Abstract
To evaluate the effects of long-term fallow tillage on soil microbial community structure in different soil layers and winter wheat yield, we conducted a 5-year long-term field experiment in the Loess Plateau, China, using three fallow tillage methods: no-tillage (NT), subsoiling tillage (ST), and deep plowing (DP). The soil physical and chemical properties, community structure, and composition of soil bacteria and fungi in the 0–20 cm and 20–40 cm soil layers, and winter wheat yield were analyzed. The results showed that, compared with DP, NT and ST significantly increased soil moisture content (SWC), soil organic carbon (SOC) content, and dissolved organic carbon (DOC) contents in 0–20 cm soil layer (p < 0.05), and significantly increased soil microbial community Shannon and Simpson index in 0–40 cm soil layer (p < 0.05). Compared with NT, ST and DP significantly increased SWC and SOC contents in 20–40 cm soil layer (p < 0.05). Actinobacteria and Ascomycota were the most abundant bacteria and fungi in the soil of the experimental site. Redundancy analysis further showed that soil physicochemical properties (SWC, SOC, DOC, and DON) were closely related to the microbial community. PICRUSt2 prediction results showed that DP increased the metabolic functional diversity of bacteria and fungi. ST and DP significantly increased the yield of winter wheat, and DP had the best effect. In conclusion, subsoiling tillage and deep plowing were beneficial to the accumulation and utilization of natural precipitation and the improvement of soil microbial community structure. Deep plowing was beneficial to the decomposition and metabolism of straw and organic fertilizer, and improved the catabolic ability of microbial community, thus increasing the yield of winter wheat.
1. Introduction
As an important part of the terrestrial ecosystem, agricultural land has suffered serious nutrient loss under the long-term strong disturbance caused by human activities [1], and poor farming practices are one of the main reasons for the decline in farmland productivity [2,3]. In addition, farmland productivity largely depends on ecosystem services provided by microorganisms [4]. As the core of the farmland ecosystem, soil microorganisms not only play a key role in the decomposition of organic matter and the degradation of pollutants [5,6], but also play an important role in stabilizing soil structure and promoting crop growth [7,8,9]. Therefore, reasonable tillage measures are of great importance for improving the farmland ecosystem.
Frequent disturbance of soil by traditional tillage deteriorates soil quality and results in soil nutrient loss [10,11]. Long-term traditional-tillage, shallow-tillage, and no-tillage measures can promote the formation of a soil-hardened layer, which will reduce soil permeability and organic matter content, leading to changes in soil chemical and biological characteristics [12,13], affecting the structural composition and stability of soil microbial community, and reducing the metabolic function diversity of soil microorganisms [14,15]. Numerous studies are showing that conservation tillage improves soil enzyme activity by reducing soil disturbance and creating a stable soil surface [16,17]. It has also been reported that cover cropping and no-tillage can increase the richness of beneficial bacterial species and increase the ratio of symbiotic and saprophytic bacteria in soil communities [18,19]. In addition, many studies have shown that tillage measures, such as subsoiling tillage and deep plowing, can improve soil physical properties, increase soil porosity, and stabilize water, fertilizer, air, and temperature in soil [20,21,22]. At the same time, subsoiling tillage and deep plowing can crush straw and return it to the field, increasing the content of organic matter in deep soil, creating a good environment for soil microbial activities, and providing favorable conditions for crops to achieve high yield [23,24]. A few studies also showed that tillage seriously affected the microbial community structure and distribution in different soil layers and changed the vertical distribution of soil bacteria and fungi, and this change would affect the effect of microorganisms on soil carbon distribution and further affect soil fertility [25]. Therefore, effective farmland management measures play a key role in the storage and transformation of soil nutrients [26,27], and are of great significance in improving soil microbial structure and increasing crop yield [28].
Soil microorganisms live together in an ecological network, affecting the soil’s ecological environment, and the core flora plays a role in promoting the presence of functional genes in the soil [29]. It is important to understand the response of soil microorganisms to tillage methods and to adopt reasonable tillage methods based on the abundance and diversity of microbial communities [30]. Many studies have shown that conservation tillage measures, such as no-tillage and reduced tillage, can reduce soil disturbance and increase soil organic carbon content and microbial activity and diversity, thereby reducing soil–air exchange and stabilizing microbial community structure [6,31]. However, some research results show that subsoiling-tillage and deep-plowing measures can retain straw in soil, promote organic matter storage, provide favorable conditions for microbial propagation, stabilize microbial community structure, and increase the abundance of functional genes [8,32]. The effects of different tillage methods on soil microbial community structure and metabolic function are different, which may be related to natural environmental factors, such as temperature, precipitation, and drought index [33,34], and physical and chemical factors, such as soil bulk density, soil water content, and pH [35,36]. Among them, precipitation, as a key factor, will affect the soil microbial community and its metabolic function, as well as the soil nutrient cycle [37]. After tillage treatment, the degree of precipitation determines the soil water content, affects the yield of wheat in the next season, further affects the amount of straw returned to the field, and provides sufficient organic matter for the soil microbial community [38,39].
Although many researchers have studied how soil physicochemical properties, soil microbial communities, and metabolic functions vary with different tillage methods, it is still unclear how to apply the best tillage methods in farmland without irrigation conditions on the Loess Plateau. For this reason, we changed the tillage time from before the winter wheat sowing to 10–15 days after the wheat harvest of the last season. The purpose of this kind of fallow tillage method is to cultivate, fertilize, and return straw to the field at a time when the soil water and heat changes the most, so as to effectively remove weeds and collect rainwater. At present, studies on the effects of fallow tillage on soil nutrient environment and microbial community are limited. To this end, we conducted a long-term field experiment in the Loess Plateau, a typical semi-arid region in northern China, and used Illumina sequencing technology to further understand the effects of fallow tillage practices on soil microbial communities and their metabolic functions. The objectives of this study are: (1) to analyze the effects of tillage methods on soil physical and chemical properties in different soil layers; (2) to compare the differences of microbial community structure in different soil layers after long-term tillage methods; (3) to use PICRUSt2 to predict the effects of different tillage methods on the metabolic function of microbial communities in different soil layers. This study can deepen our understanding of the effects that fallow tillage has on the soil microbial community. It contributes to further improvements in agricultural practices of dryland agriculture and establishing a stable and functional soil ecological environment. In addition, this knowledge can contribute to promoting sustainable crop production in the arid regions of northern China.
2. Materials and Methods
2.1. Description of the Study Site
The experimental site is located in the dryland wheat experimental demonstration base of Shanxi Agricultural University (35°23′ N, 111°25′ E, altitude 810 m), Hougong Township, Wenxi County, Yuncheng City, Shanxi Province, China. This area is in a temperate continental climate zone, with an average annual temperature of 12.4 °C and an average annual precipitation of 461.3 mm. About 60% of the precipitation is concentrated between July and September. The planting system is rainfed, winter wheat and summer fallow, without irrigation. The experimental field is hilly dry land. The soil is yellow loessial soil. The parent material is deep middle loamy Malan loess, which belongs to the calcareous cinnamon subclass fire brown loess with a deep soil body, good permeability, weak alkalinity, and medium fertility. The physical and chemical properties of the 0–40 cm deep soil layer before tillage in 2016 are shown in Table 1.

Table 1.
Basic physicochemical properties of soil in 2016.
2.2. Experimental Design
This experiment was a long-term field experiment that began in July 2016. The winter wheat (Triticum aestivum L.) variety tested was Yunhan 618 (provided by the Wenxi County Agriculture Bureau). Agricultural management practices were arranged in a completely randomized block design with three replicates. Three agricultural managements were set up in the present study: (i) NT: no-tillage (Figure 1c); (ii) ST: subsoiling tillage (Figure 1d); (iii) DP: deep plowing (Figure 1e). The different tillage practices are shown in Table 2. All the treatments were shallowly rotated at the end of August. The land was leveled, soil moisture was harvested by harrowing, and sowing was carried out at the end of September or early October. The 2BMFD-7/14 type full-return anti-winding no-tillage fertilizer seeder (Figure 1f) was used for sowing in autumn. The ridge width was about 20 cm, the ridge height was 4 cm, and the furrow depth was 8 cm, narrow row space was 12 cm (Figure 1g). Before sowing, nitrogen fertilizer (urea, 46%), phosphorus (superphosphate, 16% P2O5), and potassium (potassium chloride, 52% K2O) were applied with 150 kg·ha−2 each. The sowing rate was 165 kg·ha−1. Suppression and spraying management measures are the same as those of local farmers.
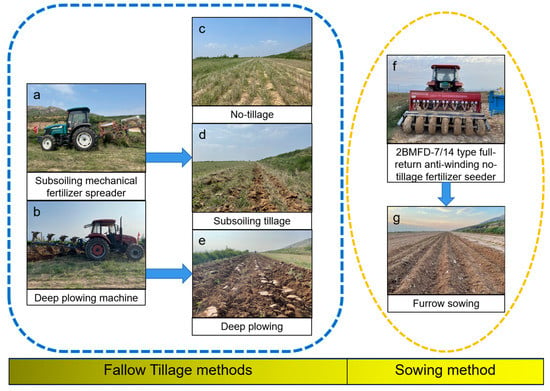
Figure 1.
Two fallow tillage machines (a,b), three fallow tillage methods (c–e), sowing machine (f), and sowing method (g).

Table 2.
Description of different tillage practices.
2.3. Measurements and Methods
2.3.1. Determination and Analysis of Winter Wheat Yield
Winter wheat was machine-harvested on 10 June 2017, 11 June 2018, 7 June 2019, 8 June 2020, and 3 June 2021. The yield of the crops was determined by manual harvesting, threshing, and air-drying grain from three 3 m2 areas taken at random in each plot for winter wheat.
2.3.2. Soil Sampling and Analysis
On 2 May 2021 (the flowering period of winter wheat), (i) soil samples were collected at 0–20 cm and 20–40 cm soil layers at five evenly distributed points in three plots of each treatment, with each point taken from between two rows of winter wheat. Samples from five points in each plot were mixed and passed through a 2 mm mesh screen to remove sand and stone impurities. During collection, the samples were temporarily placed in an ice box at 4 °C, transported back to the laboratory, and kept in the refrigerator at –80 °C. This portion of the sample was used to extract total DNA from soil microorganisms and sent to the sequencing company on 3 May for sequencing. (ii) Soil samples were collected by drilling in the 0–20 cm and 20–40 cm soil layers in the three plots of each treatment and divided into three parts. The first part was put into an aluminum box and brought back to the laboratory for the determination of soil water content (SWC). The second part was screened with a 2 mm mesh screen to remove sand and stone impurities and air-dried to determine the content of soil organic carbon (SOC) and pH. The third part was temporarily placed in an ice box at 4 °C and was promptly transported back to the laboratory and stored in a refrigerator at 4 °C for determination of dissolved organic carbon (DOC) and dissolved organic nitrogen (DON). (iii) Soil samples from 0–20 cm and 20–40 cm soil layers were collected from the soil profiles of the three plots in each treatment for the determination of soil bulk density (BD).
The BD was determined by the ring knife method [40]. Soil pH was measured using a pH meter (Mettler-Toledo FE 28, Berne, Switzerland) at a soil:water ratio of 1:5 (weight: volume). The SWC was measured by oven drying [41]. The SOC content was determined by the potassium dichromate-sulfuric acid external heating method [42]. The DOC and DON were determined using the method described by Berthrong et al., with the soil:water being 1:5. The samples were oscillated for 1 h at 250 rpm at 25 °C, and then centrifuged for 10 min at 15,000 rpm. The upper suspension was passed through a 0.45 μm thin filter membrane, and the filtrate was determined by a total carbon and nitrogen automatic analyzer (multi N/C 3100, Analytik Jena GmbH, Jena, Germany) [43].
2.4. DNA Extraction, PCR Amplicon Sequencing of Bacterial 16S and Fungal ITS Genes
Total genomic DNA was extracted from the soil samples using the OMEGA Soil DNA Kit (M5635-02) (Omega Bio-Tek, Norcross, GA, USA), following the manufacturer’s instructions, and the DNA was stored at −20 °C before further analysis. The quantity and quality of extracted DNA were measured using a NanoDrop NC2000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA) and agarose gel electrophoresis, respectively.
PCR amplification of the 16S rRNA genes V3—V4 region was performed using the forward primer 338F (5′-ACTCCTACGGGAGGCAGCA-3′) and the reverse primer 806R (5′-GGACTACHVGGGTWTCTAAT-3′). The fungal ITS1 region was performed using the forward primer ITS5F (5′-GGAAGTAAAAGTCGTAACAAGG-3′) and the reverse primer ITS2R (5′-GCTGCGTTCTTCATGC-3′). Sample-specific 7-bp barcodes were incorporated into the primers for multiplex sequencing. The PCR mixture contained 5 μL of buffer (5×), 0.25 μL of FastPfu DNA Polymerase (5 U/μL), 2 μL (2.5 mmol·L−1) of dNTPs, 1 μL (10 umol·L−1) of each forward and reverse primer, 1 μL of DNA template, and 14.75 μL of ddH2O. The thermal cycling conditions were initial denaturation at 98 °C for 5 min, followed by 25 (bacterial)/28 (fungal) cycles of additional denaturation at 98 °C for 30 s, annealing at 53 (bacterial)/55 (fungal) °C for 30 s, and extension at 72 °C for 45 s, with a final extension of 5 min at 72 °C. The PCR amplicons were purified with Vazyme VAHTSTM DNA Clean Beads (Vazyme, Nanjing, China) and quantified using the Quant-iT PicoGreen dsDNA Assay Kit (Invitrogen, Carlsbad, CA, USA). After the individual quantification step, the amplicons were pooled in equal amounts, and pair-end 2 × 250 bp sequencing was performed using the Illumina MiSeq platform with MiSeq Reagent Kit v3 at Shanghai Personal Biotechnology Co., Ltd. (Shanghai, China).
2.5. Sequence Analysis
Microbiome bioinformatics were performed with QIIME2 2019.4 [44] with slight modification according to the official tutorials (https://docs.qiime2.org/2019.4/tutorials/) (accessed on 29 October 2021). Briefly, raw sequence data were demultiplexed using the demux plugin following by primer cutting with the cutadapt plugin [45]. Sequences were then quality filtered, denoised, merged, and chimeras removed using the DADA2 plugin [46]. Non-singleton amplicon sequence variants (ASVs) were aligned with Mafft [47] and used to construct a phylogeny with Fasttree2 [48].
Alpha-diversity metrics (Chao1 [49], Observed species, Shannon [50], and Simpson [51]) were estimated using the diversity plugin. Taxonomy was assigned to ASVs using the sklearn-based naïve Bayes taxonomy classifier in the feature-classifier plugin [52] against the SILVA Release 132 (bacterial) and UNITE Release 8.0 (fungal) databases [53].
2.6. Bioinformatics and Statistical Analysis
Two-way analysis of variance (ANOVA) was conducted with soil layer (S), tillage (T), and their interaction as the main effects using the SPSS 26.0 software package for Windows (SPSS, Inc., Chicago, IL, USA). When the differences in the main effects were significant, the mean differences of all treatments were compared based on the least significant difference (Duncan test) at the 0.05 level of probability. To predict the metabolic function profiles of bacteria and fungi under different treatments, 16S rRNA and ITS gene sequences were compared with MetaCyc database (https://metacyc.org/) (accessed on 15 November 2021), and Phylogeneration Investigation of Community, Reconstruction of Unobservation States (PICRUSt2) [54] software was used for functional inference analysis. In addition, Pearson correlations of soil physicochemical properties, alpha diversity, and relative abundance of soil microbial community composition and metabolic functions under different tillage practices were analyzed using SPSS 26.0 software.
3. Results
3.1. Physical and Chemical Properties of the Soil
As shown in Table 3, all the tested indicators in the soil were significantly influenced by soil layer and tillage (p < 0.05), and the SWC, SOC, DOC, and DON in the soil were significantly affected by the interaction between soil layer and tillage practices (T × S) (p < 0.01). In the 0–20 cm soil layer, compared with DP treatment, SWC, SOC, and DOC contents in NT and ST treatment increased significantly by 7.73% and 13.37%, 22.47% and 7.60%, 23.36% and 19.67%, respectively. In the 20–40 cm soil layer, compared with NT treatment, SWC and SOC contents in ST and DP treatment increased significantly by 30.58% and 28.33%, 13.02% and 12.90%, respectively.

Table 3.
Effects of tillage methods on soil physical and chemical properties in the 0–40 cm soil layer.
3.2. Alpha Diversity
The richness and diversity index of bacteria and fungi are shown in Table 4. The results of ANOVA showed that Chao1, Observed_species, and Shannon index of bacteria were significantly affected by soil layer and tillage interaction benefit (T × S) (p < 0.01). The Chao1 and Observed_species indices of fungi were significantly affected by soil layer and tillage interaction benefit (T × S) (p < 0.05). In the 0–20 cm soil layer, the Shannon and Simpson indices of bacterial and fungal communities under NT and ST treatments were significantly higher than those under DP treatment. In the 20–40 cm soil layer, the Chao1, Observed_species, Shannon, and Simpson indices of bacterial communities under NT and ST treatments were significantly higher than those under DP treatment. It is worth noting that the Shannon index of ST treatment in the 20–40 cm soil layer was significantly higher than those of the NT and DP treatments.

Table 4.
Effects of tillage methods on the Alpha diversity index of soil bacteria in the 0–40 cm soil layer.
3.3. Dominant Bacteria and Fungi
Actinobacteria (44.50% ± 3.94%), Proteobacteria (19.21% ± 2.54%), Acidobacteria (10.51% ± 0.99%), Chloroflexi (9.10% ± 0.85%), and Gemmatimonadetes (5.59% ± 1.49%) were the dominant phyla of the soil bacterial community in the 0–20 and the 20–40 cm soil layers (Figure 2a). Rokubacteria, Firmicutes, Bacteroidetes, GAL15, and Planctomycetes were found in low relative abundance in all soils examined. In the 0–20 cm soil layer, NT and ST treatments significantly increased the relative abundance of Acidobacteria, Chloroflexi, and Gemmatimonadetes, as compared with the DP treatment. In the 20–40 cm soil layer, compared with the NT treatment, ST and DP treatment significantly increased the relative abundances of Rokubacteria, Firmicutes, and GAL15. However, the relative abundances of Proteobacteria, Chloroflexi, and Gemmatimonadetes were significantly decreased. In addition, compared with the 0–20 cm soil layer, the relative abundance of Proteobacteria in the 20–40 cm soil layer under the three cropping methods was significantly decreased, while that of Rokubacteria was significantly increased.
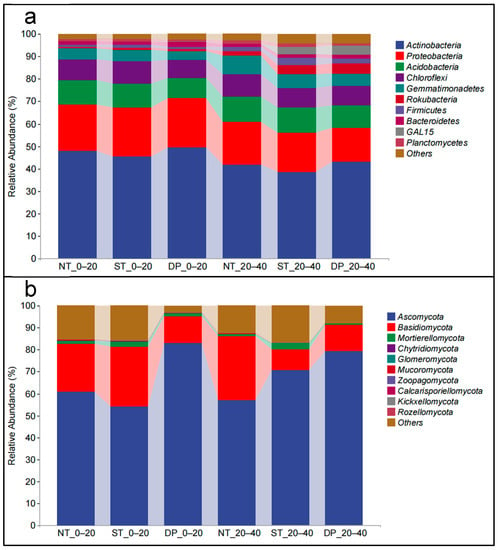
Figure 2.
Effect of fallow tillage on relative abundance of phylum taxonomic levels of bacteria (a) and fungi (b) in the 0–40 cm soil layer. NT: no-tillage; ST: subsoiling tillage; DP: deep plowing.
Ascomycota (67.60% ± 13.13%), Basidiomycota (18.60% ± 8.47%), and Mortierellomycota (1.48% ± 0.98%) accounted for 87.65% (±5.04%) of the dominant phyla of the soil fungal community in the 0–40 cm soil layer (Figure 2b). The relative abundances of the remaining species were all less than 1%. In the 0–20 cm soil layer, NT and ST treatments significantly increased the relative abundance of Basidiomycota compared with the DP treatment. In the 20–40 cm soil layer, ST and DP treatments significantly increased the relative abundance of Ascomycota compared with the NT treatment.
3.4. Principal Coordinate Analysis
The results of principal coordinate analysis based on Bray–Curtis Distance revealed that there were differences in bacterial and fungal community structures in different soil layers and under different tillage methods (Figure 3). The PCo1 and PCo2 axes explained 36.9% and 15.0% of the bacterial community and 43.3% and 13.9% of the fungal community in the 0–40 cm soil layer, respectively (Figure 3a). In addition, in bacteria, the community composition structure of the 0–20 cm soil layer in NT and ST treatments was similar, and the community composition structure of the 20–40 cm soil layer in ST and DP treatments was similar. Among fungi, NT and ST showed less difference in community structure in the 0–20 and 20–40 cm soil layers.
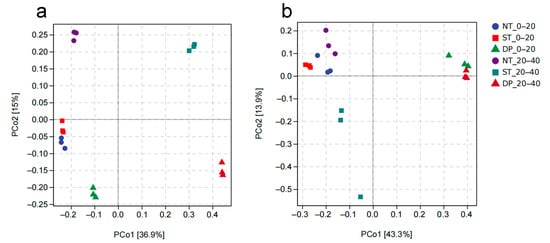
Figure 3.
Principal coordinate analysis of bacterial (a) and fungal (b) community structure based on Bray–Curtis Distance in the 0–40 cm soil layer during the fallow period. NT: no-tillage; ST: subsoiling tillage; DP: deep plowing.
3.5. Redundancy Analysis of Physicochemical Properties and Dominant Microbial Communities
Redundancy analysis showed the contribution of each environmental factor to the total variance of the microbiome, as well as the relationship between the environmental factor, sample, and microbiome (Figure 4). The treatments of the 0–20 and the 20–40 cm soil layers under the three tillage methods were dispersed in each quadrant, which indicated changes in microbial community structure. The angle between the arrow and the center line of the sample is an acute angle, which indicates a positive correlation between the sample and environmental factors. The angle is an obtuse angle, which indicates a negative correlation, and the cosine value of the angle represents the correlation between the two. The RDA1 and RDA2 axes together explained 79.52% (Figure 4a) and 65.41% (Figure 4b) of the total structural variation at the taxonomic level of bacterial and fungal phyla.
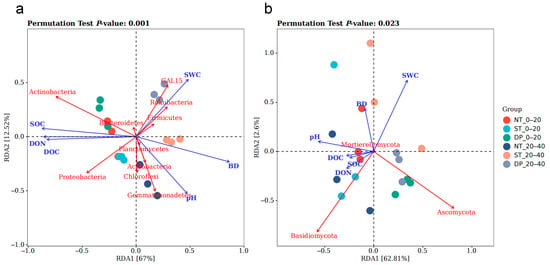
Figure 4.
Redundancy analysis of soil physicochemical properties and communities of bacteria (a) and fungi (b) in the 0–40 cm soil layer under fallow tillage (select the top ten species of bacteria and the top three species of fungi). The blue arrows indicate the relative positions of the different environmental factors in the plane, with a longer arrow indicating a more significant effect. The red arrows indicate the relative positions of the major bacterial and fungal communities on the plane. NT: no-tillage; ST: subsoiling tillage; DP: deep plowing.
In the bacterial community (Figure 4a), SOC, DOC, and DON were positively correlated with Actinobacteria and Proteobacteria, and had a positive effect on the 0–20 cm soil layer under the three tillage treatments. SWC had a significant impact on Firmicutes, GAL15, and Rokubacteria, and had a positive effect on the 20–40 cm soil layer of ST and DP treatments. PH plays a key role in Acidobacteria, Chloroflexi, and Gemmatimonadetes. BD had a large positive value in RDA1 but had no significant effect on bacterial community structure. In fungal communities (Figure 4b), SOC, DOC, and DON were positively correlated with Basidiomycota, and had a positive effect on the NT 0–40 cm soil layer and the ST 0–20 cm soil layer.
3.6. Metabolic Function Diversity of Microorganisms
Based on the MetaCyc database, an analysis was undertaken with PICRUSt2, and a heat map was used to show the genes associated with metabolic functions of soil microbial communities in different soil layers under fallow tillage (Figure 5). Level 1 of functional metabolism of the bacterial community has Biosynthesis and Degradation/Utilization/Assimilation, Detoxification, Generation of Precursor Metabolite and Energy, Glycan Pathways, Macromolecule Modification, and Metabolic Clusters (Figure 5a). Level 1 of functional metabolism of the fungal community has Biosynthesis, Degradation/Utilization/Assimilation, Generation of Precursor Metabolite and Energy, Glycan Pathways, and Metabolic Clusters (Figure 5b). The results showed that at level 2, compared with NT and ST treatments, DP treatment significantly enhanced the relative abundance of Alcohol Degradation, Carbohydrate Degradation, Chlorinated Compound Degradation, Secondary Metabolite Degradation, Methanol Oxidation to Carbon Dioxide, Glycolysis, Superpathway of Glycolysis and Entner–Doudoroff, Superpathway of Glycolysis, Pyruvate Dehydrogenase, TCA, and Glyoxylate Bypass, Glycan Biosynthesis, Pyrimidine Deoxyribonucleotides de novo Biosynthesis I, and Superpathway of L-aspartate and L-asparagine Biosynthesis of bacterial communities in the 0–40 cm soil layer. Compared with NT and ST treatments, DP treatment significantly improved the relative abundance of Aminoacyl-tRNA Charging, Degradation/Utilization/Assimilation-Other, and tRNA charging of fungal communities in the 0–40 cm soil layer.
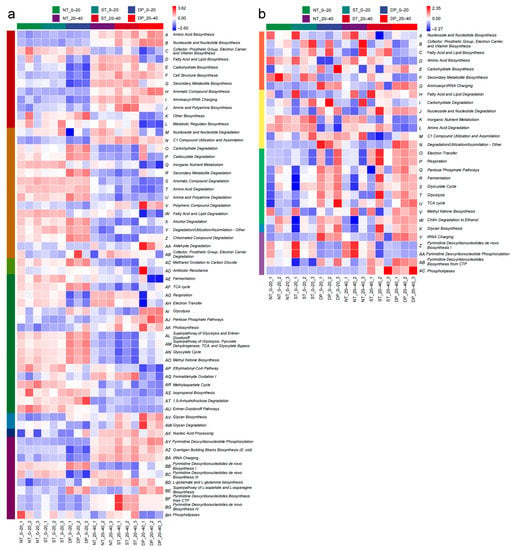
Figure 5.
The interactive heatmap shows metabolic functional pathways at level 2 of soil bacterial (a) and fungal (b) communities affected by tillage and soil layer as predicted based on the MetaCyc database. Among the metabolic functions of bacterial communities, the level 1 metabolic pathways are as follows: Biosynthesis: A-L; Degradation/Utilization/Assimilation: M-AB; Detoxification: AC-AD; Generation of Precursor Metabolite and Energy: AE-AU; Glycan Pathways: AV-AW; Macromolecule Modification: AX; Metabolic Clusters: AY-BH. Among the metabolic functions of fungal communities, the level 1 metabolic pathways are as follows: Biosynthesis: A-G; Degradation/Utilization/Assimilation: H-N; Generation of Precursor Metabolite and Energy: O-W; Glycan Pathways: X; Metabolic Clusters: Y-AC. NT: no-tillage; ST: subsoiling tillage; DP: deep plowing.
3.7. Winter Wheat Yield from 2017 to 2021
During 2017–2021, compared with NT and ST treatments, DP treatments increased winter wheat yield by 21.78% and 3.29%, 35.92% and 11.99%, 25.78% and 8.49%, 24.60% and 8.04%, 25.55% and 1.86%, respectively (Figure 6). In addition, both ST- and DP-treated winter wheat yields were above average between 2017 and 2021.
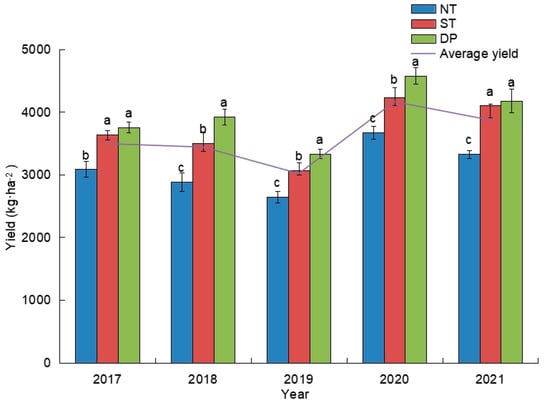
Figure 6.
Effects of fallow tillage on winter wheat yield from 2017 to 2021. Different letters above bars indicate significant differences at the 0.05 level.
4. Discussion
4.1. Effects of Fallow Tillage on Soil Physical and Chemical Properties in the 0–40 cm Soil Layer
Soil nutrients on the Loess Plateau are poor and water resources are scarce, which is exacerbated by a long history of irrational farming practices. This study showed that compared with DP treatment, the SWC of NT and ST treatment was significantly increased by 7.73% and 13.37% in the 0–20 cm soil layer, respectively. The SWC of ST and DP increased by 30.58% and 28.33% in the 20–40 cm soil layer, respectively. This is consistent with previous findings by our team [55,56]. The main reasons for this result are as follows: (i) no-tillage in successive years will lead to the formation of a soil-hardened layer, leading to water being retained in the soil surface layer, which is not conducive to the infiltration of water into the deep soil, and promotes water loss by ineffective evaporation; (ii) subsoiling tillage and deep plowing are cultivated in advance during the fallow period, which effectively breaks the plow bottom layer, increases soil porosity, and enables the soil to hold more water, so as to coordinate crop water requirements and soil water supply [28]. It is well known that soil nutrients are strongly influenced by tillage practices and vary from soil layer to soil layer. In this study, NT and ST treatments significantly increased SOC and DOC contents by 22.47% and 7.6%, 23.36% and 19.67% in the 0–20 cm soil layer, respectively, which is supported by the findings of Wang et al. [57]. Basic et al. have also shown that no-tillage and subsoiling tillage are effective measures to improve soil nutrients and prevent soil erosion [58]. In addition, the previous crop straw and the biomass of winter wheat during the growth period also affected soil water content and nutrients. On the one hand, ST and DP treatments can increase crop yield [59], thus affecting the amount of straw returned to the field of winter wheat and promoting the water retention capacity of soil and adequate nutrients in the subsurface of soil. During the whole growth period of winter wheat, the higher the biomass of winter wheat, the greater the demand for soil water and nutrients [60]. Although the SOC content in the 0–20 cm soil layer was low under DP treatment, the SOC content in the 20–40 cm soil layer was significantly increased (12.90%) under DP treatment. This is because deep plowing during the fallow period flips the soil profile, turning the SOC-rich topsoil and the wheat straw from the previous crop into the bottom soil layer and exposing the SOC-deficient subsoil, and finally forming the topsoil with a low SOC content and the subsoil with a high SOC content [61].
4.2. Effects of Fallow Tillage on Soil Microbial Communities Structure in the 0–40 cm Soil Layer
Fallow tillage affects the habitat of soil microorganisms by impacting soil physical and chemical properties, which helps stabilize the microbial community structure and promote the diversity of microbial communities [62]. Our study showed that NT and ST treatment during the fallow period significantly increased the diversity of bacterial and fungal communities in the 0–20 cm soil layer and the richness and diversity of bacterial communities in the 20–40 cm soil layer. NT and ST cause little disturbance to the soil, which can improve soil nutrients and provide a more suitable soil microenvironment for the survival of microbial communities [63]. However, the disturbance of the soil caused by continuous deep foraging may lead to the reduction in surface soil nutrients and the enrichment of competitive bacterial communities, resulting in the reduction in the richness and diversity of soil microorganisms [64]. In addition, compared with the 20–40 cm soil layer, the richness of the bacterial community was higher in the 0–20 cm soil layer, which may be due to the re-fertilization of the surface layer by the three tillage methods before sowing, which enriched the nutrients in the soil surface layer and provided better living conditions for the bacterial community [65]. From the principal coordinate analysis of microbial beta diversity and the top ten bacteria and fungi with the highest relative abundance, bacteria and fungi in soil respond differently to long-term tillage and changes in soil layers. The microbial communities of NT and ST treatments were more similar, and both were distant from those of DP treatment. Notably, bacteria were more significantly affected by the soil layer, while tillage practices had a greater impact on fungi. This suggests that fungal communities are more unstable and easily dispersed than bacterial communities. Several studies have also shown that tillage practices affect fungal communities by causing soil changes [66,67].
In this study, Actinobacteria was found to be the most abundant bacterium in soil, followed by Proteobacteria, Acidobacteria, Chloroflexi, and Gemmatimonadetes. Some studies have indicated that Proteobacteria is the most abundant species in soil [68]. The relative abundance of Acidobacteria, Chloroflexi, and Gemmatimonadetes in 0–20 cm soil layer under NT and ST treatments was significantly higher than that under DP treatment. The relative abundances of Rokubacteria and GAL15 in the 20–40 cm soil layer under ST and DP treatments were significantly higher than those under NT. Hao et al. pointed out that Gemmatimonadetes are generally considered to be enriched in environments with high soil nutrients [69]. They also have the function of secreting antibiotics and a variety of active enzymes, which can inhibit or kill pathogenic bacteria, improve crop resistance to disease and drought, increase basal soil nutrients, and improve soil microbial activity. Among fungi, Ascomycota occupies an absolute advantage, which is greatly affected by the degradation of plant species and straw residues, and its abundance increases with the addition of nutrients [70]. In this study, NT and ST treatments significantly increased the relative abundance of Basidiomycota in the 0–20 cm soil layer, which is considered to be a nutrient-rich microbiota and can break down stubborn lignocellulose with increasing soil nutrients [71]. Other studies have shown that reduced tillage and straw return contribute to the improvement of the relative abundance of Basidiomycota [72,73]. This is consistent with the results of this study. In addition, ST and DP treatments significantly increased the relative abundance of Ascomycota in the 20–40 cm soil layer. This may be because subsoiling tillage and deep plowing in the fallow period extended the tillage depth to the bottom soil layer, which directly improved the air permeability and permeability of the subsoil, and mixed straw into a large area of soil to provide more abundant nutrients for microorganisms, thus changing the transformation and accumulation of soil organic carbon [74,75]. Ascomycota is a saprophytic soil fungus strongly affected by straw residues, and its relative abundance can be significantly increased by tillage during the fallow period. Zhu et al. also found that deep tillage treatment helped to break the soil-hardened layer and increased the relative abundance of Ascomycota but decreased the relative abundance of Basidiomycota [76].
Soil microbial communities play a key role in regulating soil physicochemical properties. The structure and composition of soil microorganisms are strongly responsive to farming practices, and reasonable agricultural measures are of great significance to soil sustainability [77]. The redundancy analysis further shows that tillage and different soil layers had significant effects on soil microbial structure. SOC, DOC, and DON were positively correlated with Actinobacteria and Proteobacteria. Among them, Actinobacteria is very important for the decomposition of hard-to-degrade organic matter, and the aerobic conditions created by deep plowing measures and the incorporation of straw can provide good conditions for its growth [78]. Proteobacteria, one of the bacterial communities with the highest relative abundance, grows rapidly in soil environments with rich carbon sources [79]. In addition, pH had a significant effect on the differential species of bacterial communities in the 0–20 cm soil layer. Many studies have shown that soil pH is an important factor affecting microbial community structure. Liu et al. also pointed out that soil pH is the main factor regulating the structure and formation process of bacterial communities under tillage [80]. It is worth noting that although Acidobacteria has been regarded as a low-trophic bacteria in many studies [81,82], in our study it exists in a nutritionally high environment and is regulated by pH value [83]. This is consistent with the results of Liu et al.’s research on black land in Northeast China [84]. Interestingly, we found that SWC had a significant effect on Firmicutes, which are considered to be eutrophic bacteria and capable of rapid growth in environments with high available carbon [85]. Subsoiling tillage and deep plowing during the fallow period can improve the aeration and nutrient status of the subsoil, which is conducive to increasing the relative abundance of Firmicutes. The results of our team show that SWC is one of the indicators most affected by fallow tillage, and this effect can be carried through the whole growth period of winter wheat [86]. Subsoiling tillage and deep plowing during the fallow period can significantly improve the capacity of soil to collect rainwater, coordinate soil water status, and allow microorganisms to exchange nutrients in an environment with sufficient soil water, thus giving greater play to the decomposition of soil microorganisms [87]. This study also found that Basidiomycota was affected by SOC, DOC, and DON content. This may be because Basidiomycota plays a major role in the later stage of straw decomposition and can degrade refractory organic matter [88].
4.3. Effects of Fallow Tillage on Metabolic Diversity of Microorganisms in the 0–40 cm Soil Layer
PICRUSt2 has been applied to predict metabolic pathways in bacterial and fungal communities. Our results showed that the metabolic functions of bacterial communities were significantly affected by the soil layer (Figure 3a). Although numerous studies have shown that relatively high anabolic activity is observed in no-tillage soils, and that the potential for carbon and nitrogen fixation is greater in these soils [89], this is mainly because the stable soil environment of no-tillage is conducive to conditions that improve the metabolic rates of microorganisms involved in major nutrient cycling [90]. Microbial absorption and utilization are the main transformation pathways of low molecular weight organic matter in soil [91]. In this study, the anabolic ability of soil bacteria and fungi in the 0–40 cm soil layer was stronger under DP treatment. Specifically, soil treated by DP shows a high abundance of genes involved in Degradation/Utilization/Assimilation (Alcohol Degradation, Carbohydrate Degradation, Chlorinated Compound Degradation, and Secondary Metabolite Degradation). One reason for this is that there are large amounts of straw cover on no-tillage and deep loose surfaces, which cannot be degraded and utilized by microorganisms in the soil. Further, deep plowing of the previous straw and fertilizer into the soil during the fallow period can provide nutrients for most heterotrophic microbial populations involved in the biological metabolism process, and thus affect the metabolic function diversity of soil microorganisms by changing their biochemical properties [92]. The study also found that DP treatment significantly improved the relative abundance of Methanol Oxidation to Carbon Dioxide, Glycolysis, Superpathway of Glycolysis and Entner–Doudoroff, Superpathway of Glycolysis, Pyruvate Dehydrogenase, TCA, and Glyoxylate Bypass, Glycan Biosynthesis, Pyrimidine Deoxyribonucleotides de novo Biosynthesis I, and Superpathway of L-aspartate and L-asparagine Biosynthesis of bacterial communities in the 0–40 cm soil layer. Soil sampling for this study was carried out approximately 310 days after tillage, during which microbial decomposition and metabolism of organic matter in the soil continued for a long time. Although the richness and diversity of soil microbial communities under the deep-plowing conditions were low, more and more competitive microbial communities were enriched in the soil during long-term competition. These microorganisms formed rapidly cycling SOC through Glycolysis and released it into the soil, which promoted the increase in SOC content in the 20–40 cm soil layer under deep-plowing treatment.
4.4. Effects of Fallow Tillage on Winter Wheat Yield
In this study, both ST and DP treatments increased winter wheat yield from 2017 to 2021. By tillage in the fallow period of winter wheat, the water storage capacity of soil was improved, the water supply of winter wheat growth was guaranteed, and the yield of winter wheat was significantly increased [55,56]. In addition, we speculated that the reduction in nutrients in the 0–20 cm soil layer under DP treatment might be due to the absorption of a large amount of soil surface nutrients by winter wheat roots during the growing period, which eventually resulted in high yield. However, the relationship between winter wheat yield and soil microbial community abundance needs further study.
4.5. Insights and Future Prospects
In this research, the soil microbial community structure was measured by Illumina sequencing technology. Our results not only support previous studies, but also provide an important reference value for assessing the effects of tillage practices on soil microbial community structure in dryland wheat fields. However, although PICRUSt2 analysis showed a 10-fold increase in the genome abundance and a 1.5-fold increase in the abundance of direct Kegg orthologs compared with PICRUSt1, this method has limitations and cannot completely and accurately reflect the effects of tillage on soil microbial metabolism and the mechanisms at play. Moreover, the prediction of metabolic function by this method depends on existing reference genomes and cannot provide functional prediction results at the strain level. Therefore, in future work, we will use metagenomics as needed to dig deeper into the “tilling-soil-microbial-plant” interactions and analyze their mechanisms.
5. Conclusions
Soil physicochemical properties, microbial communities, and metabolic functions were significantly changed after five consecutive years of fallow cultivation on dryland farmland in the Loess Plateau. No-tillage and subsoiling tillage significantly increased soil water content and soil nutrients in the soil surface layer, and promoted the diversity of the soil microbial community, thus stabilizing the soil microbial community structure and increasing the abundance of the eutrophic microbial community. Deep plowing can bring organic fertilizer and straw into close contact with the soil, increase the organic matter in the soil subsurface layer, enrich the soil microbial community with stronger competitiveness, and promote the metabolic functions of the soil microbial community. Fallow tillage significantly increased winter wheat yield for five consecutive years, and deep plowing was most effective. Our study has important implications for assessing the effects of fallow tillage on soil microbial community structure and metabolic functions in dryland farmland, as well as for the sustainable development of dryland agriculture.
Author Contributions
Conceptualization, R.Z. and Y.R.; investigation, R.Z. and Z.Z.; methodology, H.N.; software, P.W.; data curation, A.R.; validation, R.Z., Y.R. and P.W.; formal analysis, P.W. and Z.Z.; resources, Z.G. and A.R.; writing—original draft, R.Z.; writing—review and editing, Y.R. and H.N.; supervision, M.S. and Z.G.; project administration, M.S.; funding acquisition, Y.R. and Z.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Shanxi Province Graduate Education Innovation Project (2021Y337), the China Agriculture Research System (CARS-03-01-24), the Central Government guides local science and Technology Development Fund projects (YDZJSX2021C016), the General Project of SCO Institute of Modern Agricultural Development (SC021B004), the State Key Laboratory of Sustainable Dryland Agriculture, Shanxi Agricultural University (202003-2), the Basic Research Program Project of Shanxi Province (20210302123410), the technology innovation team of Shanxi Province (201605D131041), the “1331” Engineering Key Laboratory of Shanxi Province, and the Shanxi Province Foundation for Returness (2022-105).
Data Availability Statement
The raw sequencing data have been deposited in NCBI SRA (Sequence ReadArchive) database with the accession number of PRJNA790651 (https://www.ncbi.nlm.nih.gov) (accessed on 12 December 2021).
Conflicts of Interest
The authors declare that there is no conflict of interest.
References
- West, P.C.; Gerber, J.S.; Engstrom, P.M.; Mueller, N.D.; Brauman, K.A.; Carlson, K.M.; Cassidy, E.S.; Johnston, M.; MacDonald, G.K.; Ray, D.K.; et al. Leverage points for improving global food security and the environment. Science 2014, 345, 325–328. [Google Scholar] [CrossRef]
- Ernst, O.R.; Kemanian, A.R.; Mazzilli, S.R.; Cadenazzi, M.; Dogliotti, S. Depressed attainable wheat yields under continuous annual no-till agriculture suggest declining soil productivity. Field Crops Res. 2016, 186, 107–116. [Google Scholar] [CrossRef]
- Yuan, M.; Fernández, F.G.; Pittelkow, C.M.; Kristin, K.D.; Schaefer, D. Soil and crop response to phosphorus and potassium management under conservation tillage. Agronomy 2020, 112, 2302–2316. [Google Scholar] [CrossRef]
- Altieri, M.A. The ecological role of biodiversity in agroecosystems. Agric. Ecosyst. Environ. 1999, 74, 19–31. [Google Scholar] [CrossRef]
- Hou, R.; Ouyang, Z.; Maxim, D.; Wilson, G.; Kuzyakov, Y. Lasting effect of soil warming on organic matter decomposition depends on tillage practices. Soil Biol. Biochem. 2016, 95, 243–249. [Google Scholar] [CrossRef]
- Wang, Y.; Li, C.; Tu, C.; Hoyt, G.D.; DeForest, J.L.; Hu, S. Long-term no-tillage and organic input management enhanced the diversity and stability of soil microbial community. Sci. Total. Environ. 2017, 609, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, Z.; Zhang, Q.; Hu, N.; Li, Z.; Lou, Y.; Li, Y.; Xue, D.; Chen, Y.; Wu, C.; et al. Long-term effects of nitrogen fertilization on aggregation and localization of carbon, nitrogen and microbial activities in soil. Sci. Total. Environ. 2018, 624, 1131–1139. [Google Scholar] [CrossRef] [PubMed]
- Hao, M.; Hu, H.; Liu, Z.; Dong, Q.; Sun, K.; Feng, Y.; Li, G.; Ning, T. Shifts in microbial community and carbon sequestration in farmland soil under long-term conservation tillage and straw returning. Appl. Soil Ecol. 2019, 136, 43–54. [Google Scholar] [CrossRef]
- Latifmanesh, H.; Deng, A.; Nawaz, M.M.; Li, L.; Chen, Z.; Zheng, Y.; Wang, P.; Zhang, J.; Zheng, C.; Zhang, W. Integrative impacts of rotational tillage on wheat yield and dry matter accumulation under corn-wheat cropping system. Soil Till. Res. 2018, 184, 100–108. [Google Scholar] [CrossRef]
- Mikha, M.M.; Vigil, M.F.; Benjamin, J.G. Long-term tillage impacts on soil aggregation and carbon dynamics under wheat-fallow in the Central Great Plains. Soil Sci. Soc. Am. J. 2013, 77, 594–605. [Google Scholar] [CrossRef]
- Kahlon, M.S.; Lal, R.; Ann-Varughese, M. Twenty two years of tillage and mulching impacts on soil physical characteristics and carbon sequestration in Central Ohio. Soil Till. Res. 2013, 126, 151–158. [Google Scholar] [CrossRef]
- Ernst, G.; Emmerling, C. Impact of five different tillage systems on soil organic carbon content and the density, biomass, and community composition of earthworms after a ten year period. Eur. J. Soil Biol. 2009, 45, 247–251. [Google Scholar] [CrossRef]
- Moinfar, A.; Shahgholi, G.; Abbaspour-Gilandeh, Y.; Herrera-Miranda, I.; Hernández-Hernández, J.L.; Herrera-Miranda, M.A. Investigating the effect of the tractor drive system type on soil behavior under tractor tires. Agronomy 2021, 11, 696. [Google Scholar] [CrossRef]
- Liu, C.; Li, L.; Xie, J.; Coulter, J.A.; Zhang, R.; Luo, Z.; Cai, L.; Wang, L.; Gopalakrishnan, S. Soil bacterial diversity and potential functions are regulated by long-term conservation tillage and straw mulching. Microorganisms 2020, 8, 836. [Google Scholar] [CrossRef] [PubMed]
- Cao, Q.; Li, G.; Yang, F.; Kong, F.; Cui, Z.; Jiang, X.; Lu, Y.; Zhang, E. Eleven-year mulching and tillage practices alter the soil quality and bacterial community composition in Northeast China. Arch. Agron. Soil Sci. 2022, 68, 1274–1289. [Google Scholar] [CrossRef]
- Bielińska, E.; Mocek-Płóciniak, A. Impact of the tillage system on the soil enzymatic activity. Arch. Environ. Prot. 2012, 38, 75–82. [Google Scholar] [CrossRef]
- Pittarello, M.; Chiarini, F.; Menta, C.; Furlan, C.; Carletti, P. Changes in Soil Quality through Conservation Agriculture in North-Eastern Italy. Agriculture 2022, 12, 1007. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, L.; Chen, Q.; Wen, X.; Liao, Y. Conservation tillage increases soil bacterial diversity in the dryland of northern China. Agron. Sustain. Dev. 2016, 36, 1–9. [Google Scholar] [CrossRef]
- Sanchez, I.I.; Fultz, L.M.; Lofton, J.; Haggard, B. Soil biological response to integration of cover crops and nitrogen rates in a conservation tillage corn production system. Soil Sci. Soc. Am. J. 2019, 83, 1356–1367. [Google Scholar] [CrossRef]
- Sun, M.; Wen, F.; Gao, Z.; Ren, A.; Deng, Y.; Zhao, W.; Zhao, H.; Yang, Z.; Hao, X.; Miao, G. Effects of farming practice during fallow period on soil water storage and yield of dryland wheat in different rainfall years. Acta Agron. Sin. 2014, 40, 1459–1469. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, J.; Feng, X.; Zhang, L.; Deng, X.; Ma, E.; Tong, W. Effects of tillage methods on soil physical properties and spatial distribution of flue-cured tobacco (Nicotiana tabacum) roots in mountainous tobacco fields. Chin. J. Eco-Agric. 2019, 27, 1673–1681. [Google Scholar]
- Yan, Q.; Dong, F.; Jia, Y.; Li, F.; Yang, F.; Lu, J.; Gao, L.; Zhang, J.; Wang, M. Effects of tillage patterns on soil water storage and wheat yield in dryland wheat field. J. Soil Water Conserv. 2021, 35, 222–228. [Google Scholar]
- Liu, X.; Peng, C.; Zhang, W.; Li, S.; An, T.; Xu, Y.; Ge, Z.; Xie, N.; Wang, J. Subsoiling tillage with straw incorporation improves soil microbial community characteristics in the whole cultivated layers: A one-year study. Soil Till. Res. 2022, 215, 105188. [Google Scholar] [CrossRef]
- Cong, P.; Li, Y.; Wang, J.; Gao, Z.; Pang, H.; Zhang, L.; Liu, N.; Dong, J. Increasing straw incorporation rates improves subsoil fertility and crop yield in the Huang-Huai-Hai Plain of China. Arch. Agron. Soil Sci. 2020, 66, 1976–1990. [Google Scholar] [CrossRef]
- Sun, R.; Li, W.; Dong, W.; Tian, Y.; Hu, C.; Liu, B. Tillage changes vertical distribution of soil bacterial and fungal communities. Front. Microbiol. 2018, 9, 699. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, R.; Xue, J.; Pu, C.; Zhang, X.; Liu, S.; Chen, F.; Lal, R.; Zhang, H. Management-induced changes to soil organic carbon in China: A meta-analysis. Adv. Agron. 2015, 134, 1–50. [Google Scholar]
- Kopittke, P.M.; Dalal, R.C.; Finn, D.; Menzies, N.W. Global changes in soil stocks of carbon, nitrogen, phosphorus, and sulphur as influenced by long-term agricultural production. Global Chang. Biol. 2016, 23, 2509–2519. [Google Scholar] [CrossRef]
- Yan, Q.; Wu, L.; Dong, F.; Zhang, Q.; Yang, F.; Yan, S.; Dong, J. Continuous tillage practices improve soil water storage and yields of dryland winter wheat grown for three consecutive years in North China. Arch. Agron. Soil Sci. 2021, 1–15. [Google Scholar] [CrossRef]
- Shi, Y.; Delgado-Baquerizo, M.; Li, Y.; Yang, Y.; Zhu, Y.; Peñuelas, J.; Chu, H. Abundance of kinless hubs within soil microbial networks are associated with high functional potential in agricultural ecosystems. Environ. Int. 2020, 142, 105869. [Google Scholar] [CrossRef]
- Zhang, H.; Shi, Y.; Dong, Y.; Lapen, D.R.; Liu, J.; Chen, W. Subsoiling and conversion to conservation tillage enriched nitrogen cycling bacterial communities in sandy soils under long-term maize monoculture. Soil Tillage Res. 2022, 215, 105197. [Google Scholar] [CrossRef]
- Anna, M.G.; Ewa, A.C.; Jadwiga, S.-T.; Anthony, R.D.; Karolina, M.F.; Jarosław, G. Effects of long-term tillage practices on the quality of soil under winter wheat. Plant Soil Environ. 2017, 63, 236–242. [Google Scholar] [CrossRef]
- Shen, X.; Wang, L.; Zhao, J.; Li, G.; Xiu, W.; Yang, Q.; Zhang, G. Effects of tillage managements on soil microbial community structure in soil aggregates of fluvo-aquic soil. Chin. J. Appl. Ecol. 2021, 32, 2713–2721. [Google Scholar]
- Chen, J.; Li, S.; Zhang, Y.; Chen, F.; Zhang, H. Characteristics of soil temperature and response to air temperature under different tillage systems diurnal dynamic of soil temperature and its response to air temperature. Sci. Agric. Sin. 2009, 42, 2592–2600. [Google Scholar]
- Ren, C.; Chen, J.; Lu, X.; Doughty, R.; Zhao, F.; Zhong, Z.; Han, X.; Yang, G.; Feng, Y.; Ren, G. Responses of soil total microbial biomass and community compositions to rainfall reductions. Soil Biol. Biochem. 2018, 116, 4–10. [Google Scholar] [CrossRef]
- Essel, E.; Xie, J.; Deng, C.; Peng, Z.; Wang, J.; Shen, J.; Xie, J.; Coulter, J.A.; Li, L. Bacterial and fungal diversity in rhizosphere and bulk soil under different long-term tillage and cereal/legume rotation. Soil Tillage Res. 2019, 194, 104302. [Google Scholar] [CrossRef]
- Legrand, F.; Picot, A.; Cobo-Díaz, J.F.; Carof, M.; Chen, W.; Floch, G.L. Effect of tillage and static abiotic soil properties on microbial diversity. Appl. Soil Ecol. 2018, 132, 135–145. [Google Scholar] [CrossRef]
- Barnard, R.L.; Osborne, C.A.; Firestone, M.K. Responses of soil bacterial and fungal communities to extreme desiccation and rewetting. ISME J. 2013, 7, 2229–2241. [Google Scholar] [CrossRef]
- Chu, H.; Grogan, P. Soil microbial biomass, nutrient availability and nitrogen mineralization potential among vegetation-types in a low arctic tundra landscape. Plant Soill 2010, 329, 411–420. [Google Scholar] [CrossRef]
- Chen, J.; Zheng, M.; Pang, D.; Yin, Y.; Han, M.; Li, Y.; Luo, Y.; Xu, X.; Li, Y.; Wang, Z. Straw return and appropriate tillage method improve grain yield and nitrogen efficiency of winter wheat. J. Integr. Agric. 2017, 16, 1708–1719. [Google Scholar] [CrossRef]
- Bi, Y.; Zou, H.; Zhu, C. Dynamic monitoring of soil bulk density and infiltration rate during coal mining in sandy land with different vegetation. Int. J. Coal Sci. Techn. 2014, 1, 198–206. [Google Scholar] [CrossRef]
- Mulumba, L.N.; Lal, R. Mulching effects on selected soil physical properties. Soil Tillage Res. 2008, 98, 106–111. [Google Scholar] [CrossRef]
- Xue, J.; Pu, C.; Liu, S.; Chen, Z.; Chen, F.; Xiao, X.; Lal, R.; Zhang, H. Effects of tillage systems on soil organic carbon and total nitrogen in a double paddy cropping system in Southern China. Soil Tillage Res. 2015, 153, 161–168. [Google Scholar] [CrossRef]
- Berthrong, S.T.; Buckley, D.H.; Drinkwater, L.E. Agricultural management and labile carbon additions affect soil microbial community structure and interact with carbon and nitrogen cycling. Microb. Ecol. 2013, 66, 158–170. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet. J. 2011, 17, 10–12. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Katoh, K.; Misawa, K.; Kuma, K.; Miyata, T. MAFFT: A novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 2002, 30, 3059–3066. [Google Scholar] [CrossRef] [PubMed]
- Price, M.N.; Dehal, P.S.; Arkin, A.P. FastTree: Computing large minimum evolution trees with profiles instead of a distance matrix. Mol. Biol. Evol. 2009, 26, 1641–1650. [Google Scholar] [CrossRef]
- Chao, A. Nonparametric Estimation of the Number of Classes in a Population. Scand. J. Stat. 1984, 11, 265–270. [Google Scholar]
- Shannon, C.E. A mathematical theory of communication. Bell Syst. Tech. J. 1948, 27, 379–423; 623–656. [Google Scholar] [CrossRef]
- Simpson, E.H. Measurement of Diversity. Nature 1949, 163, 688. [Google Scholar] [CrossRef]
- Bokulich, N.A.; Kaehler, B.D.; Rideout, J.R.; Dillon, M.; Bolyen, E.; Knight, R.; Huttley, G.A.; Gregory Caporaso, J. Optimizing taxonomic classification of marker-gene amplicon sequences with QIIME 2’s q2-feature-classifier plugin. Microbiome 2018, 6, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Kõljalg, U.; Nilsson, R.H.; Abarenkov, K.; Tedersoo, L.; Taylor, A.F.S.; Bahram, M.; Bates, S.T.; Bruns, T.D.; Bengtsson-Palme, J.; Callaghan, T.M.; et al. Towards a unified paradigm for sequence-based identification of fungi. Mol. Ecol. 2013, 22, 5271–5277. [Google Scholar] [CrossRef] [PubMed]
- Douglas, G.M.; Maffei, V.J.; Zaneveld, J.R.; Yurgel, S.N.; Brown, J.R.; Taylor, C.M.; Huttenhower, C.; Langille, M.G.I. PICRUSt2 for prediction of metagenome functions. Nat. Biotechnol. 2020, 38, 685–688. [Google Scholar] [CrossRef]
- Xue, L.; Khan, S.; Sun, M.; Anwar, S.; Ren, A.; Gao, Z.; Lin, W.; Xue, J.; Yang, Z.P.; Deng, Y. Effects of tillage practices on water consumption and grain yield of dryland winter wheat under different precipitation distribution in the loess plateau of China. Soil Tillage Res. 2019, 191, 66–74. [Google Scholar] [CrossRef]
- Khan, S.; Anwar, S.; Sun, M.; Shafiq, F.; Khalilzadeh, R.; Gao, Z. Characterizing differences in soil water content and wheat yield in response to tillage and precipitation in the Dry, Normal, and wet years at the Loess Plateau. Int. J. Plant Prod. 2021, 15, 655–668. [Google Scholar] [CrossRef]
- Wang, Z.; Li, T.; Li, Y.; Zhao, D.; Han, J.; Liu, Y.; Liao, Y. Relationship between the microbial community and catabolic diversity in response to conservation tillage. Soil Tillage Res. 2020, 196, 104431. [Google Scholar] [CrossRef]
- Basic, F.; Kisic, I.; Mesic, M.; Nestroy, O.; Butorac, A. Tillage and crop management effects on soil erosion in central Croatia. Soil Tillage Res. 2004, 78, 197–206. [Google Scholar] [CrossRef]
- Schneider, F.; Don, A.; Hennings, I.; Schmittmann, O.; Seidel, S.J. The effect of deep tillage on crop yield–What do we really know? Soil Tillage Res. 2017, 174, 193–204. [Google Scholar] [CrossRef]
- Büchi, L.; Wendling, M.; Amossé, C.; Necpalova, M.; Charles, R. Importance of cover crops in alleviating negative effects of reduced soil tillage and promoting soil fertility in a winter wheat cropping system. Agric. Ecosyst. Environ. 2018, 256, 92–104. [Google Scholar] [CrossRef]
- Feng, Q.; An, C.; Chen, Z.; Wang, Z. Can deep tillage enhance carbon sequestration in soils? A meta-analysis towards GHG mitigation and sustainable agricultural management. Renew. Sustain. Energy Rev. 2020, 133, 110293. [Google Scholar] [CrossRef]
- Villamil, M.B.; Little, J.; Nafziger, E.D. Corn residue, tillage, and nitrogen rate effects on soil properties. Soil Tillage Res. 2015, 151, 61–66. [Google Scholar] [CrossRef]
- Ceja-Navarro, J.A.; Rivera-Orduna, F.N.; Patiño-Zúñiga, L.; Vila-Sanjurjo, A.; Crossa, B.; Govaerts, B.; Dendooven, L. Phylogenetic and multivariate analyses to determine the effects of different tillage and residue management practices on soil bacterial communities. Appl. Environ. Microbiol. 2010, 76, 3685–3691. [Google Scholar] [CrossRef] [PubMed]
- Lienhard, P.; Terrat, S.; Prévost-Bouré, N.C.; Nowak, V.; Régnier, T.; Sayphoummie, S.; Panyasiri, K.; Tivet, F.; Mathieu, O.; Levêque, J. Pyrosequencing evidences the impact of cropping on soil bacterial and fungal diversity in Laos tropical grassland. Agron. Sustain. Dev. 2014, 34, 525–533. [Google Scholar] [CrossRef]
- Zhou, J.; Xia, B.; Treves, D.S.; Wu, L.; Marsh, T.L.; O’Neill, R.V.; Palumbo, A.V.; Tiedje, J.M. Spatial and resource factors influencing high microbial diversity in soil. Appl. Environ. Microbiol. 2002, 68, 326–334. [Google Scholar] [CrossRef]
- Lauber, C.L.; Strickland, M.S.; Bradford, M.A.; Fierer, N. The influence of soil properties on the structure of bacterial and fungal communities across land-use types. Soil Biol. Biochem. 2008, 40, 2407–2415. [Google Scholar] [CrossRef]
- Hong, Y.; Zhao, D.; Zhang, F.; Shen, G.; Yuan, Y.; Gao, Y.; Yan, L.; Wei, D.; Wang, W. Soil water-stable aggregates and microbial community under long-term tillage in black soil of Northern China. Ecotoxicology 2021, 30, 1754–1768. [Google Scholar] [CrossRef]
- Ashworth, A.J.; DeBruyn, J.M.; Allen, F.L.; Radosevich, M.; Owens, P.R. Microbial community structure is affected by cropping sequences and poultry litter under long-term no-tillage. Soil Biol. Biochem. 2017, 114, 210–219. [Google Scholar] [CrossRef]
- Hao, J.; Lin, Y.; Ren, G.; Yang, G.; Han, X.; Wang, X.; Ren, C.; Feng, Y. Comprehensive benefit evaluation of conservation tillage based on BP neural network in the Loess Plateau. Soil Tillage Res. 2021, 205, 104784. [Google Scholar] [CrossRef]
- Somenahally, A.; DuPont, J.I.; Brady, J.; McLawrence, J.; Northup, B.; Gowda, P. Microbial communities in soil profile are more responsive to legacy effects of wheat-cover crop rotations than tillage systems. Soil Biol. Biochem. 2018, 123, 126–135. [Google Scholar] [CrossRef]
- Blackwood, C.B.; Waldrop, M.P.; Zak, D.R.; Sinsabaugh, R.L. Molecular analysis of fungal communities and laccase genes in decomposing litter reveals differences among forest types but no impact of nitrogen deposition. Environ. Microbiol. 2007, 9, 1306–1316. [Google Scholar] [CrossRef]
- Lv, B.; Zhang, L.; Zhang, G.; Liu, Z.; Fan, Q.; Yao, Z.; Chang, F. Response of soil fungal diversity to long-term conservation tillage in dryland wheat soils on the Loess Plateau, China. Chin. J. Eco-Agric. 2021, 29, 1215–1223. [Google Scholar]
- Ge, Z.; Li, S.; Bol, R.; Zhu, P.; Peng, C.; An, T.; Cheng, N.; Liu, X.; Li, T.; Xu, Z.; et al. Differential long-term fertilization alters residue-derived labile organic carbon fractions and microbial community during straw residue decomposition. Soil Tillage Res. 2021, 213, 105120. [Google Scholar] [CrossRef]
- Wu, J.; Liu, J.; Liu, X.; Yang, H.; Wang, X.; Xu, M.; Wei, Y.; Bian, X. Effects of rice and wheat straw ditch-buried returns on the soil physical properties of wheat fields. Acta Ecol. Sin. 2016, 36, 2066–2075. [Google Scholar]
- Carter, M.R. Microbial biomass as an index for tillage-induced changes in soil biological properties. Soil Tillage Res. 1986, 7, 29–40. [Google Scholar] [CrossRef]
- Zhu, F.; Lin, X.; Guan, S.; Dou, S. Deep incorporation of corn straw benefits soil organic carbon and microbial community composition in a black soil of Northeast China. Soil Use Manage. 2022, 38, 1266–1279. [Google Scholar] [CrossRef]
- Segal, L.M.; Miller, D.N.; McGhee, R.P.; Loecke, T.D.; Cook, K.L.; Shapiro, C.A.; Drijber, R.A. Bacterial and archaeal ammonia oxidizers respond differently to long-term tillage and fertilizer management at a continuous maize site. Soil Tillage Res. 2017, 168, 110–117. [Google Scholar] [CrossRef]
- McCarthy, A.J.; Williams, S.T. Actinomycetes as agents of biodegradation in the environment—A review. Gene 1992, 115, 189–192. [Google Scholar] [CrossRef]
- Jenkins, S.N.; Rushton, S.P.; Lanyon, C.V.; Whiteley, A.S.; Waite, I.S.; Brookes, P.C.; Kemmitt, S.; Evershed, R.P.; O’Donnell, A.G. Taxon-specific responses of soil bacteria to the addition of low level C inputs. Soil Biol. Biochem. 2010, 42, 1624–1631. [Google Scholar] [CrossRef]
- Liu, Z.; Gu, H.; Liang, A.; Li, L.; Yao, Q.; Xu, Y.; Liu, J.; Jin, J.; Liu, X.; Wang, G. Conservation tillage regulates the assembly, network structure and ecological function of the soil bacterial community in black soils. Plant Soil 2022, 472, 207–223. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, S.; Li, R.; Zhang, J.; Liu, Y.; Lv, L.; Zhu, H.; Wu, W.; Li, W. Plant cultivars imprint the rhizosphere bacterial community composition and association networks. Soil Biol. Biochem. 2017, 109, 145–155. [Google Scholar] [CrossRef]
- Wang, H.; Li, X.; Li, X.; Wang, J.; Li, X.; Guo, Q.; Yu, Z.; Yang, T.; Zhang, H. Long-term no-tillage and different residue amounts alter soil microbial community composition and increase the risk of maize root rot in northeast China. Soil Tillage Res. 2020, 196, 104452. [Google Scholar] [CrossRef]
- Dong, W.; Liu, E.; Yan, C.; Zhang, H.; Zhang, Y. Changes in the composition and diversity of topsoil bacterial, archaeal and fungal communities after 22 years conventional and no-tillage managements in Northern China. Arch. Agron. Soil Sci. 2017, 63, 1369–1381. [Google Scholar] [CrossRef]
- Liu, J.; Sui, Y.; Yu, Z.; Yao, Q.; Shi, Y.; Chu, H.; Jin, J.; Liu, X.; Wang, G. Diversity and distribution patterns of acidobacterial communities in the black soil zone of northeast China. Soil Biol. Biochem. 2016, 95, 212–222. [Google Scholar] [CrossRef]
- Rodrigues, J.L.M.; Pellizari, V.H.; Mueller, R.; Baek, K.; Jesus, E.C.; Paula, F.S.; Mirza, B.; Hamaoui, G.S., Jr.; Tsai, S.M.; Feigl, B.; et al. Conversion of the Amazon rainforest to agriculture results in biotic homogenization of soil bacterial communities. Proc. Natl. Acad. Sci. USA 2013, 110, 988–993. [Google Scholar] [CrossRef]
- Ren, A.; Zhao, W.; Sumera, A.; Lin, W.; Ding, P.; Hao, R.; Wang, P.; Zhong, R.; Tong, J.; Gao, Z.; et al. Effects of tillage and seasonal variation of rainfall on soil water content and root growth distribution of winter wheat under rainfed conditions of the Loess Plateau, China. Agric. Water Manage. 2022, 268, 107533. [Google Scholar]
- Chen, Q.; Zhang, X.; Sun, L.; Ren, J.; Yuan, Y.; Zang, S. Influence of Tillage on the Mollisols Physicochemical Properties, Seed Emergence and Yield of Maize in Northeast China. Agriculture 2021, 11, 939. [Google Scholar] [CrossRef]
- Bastian, F.; Bouziri, L.; Nicolardot, B.; Ranjard, L. Impact of wheat straw decomposition on successional patterns of soil microbial community structure. Soil Biol. Biochem. 2009, 41, 262–275. [Google Scholar] [CrossRef]
- Smith, C.R.; Blair, P.L.; Boyd, C.; Cody, B.; Hazel, A.; Hedrick, A.; Kathuria, P.; Kramer, B.; Muterspaw, K.; Peck, C.; et al. Microbial community responses to soil tillage and crop rotation in a corn/soybean agroecosystem. Ecol. Evol. 2016, 6, 8075–8084. [Google Scholar] [CrossRef]
- Srour, A.Y.; Ammar, H.A.; Subedi, A.; Pimentel, M.; Cook, R.L.; Bond, J.; Fakhoury, A.M. Microbial communities associated with long-term tillage and fertility treatments in a corn-soybean cropping system. Front. Microbiol. 2020, 11, 1363. [Google Scholar] [CrossRef]
- Gunina, A.; Dippold, M.A.; Glaser, B.; Kuzyakov, Y. Fate of low molecular weight organic substances in an arable soil: From microbial uptake to utilisation and stabilisation. Soil Biol. Biochem. 2014, 77, 304–313. [Google Scholar] [CrossRef]
- Tian, J.; McCormack, L.; Wang, J.; Guo, D.; Wang, Q.; Zhang, X.; Yu, G.; Blagodatskaya, Y. Linkages between the soil organic matter fractions and the microbial metabolic functional diversity within a broad-leaved Korean pine forest. Eur. J. Soil Biol. 2015, 66, 57–64. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).