Abstract
Orthodox seeds deteriorate even when stored in the best of conditions; hence, it is very important to monitor germination in stored seeds. To assess orthodox seed deterioration, a germination test is usually employed. This study assessed and compared seed deterioration in five orthodox species using electrolyte leakage and Fourier transform infrared spectroscopy (FTIR). The study also compared water imbibition by the test orthodox seeds. To achieve this, the seeds from three wild (Bolusanthus speciosus; Combretum erythrophyllum; Erythrina caffra) and two agricultural (Pisum sativum and Cucurbita pepo) species were imbibed between 20 layers of single-ply paper towel. The other set was subjected to controlled deterioration at 40 °C and 100% relative humidity for 32 d, with samples taken for germination and electrolyte leakage measurement at 4 d intervals. FTIR measurements were performed at 0, 20, and 32 d of controlled deterioration. The results indicated that there were some significant interspecies differences in the imbibition times and seed water contents but these were not large. In all species, uptake of water was complete between about 15 and 25 h. The wild species showed higher sensitivity to controlled deterioration. Complete loss of germinability occurred much earlier in the wild species (20 d in B. speciosus and E. caffra, and 16 d in Co. erythrophyllum) compared with 36 d for the agricultural species P. sativum and Cu. pepo. There was a negative correlation between electrolyte leakage and seed germination in all wild and agricultural species. A strong positive correlation was observed between the time of controlled deterioration, electrolyte leakage, and FTIR transmission in all the species. While controlled deterioration may help in decisions relating to the storage of orthodox seeds, the water imbibition results from this study will help to set the priming time of the species. The study reaffirms electrolyte leakage as an indicator of seed viability in P. sativum and Cu. pepo; it also recommends the use of electrolyte leakage as an indicator of seed deterioration in B. speciosus, Co. erythrophyllum, and E. caffra. The study also recommends FTIR as a tool for monitoring the germination of stored seeds of all the test species.
1. Introduction
Seeds are the genetic resources by means of which most higher plants are propagated [1]. Based on their desiccation tolerance, seeds are classified into recalcitrant and orthodox seeds. While recalcitrant seeds are desiccation-sensitive and cannot be stored for a long time, orthodox seeds are tolerant of desiccation [2,3] and can be stored for a long time, especially at low water contents and temperatures [4]. Of all the plants that have been studied, about 90% produce orthodox seeds [1]. Orthodox seeds are either stored in the short or the long term. Short-term orthodox seed storage provides high-quality material for planting, re-introduction, and rehabilitation [5]. Seeds are also stored longer-term in gene banks, for distribution (active collections) and to conserve genetic resources and the diversity of orthodox-seeded species (base collections) [6,7].
Natural ageing is a key factor affecting the germination of stored orthodox seeds [1,8]. Seed ageing and eventual death are complex biological traits. They involve the interaction of metabolic, biochemical, physiological, and molecular processes. As ageing time increases, organic compounds present in seeds break down, germination and seed vigour are reduced, and ultimately the complete death of seeds occurs [8,9,10]. Although significant efforts are being made to understand the causes and mechanisms involved in seed deterioration, the causes are poorly understood. Seed deterioration may be slow under normal, ambient conditions. However, for experimental purposes, there is a need to shorten the time required for seeds to deteriorate. Hence, seed deterioration can be accelerated by subjecting seeds to predetermined and aggravated conditions of heat and humidity [11,12]. Controlled deterioration of seeds has been used in the study of Capsicum annumm [13], Phaseolus vulgaris [8], Hordeum vulgare [14], and Vigna radiata [12].
Gene banking of seeds is the ideal means of ex situ conservation of the plant genetic resources of orthodox seeds [15,16,17]. However, as indicated above, during long-term storage, even under very good conditions, orthodox seeds deteriorate. The best that can be done is to reduce the rate of deterioration during storage [17]. The deterioration of seeds during long-term storage is known as seed ageing, and, in the process, both vigour and viability are gradually compromised [1,18]. Seed deterioration and its consequence of reduction in seed ‘performance’ is of significant financial concern to the seed industry. On a global scale, such concerns relate to the long-term conservation of the genetic diversity of wild, agricultural, and horticultural plants [5]. Seed deterioration has been implicated in the loss of diversity in natural ecosystems [19]. It is a threat to global efforts to conserve the genetic resources of plants [16,20].
Seed deterioration is directly related to seed longevity. However, the rate of seed deterioration differs between and within species [12,21]. As seed deterioration progresses, seed longevity and, by extension, seed viability is compromised [12]. As ageing time increases, a point is reached where the regeneration of stored seeds becomes inevitable [16,17,22]. Seed regeneration is costly and may affect the genetic integrity of an accession. It is therefore crucial that ex situ conserved seeds must be managed in a way that ensures maximum longevity. Assessment of deterioration in stored seeds is the basic tool for managing the germplasm of ex situ conserved seeds [23].
Testing germination is currently the standard method of assessing the viability of ex situ conserved seeds [23,24]. However, the conventional germination method is destructive, labour-intensive, and time-consuming, especially when large amounts of plant germplasm present in gene banks are considered. Furthermore, the traditional germination method does not elucidate the mechanisms involved in seed deterioration. Therefore, it is important to develop new and equally reliable tools which are time-efficient, low-cost, and non-destructive in order to assess the deterioration of stored seeds and to supplement the traditional germination tests for more effective ex situ conservation of seed germplasm [23,25,26]. Among the recently developed tools used for seed deterioration assessment are electrolyte leakage, together with genomic and biochemical markers. Recent advances have shed some light on the complexities involved in seed ageing. It should be noted that most of the tools are species-dependent; hence, it is important to test the tools on a particular species to know if they are suitable. This study used both electrolyte leakage and Fourier transform infrared spectroscopy (FTIR) to assess and compare controlled deterioration in the seeds of five orthodox species.
Generally, the starting process of germination is water imbibition. Under optimal conditions, water uptake by a dry seed is divided into three phases. The first phase, known as phase I, is mainly characterised by rapid water imbibition. The rapid water intake which occurs in phase I is largely due to the matric forces exerted by the seed. In phase I, biochemical, genetic, and physiological activities, such as DNA and mitochondria repair, take place; protein synthesis also occurs using existing messenger ribonucleic acid (mRNA) [27,28]. Immediately after phase I, the seeds move to phase II, also known as the lag phase or activation phase. In phase II, only a small net gain of water takes place. However, considerable metabolic activities occur which prepare viable non-dormant seeds for radicle emergence. A major occurrence in phase II is the synthesis of mitochondria and proteins by new mRNA [29]. In the final phase, otherwise called phase III, water uptake increases coupled with radicle elongation [30,31,32].
Water uptake into the outer part of the seed cotyledons occurs because of damaged or broken testae, causing some damage to the cell membrane. Cell membrane damage results in increased electrolyte leakage from the seed embryo and ultimately leads to increased imbibitional stress and cell death [31,33]. The loss of seed quality due to water uptake is called imbibition damage and it has been described as a leading cause of loss in seed quality [31,33]. Imbibition damage reduces seed respiratory activities and germination and lowers seedling emergence and growth. The negative influence of imbibitional damage on seedling growth is due to disruption in the transfer of food from the cotyledon(s) to the growing embryos of the seed. Electrolyte leakage and seed germination are positively correlated. Therefore, the electrolyte conductivity test, which is an index of membrane stability, has been recommended for the evaluation of seed vigour, for example in Pisum sativum [34] and Cucurbita pepo [35].
These concepts of water imbibition, artificial ageing (or controlled deterioration), and electrolyte leakage are very important in seed storage, seed priming, and seed germination studies. Some studies have reported a positive correlation between seed deterioration and electrolyte leakage and have therefore recommended electrolyte leakage to measure seed deterioration in P. sativum and Cu. pepo. However, similar recommendations have not been reported for B. speciosus, Co. erythrophyllum, or E. caffra. The use of FTIR as a tool for monitoring seed deterioration is also novel. This study aimed at assessing and comparing seed deterioration in five orthodox species using electrolyte leakage and Fourier transform infrared spectroscopy (FTIR). The study also compared water imbibition by the test orthodox seeds.
2. Materials and Methods
2.1. Plant Material
The orthodox seeds from five species (Bolusanthus speciosus (Bolus) Harms, Combretum erythrophyllum (Burch.) Sond, Erythrina caffra Thumb., Pisum sativum L. (pea), and Cucurbita pepo L. (pumpkin)) were used in this study. In terms of economic usage, P. sativum and Cu. pepo are agricultural species and were purchased from a local seed company, Grovida Seeds, Durban, South Africa. The other three species can be regarded as wild or horticultural species. They were purchased from Silverhill Seeds, Cape Town, South Africa. The seeds were collected in paper bags from around the city of Cape Town, kept in airtight containers, and stored at 4 °C until use.
Initial testing indicated that all the wild species had physical dormancy due to their seed coats. While the dormancy in B. speciosus and E. caffra was broken by mechanical scarification, in Co. erythrophyllum dormancy was broken by removing the samara covering the seeds with the aid of a scalpel. Seeds sizes were examined and only seeds of similar sizes were used in the study [36]. Although both commercial and wild species were used in this study, it was not the intention of this study to generalise the results.
2.2. Determination of Seeds’ Water Imbibition
The initial water contents of the seeds were determined gravimetrically. Thereafter, ten seeds of each test species were hydrated between 20 plies of single-layer paper towels. The seeds were initially weighed at intervals of 2 h as they imbibed water. The interval was then increased to 4 h for P. sativum and Cu. pepo, E. caffra, and B. speciosus, and 12 h for Co. erythrophylum until germination started. Imbibition curves were fitted to determine the triphasic pattern of seed water imbibition.
2.3. Controlled Deterioration of Seeds
Scarification of seeds was carried out for B. speciosus, E. caffra, and Co. erythrophyllum before controlled deterioration was carried out. The water content for all the species was raised to 14% using a vapour chamber. The seeds were then sealed in airtight glass jars and kept in a digital oven (Series 2000, Scientific, Industria, South Africa) at 40 °C and 100% relative humidity. Samples (100 seeds per species) were retrieved at 4 d intervals until 36 d, and germination and electrolyte leakages were measured. The experiment was repeated twice.
2.4. Seed Germination
Germination was carried out in 90 mm Petri dishes with five layers of germination paper placed inside. Another layer of germination paper was placed on top of the seeds. The germination papers were kept moist with distilled water. As indicated above, at each sampling occasion, 100 seeds were retrieved and then divided into four replicates of 25 seeds [37]. The Petri dishes were placed on shelves in the germination room. Each replicate of 25 seeds was separated into five Petri dishes with five seeds each to minimize competition between the seedlings. The photoperiod of the germination room was 16 h light (52 µmol m−2 s−1)/8 h dark and the temperature was 25 ± 2 °C. A seed was considered germinated when 1 mm radicle protrusion was observed.
2.5. Determination of Seed Electrolyte Leakage
Electrolyte leakage (S m−1 g−1) was measured using the method of Hampton and Tekrony (1995). Three seeds (c. 1 g) were immersed in 50 mL of deionized water in glass tubes. The glass tubes were placed in a water bath for a period of 24 h at 25 °C. Thereafter, the electrolyte conductivity of the leachate was measured using a conductivity meter: CM 100-2 multi-cell conductivity meter (Reid and Associates, Durban, South Africa).
2.6. Fourier Transform Infrared Spectroscopy (FTIR) Spectra Acquisition
Forty seeds of each test species were ground to prepare seed meals. The spectra of the seed meals were acquired using a Fourier transform infrared spectrometer (Bruker Tensor 27 FTIR Spectrometer with an ATR measurement chamber). The FTIR had a spectral range of 400–10,000 cm−1 (1000–2500 nm) and a resolution of 4 cm−1. The reflectance spectra of the seeds were acquired by placing about 0.01 g of seed meal at the centre of the FTIR scanning glass window and covering it with the instrument lid. The instrument lid had a black background. Each treatment was replicated three times; each sample was scanned two times. The mean of the two scans was reported as the spectra of the sample. The FTIR provided some semi-quantitative data that were used to characterise the compounds present in the seeds at the various ageing times investigated in this study.
2.7. Statistical Analyses
Data were subjected to analyses of variance. Means of replicates were separated using 5% LSD. Post hoc testing was performed using the Tukey test. FTIR wave numbers and the corresponding transmittances were extrapolated with the aid of Sigma plot.
3. Results and Discussion
3.1. Seed Water Imbibition
When expressed as percentage water content, there were some interspecies differences noted across the imbibition times and seed water contents. Although the seeds used for this study had initial water contents of 11.8% (B. speciosus), 7.5% (Co. erythrophyllum), 11.2% (E. caffra), 11.7% (P. sativum), and 6.7% (Cu. pepo), the actual critical water contents of the various species did not vary widely: 63% (B. speciosus), 57% (Co. erythrophyllum), 60% (E. caffra), 62% (P. sativum), and 56% (Cu. Pepo) (Figure 1). In all species, uptake of water was complete between about 15 and 25 h. However, it must be noted that these rates of water uptake occurred only after scarification of the seeds of B. speciosus, E. caffra, and Co. erythrophyllum, resulting in similar rates for all species (Figure 1).
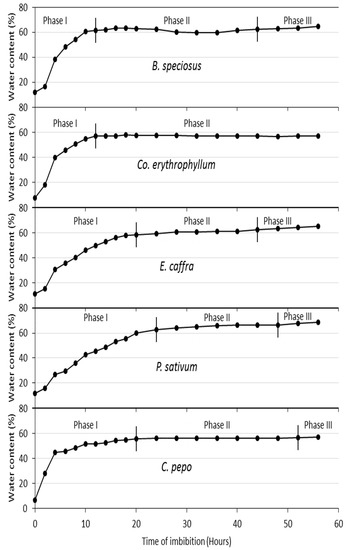
Figure 1.
Water imbibition curves for B. speciosus, P. sativum, Cu. pepo, E. caffra, and Co. erythrophyllum over 56 h. Co. erythrophyllum transited to Phase III at 248 h (data not shown).
Seed water content and time of imbibition were highly positively correlated in all the species (Figure 1). As proposed by [38], seed water intake is triphasic in nature and can be divided into phases I, II, and III. The triphasic pattern of seed water uptake observed in this study has been reported by many authors, for example, Nicotiana tabacum [39], Zea mays [40], and Annona emarginata [41]. In priming, determination of seed imbibition curves helped in setting the times of invigoration treatments, which were 18 h (B. speciosus), 20 h (Co. erythrophyllum), 20 h (E. caffra), 24 h (P. sativum), and 24 h (Cu. pepo) (Figure 2). Basically, the duration of seed invigoration treatments is defined according to the duration of phase I [32].
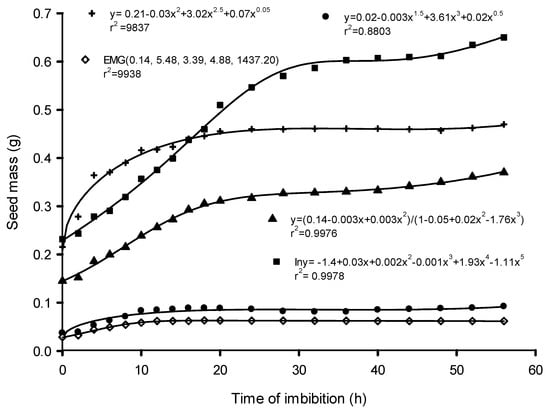
Figure 2.
Changes in the seed mass of Bolusanthus speciosus (●), Combretum erythrophyllum (◊), Erythrina caffra (▲), Pisum sativum (■), and Cucurbita pepo (+) as the species imbibed water.
The time required for the seeds to reach the critical water level (the point beyond which the seeds transit from phase I to phase II or minimum seed water content and below which germination is blocked) varied among the species, ranging from 18 h in B. speciosus and Co. erythrophyllum to 24 h in E. caffra and Cu. pepo (Figure 1). P. sativum reached the critical water level at 32 h of imbibition. The variation in the time required for the seeds to reach the critical water level may have been due to variations in the seed tissue water potential of the test species and/or the resistance of the seed coat. It has been reported that rapid water intake occurs in seeds because of the huge difference between seed tissue water potential and the ambient water potential of pure water [42]. These huge differences between dry seed tissue water potential and the ambient water potential (in the case of pure water) result in rapid influxes of water during phase I of water imbibition (Figure 1).
Phase II is the lag period (Figure 1). During this phase, there was little or no uptake of water, resulting in only a small change in fresh seed mass and a slight decline in mass in some instances (Figure 2). The slight loss of seed mass may have been due to the loss of mucilage surrounding the seeds. Water always flows from an area of higher to lower water potential and the net flow stops when the difference in water potential becomes zero—an indication of the beginning of phase II of the water imbibition process. While dry seed tissues have been reported to have a water potential (Ψ) of between −350 and −50 MPa, pure water has a water potential of zero. Uptake of water by seeds is a physical process and results in seed mass increase (Figure 2). Although not investigated in this study, as water imbibition increases, a series of physiological, metabolic, and biochemical processes are triggered [42]. The activation of different metabolic processes with water uptake by seeds may lead to an increase in respiration and the beginning of sugar consumption [42]. The sugar serves as a substrate for seed embryo respiration. Sugars such as sucrose and glucose are carbon sources for the production of metabolites, including amino acids, lipids, proteins, and complex carbohydrates such as starch and cellulose [30,43]. All these lead to initial radicle extension due to reversible (“elastic”) growth driven by osmotic water uptake [32]. The small amount of water uptake by seeds in phase II may also be due to changes in osmotic potential resulting from reserve degradation [30,32].
There were wide variations in the duration of phase II among the species, with Co. erythrophyllum having the longest duration of 230 h (data not shown). The durations of phase II for the other species were 22 h (B. speciosus), 8 h (E. caffra), 12 h (P. sativum), and 8 h (Cu. pepo) (Figure 1). The wide variations in the duration of phase II among the species may have been due to differences in the activation and duration of the metabolic activities required for seed germination. Although there is little or no increase in water uptake in phase II, this phase is associated with considerable levels of metabolic activity [32,44]. Stored reserves in the endosperm, such as proteins, fats, and lipids, are converted into compounds such as sugars needed for germination [44,45]. The tissues surrounding the embryos are weakened by enzymes that are activated by gibberellin [46]. Similarly, smaller compounds needed to supply the required energy for embryo growth are released because of seed reserve degradation in the endosperm [44,45]. All these biochemical and physiological changes facilitate the protrusion of the radicle [46], which indicates the start of phase III of the imbibition process.
Phase III, in which radicle protrusion takes place, is characterised by the resumption of water uptake by the germinating seed (Figure 1). The higher water intake in this phase may be associated with activities such as respiration and mitochondrial functions of the embryo [30,42]. Further growth of the embryo after the completion of seed germination requires cell wall loosening that allows phase III water uptake. Suitable water potentials and temperatures are of utmost importance at this stage of the germination process. The growth potential of the embryo and the constraining force of the endosperm and testa layers determine the completion of germination.
3.2. Controlled Deterioration of Seeds and Electrolyte Leakage
There were marked differences in the response of the test agricultural species and the wild species to controlled deterioration (CD). In the agricultural species, a gradual decline in germination started at 8 d for P. sativum, with a 5% loss in germination (Figure 3). In Cu. pepo, germination loss began later, with a 7% loss in germination after 12 d of controlled deterioration (Figure 3). The relationships in all the species between ageing time and seed germination were represented by polynomial equations (Figure 3). The wild species showed greater sensitivity to controlled deterioration, and germination loss began much earlier than in the agricultural species, with loss beginning at 4 d for E. caffra, B. speciosus, and Co. erythrophyllum. As the ageing time increased, gradual but continuous decline in germination continued in all the species. The critical periods for rapid decrease in percentage of germination (in d) were determined to be 0.5 for B. speciosus, 3 for Co. erythrophyllum, 1 for E. caffra, 10 for P. sativum, and 14 d for Cu. pepo (Figure 3).
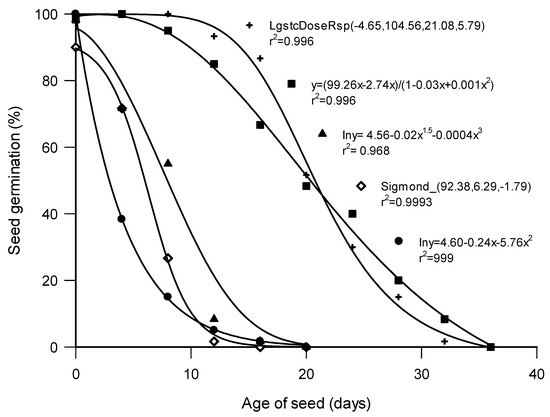
Figure 3.
Loss of germination in Bolusanthus speciosus (●), Combretum erythrophyllum (◊), Erythrina caffra (▲), Pisum sativum (■), and Cucurbita pepo (+) subjected to controlled deterioration at 40 °C and 100% relative humidity.
Consistent with their greater sensitivity to controlled deterioration, total inhibition of seed germinability occurred much earlier in the wild species, at 20 d in B. speciosus and E. caffra, and 16 d in Co. erythrophyllum. In the agricultural species, complete loss of germination occurred at 36 d for both P. sativum and Cu. pepo. Unlike the agricultural species, which had their seed coats unbroken, the greater sensitivity of the wild species may have been due to the scarification that took place before the controlled deterioration treatment. Factors such as seed coat permeability, physical damage, and seed dormancy have been reported to predispose seeds to deterioration [47]. For example, [48] demonstrated the role of testa damage in seed quality by scarifying some pea seeds and using unscarified seeds as controls. The scarified seeds, which imbibed water rapidly, showed increased dead tissue and higher electrolyte leakage (which are indications of imbibition damage) and emerged poorly in the field. Other crops for which similar reports exist include grain legumes and some other species, such as Phaseolus vulgaris [33], Glycine max [49], Pisum sativum [50], Arabidopsis thaliana [51], Senna multijuga [52], and Triticum aestivum [31]. Other factors that predispose seeds to deterioration are genetic factors, seed size, and seed maturity [47,52,53]. Hence, the differences observed germination/tolerance to CD among species in this study might be attributable to some of these factors.
In all species, germination was negatively correlated with the time of controlled deterioration (Figure 3). It seems likely that a major cause of seed deterioration is free radical attack. Production of reactive oxygen species—the hydroxyl radical, in particular—is a major cause of peroxidation of the unsaturated fatty acids of cell membranes [54], leading to membrane damage and electrolyte leakage [54,55]. Other biochemical changes during seed deterioration leading to a loss in germination include chromosomal aberrations and damage to DNA, changes in the synthesis of RNA and proteins, changes in enzymes, differences in respiratory activity caused by ATP production, and membrane alteration [12,14,53]. Seeds are protected against free radicals by an array of protective enzymes, such as superoxide dismutase, catalase, glutathione peroxidase, and non-enzymatic compounds (glutathione, ascorbic acid, tocopherol) and other antioxidants that react with free radicals [21,53]. Despite this protection, seed deterioration is cumulative; as seed CD increases, seed performance is compromised [55]. In each of the species investigated, the point at which decline in germination started may have been the point at which the seeds’ endogenous antioxidants became insufficient to protect the seeds. It has been reported that when the strength of antioxidant protection becomes insufficient, oxidation occurs, resulting in free radical accumulation and the subsequent deterioration of cells and seeds [25].
In general, there were steeper increases in electrolyte leakage in the early stages of controlled deterioration in the wild species when compared with the agricultural species (Figure 4). These were followed by smaller increases as ageing time increased. In the agricultural species, increases in electrolyte leakage in the early stages of CD were small when compared with the wild species. Electrolyte leakage was highly positively correlated (r2 = 0.956) with days of deterioration (Figure 4). However, a negative correlation occurred between electrolyte leakage and seed germination in all wild and agricultural species (Figure 4). The relatively higher leakage at the early stages of CD in the wild species may explain the earlier loss of germination in these species. In the agricultural species, increases in electrolyte leakage in the early stages of CD were small when compared with the wild species. Many authors have implicated cell membrane damage in the leakage of electrolytes from seeds because the cell membrane is the first part of the cells to interact with the environment [25,55]. In the case of the wild species, physical damage was done to the seed coat while breaking dormancy. Lipid peroxidation of cell membranes, which occurs during the CD of seeds, is species-dependent; the differences in the rates of lipid peroxidation between the species may have been responsible for the differences in electrolyte leakage and, consequently, the differences in the rates and ages of germination loss [25,55].
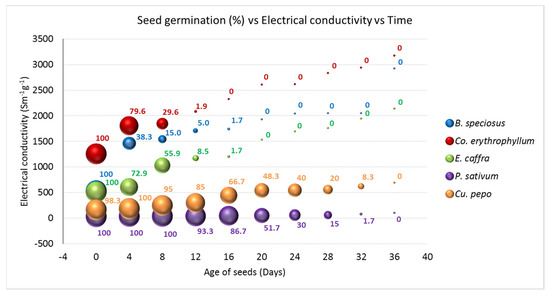
Figure 4.
Germination and electrolyte leakage in Pisum sativum, Cucurbita pepo, Erythrina caffra, Bolusanthus speciosus, and Combretum erythrophyllum subjected to controlled deterioration at 40 °C and 100% relative humidity for 36 d. Samples were taken at an interval of 4 d. The samples were subjected to both germination and electrolyte conductivity measurements.
3.3. Fourier Transform Infrared Spectroscopy (FTIR)
There was a positive correlation between the transmittance and the age of seeds in both the agricultural (Figure 5) and the wild species (Figure 6). Compared to the controls, there were significant reductions in transmittance at 20 d of controlled deterioration in all the test species. This is an indication of loss of seed integrity. As the ageing time increased from 20 to 32 d, the transmittance increased significantly in both P. sativum and Cu. pepo; however, in B. speciosus and E. caffra the changes were benign. This is an indication that further exposure to CD beyond 20 d was no longer significantly affecting the organic compounds present in the seeds of B. speciosus and E. caffra. This observation is consistent with the results of the germination and electrolyte leakage tests conducted; at 20 d of ageing, there was complete loss of germination in the wild species (Figure 3 and Figure 4).
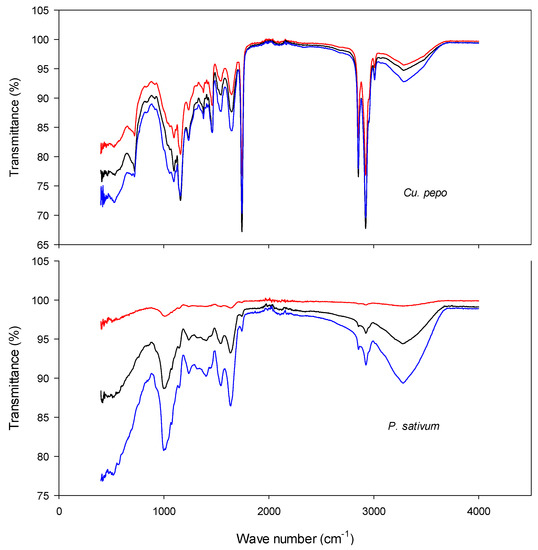
Figure 5.
FTIR of Cucurbita pepo and Pisum sativum at 0 (blue), 20 (black), and 32 (red) d of controlled deterioration. Seeds were subjected to controlled deterioration at 40 °C and 100% relative humidity. Samples were taken at an interval of 4 d intervals, and the samples were subjected to both germination and electrolyte conductivity measurements.
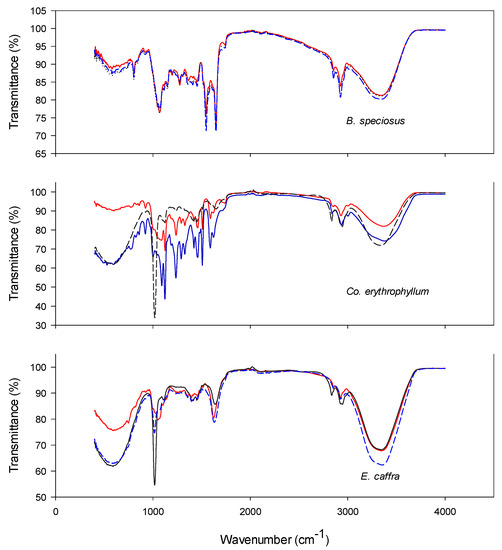
Figure 6.
FTIR of Bolusanthus speciosus, Combretum erythrophyllum, and Erythrina caffra at 0 (blue), 20 (black), and 32 (red) d of controlled deterioration. Seeds were subjected to controlled deterioration at 40 °C and 100% relative humidity. Samples were taken at intervals of 4 d and the samples were subjected to both germination and electrolyte conductivity measurements.
The numbers of peaks present in the spectra vary according to species, ranging from 6 in E. caffra to 11 in Cu. pepo. The numbers for the other species were as follows: P. sativum, 8; B. speciosus, 9; and Co. erythrophyllum, 10. Table 1 and Table 2 present the classes of compounds identified for each species. Generally, for all test species, it was the amines, alkanes and halo compounds that were degraded as ageing time increased (Table 1 and Table 2). Other identified compound classes which were present in one or more of the test species included anhydrides, sulphates, isothiocyanates, carboxylic acids, alcohols, alkynes, phenols, α,β-unsaturated esters, and nitro and aromatic compounds (Table 1 and Table 2). For most of these compounds, transmittance was negatively correlated with the time of seed ageing. The decline in the transmittance of these compounds indicates that deterioration progressively reduced their concentrations. The most likely explanation is that deterioration increases the production of ROS, such as the superoxide anion, hydrogen peroxide, hydroxyl radicals, and singlet oxygen. These ROS can attack the organic molecules present in seeds, particularly polyunsaturated fatty acids [4]. Seed deterioration involves structural, cytological, physiological, biochemical, and physical changes, such as lipid peroxidation, membrane disruption, DNA damage, and impairment of RNA and protein synthesis. ROS attacks on the polyunsaturated fatty acids in cell membranes increase permeability, increasing solute leakage. Additionally, ageing-induced ROS can attack other biomolecules such as enzymes, altering their structure and reducing activity [4,56]. Furthermore, reductions in enzyme activity will reduce respiration, which will in turn reduce energy (ATP) production. Taken together, these effects will reduce germination and result in weaker seedling growth [4,56].

Table 1.
FTIR table including the compounds present in Pisum sativum and Cucurbita pepo at 0 (control), 20, and 32 d of controlled deterioration.

Table 2.
FTIR table including the compounds present in Bolusanthus speciosus, Combretum erythrophyllum, and Erythrina caffra at 0 (control), 20, and 32 d of controlled deterioration.
Interestingly, after 21 and 32 d of controlled deterioration, transmittance peaks appeared that were not observed for the controls (i.e., seeds that had not been aged; Figure 5 and Figure 6). The peaks may have appeared because of the breakdown of large biomolecules or compounds into smaller degradation products. Peroxidation resulting from ROS attack during seed deterioration can lead to the formation of reactive smaller molecules. These molecules can readily diffuse through cell membranes, thereby increasing electrolyte leakage. The smaller biomolecules may also cause intermolecular cross-linking between carbohydrates, proteins, and nucleic acids. These molecules can further degrade into advanced glycation end-products [17]. These biochemical changes will reduce seed quality and, hence, germination is compromised.
Potentially harmful volatile compounds are present in seeds and have been implicated in seed deterioration. For example, aldehydes have been reported to non-enzymatically attack proteins and DNA [57]. The concentrations of these compounds can serve as biochemical markers of seed ageing. However, while there were significant differences in the peaks of some spectra, others either overlapped or showed no differences from the controls (Table 1 and Table 2), indicating that not all compound concentrations change during ageing. Considering that the seeds were aged at 100% RH, it was perhaps surprising that larger changes were not observed. Nevertheless, the results presented here support the findings of some earlier studies that clear differences can occur in the chemical compositions of viable and non-viable seeds [58]. In other words, FTIR can enable workers to establish a clear “signature of ageing” in seeds that can be used to monitor deterioration.
In conclusion, this study was able to show that in orthodox seeds significant correlations exist between the patterns of water imbibition, seed deterioration, electrolyte leakage, and FTIR spectra. This study reaffirms electrolyte leakage as an indicator of seed viability in P. sativum [34] and Cu. Pepo [35]. Furthermore, this study recommends electrolyte leakage as an indicator of seed quality in B. speciosus, Co. erythrophyllum, and E. caffra; to the best of our knowledge, these species have not previously been studied in this regard. The imbibition curves may assist interested farmers, seed scientists, and workers in gene banks in setting imbibition times while invigorating debilitated seeds of the test species. The study also recommends FTIR as a tool for monitoring the deterioration of ex situ stored seeds of all the test species.
Author Contributions
Conceptualization, B.V.; methodology, K.F. and B.V.; supervision, R.P.B.; investigation, K.F.; formal analysis, K.F.; writing—original draft, K.F.; writing—review and editing, R.P.B.; funding acquisition, B.V.; resources, B.V.; validation, B.V. All authors have read and agreed to the published version of the manuscript.
Funding
A National Research Foundation of South Africa grant to NW Pammenter (grant number CPRR13092145823, entitled “Cathodic quenching of oxidative stress in explants processed for cryostorage and in vitro procedures”) and was partially funded by the University of KwaZulu-Natal, South Africa. Richard Beckett acknowledges that the work presented here was partly supported by the Kazan Federal University Strategic Academic Leadership Program (PRIORITY-2030).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Volk, G.M.; Henk, A.D.; Forsline, P.L.; Szewc-McFadden, A.K.; Fazio, G.; Aldwinckle, H.; Richards, C.M. Seeds capture the diversity of genetic resource collections of Malus sieversii maintained in an orchard. Genet. Resour. Crop Evol. 2017, 64, 1513–1528. [Google Scholar] [CrossRef]
- Athugala, Y.S.; Jayasuriya, K.G.; Gunaratne, A.M.T.A.; Baskin, C.C. Desiccation tolerance and sensitivity of selected tropical montane species in Sri Lanka. Seed Sci. Res. 2021, 31, 98–104. [Google Scholar] [CrossRef]
- Engelmann, F. Use of biotechnologies for the conservation of plant biodiversity. Vitr. Cell. Dev. Biol.-Plant 2011, 47, 5–16. [Google Scholar] [CrossRef]
- McDonald, M.B. Orthodox seed deterioration and its repair. In Handbook of Seed Physiology: Applications to Agriculture; Benech-Arnold, R.L., Sanchez, R.A., Eds.; Food Products Press: New York, NY, USA, 2004; pp. 273–304. ISBN 9781560229292. [Google Scholar]
- De Vitis, M.; Hay, F.R.; Dickie, J.B.; Trivedi, C.; Choi, J.; Fiegener, R. Seed storage: Maintaining seed viability and vigor for restoration use. Restor. Ecol. 2020, 28, S249–S255. [Google Scholar] [CrossRef]
- Singh, R.J.; Jauhar, P.P. Genetic Resources, Chromosome Engineering, and Crop Improvement. Grain Legumes. Vol. 1; CRC Press: Boca Raton, FL, USA, 2005; ISBN 0-8493-1430-5. [Google Scholar]
- Solberg, S.Ø.; Yndgaard, F.; Andreasen, C. Long-Term Storage and Longevity of Orthodox Seeds: A Systematic review. Front. Plant Sci. 2020, 11, 1007. [Google Scholar] [CrossRef]
- Amanpour-Balaneji, B.; Sedghi, M. Effect of aging and priming on physiological and biochemical traits of common bean (Phaseolus vulgaris L.). Not. Sci. Biol. 2012, 4, 95–100. [Google Scholar] [CrossRef]
- Mohammadi, H.; Soltani, A.; Sadeghipour, H.R.; Zeinali, E. Effects of seed aging on subsequent seed reserve utilization and seedling growth in soybean. Int. J. Plant Prod. 2012, 5, 65–70. [Google Scholar]
- Rajjou, L.; Lovigny, Y.; Groot, S.P.; Belghazi, M.; Job, C.; Job, D. Proteome-wide characterization of seed aging in Arabidopsis: A comparison between artificial and natural aging protocols. Plant Physiol. 2008, 148, 620–641. [Google Scholar] [CrossRef]
- Ghahfarokhi, M.; Ghasemi, E.; Saeidi, M.; Kazafi, Z. The Effect of accelerated ageing on germination characteristics, seed reserve utilization and malondialdehyde content of two wheat cultivars. J. Stress Physiol. Biochem. 2014, 10, 15–23. [Google Scholar]
- Sharma, S.N.; Maheshwari, A.; Sharma, C.; Shukla, N. Gene expression patterns regulating the seed metabolism in relation to deterioration/ageing of primed mung bean (Vigna radiata L.) seeds. Plant Physiol. Biochem. 2018, 124, 40–49. [Google Scholar] [CrossRef]
- Kaewnaree, P.; Vichitphan, S.; Klanrit, P.; Siri, B.; Vichitphan, K. Effect of accelerated ageing process on seed quality and biochemical changes in sweet pepper (Capsicum annuum Linn.) seeds. Biotechnology 2011, 10, 175–182. [Google Scholar] [CrossRef]
- Nagel, M.; Kodde, J.; Pistrick, S.; Mascher, M.; Börner, A.; Groot, S.P. Barley seed aging: Genetics behind the dry elevated pressure of oxygen aging and moist controlled deterioration. Front. Plant Sci. 2016, 7, 388. [Google Scholar] [CrossRef] [PubMed]
- Berjak, P.; Villiers, T.A. Ageing in plant embryos II. Age-induced damage and its repair during early germination. New Phytol. 1972, 71, 135–144. [Google Scholar] [CrossRef]
- Chacko, X.S. Creative practices of care: The subjectivity, agency, and affective labor of preparing seeds for long-term banking. Cult. Agric. Food Environ. 2019, 41, 97–106. [Google Scholar] [CrossRef]
- Walters, C.; Ballesteros, D.; Vertucci, V.A. Structural mechanics of seed deterioration: Standing the test of time. Plant Sci. 2010, 179, 565–573. [Google Scholar] [CrossRef]
- Garza-Caligaris, L.; Avendaño-Vázquez, A.; Alvarado-López, S.; Zúñiga-Sánchez, E.; Orozco-Segovia, A.; Pérez-Ruíz, R.; Gamboa-Debuen, A. At3g08030 transcript: A molecular marker of seed ageing. Ann. Bot. 2012, 110, 1253–1260. [Google Scholar] [CrossRef]
- Hortal, J.; Santos, A. Rethinking extinctions that arise from habitat loss. Nature 2020, 584, 194–196. [Google Scholar] [CrossRef]
- Frankel, O.H.; Bennett, E. Genetic Resources in Plants-Their Exploration and Conservation; Blackwell Scientific Publications: Oxford, UK, 1970; ISBN 0632057300. [Google Scholar]
- Santos, H.O.D.; Carvalho, M.L.M.D.; Caldeira, C.M.; Coelho, S.V.B.; Pinho, E.V.D.R.V.; Oliveira, J.A. Physiological and biochemical aspects of castor beans seeds deterioration stored in different packaging conditions and temperatures. J. Seed Sci. 2016, 38, 241–247. [Google Scholar] [CrossRef]
- Van Treuren, R.; de Groot, E.C.; van Hintum, T.J. Preservation of seed viability during 25 years of storage under standard genebank conditions. Genet. Resour. Crop Evol. 2013, 60, 1407–1421. [Google Scholar] [CrossRef]
- Engels, J.M.M.; Visser, L. Genebank management procedures. In A Guide to Effective Management of Germplasm Collections; International Plant Genetic Resources Institute: Rome, Italy, 2003; Volume 6, pp. 60–79. ISBN 92-9043-582-8. [Google Scholar]
- Fatokun, K.; Beckett, R.P.; Naidoo, S.; Cloete, J.; Pammenter, N.W. Influence of cathodic water invigoration on the emergence and subsequent growth of controlled deteriorated pea and pumpkin seeds. Plants 2020, 9, 955. [Google Scholar] [CrossRef]
- Colville, L.; Bradley, E.L.; Lloyd, A.S.; Pritchard, H.W.; Castle, L.; Kranner, I. Volatile fingerprints of seeds of four species indicate the involvement of alcoholic fermentation, lipid peroxidation, and Maillard reactions in seed deterioration during ageing and desiccation stress. J. Exp. Bot. 2012, 63, 6519–6530. [Google Scholar] [CrossRef] [PubMed]
- Donà, M.; Balestrazzi, A.; Mondoni, A.; Rossi, G.; Ventura, L.; Buttafava, A.; Carbonera, D. DNA profiling, telomere analysis and antioxidant properties as tools for monitoring ex situ seed longevity. Ann. Bot. 2013, 111, 987–998. [Google Scholar] [CrossRef] [PubMed]
- Balestrazzi, A.; Confalonieri, M.; Macovei, A.; Carbonera, D. Seed imbibition in Medicago truncatula Gaertn.: Expression profiles of DNA repair genes in relation to PEG-mediated stress. J. Plant Physiol. 2011, 168, 706–713. [Google Scholar] [CrossRef] [PubMed]
- Ventura, L.; Donà, M.; Macovei, A.; Carbonera, D.; Buttafava, A.; Mondoni, A.; Balestrazzi, A. Understanding the molecular pathways associated with seed vigor. Plant Physiol. Biochem. 2012, 60, 196–206. [Google Scholar] [CrossRef] [PubMed]
- Sano, N.; Ono, H.; Murata, K.; Yamada, T.; Hirasawa, T.; Kanekatsu, M. Accumulation of long-lived mRNAs associated with germination in embryos during seed development of rice. J. Exp. Bot. 2015, 66, 4035–4046. [Google Scholar] [CrossRef]
- da Silva Moura, M.L.; Chagas, E.A.; Smiderle, O.J.; Vilaça, R.; Chagas, P.C.; de Moura, E.A.; Farias, E.E. Biometric characterization, water absorption curve and vigor on araçá-boi seeds. Int. J. Plant Biol. 2016, 7, 1. [Google Scholar] [CrossRef]
- Lev, J.; Blahovec, J. Effect of I2/KI water solution to wheat seeds imbibition assessed by image analysis. Agron. Res. 2018, 16, 492–499. [Google Scholar] [CrossRef]
- Varier, A.; Vari, A.K.; Dadlani, M. The subcellular basis of seed priming. Curr. Sci. 2010, 99, 450–456. [Google Scholar]
- Powell, A.A.; Oliveira, M.D.A.; Matthews, S. The role of imbibition damage in determining the vigour of white and coloured seed lots of dwarf French beans (Phaseolus vulgaris). J. Exp. Bot. 1986, 37, 716–722. [Google Scholar] [CrossRef]
- Panobianco, M.V.; Daiton, R.; Dilermando, P. Electrical conductivity as an indicator of pea seed aging of stored at different temperatures. Sci. Agric. 2007, 64, 119–124. [Google Scholar] [CrossRef]
- Vieira, R.D.; Dutra, A.S. Condutividade elétrica em sementes de abóbora, híbrido Bárbara. Hortic. Bras. 2006, 24, 305–308. [Google Scholar] [CrossRef][Green Version]
- Saha, D.; Mandal, A.K. Seed invigoration treatments in different seed sizes of sunflower (Helianthus annuus L.) for maintenance of vigour, viability and yield potential. Indian J. Agric. Res. 2016, 50, 22–26. [Google Scholar] [CrossRef]
- Hampton, J.G.; TeKrony, D.M. Handbook of Vigour Test Methods; The International Seed Testing Association: Zurich, Switzerland, 1995. [Google Scholar]
- Bewley, J.D.; Black, M. Seeds Physiology of Development and Germination, 3rd ed.; Plenum Press: New York, NY, USA, 1994. [Google Scholar]
- Manz, B.; Müller, K.; Kucera, B.; Volke, F.; Leubner-Metzger, G. Water uptake and distribution in germinating tobacco seeds investigated in vivo by nuclear magnetic resonance imaging. Plant Physiol. 2005, 138, 1538–1551. [Google Scholar] [CrossRef] [PubMed]
- Mei, Y.Q.; Song, S.Q. Early morphological and physiological events occurring during germination of maize seeds. Agric. Sci. China 2008, 7, 950–957. [Google Scholar] [CrossRef]
- Gimenez, J.I.; Ferreira, G.; Corsato, J.M. Soluble sugars and germination of Annona emarginata (Schltdl.) H. Rainer seeds submitted to immersion in GA3 up to different water contents. Rev. Bras. Frutic. 2014, 36, 281–287. [Google Scholar] [CrossRef]
- Tonini, P.P.; Purgatto, E.; Buckeridge, M.S. Effects of abscisic acid, ethylene and sugars on the mobilization of storage proteins and carbohydrates in seeds of the tropical tree Sesbania virgata (Leguminosae). Ann. Bot. 2010, 106, 607–616. [Google Scholar] [CrossRef]
- Min, C.W.; Lee, S.H.; Cheon, Y.E.; Han, W.Y.; Ko, J.M.; Kang, H.W.; Kim, Y.C.; Agrawal, G.K.; Rakwal, R.; Gupta, R.; et al. In-depth proteomic analysis of Glycine max seeds during controlled deterioration treatment reveals a shift in seed metabolism. J. Proteome 2017, 169, 125–135. [Google Scholar] [CrossRef]
- Reis, R.C.R.; Dantas, B.F.; Castro, R.D.D.; Antunes, C.G.C.; Silva, F.F.S.D.; Pelacani, C.R. Reserve mobilization during imbibition of stored Gliricidia sepium (Jacq.) Steud. (Leguminosae-Papilionoideae) seeds. Rev. Bras. Sementes 2011, 33, 549–560. [Google Scholar] [CrossRef]
- Rajjou, L.; Duval, M.; Gallardo, K.; Catusse, J.; Bally, J.; Job, C.; Job, D. Seed germination and vigor. Annu. Rev. Plant Biol. 2012, 63, 507–533. [Google Scholar] [CrossRef]
- Ogawa, M.; Hanada, A.; Yamauchi, Y.; Kuwahara, A.; Kamiya, Y.; Yamaguchi, S. Gibberellin biosynthesis and response during Arabidopsis seed germination. Plant Cell 2003, 15, 1591–1604. [Google Scholar] [CrossRef]
- Baskin, C.C.; Baskin, J.M. Seeds: Ecology, Biogeography, and Evolution of Dormancy and Germination, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2014; ISBN 978-0-12-416677-6. [Google Scholar]
- Powell, A.A.; Matthews, S. The significance of damage during imbibition to the field emergence of pea (Pisum sativum L.) seeds. J. Agric. Sci. 1980, 95, 35–38. [Google Scholar] [CrossRef]
- Blackman, S.; Obendorf, R.; Leopold, A. Desiccation tolerance in developing soybean seeds: The role of stress proteins. Physiol. Plant. 1995, 93, 630–638. [Google Scholar] [CrossRef]
- Wang, W.Q.; Cheng, H.Y.; Møller, I.M.; Song, S.Q. The role of recovery of mitochondrial structure and function in desiccation tolerance of pea seeds. Physiol. Plantarum 2012, 144, 20–34. [Google Scholar] [CrossRef] [PubMed]
- Maia, J.; Dekkers, B.J.; Dolle, M.J.; Ligterink, W.; Hilhorst, H.W. Abscisic acid (ABA) sensitivity regulates desiccation tolerance in germinated Arabidopsis seeds. New Phytol. 2014, 203, 81–93. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues-Junior, A.G.; Faria, J.M.R.; Vaz, T.A.A.; José, A.C. Loss of desiccation tolerance and storage behavior in germinating seeds of Senna multijuga: Implications for seed germination and conservation. New Forests 2015, 46, 283–291. [Google Scholar] [CrossRef]
- Boniecka, J.; Kotowicz, K.; Skrzypek, E.; Dziurka, K.; Rewers, M.; Jedrzejczyk, I.; Dąbrowska, G.B. Potential biochemical, genetic and molecular markers of deterioration advancement in seeds of oilseed rape (Brassica napus L.). Ind. Crop. Prod. 2019, 130, 478–490. [Google Scholar] [CrossRef]
- Ratajczak, E.; Małecka, A.; Bagniewska-Zadworna, A.; Kalemba, E.M. The production, localization and spreading of reactive oxygen species contributes to the low vitality of long-term stored common beech (Fagus sylvatica L.) seeds. J. Plant Physiol. 2015, 174, 147–156. [Google Scholar] [CrossRef]
- Lazar, S.L.; Mira, S.; Pamfil, D.; Martinez-Laborde, J.B. Germination and electrical conductivity tests on artificially aged seed lots of 2 wall-rocket species. Turk. J. Agric. For. 2014, 38, 857–864. [Google Scholar] [CrossRef]
- Lehner, A.; Mamadou, N.; Poels, P.; Come, D.; Bailly, C.; Corbineau, F. Changes in soluble carbohydrates, lipid peroxidation and antioxidant enzyme activities in the embryo during ageing in wheat grains. J. Cereal Sci. 2008, 47, 555–565. [Google Scholar] [CrossRef]
- Taylor, S.J.; Morken, J.P. Catalytic diastereoselective reductive aldol reaction: Optimization of interdependent reaction variables by arrayed catalyst evaluation. J. Am. Chem. Soc. 1999, 121, 12202–12203. [Google Scholar] [CrossRef]
- Mukasa, Y.; Kyamanywa, S.; Serumaga, J.P.; Otim, M.; Tumuhaise, V.; Erbaugh, M.; Egonyu, J.P. An atoxigenic L-strain of Aspergillus flavus (Eurotiales: Trichocomaceae) is pathogenic to the coffee twig borer, Xylosandrus compactus (Coleoptera: Curculionidea: Scolytinae). Environ. Microbiol. Rep. 2019, 11, 508–517. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).