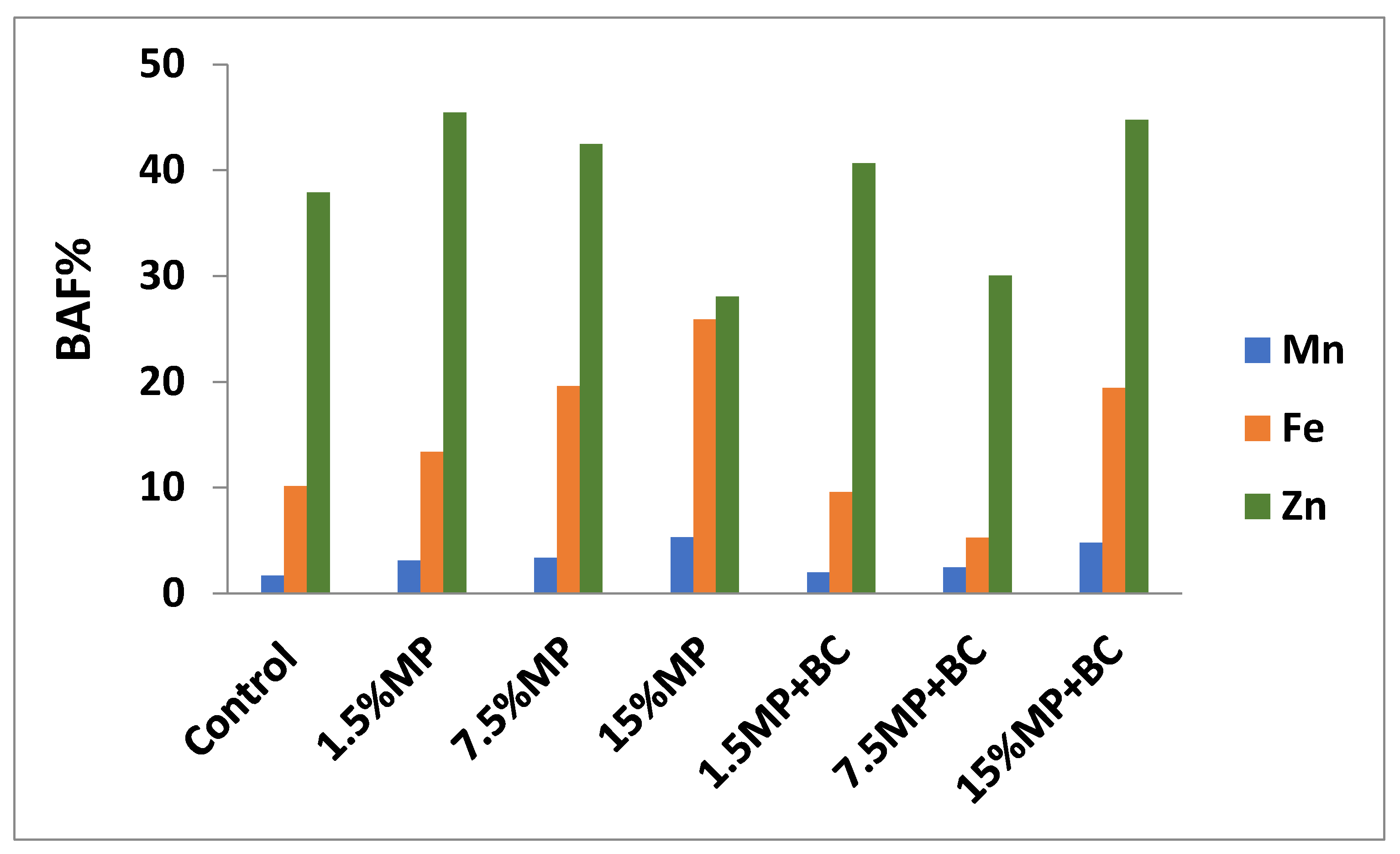1. Introduction
Plastics have become an indispensable aspect of modern life. Plastic is frequently utilized in modern civilization. Current production and consumption, however, are unsustainable. The global usage of plastics in 2019 was estimated to be 368 million tons [
1,
2] but is expected to be doubled within 20 years [
2]. Plastic waste is recognized as toxic, and the risk grows when polymers degrade in nature to secondary microplastics (MPs) or even nanoplastics [
3]. Microplastics are often described as plastic trash with dimensions less than 5 mm. They either are the product of larger plastic fragmentation (secondary MPs) or the direct release of small plastic fragments from anthropogenic activities, which are considered the major contributor to global contamination [
1,
4]. The primary causes of MP accumulation in diverse ecosystems of the environment include urban sprawl, industrialization, uncontrolled usage and inadequate waste management of plastic items. Microplastics in the soil are seen as an emerging danger to agro-ecosystems. The majority of MPs research has been conducted in aquatic environments, while the eco-toxicological impacts of these pollutants in terrestrial ecosystems, particularly agro-ecosystems, are still little understood. The harmful impacts of MPs on soil’s physical, chemical and biological characteristics are gradually becoming clear. [
5]. Thus, MP and residual plastic in soil can effect soil fertility, plant health and plant production [
6,
7,
8]. Soil contamination with MPs implies suffering plant communities in this soil from various degrees of MP contamination. Because of the importance of plants in terrestrial ecosystems and the continued release of MPs, the potential effects of MPs on terrestrial plants are causing significant worry. The MP exposure can have a number of effects on terrestrial plants’ physiology, morphology and community structure. Mulching agricultural soil with plastic films, for example, might enhance water evaporation from this soil, resulting in more severe droughts. They can also be absorbed by the roots, fruits and vegetables or adsorbed onto the surface of the roots [
9,
10,
11].
Microplastics pose significant threats to terrestrial ecosystems due to their abundance and persistence [
12]. The broad presence of MPs in many ecosystems makes them accessible to a diverse spectrum of species living in those ecosystems, including plants, animals, and then, even humans. As a result, their existence and possible detrimental impacts on all living species should be carefully examined [
13]. Higher plants, which constitute an essential component of the terrestrial ecosystem, are invariably subjected to MPs [
12]. Given that the great majority of higher plants are terrestrial plants and that soil is a significant sink for MPs, soil cultivation experiments are required to expose the impacts of MPs on plants. In most soil cultivation experiments, the investigated MPs were typically applied to soils [
12]. Knowledge of the ecotoxicology of MP is advancing as well; however, information regarding its impacts on plants is still lacking [
3].
In addition, contamination of soil by MP has many different impacts on the microbial community structure and activity by changing soil parameters [
14]. Changes of microbial communities because of MP may alter biogeochemical cycling, which possibly results in the influence on the whole functions and services of soil ecosystem [
15]. As well, altering the microbial soil ecosystems due to MP may also increase or decrease enzymatic processes based on MP type [
16].
MPs can be degraded naturally by some processes, such as photodegradation and thermo-oxidative degradation. During these processes, the MPs’ polymer can be degraded into smaller pieces with a molecular weight that is favorable to microbial decomposition. However, the C in the polymer may be converted by microbes into CO
2. In addition, the long period (more than 50 years) for totally decomposing the MPs is another disadvantage of these processes [
17]. Furthermore, some physical techniques and synthesis of some chemicals are used in this purpose; however, complexity, non-greener character and polymer and environmental variability are among other disadvantages for MPs’ remediation [
18]. Thus, it is critical to develop MPs’ removal technologies; for instance, including MPs’ removal procedures in wastewater treatment will decrease the quantity of MPs that reach soil ecosystems as a result of sewage irrigation [
19].
Biochar is one of the most effective and widely available biomass adsorbents. It has a stable and unique structure and many properties, such as abundance, carbon-rich and high porosity. In addition, BC is less expensive, more eco-friendly and needs less energy-intensive manufacturing technology. All of these features make it effective in the removal of numerous types of pollutants, including MPs. As a result, investigating approaches for removal of MPs utilizing BC can assist researchers in developing and improving novel mitigation technologies [
20]. Some researchers addressed the using of BC or modified BC for MP removal with high efficiency; however, most of these trials were conducted on water such as [
21,
22,
23]. Wang et al. [
22] recommended further research for more confidence on the effectiveness of BC to remove MP. Little is known about using BC in this purpose in soil because of some experiments performed as incubation experiments with the acceleration of MP removal of BC such as [
24,
25,
26] or microcosm experiment [
15] and little others on cultivated plants. Palansooriya et al. [
16] and Dissanayake et al. [
26] reported that the potential use of BC as a soil amendment can generally improve the soil quality of contaminated soil with MP particles; however, this impact differs depending on the temperature and feedstock type.
Although the quantity of research on the toxicological effects of MPs on organisms has increased dramatically, there is a need to better understand the potential mitigating effect of BC of date palm nuclei in this approach. This will achieve two benefits: utilizing the date palm nuclei and avoiding their accumulation in the arid environments especially and ecofriendly remediation of MP-contaminated soil. Thus, this study aims to: (1) study the effects of MPs and the toxicity, enzyme activity and properties of Vicia faba planted in pots; (2) examine the effects of MPs on the soil properties; (3) investigate the effects of MPs on microbial count and microbial biomass C and N; and (4) study the remediating effect of BC on the harmful effects on soil, plant and microbial activity. Thus, the novelty of this study is to better understand the effect of BC on mitigating the impacts of MPs on soil, plant and microbes, focusing on some aspects that have not been studied well before, such as cytogenetic effect of MPs in Vicia faba and investigating the effect of MP on soil C and N biomass.
2. Materials and Methods
The experiment was carried out at the Environmental and Biological Sciences Dept., Fac. of Home Economics, Al-Azhar Univ., and Tanta, Egypt.
2.1. Microplastic and Biochar Preparation
The residues of plastic banners (acrylic plastic) were obtained locally, manually cut with scissors into small pieces and ground by special grind into pieces less than 5 mm. The Biochar used in this study was made from date nuclei which were washed after collection, oven dried at 105°C and then pyrolyzed at 500°C in a muffle furnace for 4 h.
2.2. Soil and Seeds Preparation
Vicia Faba bean (sakha 1) were obtained from the agricultural research center, Sakha, Kafr El-Sheikh, Egypt, sorted in equal size and used in the experiment. The seeds were soaked for 24 h in water and dried by filter paper. Bulk soil (0–30 cm) was collected from agricultural soil from El-Gharbia governorate, Egypt, air-dried, ground, sieved (2 mm mesh size) and kept in plastic bags until analysis and use in the experiment.
2.3. Experimental Design
The current experiment was designed using a completely randomized design. First, microplastics were mixed with soil before being placed into each individual pot to help provide an equal distribution of MPs particles in the soil. Seven treatments with three replicates for each concentration were established: control (soil without MPs), 1.5% MPs, 7.5% MPs, 15% MPs, 1.5% MPs + 2% BC, 7.5% MPs + 2% BC, 15% MPs + 2% BC. Each pot contained 300 g of soil mixed with a treatment and four seeds of Vicia Faba beans.
In December 2021, the seeds were planted in pots and watered with 100 mL twice a week (approximately 70% of soil field capacity) during the first 3 weeks of growth and then every week. Pots were watered gently by hand by spraying distilled water on the soil surface. After 45 days, plants were carefully removed from the soil, washed by tap water and rinsed many times with distilled water.
2.4. Soil, Biochar and Microplastic Analysis
The physiochemical soil properties were performed. Soil pH, EC, TDS and organic matter were measured according to standard method. Soil pH was determined by pH-meter (JENWAY 3510, UK) in 1: 2.5 (soil: water) (
w/v) suspensions [
27]. Electrical conductivity (EC) of soil and TDS were measured in 1: 5 (soil: water extract) (
w/v) using EC meter (Mi170, Milwaukee, Italy) [
27]. Organic matter (OM) in soil (OM) was determined using a muffle furnace at 400 °C for 4 h by the loss on ignition method [
28,
29]. Total CaCO
3 was measured by a Collins calcimeter. Soil available phosphorus (P) was extracted using ammonium bicarbonate–diethylene triaminepentaacetic (AB–DTPA) and calorimetrically determined by the ascorbic acid method. Available Fe, Mn and Zn were extracted using AB–DTPA [
30]. The total metals were measured after extraction by HNO
3 and HCl acids and 30% hydrogen peroxide [
31]. The metals concentration was measured by atomic absorption spectrometry AAS (GBC Avanta E, Victoria, Australia).
The functional groups of BC and MPs and treated soil samples were analyzed by fourier transform infrared (FTIR) spectra of specimens, Jasco FT/IR 4100 spectrometer in the wavelength range of 4000–400 cm−0 with equipping all the samples on KBr tablets.
2.5. Plant Analysis
2.5.1. Germination Percentage
The germination percentage was calculated as in the following equation:
2.5.2. Phytotoxicity
The phytotoxicity percentage is described as a percentage of phytotoxicity compared to phytotoxicity of untreated controls and is defined as follows [
32,
33,
34]:
2.5.3. Morphological Traits
Morphological traits were directly determined (after plant removing from soil and washing) for fine roots (i.e., <2 mm in diameter), root length, shoot length, surface area and number of root nodules on a fresh sample of the plants. Fresh weight was recorded and plants were dried at 60°C for 72 h for recording dry weight. Plant leaves were randomly sampled from different parts of the plants. Maximum leaflet width (W) and length (L) were measured, and leaf area was calculated as described by [
35].
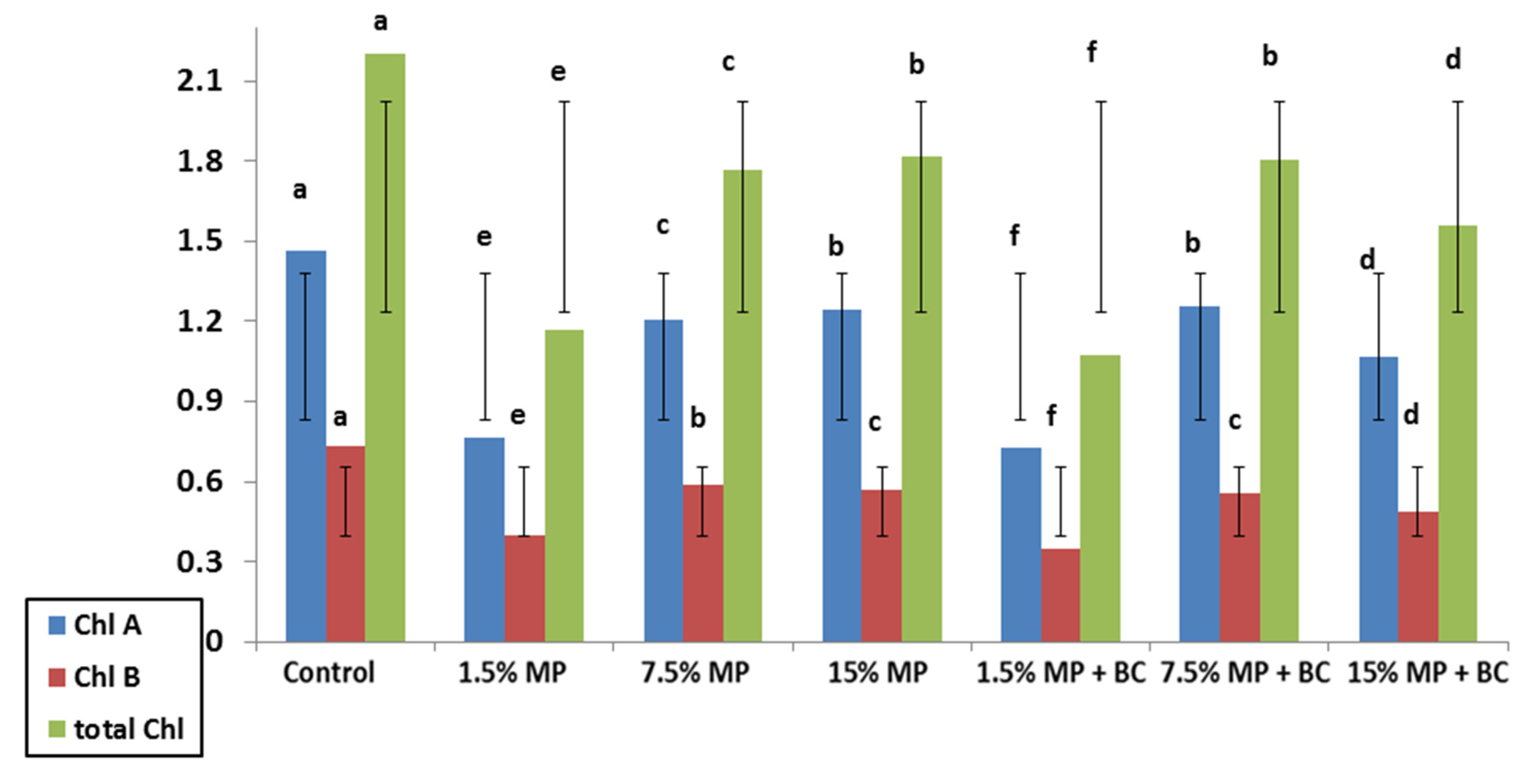
2.5.4. Chlorophyll Content Measurement
Equal circle pieces of leaves were treated by 1: 1
v/v of 80% acetone and absolute ethyl alcohol to extract chlorophyll. Chlorophyll content was spectrophotometrically determined using a chlorophyll meter (Konica-Minolta, Osaka, Japan) [
36].
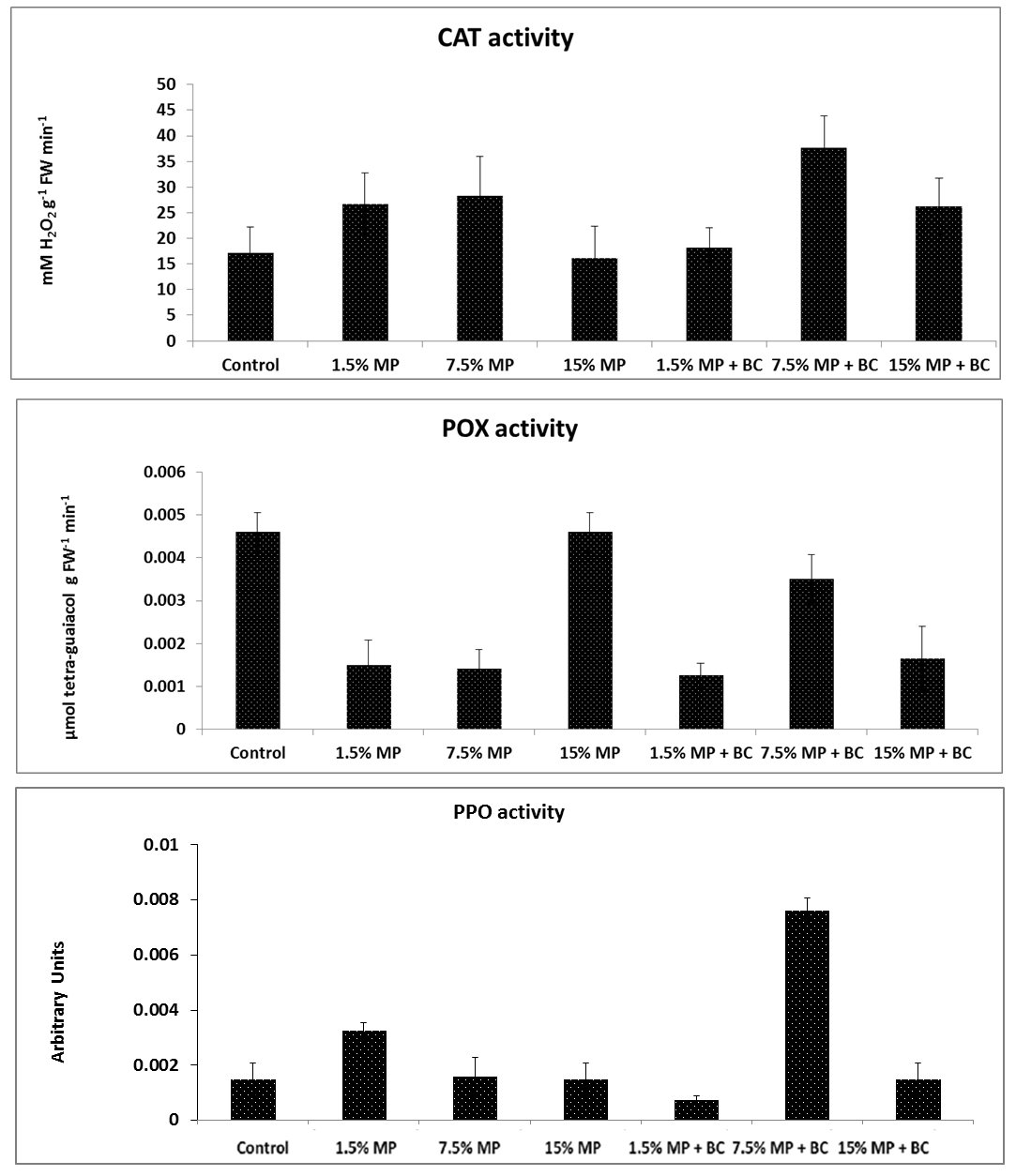
2.5.5. Enzymes Activity
After the experiment, samples from fresh leaves were collected to measure the total soluble enzymes activity of Catalase (CAT) activity [
37], peroxidases (POD) [
38] and polyphenol oxidase [
39].
2.5.6. Metals in Plants
Metals in plants were determined by the ash drying of 1 g of plant in a muffle furnace at 450 °C for 4 h, extracting with 20% HCl [
40] and then the metal measured by the same previous ASS.
Bioaccumulation factor (BAF) was determined as an indicator to plat efficiency to accumulate metals from the soil and was calculated as follow [
30]:
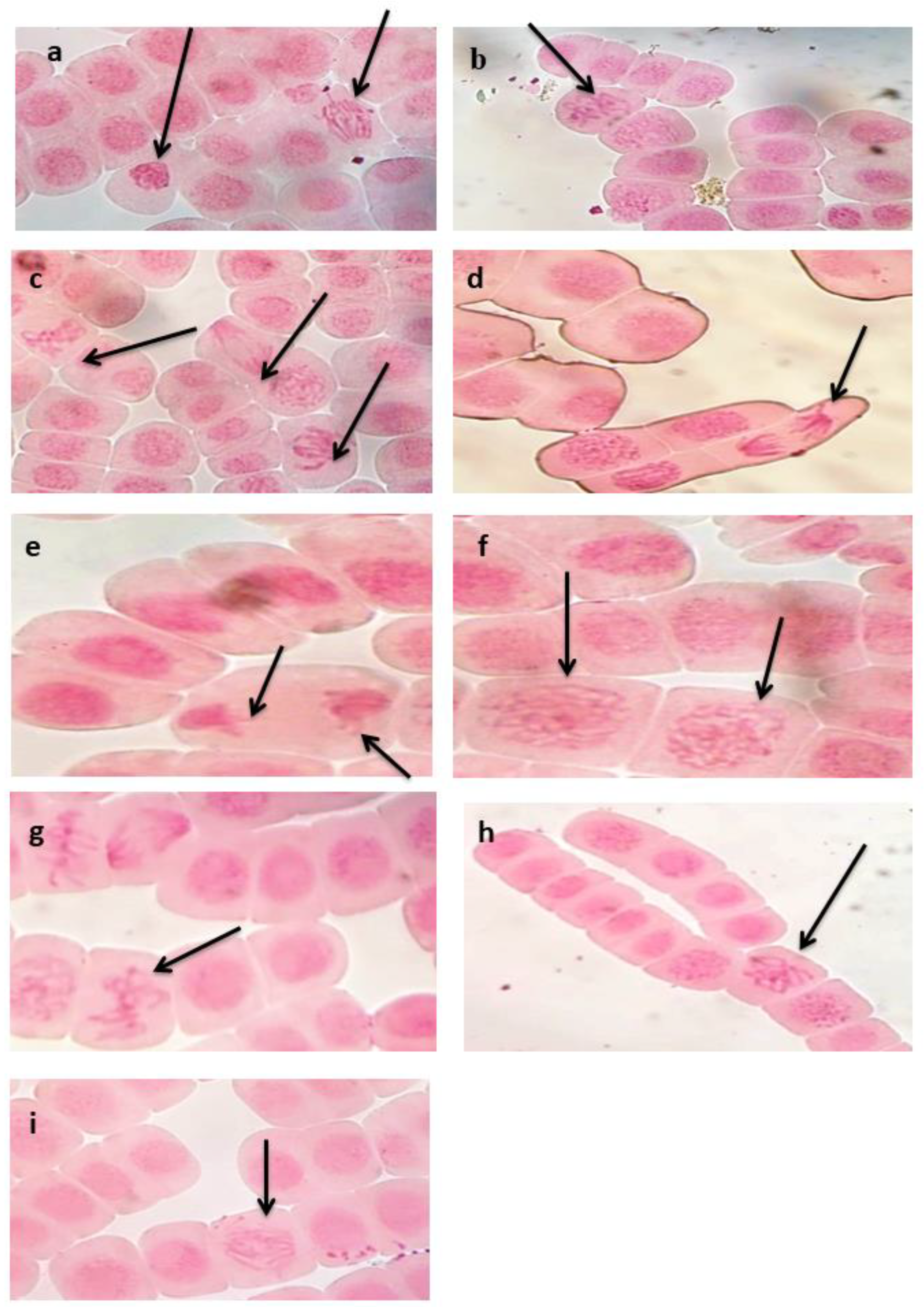
2.5.7. Cytological Analysis
Root tips of germinated seeds (1.5–2 cm) were cut and fixed in Carnoy’s fixative solution (1:3 glacial acetic acid: ethyl alcohol absolute) for 24 h. Carnoy’s fixed root tips were kept in ethyl alcohol (70%) at 4°C till using for cytological analysis.
Aceto–carmine stain (2%) was used for cytological preparation [
41]. Mitotic index, numbers and types of abnormalities were scored in at least 3000 examined cells/treatment (1000 cell/replicate) using light microscope.
Percentage of abnormal cells and mitotic index (MI) were estimated by the following equation:
2.6. Microbial Activity
2.6.1. Total Count of Bacteria and Fungi
Standard dilution technique was used in which 10 g of soil were suspended in 90 mL of sterile distilled water and shaken for 20 min. Tenfold serial dilution was used for bacterial and fungal counts, as follows:
Total count of bacteria was estimated through plate count on media of nutrient agar [
42]. This media were made at pH 7.4 ± 0.2 and incubated at 30 °C for 2 days.
Total count of fungi was estimated by plate count on media of Potato-dextrose agar after incubation of 5 days of incubation at 28°C [
42] and adjusting pH at 5.2.
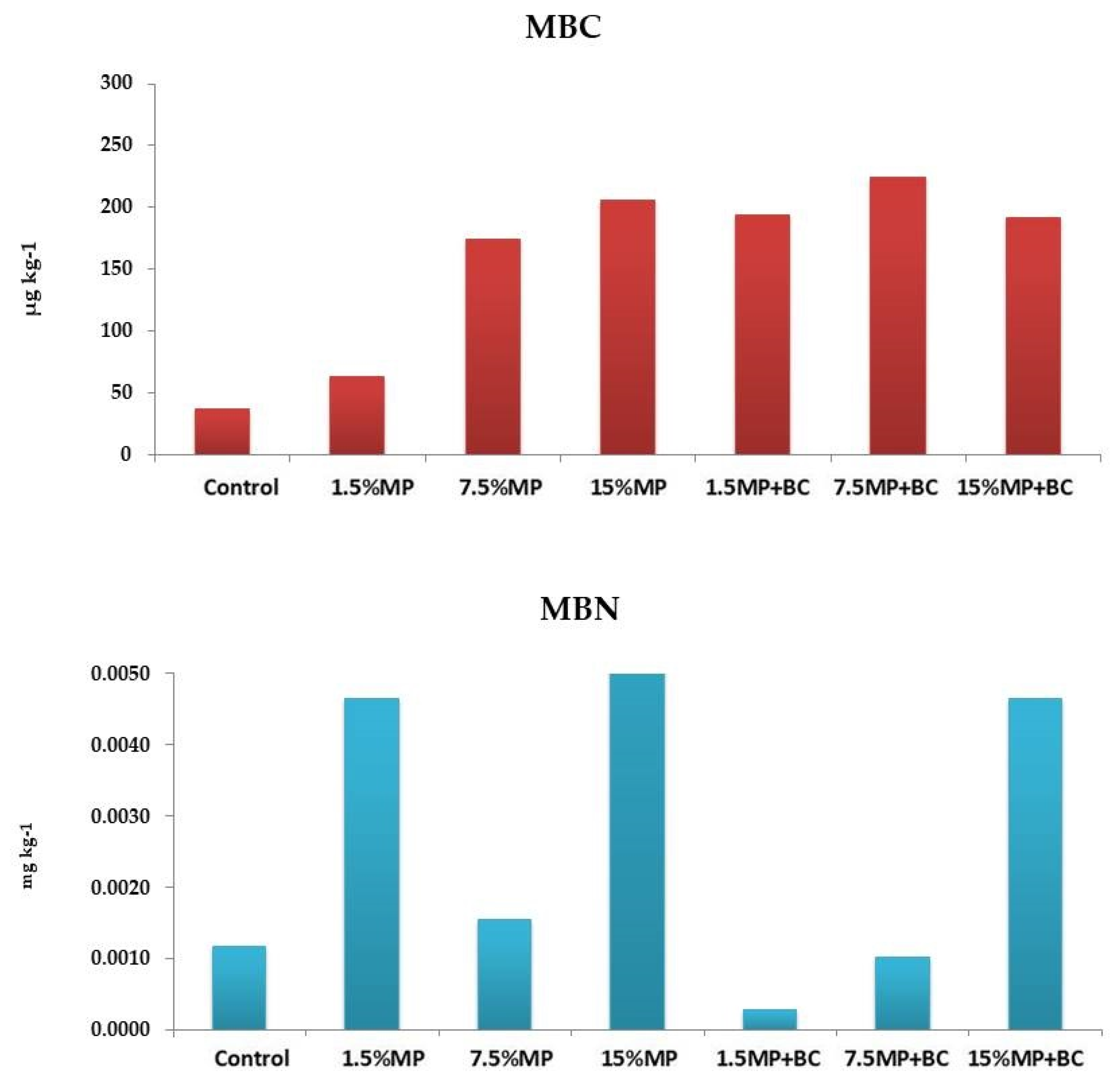
2.6.2. Soil Microbial Biomass Carbon and Nitrogen
Exactly 5 g of microwaved and field-moist soils were placed into 50 mL centrifuge tubes with 20 mL 0.5 M K
2SO
4 (adjusted at pH 7.0) and extracted by horizontal shaking at 250×
g rpm for 60 min. The soil suspension was filtered to obtain soil-free filtrate. A few drops of concentrated sulfuric acid were added to the filtrates to inhibit microbial breakdown of organic C; then the samples were frozen until analyzed. Total organic C by TOC analyzer, Sievers 5310 C, while total organic N in these extracts were analyzed by Kjeldahl digestion and both calculated as in [
43].
2.6.3. Statistical Analysis
The obtained data were statistically analyzed for One-Way Analysis of Variance and Duncan test. The results significance was considered at p < 0.05 by statistical package for social sciences (SPSS) software (for windows version 18). The results are presented as a mean ± standard deviation (SD).