Response of Tagetes patula L. and Ageratum houstonianum Mill. to Microbial Biostimulant Inoculation and Organic Fertilization
Abstract
1. Introduction
2. Materials and Methods
3. Results
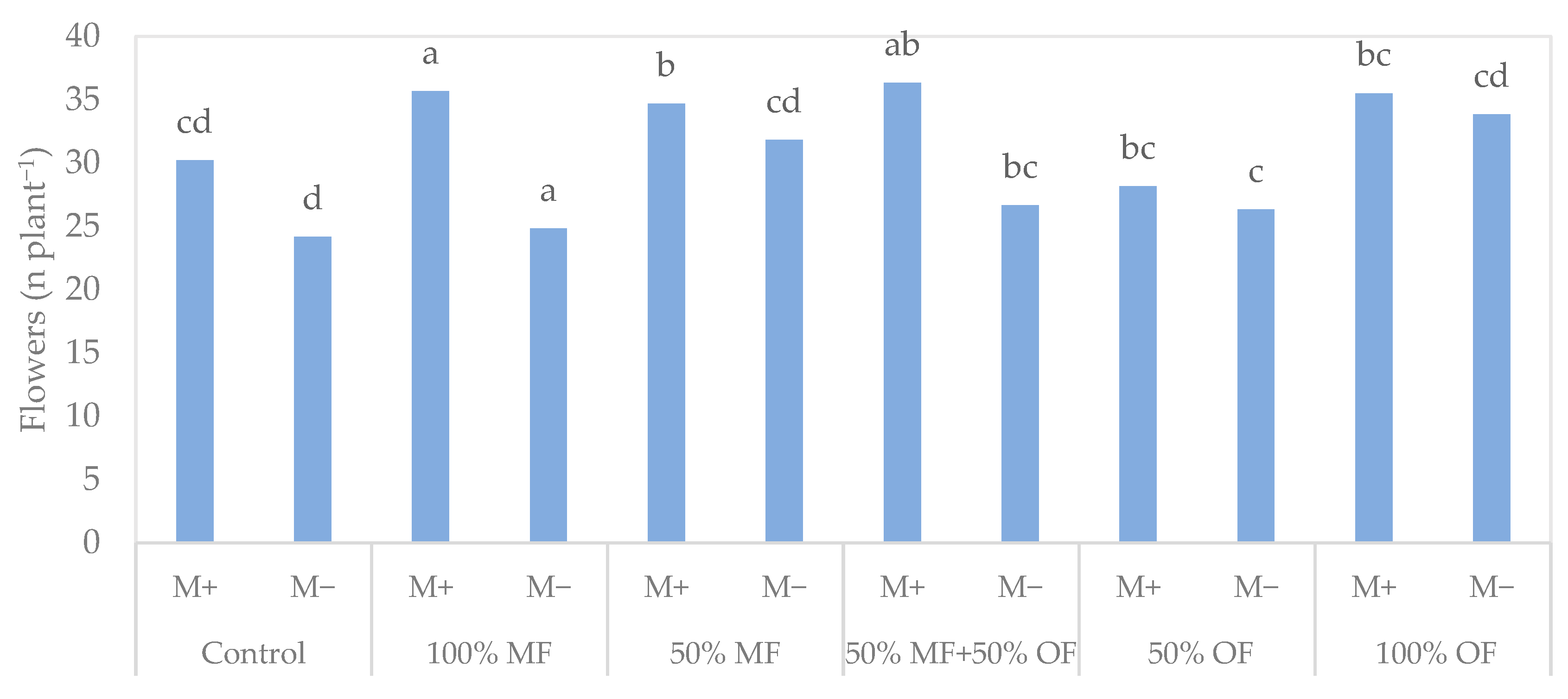
3.1. Tagetes patula
3.2. Ageratum houstonianum
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xia, L.; Hao, W.; Qin, J.; Ji, F.; Yue, X. Carbon emission reduction and promotion policies considering social preferences and consumers’ low-carbon awareness in the cap-and-trade system. J. Clean. Prod. 2018, 195, 1105–1124. [Google Scholar] [CrossRef]
- Stewart, A. Flower Confidential: The Good, the Bad, and the Beautiful; Algonquin Books: Chapel Hill, NC, USA, 2008; ISBN 1565126459. [Google Scholar]
- Choi, S.; Feinberg, R.A. The LOHAS (lifestyle of health and sustainability) scale development and validation. Sustainability 2021, 13, 1598. [Google Scholar] [CrossRef]
- Wani, M.A.; Nazki, I.T.; Din, A.; Iqbal, S.; Wani, S.A.; Khan, F.U. Neelofar Floriculture Sustainability Initiative: The Dawn of New Era BT—Sustainable Agriculture Reviews 27; Lichtfouse, E., Ed.; Springer International Publishing: Cham, Switzerland, 2018; pp. 91–127. ISBN 978-3-319-75190-0. [Google Scholar]
- Krug, B.A.; Burnett, S.E.; Dennis, J.H.; Lopez, R.G. Growers look at operating a sustainable greenhouse. GMPro 2008, 28, 43–45. [Google Scholar]
- Lopez, R.G.; Burnett, S.E.; Dennis, J.H.; Krug, B.A. 8 steps to take to become sustainable. GMPro 2008, 28, 26–29. [Google Scholar]
- Hattam, C. Adopting organic agriculture: An investigation using the theory of planned Behaviour. In Proceedings of the International Association of Agricultural Economics Conference, Gold Coast, Australia, 12–18 August 2006. [Google Scholar]
- D’Souza, G.; Cyphers, D.; Phipps, T. Factors affecting the adoption of sustainable agricultural practices. Agric. Resour. Econ. Rev. 1993, 22, 159–165. [Google Scholar] [CrossRef]
- Gillespie, J.; Lewis, D. Processor willingness to adopt a crawfish peeling machine: An application of technology adoption under uncertainty. J. Agric. Appl. Econ. 2008, 40, 369–383. [Google Scholar] [CrossRef]
- Barreiro-Hurle, J.; Espinosa-Goded, M.; Dupraz, P. Does intensity of change matter? Factors affecting adoption in two agri-environmental schemes. In Proceedings of the EAAE Seminar: Modeling of Agricultural and Rural Development Policies, Seville, Spain, 29 January–1 February 2008. [Google Scholar]
- Paudel, K.P.; Gauthier, W.M.; Westra, J.V. Hall Factors influencing and steps leading to the adoption of best management practices by Louisiana dairy farmers. J. Agr. Appl. Econ. 2008, 40, 203–222. [Google Scholar] [CrossRef]
- Banerjee, S.; Martin, S.W.; Roberts, R.K.; Larkin, S.L.; Larson, J.A.; Paxton, K.W.; English, B.C.; Marrar, M.C.; Reeves, J.M. A binary logit estimation of factors affecting adoption of GPS guidance systems by cotton producers. J. Agr. Appl. Econ. 2008, 40, 345–355. [Google Scholar] [CrossRef]
- Bethlenfalvay, G.J.; Cantrell, I.C.; Mihara, K.L.; Schreiner, R.P. Relationships between soil aggregation and mycorrhizae as influenced by soil biota and nitrogen nutrition. Biol. Fertil. Soils 1999, 28, 356–363. [Google Scholar] [CrossRef]
- Johansen, A.; Jakobsen, I.; Jensen, E.S. Hyphal N transport by a vesicular-arbuscular mycorrhizal fungus associated with cucumber grown at three nitrogen levels. Plant Soil 1994, 160, 1–9. [Google Scholar] [CrossRef]
- Paszkowski, U.; Boller, T. The growth defect of lrt1, a maize mutant lacking lateral roots, can be complemented by symbiotic fungi or high phosphate nutrition. Planta 2002, 214, 584–590. [Google Scholar] [CrossRef]
- St-Arnaud, M.; Vimard, B.; Fortin, J.A.; Hamel, C.; Caron, M. Inhibition of Fusarium oxysporum f-sp. dianthi in the non-VAM species Dianthus caryophyllus by co-culture with Tagetes patula companion plants colonized by Glomus intraradices. Can. J. Bot. 1997, 75, 998–1005. [Google Scholar] [CrossRef]
- Larsen, J.; Ravnskov, S.; Jakobsen, I. Combined effect of an arbuscular mycorrhizal fungus and a biocontrol bacterium against Pythium ultimum in soil. Folia Geobot. 2003, 38, 145–154. [Google Scholar] [CrossRef]
- Miceli, A.; Moncada, A.; Vetrano, F. Use of Microbial Biostimulants to Increase the Salinity Tolerance of Vegetable Transplants. Agronomy 2021, 11, 1143. [Google Scholar] [CrossRef]
- Miceli, A.; Vetrano, F.; Torta, L.; Esposito, A.; Moncada, A. Effect of Mycorrhizal Inoculation on Melon Plants under Deficit Irrigation Regimes. Agronomy 2023, 13, 440. [Google Scholar] [CrossRef]
- Gaur, A.; Adholeya, A. Diverse response of five ornamental plant species to mixed indigenous and single isolate arbuscular-mycorrhizal inocula in marginal soil amended with organic matter. J. Plant Nutr. 2005, 28, 707–723. [Google Scholar] [CrossRef]
- Sohn, B.K.; Kim, K.Y.; Chung, S.J.; Kim, W.S.; Park, S.M.; Kang, J.G.; Rim, Y.S.; Cho, J.S.; Kim, T.H.; Lee, J.H. Effect of the different timing of AMF inoculation on plant growth and flower quality of chrysanthemum. Sci. Hortic. 2003, 98, 173–183. [Google Scholar] [CrossRef]
- Dole, J.; Wilkins, H. Floriculture: Principles and Species; Pearson: Hoboken, NJ, USA, 2004. [Google Scholar]
- El Hadidy, D.; El Sayed, A.M.; El Tantawy, M.; El Alfy, T. Phytochemical Analysis and Biological Activities of Essential Oils of the Leaves and Flowers of Ageratum houstonianum Mill. Cultivated in Egypt. J. Essent. Oil Bear. Plants 2019, 22, 1241–1251. [Google Scholar] [CrossRef]
- Kanfra, X.; Obawolu, T.; Wrede, A.; Strolka, B.; Winkelmann, T.; Hardeweg, B.; Heuer, H. Alleviation of nematode-mediated apple replant disease by pre-cultivation of Tagetes. Horticulturae 2021, 7, 433. [Google Scholar] [CrossRef]
- Krueger, R.; Dover, K.E.; McSorley, R.; Wang, K.H. Marigolds (Tagetes spp.) for Nematode Management: ENY-056/NG045, 8/2007. EDIS 2007, 2007, ENY-056. [Google Scholar] [CrossRef]
- Hooks, C.R.R.; Wang, K.-H.; Ploeg, A.; McSorley, R. Using marigold (Tagetes spp.) as a cover crop to protect crops from plant-parasitic nematodes. Appl. Soil Ecol. 2010, 46, 307–320. [Google Scholar] [CrossRef]
- Natarajan, N.; Cork, A.; Boomathi, N.; Pandi, R.; Velavan, S.; Dhakshnamoorthy, G. Cold aqueous extracts of African marigold, Tagetes erecta for control tomato root knot nematode, Meloidogyne incognita. Crop Prot. 2006, 25, 1210–1213. [Google Scholar] [CrossRef]
- Kurade, N.P.; Jaitak, V.; Kaul, V.K.; Sharma, O.P. Chemical composition and antibacterial activity of essential oils of Lantana camara, Ageratum houstonianum and Eupatorium adenophorum. Pharm. Biol. 2010, 48, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Asrar, A.-W.A.; Elhindi, K.M. Alleviation of drought stress of marigold (Tagetes erecta) plants by using arbuscular mycorrhizal fungi. Saudi J. Biol. Sci. 2011, 18, 93–98. [Google Scholar] [CrossRef]
- Sonneveld, C.; Voogt, W.; Sonneveld, C.; Voogt, W. Nutrient solutions for soilless cultures. In Plant Nutrition of Greenhouse Crops; Springer: Dordrecht, The Netherlands, 2009; pp. 257–275. [Google Scholar]
- Bakker, J.; Bridle, P.; Timberlake, C. Tristimulus measurements (CIELAB 76) of port wine colour. Vitis 1986, 25, 67–78. [Google Scholar]
- McLellan, M.R.; Lind, L.R.; Kime, R.W. Hue angle determinations and statistical analysis for multiquadrant Hunter L, a, b data. J. Food Qual. 1995, 18, 235–240. [Google Scholar] [CrossRef]
- Linderman, R.G.; Davis, E.A. Varied response of marigold (Tagetes spp.) genotypes to inoculation with different arbuscular mycorrhizal fungi. Sci. Hortic. 2004, 99, 67–78. [Google Scholar] [CrossRef]
- Scaramuzzi, S.; Gabellini, S. Il comparto floricolo e ornamentale. In Florovivaismo. Principi e Tecniche; New Business Media Srl: Milan, Italy, 2022; pp. 13–28. ISBN 8850655177. [Google Scholar]
- Pramuk, L.A.; Runkle, E. Temperature and light on bedding plants. Greenh. Prod. News 2003, 13, 32–41. [Google Scholar]
- Bachman, G.R.; Metzger, J.D. Growth of bedding plants in commercial potting substrate amended with vermicompost. Bioresour. Technol. 2008, 99, 3155–3161. [Google Scholar] [CrossRef]
- Herencia, J.F.; Ruiz-Porras, J.C.; Melero, S.; Garcia-Galavis, P.A.; Morillo, E.; Maqueda, C. Comparison between organic and mineral fertilization for soil fertility levels, crop macronutrient concentrations, and yield. Agron. J. 2007, 99, 973–983. [Google Scholar] [CrossRef]
- Akoumianaki-Ioannidou, A.; Rasouli, M.; Podaropoulou, L.; Karapanos, I.; Bilalis, D. Effects of Cultivation System and Fertilization on Seedling Production of Ocimum basilicum L. and Mentha spicata L. Not. Bot. Horti Agrobot. Cluj-Napoca 2015, 43, 131–137. [Google Scholar] [CrossRef]
- Treadwell, D.D.; Hochmuth, G.J.; Hochmuth, R.C.; Simonne, E.H.; Sargent, S.A.; Davis, L.L.; Laughlin, W.L.; Berry, A. Organic Fertilization Programs for Greenhouse Fresh-cut Basil and Spearmint in a Soilless Media Trough System. Horttechnology 2011, 21, 162–169. [Google Scholar] [CrossRef]
- Succop, C.E.; Newman, S.E. Organic Fertilization of Fresh Market Sweet Basil in a Greenhouse. Horttechnology 2004, 14, 235–239. [Google Scholar] [CrossRef]
- Warman, P.R.; Havard, K.A. Yield, vitamin and mineral contents of organically and conventionally grown carrots and cabbage. Agric. Ecosyst. Environ. 1997, 61, 155–162. [Google Scholar] [CrossRef]
- Warman, P.R.; Havard, K.A. Yield, vitamin and mineral contents of organically and conventionally grown potatoes and sweet corn. Agric. Ecosyst. Environ. 1998, 68, 207–216. [Google Scholar] [CrossRef]
- Foth, H.D.; Ellis, B.G. Soil Fertility; John Wiley & Sons: New York, NY, USA, 1988. [Google Scholar]
- Bi, G.; Evans, W.B.; Spiers, J.M.; Witcher, A.L. Effects of Organic and Inorganic Fertilizers on Marigold Growth and Flowering. HortScience 2010, 45, 1373–1377. [Google Scholar] [CrossRef]
- Berry, P.M.; Sylvester-Bradley, R.; Philipps, L.; Hatch, D.J.; Cuttle, S.P.; Rayns, F.W.; Gosling, P. Is the productivity of organic farms restricted by the supply of available nitrogen? Soil Use Manag. 2002, 18, 248–255. [Google Scholar] [CrossRef]
- Shammas, N.K. Interactions of temperature, pH, and biomass on the nitrification process. J. Water Pollut. Control Fed. 1986, 58, 52–59. [Google Scholar]
- Cure, J.D.; Acock, B. Crop responses to carbon dioxide doubling: A literature survey. Agric. For. Meteorol. 1986, 38, 127–145. [Google Scholar] [CrossRef]
- Lawson, T.; Blatt, M.R. Stomatal Size, Speed, and Responsiveness Impact on Photosynthesis and Water Use Efficiency. Plant Physiol. 2014, 164, 1556–1570. [Google Scholar] [CrossRef]
- Ruiz-Lozano, J.M.; Aroca, R. Host Response to Osmotic Stresses: Stomatal Behaviour and Water Use Efficiency of Arbuscular Mycorrhizal Plants. In Arbuscular Mycorrhizas: Physiology and Function; Koltai, H., Kapulnik, Y., Eds.; Springer: Dordrecht, The Netherlands, 2010; pp. 239–256. ISBN 9789048194896. [Google Scholar]
- Faber, B.A.; Zasoski, R.J.; Munns, D.N.; Shackel, K. A method for measuring hyphal nutrient and water uptake in mycorrhizal plants. Can. J. Bot. 1991, 69, 87–94. [Google Scholar] [CrossRef]
- Hardie, K.A.Y.; Leyton, L. The influence of vesicular-arbuscular mycorrhiza on growth and water relations of red clover: I. in phosphate deficient soil. New Phytol. 1981, 89, 599–608. [Google Scholar] [CrossRef]
- Kothari, S.K.; Marschner, H.; George, E. Effect of VA mycorrhizal fungi and rhizosphere microorganisms on root and shoot morphology, growth and water relations in maize. New Phytol. 1990, 116, 303–311. [Google Scholar] [CrossRef]
- Stefan, M.; Munteanu, N.; Stoleru, V.; Mihasan, M. Effects of inoculation with plant growth promoting rhizobacteria on photosynthesis, antioxidant status and yield of runner bean. Rom. Biotechnol. Lett. 2013, 18, 8132–8143. [Google Scholar]
- Stefan, M.; Munteanu, N.; Stoleru, V.; Mihasan, M.; Hritcu, L. Seed inoculation with plant growth promoting rhizobacteria enhances photosynthesis and yield of runner bean (Phaseolus coccineus L.). Sci. Hortic. 2013, 151, 22–29. [Google Scholar] [CrossRef]
- Han, H.S.; Lee, K.D. Plant growth promoting rhizobacteria effect on antioxidant status, photosynthesis, mineral uptake and growth of lettuce under soil salinity. Res. J. Agric. Biol. Sci. 2005, 1, 210–215. [Google Scholar]
- Ibrahim, M.A.; Campbell, W.F.; Rupp, L.A.; Allen, E.B. Effects of mycorrhizae on sorghum growth, photosynthesis, and stomatal conductance under drought conditions. Arid Soil Res. Rehabil. 1990, 4, 99–107. [Google Scholar] [CrossRef]
- Khandaker, M.M.; Rohani, F.; Dalorima, T.; Mat, N. Effects of different organic fertilizers on growth, yield and quality of Capsicum annuum L. Var. Kulai (Red Chilli Kulai). Biosci. Biotechnol. Res. Asia 2017, 14, 185–192. [Google Scholar] [CrossRef]
- Khandaker, M.M.; Nor, M.F.; Dalorima, T.; Sajili, M.H.; Mat, N. Effect of different rates of inorganic fertilizer on physiology, growth and yield of okra (‘Abelmoschus esculentus’) cultivated on Bris soil of Terengganu, Malaysia. Aust. J. Crop Sci. 2017, 11, 880–887. [Google Scholar] [CrossRef]
- Miceli, A.; Vetrano, F.; Moncada, A. Influence of Ecklonia maxima Extracts on Growth, Yield, and Postharvest Quality of Hydroponic Leaf Lettuce. Horticulturae 2021, 7, 440. [Google Scholar] [CrossRef]
- Candido, V.; Cantore, V.; Castronuovo, D.; Denora, M.; Schiattone, M.I.; Sergio, L.; Todorovic, M.; Boari, F. Effect of Water Regime, Nitrogen Level, and Biostimulant Application on the Water and Nitrogen Use Efficiency of Wild Rocket [Diplotaxis tenuifolia (L.) DC]. Agronomy 2023, 13, 507. [Google Scholar] [CrossRef]
- Alfosea-Simón, M.; Simón-Grao, S.; Zavala-Gonzalez, E.A.; Cámara-Zapata, J.M.; Simón, I.; Martínez-Nicolás, J.J.; Lidón, V.; Rodríguez-Ortega, W.M.; García-Sánchez, F. Application of Biostimulants Containing Amino Acids to Tomatoes Could Favor Sustainable Cultivation: Implications for Tyrosine, Lysine, and Methionine. Sustainability 2020, 12, 9729. [Google Scholar] [CrossRef]
- Vetrano, F.; Miceli, C.; Angileri, V.; Frangipane, B.; Moncada, A.; Miceli, A. Effect of bacterial inoculum and fertigation management on nursery and field production of Lettuce Plants. Agronomy 2020, 10, 1477. [Google Scholar] [CrossRef]
- Pavithra, D.; Yapa, N. Arbuscular mycorrhizal fungi inoculation enhances drought stress tolerance of plants. Groundw. Sustain. Dev. 2018, 7, 490–494. [Google Scholar] [CrossRef]
- Asrar, A.A.; Abdel-Fattah, G.M.; Elhindi, K.M. Improving growth, flower yield, and water relations of snapdragon (Antirhinum majus L.) plants grown under well-watered and water-stress conditions using arbuscular mycorrhizal fungi. Photosynthetica 2012, 50, 305–316. [Google Scholar] [CrossRef]
- Xiukang, W.; Yingying, X. Evaluation of the effect of irrigation and fertilization by drip fertigation on tomato yield and water use efficiency in greenhouse. Int. J. Agron. 2016, 2016, 3961903. [Google Scholar] [CrossRef]
- Augé, R.M.; Toler, H.D.; Saxton, A.M. Arbuscular mycorrhizal symbiosis alters stomatal conductance of host plants more under drought than under amply watered conditions: A meta-analysis. Mycorrhiza 2015, 25, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Gemin, L.G.; Mógor, Á.F.; Amatussi, J.D.O.; Mógor, G. Microalgae associated to humic acid as a novel biostimulant improving onion growth and yield. Sci. Hortic. 2019, 256, 108560. [Google Scholar] [CrossRef]
- Fei, H.; Crouse, M.; Papadopoulos, Y.A.; Vessey, J.K. Improving biomass yield of giant Miscanthus by application of beneficial soil microbes and a plant biostimulant. Can. J. Plant Sci. 2019, 100, 29–39. [Google Scholar] [CrossRef]
- Jiménez-Arias, D.; Morales-Sierra, S.; Borges, A.A.; Herrera, A.J.; Luis, J.C. New biostimulants screening method for crop seedlings under water deficit stress. Agronomy 2022, 12, 728. [Google Scholar] [CrossRef]
- Geng, Y.; Cao, G.; Wang, L.; Wang, S. Effects of equal chemical fertilizer substitutions with organic manure on yield, dry matter, and nitrogen uptake of spring maize and soil nitrogen distribution. PLoS ONE 2019, 14, e0219512. [Google Scholar] [CrossRef]
- St. John, T.V.; Coleman, D.C.; Reid, C.P.P. Association of vesicular-arbuscular mycorrhizal hyphae with soil organic particles. Ecology 1983, 64, 957–959. [Google Scholar] [CrossRef]
- Joner, E.J.; Jakobsen, I. Growth and extracellular phosphatase activity of arbuscular mycorrhizal hyphae as influenced by soil organic matter. Soil Biol. Biochem. 1995, 27, 1153–1159. [Google Scholar] [CrossRef]
- Gryndler, M.; Hršelová, H.; Vosatka, M.; Votruba, J.; Klir, J. Organic fertilization changes the response of mycelium of arbuscular mycorrhizal fungi and their sporulation to mineral NPK supply. Folia Microbiol. 2001, 46, 540–542. [Google Scholar] [CrossRef]
- Joner, E.J. The effect of long-term fertilization with organic or inorganic fertilizers on mycorrhiza-mediated phosphorus uptake in subterranean clover. Biol. Fertil. Soils 2000, 32, 435–440. [Google Scholar] [CrossRef]
- Ravnskov, S.; Larsen, J.; Olsson, P.Å.L.A.; Jakobsen, I. Effects of various organic compounds on growth and phosphorus uptake of an arbuscular mycorrhizal fungus. New Phytol. 1999, 141, 517–524. [Google Scholar] [CrossRef]
- Harman, G.E.; Howell, C.R.; Viterbo, A.; Chet, I.; Lorito, M. Trichoderma species—Opportunistic, avirulent plant symbionts. Nat. Rev. Microbiol. 2004, 2, 43–56. [Google Scholar] [CrossRef]
- Hermosa, R.; Viterbo, A.; Chet, I.; Monte, E. Plant-beneficial effects of Trichoderma and of its genes. Microbiology 2012, 158, 17–25. [Google Scholar] [CrossRef]
- Studholme, D.J.; Harris, B.; Le Cocq, K.; Winsbury, R.; Perera, V.; Ryder, L.; Ward, J.L.; Beale, M.H.; Thornton, C.R.; Grant, M. Investigating the beneficial traits of Trichoderma hamatum GD12 for sustainable agriculture—Insights from genomics. Front. Plant Sci. 2013, 4, 258. [Google Scholar] [CrossRef]
- Mendoza-Mendoza, A.; Zaid, R.; Lawry, R.; Hermosa, R.; Monte, E.; Horwitz, B.A.; Mukherjee, P.K. Molecular dialogues between Trichoderma and roots: Role of the fungal secretome. Fungal Biol. Rev. 2018, 32, 62–85. [Google Scholar] [CrossRef]
- Altomare, C.; Norvell, W.A.; Björkman, T.; Harman, G. Solubilization of phosphates and micronutrients by the plant-growth-promoting and biocontrol fungus Trichoderma harzianum Rifai 1295-22. Appl. Environ. Microbiol. 1999, 65, 2926–2933. [Google Scholar] [CrossRef]
- Vinale, F.; Sivasithamparam, K. Beneficial effects of Trichoderma secondary metabolites on crops. Phyther. Res. 2020, 34, 2835–2842. [Google Scholar] [CrossRef]
- Hermosa, R.; Rubio, M.B.; Cardoza, R.E.; Nicolás, C.; Monte, E.; Gutiérrez, S. The contribution of Trichoderma to balancing the costs of plant growth and defense. Int. Microbiol. 2013, 16, 69–80. [Google Scholar] [PubMed]
- Contreras-Cornejo, H.A.; López-Bucio, J.S.; Méndez-Bravo, A.; Macías-Rodríguez, L.; Ramos-Vega, M.; Guevara-García, Á.A.; López-Bucio, J. Mitogen-activated protein kinase 6 and ethylene and auxin signaling pathways are involved in Arabidopsis root-system architecture alterations by Trichoderma atroviride. Mol. Plant-Microbe Interact. 2015, 28, 701–710. [Google Scholar] [CrossRef] [PubMed]
- Woo, S.L.; Pepe, O. Microbial consortia: Promising probiotics as plant biostimulants for sustainable agriculture. Front. Plant Sci. 2018, 9, 1801. [Google Scholar] [CrossRef] [PubMed]
- Bradáčová, K.; Florea, A.S.; Bar-Tal, A.; Minz, D.; Yermiyahu, U.; Shawahna, R.; Kraut-Cohen, J.; Zolti, A.; Erel, R.; Dietel, K. Microbial consortia versus single-strain inoculants: An advantage in PGPM-assisted tomato production? Agronomy 2019, 9, 105. [Google Scholar] [CrossRef]
- de Fretes, C.E.; Sembiring, L.; Purwestri, Y.A. Characterization of Streptomyces spp. producing indole-3-acetic acid as biostimulant agent. Indones. J. Biotechnol. 2013, 18, 83–91. [Google Scholar] [CrossRef][Green Version]
- Alkaabi, A.K.; Ramadan, G.A.; Elddin, A.M.T.; El-Tarabily, K.A.; AbuQamar, S.F. The multifarious endophytic actinobacterial isolate, Streptomyces tubercidicus UAE1, combined with the seaweed biostimulant further promotes growth of Avicennia marina. Front. Mar. Sci. 2022, 9, 896461. [Google Scholar] [CrossRef]
- Poveda, J.; González-Andrés, F. Bacillus as a source of phytohormones for use in agriculture. Appl. Microbiol. Biotechnol. 2021, 105, 8629–8645. [Google Scholar] [CrossRef]
- Glick, B.R. Bacteria with ACC deaminase can promote plant growth and help to feed the world. Microbiol. Res. 2014, 169, 30–39. [Google Scholar] [CrossRef]
- Hariprasad, P.; Niranjana, S.R. Isolation and characterization of phosphate solubilizing rhizobacteria to improve plant health of tomato. Plant Soil. 2009, 316, 13–24. [Google Scholar] [CrossRef]
- Rojas-Tapias, D.; Moreno-Galván, A.; Pardo-Díaz, S.; Obando, M.; Rivera, D.; Bonilla, R. Effect of inoculation with plant growth-promoting bacteria (PGPB) on amelioration of saline stress in maize (Zea mays). Appl. Soil Ecol. 2012, 61, 264–272. [Google Scholar] [CrossRef]
- Verma, M.; Singh, A.; Dwivedi, D.H.; Arora, N.K. Zinc and phosphate solubilizing Rhizobium radiobacter (LB2) for enhancing quality and yield of loose leaf lettuce in saline soil. Environ. Sustain. 2020, 3, 209–218. [Google Scholar] [CrossRef]
- Rizvi, A.; Khan, M.S. Heavy metal induced oxidative damage and root morphology alterations of maize (Zea mays L.) plants and stress mitigation by metal tolerant nitrogen fixing Azotobacter chroococcum. Ecotoxicol. Environ. Saf. 2018, 157, 9–20. [Google Scholar] [CrossRef]
- Viscardi, S.; Ventorino, V.; Duran, P.; Maggio, A.; De Pascale, S.; Mora, M.L.; Pepe, O. Assessment of plant growth promoting activities and abiotic stress tolerance of Azotobacter chroococcum strains for a potential use in sustainable agriculture. J. Soil Sci. Plant Nutr. 2016, 16, 848–863. [Google Scholar] [CrossRef]
- Van Oosten, M.J.; Di Stasio, E.; Cirillo, V.; Silletti, S.; Ventorino, V.; Pepe, O.; Raimondi, G.; Maggio, A. Root inoculation with Azotobacter chroococcum 76A enhances tomato plants adaptation to salt stress under low N conditions. BMC Plant Biol. 2018, 18, 205. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Huang, G.; Li, J.; Zheng, J.; Huang, Q.; Liu, H. Effect of soil moisture-based furrow irrigation scheduling on melon (Cucumis melo L.) yield and quality in an arid region of Northwest China. Agric. Water Manag. 2017, 179, 167–176. [Google Scholar] [CrossRef]
- Zhang, X.; Myrold, D.D.; Shi, L.; Kuzyakov, Y.; Dai, H.; Hoang, D.T.T.; Dippold, M.A.; Meng, X.; Song, X.; Li, Z. Resistance of microbial community and its functional sensitivity in the rhizosphere hotspots to drought. Soil Biol. Biochem. 2021, 161, 108360. [Google Scholar] [CrossRef]
- Haramija, J. Nutrient status, growth and proline concentration of French marigold (Tagetes patula L.) as affected by biostimulant treatment. J. Food Agric. Environ. 2013, 11, 2324–2327. [Google Scholar]
- Castiglione, A.M.; Mannino, G.; Contartese, V.; Bertea, C.M.; Ertani, A. Microbial biostimulants as response to modern agriculture needs: Composition, role and application of these innovative products. Plants 2021, 10, 1533. [Google Scholar] [CrossRef]
- Ferrante, A.; Spinardi, A.; Maggiore, T.; Testoni, A.; Gallina, P.M. Effect of nitrogen fertilisation levels on melon fruit quality at the harvest time and during storage. J. Sci. Food Agric. 2008, 88, 707–713. [Google Scholar] [CrossRef]
- Fernández-Vázquez, R.; Stinco, C.M.; Meléndez-Martínez, A.J.; Heredia, F.J.; Vicario, I.M. Visual and Instrumental evaluation of orange juice color: A consumers’ preference study. J. Sens. Stud. 2011, 26, 436–444. [Google Scholar] [CrossRef]
- Jeyalakshmi, S.; Radha, R. A review on diagnosis of nutrient deficiency symptoms in plant leaf image using digital image processing. ICTACT J. Image Video Process. 2017, 7, 1515–1524. [Google Scholar] [CrossRef]
- McCauley, A.; Jones, C.; Jacobsen, J. Functions and Deficiency and Toxicity Symptoms. Nutr. Manag. Modul. 2009, 9, 1–16. [Google Scholar]
- Moncada, A.; Miceli, A.; Vetrano, F. Use of plant growth-promoting rhizobacteria (PGPR) and organic fertilization for soilless cultivation of basil. Sci. Hortic. 2021, 275, 109733. [Google Scholar] [CrossRef]
- Adekunte, A.O.; Tiwari, B.K.; Cullen, P.J.; Scannell, A.G.M.; O’Donnell, C.P. Effect of sonication on colour, ascorbic acid and yeast inactivation in tomato juice. Food Chem. 2010, 122, 500–507. [Google Scholar] [CrossRef]
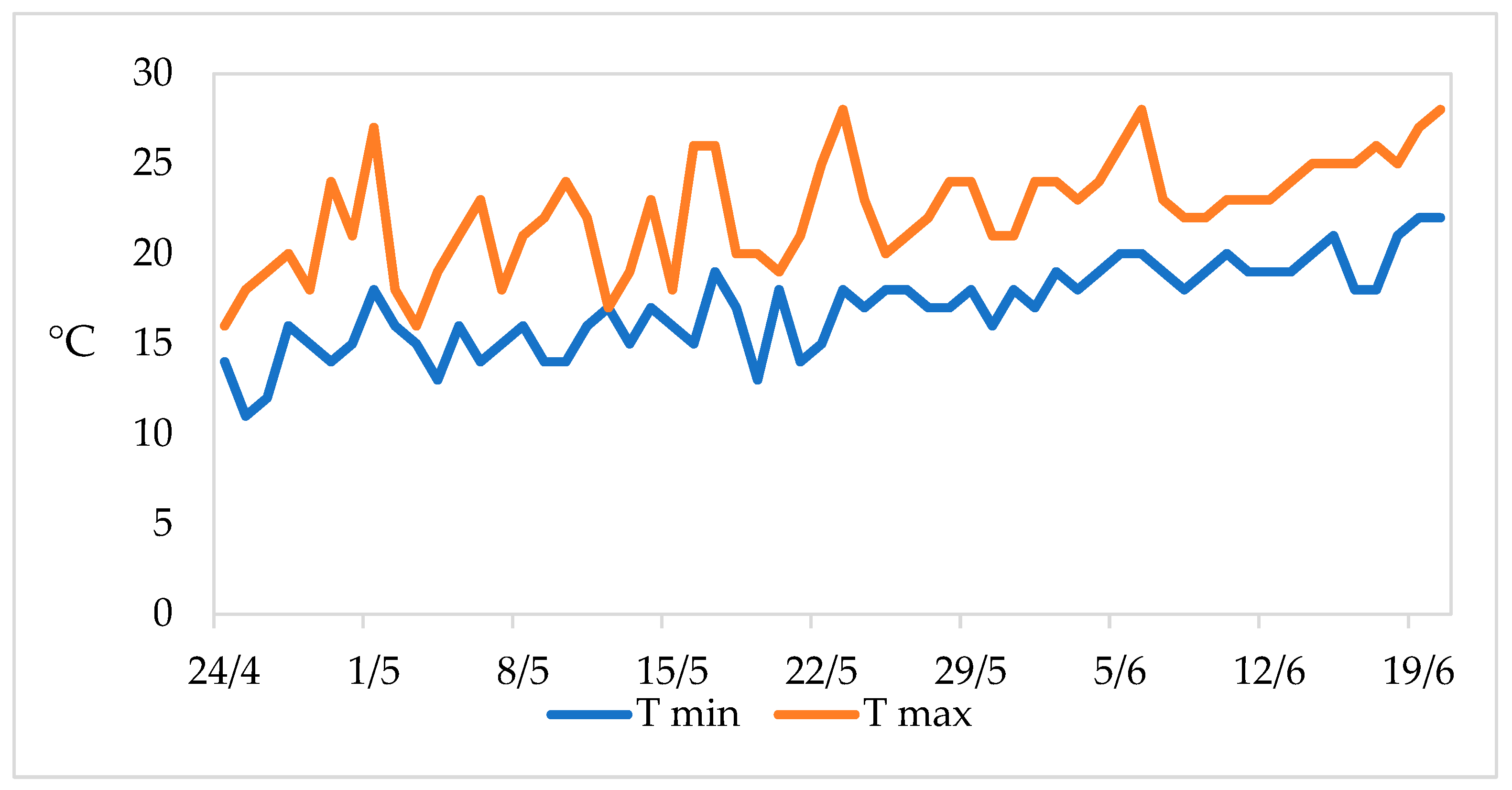
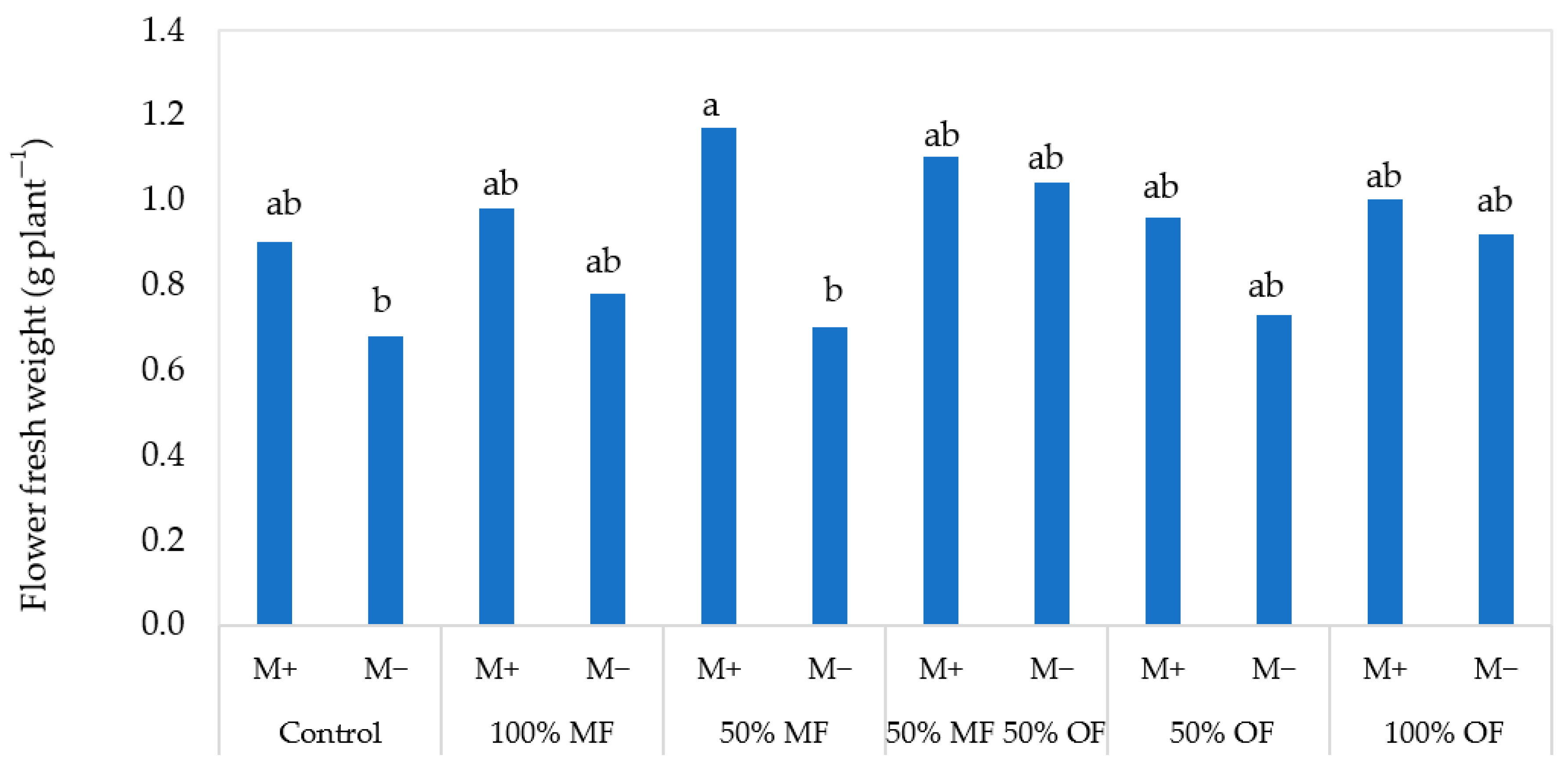




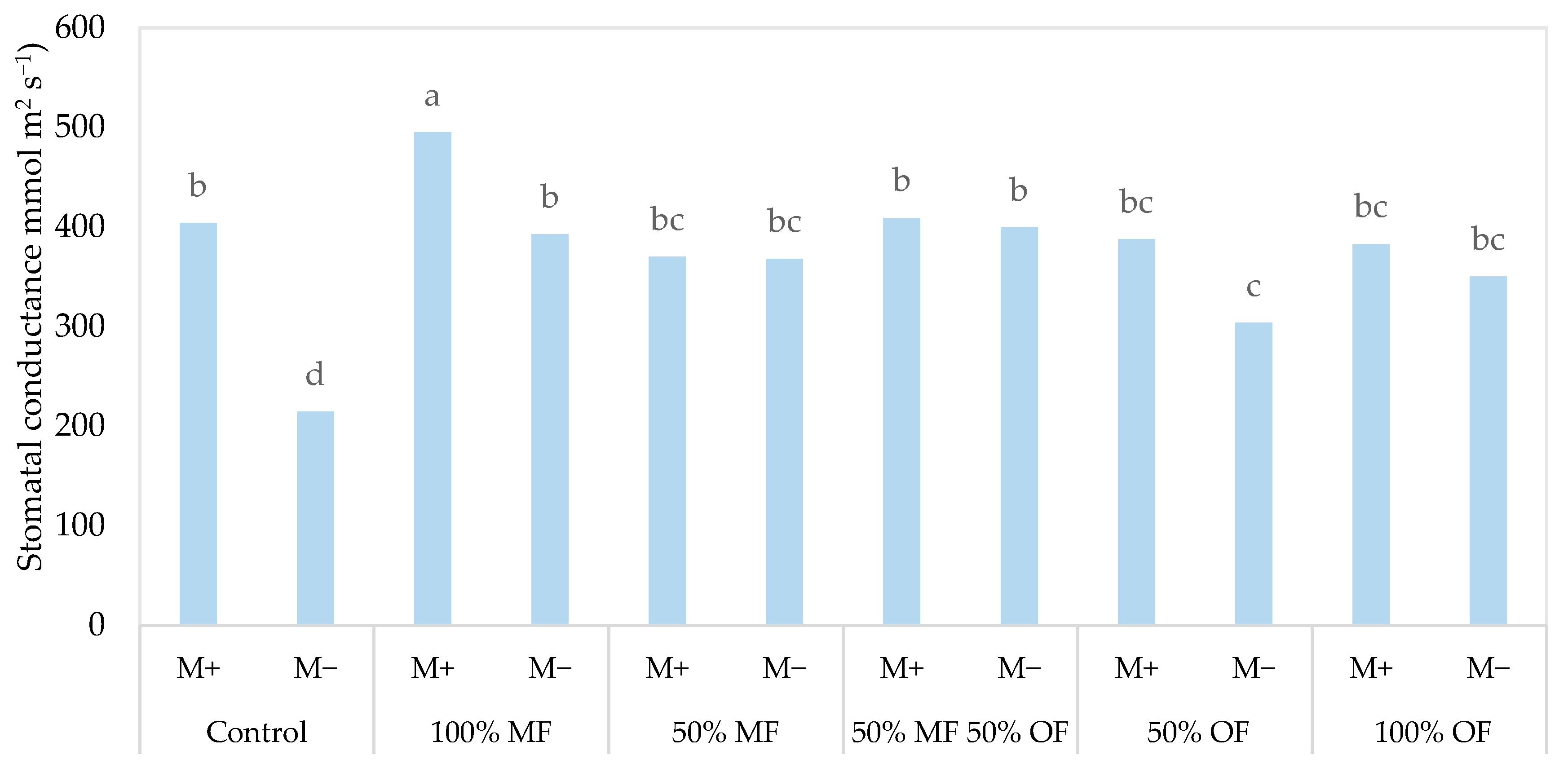
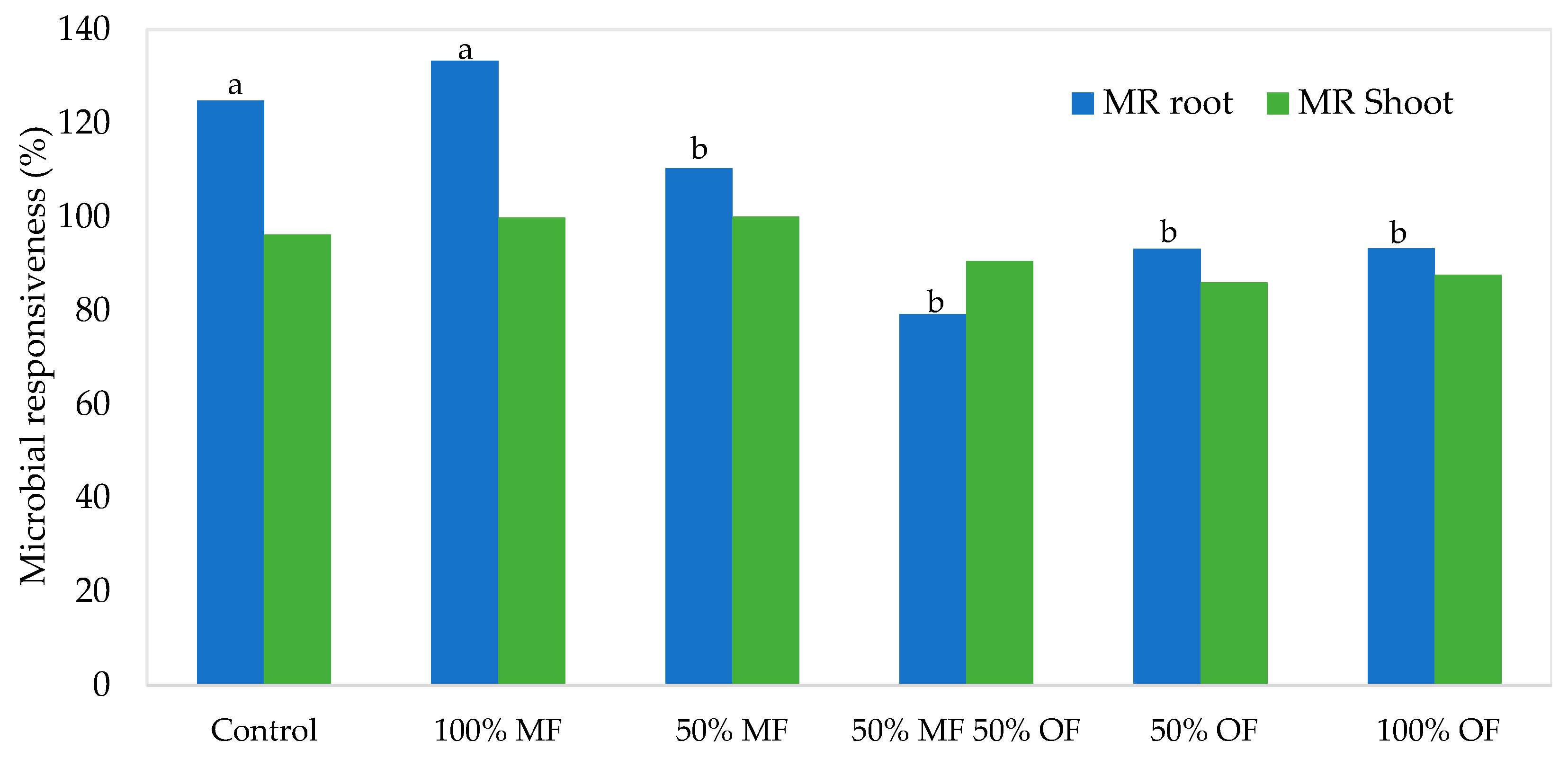
| Source of Variance | Plant Height (cm) | Stem Diameter (mm) | Shoot Number (n plant−1) | |||
|---|---|---|---|---|---|---|
| Microbial inoculum | ||||||
| M+ | 12.1 | a | 4.1 | a | 9.4 | a |
| M− | 11.0 | b | 3.9 | b | 8.4 | b |
| Fertigation | ||||||
| Control | 10.1 | d | 3.7 | d | 6.9 | b |
| 100% MF | 11.3 | c | 4.3 | a | 9.3 | a |
| 50% MF | 11.0 | cd | 4.1 | b | 8.3 | ab |
| 50% MF + 50% OF | 11.6 | bc | 4.3 | a | 10.2 | a |
| 50% OF | 12.4 | ab | 3.8 | dc | 8.9 | a |
| 100% OF | 12.8 | a | 4.0 | bc | 9.6 | a |
| Significance | ||||||
| Microbial inoculum (M) | ** | *** | * | |||
| Fertigation (F) | ** | ** | *** | |||
| M × F | ns | ns | ns | |||
| Source of Variance | Fresh Weight (g plant−1) | Dry Weight (g plant−1) | Dry Matter (%) | |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Plant | Stem | Leaves | Flower | Roots | Shoot/ Root | Plant | Stem | Leaves | Flower | Roots | Shoot/ Root | Shoot | Root | |||||||||||||||
| Microbial inoculum | ||||||||||||||||||||||||||||
| M+ | 24.66 | 2.69 | a | 6.84 | 1.01 | a | 14.12 | 0.74 | 2.34 | 0.41 | a | 0.85 | a | 0.14 | 1.01 | 1.39 | 13.3 | a | 7.2 | |||||||||
| M− | 22.81 | 2.53 | b | 6.64 | 0.80 | b | 12.82 | 0.77 | 2.26 | 0.37 | b | 0.76 | b | 0.15 | 0.95 | 1.35 | 12.8 | b | 7.4 | |||||||||
| Fertigation | ||||||||||||||||||||||||||||
| Control | 19.43 | d | 1.88 | e | 4.88 | e | 0.79 | b | 11.89 | c | 0.63 | b | 1.88 | d | 0.30 | c | 0.63 | d | 0.13 | b | 0.86 | b | 1.23 | b | 14.0 | a | 7.2 | |
| 100% MF | 27.62 | a | 3.15 | a | 8.72 | a | 0.88 | ab | 14.87 | a | 0.86 | a | 2.58 | a | 0.46 | a | 0.99 | a | 0.15 | ab | 1.02 | ab | 1.57 | a | 12.6 | b | 6.9 | |
| 50% MF | 24.56 | b | 2.79 | bc | 7.18 | b | 0.93 | ab | 13.66 | b | 0.81 | a | 2.41 | ab | 0.41 | ab | 0.85 | b | 0.15 | ab | 1.00 | ab | 1.41 | ab | 12.9 | b | 7.3 | |
| 50% MF + 50% OF | 25.87 | b | 2.88 | b | 7.60 | b | 1.07 | a | 14.32 | ab | 0.81 | a | 2.48 | ab | 0.41 | ab | 0.86 | b | 0.17 | a | 1.06 | a | 1.36 | ab | 12.5 | b | 7.4 | |
| 50% OF | 21.39 | cd | 2.38 | d | 5.67 | d | 0.85 | ab | 12.49 | bc | 0.72 | ab | 2.17 | c | 0.37 | b | 0.73 | c | 0.13 | b | 0.95 | ab | 1.29 | ab | 13.8 | a | 7.6 | |
| 100% OF | 23.58 | bc | 2.58 | cd | 6.40 | c | 0.96 | ab | 13.64 | b | 0.74 | ab | 2.27 | bc | 0.37 | b | 0.78 | bc | 0.14 | ab | 0.99 | ab | 1.30 | ab | 13.0 | b | 7.3 | |
| Significance | ||||||||||||||||||||||||||||
| Microbial inocul. (M) | ns | * | ns | * | ns | ns | ns | *** | *** | ns | ns | ns | *** | ns | ||||||||||||||
| Fertigation (F) | *** | *** | *** | * | * | ** | *** | *** | *** | ** | ** | ** | *** | ns | ||||||||||||||
| M × F | ns | ns | ns | ** | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ||||||||||||||
| Source of Variance | PWU (g FW L−1 H2O) | WUE (g DW L−1 H2O) | ||
|---|---|---|---|---|
| Microbial inoculum | ||||
| M+ | 27.8 | a | 2.6 | a |
| M− | 25.6 | b | 2.5 | b |
| Fertigation | ||||
| Control | 23.4 | c | 2.1 | b |
| 100% MF | 30.5 | a | 2.9 | a |
| 50% MF | 27.7 | ab | 2.7 | a |
| 50% MF 50% OF | 27.1 | b | 2.7 | a |
| 50% OF | 23.9 | c | 2.3 | b |
| 100% OF | 27.7 | ab | 2.7 | a |
| Significance | ||||
| Microbial inoculum (M) | * | * | ||
| Fertigation (F) | *** | *** | ||
| M × F | ns | ns | ||
| Source of Variance | Leaf Number | Leaf Area (cm2 plant−1) | Leaf Color | Flower Diameter (mm) | Flower Color | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| L* | Chroma | Hue | ΔE | L* | Chroma | Hue | ΔE | |||||||||||||||
| Microbial inoculum | ||||||||||||||||||||||
| M+ | 59.5 | 367.9 | a | 45.0 | 26.3 | a | 124.5 | 3.8 | 30.6 | 83.2 | 94.0 | 97.4 | 6.7 | a | ||||||||
| M− | 57.3 | 344.1 | b | 43.9 | 24.7 | b | 125.5 | 3.1 | 29.6 | 81.5 | 92.7 | 96.7 | 6.0 | b | ||||||||
| Fertigation | ||||||||||||||||||||||
| Control | 47.8 | c | 258.3 | d | 45.8 | 26.1 | ab | 123.8 | 5.5 | a | 29.0 | 81.8 | 99.6 | a | 96.2 | 4.9 | b | |||||
| 100% MF | 68.6 | a | 468.6 | a | 43.1 | 22.1 | c | 123.8 | 1.8 | d | 30.1 | 80.9 | 97.8 | a | 95.9 | 3.6 | c | |||||
| 50% MF | 61.9 | ab | 383.4 | b | 44.5 | 25.8 | ab | 126.4 | 3.3 | c | 30.2 | 84.3 | 91.8 | b | 98.6 | 6.4 | bc | |||||
| 50% MF 50% OF | 65.4 | ab | 384.8 | b | 44.6 | 26.8 | ab | 126.7 | 3.1 | c | 31.4 | 82.8 | 97.4 | a | 96.9 | 4.6 | c | |||||
| 50% OF | 48.3 | c | 293.0 | cd | 45.4 | 28.2 | a | 125.0 | 4.4 | b | 30.0 | 80.9 | 89.6 | b | 96.3 | 7.3 | b | |||||
| 100% OF | 58.4 | b | 317.8 | c | 43.3 | 24.1 | bc | 124.2 | 2.9 | c | 29.9 | 83.4 | 84.1 | c | 98.4 | 11.5 | a | |||||
| Significance | ||||||||||||||||||||||
| Microbial inoculum (M) | ns | *** | ns | * | ns | ns | ns | ns | ns | ns | ** | |||||||||||
| Fertigation (F) | *** | *** | ns | ** | ns | ** | ns | ns | * | ns | ** | |||||||||||
| M × F | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | |||||||||||
| Source of Variance | Plant Height (cm) | Stem Diameter (mm) | Shoot Number (n. plant−1) | |||
|---|---|---|---|---|---|---|
| Microbial inoculum | ||||||
| M+ | 19.5 | 5.8 | a | 4.7 | ||
| M− | 17.3 | 5.2 | b | 4.6 | ||
| Fertigation | ||||||
| Control | 17.8 | ab | 5.1 | c | 3.3 | |
| 100% MF | 16.6 | b | 5.8 | a | 5.9 | |
| 50% MF | 17.9 | ab | 5.6 | ab | 4.3 | |
| 50% MF 50% OF | 19.3 | ab | 5.8 | a | 4.9 | |
| 50% OF | 19.8 | a | 5.3 | bc | 3.9 | |
| 100% OF | 18.9 | ab | 5.5 | ac | 4.0 | |
| Significance | ||||||
| Microbial inoculum (M) | ns | *** | * | |||
| Fertigation (F) | * | ** | *** | |||
| M × F | ns | ns | * | |||
| Source of Variance | Fresh Weight (g plant−1) | Dry Weight (g plant−1) | Dry Matter (%) | |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Plant | Stem | Leaves | Flower | Roots | Shoot/ Root | Plant | Stem | Leaves | Flower | Roots | Shoot/ Root | Shoot | Root | |||||||||||||||
| Microbial inoculum | ||||||||||||||||||||||||||||
| M+ | 28.38 | a | 5.62 | a | 10.11 | a | 1.90 | 11.71 | 1.52 | 3.10 | 0.90 | 1.07 | 0.23 | 1.01 | 2.18 | 12.5 | 8.6 | |||||||||||
| M− | 26.45 | b | 4.82 | b | 8.71 | b | 1.44 | 10.69 | 1.40 | b | 2.89 | b | 0.81 | b | 0.96 | b | 0.17 | 0.86 | 2.25 | 13.0 | 8.0 | |||||||
| Fertigation | ||||||||||||||||||||||||||||
| Control | 21.20 | d | 3.92 | c | 7.01 | d | 1.32 | b | 8.95 | d | 1.37 | c | 2.42 | d | 0.65 | c | 0.84 | b | 0.16 | 0.80 | c | 2.06 | ab | 13.4 | a | 8.9 | ab | |
| 100% MF | 29.75 | ab | 5.72 | a | 11.92 | a | 1.84 | ab | 10.28 | cd | 1.89 | a | 3.18 | ab | 0.92 | ab | 1.22 | a | 0.22 | 0.84 | bc | 2.80 | a | 12.1 | c | 8.2 | b | |
| 50% MF | 28.20 | b | 5.44 | a | 10.10 | b | 1.92 | a | 10.74 | bd | 1.63 | b | 3.22 | ab | 0.91 | ab | 1.10 | a | 0.24 | 0.97 | ab | 2.33 | ab | 12.9 | ac | 9.0 | a | |
| 50% MF 50% OF | 31.69 | a | 5.93 | a | 10.47 | b | 1.78 | ab | 13.51 | a | 1.37 | c | 3.38 | a | 0.98 | a | 1.14 | a | 0.21 | 1.07 | a | 2.17 | ab | 12.8 | ac | 7.9 | b | |
| 50% OF | 25.39 | c | 4.73 | b | 7.83 | d | 1.46 | ab | 11.38 | bc | 1.23 | c | 2.76 | c | 0.80 | b | 0.84 | b | 0.18 | 0.94 | ab | 1.93 | b | 13.0 | ab | 8.3 | ab | |
| 100% OF | 28.80 | b | 5.57 | a | 9.13 | c | 1.72 | ab | 12.39 | ab | 1.32 | c | 3.02 | bc | 0.88 | ab | 0.95 | b | 0.21 | 0.98 | ab | 2.08 | ab | 12.4 | bc | 7.9 | b | |
| Significance | ||||||||||||||||||||||||||||
| Microbial inoculum (M) | ** | *** | *** | ns | ns | *** | * | * | *** | ns | ns | ns | ns | ns | ||||||||||||||
| Fertigation (F) | *** | *** | *** | * | *** | *** | *** | *** | *** | ns | ** | ** | ** | ** | ||||||||||||||
| M × F | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ** | ns | ns | ||||||||||||||
| Source of Variance | PWU (g FW L−1 H2O) | WUE (g DW L−1 H2O) | ||
|---|---|---|---|---|
| Microbial inoculum | ||||
| M+ | 31.4 | 3.4 | ||
| M− | 30.8 | 3.4 | ||
| Fertigation | ||||
| Control | 26.5 | c | 3.1 | d |
| 100% MF | 33.8 | a | 3.6 | a |
| 50% MF | 31.7 | ab | 3.6 | a |
| 50% MF 50% OF | 33.2 | a | 3.6 | ab |
| 50% OF | 29.7 | b | 3.2 | cd |
| 100% OF | 31.9 | ab | 3.3 | bc |
| Significance | ||||
| Microbial inoculum (M) | ns | ns | ||
| Fertigation (F) | *** | *** | ||
| M × F | ns | ns | ||
| Source of Variance | Leaves (n. plant−1) | Leaf Area (cm2 plant−1) | Leaf Color | Flower (n. plant−1) | Flower Color | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| L* | Chroma | Hue | ΔE | L* | Chroma | Hue | ΔE | |||||||||||||||
| Microbial inoculum | ||||||||||||||||||||||
| M+ | 38.4 | a | 399.1 | a | 42.7 | 28.5 | 125.9 | 2.6 | 33.4 | 37.4 | ns | 36.8 | a | 324.7 | b | 18.2 | a | |||||
| M− | 34.5 | b | 353.2 | b | 43.3 | 28.4 | 126.3 | 2.6 | 27.9 | 38.1 | 23.7 | b | 332.9 | a | 8.8 | b | ||||||
| Fertigation | ||||||||||||||||||||||
| Control | 27.8 | d | 275.9 | f | 42.5 | 28.4 | 126.2 | 3.9 | a | 27.2 | b | 41.8 | a | 28.2 | b | 331.5 | a | 19.3 | a | |||
| 100% MF | 43.1 | a | 491.9 | a | 41.1 | 26.5 | 127.4 | 1.2 | c | 30.3 | ab | 38.8 | a | 33.4 | ab | 325.1 | b | 14.0 | b | |||
| 50% MF | 35.5 | c | 392.0 | c | 43.4 | 28.6 | 126.1 | 2.3 | b | 33.3 | a | 38.1 | a | 36.6 | a | 325.2 | b | 17.6 | a | |||
| 50% MF 50% OF | 39.3 | b | 432.7 | b | 42.8 | 28.8 | 126.0 | 1.2 | c | 31.5 | ab | 37.2 | ab | 37.7 | a | 330.6 | a | 7.5 | bc | |||
| 50% OF | 33.8 | c | 312.1 | e | 44.1 | 29.7 | 125.2 | 3.4 | a | 27.3 | b | 35.6 | b | 26.8 | b | 323.9 | b | 18.3 | a | |||
| 100% OF | 39.2 | b | 352.2 | d | 44.1 | 28.7 | 125.7 | 3.4 | a | 34.7 | a | 35.1 | b | 18.8 | c | 336.6 | a | 4.3 | c | |||
| Significance | ||||||||||||||||||||||
| Microbial inoculum (M) | ** | * | ns | ns | ns | ns | ns | ns | * | * | * | |||||||||||
| Fertigation (F) | *** | ** | ns | ns | ns | * | ** | * | * | * | ** | |||||||||||
| M × F | ns | ns | ns | ns | ns | ns | *** | ns | ns | ns | ns | |||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miceli, A.; Moncada, A.; Vetrano, F.; Esposito, A. Response of Tagetes patula L. and Ageratum houstonianum Mill. to Microbial Biostimulant Inoculation and Organic Fertilization. Agronomy 2023, 13, 2522. https://doi.org/10.3390/agronomy13102522
Miceli A, Moncada A, Vetrano F, Esposito A. Response of Tagetes patula L. and Ageratum houstonianum Mill. to Microbial Biostimulant Inoculation and Organic Fertilization. Agronomy. 2023; 13(10):2522. https://doi.org/10.3390/agronomy13102522
Chicago/Turabian StyleMiceli, Alessandro, Alessandra Moncada, Filippo Vetrano, and Alessandro Esposito. 2023. "Response of Tagetes patula L. and Ageratum houstonianum Mill. to Microbial Biostimulant Inoculation and Organic Fertilization" Agronomy 13, no. 10: 2522. https://doi.org/10.3390/agronomy13102522
APA StyleMiceli, A., Moncada, A., Vetrano, F., & Esposito, A. (2023). Response of Tagetes patula L. and Ageratum houstonianum Mill. to Microbial Biostimulant Inoculation and Organic Fertilization. Agronomy, 13(10), 2522. https://doi.org/10.3390/agronomy13102522






