Evaluation of the Reproductive Toxicity of Fluopimomide in Meloidogyne incognita and Caenorhabditis elegans
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Nematodes
2.1.1. Chemicals
2.1.2. Nematodes
2.2. Pot Experiments
2.3. Effects of Fluopimomide on Reproduction of C. elegans
2.4. Germ Cells Assay
2.5. Germ Cell Apoptosis Assay
2.6. RNA Isolation and qPCR
2.7. Statistical Analyses
3. Results
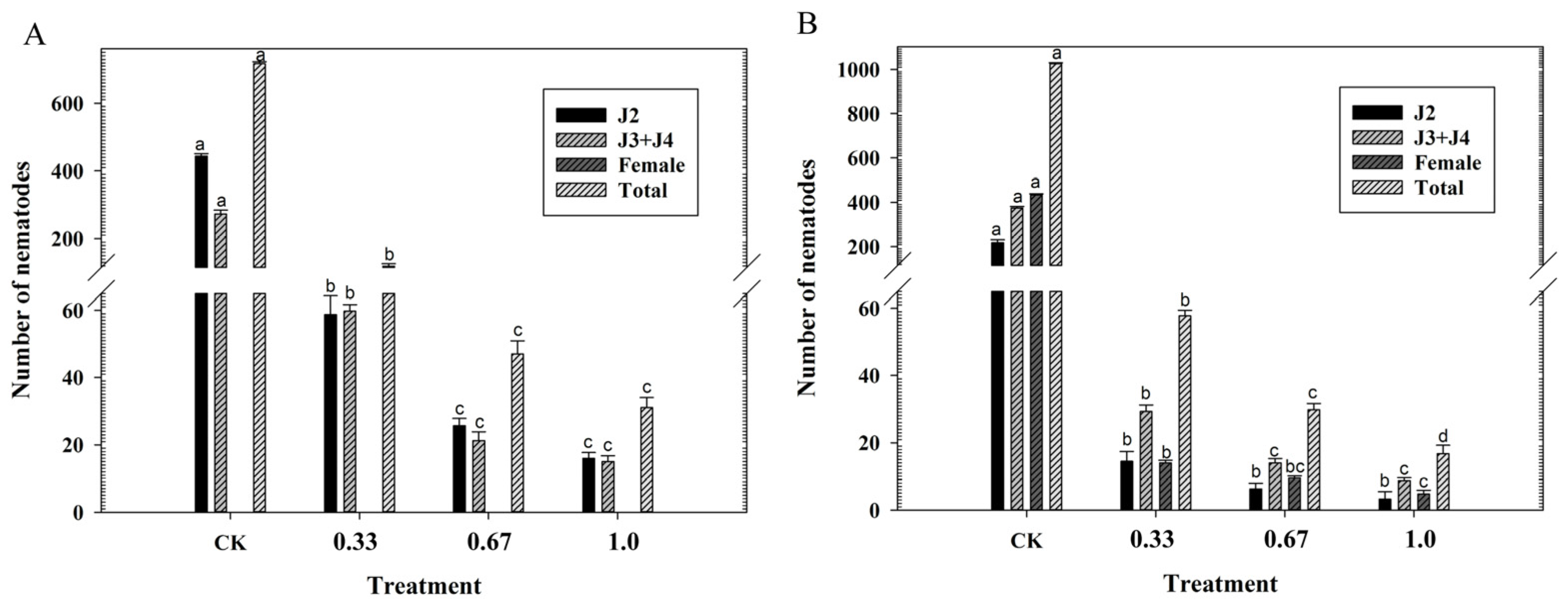
3.1. Effects of Fluopimomide on the Development of M. incognita
3.2. Effects of Fluopimomide on M. incognita Reproduction
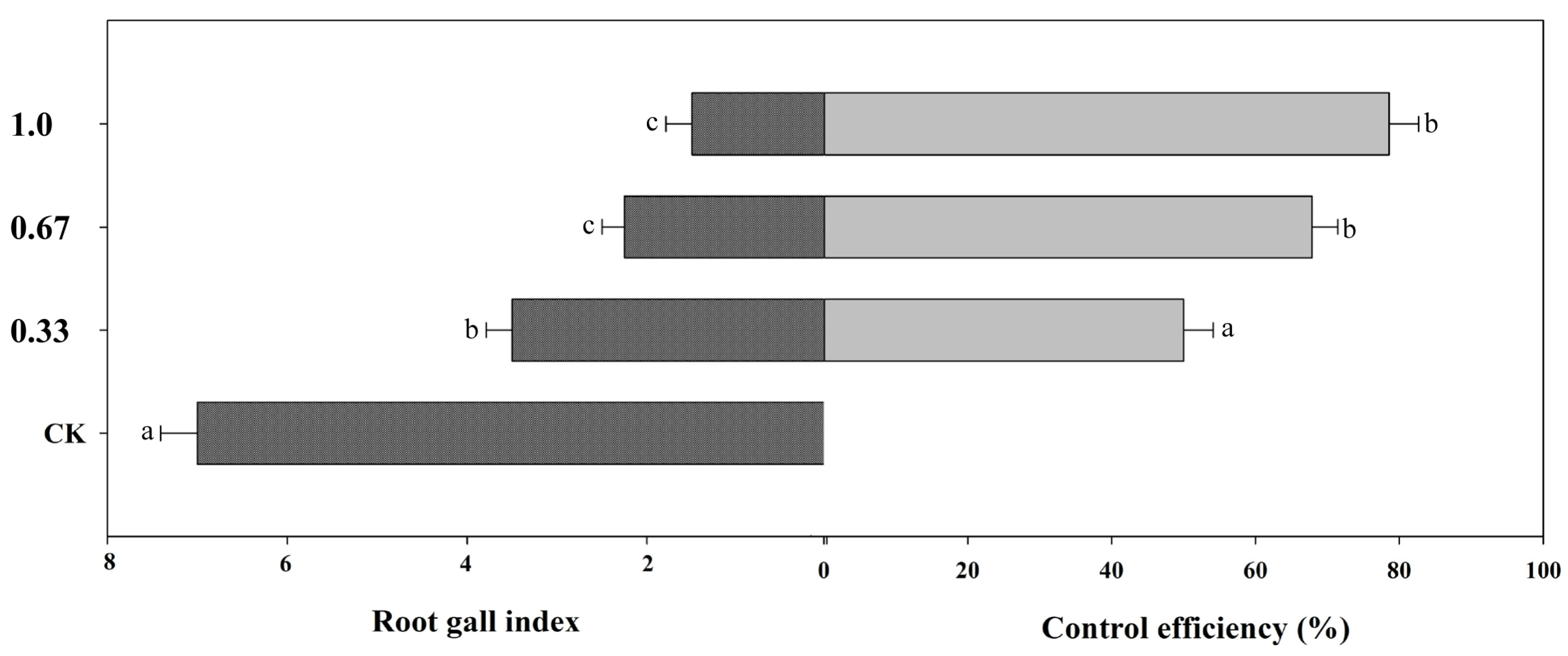
3.3. Effects of Fluopimomide on the Root Gall Index of Tomato
3.4. Effects of Fluopimomide on the Brood Size and Germ Cell Number of C. elegans
3.5. Effects of Fluopimomide on the Germ Cell Apoptosis of C. elegans
3.6. Effects of Fluopimomide on the DNA Damage of C. elegans
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rutter, W.B.; Franco, J.; Gleason, C. Rooting out the mechanisms of root-knot nematode-plant interactions. Annu. Rev. Phytopathol. 2022, 60, 43–76. [Google Scholar] [CrossRef] [PubMed]
- Qiao, K.; Liu, X.; Wang, H.; Xia, X.; Ji, X.; Wang, K. Effect of abamectin on root-knot nematodes and tomato yield. Pest Manag. Sci. 2012, 68, 853–857. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Meng, Z.; Li, N.; Ji, X.; Dong, B.; Zhang, S.; Qiao, K. Evaluating a new non-fumigant nematicide fluopimomide for management of southern root-knot nematodes in tomato. Crop. Prot. 2020, 129, 105040. [Google Scholar] [CrossRef]
- Jones, J.; Haegeman, A.; Danchin, E.; Gaur, H.; Helder, J.; Jones, M.; Kikuchi, T.; Manzanilla-López, R.; Palomares-Rius, J.; Wesemael, W.; et al. Top 10 plant-parasitic nematodes in molecular plant pathology. Mol. Plant Pathol. 2013, 14, 946–961. [Google Scholar] [CrossRef]
- Mao, L.; Wang, Q.; Yan, D.; Liu, P.; Shen, J.; Fang, W.; Hu, X.; Li, Y.; Ouyang, C.; Guo, M.; et al. Application of the combination of 1,3-dichloropropene disulfide by soil injection or chemigation: Effects against soilborne pests in cucumber in China. J. Integr. Agric. 2016, 15, 145–152. [Google Scholar] [CrossRef]
- Desaeger, J.; Wram, C.; Zasada, I. New reduced-risk agricultural nematicides-rationale and review. J. Nematol. 2020, 52, 1–16. [Google Scholar] [CrossRef]
- Cao, J.; Guenther, R.; Sit, T.; Lommel, S.; Opperman, C.; Willoughby, J. Development of abamectin loaded lignocellulosic matrices for the controlled release of nematicide for crop protection. Cellulose 2016, 23, 673–687. [Google Scholar] [CrossRef]
- Huang, B.; Qian, W.; Guo, M.; Fang, W.; Wang, X.; Wang, Q.; Yan, D.; Ouyang, C.; Li, Y.; Cao, A. The synergistic advantage of combining chloropicrin or dazomet with fosthiazate nematicide to control root-knot nematode in cucumber production. J. Integr. Agric. 2019, 18, 2093–2106. [Google Scholar] [CrossRef]
- Qiao, K.; Liu, Q.; Zhang, S. Evaluation of fluazaindolizine, a new nematicide for management of Meloidogyne incognita in squash in calcareous soils. Crop Prot. 2021, 143, 105469. [Google Scholar] [CrossRef]
- Watson, T.T. Sensitivity of Meloidogyne enterolobii and M. incognita to fluorinated nematicides. Pest Manag. Sci. 2022, 78, 1398–1406. [Google Scholar] [CrossRef]
- Giannakou, I.O.; Panopoulou, S. The use of fluensulfone for the control of root-knot nematodes in greenhouse cultivated crops: Efficacy and phytotoxicity effects. Cogent Food Agric. 2019, 5, 1643819. [Google Scholar] [CrossRef]
- Rathod, P.H.; Shah, P.G.; Parmar, K.D.; Kalasariya, R.L. The fate of fluopyram in the Soil-Water-Plant ecosystem: A Review. Rev. Environ. Contam. Toxicol. 2022, 260, 1. [Google Scholar] [CrossRef]
- Zhang, W.; Sun, W.; Wang, Y.; Liu, H.; Zhang, S.; Dong, B.; Qiao, K. Management of Meloidogyne incognita on cucumber with a new nonfumigant nematicide fluopimomide. Plant Dis. 2022, 106, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Li, J.; Meng, Z.; Li, N.; Dong, B.; Zhang, S.; Qiao, K. Fluopimomide effectively controls Meloidogyne incognita and shows a growth promotion effect in cucumber. J. Pest Sci. 2020, 93, 1421–1430. [Google Scholar] [CrossRef]
- Yu, Y.; Chen, H.; Hua, X.; Wang, C.; Dong, C.; Xie, D.; Tan, S.; Xiang, M.; Li, H. A review of the reproductive toxicity of environmental contaminants in Caenorhabditis elegans. Hyg. Environ. Health Adv. 2022, 3, 100007. [Google Scholar] [CrossRef]
- El-Nahhal, Y. Pesticide residues in honey and their potential reproductive toxicity. Sci. Total Environ. 2020, 741, 139953. [Google Scholar] [CrossRef]
- Cooper, R.L. Current developments in reproductive toxicity testing of pesticides. Reprod. Toxicol. 2009, 28, 180–187. [Google Scholar] [CrossRef]
- Kaletta, T.; Hengartner, M. Finding function in novel targets: C. elegans as a model organism. Nat. Rev. Drug Discov. 2006, 5, 387–399. [Google Scholar] [CrossRef]
- Moya, A.; Tejedor, D.; Manetti, M.; Clavijo, A.; Pagano, E.; Munarriz, E.; Kronberg, M. Reproductive toxicity by exposure to low concentrations of pesticides in Caenorhabditis elegans. Toxicology 2022, 475, 153229. [Google Scholar] [CrossRef]
- Yin, J.; Hong, X.; Wang, J.; Li, W.; Shi, Y.; Wang, D.; Liu, R. DNA methylation 6mA and histone methylation involved in multi-/trans-generational reproductive effects in Caenorhabditis elegans induced by atrazine. Ecotoxicol. Environ. Saf. 2023, 249, 114348. [Google Scholar] [CrossRef]
- Yu, C.W.; Luk, T.C.; Liao, V.H.C. Long-term nanoplastics exposure results in multi and trans-generational reproduction decline associated with germline toxicity and epigenetic regulation in Caenorhabditis elegans. J. Hazard. Mater. 2021, 412, 125173. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Yang, X.; Xue, H.; Huang, D.; Cai, M.; Huang, F.; Zheng, L.; Yu, Z.; Zhang, J. Reproductive toxicity of furfural acetone in Meloidogyne incognita and Caenorhabditis elegans. Cells 2022, 11, 401. [Google Scholar] [CrossRef] [PubMed]
- Wamucho, A.; Unrine, J.; May, J.; Tsyusko, O. Global DNA adenine methylation in Caenorhabditis elegans after multigenerational exposure to silver nanoparticles and silver nitrate. Int. J. Mol. Sci. 2023, 24, 6168. [Google Scholar] [CrossRef]
- Brenner, S. The genetics of Caenorhabditis elegans. Genetics 1974, 77, 71–94. [Google Scholar] [CrossRef] [PubMed]
- Bridge, J.; Page, L. Estimation of root-knot nematode infestation levels on roots using a rating chart. Int. J. Pest Manag. 1980, 26, 296–298. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, H.; Fu, G.; Li, Y.; Ji, X.; Zhang, S.; Qiao, K. Exposure to fluopimomide at sublethal doses causes oxidative stress in Caenorhabditis elegans regulated by insulin/IGF-1-like signaling pathway. Environ. Toxicol. 2022, 37, 2529–2539. [Google Scholar] [CrossRef] [PubMed]
- Hou, L.; Jiang, M.; Guo, Q.; Shi, W. Zymolytic grain extract (ZGE) significantly extends the lifespan and enhances the environmental stress resistance of Caenorhabditis elegans. Int. J. Mol. Sci. 2019, 20, 3489. [Google Scholar] [CrossRef]
- Qu, Z.; Ji, S.; Zheng, S. BRAF controls the effects of metformin on neuroblast cell divisions in C. elegans. Int. J. Mol. Sci. 2021, 22, 178. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, W.; Wang, Y.; Liu, H.; Zhang, S.; Ji, X.; Qiao, K. Oxidative stress, intestinal damage, and cell apoptosis: Toxicity induced by fluopyram in Caenorhabditis elegans. Chemosphere 2022, 286, 131830. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Ji, H.; Kyndt, T.; He, W.; Vanholme, B.; Gheysen, G. β-Aminobutyric acid-induced resistance against root-knot nematodes in rice is based on increased basal defense. Mol. Plant Microbe Interact. 2015, 28, 519–533. [Google Scholar] [CrossRef] [PubMed]
- Zhan, L.; Peng, D.; Wang, X.; Kong, L.; Peng, H.; Liu, S.; Liu, Y.; Huang, W. Priming effect of root-applied silicon on the enhancement of induced resistance to the root-knot nematode Meloidogyne graminicola in rice. BMC Plant Biol. 2018, 18, 50. [Google Scholar] [CrossRef] [PubMed]
- Shivakumara, T.; Dutta, T.; Chaudhary, S.; von Reuss, S.; Williamson, V.; Rao, U. Homologs of Caenorhabditis elegans chemosensory genes have roles in behavior and chemotaxis in the root-knot nematode Meloidogyne incognita. Mol. Plant-Microbe Interact. 2019, 32, 876–887. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Wang, C.; Li, H.; Ma, R.; Yu, Z.; Li, L.; Xiang, M.; Chen, X.; Hua, X.; Yu, Y. A review of toxicity induced by persistent organic pollutants (POPs) and endocrine-disrupting chemicals (EDCs) in the nematode Caenorhabditis elegans. J. Environ. Manag. 2019, 237, 519–525. [Google Scholar] [CrossRef]
- Lu, Q.; Bu, Y.; Ma, L.; Liu, R. Transgenerational reproductive and developmental toxicity of tebuconazole in Caenorhabditis elegans. J. Appl. Toxicol. 2020, 40, 578–591. [Google Scholar] [CrossRef]
- Yin, J.; Liu, R.; Jian, Z.; Yang, D.; Pu, Y.; Yin, L.; Wang, D. Di (2-ethylhexyl) phthalate-induced reproductive toxicity involved in dna damage-dependent oocyte apoptosis and oxidative stress in Caenorhabditis elegans. Ecotoxicol. Environ. Saf. 2018, 163, 298–306. [Google Scholar] [CrossRef]
- Du, H.; Wang, M.; Dai, H.; Hong, W.; Wang, M.; Wang, J.; Weng, N.; Nie, Y.; Xu, A. Endosulfan isomers and sulfate metabolite induced reproductive toxicity in Caenorhabditis elegans involves genotoxic response genes. Environ. Sci. Technol. 2015, 49, 2460–2468. [Google Scholar] [CrossRef]
- Salinas, L.S.; Maldonado, E.; Navarro, R.E. Stress-induced germ cell apoptosis by a p53 independent pathway in Caenorhabditis elegans. Cell Death Differ. 2006, 13, 2129–2139. [Google Scholar] [CrossRef]
- Boag, P.; Nakamura, A.; Blackwell, T. A conserved RNA-protein complex component involved in physiological germline apoptosis regulation in C. elegans. Development 2005, 132, 4975–4986. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, L.; Luo, X.; Wang, S.; Wang, Y. Bisphenol A exposure triggers apoptosis via three signaling pathways in Caenorhabditis elegans. RSC Adv. 2017, 7, 32624–32631. [Google Scholar] [CrossRef]
- Jackson, S.P.; Bartek, J. The DNA-damage response in human biology and disease. Nature 2009, 461, 1071–1078. [Google Scholar] [CrossRef] [PubMed]
- Norbury, C.J.; Hickson, I.D. Cellular responses to DNA damage. Annu. Rev. Pharmacol. Toxicol. 2001, 41, 367–401. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Hartford, S.A.; Zeng, R.; Southard, T.L.; Shima, N.; Schimenti, J.C. Hypersensitivity of primordial germ cells to compromised replication-associated DNA repair involves ATM-p53-p21 signaling. PLoS Genet. 2014, 10, e1004471. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, B.; Hofmann, K.; Boulton, S.; Gartner, A. The C. elegans homolog of the p53 tumor suppressor is required for DNA damage-induced apoptosis. Curr. Biol. 2001, 11, 1722–1727. [Google Scholar] [CrossRef]
- Conradt, B.; Horvitz, H.R. The C. elegans protein EGL-1 is required for programmed cell death and interacts with the Bcl-2-like protein CED-9. Cell 1998, 93, 519–529. [Google Scholar] [CrossRef]
- Lettre, G.; Hengartner, M.O. Developmental apoptosis in C. elegans: A complex CEDnario. Nat. Rev. Mol. Cell Biol. 2006, 7, 97–108. [Google Scholar] [CrossRef]
- Lans, H.; Vermeulen, W. Tissue specific response to DNA damage: C. elegans as role model. DNA Repair 2015, 32, 141–148. [Google Scholar] [CrossRef]
- Ni, S.; Zhang, H.; Sun, L.; Zhao, Y.; Pei, C.; Nie, Y.; Liu, X.; Wu, L.; Xu, A. Transgenerational reproductive toxicity of 2, 4, 6-trinitrotoluene (TNT) and its metabolite 4-ADNT in Caenorhabditis elegans. Environ. Toxicol. Pharmacol. 2022, 92, 103865. [Google Scholar] [CrossRef]




| Genes | Primers | |
|---|---|---|
| Forward | Reverse | |
| act-1 | CCAGGAATTGCTGATCGTATGCAGAA | TGGAGAGGGAAGCGAGGATAGA |
| cep-1 | TACCCGATTCGCAGGACATC | GCATCGGAAATCTTTGGCGT |
| egl-1 | GCCTCAACCTCTTCGGATCT | GCACATTGCTGCTAGCTTGG |
| hus-1 | GCGGCAATCGACGTGTTTAT | CCGGGCAGAACACGTACTAA |
| clk-2 | CACAGTGCCCAACAAAGTCG | TGACATGCTCGCCAGACAAT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, B.; Liu, H.; Zhang, S.; Ji, X.; Zhang, S.; Wang, Z.; Qiao, K. Evaluation of the Reproductive Toxicity of Fluopimomide in Meloidogyne incognita and Caenorhabditis elegans. Agronomy 2023, 13, 2471. https://doi.org/10.3390/agronomy13102471
Liu B, Liu H, Zhang S, Ji X, Zhang S, Wang Z, Qiao K. Evaluation of the Reproductive Toxicity of Fluopimomide in Meloidogyne incognita and Caenorhabditis elegans. Agronomy. 2023; 13(10):2471. https://doi.org/10.3390/agronomy13102471
Chicago/Turabian StyleLiu, Bingjie, Huimin Liu, Siqi Zhang, Xiaoxue Ji, Shouan Zhang, Zhongtang Wang, and Kang Qiao. 2023. "Evaluation of the Reproductive Toxicity of Fluopimomide in Meloidogyne incognita and Caenorhabditis elegans" Agronomy 13, no. 10: 2471. https://doi.org/10.3390/agronomy13102471
APA StyleLiu, B., Liu, H., Zhang, S., Ji, X., Zhang, S., Wang, Z., & Qiao, K. (2023). Evaluation of the Reproductive Toxicity of Fluopimomide in Meloidogyne incognita and Caenorhabditis elegans. Agronomy, 13(10), 2471. https://doi.org/10.3390/agronomy13102471







