Land use intensification affects soil microbial populations, functional diversity and related suppressiveness of cucumber Fusarium wilt in China’s Yangtze River Delta. Plant Soil 2008, 306, 117–127. [Google Scholar] [CrossRef]
- Chen, L.; Liu, S.; Chen, Q.; Zhu, G.; Wu, X.; Wang, J.; Li, X.; Hou, L.; Ni, J. Anammox response to natural and anthropogenic impacts over the Yangtze River. Sci. Total Environ. 2019, 665, 171–180. [Google Scholar] [CrossRef] [PubMed]
- White, H.J.; León-Sánchez, L.; Burton, V.J.; Cameron, E.K.; Caruso, T.; Cunha, L.; Dirilgen, T.; Jurburg, S.D.; Kelly, R.; Kumaresan, D. Methods and approaches to advance soil macroecology. Glob. Ecol. Biogeogr. 2020, 29, 1674–1690. [Google Scholar] [CrossRef]
- Reese, E.G.; Batzer, D.P. Do invertebrate communities in floodplains change predictably along a river’s length? Freshw. Biol. 2007, 52, 226–239. [Google Scholar] [CrossRef]
- Colwell, R.K.; Lees, D.C. The mid-domain effect: Geometric constraints on the geography of species richness. Trends Ecol. Evol. 2000, 15, 70–76. [Google Scholar] [CrossRef]
- Convey, P. Latitudinal variation in allocation to reproduction by the Antarctic oribatid mite, Alaskozetes antarcticus. Appl. Soil Ecol. 1998, 9, 93–99. [Google Scholar] [CrossRef]
- Maraun, M.; Schatz, H.; Scheu, S. Awesome or ordinary? Global diversity patterns of oribatid mites. Ecography 2007, 30, 209–216. [Google Scholar] [CrossRef]
- Marshall, D.J.; Convey, P. Latitudinal variation in habitat specificity of ameronothrid mites (Oribatida). Exp. Appl. Acarol. 2002, 34, 21–35. [Google Scholar] [CrossRef]
- Condamine, F.L.; Sperling, F.A.H.; Wahlberg, N.; Rasplus, J.Y.; Kergoat, G.J. What causes latitudinal gradients in species diversity? Evolutionary processes and ecological constraints on swallowtail biodiversity. Ecol. Lett. 2012, 15, 267–277. [Google Scholar] [CrossRef]
- Yang, B.; Liu, X.; Chen, H.; Ge, F. The specific responses of Acari community to Bt cotton cultivation in agricultural soils in northern China. Appl. Soil Ecol. 2013, 66, 1–7. [Google Scholar] [CrossRef]
- Song, D.; Pan, K.; Tariq, A.; Sun, F.; Li, Z.; Sun, X.; Zhang, L.; Olusanya, O.A.; Wu, X. Large-scale patterns of distribution and diversity of terrestrial nematodes. Appl. Soil Ecol. 2017, 114, 161–169. [Google Scholar] [CrossRef]
- Osler, G.H.R.; Westhorpe, D.; Oliver, I. The short-term effects of endosulfan discharges on eucalypt floodplain soil microarthropods. Appl. Soil Ecol. 2001, 16, 263–273. [Google Scholar] [CrossRef]
- Faleńczyk-Koziróg, K.; Skubała, P.; Habel, M.; Waldon-Rudzionek, B.; Szatten, D. River islands as habitats for soil mites (Acari). River Res. Appl. 2019, 35, 736–748. [Google Scholar] [CrossRef]
- Acharya, S.; Datta, T.K. Diversity of soil cryptostigmatid mites (Acari: Oribatida) of Himachal Pradesh, India, from an altitudinal perspective. J. Asia-Pac. Biodivers. 2019, 12, 357–362. [Google Scholar] [CrossRef]
- Sterzynska, M.; Shrubovych, J.; Nicia, P. Impact of plant invasion (Solidago gigantea L.) on soil mesofauna in a riparian wet meadows. Pedobiologia 2017, 64, 1–7. [Google Scholar] [CrossRef]
- Hernández, P.; Fernández, R.; Novo, M.; Trigo, D.; Díaz Cosín, D.J. Geostatistical and multivariate analysis of the horizontal distribution of an earthworm community in El Molar (Madrid, Spain). Pedobiologia 2007, 51, 13–21. [Google Scholar] [CrossRef][Green Version]
- Yu, X.; Li, C.; Huo, T.; Ji, Z. Information diffusion theory-based approach for the risk assessment of meteorological disasters in the Yangtze River Basin. Nat. Hazards 2021, 107, 2337–2362. [Google Scholar] [CrossRef]
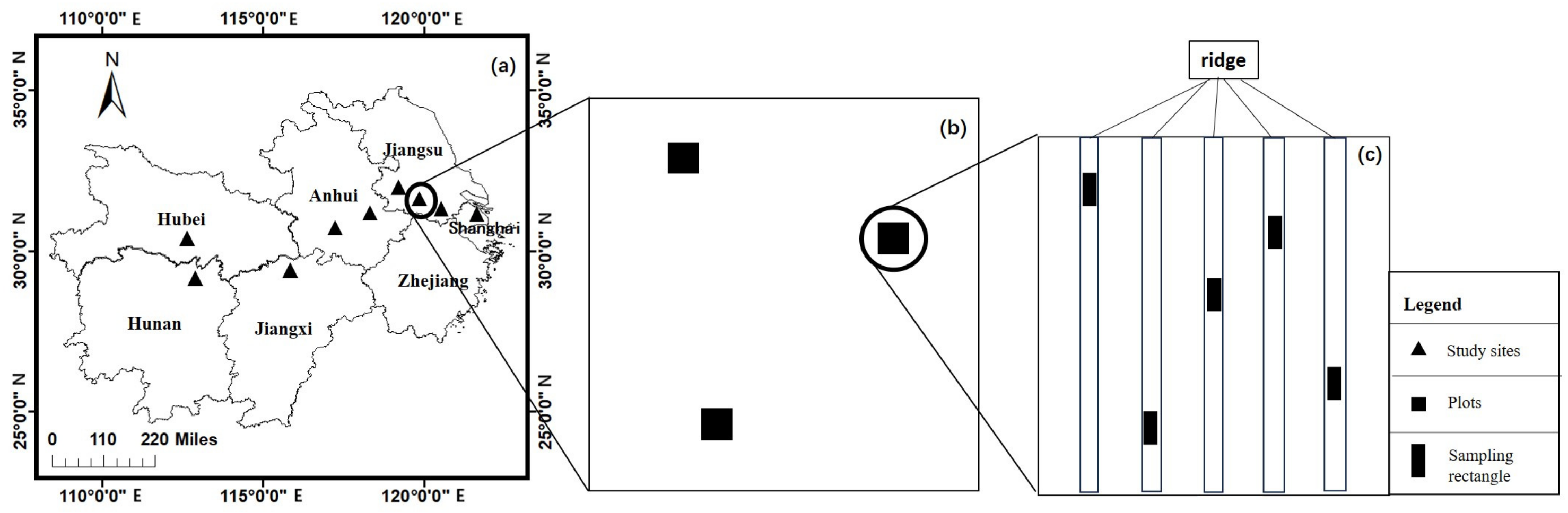
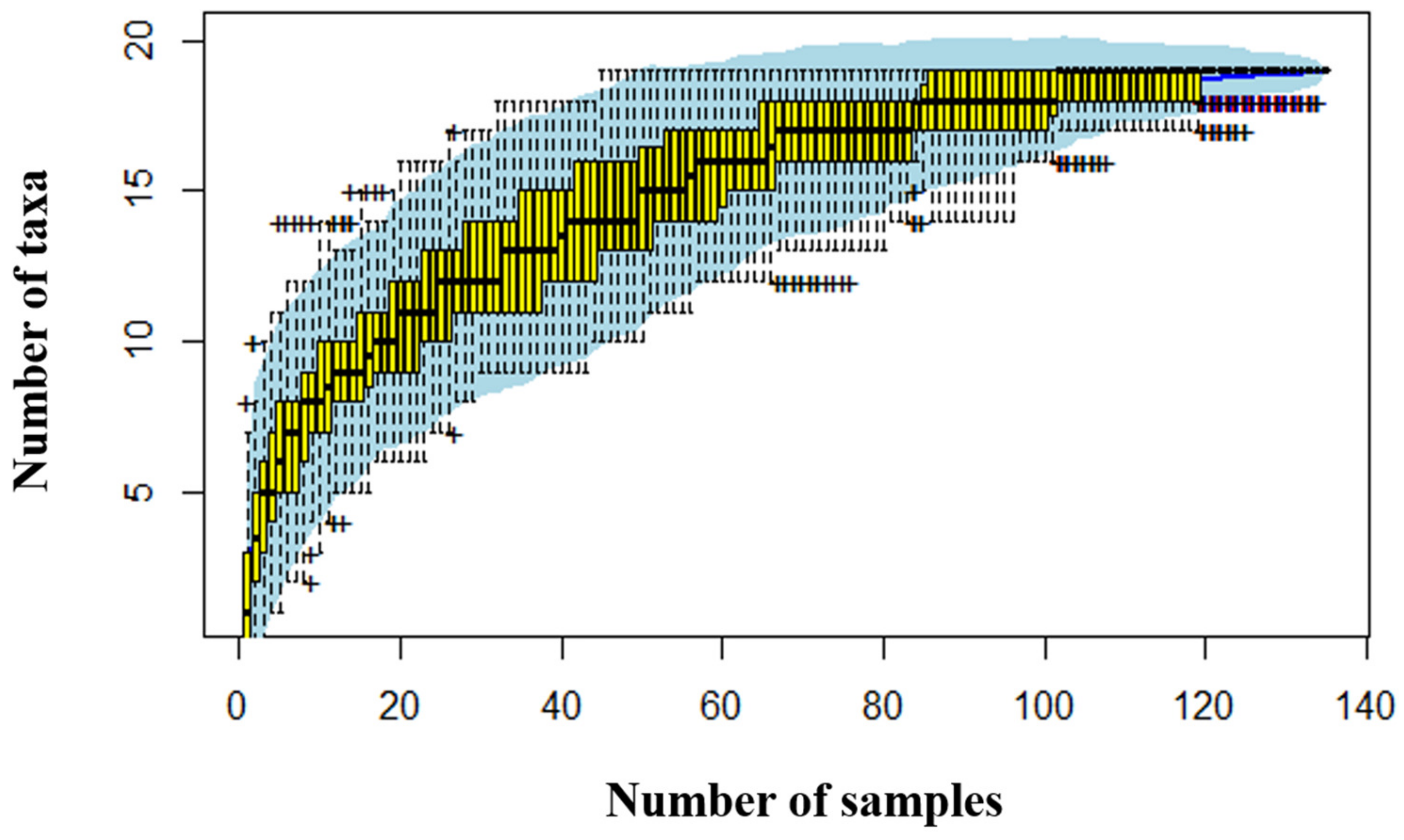
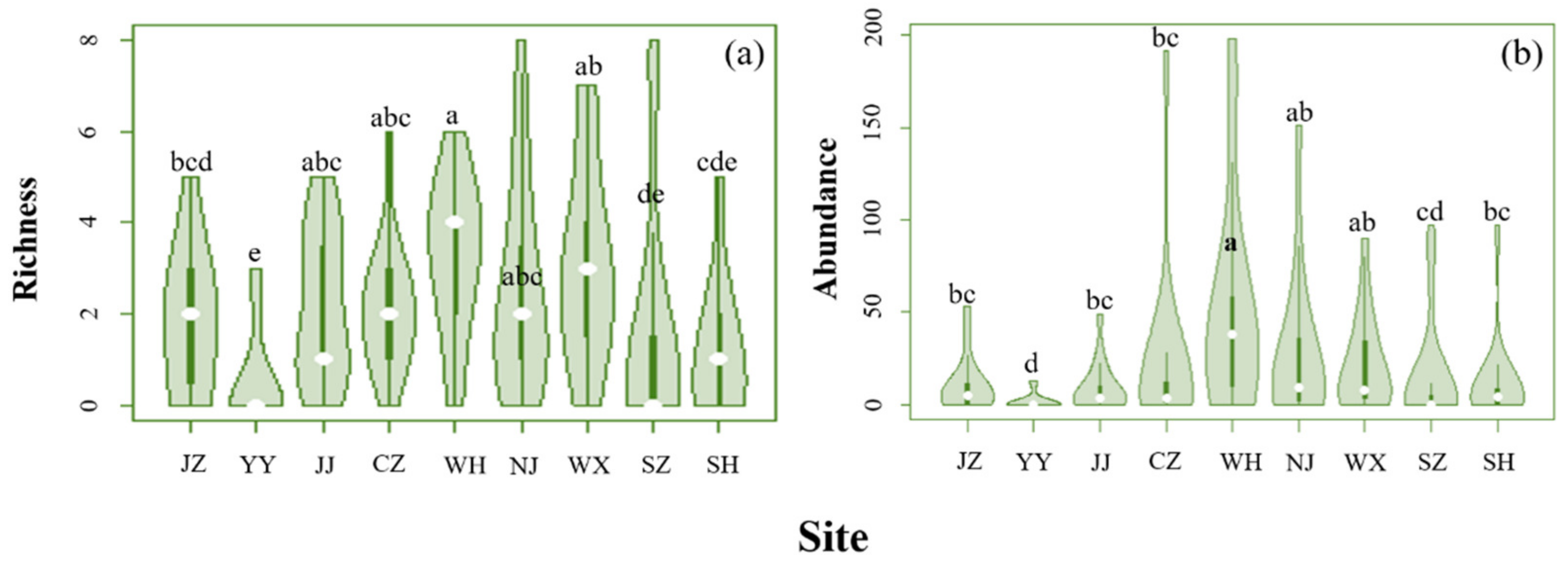

| Site (Abbr.) | Province (Municipality) | Longitude (°, E) a | Latitude (°, N) b | MAT (°C) | MAP (mm) |
|---|---|---|---|---|---|
| Jingzhou (JZ) | Hubei | 112.312 ± 0.012 | 30.211 ± 0.003 | 17.0 | 886.4 |
| Yueyang (YY) | Hunan | 113.027 ± 0.001 | 29.484 ± 0.012 | 18.9 | 1310.6 |
| Jiujiang (JJ) | Jiangxi | 115.817 ± 0.004 | 29.606 ± 0.002 | 20.4 | 1485.9 |
| Chizhou (CZ) | Anhui | 117.571 ± 0.014 | 30.524 ± 0.005 | 17.7 | 1556 |
| Wuhu (WH) | Anhui | 118.263 ± 0.002 | 31.468 ± 0.002 | 17.9 | 1248.1 |
| Nanjing (NJ) | Jiangsu | 118.680 ± 0.001 | 31.827 ± 0.001 | 17.6 | 1267.1 |
| Wuxi (WX) | Jiangsu | 120.541 ± 0.001 | 31.765 ± 0.001 | 18.0 | 1142.0 |
| Suzhou (SZ) | Jiangsu | 120.388 ± 0.001 | 31.369 ± 0.003 | 18.3 | 1318.6 |
| Shanghai (SH) | Shanghai | 121.170 ± 0.018 | 31.204 ± 0.002 | 18.1 | 1388.2 |
| Community Type | Abbr. | Requirements |
|---|---|---|
| Macrophylina type | M | The number of M type mites is more than 50%. |
| Gymnonota type | G | The number of G type mites is more than 50%. |
| Poronota type | P | The number of P type mites is more than 50%. |
| Overall type | O | The individual numbers of M, G, and P type mites are all in the range of 20–50%. |
| Macrophylina-Gymnonota type | MG | The individual numbers of type M and G type mites are in the range of 20–50%, while the individual numbers of P type mites are less than 20%. |
| Gymnonota-Poronota type | GP | The individual numbers of type G and P type mites are in the range of 20–50%, while the individual numbers of M type mites are less than 20%. |
| Macropylina-Poronota type | MP | The individual numbers of type M and P type mites are in the range of 20–50%, while the individual numbers of G type mites are less than 20%. |
| CZ | JZ | JJ | NJ | SH | SZ | WH | WX | YY | Abundance | Percentage (%) | DD | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Protoribates Berlese, 1908 | 125 | 79 | 59 | 68 | 18 | 97 | 273 | 62 | 10 | 791 | 33.07 | +++ |
| Eremobelba Berlese, 1908 | 3 | 0 | 5 | 2 | 0 | 0 | 4 | 1 | 0 | 15 | 0.63 | + |
| Ceratozetes Berlese, 1908 | 89 | 59 | 40 | 179 | 114 | 71 | 403 | 129 | 6 | 1090 | 45.57 | +++ |
| Oppiella Jacot, 1937 | 44 | 3 | 1 | 92 | 1 | 1 | 2 | 7 | 0 | 151 | 6.31 | ++ |
| Pergalumna Grandjean, 1936 | 31 | 5 | 7 | 4 | 3 | 9 | 3 | 26 | 0 | 88 | 3.68 | ++ |
| Tectocepheus Berlese, 1896 | 4 | 1 | 4 | 9 | 8 | 3 | 19 | 11 | 0 | 59 | 2.47 | ++ |
| Lohmannia Michael, 1898 | 0 | 5 | 1 | 1 | 0 | 4 | 0 | 0 | 0 | 11 | 0.46 | + |
| Perxylobates Hammer, 1972 | 1 | 0 | 11 | 21 | 3 | 1 | 0 | 63 | 1 | 101 | 4.22 | ++ |
| Scheloribates Berlese, 1908 | 0 | 0 | 1 | 14 | 0 | 0 | 7 | 0 | 0 | 22 | 0.92 | + |
| Eremaeus Koch, 1835 | 0 | 0 | 0 | 1 | 0 | 0 | 4 | 0 | 7 | 12 | 0.50 | + |
| Oppiidae Grandjean, 1954 | 0 | 0 | 0 | 0 | 0 | 5 | 0 | 0 | 2 | 7 | 0.29 | + |
| Punctoribatidae Thor, 1937 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0.04 | + |
| Mochlozetidae Grandjean, 1960 | 0 | 1 | 0 | 0 | 0 | 8 | 0 | 0 | 0 | 9 | 0.38 | + |
| Epilohmanniidae Oudemans, 1923 | 0 | 0 | 0 | 0 | 0 | 1 | 2 | 0 | 0 | 3 | 0.13 | + |
| Parakalummidae Grandjean, 1936 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 11 | 0 | 11 | 0.46 | + |
| Damaeidae Berlese, 1896 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 3 | 0.13 | + |
| Tetracondylidae Aoki, 1961 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0.04 | + |
| Trhypochthoniidae Willmann, 1931 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 0 | 4 | 0.17 | + |
| Euphthiracaridae Jacot, 1930 | 0 | 0 | 2 | 0 | 0 | 0 | 11 | 0 | 0 | 13 | 0.54 | + |
| Abundance | 297 | 153 | 131 | 391 | 147 | 201 | 728 | 318 | 26 | 2392 | ||
| Richness | 7 | 7 | 10 | 10 | 6 | 11 | 10 | 11 | 5 | 19 | ||
| Density Individual/m2 | 5147.54 | 2651.76 | 2270.46 | 6776.72 | 2547.77 | 3483.69 | 12,617.53 | 5511.50 | 450.63 | 1535.47 |
| Response Variable | Model | R2 | Slope | |||
|---|---|---|---|---|---|---|
| Estimate | SE | t Value | p-Value | |||
| Abundance | longitude | 0.021 | 183,900 | 87,030 | 2.114 | 0.036 * |
| longitude2 | 0.021 | −5282 | 2499 | −2.114 | 0.036 * | |
| latitude | 0.069 | 24.9 | 7.377 | 3.375 | <0.001 *** | |
| Richness | longitude | 0.033 | 123,300 | 38,190 | 3.228 | 0.002 ** |
| longitude2 | 0.033 | −3540 | 1096 | −3.228 | 0.002 ** | |
| latitude | 0.039 | 9.154 | 3.237 | 2.828 | 0.005 ** | |
| H′ | longitude | 0.014 | 46,340 | 19,400 | 2.389 | 0.018 * |
| longitude2 | 0.014 | −1331 | 556.9 | −2.389 | 0.018 * | |
| latitude | 0.016 | 3.579 | 1.644 | 2.177 | 0.031 * | |
| C | longitude | 0.003 | 16,687.1 | 18,083 | 0.923 | 0.358 |
| longitude2 | 0.003 | −479.2 | 519.1 | −0.923 | 0.358 | |
| J | longitude | 0.045 | 66,700 | 18,730 | 3.561 | <0.001 *** |
| longitude2 | 0.045 | −1915 | 537.8 | −3.561 | <0.001 *** | |
| latitude | 0.010 | 3.092 | 1.617 | 1.912 | 0.059 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, J.; Liu, Y.; Ye, Y.; Lai, J.; Zheng, Y.; Liu, D.; Gao, M. Preliminary Study on the Diversity of Soil Oribatid Mite (Acari: Oribatida) Community Reveals Both Longitudinal and Latitudinal Patterns in Paddy Fields along the Middle and Lower Reaches of Yangtze River, China. Agronomy 2023, 13, 2718. https://doi.org/10.3390/agronomy13112718
Sun J, Liu Y, Ye Y, Lai J, Zheng Y, Liu D, Gao M. Preliminary Study on the Diversity of Soil Oribatid Mite (Acari: Oribatida) Community Reveals Both Longitudinal and Latitudinal Patterns in Paddy Fields along the Middle and Lower Reaches of Yangtze River, China. Agronomy. 2023; 13(11):2718. https://doi.org/10.3390/agronomy13112718
Chicago/Turabian StyleSun, Jiahuan, Yifei Liu, Yanyan Ye, Jiangshan Lai, Ye Zheng, Dong Liu, and Meixiang Gao. 2023. "Preliminary Study on the Diversity of Soil Oribatid Mite (Acari: Oribatida) Community Reveals Both Longitudinal and Latitudinal Patterns in Paddy Fields along the Middle and Lower Reaches of Yangtze River, China" Agronomy 13, no. 11: 2718. https://doi.org/10.3390/agronomy13112718
APA StyleSun, J., Liu, Y., Ye, Y., Lai, J., Zheng, Y., Liu, D., & Gao, M. (2023). Preliminary Study on the Diversity of Soil Oribatid Mite (Acari: Oribatida) Community Reveals Both Longitudinal and Latitudinal Patterns in Paddy Fields along the Middle and Lower Reaches of Yangtze River, China. Agronomy, 13(11), 2718. https://doi.org/10.3390/agronomy13112718






