Physiological Mechanism of Abscisic Acid-Induced Heat-Tolerance Responses to Cultivation Techniques in Wheat and Maize—Review
Abstract
:1. Introduction
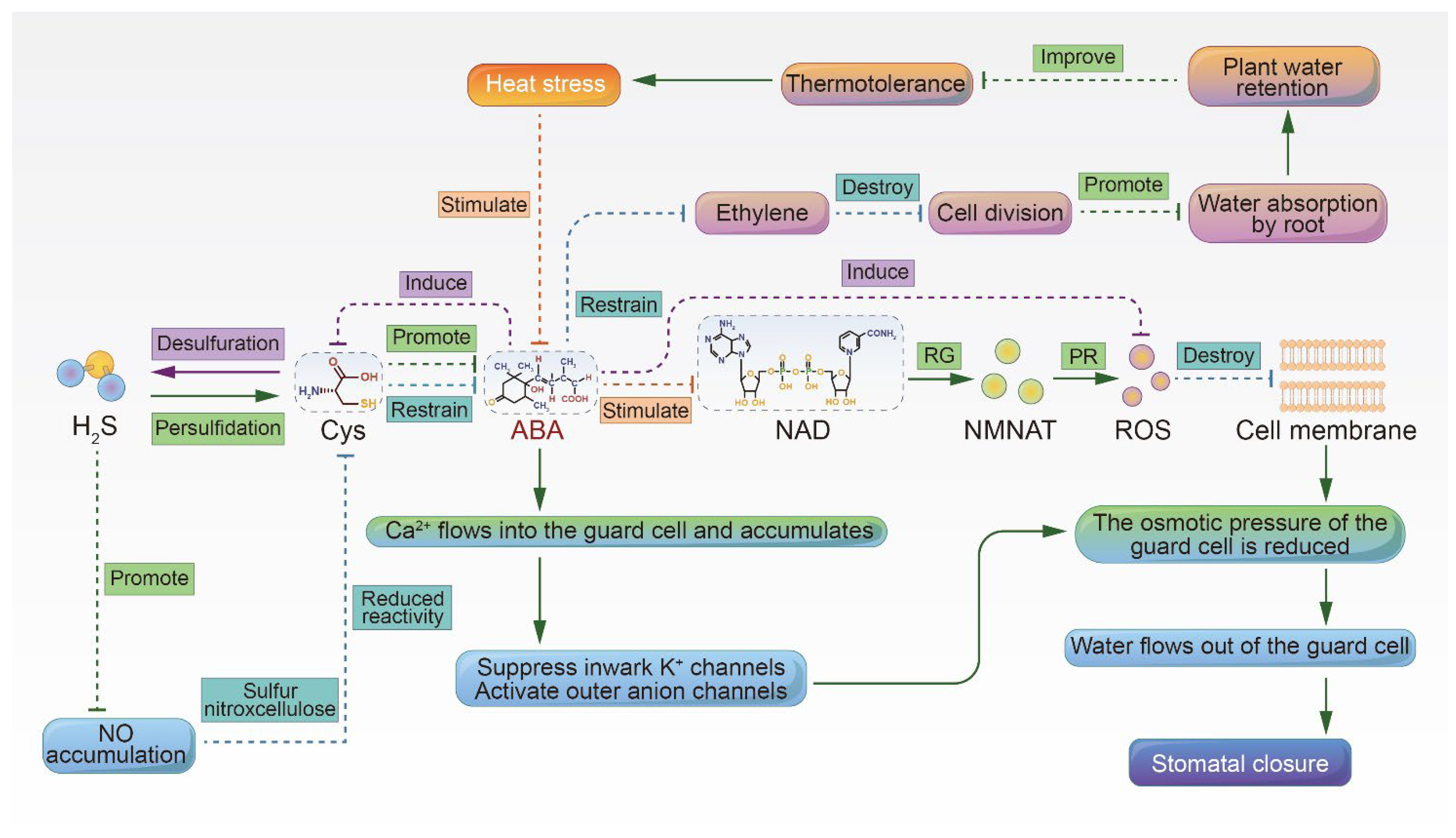
2. Improved Heat Resistance through ABA-Induced Stomatal Closure, Promotion of Root Growth, and Maintenance of Plant Water Status
2.1. Hydrogen Sulfide Promotes ABA-Induced Stomatal Closure
2.2. ABA Promotes Root Growth and Soil Water Absorption, Which in Turn Maintains the Plant Water Status
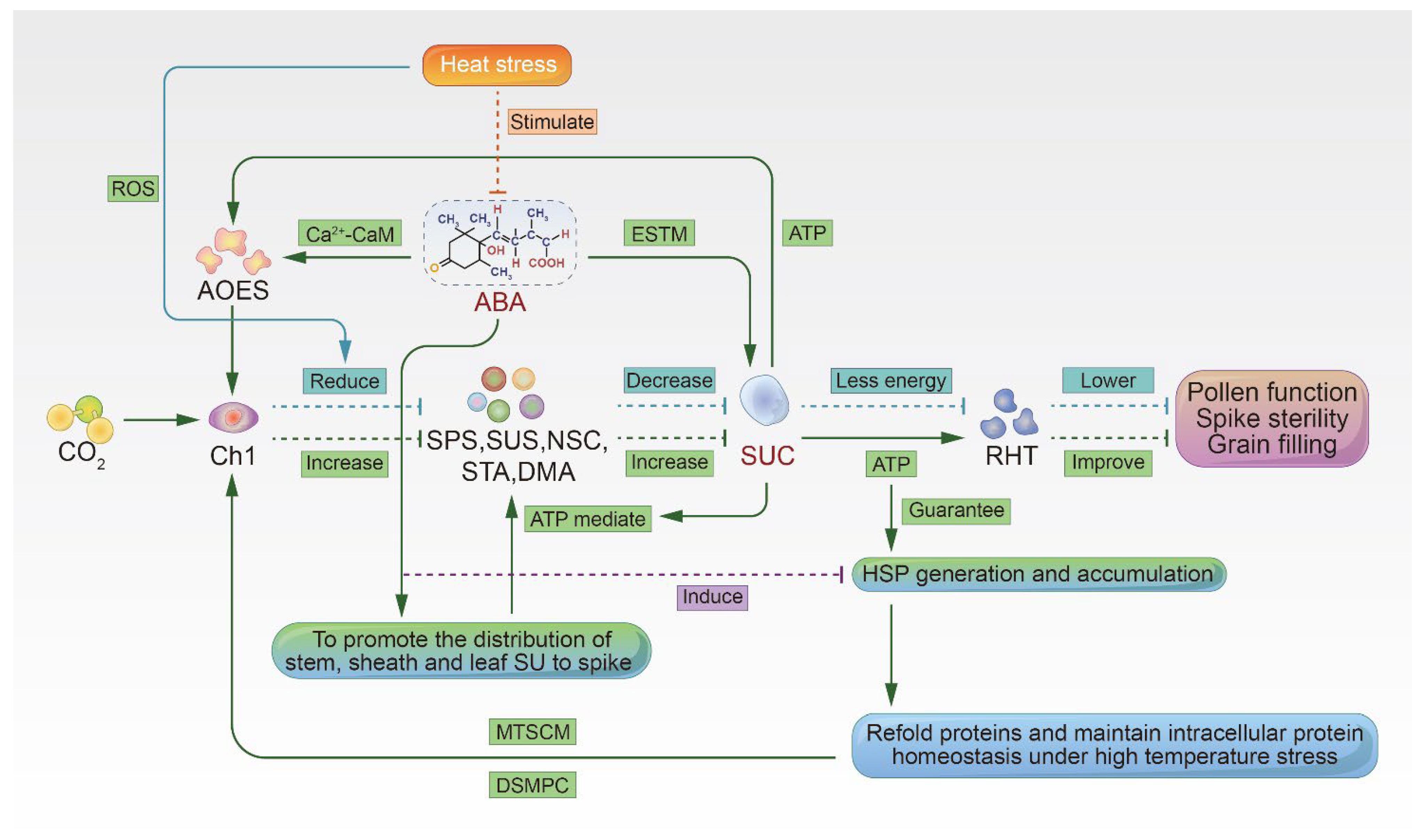
3. ABA Regulates Sugar Metabolism in the Spike and Improves Heat Tolerance
3.1. ABA Enhances Sucrose Transport and Metabolism
3.2. ABA Induces Antioxidant Defenses in Cells
3.3. ABA Induces the Accumulation of Heat Shock Proteins
3.4. Adenosine Triphosphate Provides Energy to Support the Accumulation of HSPs
4. The Mechanism of How Cultivation Techniques Respond to High Temperature in Wheat and Maize
4.1. Cultivars
4.2. Sowing Date
4.3. Chemical Regulation
4.4. Fertilization
4.4.1. Nitrogen Fertilization
4.4.2. Sulfur Fertilization
4.4.3. Zinc Fertilization
4.4.4. Arbuscular Mycorrhizal Fungi Fertilization
4.5. Carbon Dioxide
4.6. Irrigation
4.7. Subsoiling
4.8. Heat Acclimation
5. Concluding Remarks and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Antoni, R.; Gonzalez-Guzman, M.; Rodriguez, L.; Peirats-Llobet, M.; Pizzio, G.A.; Fernandez, M.A.; De Winne, N.; De Jaeger, G.; Dietrich, D.; Bennett, M.J.; et al. PYRABACTIN RESISTANCE1-LIKE8 Plays an Important Role for the Regulation of Abscisic Acid Signaling in Root. Plant Physiol. 2013, 161, 931–941. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barberon, M.; Vermeer, J.E.M.; De Bellis, D.; Wang, P.; Naseer, S.; Andersen, T.G.; Humbel, B.M.; Nawrath, C.; Takano, J.; Salt, D.E.; et al. Adaptation of Root Function by Nutrient-Induced Plasticity of Endodermal Differentiation. Cell 2016, 164, 447–459. [Google Scholar] [CrossRef] [Green Version]
- Kuromori, T.; Seo, M.; Shinozaki, K. ABA Transport and Plant Water Stress Responses. Trends Plant Sci. 2018, 23, 513–522. [Google Scholar] [CrossRef] [PubMed]
- Christmann, A.; Weiler, E.W.; Steudle, E.; Grill, E. A hydraulic signal in root-to-shoot signalling of water shortage. Plant J. 2007, 52, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Umezawa, T.; Okamoto, M.; Kushiro, T.; Nambara, E.; Oono, Y.; Seki, M.; Kobayashi, M.; Koshiba, T.; Kamiya, Y.; Shinozaki, K. CYP707A3, a major ABA 8′-hydroxylase involved in dehydration and rehydration response in Arabidopsis thaliana. Plant J. 2006, 46, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Yaaran, A.; Negin, B.; Moshelion, M. Role of guard-cell ABA in determining steady-state stomatal aperture and prompt vapor-pressure-deficit response. Plant Sci. 2019, 281, 31–40. [Google Scholar] [CrossRef]
- Ruan, Y.L.; Jin, Y.; Yang, Y.J.; Li, G.J.; Boyer, J.S. Sugar input, metabolism, and signaling mediated by invertase: Roles in development, yield potential, and response to drought and heat. Mol. Plant 2010, 3, 942–955. [Google Scholar] [CrossRef]
- Yang, J.C.; Zhang, J.H.; Wang, Z.Q.; Xu, G.W.; Zhu, Q.S. Activities of key enzymes in sucrose-to-starch conversion in wheat grains subjected to water deficit during grain filling. Plant Physiol. 2004, 135, 1621–1629. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Dun, X.L.; Zhou, Z.F.; Xia, S.Q.; Yi, B.; Wen, J.; Shen, J.X.; Ma, C.Z.; Tu, J.X.; Fu, T.D. A separation defect of tapetum cells and microspore mother cells results in male sterility in Brassica napus: The role of abscisic acid in early anther development. Plant Mol. Biol. 2010, 72, 111–123. [Google Scholar] [CrossRef]
- Yadav, M.R.; Choudhary, M.; Singh, J.; Lal, M.K.; Jha, P.K.; Udawat, P.; Gupta, N.K.; Rajput, V.D.; Garg, N.K.; Maheshwari, C.; et al. Impacts, Tolerance, Adaptation, and Mitigation of Heat Stress on Wheat under Changing Climates. Int. J. Mol. Sci. 2022, 23, 2838. [Google Scholar] [CrossRef]
- Hu, X.L.; Jiang, M.Y.; Zhang, J.H.; Zhang, A.Y.; Lin, F.; Tan, M.P. Calcium-calmodulin is required for abscisic acid-induced antioxidant defense and functions both upstream and downstream of H2O2 production in leaves of maize (Zea mays) plants. New Phytol. 2007, 173, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Rezaul, I.M.; Feng, B.H.; Chen, T.T.; Fu, W.M.; Zhang, C.X.; Tao, L.X.; Fu, G.F. Abscisic acid prevents pollen abortion under high-temperature stress by mediating sugar metabolism in rice spikelets. Physiol. Plant. 2019, 165, 644–663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xue, G.P.; Sadat, S.; Drenth, J.; McIntyre, C.L. The heat shock factor family from Triticum aestivum in response to heat and other major abiotic stresses and their role in regulation of heat shock protein genes. J. Exp. Bot. 2014, 65, 539–557. [Google Scholar] [CrossRef] [Green Version]
- Gouache, D.; Le Bris, X.; Bogard, M.; Deudon, O.; Page, C.; Gate, P. Evaluating agronomic adaptation options to increasing heat stress under climate change during wheat grain filling in France. Eur. J. Agron. 2012, 39, 62–70. [Google Scholar] [CrossRef]
- Zhu, Y.G.; Chu, J.P.; Dai, X.L.; He, M.R. Delayed sowing increases grain number by enhancing spike competition capacity for assimilates in winter wheat. Eur. J. Agron. 2019, 104, 49–62. [Google Scholar] [CrossRef]
- Tao, Q.; Zhou, Y.Y.; Guo, Q.; Liu, Y.R.; Yu, S.; Yu, C.X.; Zhang, M.C.; Li, Z.H.; Duan, L.S. A novel plant growth regulator alleviates high-temperature stress in maize. Agron. J. 2018, 110, 2350–2359. [Google Scholar] [CrossRef]
- Brunel-Muguet, S.; D’Hooghe, P.; Bataille, M.P.; Larre, C.; Kim, T.H.; Trouverie, J.; Avice, J.C.; Etienne, P.; Durr, C. Heat stress during seed filling interferes with sulfur restriction on grain composition and seed germination in oilseed rape (Brassica napus L.). Front. Plant Sci. 2015, 6, 213. [Google Scholar] [CrossRef] [Green Version]
- Ito, S.; Hara, T.; Kawanami, Y.; Watanabe, T.; Thiraporn, K.; Ohtake, N.; Sueyoshi, K.; Mitsui, T.; Fukuyama, T.; Takahashi, Y.; et al. Carbon and Nitrogen Transport during Grain Filling in Rice Under High-temperature Conditions. J. Agron. Crop Sci. 2009, 195, 368–376. [Google Scholar] [CrossRef]
- Pleijel, H.; Broberg, M.C.; Hogy, P.; Uddling, J. Nitrogen application is required to realize wheat yield stimulation by elevated CO2 but will not remove the CO2-induced reduction in grain protein concentration. Glob. Chang. Biol. 2019, 25, 1868–1876. [Google Scholar] [CrossRef]
- Tao, Z.Q.; Wang, D.M.; Chang, X.H.; Wang, Y.J.; Yang, Y.S.; Zhao, G.C. Effects of zinc fertilizer and short-term high temperature stress on wheat grain production and wheat flour proteins. J. Integr. Agric. 2018, 17, 1979–1990. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.C.; Song, F.B.; Liu, S.Q.; Liu, T.D. Effects of arbuscular mycorrhizal fungus on photosynthesis and water status of maize under high temperature stress. Plant Soil 2011, 346, 189–199. [Google Scholar] [CrossRef]
- Duan, H.; Yu, Z.H.; Xu, Y.J.; Wang, Z.Q.; Liu, L.J.; Yang, J.C. Role of irrigation patterns in reducing harms of high temperature to rice. Acta Agron. Sin. 2013, 38, 107–120, (In Chinese with English Abstract). [Google Scholar] [CrossRef]
- Tao, Z.Q.; Sui, P.; Chen, Y.Q.; Li, C.; Nie, Z.J.; Yuan, S.F.; Shi, J.T.; Gao, W.S. Subsoiling and Ridge Tillage Alleviate the High Temperature Stress in Spring Maize in the North China Plain. J. Integr. Agric. 2013, 12, 2179–2188. [Google Scholar] [CrossRef]
- Scuffi, D.; Alvarez, C.; Laspina, N.; Gotor, C.; Lamattina, L.; Garcia-Mata, C. Hydrogen Sulfide Generated by L-Cysteine Desulfhydrase Acts Upstream of Nitric Oxide to Modulate Abscisic Acid-Dependent Stomatal Closure(1[C][W]). Plant Physiol. 2014, 166, 2065–2076. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hou, Z.; Wang, L.; Liu, J.; Hou, L.; Liu, X. Hydrogen sulfide regulates ethylene-induced stomatal closure in Arabidopsis thaliana. J. Integr. Plant Biol. 2013, 55, 277–289. [Google Scholar] [CrossRef]
- Kopriva, S. Regulation of sulfate assimilation in Arabidopsis and beyond. Ann. Bot. 2006, 97, 479–495. [Google Scholar] [CrossRef] [Green Version]
- Papenbrock, J.; Riemenschneider, A.; Kamp, A.; Schulz-Vogt, H.N.; Schmidt, A. Characterization of cysteine-degrading and H2S-releasing enzymes of higher plants—From the field to the test tube and back. Plant Biol. 2007, 9, 582–588. [Google Scholar] [CrossRef]
- Suwa, R.; Hakata, H.; Hara, H.; El-Shemy, H.A.; Adu-Gyamfi, J.J.; Nguyen, N.T.; Kanai, S.; Lightfoot, D.A.; Mohapatra, P.K.; Fujita, K. High temperature effects on photosynthate partitioning and sugar metabolism during ear expansion in maize (Zea mays L.) genotypes. Plant Physiol. Biochem. 2010, 48, 124–130. [Google Scholar] [CrossRef]
- Chen, S.S.; Jia, H.L.; Wang, X.F.; Shi, C.; Wang, X.; Ma, P.Y.; Wang, J.; Ren, M.J.; Li, J.S. Hydrogen Sulfide Positively Regulates Abscisic Acid Signaling through Persulfidation of SnRK2.6 in Guard Cells. Mol. Plant 2020, 13, 732–744. [Google Scholar] [CrossRef]
- Hashida, S.N.; Itami, T.; Takahashi, H.; Takahara, K.; Nagano, M.; Kawai-Yamada, M.; Shoji, K.; Goto, F.; Yoshihara, T.; Uchimiya, H. Nicotinate/nicotinamide mononucleotide adenyltransferase-mediated regulation of NAD biosynthesis protects guard cells from reactive oxygen species in ABA-mediated stomatal movement in Arabidopsis. J. Exp. Bot. 2010, 61, 3813–3825. [Google Scholar] [CrossRef]
- Acharya, B.R.; Jeon, B.W.; Zhang, W.; Assmann, S.M. Open Stomata 1 (OST1) is limiting in abscisic acid responses of Arabidopsis guard cells. New Phytol. 2013, 200, 1049–1063. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wan, R.J.; Shi, Y.H.; Xue, S.W. Hydrogen Sulfide Activates S-Type Anion Channel via OST1 and Ca2+ Modules. Mol. Plant 2016, 9, 489–491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hubbard, K.E.; Siegel, R.S.; Valerio, G.; Brandt, B.; Schroeder, J.I. Abscisic acid and CO2 signalling via calcium sensitivity priming in guard cells, new CDPK mutant phenotypes and a method for improved resolution of stomatal stimulus-response analyses. Ann. Bot. 2012, 109, 5–17. [Google Scholar] [CrossRef] [PubMed]
- Corpas, F.J.; Gonzalez-Gordo, S.; Canas, A.; Palma, J.M. Nitric oxide and hydrogen sulfide in plants: Which comes first? J. Exp. Bot. 2019, 70, 4391–4404. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.C.; Dua, Y.Y.; Hou, Y.J.; Zhao, Y.; Hsu, C.C.; Yuan, F.J.; Zhu, X.H.; Tao, W.A.; Song, C.P.; Zhu, J.K. Nitric oxide negatively regulates abscisic acid signaling in guard cells by S-nitrosylation of OST1. Proc. Natl. Acad. Sci. USA 2015, 112, 613–618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.M.; Han, W.; De Smet, I.; Talboys, P.; Loya, R.; Hassan, A.; Rong, H.L.; Jurgens, G.; Knox, J.P.; Wang, M.H. ABA promotes quiescence of the quiescent centre and suppresses stem cell differentiation in the Arabidopsis primary root meristem. Plant J. 2010, 64, 764–774. [Google Scholar] [CrossRef]
- Ortega-Martinez, O.; Pernas, M.; Carol, R.J.; Dolan, L. Ethylene modulates stem cell division in the Arabidopsis thaliana root. Science 2007, 317, 507–510. [Google Scholar] [CrossRef]
- Zhao, Y.; Xing, L.; Wang, X.G.; Hou, Y.J.; Gao, J.H.; Wang, P.C.; Duan, C.G.; Zhu, X.H.; Zhu, J.K. The ABA Receptor PYL8 Promotes Lateral Root Growth by Enhancing MYB77-Dependent Transcription of Auxin-Responsive Genes. Sci. Signal. 2014, 7, ra53. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.F.; Zhang, L.X.; Yu, Y.W.; Quan, R.D.; Zhang, Z.J.; Zhang, H.W.; Huang, R.F. The ethylene response factor AtERF11 that is transcriptionally modulated by the bZIP transcription factor HY5 is a crucial repressor for ethylene biosynthesis in Arabidopsis. Plant J. 2011, 68, 88–99. [Google Scholar] [CrossRef]
- Dietrich, D.; Pang, L.; Kobayashi, A.; Fozard, J.A.; Boudolf, V.; Bhosale, R.; Antoni, R.; Nguyen, T.; Hiratsuka, S.; Fujii, N.; et al. Root hydrotropism is controlled via a cortex-specific growth mechanism. Nat. Plants 2017, 3, 17057. [Google Scholar] [CrossRef]
- Smeekens, S.; Ma, J.; Hanson, J.; Rolland, F. Sugar signals and molecular networks controlling plant growth. Curr. Opin. Plant Biol. 2010, 13, 274–279. [Google Scholar] [CrossRef] [PubMed]
- Granot, D.; David-Schwartz, R.; Kelly, G. Hexose kinases and their role in sugar-sensing and plant development. Front. Plant Sci. 2013, 4, 44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parrotta, L.; Faleri, C.; Cresti, M.; Cai, G. Heat stress affects the cytoskeleton and the delivery of sucrose synthase in tobacco pollen tubes. Planta 2016, 243, 43–63. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.P.; Yuan, Y.Z.; Zhou, W.; Zhang, C.F. Effects of exogenously supplied sucrose on OsSUTs and OsSPSs transcript abundances and rice root ammonium assimilation. Acta Physiol. Plant. 2016, 38, 274. [Google Scholar] [CrossRef]
- Koch, K. Sucrose metabolism: Regulatory mechanisms and pivotal roles in sugar sensing and plant development. Curr. Opin. Plant Biol. 2004, 7, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Gong, M.; Li, Y.J.; Chen, S.Z. Abscisic acid-induced thermotolerance in maize seedlings is mediated by calcium and associated with antioxidant systems. J. Plant Physiol. 1998, 153, 488–496. [Google Scholar] [CrossRef]
- Mirza, H.; Nahar, K.; Alam, M.M.; Rajib, R.; Fujita, M. Physiological, biochemical, and molecular mechanisms of heat stress tolerance in plants. Int. J. Mol. Sci. 2013, 14, 9643–9684. [Google Scholar] [CrossRef]
- Li, H.; Liu, S.S.; Yi, C.Y.; Wang, F.; Zhou, J.; Xia, X.J.; Shi, K.; Zhou, Y.H.; Yu, J.Q. Hydrogen peroxide mediates abscisic acid-induced HSP70 accumulation and heat tolerance in grafted cucumber plants. Plant Cell Environ. 2014, 37, 2768–2780. [Google Scholar] [CrossRef]
- Gechev, T.S.; Hille, J. Hydrogen peroxide as a signal controlling plant programmed cell death. J. Cell Biol. 2005, 168, 17–20. [Google Scholar] [CrossRef] [Green Version]
- Bolouri-Moghaddam, M.R.; Le Roy, K.; Xiang, L.; Rolland, F.; Van den Ende, W. Sugar signalling and antioxidant network connections in plant cells. FEBS J. 2010, 277, 2022–2037. [Google Scholar] [CrossRef]
- Zou, J.; Liu, A.L.; Chen, X.B.; Zhou, X.Y.; Gao, G.F.; Wang, W.F.; Zhang, X.W. Expression analysis of nine rice heat shock protein genes under abiotic stresses and ABA treatment. J. Plant Physiol. 2009, 166, 851–861. [Google Scholar] [CrossRef] [PubMed]
- Ozga, J.A.; Kaur, H.; Savada, R.P.; Reinecke, D.M. Hormonal regulation of reproductive growth under normal and heat-stress conditions in legume and other model crop species. J. Exp. Bot. 2017, 68, 1885–1894. [Google Scholar] [CrossRef] [PubMed]
- Fragkostefanakis, S.; Roeth, S.; Schleiff, E.; Scharf, K.-D. Prospects of engineering thermotolerance in crops through modulation of heat stress transcription factor and heat shock protein networks. Plant Cell Environ. 2015, 38, 1881–1895. [Google Scholar] [CrossRef]
- Baena-Gonzalez, E.; Sheen, J. Convergent energy and stress signaling. Trends Plant Sci. 2008, 13, 474–482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buesa, C.; Dominguez, M.; Vendrell, M. Abscisic acid effects on ethylene production and respiration rate in detached apple fruits at different stages of development. Rev. Esp. Cienc. Tecnol. Aliment. 1994, 34, 495–506. [Google Scholar]
- Tetteroo, F.A.A.; Peters, A.; Hoekstra, F.A.; Van der Plas, L.H.W.; Hagendoorn, M.J.M. ABA reduces respiration and sugar metabolism in developing carrot (Daucus-carota L.) embryoids. J. Plant Physiol. 1995, 145, 477–482. [Google Scholar] [CrossRef]
- Mohammed, R.; Cothren, J.T.; Tarpley, L. High night temperature and abscisic acid affect rice productivity through altered photosynthesis, respiration and spikelet fertility. Crop Sci. 2013, 53, 2603–2612. [Google Scholar] [CrossRef]
- Finka, A.; Cuendet, A.F.H.; Maathuis, F.J.M.; Saidi, Y.; Goloubinoff, P. Plasma membrane cyclic nucleotide gated calcium channels control land plant thermal sensing and acquired thermotolerance. Plant Cell 2012, 24, 3333–3348. [Google Scholar] [CrossRef] [Green Version]
- Mittler, R.; Finka, A.; Goloubinoff, P. How do plants feel the heat? Trends Biochem. Sci. 2012, 37, 118–125. [Google Scholar] [CrossRef]
- Liu, Y.H.; Offler, C.E.; Ruan, Y.L. Regulation of fruit and seed response to heat and drought by sugars as nutrients and signals. Front. Plant Sci. 2013, 4, 282. [Google Scholar] [CrossRef] [Green Version]
- Zheng, B.Y.; Chenu, K.; Dreccer, M.F.; Chapman, S.C. Breeding for the future: What are the potential impacts of future frost and heat events on sowing and flowering time requirements for Australian bread wheat (Triticum aestivium) varieties? Glob. Chang. Biol. 2012, 18, 2899–2914. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Liu, L.; Tian, L.; Cao, W.; Zhu, Y.; Asseng, S. Post-heading heat stress and yield impact in winter wheat of China. Glob. Chang. Biol. 2014, 20, 372–381. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.H.; Yang, Y.M.; Cao, L.; Hao, Y.F.; Huang, Q.; Li, J.P.; Yao, D.X.; Wang, Z.M. Effect of high temperature on photosynthetic capability and antioxidant enzyme activity of flag leaf and non-leaf organs in wheat. Acta Agron. Sin. 2015, 41, 136–144, (In Chinese with English Abstract). [Google Scholar] [CrossRef]
- Hu, Y.Y.; Lu, H.F.; Liu, W.X.; Kang, J.; Ma, G.; Meng, S.S.; Chu, Y.Y.; Wang, C.Y. Effects of high temperature and water deficiency during grain filling on activities of key starch synthesis enzymes and starch accumulation in wheat. Acta Agron. Sin. 2018, 44, 591–600, (In Chinese with English Abstract). [Google Scholar] [CrossRef]
- Abdelrahman, M.; Burritt, D.J.; Gupta, A.; Tsujimoto, H.; Tran, L.P. Heat stress effect on source-sink relationships and metabolome dynamics in wheat. J. Exp. Bot. 2020, 71, 543–554. [Google Scholar] [CrossRef] [PubMed]
- Arena, S.; D’Ambrosio, C.; Vitale, M.; Mazzeo, F.; Mamone, G.; Di Stasio, L.; Maccaferri, M.; Curci, P.L.; Sonnante, G.; Zambrano, N.; et al. Differential representation of albumins and globulins during grain development in durum wheat and its possible functional consequences. J. Proteom. 2017, 162, 86–98. [Google Scholar] [CrossRef]
- Sehgal, A.; Sita, K.; Siddique, K.H.M.; Kumar, R.; Bhogireddy, S.; Varshney, R.K.; HanumanthaRao, B.; Nair, R.M.; Prasad, P.V.V.; Nayyar, H. Drought or/and heat-stress effects on seed filling in food crops: Impacts on functional biochemistry, seed yields, and nutritional quality. Front. Plant Sci. 2018, 9, 1705. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.M.; Hou, L.J.; Lu, Y.Z.; Wu, B.J.; Gong, X.; Liu, M.S.; Wang, J.; Sun, Q.X.; Vierling, E.; Xu, S.B. Metabolic adaptation of wheat grain contributes to a stable filling rate under heat stress. J. Exp. Bot. 2018, 69, 5531–5545. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.F.; Wang, C.Y.; Ma, D.Y.; Lu, H.F.; Zhu, Y.J.; Xie, Y.X.; Guo, T.C. Effects of waterlogging, high temperature and their interaction after anthesis on grain protein components and flour color in wheat. Acta Agron. Sin. 2014, 40, 1102–1108. [Google Scholar] [CrossRef]
- Wang, Y.; Tao, H.; Tian, B.; Sheng, D.; Xu, C.; Zhou, H.; Huang, S.; Wang, P. Flowering dynamics, pollen, and pistil contribution to grain yield in response to high temperature during maize flowering. Environ. Exp. Bot. 2019, 158, 80–88. [Google Scholar] [CrossRef]
- Zhao, L.X.; Zhang, P.; Wang, R.N.; Wang, P.; Tao, H.B. Effect of high temperature after flowering on growth and development of superior and inferior maize kernels. Acta Agron. Sin. 2014, 40, 1839–1845, (In Chinese with English Abstract). [Google Scholar] [CrossRef]
- Zhao, F.C.; Jing, L.Q.; Yan, F.B.; Lu, D.L.; Wang, G.Y.; Lu, W.P. Effects of heat stress during grain filling on sugar accumulation and enzyme activity associated with sucrose metabolism in sweet corn. Acta Agron. Sin. 2013, 39, 1644–1651, (In Chinese with English Abstract). [Google Scholar] [CrossRef]
- Zhao, L.F.; Li, C.H.; Liu, T.X.; Wang, X.P.; Zeng, S.S.; Pan, X. Genotypic responses and physiological mechanisms of maize (Zea mays L.) to high temperature stress during flowering. Acta Agron. Sin. 2012, 38, 857–864, (In Chinese with English Abstract). [Google Scholar] [CrossRef]
- Yan, P.; Tao, Z.Q.; Chen, Y.Q.; Zhang, X.P.; Sui, P. Spring maize kernel number and assimilate supply responses to high-temperature stress under field conditions. Agron. J. 2017, 109, 1433–1442. [Google Scholar] [CrossRef]
- Lv, X.K.; Han, J.; Liao, Y.C.; Liu, Y. Effect of phosphorus and potassium foliage application post-anthesis on grain filling and hormonal changes of wheat. Field Crops Res. 2017, 214, 83–93. [Google Scholar] [CrossRef]
- Zörb, C.; Ludewig, U.; Hawkesford, M.J. Perspective on wheat yield and quality with reduced nitrogen supply. Trends Plant Sci. 2018, 23, 1029–1037. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.T.; Hu, C.S.; Olesen, J.E.; Ju, Z.Q.; Yang, P.P.; Zhang, Y.M. Warming and nitrogen fertilization effects on winter wheat yields in northern China varied between four years. Field Crops Res. 2013, 151, 56–64. [Google Scholar] [CrossRef]
- Elia, M.; Slafer, G.A.; Savin, R. Yield and grain weight responses to post-anthesis increases in maximum temperature under field grown wheat as modified by nitrogen supply. Field Crops Res. 2018, 221, 228–237. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Fu, P.X.; Lu, W.P.; Lu, D.L. Application of moderate nitrogen levels alleviates yield loss and grain quality deterioration caused by post-silking heat stress in fresh waxy maize. Crop J. 2020, 8, 1081–1092. [Google Scholar] [CrossRef]
- Tao, Z.Q.; Chen, Y.Q.; Li, C.; Zou, J.X.; Yan, P.; Yuan, S.F.; Wu, X.; Sui, P. The causes and impacts for heat stress in spring maize during grain filling in the North China Plain—A review. J. Integr. Agric. 2016, 15, 2677–2687. [Google Scholar] [CrossRef] [Green Version]
- Luo, Y.L.; Tang, Y.H.; Zhang, X.; Li, W.Q.; Chang, Y.L.; Pang, D.W.; Xu, X.; Li, Y.; Wang, Z.L. Interactions between cytokinin and nitrogen contribute to grain mass in wheat cultivars by regulating the flag leaf senescence process. Crop J. 2018, 6, 538–551. [Google Scholar] [CrossRef]
- Yang, D.Q.; Luo, Y.L.; Ni, Y.L.; Yin, Y.P.; Yang, W.B.; Peng, D.L.; Cui, Z.Y.; Wang, Z.L. Effects of exogenous ABA application on post-anthesis dry matter redistribution and grain starch accumulation of winter wheat with different staygreen characteristics. Crop J. 2014, 2, 144–153. [Google Scholar] [CrossRef] [Green Version]
- Jiang, W.W.; Yin, Y.P.; Wang, Z.L.; Li, Y.; Yang, W.B.; Peng, D.L.; Yang, D.Q.; Cui, Z.Y.; Lu, K.L.; Li, Y.X. Effects of postponed application of nitrogen fertilizer on yield and physiological characteristics of flag leaf in wheat under post-anthesis heat stress. Acta Agron. Sin. 2014, 40, 942–949, (In Chinese with English Abstract). [Google Scholar] [CrossRef]
- Yan, P.; Chen, Y.Q.; Dadouma, A.; Tao, Z.Q.; Sui, P. Effect of nitrogen regimes on narrowing the magnitude of maize yield penalty caused by high temperature stress in North China Plain. Plant Soil Environ. 2017, 63, 131–138. [Google Scholar] [CrossRef] [Green Version]
- Ordonez, R.A.; Savin, R.; Mariano Cossani, C.; Slafer, G.A. Yield response to heat stress as affected by nitrogen availability in maize. Field Crops Res. 2015, 183, 184–203. [Google Scholar] [CrossRef]
- Xie, Y.X.; Zhang, H.; Zhu, Y.J.; Zhao, L.; Yang, J.H.; Cha, F.N.; Liu, C.; Wang, C.Y.; Guo, T.C. Grain yield and water use of winter wheat as affected by water and sulfur supply in the North China Plain. J. Integr. Agric. 2017, 16, 614–625. [Google Scholar] [CrossRef] [Green Version]
- Zörb, C.; Dorothee, S.; Gödde, V.; Niehaus, K.; Mühling, K.H. Metabolite profiling of wheat flag leaf and grains during grain filling phase as affected by sulfur fertilisation. Funct. Plant Biol. 2012, 39, 156–166. [Google Scholar] [CrossRef] [PubMed]
- D’Hooghe, P.; Dubousset, L.; Gallardo, K.; Kopriva, S.; Avice, J.-C.; Trouverie, J. Evidence for proteomic and metabolic adaptations associated with alterations of seed yield and quality in sulfur- limited Brassica napus L. Mol. Cell. Proteom. 2014, 13, 1165–1183. [Google Scholar] [CrossRef] [Green Version]
- Tao, Z.Q.; Chang, X.H.; Wang, D.M.; Wang, Y.J.; Ma, S.K.; Yang, Y.S.; Zhao, G.C. Effects of sulfur fertilization and short-term high temperature on wheat grain production and wheat flour proteins. Crop J. 2018, 6, 413–425. [Google Scholar] [CrossRef]
- Liu, W.H.; Li, S.N.; Hou, G.G.; Yang, J.H.; Duan, J.Z.; Zhu, Y.J. Effects of foliar-spraying of different nutritional mixtures on stress tolerance to dry-hot wind and yield in winter wheat. J. Plant Nutr. Fertilizer 2019, 25, 1600–1606. [Google Scholar]
- Zhang, J.; Liang, Z.K.; Wang, X.P.; Hu, L.H.; Li, Y.J. Effects of zinc fertilizer on root growth and yield of winter wheat under drought stress. Acta Agric. Boreali-Sin. 2019, 34, 126–136, (In Chinese with English Abstract). [Google Scholar] [CrossRef]
- Wang, Z.Q.; Zhang, L.T.; Peng, L.X.; Zhang, Z.W.; Hu, Y.B.; Lin, T.B. Effect of zinc fertilizer on yield formation of maize under water stresses. J. Henan Agric. Univ. 2014, 48, 674–679, (In Chinese with English Abstract). [Google Scholar]
- Mathur, S.; Jajoo, A. Arbuscular mycorrhizal fungi protects maize plants from high temperature stress by regulating photosystem II heterogeneity. Ind. Crops Prod. 2020, 143, 111934. [Google Scholar] [CrossRef]
- Qaderi, M.M.; Kurepin, L.V.; Reid, D.M. Growth and physiological responses of canola (Brassica napus) to three components of global climate change: Temperature, carbon dioxide and drought. Physiol. Plant. 2006, 128, 710–721. [Google Scholar] [CrossRef]
- Li, X.; Ahammed, G.J.; Zhang, Y.Q.; Zhang, G.Q.; Sun, Z.H.; Zhou, J.; Zhou, Y.H.; Xia, X.J.; Yu, J.Q.; Shi, K. Carbon dioxide enrichment alleviates heat stress by improving cellular redox homeostasis through an ABA-independent process in tomato plants. Plant Biol. 2015, 17, 81–89. [Google Scholar] [CrossRef]
- Zaveri, E.; Lobell, D.B. The role of irrigation in changing wheat yields and heat sensitivity in India. Nat. Commun. 2019, 10, 4144. [Google Scholar] [CrossRef]
- Wang, D.; Xu, X.X.; Zhang, H.B.; Lin, X.; Zhao, Y. Effects of irrigation with micro-sprinkling hoses on canopy temperature and humidity at filling stage and grain weight of wheat. Acta Agron. Sin. 2015, 41, 1564–1574, (In Chinese with English Abstract). [Google Scholar] [CrossRef]
- Xiao, G.J.; Liu, W.X.; Xu, Q.; Sun, Z.J.; Wang, J. Effects of temperature increase and elevated CO2 concentration, with supplemental irrigation, on the yield of rain-fed spring wheat in a semiarid region of China. Agric. Water Manag. 2005, 74, 243–255. [Google Scholar] [CrossRef]
- Kang, S.Z.; Zhang, J.H.; Liang, Z.S.; Hu, X.T.; Cai, H.J. The controlled alternative irrigation—A new approach for water saving regulation in farmland. Agric. Res. Arid. Areas 1997, 15, 1–6, (In Chinese with English Abstract). [Google Scholar]
- Chai, Q.; Yang, C.H.; Huang, G.B. Water use characteristics of alternately irrigated wheat/maize intercropping in oasis region of Northwestern China. Acta Agron. Sin. 2011, 37, 1623–1630, (In Chinese with English Abstract). [Google Scholar] [CrossRef]
- Tao, Z.Q. Technologic Solutions of High Temperature Stress in Spring Maize during the Filling Stage in the North China Plain. Ph.D. Thesis, China Agriculture University, Beijing, China, 2013. (In Chinese with English Abstract). [Google Scholar]
- Rashid, F.A.A.; Crisp, P.A.; Zhang, Y.; Berkowitz, O.; Pogson, B.J.; Day, D.A.; Masle, J.; Dewar, R.C.; Whelan, J.; Atkin, O.K.; et al. Molecular and physiological responses during thermal acclimation of leaf photosynthesis and respiration in rice. Plant Cell Environ. 2020, 43, 594–610. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.G.; Fan, Z.H.; Li, X.Z.; Wang, Z.W.; Han, W. The effect of heat acclimation on celluar membrane thermostability in wheat. Acta Agric. Boreali-Sin. 1993, 8, 33–37, (In Chinese with English Abstract). [Google Scholar]
- Zhou, R.G.; Fan, Z.H.; Li, X.Z.; Wang, Z.W.; Han, W. The effect of heat acclimation on membrane thermostability and relative enzyme activity. Acta Agron. Sin. 1995, 21, 568–572, (In Chinese with English Abstract). [Google Scholar]
- Chen, X.Y.; Li, Y.J.; Gao, Z.Y.; Tian, S.M. Relationship of acquired heat tolerance and performance of heat tolerance in wheat. Acta Agric. Boreali-Sin. 2003, 18, 52–55, (In Chinese with English Abstract). [Google Scholar]
- Li, X.Z.; Zhou, R.G.; Fan, Z.H.; Bai, J. A Study on relationship between heat acclimation and calmodulin level in wheat. Acta Agric. Boreali-Sin. 2000, 15, 20–23, (In Chinese with English Abstract). [Google Scholar] [CrossRef]
- Yoshida, T.; Christmann, A.; Yamaguchi-Shinozaki, K.; Grill, E.; Fernie, A.R. Revisiting the Basal Role of ABA—Roles Outside of Stress. Trends Plant Sci. 2019, 24, 625–635. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tao, Z.; Yan, P.; Zhang, X.; Wang, D.; Wang, Y.; Ma, X.; Yang, Y.; Liu, X.; Chang, X.; Sui, P.; et al. Physiological Mechanism of Abscisic Acid-Induced Heat-Tolerance Responses to Cultivation Techniques in Wheat and Maize—Review. Agronomy 2022, 12, 1579. https://doi.org/10.3390/agronomy12071579
Tao Z, Yan P, Zhang X, Wang D, Wang Y, Ma X, Yang Y, Liu X, Chang X, Sui P, et al. Physiological Mechanism of Abscisic Acid-Induced Heat-Tolerance Responses to Cultivation Techniques in Wheat and Maize—Review. Agronomy. 2022; 12(7):1579. https://doi.org/10.3390/agronomy12071579
Chicago/Turabian StyleTao, Zhiqiang, Peng Yan, Xuepeng Zhang, Demei Wang, Yanjie Wang, Xinglin Ma, Yushuang Yang, Xiwei Liu, Xuhong Chang, Peng Sui, and et al. 2022. "Physiological Mechanism of Abscisic Acid-Induced Heat-Tolerance Responses to Cultivation Techniques in Wheat and Maize—Review" Agronomy 12, no. 7: 1579. https://doi.org/10.3390/agronomy12071579
APA StyleTao, Z., Yan, P., Zhang, X., Wang, D., Wang, Y., Ma, X., Yang, Y., Liu, X., Chang, X., Sui, P., & Chen, Y. (2022). Physiological Mechanism of Abscisic Acid-Induced Heat-Tolerance Responses to Cultivation Techniques in Wheat and Maize—Review. Agronomy, 12(7), 1579. https://doi.org/10.3390/agronomy12071579





