Comparison of System of Rice Intensification Applications and Alternatives in India: Agronomic, Economic, Environmental, Energy, and Other Effects
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAOSTAT. FAO Food and Agriculture Database 2019; UN Food and Agriculture Organization: Rome, Italy, 2021; Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 10 October 2022).
- Agricultural Statistics at a Glance; Directorate of Economics and Statistics, Ministry of Agriculture and Farmers Welfare, Government of India: New Delhi, India, 2022.
- Yadav, S.N.; Chandra, R.; Khura, T.K.; Chauhan, N.S. Energy input-output analysis and mechanisation status for the cultivation of rice and maize crops in Sikkim. Agric. Engin. Int. CIGR J. 2013, 15, 108–116. [Google Scholar]
- Gujja, B.; Thiyagarajan, T.M. New Hope for Indian Food Security? The System of Rice Intensification; Gatekeeper Series; International Institute for Environment and Development: London, UK, 2009. [Google Scholar]
- SRI-Rice Website, Cornell University. Available online: http://sri.cals.cornell.edu/countries/index.html (accessed on 22 November 2022).
- Awan, T.H.; Ali, I.; Safdar, M.E.; Ahmad, M.; Akhtar, M.S. Comparison of parachute, line, and traditional rice transplanting methods at farmer’s field in rice growing area. Pak. J. Agric. Sci. 2008, 45, 432–438. [Google Scholar]
- Swain, S.K.; Nayak, B.R.; Khanda, C.M.; Dash, A.K. Effect of establishment methods on yield and economics of rice (Oryza sativa). Madras Agric. J. 2013, 100, 803–805. [Google Scholar]
- Singh, T.V.; Kumar, M.R.; Viraktamath, B.C. Selective mechanization in rice cultivation for energy saving and enhancing profitability. In Rice Knowledge Management Portal; Indian Institute of Rice Research: Hyderabad, India, 2011. [Google Scholar]
- International Rice Research Institute (IRRI). What Is DSR? DSR Consortium Website. Available online: https://dsrc.irri.org/our-work/what-is-dsr (accessed on 29 August 2023).
- Sooksa-Nguan, T.; Thies, J.E.; Gypmantsiri, P.; Boonkerd, N.; Teaumroong, N. Effect of rice cultivation system on nitrogen cycling and nitrifying bacterial community structure. Appl. Soil Ecol. 2009, 43, 139–149. [Google Scholar] [CrossRef]
- Anas, I.; Rupela, O.P.; Thiyagarajan, T.M.; Uphoff, N. A review of studies on SRI effects on beneficial organisms in rice soil rhizospheres. Paddy Water Envir. 2011, 9, 53–64. [Google Scholar] [CrossRef]
- Doni, F.; Mispan, M.S.; Shamsinah, N.; Suhaimi, M.; Ishak, N.; Uphoff, N. Roles of microbes in supporting sustainable rice production using the system of rice intensification. Appl. Microbiol. Biotechnol. 2019, 103, 5131–5142. [Google Scholar] [CrossRef] [PubMed]
- Thakur, A.K.; Uphoff, N.; Stoop, W.A. Scientific underpinnings of the System of Rice Intensification (SRI): What is known so far? Adv. Agron. 2016, 135, 147–179. [Google Scholar]
- Uphoff, N. SRI 1.0 and beyond: Understanding the System of Crop Intensification as SRI 3.0. J. Rice Res. 2022, 15, 3–9. [Google Scholar] [CrossRef]
- Mittal, J.P.; Dhawan, K.C. Research Manual on Energy Requirements in Agricultural Sector; ICAR: New Delhi, India, 1989; pp. 20–23. [Google Scholar]
- Yadav, S.K.; Babu, S.; Singh, Y.; Yadav, G.S.; Singh, K.; Singh, R.; Singh, H. Effect of organic nitrogen sources and biofertilizers on production potential and energy budgeting of rice (Oryza sativa)-based cropping systems. Indian J. Agron. 2014, 58, 459–464. [Google Scholar]
- Devasenapathy, P.; Senthilkumar, G.; Shanmugam, P.M. Energy management in crop production. Indian J. Agron. 2009, 54, 80–90. [Google Scholar]
- Islam, A.K.M.; Rahman, S.M.A.; Sarker, R.I.; Ahiduzzaman, M.; Baqui, M.A. Energy audit for rice production under Bangladesh. Online J. Biol. Sci. 2001, 1, 873–876. [Google Scholar]
- Cervinka, V. Fuel and energy efficiency. In Handbook of Energy Utilization in Agriculture; Pimentel, D., Ed.; CRC Press: Boca Raton, FL, USA, 1980; pp. 15–20. [Google Scholar]
- Singh, S.; Mittal, J.P. Energy in Production Agriculture; Mittal Publications: New Delhi, India, 1992. [Google Scholar]
- Singh, H.; Mishra, D.; Nahar, N.M. Energy use pattern in production agriculture of a typical village in arid zone India, Part I. Energy Convers. Manag. 2002, 43, 2275–2286. [Google Scholar] [CrossRef]
- Alipour, A.; Veisi, H.; Darijani, F.; Mirbagheri, B.; Behbahani, A.G. Study and determination of energy consumption to produce conventional rice of the Guilan province. Res. Agric. Eng. 2012, 58, 99–106. [Google Scholar] [CrossRef]
- Ozkan, B.; Akcaoz, H.; Fert, C. Energy input-output analysis in Turkish agriculture. Renew. Energy 2004, 29, 39–51. [Google Scholar] [CrossRef]
- Iturbide, M.; Fernández, J.; Gutiérrez, J.M.; Pirani, A.; Huard, D.; Al Khourdajie, A.; Baño-Medina, J.; Bedia, J.; Casanueva, A.; Cimadevilla, E.; et al. Implementation of FAIR principles in the IPCC: The WGI AR6 Atlas repository. Sci. Data 2022, 9, 629. [Google Scholar] [CrossRef] [PubMed]
- Biswas, J.C.; Haque, M.M.; Hossain, M.B.; Maniruzzaman, M.; Zahan, T.; Rahman, M.M.; Sen, R.; Ishtiaque, S.; Chaki, A.K.; Ahmed, I.M.; et al. Seasonal variations in grain yield, greenhouse gas emissions, and carbon sequestration for maize cultivation in Bangladesh. Sustainability 2022, 14, 9144. [Google Scholar] [CrossRef]
- Allen, O.N. Experiments in Soil Bacteriology; Burgess Publishing: Minneapolis, MN, USA, 1953. [Google Scholar]
- Martin, J.P. Use of acid, rose bengal, and streptomycin in the plate method for estimating soil fungi. Soil Sci. 1953, 69, 215–232. [Google Scholar] [CrossRef]
- Kuster, E.; Williams, S.T. Selection of media for isolation of Streptomycetes. Nature 1964, 202, 928–929. [Google Scholar] [CrossRef]
- Adam, G.; Duncan, H. Development of a sensitive and rapid method for the measurement of total microbial activity using fluorescein diacetate (FDA) in a range of soils. Soil Biol. Biochem. 2001, 33, 943–951. [Google Scholar] [CrossRef]
- Bottomley, P.S.; Angle, J.S.; Weaver, R.W.; Tabatabai, M.A. Soil enzymes. In Methods of Soil Analysis, Pt. 2. Microbiological and Biochemical Properties; Weaver, R.W., Ed.; Soil Science Society of America: Madison, WI, USA, 1994; pp. 775–883. [Google Scholar]
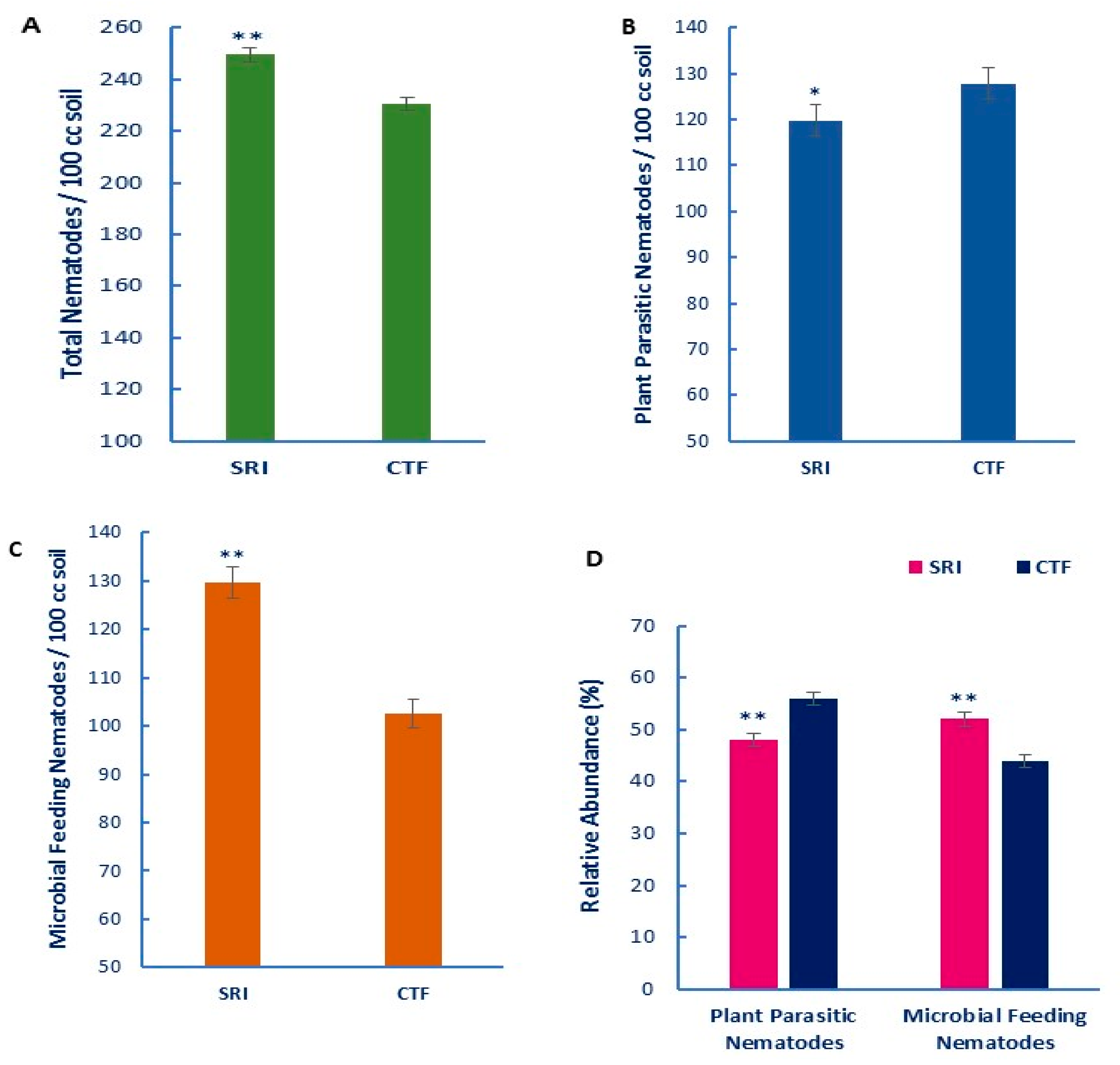
- Yeates, G.W.; Bongers, T.; De Goede, R.G.M.; Freckman, D.W.; Georgieva, S.S. Feeding habits in soil nematode families and genera: An outline for soil ecologists. J. Nematol. 1993, 25, 315–331. [Google Scholar]
- Tuti, M.D.; Rapolu, M.K.; Sreedevi, B.; Bandumula, N.; Kuchi, S.; Bandeppa, S.; Saha, S.; Parmar, B.; Rathod, S.; Ondrasek, G.; et al. Sustainable intensification of a rice–maize system through Conservation Agriculture to enhance system productivity in Southern India. Plants 2022, 11, 1229. [Google Scholar] [CrossRef] [PubMed]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016; Available online: https://ggplot2.tidyverse.org (accessed on 12 November 2022).
- Statistical Analysis Software (SAS), version 9.3; SAS Institute: Cary, NC, USA, 2011.
- Gathorne-Hardy, A.; Reddy, D.N.; Venkatanarayana, M.; Harriss-White, B. A Life Cycle Assessment (LCA) of greenhouse gas emissions from SRI and flooded rice production in SE India. Taiwan Water Conserv. 2013, 61, 110–112. [Google Scholar]
- Jain, N.; Dubey, R.; Dubey, D.S.; Singh, J.; Khanna, M.; Pathak, H.; Bhatia, A. Mitigation of greenhouse gas emissions with system of rice intensification in the Indo-Gangetic plains. Paddy Water Environ. 2014, 12, 355–363. [Google Scholar] [CrossRef]
- Suryavanshi, P.; Singh, Y.V.; Prasanna, R.; Bhatia, A.; Shivay, Y.S. Pattern of methane emission and water productivity under different methods of rice crop establishment. Paddy Water Environ. 2012, 11, 321–332. [Google Scholar] [CrossRef]
- Choi, J.; Kim, G.; Park, W.; Shin, M.; Choi, Y.; Lee, S.; Lee, D.; Yun, D. Effect of SRI methods on water use, NPS pollution discharge, and greenhouse gas emissions in Korean trials. Paddy Water Environ. 2015, 13, 205–213. [Google Scholar] [CrossRef]
- Wang, Y. N2O emission from paddy field under different rice planting modes. Wuhan Univ. J. Nat. Sci. 2006, 11, 989–996. [Google Scholar]
- Dill, J.; Deichert, G.; Le, T.N.T. Promoting the System of Rice Intensification: Lessons Learned from Trà Vinh Province, Vietnam; GIZ and IFAD: Hanoi, Vietnam, 2013; Available online: https://wocatpedia.net/images/f/f2/Giz2013-0503en-rice-lessons-learned-vietnam.pdf (accessed on 30 August 2023).
- Thakur, A.K.; Uphoff, N. How the system of rice intensification can contribute to climate-smart agriculture. Agron. J. 2017, 109, 1163–1182. [Google Scholar] [CrossRef]
- Laulanié, H. Le système de riziculture intensive malgache. Tropicultura 1993, 11, 110–114, republished in English, 2011, 29, 183–187. Available online: http://www.tropicultura.org/text/v29n3.pdf#page=57 (accessed on 4 September 2023).
- Uphoff, N. SRI 2.0 and beyond: Sequencing the protean evolution of the System of Rice Intensification. Agronomy 2023, 13, 1253. [Google Scholar] [CrossRef]
- Bouman, B.A.M.; Tuong, T.P. Field water management to save water and increase its productivity in irrigated lowland rice. Agric. Water Manag. 2001, 49, 11–30. [Google Scholar] [CrossRef]
- Jayadeva, H.M.; Setty, T.K.P.; Bandi, A.G.; Gowda, R.C. Water use efficiency, energetics, and economics of rice as influenced by crop establishment techniques and sources of nitrogen. Crop Res. 2010, 39, 14–19. [Google Scholar]
- Uphoff, N.; Kassam, A.; Harwood, R. SRI as a methodology for raising crop and water productivity: Productive adaptations in rice agronomy and irrigation water management. Paddy Water Environ. 2011, 9, 3–11. [Google Scholar] [CrossRef]
- Ambani, S.; Selvakumar, S.; Thirukumaran, K. Mechanized transplanting in system of rice intensification and its evaluation. Int. J. Chem. Stud. 2020, 8, 2301–2305. [Google Scholar]
- Satyanarayana, A.; Thiyagarajan, T.M.; Uphoff, N. Opportunities for water saving with higher yield from the system of rice intensification. Irrig. Sci. 2007, 25, 99–115. [Google Scholar] [CrossRef]
- Nirmala, B.; Tuti, M.D.; Mahender Kumar, R.; Waris, A.; Muthuraman, P.; Parmar, B.; Vidhan Singh, T. Integrated assessment of system of rice intensification vs conventional method of transplanting for economic benefit, energy efficiency and lower global warming potential in India. Agroecol. Sustain. Food Syst. 2021, 45, 745–766. [Google Scholar] [CrossRef]
- Reuben, P.; Katambara, Z.; Kahimba, F.C.; Mahoo, H.F.; Mbungu, W.B.; Mhenga, F.; Nyarubamba, A.; Maugo, M. Influence of transplanting age on paddy yield under the system of rice intensification. Agric. Sci. 2016, 7, 154–163. [Google Scholar] [CrossRef][Green Version]
- Cai, Z.C.; Shan, Y.H.; Xu, H. Effects of nitrogen fertilization on CH4 emissions from rice fields. Soil Sci. Plant Nutr. 2007, 53, 353–361. [Google Scholar] [CrossRef]
- Gathorne-Hardy, A.; Narasimha Reddy, D.; Venkatanarayana, M.; Barbara Harriss-White, B. System of Rice Intensification provides environmental and economic gains but at the expense of social sustainability—A multidisciplinary analysis in India. Agric. Syst. 2016, 143, 159–168. [Google Scholar] [CrossRef]
- Gayathry, G. Studies on Dynamics of Soil Microbes in Rice Rhizosphere with Water Saving Irrigation and In Situ Weed Incorporation. Master’s Thesis, Tamil Nadu Agricultural University, Coimbatore, India, 2002. [Google Scholar]
- Gopalakrishnan, S.; Mahender Kumar, R.; Humayun, P.; Srinivas, V.; Ratna Kumari, B.; Vijayabharathi, R.; Singh, A.; Surekha, K.; Padmavathi, C.; Somashekar, N.; et al. Assessment of different methods of rice (Oryza sativa L.) cultivation affecting growth parameters, soil chemical, biological, and microbiological properties, water saving, and grain yield in rice–rice system. Paddy Water Environ. 2013, 12, 79–87. [Google Scholar] [CrossRef]
- Ma, L.; Guo, C.; Lü, X.; Yuan, S.; Wang, R. Soil moisture and land use are major determinants of soil microbial community composition and biomass at a regional scale in northeastern China. Biogeosciences 2015, 12, 2585–2596. [Google Scholar] [CrossRef]
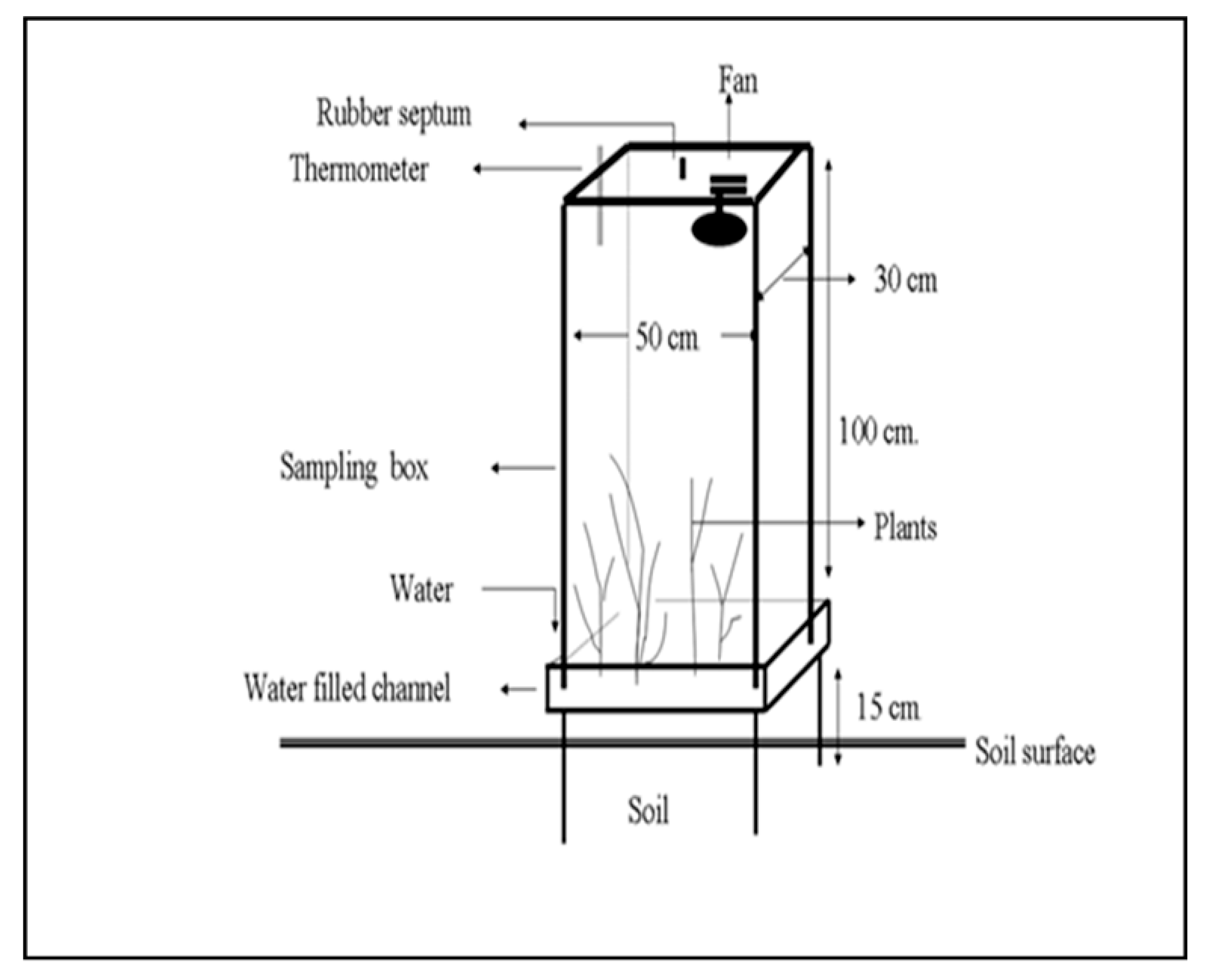

| Parameters | SRI | MSRI | DSR | CTF |
|---|---|---|---|---|
| Seed rate (kg ha−1) | 5 | 12 | 15 | 45 |
| No. of hills m−2 | 16 | 42 | 83 | 33 |
| No. of seedlings hill−1 | 1 | 3–4 | 2–3 | 3–4 |
| Plant density (m−2) | 16 | 125–170 | 165–250 | 100–132 |
| Nursery for raising seedlings | Raised bed, not flooded | Raised bed, mat nursery | No nursery needed | Flooded nursery |
| Nursery (m2 ha−1) | 100 m2 | 100 m2 | Nil | 1000 m2 |
| Seedling age at transplanting (days) | 12–14 | 16–18 | Direct sowing by drum seeder in the main field | 30–35 |
| Spacing (cm) | 25 × 25 cm | 24 cm between rows; 10–12 cm between plants | 20 cm between rows; 6 cm between plants | 20 × 15 cm |
| Water management | Alternate wetting & drying (AWD) | AWD method | AWD method | Continuous flooding |
| Weed management | Cono-weeder used 3 times in two directions | Cono-weeder used 3 times in one direction | Cono-weeder used 3 times in one direction | Manual weeding (3 times) |
| Weather Parameters | Wet Season | |||||
|---|---|---|---|---|---|---|
| 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | |
| Average temperature (°C) | 25.5 | 25.1 | 26.1 | 26.8 | 25.4 | 24.8 |
| Maximum temperature (°C) | 29.9 | 29.1 | 31.4 | 32.0 | 29.9 | 30.3 |
| Minimum temperature (°C) | 21.0 | 20.7 | 21.8 | 21.6 | 20.8 | 19.4 |
| Total rainfall (mm) | 584.8 | 710.5 | 432.5 | 373.1 | 749.1 | 969.8 |
| Dry season | ||||||
| 2012–2013 | 2013–2014 | 2014–2015 | 2015–2016 | 2016–2017 | 2017–2018 | |
| Average temperature (°C) | 25.7 | 24.1 | 23.9 | 26.1 | 24.2 | 26.1 |
| Maximum temperature (°C) | 33.0 | 31.7 | 31.5 | 34.1 | 33.0 | 34.8 |
| Minimum temperature (°C) | 18.4 | 16.4 | 16.3 | 18.1 | 15.3 | 17.5 |
| Total rainfall (mm) | 74.2 | 129.4 | 159.1 | 7.0 | 10.2 | 64.7 |
| Method of Establishment | Grain Yield (t ha−1) | ||||||
|---|---|---|---|---|---|---|---|
| Experiment 1 | Experiment 2 | Experiment 3 | Experiment 4 | ||||
| Wet Season | Dry Season | Wet Season | Dry Season | Wet Season | Dry Season | Wet Season | |
| SRI | 6.23 a | 6.47 a | 6.09 a | 6.23 a | - | - | - |
| MSRI | 4.75 b | 5.02 b | 5.72 b | 5.65 b | 6.27 a | 6.41 a | 5.07 a |
| DSR | - | - | - | - | 6.02 a | 6.09 a | - |
| CTF | 4.10 c | 4.44 c | - | - | 5.59 b | 5.36 b | 4.64 b |
| SEm± | 0.05 | 0.01 | 0.03 | 0.04 | 0.06 | 0.10 | 0.32 |
| CD (p ≤ 0.05) | 0.28 | 0.15 | 0.24 | 0.28 | 0.33 | 0.41 | 0.09 |
| p ≥ F | 0.07 | 0.0002 | 0.36 | 0.07 | 0.11 | 0.54 | 0.23 |
| Method of Establishment | A. Water productivity (kg ha-mm−1) | ||||||
| Experiment 1 | Experiment 2 | Experiment 3 | Experiment 4 | ||||
| Wet Season | Dry Season | Wet Season | Dry Season | Wet Season | Dry Season | Wet Season | |
| SRI | 5.53 a | 6.83 a | 5.32 a | 5.32 a | - | - | - |
| MSRI | 4.14 b | 5.12 b | 5.16 b | 5.16 b | 5.48 a | 5.67 a | 5.72 a |
| DSR | - | - | - | - | 5.06 b | 5.11 b | - |
| CTF | 3.52 c | 4.50 c | - | - | 4.42 c | 4.56 c | 4.18 b |
| SEm± | 0.05 | 0.08 | 0.003 | 0.01 | 0.02 | 0.02 | 0.13 |
| CD (p ≤ 0.05) | 0.3 | 0.37 | 0.08 | 0.08 | 0.16 | 0.17 | 0.46 |
| p ≥ F | 0.10 | 0.05 | 0.0008 | 0.08 | 0.05 | 0.41 | 0.0003 |
| Method of Establishment | B. Economic Productivity (Benefit/Cost Ratio) | ||||||
| Experiment 1 | Experiment 2 | Experiment 3 | Experiment 4 | ||||
| Wet Season | Dry Season | Wet Season | Dry Season | Wet Season | Dry Season | Wet Season | |
| SRI | 3.12 a | 2.93 a | 1.42 a | 1.44 a | - | - | - |
| MSRI | 2.69 b | 2.67 b | 1.34 b | 1.31 b | 1.48 a | 1.52 a | 1.91 a |
| DSR | - | - | - | - | 1.33 b | 1.21 b | - |
| CTF | 2.21 c | 2.14 c | - | - | 1.15 c | 1.16 c | 1.63 b |
| SEm± | 0.02 | 0.02 | 0.004 | 0.001 | 0.01 | 0.01 | 0.13 |
| CD (p ≤ 0.05) | 0.17 | 0.2 | 0.03 | 0.05 | 0.13 | 0.15 | 0.04 |
| p ≥ F | 0.002 | 0.003 | 0.02 | 0.01 | 0.02 | 0.89 | 0.45 |
| Method of Establishment | Energy Use Efficiency (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Experiment 1 | Experiment 2 | Experiment 3 | Experiment 4 | Wet Season Mean | Dry Season Mean | Total Mean | ||||
| Wet Season | Dry Season | Wet Season | Dry Season | Wet Season | Dry Season | Wet Season | ||||
| SRI | 11.44 a | 11.76 a | 12.30 a | 11.79 a | - | - | - | 11.9 | 11.8 | 11.8 |
| MSRI | 8.94 b | 9.25 b | 10.55 b | 10.49 b | 10.45 a | 10.84 a | 7.09 a | 9.7 | 10.2 | 9.7 |
| DSR | - | - | - | - | 10.08 b | 10.15 b | - | 10.1 | 10.2 | 10.1 |
| CTF | 7.02 c | 7.48 c | - | - | 9.82 c | 10.08 c | 6.34 b | 7.7 | 8.8 | 8.3 |
| SEm± | 0.12 | 0.04 | 0.002 | 0.003 | 0.02 | 0.04 | 0.1 | |||
| CD (p ≤ 0.05) | 0.44 | 0.27 | 0.02 | 0.02 | 0.20 | 0.25 | 0.33 | |||
| p ≥ F | 0.004 | 0.01 | 0.0001 | 0.0001 | 0.001 | 0.86 | 0.15 | |||
| Method of Establishment | A. Methane (CH4) Emissions (kg ha−1 Season−1) | |||||||||
| Experiment 1 | Experiment 3 | Wet Season Mean | Dry Season Mean | |||||||
| Wet Season 2012 | Dry Season 2012–2013 | Wet Season 2013 | Dry Season 2013–2014 | Wet Season 2015 | Wet Season 2016 | |||||
| SRI | 20.6 b | 18.9 c | 21.6 b | 20.9 b | - | - | 21.1 | 19.9 | ||
| MSRI | 25.0 a | 22.1 b | 21.6 b | 23.9 b | 11.6 b | 20.6 c | 19.7 | 22.9 | ||
| DSR | - | - | - | - | 26.0 a | 32.4 b | 29.2 | - | ||
| CTF | 26.9 a | 27.1 a | 29.6 a | 28.3 a | 27.8 a | 36.6 a | 30.2 | 27.7 | ||
| SEm± | 0.71 | 0.74 | 0.8 | 1.04 | 0.85 | 0.74 | ||||
| CD (p ≤ 0.05) | 2.77 | 2.92 | 3.13 | 4.08 | 3.98 | 2.92 | ||||
| Probability of significance | 0.0008 | <0.0001 | <0.0001 | 0.0001 | <0.0001 | <0.0001 | ||||
| Method of Establishment | B. Nitrous Oxide (N2O) Emissions (kg ha−1 Season−1) | |||||||||
| Experiment 1 | Experiment 3 | Wet Season Mean | Dry Season Mean | |||||||
| Wet Season 2012 | Dry Season 2012–2013 | Wet Season 2013 | Dry Season 2013–2014 | Wet Season 2015 | Wet Season 2016 | |||||
| SRI | 10.1 | 10.3 | 10.3 | 10.3 | 10.2 | 10.3 | ||||
| MSRI | 10.2 | 10.5 | 10.5 | 10.6 | 10.7 | 7.3 | 9.7 | 10.5 | ||
| DSR | - | - | - | - | 10.2 | 7.3 | 8.8 | - | ||
| CTF | 9.9 | 10.0 | 10.1 | 10.1 | 10.1 | 6.5 | 9.2 | 10.0 | ||
| SEm± | 0.36 | 0.41 | 0.41 | 0.22 | 0.36 | 0.28 | ||||
| CD (p ≤ 0.05) | NS | NS | NS | NS | NS | 0.33 | ||||
| Probability of significance | 0.72 | 0.41 | 0.42 | 0.52 | 0.083 | 0.0004 | ||||
| C. Global Warming Potential (GWP) (kg CO2-eq ha−1) | ||||||||||
| Method of Establishment | Experiment 1 | Experiment 3 | Wet Season Mean | Dry Season Mean | GHG kg−1 Grain Wet Season | GHG kg−1 Grain Dry Season | ||||
| Wet Season 2012 | Dry Season 2012–2013 | Wet Season 2013 | Dry Season 2013–2014 | Wet Season 2015 | Wet Season 2016 | |||||
| SRI | 3512 | 3552 | 3619 | 3602 | - | - | 3565 | 3577 | 0.58 | 0.56 |
| MSRI | 3671 | 3680 | 3710 | 3742 | 3488 b | 2692 b | 3390 | 3711 | 0.62 | 0.65 |
| DSR | - | - | - | - | 3705 a | 2986 a | 3346 | - | 0.56 | - |
| CTF | 3635 | 3657 | 3735 | 3705 | 3720 a | 2861 a | 3488 | 3681 | 0.73 | 0.75 |
| SEm± | 107.8 | 112.9 | 130.8 | 86.2 | 43 | 39 | ||||
| CD (p ≤ 0.05) | NS | NS | NS | NS | 170 | 154 | ||||
| Probability of significance | 0.24 | 0.12 | 0.34 | 0.29 | 0.008 | 0.0004 | ||||
| Parameters | SRI | CTF |
|---|---|---|
| Microbial populations (log CFU g−1 dry soil) * | ||
| Bacteria | 7.20 a | 6.67 b |
| Fungi | 5.22 a | 4.66 b |
| Actinomycetes | 4.62 a | 3.86 b |
| Soil enzyme activities | ||
| Dehydrogenase (µg TPF g−1 soil 24 h−1) | 196.08 a | 180.73 b |
| Fluorescein diacetate hydrolytic activity (μg g−1 dry soil 0.5 h−1) | 51.06 a | 44.08 b |
| Glucosidase activity (µg p-nitrophenol g−1 soil h−1) | 91.24 a | 51.18 b |
| Phosphatase activity (mg p-nitrophenol g−1 soil h−1) | 1.23 a | 1.18 a |
| Arylsulfatase activity (mg p-nitrophenol g−1 soil h−1) | 7.61 a | 7.35 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, R.M.; Chintalapati, P.; Rathod, S.; Vidhan Singh, T.; Kuchi, S.; Mannava, P.B.B.B.; Latha, P.C.; Somasekhar, N.; Bandumula, N.; Madamsetty, S.P.; et al. Comparison of System of Rice Intensification Applications and Alternatives in India: Agronomic, Economic, Environmental, Energy, and Other Effects. Agronomy 2023, 13, 2492. https://doi.org/10.3390/agronomy13102492
Kumar RM, Chintalapati P, Rathod S, Vidhan Singh T, Kuchi S, Mannava PBBB, Latha PC, Somasekhar N, Bandumula N, Madamsetty SP, et al. Comparison of System of Rice Intensification Applications and Alternatives in India: Agronomic, Economic, Environmental, Energy, and Other Effects. Agronomy. 2023; 13(10):2492. https://doi.org/10.3390/agronomy13102492
Chicago/Turabian StyleKumar, Rapolu Mahender, Padmavathi Chintalapati, Santosha Rathod, Tapeshwar Vidhan Singh, Surekha Kuchi, Prasad Babu B. B. Mannava, Patharath Chandran Latha, Nethi Somasekhar, Nirmala Bandumula, Srinivas Prasad Madamsetty, and et al. 2023. "Comparison of System of Rice Intensification Applications and Alternatives in India: Agronomic, Economic, Environmental, Energy, and Other Effects" Agronomy 13, no. 10: 2492. https://doi.org/10.3390/agronomy13102492
APA StyleKumar, R. M., Chintalapati, P., Rathod, S., Vidhan Singh, T., Kuchi, S., Mannava, P. B. B. B., Latha, P. C., Somasekhar, N., Bandumula, N., Madamsetty, S. P., Prasad, J. V. N. S., Vijayakumar, S., Srinivas, D., Sreedevi, B., Tuti, M. D., Arun, M. N., Sailaja, B., & Sundaram, R. M. (2023). Comparison of System of Rice Intensification Applications and Alternatives in India: Agronomic, Economic, Environmental, Energy, and Other Effects. Agronomy, 13(10), 2492. https://doi.org/10.3390/agronomy13102492







