Abstract
Rice is the main staple food worldwide, yet paddy fields are a primary source of artificial methane (CH4) emissions. Phosphorus (P) is a key element in the growth of plants and microbes, and P fertilizer input is a conventional agricultural practice adopted to improve rice yield. However, the impact of long-term P fertilizer addition on CH4 emissions in rice paddies is still unclear. To test this impact, a 36-yr field experiment with and without P fertilizer application treatments under a double-rice cropping system was used in this study to explore how continuous P application affects CH4 emissions and related plant and soil properties. The cumulative CH4 emissions were 21.2% and 28.6% higher without P fertilizer application treatment than with P fertilizer application treatment during the early and late season, respectively. Long-term P fertilizer application increased the rice aboveground biomass by 14.7–85.1% and increased grain yield by 24.5–138.7%. However, it reduced the ratio of root biomass to aboveground biomass. Long-term P fertilizer input reduced the soil NH4+ concentrations in both rice seasons but increased the soil DOC concentrations in the late season. The soil methanogenic abundance and CH4 production potential were similar without and with P fertilizer application treatments; however, the methanotrophic abundance and soil CH4 oxidation potential with P fertilizer application treatment were significantly higher than without P fertilizer application treatment. Our findings indicate that long-term P fertilizer input reduces CH4 emissions in rice fields, mainly by improving CH4 oxidation, which highlights the need for judicious P management to increase rice yield while reducing CH4 emissions.
1. Introduction
Methane (CH4) is the second most important greenhouse gas after CO2, and the atmospheric CH4 concentrations increased by 156%, from 729.3 ppb to 1866.3 ppb, in 2019 [1]. Paddy fields are a major source of CH4 emissions, accounting for 9% of human-caused CH4 emissions [1]. Meanwhile, rice is a staple crop of importance to humans, and rice yields need to increase by ~28% from 2007 to 2050 to fulfill the growing global food demand [2]. However, due to soil fertility depletion (e.g., decreased nutrient availability and lower soil acidification) across 35% of global rice-growing areas, yields either collapse, or stagnate [3,4,5]. Therefore, it is urgent to increase soil fertility while reducing CH4 emissions. Because phosphorus (P) is a key component for plants and a widespread lack of P occurs in paddy fields, the application of P fertilizer exerts an important role in the improvement of rice yields [6,7]. Yet, the impact of P fertilizer management on CH4 emissions from paddy fields is still unclear.
CH4 emissions from paddy soils were determined by the net impact of the CH4 production and consumption processes [8]. CH4 is mainly produced by methanogenic archaea in flooded paddy soil, and this procedure has been greatly influenced by the soil C supply [9,10]. In flooded rice fields, CH4 was consumed mainly by aerobic methanotrophic bacteria, and the growth of methanotrophs was influenced by soil-available nutrients (e.g., N and P) [11,12,13]. Indeed, the soil-available P concentration can directly affect CH4 production and oxidation [14]. Microcosm incubation studies have indicated that the negative correlation between CH4 formation, oxidation and available P concentrations [15,16,17] is because a high concentration of available P can restrain CH4 production via acetoclastic methanogenesis [18]. Moreover, P fertilizer application can indirectly affect CH4 emissions through rice-plant growth and soil properties. For example, P fertilizer amendment often enhances rice-plant growth, which may provide more substrates for methanogens and more oxygen for methanotrophs [19,20]. The P fertilizer application can stimulate carbon mineralization that affects soil C availability [21]. Taken together, these findings suggest that P fertilizer application may largely affect CH4 emission.
Since the 1990s, several studies have been performed to clarify the impact of P fertilizer addition on CH4 emissions in rice fields. Adhya et al. (1998) [22] showed that P amendment decreased CH4 emissions from paddy fields. Yang et al. (2010) [23] indicated that P addition had no influence on CH4 emissions in rice fields. Datta et al. (2013) [24] found that P addition could result in higher CH4 emissions during the wet season. The high variation of results across individual experiments suggests that more experiments are needed to investigate the influence of P fertilizer application under different conditions. Furthermore, because the effect of P fertilizer input on soil traits operates at different time scales [23,25], it is urgent to focus on the impact of long-term P fertilizer amendment on CH4 emissions. However, the effect of long-term P fertilizer addition on CH4 emissions in paddies is rarely evaluated. Furthermore, an exploration of how P fertilizer application affects CH4 emissions through changing CH4 production and CH4 oxidation is needed.
China is the largest rice-producing country, contributing to 21.9% of global paddies’ CH4 emissions [26]. Rice yields from the double-rice system account for 33.3% of China’s total rice production [27]. Furthermore, the double-rice cropping system has the highest area-scaled and yield-scaled CH4 emissions among China’s rice cropping systems [28]. Therefore, based on a 36-yr field experiment, we investigated the CH4 emissions, plant growth, and soils microbes involved in CH4 emissions without and with P fertilizer application treatments in a double-rice system. The purposes of this study were: (1) to determine the effect of continuous P fertilizer application on CH4 emissions; and (2) to learn the underlying mechanisms of continuous P fertilizer addition effects on CH4 emissions. We hypothesized that long-term P fertilizer application would affect soil P availability and soil microbes relative to CH4 production and oxidation, and thus change CH4 emissions in paddy field.
2. Materials and Methods
2.1. Site Description
The long-term field experiment began in 1984 at Nanchang (28°06′ N, 115°09′ E), Jiangxi province, China. The local climate is characterized by a subtropical monsoonal humid climate. Meteorological data, including the average daily temperature and rainfall during double-rice-growing periods in 2021, are shown in Figure 1. The early rice was transplanted on 30 April and harvested on 16 July; the late rice was transplanted on 26 July and harvested on 17 October in 2021. The soil of the experimental site is classified as red clay soil. The initial soil (0–20 cm) characteristics were pH (H2O) 6.5, soil organic matter 25.6 g kg−1, total nitrogen (N) 1.36 g kg−1, available N 81.6 mg kg−1, Olsen P 20.8 mg kg−1, and exchangeable potassium (K) 35.0 mg kg−1.
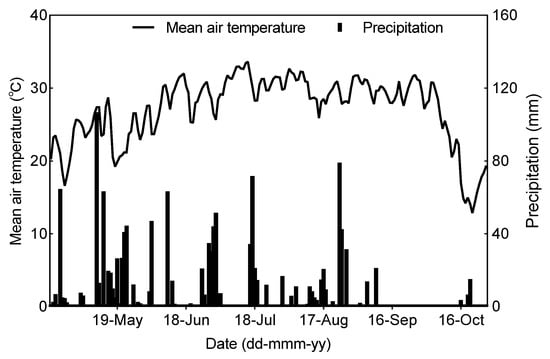
Figure 1.
The average daily air temperature and precipitation in early and late rice seasons in 2021 at the experimental site.
2.2. Experiment Design
A randomized block design with three replicates was conducted, including two long-term P fertilizer treatments, unit plots of 33.3 m2 size, and fertilizer treatments as follows: N, P, and K fertilizers applied simultaneously (NPK), and only N and K fertilizers applied (NK). In the NPK treatment, the N application rate was 150 kg N ha−1 in the early season and 180 kg N ha−1 in the late season: the same amount of P (60 kg P2O5 ha−1) and K (150 kg K2O ha−1) fertilizers were applied in both the early and late rice seasons. The amount of N and K fertilizers were kept the same between the NPK and NK treatments. Urea (46.7% N) for N fertilizer, calcium superphosphate (12.5% P2O5) for P fertilizer, and potassium chloride (60.0% K2O) for K fertilizer were used throughout the experiments. After rice transplanting, each plot was flooded with 3–5 cm water until midseason drainage at the jointing stage. Afterwards, all plots were flooded before the anthesis stage, and were then intermittently flooded until harvest. Herbicides and pesticides were applied according to standard commercial practice.
2.3. Sampling and Measurement Methods
The CH4 flux was measured once every 5–7 days using the static closed chamber gas chromatography method during both rice-growing periods in 2021. The PVC chamber with a size of 50 × 50 × 15 cm was fixed into each plot before rice transplanting. The gas samples were taken at five minute intervals between 09:00 and 11:00 AM and measured by gas chromatography (Agilent 7890B, Santa Clara, CA, USA). The CH4 fluxes (F) were calculated by the following equation:
where ∆C/∆T is the increase rate of CH4 concentration (mg m−3 h−1) in the chamber, V is the volume of the chamber (m3), and A indicates the enclosed area of the chamber (m2). Only measurements for which R2 > 0.90 were used to calculate ∆C/∆T. We discarded approximately 4% of the measurements. Accumulative CH4 emissions during rice-growing seasons were calculated by linear interpolation.
F = ΔC/ΔT × V/A
Topsoil (0–15 cm) samples were randomly taken from each plot before early rice transplanting in 2021. Soil organic carbon (SOC) content was measured following the method of vitriol acid potassium dichromate oxidation [29]. Soil total N and P were determined following Black (1965) [30] and Murphy and Riley (1962) [31], respectively. We also investigated the concentrations of available N [30], available P [32], and available K [33].
Fresh soil was also collected at the jointing stage in the early and late rice seasons, when CH4 flux reached a peak during the rice-growing season. CH4 potential production and oxidation rates were determined following the method of Zhu et al. (2016) [34]. Briefly, about 15 g soils (dry weight) and 15 mL of deionized sterile water were transferred into a 150 mL serum bottle. Then, some bottles were purged with N2 gas for 10 min to clear O2 and cultured in the dark at 25 °C for two days. Other bottles were cultured with 10,000 ppmv CH4 at 25 °C for 24 h and 120 r.p.m shaking condition. Gas samples in headspace were collected every 2 h (five time points in total). CH4 concentration in headspace was determined by gas chromatography. Potential CH4 production and oxidation rates of each soil sample were calculated as the slope of the changes in CH4 concentration during the incubation time. A continuous-flow injection analyzer (SKALAR SansPlus Systems, The Netherlands) was used to measure the concentrations of soil dissolved ammonium nitrogen (NH4+-N) and nitrate nitrogen (NO3−-N) concentrations. Additionally, soil dissolved organic carbon (DOC) concentration was analyzed using an organic carbon analyzer (multi N/C UV, Analytik Jena AG, JenaGermany). A real-time quantitative polymerase chain reaction (PCR) was used to quantify the copies of the mcrA gene (abundances of methanogens) and pmoA gene (abundances of methanotrophs). Soil DNA was extracted using Power soil DNA Isolation Kit (MoBio, Vancouver, BC, Canada). The detailed information of primer pairs used for PCR amplification of the mcrA genes and pmoA genes was shown in Holmes et al. (1995) [35] and Luton et al. (2002) [36], respectively.
At the maturity stage, we determined rice yield by aboveground and root biomass. All hills in each chamber were manually weighed and corrected to a moisture of 14%. At the same time, root samples were collected from 0–20 cm soil depth using a square frame (20 × 15 × 20 cm). The aboveground and root samples were placed in an oven at a constant temperature of 70 °C for 72 h until they reached a constant weight. The root/shoot ratio was calculated by aboveground biomass to root biomass.
2.4. Statistical Analysis
A two-way ANOVA (the P fertilizer management and growing season) was performed to analyze plant traits, the seasonal CH4 emissions, and soil microbes. If ANOVA showed interactive effects at a significance level of p <0.05, the method of multiple comparisons using the least significant difference (LSD) test was performed. We also used an independent sample t-test to examine the influence of long-term P fertilizer amendment on soil chemical properties. We used the statistical package SPSS 19.0 to perform all analyses.
3. Results
3.1. CH4 Emissions
The NPK and NK treatments showed similar dynamics of CH4 fluxes in early and late rice seasons (Figure 2). The CH4 fluxes peak appeared, approximately, at the first 30–40 days (jointing stage) during both seasons. The cumulative CH4 emissions were significantly influenced by the seasons and P fertilizer management, but there was no interaction between the season and P fertilizer management. The cumulative CH4 emissions of the early season were 28.1% lower than that of the late season. Compared to NK, NPK reduced cumulative CH4 emissions by 21.2% during the early season (Figure 3a) and 28.6% during the late season (Figure 3b). The yield-scaled cumulative CH4 emissions during the early season were 46.1% higher than that during the late season. We also found an interaction effect of season and P fertilizer management on yield-scaled CH4 emissions. The NPK treatment decreased yield-scaled CH4 emissions strongly during the early season (−67.8%), in comparison to the late season (−42.3%).
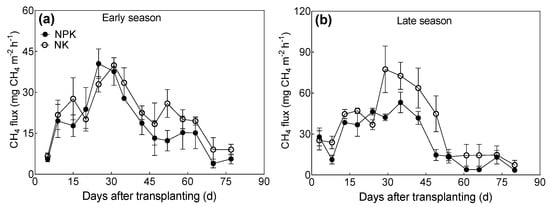
Figure 2.
CH4 fluxes from paddy fields under different long-term P addition treatments during early (a) and late (b) rice seasons. NPK: long-term N, P, and K fertilizers application; NK: long-term N and K fertilizers application. Error bars represent S.E. (n = 3).
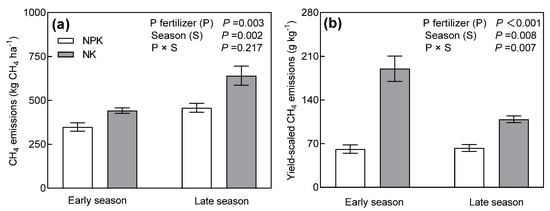
Figure 3.
Cumulative CH4 emissions (a) and yield-scaled CH4 emissions (b) under different long-term P addition treatments during early and late rice seasons. NPK: long-term N, P, and K fertilizers application; NK: long-term N and K fertilizers application. Error bars represent S.E. (n = 3).
3.2. Soil Properties
Total phosphorus, Olsen phosphorus, available potassium, and pH were significantly influenced by P fertilizer application (Table 1). The NPK treatment increased total phosphorus and Olsen phosphorus concentrations by 139.3% and 1177.8% relative to NK, respectively, but decreased available potassium concentration and pH by 28.7% and 4.1%, respectively. Soil organic C, total N, and available N concentrations were similar between the two P fertilizer treatments.

Table 1.
Influences of long-term P fertilizer amendment on soil properties.
The soil DOC concentrations during the early season were lower than that during the late season (Table 2). We found that the interaction effect of season and P fertilizer management on soil DOC concentrations by NPK increased soil DOC concentration by 34.0% in the late season but did not have an effect in the early season. The soil NH4+ concentration was not affected by season or the interaction of season and P fertilizer management (Table 2). Compared to NK, NPK increased soil NH4+ concentration by 33.3% during the early season and by 39.3% during the late season. The soil NO3− concentration during the early season was higher than that during the late season (Table 2). We also detected the interaction effect of season and P fertilizer management on soil NO3− concentrations by the NPK-stimulated soil NO3− concentrations by 165.5% during the early season, but this had no influence during the late season.

Table 2.
The effect of long-term P addition on soil DOC, NH4+, and NO3− concentrations during early and late rice seasons.
The CH4 production and oxidation potential during the early season were lower than that of the late season (Figure 4). CH4 production potential was not affected by P fertilizer management (Figure 4a). Yet, CH4 oxidation potential was significantly affected by P fertilizer management (Figure 4b). Compared to NK, NPK increased CH4 oxidation potential by 19.5% during the early season and 32.7% during the late season. The interaction effect of season and P fertilizer management on CH4 production and oxidation potential was not significant.
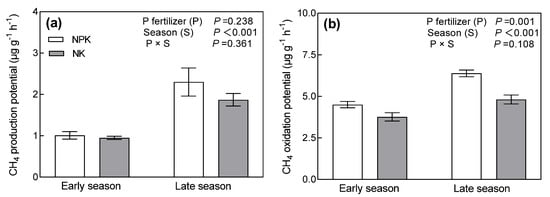
Figure 4.
The CH4 production potential (a) and oxidation potential (b) under different long-term P addition treatments during early and late rice seasons. NPK: long-term N, P, and K fertilizers application; NK: long-term N and K fertilizers application. Error bars represent S.E. (n = 3).
Both season and P fertilizer treatment had no significant influence on the abundance of methanogens (Figure 5a). Methanotrophic abundance was unaffected by seasons, but it was significantly increased by P fertilizer amendment (Figure 5b). Compared to NK, NPK increased methanotrophic abundance by 144.2% during the early season and 82.9% during the late season. Methanogenic activity of the early season was lower than that of the late season (Figure 5c), but it was not affected by P fertilizer management. Methanotrophic activity was not affected by seasons, but it was significantly decreased by P fertilizer application (Figure 5d). Compared to NK, NPK reduced methanotrophic activity by 58.6% during the early season and 52.1% during the late season. The interaction effect of season and P fertilizer treatment on the abundance and activity of methanogens and methanotrophs was not significant.
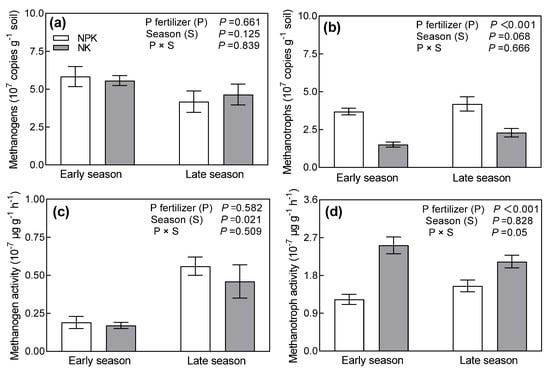
Figure 5.
The abundances (a,b) and activities (c,d) of methanogens and methanotrophs under different long-term P addition treatments during early and late rice seasons. NPK: long-term N, P, and K fertilizers application; NK: long-term N and K fertilizers application. Error bars represent S.E. (n = 3).
3.3. Plant Traits
Rice grain yield of the early season averaged 38.2% lower than that of the late season (Figure 6a). We observed a season × P fertilizer application interaction, whereby NPK improved grain yield strongly during the early season (138.7%) in comparison to the late season (24.5%). The effects of season and P fertilizer management on aboveground biomass were similar to those on grain yield (Figure 6b). The root biomass of the early season was lower than that of the late season, but the root biomass was unaffected by P fertilizer amendment (Figure 6c). The root–shoot ratio was not affected by the seasons (Figure 6d). However, we found the interaction effect of season and P fertilizer management on the root–shoot ratio. NPK decreased the root–shoot ratio more strongly during the early season (37.4%) than during late season (9.9%).
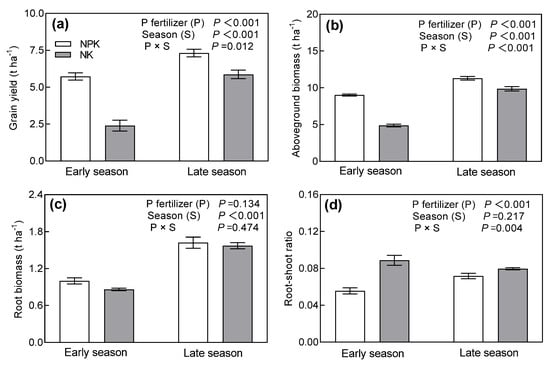
Figure 6.
Grain yield (a), aboveground biomass (b), root biomass (c) and root–shoot ratio (d) under different long-term P addition treatments during early and late rice seasons. NPK: long-term N, P, and K fertilizers application; NK: long-term N and K fertilizers application. Error bars represent S.E. (n = 3).
4. Discussion
Our results showed that long-term P addition reduced CH4 emissions in rice fields during both the early and late rice seasons, agreeing with previous studies [24,37]. On the contrary, Shang et al. (2011) [25] and Sheng et al. (2016) [38] found that long-term P addition did not affect or even increase CH4 emissions. This can be interpreted by the variations in soil pH and the water regime between our study and those studies. Soil pH is the key factor of methanogenic and methanotrophic growth [39], but the soil pH curves in CH4 production and CH4 oxidation are varied [40,41]. The optimum pH for methanogens is less than that of methanotrophs [42]. The low soil pH (i.e., 5.1–5.3) may have limited the growth of methanotrophs in those studies [41]. Furthermore, the water regime (i.e., continuous flooding) in those studies was different from our study (intermittently irrigation), and the positive influence of P application on methanotrophic growth may be limited by low O2 availability in soil under continuous flooding [43].
The balance between CH4 generation and oxidation determines CH4 emissions in paddy soil. Yet, previous studies have rarely evaluated the response of CH4 production and oxidation to P fertilizer application and its relation to CH4 emissions in a long-term field experiment. Our results indicate that long-term P fertilizer application had no impact on the methanogenic abundance and activity in a double-rice system, which was similar to several previous observations [38,44]. However, Gao et al. (2020) [45] showed that P fertilizer addition could stimulate methanogenic growth in P deficient soil, because P fertilizer application stimulates rice-plant growth that produces more C substrate for methanogens. In our study, long-term P application increased soil DOC in the late season because it stimulated the rice aboveground biomass in the early season. Indeed, aside from C substrate, soil N availability and P availability affect CH4 production. Higher NH4+ concentrations without P addition treatments may stimulate the straw decomposition and provide more substrates for methanogens [46]. Moreover, lower P concentrations can stimulate CH4 production via acetoclastic methanogenesis [18]. Thus, the effect of NH4+ and P concentrations may offset the effect of C. In addition, P fertilizer application can affect the composition of root exudates, and then exert an influence on CH4 production [47].
This study shows that long-term P fertilizer amendment stimulated the methanotrophic abundance, which agrees with [13,45]. There are several reasons for this phenomenon. First, P is the essential element for microbes, so soil P deficiency can limit the growth of methanotrophs. Moreover, P deficiency can decrease the activity of roots and thus lower radial oxygen loss from roots to the rhizosphere [47,48], which may restrict the aerobic methanotrophic growth. Furthermore, soil P deficiency increased soil NH4+ concentration in our study, which may limit CH4 consumption by fighting for CH4 monooxygenase [49]. In contrast, Sheng et al. (2016) [38] showed that P amendment decreased the abundance of methanotrophs, probably because the responses of diverse species of methanotrophs exist in different experimental soils to P deficiency are different [50]. In addition, our findings indicate that long-term P fertilizer amendment reduced methanotrophic activity, which may be due to the change in the community of methanotrophs. Recent research has shown that long-term P addition caused changes in the community of methanotrophs in paddy soils, resulting in an increase in type I methanotrophic abundance and a reduction in type II methanotrophic abundance [13]. Indeed, the CH4 oxidation capacity of type I methanotrophs was lower than type II methanotrophs [51].
As a key element for rice-plant growth, P deficiency is widespread around the world and P fertilizer application is widely used to improve rice production [6,7]. This study also showed that long-term P addition promotes rice grain yield in both the early and late rice seasons. Yet, a higher increase was observed in the early season than in late season, which may be due to higher temperatures during the reproductive stage in the early season (30.1 °C) than in the late season (27.5 °C). The average air temperature of 30.1 °C may cause heat damage during the reproductive stage [52]. The P addition can increase rice resistance to heat stress, resulting in a higher rice yield [53]. Our findings indicate that the continuous P fertilizer amendment could play a more important role in rice yields in the global warming world.
We found that long-term P amendment reduced yield-scaled CH4 emissions, indicating that P fertilizer addition can promote win–win outcomes for both food security and greenhouse gas reduction. However, long-term P amendment can affect soil N availability and ammonia oxidizing bacteria, which can directly affect N2O emissions [54]. P addition also affects arbuscular mycorrhizal fungi [55], which may affect the decomposition of soil organic matter [56,57] and soil organic C storage. Therefore, we suggest that future study is needed to extend the impact of continuous P fertilizer amendment on net greenhouse gas emissions.
5. Conclusions
We found that long-term P fertilizer amendment improved aboveground dry matter accumulation and grain yield, but that it had no influence on root dry matter accumulation and also decreased the root–shoot ratio. Continuous P fertilizer amendment decreased soil NH4+ concentrations during both rice seasons, and increased soil DOC concentrations only in the late season. Long-term P fertilizer application increased methanotrophic abundance and CH4 oxidation potential, without affecting methanogenic abundance and CH4 production potential. Continuous P fertilizer addition reduced CH4 emissions through stimulating CH4 oxidation, without affecting CH4 production. These findings suggest that long-term P fertilizer application can achieve higher rice productivity while mitigating CH4 emissions under a double-rice cropping system.
Author Contributions
Conceptualization, J.C. and X.Z.; investigation, X.C., J.J., Y.C., X.L. (Xihuan Liang) and X.L. (Xianjin Lan); Data analysis, X.Z.; writing—original draft preparation, J.L. and X.Z.; writing—review and editing, J.C. and C.P.; funding acquisition, J.L., W.X. and J.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Natural Science Foundation of China (32060431), Collaborative Innovation Special Project of Jiangxi Modern Agricultural Research (JXXTCXQN 201904, JXXTCXQN202210), Science and Technology Project of Jiangxi Provincial Education Department (GJJ191138), Doctoral Research Fund of Nanchang Normal University (090170003312), Central Guided Local Science and Technology Development Fund (20221ZDH04057), and opening project of National Engineering and Technology Research Center for Red Soil Improvement (2020NETRCRSI-8).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- IPCC. Summary for Policymakers. In Climate Change 2021: The Physical Science Basis; Working Group 1 contribution to the Sixth Assessment report of the Intergovernmental Panel on Climate Change, Ed.; Cambridge University Press: Cambridge, UK, 2021. [Google Scholar]
- Alexandratos, N.; Bruinsma, J. World Agriculture Towards 2030/2050; ESA Working Paper; Food and Agriculture Organization of the United Nations: Rome, Italy, 2012; Available online: https://www.fao.org/3/ap106e/ap106e.pdf (accessed on 18 January 2022).
- Ray, D.K.; Ramankutty, N.; Mueller, N.D.; West, P.C.; Foley, J.A. Recent patterns of crop yield growth and stagnation. Nat. Commun. 2012, 3, 1293. [Google Scholar] [CrossRef] [PubMed]
- Amelung, W.; Bossio, D.; de Vries, W.; Kogel-Knabner, I.; Lehmann, J.; Amundson, R.; Bol, R.; Collins, C.; Lal, R.; Leifeld, J.; et al. Towards a global-scale soil climate mitigation strategy. Nat. Commun. 2020, 11, 5427. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, P.; MacDonald, G.K.; Bernard de Raymond, A.; Nesme, T. Food system resilience to phosphorus shortages on a telecoupled planet. Nat Sustain. 2022, 5, 114–122. [Google Scholar] [CrossRef]
- Dhillon, J.; Torres, G.; Driver, E.; Figueiredo, B.; Raun, W.R. World phosphorus use efficiency in cereal crops. Agron. J. 2017, 109, 1670–1677. [Google Scholar] [CrossRef]
- Du, M.; Zhang, W.; Gao, J.; Liu, M.; Zhou, Y.; He, D.; Zhao, Y.; Liu, S. Improvement of Root Characteristics Due to Nitrogen, Phosphorus, and Potassium Interactions Increases Rice (Oryza sativa L.) Yield and Nitrogen Use Efficiency. Agronomy 2022, 12, 23. [Google Scholar] [CrossRef]
- Zhu, Y.; Purdy, K.J.; Eyice, Ö.; Shen, L.; Harpenslager, S.F.; Yvon-Durocher, G.; Dumbrell, A.J.; Trimmer, M. Disproportionate increase in freshwater methane emissions induced by experimental warming. Nat. Clim. Chang. 2020, 10, 685–690. [Google Scholar] [CrossRef]
- Qian, H.Y.; Chen, J.; Zhu, X.C.; Wang, L.; Liu, Y.; Zhang, J.; Deng, A.X.; Song, Z.W.; Ding, Y.F.; Jiang, Y.; et al. Intermittent flooding lowers the impact of elevated atmospheric CO2 on CH4 emissions from rice paddies. Agric. Ecosyst. Environ. 2022, 329, 107872. [Google Scholar] [CrossRef]
- Bertora, C.; Alexandra, M.; Lerda, C.; Peyron, M.; Bardi, L.; Gorra, R.; Sacco, D.; Celi, L.; Said-Pullicino, D. Dissolved organic carbon cycling, methane emissions and related microbial populations in temperate rice paddies with contrasting straw and water management. Agric. Ecosyst. Environ. 2018, 265, 292–306. [Google Scholar] [CrossRef]
- Conrad, R.; Klose, M.; Lu, Y.H.; Chidthaisong, A. Methanogenic pathway and archaeal communities in three different anoxic soils amended with rice straw and maize straw. Front. Microbiol. 2012, 3, 4. [Google Scholar] [CrossRef]
- Bodelier, P.L.E.; Laanbroek, H.J. Nitrogen as a regulatory factor of methane oxidation in soils and sediments. FEMS Microbiol. Ecol. 2004, 47, 265–277. [Google Scholar] [CrossRef]
- Gao, D.D.; Sheng, R.; Moreira-Grez, B.; Liu, S.G.; Wei, W.X. Influences of phosphorus and potassium deficiencies on the methanotrophic communities in rice rhizosphere. Appl. Soil Ecol. 2022, 170, 104265. [Google Scholar] [CrossRef]
- Veraart, A.J.; Steenbergh, A.K.; Ho, A.; Sang, Y.K.; Bodelier, P.L.E. Beyond nitrogen: The importance of phosphorus for CH4 oxidation in soils and sediments. Geoderma 2015, 259–260, 337–346. [Google Scholar] [CrossRef]
- Rath, A.K.; Ramakrishnan, B.; Rao, V.R.; Sethunathan, N. Effects of rice-straw and phosphorus application on production and emission of methane from tropical rice soil. J. Plant Nutr. Soil Sci. 2005, 168, 248–254. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhang, L.M.; He, J.Z. Immediate effects of nitrogen, phosphorus, and potassium amendments on the methanotrophic activity and abundance in a Chinese paddy soil under short-term incubation experiment. J. Soils Sed. 2013, 13, 189–196. [Google Scholar] [CrossRef]
- Alam, M.S.; Xia, W.W.; Jia, Z.J. Methane and ammonia oxidations interact in paddy soils. Int. J. Agric. Biol. 2014, 16, 365–370. [Google Scholar] [CrossRef]
- Conrad, R.; Klose, M. Selective inhibition of reactions involved in methanogenesis and fatty acid production on rice roots. FEMS Microbiol. Ecol. 2000, 34, 27–34. [Google Scholar] [CrossRef]
- Guo, T.F.; Luan, H.A.; Song, D.L.; Zhang, S.Q.; Zhou, W.; Liang, G.Q. Combined Fertilization Could Increase Crop Productivity and Reduce Greenhouse Gas Intensity through Carbon Sequestration under Rice-Wheat Rotation. Agronomy 2021, 11, 2540. [Google Scholar] [CrossRef]
- Jiang, Y.; van Groenigen, K.J.; Huang, S.; Hungate, B.A.; van Kessel, C.; Hu, S.; Zhang, J.; Wu, L.; Yan, X.; Wang, L.; et al. Higher yields and lower methane emissions with new rice cultivars. Glob. Change Biol. 2017, 23, 4728–4738. [Google Scholar] [CrossRef]
- Hui, D.; Porter, W.; Phillips, J.R.; Aidar, M.P.M.; Lebreux, S.J.; Schadt, C.W.; Mayes, M.A. Phosphorus rather than nitrogen enhances CO2 emissions in tropical forest soils: Evidence from a laboratory incubation study. Eur. J. Soil Sci. 2020, 71, 495–510. [Google Scholar] [CrossRef]
- Adhya, T.K.; Pattnaik, P.; Satpathy, S.N.; Kumaraswamy, S.; Sethunathan, N. Influence of phosphorus application on methane emission and production in flooded paddy soils. Soil Biol. Biochem. 1998, 30, 177–181. [Google Scholar] [CrossRef]
- Yang, X.; Shang, Q.Y.; Wu, P.; Liu, J.; Shen, Q.R.; Guo, S.W.; Xiong, Z.Q. Methane emissions from double rice agriculture under long-term fertilizing systems in Hunan, China. Agric. Ecosyst. Environ. 2010, 137, 308–316. [Google Scholar] [CrossRef]
- Datta, A.; Santra, S.C.; Adhya, T.K. Effect of inorganic fertilizers (N, P, K) on methane emission from tropical rice field of India. Atmos Environ. 2013, 66, 123–130. [Google Scholar] [CrossRef]
- Shang, Q.Y.; Yang, X.; Gao, C.; Wu, P.; Liu, J.; Xu, Y.C.; Shen, Q.R.; Zou, J.W.; Guo, S.W. Net annual global warming potential and greenhouse gas intensity in Chinese double rice-cropping systems: A 3-year field measurement in long-term fertilizer experiments. Glob. Chang. Biol. 2011, 17, 2196–2210. [Google Scholar] [CrossRef]
- FAOSTAT Online Statistical Service. 2020. Available online: https://www.fao.org/faostat/en/#data/GR (accessed on 18 January 2022).
- Ministry of Agriculture of China (MOA). 2017. Available online: http://zdscxx.moa.gov.cn:8080/nyb/pc/search.jsp (accessed on 18 February 2022).
- Shang, Z.Y.; Abdalla, M.; Xia, L.L.; Zhou, F.; Sun, W.J.; Smith, P. Can cropland management practices lower net greenhouse emissions without compromising yield? Glob. Change Biol. 2021, 27, 4657–4670. [Google Scholar] [CrossRef]
- Kalembas, S.J.; Jenkinso, D.S. Comparative study of titrimetric and gravimetric methods for determination of organic carbon in soil. J. Sci. Food Agr. 1973, 24, 1085–1090. [Google Scholar] [CrossRef]
- Black, C.A. Methods of soil analysis part II. In Chemical and Microbiological Properties; Norman, A.G., Ed.; American Society of Agriculture: St. Joseph, MI, USA, 1965. [Google Scholar]
- Murphy, J.; Riley, J.P.A. Modified single solution method for the determination of phosphate in natural waters. Anal. Chim. Acta 1962, 27, 31–36. [Google Scholar] [CrossRef]
- Olsen, S.R.; Cole, C.V.; Watanabe, F.S.; Dean, A. Estimation of Available Phosphorus in Soils by Extraction with Sodium Bicarbonate; No. 939; United States Department of Agriculture Circular: Washington, DC, USA, 1954.
- Helmke, P.A.; Sparks, D.L. Lithium, sodium, potassium, rubidium and cesium. In Methods of Soil Analysis. Part 3. Chemical Methods. Soil Science Society of America; Sparks, D.L., Ed.; American Society of Agronomy: Madison, WI, USA, 1966; pp. 551–574. [Google Scholar]
- Zhu, X.C.; Zhang, J.; Zhang, Z.P.; Deng, A.X.; Zhang, W.J. Dense planting with less basal nitrogen fertilization might benefit rice cropping for high yield with less environmental impacts. Eur. J. Agron. 2016, 75, 50–59. [Google Scholar] [CrossRef]
- Holmes, A.J.; Costello, A.; Lidstrom, M.E.; Murrell, J.C. Evidence that participate methane monooxygenase and ammonia monooxygenase may be evolutionarily related. FEMS Microbiol. Lett. 1995, 132, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Luton, P.E.; Wayne, J.M.; Sharp, R.J.; Riley, P.W. The mcrA gene as an alternative to 16S rRNA in the phylogenetic analysis of methanogen population in landfill. Microbiology 2002, 148, 3521–3530. [Google Scholar] [CrossRef]
- He, Z.; Xu, C.; Zhou, B.B.; Xue, L.H.; Wang, Y.; Shen, M.X.; Yang, L.Z. Effects of long-term fertilization without phosphorus on greenhouse gas emissions from paddy fields. Chin. J. Appl. Ecol. 2020, 2, 942–950. (In Chinese) [Google Scholar] [CrossRef]
- Sheng, R.; Chen, A.L.; Zhang, M.M.; Whiteley, A.S.; Kumaresan, D.; Wei, W.X. Transcriptional activities of methanogens and methanotrophs vary with methane emission flux in rice soils under chronic nutrient constraints of phosphorus and potassium. Biogeosciences 2016, 13, 6507–6518. [Google Scholar] [CrossRef]
- Liu, Z.; Li, D.; Zhang, J.; Saleem, M.; Zhang, Y.; Ma, R.; He, Y.; Yang, J.; Xiang, H.; Wei, H. Effect of simulated acid rain on soil CO2, CH4 and N2O emissions and microbial communities in an agricultural soil. Geoderma 2020, 366, 114222. [Google Scholar] [CrossRef]
- Ravi, P.P.; Lindner, J.; Oechsner, H.; Lemmer, A. Effects of Target PH-Value on Organic Acids and Methane Production in TwoStage Anaerobic Digestion of Vegetable Waste. Bioresour. Technol. 2018, 247, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Hütsch, B.W.; Webster, C.P.; Powlson, D.S. Methane oxidation in soil as affected by land use, soil pH and N fertilization. Soil Biol. Biochem. 1994, 26, 1613–1622. [Google Scholar] [CrossRef]
- Malyan, S.K.; Bhatia, A.; Kumar, A.; Gupta, D.K.; Singh, R.; Kumar, S.S.; Tomer, R.; Kumar, O.; Jain, N. Methane production, oxidation and mitigation: A mechanistic understanding and comprehensive evaluation of influencing factors. Sci. Total Environ. 2016, 572, 874–896. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Qian, H.Y.; Wang, L.; Feng, J.F.; Huang, S.; Hungate, B.A.; van Kessel, C.; Horwath, W.R.; Zhang, X.; Qin, X.; et al. Limited potential of harvest index improvement to reduce methane emissions from rice paddies. Glob. Change Biol. 2019, 25, 686–698. [Google Scholar] [CrossRef]
- Zhang, W.B.; Sheng, R.; Zhang, M.M.; Xiong, G.Y.; Hou, H.J.; Li, S.L.; Wei, W.X. Effects of continuous manure application on methanogenic and methanotrophic communities and methane production potentials in rice paddy soil. Agric. Ecosyst. Environ. 2018, 258, 121–128. [Google Scholar] [CrossRef]
- Gao, D.D.; Sheng, R.; Whiteley, A.S.; Moreiragrez, B.; Qin, H.L.; Zhang, W.B.; Zhan, Y.; Wei, W.X. Effect of phosphorus amendments on rice rhizospheric methanogens and methanotrophs in a phosphorus deficient soil. Geoderma 2020, 368, 114312. [Google Scholar] [CrossRef]
- Guo, T.F.; Zhang, Q.; Ai, C.; Liang, G.Q.; He, P.; Zhou, W. Nitrogen enrichment regulates straw decomposition and its associated microbial community in a double-rice cropping system. Sci. Rep. 2018, 81, 1847. [Google Scholar] [CrossRef]
- Chen, Y.; Li, S.Y.; Zhang, Y.J.; Li, T.T.; Ge, H.M.; Xia, S.M.; Gu, J.F.; Zhang, H.; Lu, B.; Wu, X.X.; et al. Rice root morphological and physiological traits interaction with rhizosphere soil and its effect on methane emissions in paddy fields. Soil Biol. Biochem. 2019, 129, 191–200. [Google Scholar] [CrossRef]
- Insalud, N.; Bell, R.W.; Colmer, T.D.; Rerkasem, B. Morphological and physiological responses of rice (Oryza sativa) to limited phosphorus supply in aerated and stagnant solution culture. Ann. Bot. 2006, 98, 995–1004. [Google Scholar] [CrossRef]
- He, R.; Chen, M.; Ma, R.-C.; Su, Y.; Zhang, X. Ammonium conversion and its feedback effect on methane oxidation of Methylosinus sporium. J. Biosci. Bioeng. 2017, 123, 466–473. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, A.; Pathak, A.; Ogram, A. Composition of Methaneoxidizing bacterial communities as a function of nutrient loading in the Florida Everglades. Microb. Ecol. 2012, 64, 750–759. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, M.; Shrestha, P.M.; Frenzel, P.; Conrad, R. Effect of nitrogen fertilization on methane oxidation, abundance, community structure, and gene expression of methanotrophs in the rice rhizosphere. ISME J. 2010, 4, 1545. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.C.; Chen, J.; Huang, S.; Li, W.W.; Penuelas, J.; Chen, J.; Zhou, F.; Zhang, W.J.; Li, G.H.; Liu, Z.H.; et al. Manure amendment can reduce rice yield loss under extreme temperatures. Commun. Earth Environ. 2022, 3, 147. [Google Scholar] [CrossRef]
- Xi, Y.P.; Han, X.Y.; Zhang, Z.Z.; Joshi, J.; Borza, T.; Mohammad Aqa, M.; Zhang, B.B.; Yuan, H.M.; Wang-Pruski, G. Exogenous phosphite application alleviates the adverse effects of heat stress and improves thermotolerance of potato (Solanum tuberosum L.) seedlings. Ecotoxicol. Environ. Saf. 2020, 190, 110048. [Google Scholar] [CrossRef]
- Zhou, Z.F.; Shi, X.J.; Zheng, Y.; Qin, Z.X.; Xie, D.T.; Li, Z.L.; Guo, T. Abundance and community structure of ammonia-oxidizing bacteria and archaea in purple soil under long-term fertilization. Eur. J. Soil Biol. 2014, 60, 24–33. [Google Scholar] [CrossRef]
- Chifflot, V.; Rivest, D.; Olivier, A.; Cogliastro, A.; Khasa, D. Molecular analysis of arbuscular mycorrhizal community structure and spores distribution in tree-based intercropping and forest systems. Agric. Ecosyst. Environ. 2009, 131, 32–39. [Google Scholar] [CrossRef]
- Cheng, L.; Booker, F.L.; Tu, C.; Burkey, K.O.; Zhou, L.S.; Shew, H.D.; Rufty, T.W.; Hu, S.J. Arbuscular mycorrhizal fungi increase organic carbon decomposition under elevated CO2. Science 2012, 337, 1084–1087. [Google Scholar] [CrossRef]
- Wang, L.; Liu, Y.L.; Zhu, X.C.; Zhang, Y.; Yang, H.Y.; Dobbie, S.; Zhang, X.; Deng, A.X.; Qian, H.Y.; Zhang, W.J. Effects of arbuscular mycorrhizal fungi on crop growth and soil N2O emissions in the legume system. Agric. Ecosyst. Environ. 2021, 322, 107641. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).