Identification and Characterization of the Tomato UGT Gene Family and Effects of GAME 17 Overexpression on Plants and Growth and Development under High-CO2 Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Identification of SlUGT Proteins in S. lycopersicum
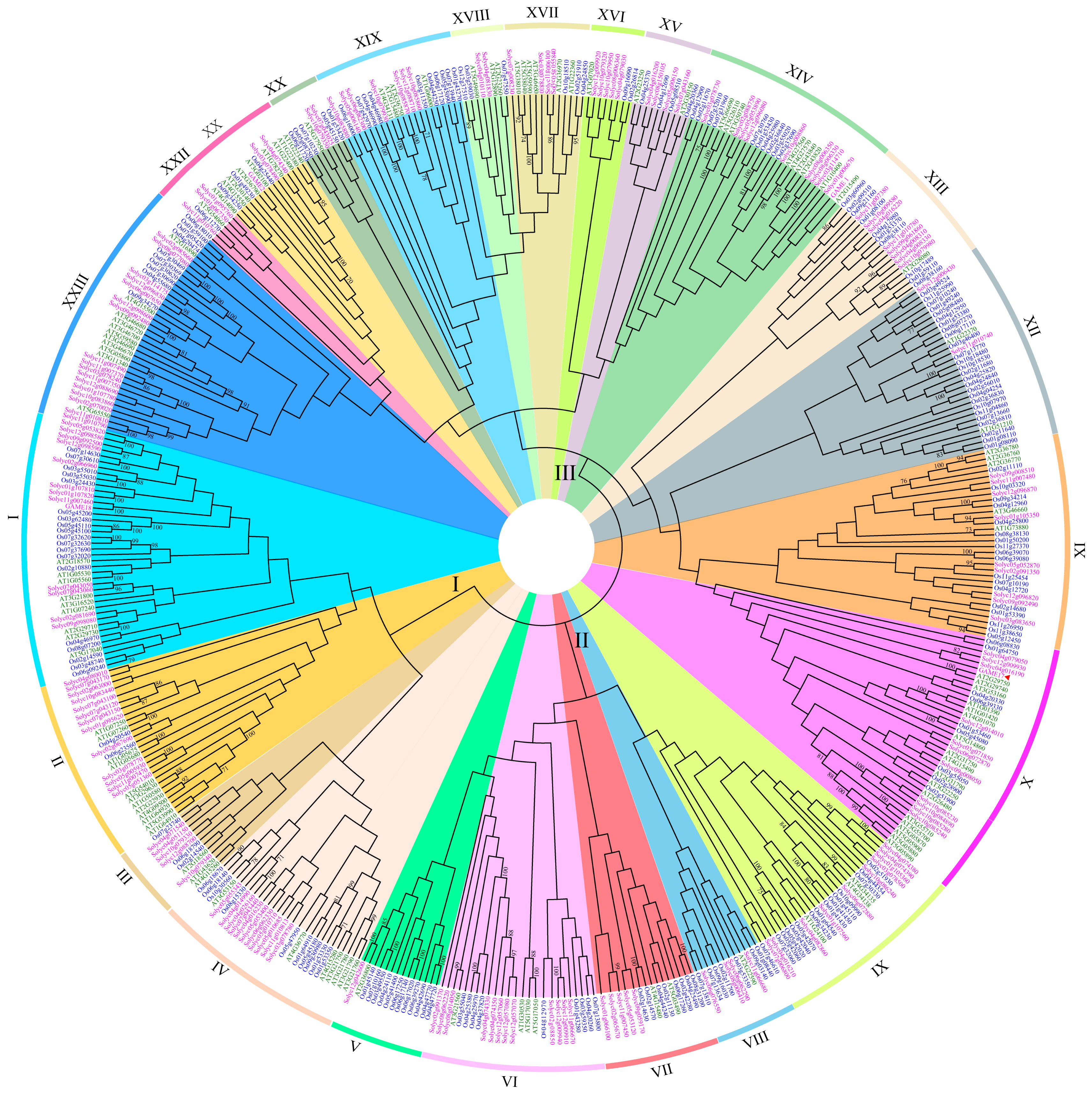
2.2. Phylogenetic Analysis
2.3. Chromosomal Localization, and Motif and Gene Structure Analyses
2.4. Expression Analysis
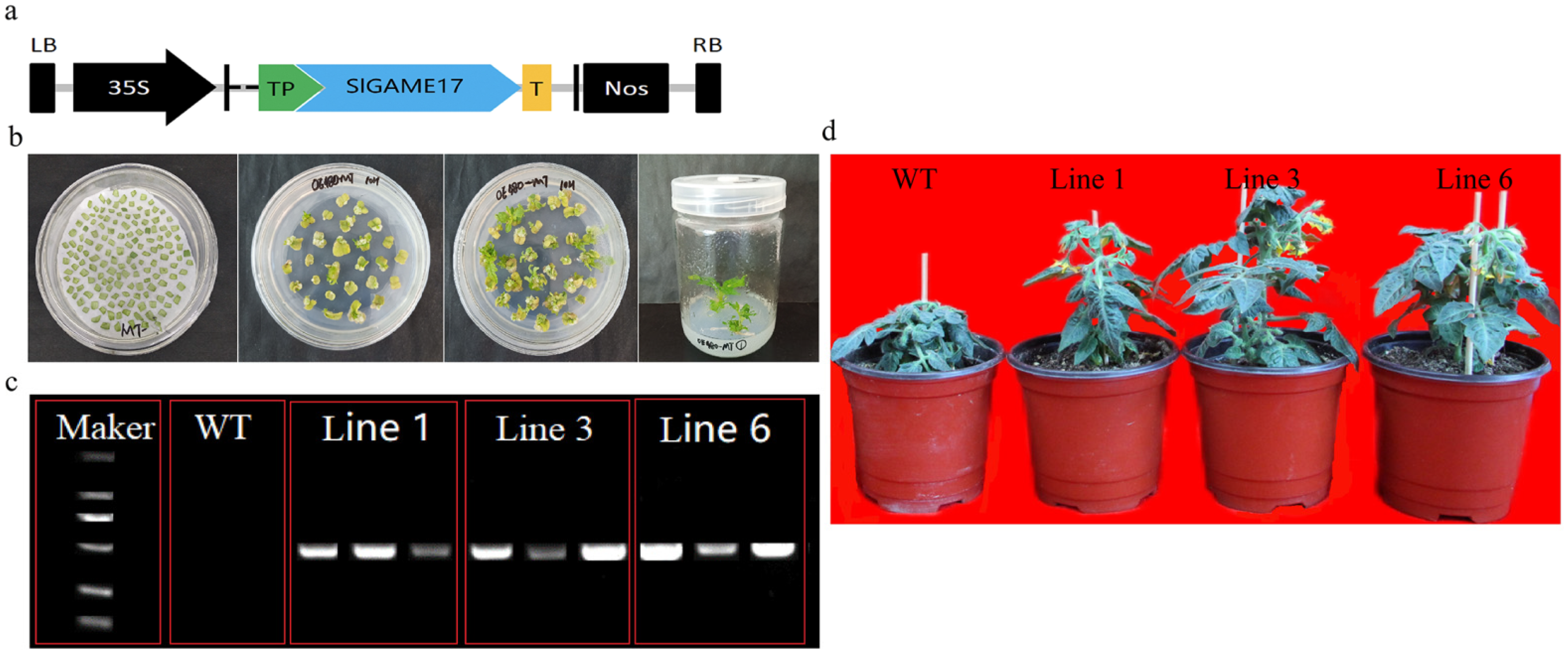
2.5. Construction of Plasmids and Plant Transformation
2.6. Detection of Transgenic Tomato Plants
2.7. RNA Isolation and Real-Time Quantitative PCR Assays
2.8. Determination of Plant Morphological Indices
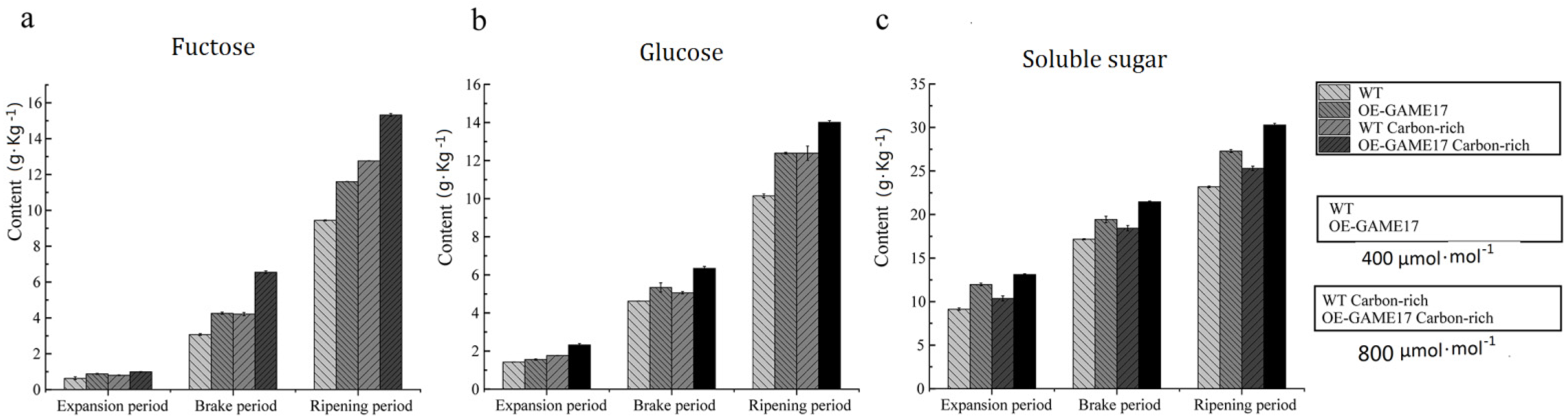
2.9. Determination of Glucose, Fructose, and Soluble Sugar Contents in Fruits
2.10. Statistical Analysis
3. Results
3.1. Genome-Wide Identification and Analysis of SlUGT Family Genes in S. lycopersicum
3.2. Phylogenetic Analysis of the UGT Gene Family among Arabidopsis, Rice, and S. lycopersicum
3.3. Gene Structure and Motif Analyses of the SlUGT Gene Family in S. lycopersicum
3.4. Expression Pattern of SlUGT Family Genes in Different Organs of S. lycopersicum
3.5. Generation and Identification of Transgenic Tomato Plants
3.6. Effects of the Constitutive Overexpression of GAME 17 on Plant Growth and Development
3.7. Effects of the Constitutive Overexpression of SlGAME 17 Gene on the Content of Glucose, Fructose, and Soluble Sugar
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Choudhary, P.; Rai, G.K.; Bagati, S.; Jamwal, D.; Kumar, P. Evaluation of genetic variability in tomato (Solanum lycopersicum L. mill) genotypes using microsatellite markers. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 2117–2124. [Google Scholar] [CrossRef]
- Mishra, A. Recent developments in breeding approaches of tomato (Solanum lycopersicum L.)—A review. Int. J. Farm. Sci. 2022, 12, 1–6. [Google Scholar] [CrossRef]
- Chaudhary, P.; Sharma, A.; Singh, B.; Nagpal, A.K. Bioactivities of phytochemicals present in tomato. J. Food Sci. Technol. 2018, 55, 2833–2849. [Google Scholar] [CrossRef]
- Salehi, B.; Sharifi-Rad, R.; Sharopov, F.; Namiesnik, J.; Roointan, A.; Kamle, M.; Kumar, P.; Martins, N.; Sharifi-Rad, J. Beneficial effects and potential risks of tomato consumption for human health: An overview. Nutrition 2019, 62, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Augustin, M.M.; Ruzicka, D.R.; Shukla, A.K.; Augustin, J.M.; Starks, C.M.; O’Neil-Johnson, M.; McKain, M.R.; Bradley, S.; Evans, B.S.; Matt, D.; et al. Elucidating steroid alkaloid biosynthesis in Veratrum californicum: Production of verazine in Sf9 cells. Plant J. 2015, 82, 991–1003. [Google Scholar] [CrossRef] [PubMed]
- Sonawane, P.D.; Jozwiak, A.; Panda, S.; Aharoni, A. “Hijacking” core metabolism: A new panache for the evolution of steroidal glycoalkaloids structural diversity. Curr. Opin. Plant Biol. 2020, 55, 118–128. [Google Scholar] [CrossRef]
- Friedman, M.; Dao, L. Distribution of glycoalkaloids in potato plants and commercial potato products. J. Agric. Food Chem. 1992, 40, 419–423. [Google Scholar] [CrossRef]
- Friedman, M. Potato glycoalkaloids and metabolites: Roles in the plant and in the diet. J. Agric. Food Chem. 2006, 54, 8655–8681. [Google Scholar] [CrossRef]
- Kozukue, N.; Yoon, K.S.; Byun, G.I.; Misoo, S.; Levin, C.E.; Friedman, M. Distribution of glycoalkaloids in potato tubers of 59 accessions of two wild and five cultivated Solanum species. J. Agric. Food Chem. 2008, 56, 11920–11928. [Google Scholar] [CrossRef]
- Iijima, Y.; Watanabe, B.; Sasaki, R.; Takenaka, M.; Ono, H.; Sakurai, N.; Umemotoe, N.; Suzukib, H.; Shibatab, D.; Aoki, K. Steroidal glycoalkaloid profiling and structures of glycoalkaloids in wild tomato fruit. Phytochemistry 2013, 95, 145–157. [Google Scholar] [CrossRef]
- Moco, S.; Bino, R.J.; Vorst, O.; Verhoeven, H.A.; de Groot, J.; van Beek, T.A.; Vervoort, J.; De Vos, C.R. A liquid chromatography-mass spectrometry-based metabolome database for tomato. Plant Physiol. 2006, 141, 1205–1218. [Google Scholar] [CrossRef] [PubMed]
- Mintz-Oron, S.; Mandel, T.; Rogachev, I.; Feldberg, L.; Lotan, O.; Yativ, M.; Wang, Z.H.; Jetter, R.; Venger, I.; Adato, A.; et al. Gene expression and metabolism in tomato fruit surface tissues. Plant Physiol. 2008, 147, 823–851. [Google Scholar] [CrossRef] [PubMed]
- Schwahn, K.; de Souza, L.P.; Fernie, A.R.; Tohge, T. Metabolomics-assisted refinement of the pathways of steroidal glycoalkaloid biosynthesis in the tomato clade. J. Integr.Plant Biol. 2014, 56, 864–875. [Google Scholar] [CrossRef] [PubMed]
- Iijima, Y.; Fujiwara, Y.; Tokita, T.; Ikeda, T.; Nohara, T.; Aoki, K.; Shibata, D. Involvement of ethylene in the accumulation of esculeoside A during fruit ripening of tomato (Solanum lycopersicum). J. Agric. Food Chem. 2009, 57, 3247–3252. [Google Scholar] [CrossRef]
- Moco, S.; Capanoglu, E.; Tikunov, Y.; Bino, R.J.; Boyacioglu, D.; Hall, R.D.; Jacques Vervoort, J.; De Vos, R.C. Tissue specialization at the metabolite level is perceived during the development of tomato fruit. J. Exp. Bot. 2007, 58, 4131–4146. [Google Scholar] [CrossRef]
- Yamanaka, T.; Vincken, J.P.; Zuilhof, H.; Legger, A.; Takada, N.; Gruppen, H. C22 isomerization in α-tomatine-to-esculeoside a conversion during tomato ripening is driven by C27 hydroxylation of triterpenoidal skeleton. J. Agric. Food Chem. 2009, 57, 3786–3791. [Google Scholar] [CrossRef]
- Itkin, M.; Heinig, U.; Tzfadia, O.; Bhide, A.J.; Shinde, B.; Cardenas, P.D.; Bocobza, S.E.; Unger, T.; Malitsky, S.; Finkers, R.; et al. Biosynthesis of antinutritional alkaloids in solanaceous crops is mediated by clustered genes. Science 2013, 341, 175–179. [Google Scholar] [CrossRef]
- Zhao, D.K.; Zhao, Y.; Chen, S.Y.; Kennelly, E.J. Solanum steroidal glycoalkaloids: Structural diversity, biological activities, and biosynthesis. Nat. Prod. Rep. 2021, 38, 1423–1444. [Google Scholar] [CrossRef]
- Nakayasu, M.; Umemoto, N.; Ohyama, K.; Fujimoto, Y.; Lee, H.J.; Watanabe, B.; Muranaka, T.; Saito, K.; Sugimoto, Y.; Mizutani, M. A dioxygenase catalyzes steroid 16α-hydroxylation in steroidal glycoalkaloid biosynthesis. Plant Physiol. 2017, 175, 120–133. [Google Scholar] [CrossRef]
- Irmisch, S.; Jancsik, S.; Man Saint Yuen, M.; Madilao, L.L.; Bohlmann, J. Complete biosynthesis of the anti-diabetic plant metabolite montbretin A. Plant Physiol. 2020, 184, 97–109. [Google Scholar] [CrossRef]
- Mekapogu, M.; Sohn, H.B.; Kim, S.J.; Lee, Y.Y.; Park, H.M.; Jin, Y.I.; Hong, S.; Suh, J.; Kweon, K.; Kim, Y.H. Effect of light quality on the expression of glycoalkaloid biosynthetic genes contributing to steroidal glycoalkaloid accumulation in potato. Am. J. Potato Res. 2016, 93, 264–277. [Google Scholar] [CrossRef]
- Kumar, A.; Fogelman, E.; Weissberg, M.; Tanami, Z.; Veilleux, R.E.; Ginzberg, I. Lanosterol synthase-like is involved with differential accumulation of steroidal glycoalkaloids in potato. Planta 2017, 246, 1189–1202. [Google Scholar] [CrossRef] [PubMed]
- Pramanik, P.; Phukan, M. Assimilating atmospheric carbon dioxide in tea gardens of northeast India. J. Environ. Manag. 2020, 256, e109912. [Google Scholar] [CrossRef] [PubMed]
- Kutschera, U.; Khanna, R. Experimental plant research and the discovery of carbon dioxide-mediated global greening: A tribute to Wilhelm Pfeffer (1845–1920). J. Plant Biochem. Biot. 2021, 30, 407–420. [Google Scholar] [CrossRef]
- Wang, C.; Ning, P. Post-silking phosphorus recycling and carbon partitioning in maize under low to high phosphorus inputs and their effects on grain yield. Front. Plant Sci. 2019, 10, e784. [Google Scholar] [CrossRef]
- Sun, L.; Wang, J.; Lian, L.; Song, J.; Du, X.; Liu, W.; Zhao, W.C.; Yang, L.; Li, C.B.; Qin, Y.; et al. Systematic analysis of the sugar accumulation mechanism in sucrose-and hexose-accumulating cherry tomato fruits. BMC Plant Biol. 2022, 22, e303. [Google Scholar] [CrossRef]
- Rehman, H.M.; Nawaz, M.A.; Shah, Z.H.; Ludwig-Müller, J.; Chung, G.; Ahmad, M.Q.; Yang, S.H.; Lee, S.I. Comparative genomic and transcriptomic analyses of family-1 UDP glycosyltransferase in three Brassica species and Arabidopsis indicates stress-responsive regulation. Sci. Rep. 2018, 8, e1875. [Google Scholar] [CrossRef]
- Michlmayr, H.; Malachová, A.; Varga, E.; Kleinová, J.; Lemmens, M.; Newmister, S.; Rayment, I.; Berthiller, F.; Adam, G. Biochemical characterization of a recombinant UDP-glucosyltransferase from rice and enzymatic production of deoxynivalenol-3-O-β-D-glucoside. Toxins 2015, 7, 2685–2700. [Google Scholar] [CrossRef]
- Eddy, S. Accelerated profile HMM searches. PLoS Comput. Biol. 2011, 7, e1002195. [Google Scholar] [CrossRef]
- Sudhir, K.; Glen, S.; Li, M.; Christina, K.; Koichiro, T. Mega x: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar]
- Keane, T.M.; Creevey, C.J.; Pentony, M.M.; Naughton, T.J.; McLnerney, J.O. Assessment of methods for amino acid matrix selection and their use on empirical data shows that ad hoc assumptions for choice of matrix are not justified. BMC Evol. Biol. 2006, 6, e29. [Google Scholar] [CrossRef] [PubMed]
- Bailey, T.L.; Johnson, J.; Grant, C.E.; Noble, W.S. The MEME suite. Nucleic Acids Res. 2015, 43, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Kesiraju, K.; Mishra, P.; Bajpai, A.; Sharma, M.; Rao, U.; Sreevathsa, R. Agrobacterium tumefaciens-mediated in planta transformation strategy for development of transgenics in cotton (Gossypium hirsutum L.) with GFP as a visual marker. Physiol. Mol. Biol. Plants 2020, 26, 2319–2327. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Mistry, J.; Chuguransky, S.; Williams, L.; Qureshi, M.; Salazar, G.A.; Sonnhammer, E.L.L.; Tosatto, S.C.E.; Paladin, L.; Raj, S.; Richardson, L.G.; et al. pfam: The protein families database in 2021. Nucleic Acids Res. 2021, 49, 412–419. [Google Scholar] [CrossRef]
- Sivasankara Pillai, S.; Dandurand, L.M. Effect of steroidal glycoalkaloids on hatch and reproduction of the potato cyst nematode Globodera pallida. Plant Dis. 2021, 105, 2975–2980. [Google Scholar] [CrossRef]
- Fogelman, E.; Oren-Shamir, M.; Hirschberg, J.; Mandolino, G.; Parisi, B.; Ovadia, R.; Tanami, Z.; Faigenboim, A.; Ginzberg, I. Nutritional value of potato (Solanum tuberosum) in hot climates: Anthocyanins, carotenoids, and steroidal glycoalkaloids. Planta 2019, 249, 1143–1155. [Google Scholar] [CrossRef]
- Alseekh, S.; Ofner, I.; Liu, Z.; Osorio, S.; Vallarino, J.; Last, R.L.; Zamir, D.; Tohge, T.; Fernie, A.R. Quantitative trait loci analysis of seed-specialized metabolites reveals seed-specific flavonols and differential regulation of glycoalkaloid content in tomato. Plant J. 2020, 103, 2007–2024. [Google Scholar] [CrossRef]
- Ofudje, E.A.; Sodiya, E.F.; Ibadin, F.H.; Ogundiran, A.A.; Alayande, S.O.; Osideko, O.A. Mechanism of Cu2+ and reactive yellow 145 dye adsorption onto eggshell waste as low-cost adsorbent. Chem. Ecol. 2021, 37, 268–289. [Google Scholar] [CrossRef]
- Krishnamurthy, P.; Tsukamoto, C.; Ishimoto, M. Reconstruction of the evolutionary histories of UGT gene superfamily in legumes clarifies the functional divergence of duplicates in specialized metabolism. Int. J. Mol. Sci. 2020, 21, 1855. [Google Scholar] [CrossRef]
- Yin, Q.; Shen, G.; Di, S.; Fan, C.; Chang, Z.; Pang, Y. Genome-wide identification and functional characterization of UDP-glucosyltransferase genes involved in flavonoid biosynthesis in Glycine max. Plant Cell Physiol. 2017, 58, 1558–1572. [Google Scholar] [CrossRef] [Green Version]
- Zhang, K.; Sun, Y.; Li, M.; Long, R. CrUGT87A1, a UDP-sugar glycosyltransferases (UGTs) gene from Carex rigescens, increases salt tolerance by accumulating flavonoids for antioxidation in Arabidopsis thaliana. Plant Physiol. Bioch. 2021, 159, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Sun, R.; Liao, X.; Aa, J.; Wang, G. UDP-glucuronosyltransferases (UGTs) and their related metabolic cross-talk with internal homeostasis: A systematic review of UGT isoforms for precision medicine. Pharmacol. Res. 2017, 121, 169–183. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Li, P.; Xia, T.; Wan, X. Exploring plant metabolic genomics: Chemical diversity, metabolic complexity in the biosynthesis and transport of specialized metabolites with the tea plant as a model. Crit. Rev. Biotechnol. 2020, 40, 667–688. [Google Scholar] [CrossRef]
- Guerriero, G.; Berni, R.; Muñoz-Sanchez, J.A.; Apone, F.; Abdel-Salam, E.M.; Qahtan, A.A.; Alatar, A.A.; Cantini, C.; Cai, C.; Hausman, J.F.; et al. Production of plant secondary metabolites: Examples, tips and suggestions for biotechnologists. Genes 2018, 9, 309. [Google Scholar] [CrossRef]
- Kohara, A.; Nakajima, C.; Hashimoto, K.; Ikenaga, T.; Tanaka, H.; Shoyama, Y.; Yoshida, S.; Muranaka, T. A novel glucosyltransferase involved in steroid saponin biosynthesis in Solanum aculeatissimum. Plant Mol. Biol. 2005, 57, 225–239. [Google Scholar] [CrossRef] [PubMed]
- Moreau, R.A.; Nyström, L.; Whitaker, B.D.; Winkler-Moser, J.K.; Baer, D.J.; Gebauer, S.K.; Hicks, K.B. Phytosterols and their derivatives: Structural diversity, distribution, metabolism, analysis, and health-promoting uses. Prog. Lipid Res. 2018, 70, 35–61. [Google Scholar] [CrossRef]
- Kotake, T.; Yamaguchi, D.; Ohzono, H.; Hojo, S.; Kaneko, S.; Ishida, H.K.; Tsumuraya, Y. UDP-sugar pyrophosphorylase with broad substrate specificity toward various monosaccharide 1-phosphates from pea sprouts. J. Biol. Chem. 2004, 279, 45728–45736. [Google Scholar] [CrossRef]
- Yu, G.; Li, C.; Zhang, L.; Zhu, G.; Munir, S.; Shi, C.; Zhang, H.; Ai, G.; Gao, S.; Ye, Z. An allelic variant of GAME9 determines its binding capacity with the GAME 17 promoter in the regulation of steroidal glycoalkaloid biosynthesis in tomato. J. Exp. Bot. 2020, 71, 2527–2536. [Google Scholar] [CrossRef]
- Wang, C.C.; Meng, L.H.; Gao, Y.; Grierson, D.; Fu, D.Q. Manipulation of light signal transduction factors as a means of modifying steroidal glycoalkaloids accumulation in tomato leaves. Front. Plant Sci. 2018, 9, e437. [Google Scholar] [CrossRef]
- Beckles, D.M. Factors affecting the postharvest soluble solids and sugar content of tomato (Solanum lycopersicum L.) fruit. Postharvest Biol. Technol. 2012, 63, 129–140. [Google Scholar] [CrossRef]
- Tang, Y.; Ren, J.; Liu, C.; Jiang, J.; Yang, H.; Li, J. Genetic characteristics and QTL analysis of the soluble sugar content in ripe tomato fruits. Sci. Hortic-Amst. 2021, 276, 109785. [Google Scholar] [CrossRef]
- Uematsu, K.; Suzuki, N.; Iwamae, T.; Inui, M.; Yukawa, H. Increased fructose 1, 6-bisphosphate aldolase in plastids enhances growth and photosynthesis of tobacco plants. J. Exp. Bot. 2012, 63, 3001–3009. [Google Scholar] [CrossRef] [PubMed]
- Bodelón, O.G.; Blanch, M.; Sanchez-Ballesta, M.T.; Escribano, M.I.; Merodio, C. The effects of high CO2 levels on anthocyanin composition, antioxidant activity and soluble sugar content of strawberries stored at low non-freezing temperature. Food Chem. 2010, 122, 673–678. [Google Scholar] [CrossRef]
- Wang, D.; Ma, Q.; Belwal, T.; Li, D.; Li, W.; Li, L.; Luo, Z. High carbon dioxide treatment modulates sugar metabolism and maintains the quality of fresh-cut pear fruit. Molecules 2020, 25, 4261. [Google Scholar] [CrossRef]
- Duan, B.; Ge, Y.; Li, C.; Gao, X.; Tang, Q.; Li, X.; Wei, M.; Chen, Y. Effect of exogenous ATP treatment on sucrose metabolism and quality of Nanguo pear fruit. Sci. Hortic. 2019, 249, 71–76. [Google Scholar] [CrossRef]
- Wang, T.; Ma, Y.Q.; Huang, X.X.; Mu, T.J.; Li, Y.J.; Li, X.K.; Liu, X.; Hou, B.K. Overexpression of OsUGT3 enhances drought and salt tolerance through modulating ABA synthesis and scavenging ROS in rice. Environ. Exp. Bot. 2021, 192, e104653. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, S.-W.; Chen, Z.-F.; Liu, T.-T.; Zhao, Z.-Y.; Li, T.-M.; Xing, G.-M. Identification and Characterization of the Tomato UGT Gene Family and Effects of GAME 17 Overexpression on Plants and Growth and Development under High-CO2 Conditions. Agronomy 2022, 12, 1998. https://doi.org/10.3390/agronomy12091998
Zheng S-W, Chen Z-F, Liu T-T, Zhao Z-Y, Li T-M, Xing G-M. Identification and Characterization of the Tomato UGT Gene Family and Effects of GAME 17 Overexpression on Plants and Growth and Development under High-CO2 Conditions. Agronomy. 2022; 12(9):1998. https://doi.org/10.3390/agronomy12091998
Chicago/Turabian StyleZheng, Shao-Wen, Zhi-Feng Chen, Ting-Ting Liu, Zi-Yao Zhao, Tian-Meng Li, and Guo-Ming Xing. 2022. "Identification and Characterization of the Tomato UGT Gene Family and Effects of GAME 17 Overexpression on Plants and Growth and Development under High-CO2 Conditions" Agronomy 12, no. 9: 1998. https://doi.org/10.3390/agronomy12091998
APA StyleZheng, S.-W., Chen, Z.-F., Liu, T.-T., Zhao, Z.-Y., Li, T.-M., & Xing, G.-M. (2022). Identification and Characterization of the Tomato UGT Gene Family and Effects of GAME 17 Overexpression on Plants and Growth and Development under High-CO2 Conditions. Agronomy, 12(9), 1998. https://doi.org/10.3390/agronomy12091998




