Characterizing Root Morphological Traits in 65 Genotypes of Foxtail Millet (Setaria italica L.) from Four Different Ecological Regions in China
Abstract
1. Introduction
2. Materials and Methods
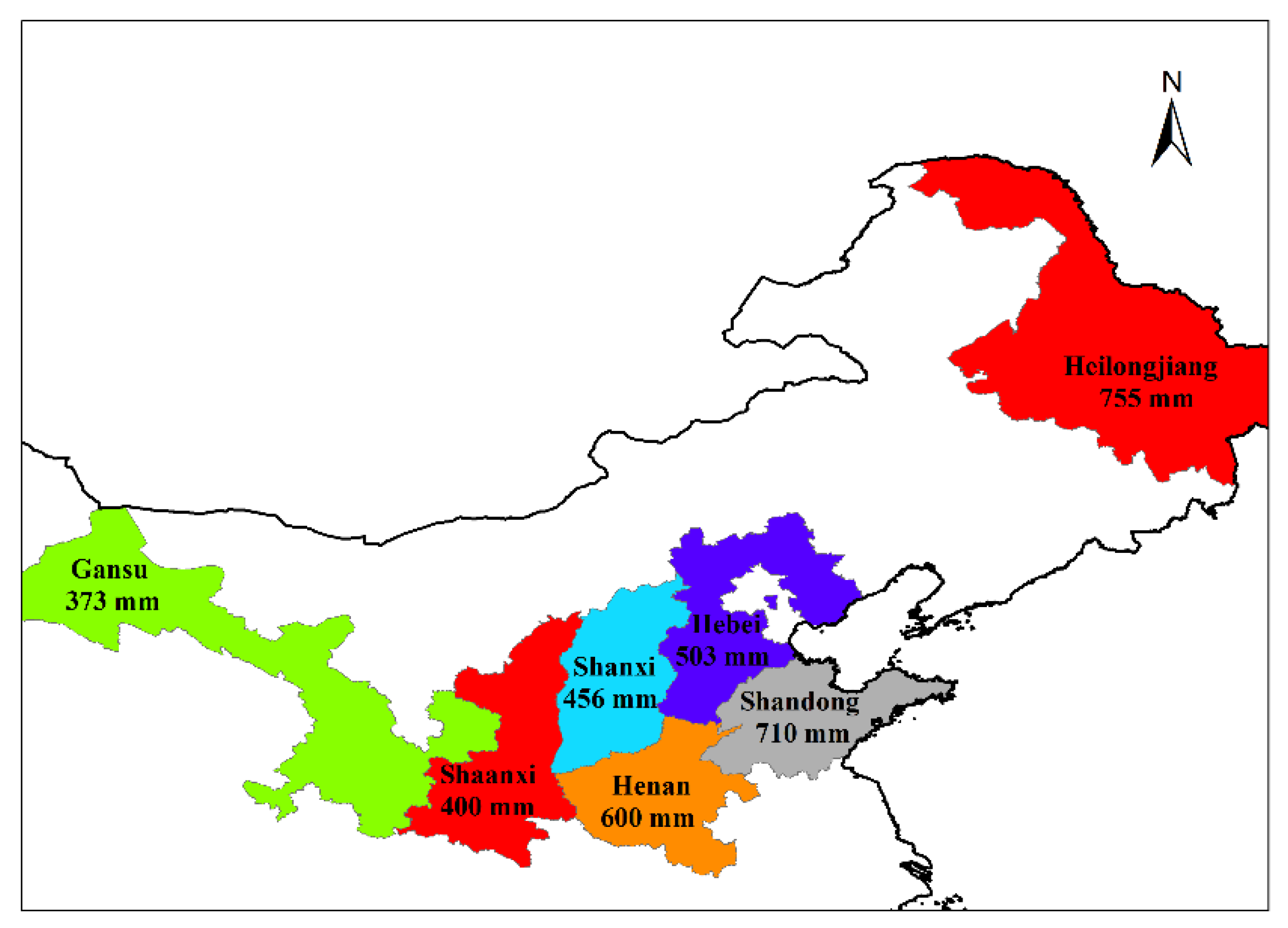
2.1. Plant Materials
2.2. Plant Culture
2.3. Root Trait Measurements and Calculations
2.4. Statistical Analysis
3. Results
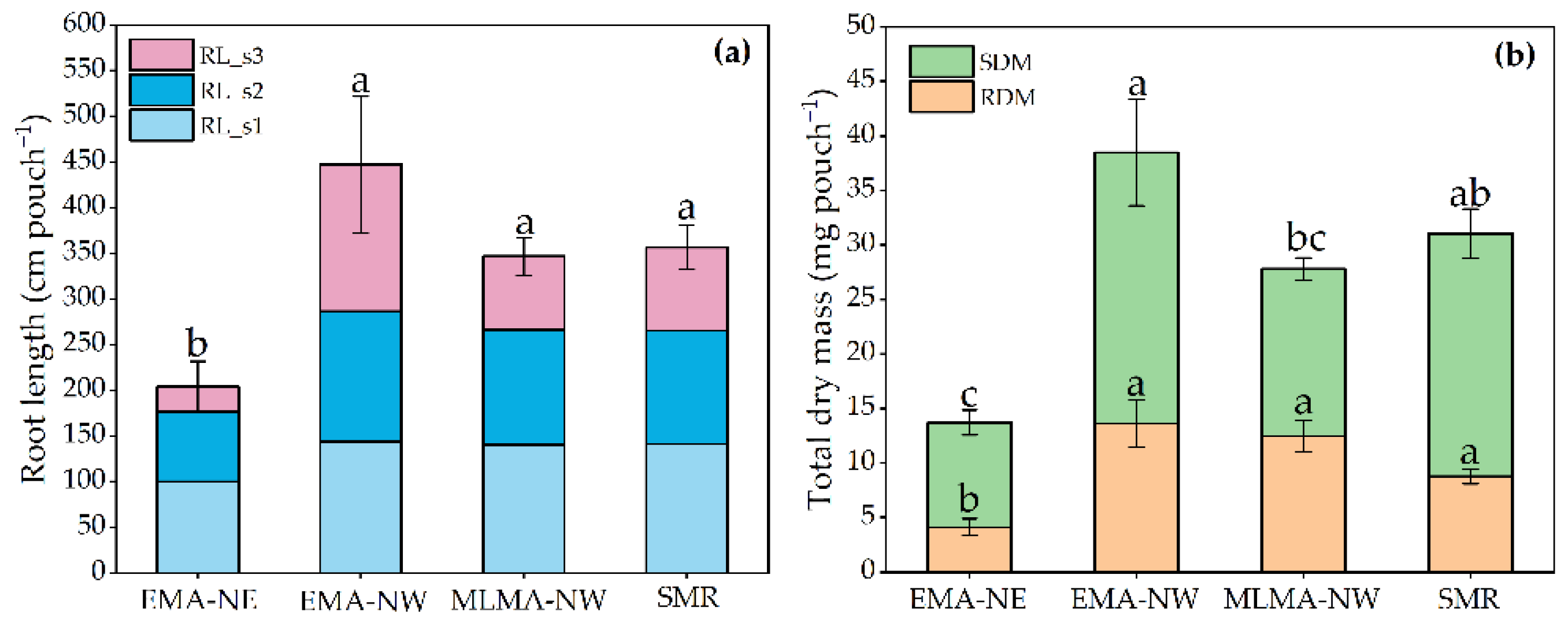
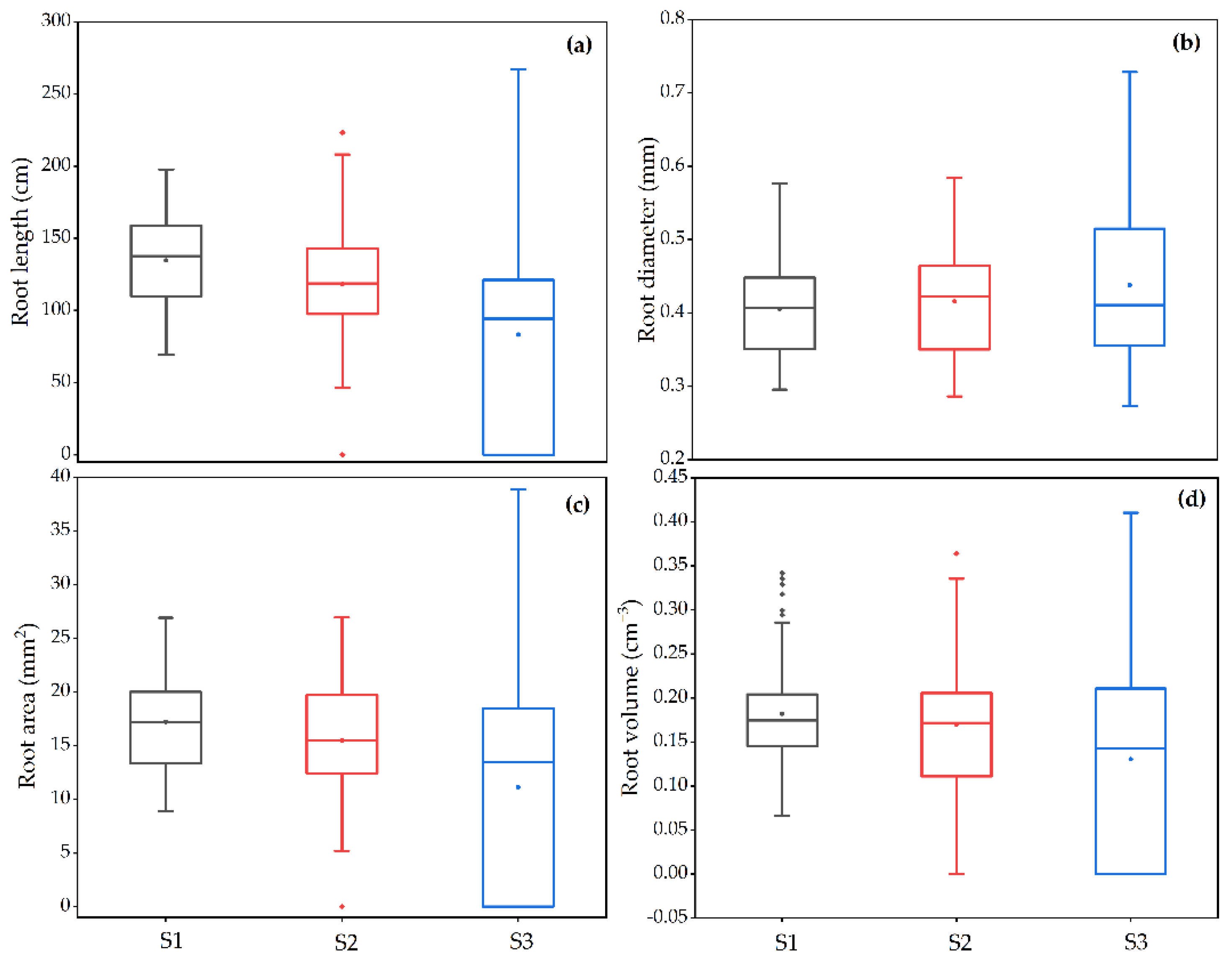
3.1. Variation of Root Phenotypic Characteristics at Seedling Stage
3.1.1. Global Traits
3.1.2. Local Traits
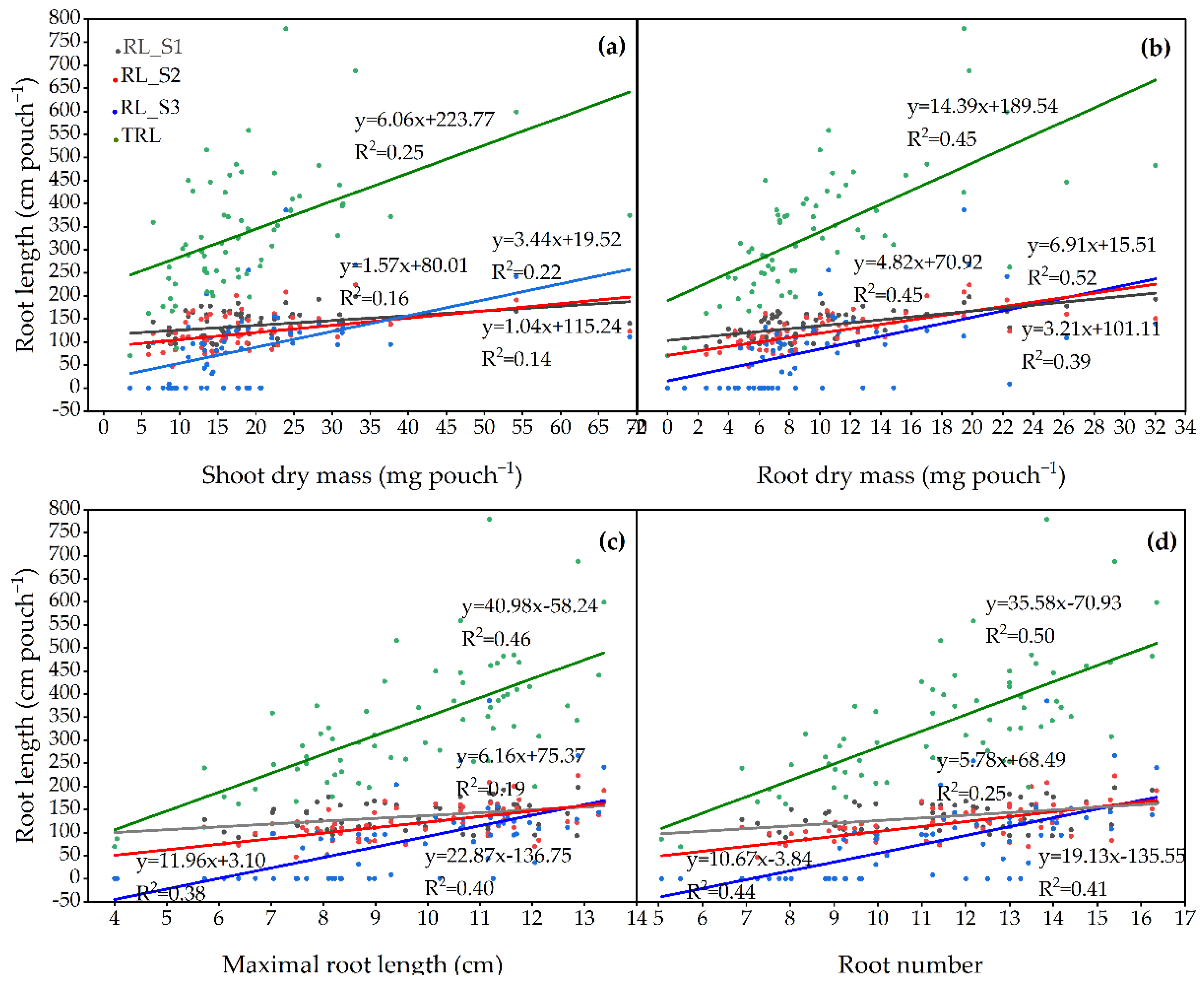
3.2. General Variation and Correlation among Traits
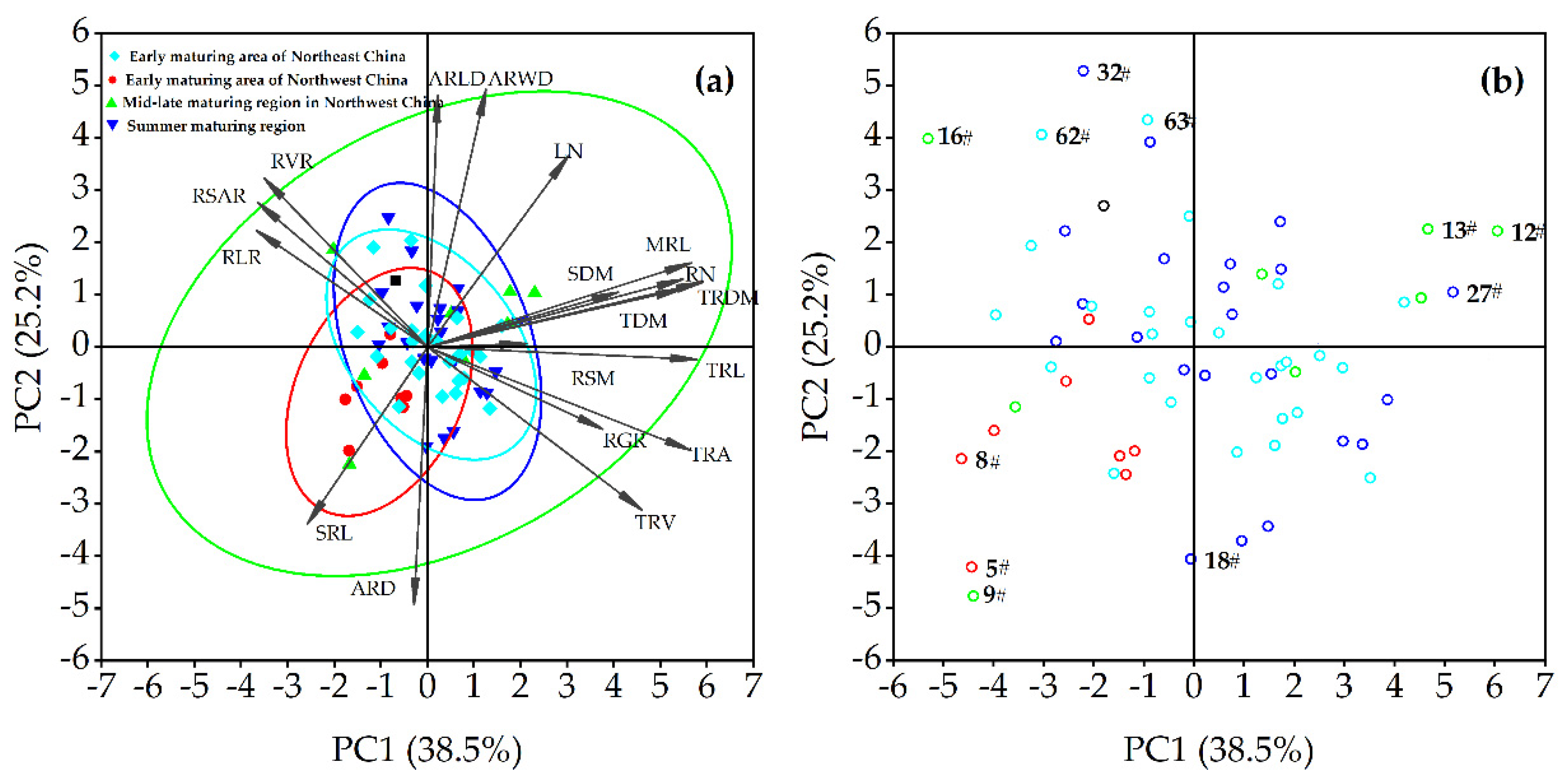
3.3. Principal Component Analysis of Selected Traits
3.4. Genotypic Population Identification Based on the Selected Traits
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Puccio, G.; Ingraffia, R.; Giambalvo, D.; Amato, G.; Frenda, A.S. Morphological and physiological root traits and their relationship with nitrogen uptake in wheat varieties released from 1915 to 2013. Agronomy 2021, 11, 1149. [Google Scholar] [CrossRef]
- Liu, M.; Qiao, Z.; Zhang, S.; Wang, Y.; Lu, P. Response of broomcorn millet (Panicum miliaceum L.) genotypes from semiarid regions of China to salt stress. Crop. J. 2015, 3, 57–66. [Google Scholar] [CrossRef]
- Zhang, M.; Yang, L.; Hao, R.; Bai, X.; Wang, Y.; Yu, X. Drought-tolerant plant growth-promoting rhizobacteria isolated from jujube (Ziziphus jujuba) and their potential to enhance drought tolerance. Plant Soil 2020, 452, 423–440. [Google Scholar] [CrossRef]
- Das, R.R.; Vinayan, M.T.; Seetharam, K.; Patel, M.; Phagna, R.K.; Singh, S.B.; Shahi, J.P.; Sarma, A.; Barua, N.S.; Babu, R.; et al. Genetic gains with genomic versus phenotypic selection for drought and waterlogging tolerance in tropical maize (Zea mays L.). Crop. J. 2021, 9, 1438–1448. [Google Scholar] [CrossRef]
- Yan, J.; Liu, P. Does complex soil enhance grain yield under cropping system? Agronomy 2021, 11, 1502. [Google Scholar] [CrossRef]
- Saenz Rodriguez, M.N.; Cassab, G.I. Primary root and mesocotyl elongation in maize seedlings: Two organs with antagonistic growth below the soil surface. Plants 2021, 10, 1274. [Google Scholar] [CrossRef] [PubMed]
- Al Azzawi, T.N.I.; Khan, M.; Hussain, A.; Shahid, M.; Imran, Q.M.; Mun, B.G.; Lee, S.U.; Yun, B.W. Evaluation of Iraqi rice cultivars for their tolerance to drought stress. Agronomy 2020, 10, 1782. [Google Scholar] [CrossRef]
- Burridge, J.D.; Rangarajan, H.; Lynch, J.P. Comparative phenomics of annual grain legume root architecture. Crop. Sci. 2020, 60, 2574–2593. [Google Scholar] [CrossRef]
- Ramirez-Villegas, J.; Molero Milan, A.; Alexandrov, N.; Asseng, S.; Challinor, A.J.; Crossa, J.; van Eeuwijk, F.; Ghanem, M.E.; Grenier, C.; Heinemann, A.B.; et al. CGIAR modeling approaches for resource-constrained scenarios: I. Accelerating crop breeding for a changing climate. Crop. Sci. 2020, 60, 547–567. [Google Scholar] [CrossRef]
- Zhang, W.; Zhi, H.; Tang, S.; Zhang, H.; Sui, Y.; Jia, G.; Wu, C.; Diao, X. Identification of no pollen 1 provides a candidate gene for heterosis utilization in foxtail millet (Setaria italica L.). Crop. J. 2021, 9, 1309–1319. [Google Scholar] [CrossRef]
- Yang, Y.; Jia, G.; Deng, L.; Qin, L.; Chen, E.; Cong, X.; Zou, R.; Wang, H.; Zhang, H.; Liu, B.; et al. Genetic variation of yellow pigment and its components in foxtail millet (Setaria italica (L.) P. Beauv.) from different eco-regions in China. J. Integr. Agric. 2017, 16, 2459–2469. [Google Scholar] [CrossRef]
- Krishnamurthy, L.; Upadhyaya, H.D.; Kashiwagi, J.; Purushothaman, R.; Dwivedi, S.L.; Vadez, V. Variation in drought tolerance components and their interrelationships in the core collection of foxtail millet (Setaria italica) germplasm. Crop. Pasture Sci. 2016, 67, 834–846. [Google Scholar] [CrossRef]
- Niu, X.; Song, L.; Xiao, Y.; Ge, W. Drought tolerant plant growth promoting rhizobacteria associated with foxtail millet in a semi-arid agroecosystem and their potential in alleviating drought stress. Front. Microbiol. 2018, 8, 2580. [Google Scholar] [CrossRef] [PubMed]
- Upadhyaya, H.D.; Ramesh, S.; Sharma, S.; Singh, S.K.; Varshney, S.K.; Sarma, N.D.R.K.; Ravishankar, C.R.; Narasimhudu, Y.; Reddy, V.G.; Sahrawat, K.L.; et al. Genetic diversity for grain nutrients contents in a core collection of finger millet (Eleusine coracana (L.) Gaertn.) germplasm. Field Crops Res. 2011, 121, 42–52. [Google Scholar] [CrossRef]
- Zhang, M.; Mo, Z.; Liao, J.; Pan, S.; Chen, X.; Zheng, L.; Luo, X.; Wang, Z. Lodging resistance related to root traits for mechanized wet-seeding of two super rice cultivars. Rice Sci. 2021, 28, 200–208. [Google Scholar]
- Wan, X.; Chen, X.; Huang, Z.; Han, Y.; Chen, H. Contribution of root traits to variations in soil microbial biomass and community composition. Plant Soil 2021, 460, 483–495. [Google Scholar] [CrossRef]
- Sun, J.; Meyer, W.; Cross, J.; Huang, B.R. Growth and physiological traits of canopy and root systems associated with drought resistance in tall fescue. Crop. Sci. 2013, 53, 575–584. [Google Scholar] [CrossRef]
- Nadeem, F.; Ahmad, Z.; Ul Hassan, M.; Wang, R.F.; Diao, X.M.; Li, X.X. Adaptation of foxtail millet (Setaria italica L.) to abiotic stresses: A special perspective of responses to nitrogen and phosphate limitations. Front. Plant Sci. 2020, 11, 187. [Google Scholar] [CrossRef]
- Jahanzad, E.; Sadeghpour, A.; Hoseini, M.B.; Barker, A.V.; Hashemi, M.; Keshavarz Afshar, R. Competition, nitrogen use efficiency, and productivity of millet–soybean intercropping in semiarid conditions. Crop. Sci. 2015, 55, 2842–2851. [Google Scholar] [CrossRef]
- Moussa, A.A.; Mandozai, A.; Jin, Y.; Qu, J.; Zhang, Q.; Zhao, H.; Anwari, G.; Khalifa, M.A.S.; Lamboro, A.; Noman, M.; et al. Genome wide association screening and verification of potential genes associated with root architectural traits in maize (Zea mays L.) at multiple seedling stages. BMC Genom. 2021, 22, 558. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.; Wang, J.; Wang, W.; Zhang, T.; Li, J. Effects of root phenotypic changes on the deep rooting of populus euphratica seedlings under drought stresses. Peer J. 2019, 7, e6513. [Google Scholar] [CrossRef] [PubMed]
- Kengkanna, J.; Jakaew, P.; Amawan, S.; Busener, N.; Bucksch, A.; Saengwilai, P. Phenotypic variation of cassava root traits and their responses to drought. Appl. Plant Sci. 2019, 7, e01238. [Google Scholar] [CrossRef] [PubMed]
- Azab, O.; Al-Doss, A.; Alshahrani, T.; El-Hendawy, S.; Zakri, A.M.; Abd-ElGawad, A.M. Root system architecture plasticity of bread wheat in response to oxidative burst under extended osmotic stress. Plants 2021, 10, 939. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Xiong, X.; Cheng, W. Root characteristics of spring wheat under drip irrigation and their relationship with aboveground biomass and yield. Sci. Rep. 2021, 11, 4913. [Google Scholar] [CrossRef] [PubMed]
- Nakhforoosh, A.; Nagel, K.A.; Fiorani, F.; Bodner, G. Deep soil exploration vs. topsoil exploitation: Distinctive rooting strategies between wheat landraces and wild relatives. Plant Soil 2021, 459, 397–421. [Google Scholar] [CrossRef]
- Chen, Y.; Palta, J.; Prasad, P.V.V.; Siddique, K.H.M. Phenotypic variability in bread wheat root systems at the early vegetative stage. BMC Plant Biol. 2020, 20, 185. [Google Scholar] [CrossRef]
- Jia, Z.; Liu, Y.; Gruber, B.D.; Neumann, K.; Kilian, B.; Graner, A.; Wiren, N. Genetic dissection of root system architectural traits in spring barley. Front. Plant Sci. 2019, 10, 400. [Google Scholar] [CrossRef]
- Zohreh; Siadat, S.A.; Abdolmehdi, B.; Abdollah; Masoud, H. Interactive effects of drought stress and chitosan application on physiological characteristics and essential oil yield of Thymus daenensis celak. Crop. J. 2017, 5, 407–415. [Google Scholar]
- Strock, C.F.; Burridge, J.; Massas, A.S.F.; Beaver, J.; Beebe, S.; Camilo, S.A.; Fourie, D.; Jochua, C.; Miguel, M.; Miklas, P.N.; et al. Seedling root architecture and its relationship with seed yield across diverse environments in Phaseolus vulgaris. Field Crops Res. 2019, 237, 53–64. [Google Scholar] [CrossRef]
- Jochua, C.; Strock, C.; Lynch, J. Root phenotypic diversity in common bean (Phaseolus vulgaris L.) reveals contrasting strategies for soil resource acquisition among gene pools and races. Crop. Sci. 2020, 60, 3261–3277. [Google Scholar] [CrossRef]
- Pan, J.; Li, Z.; Wang, Q.; Guan, Y.; Li, X.; Huangfu, Y.; Meng, F.; Li, J.; Dai, S.; Liu, W. Phosphoproteomic profiling reveals early salt responsive mechanisms in two foxtail millet cultivars. Front. Plant Sci. 2021, 12, 1934. [Google Scholar] [CrossRef] [PubMed]
- Falk, K.G.; Jubery, T.Z.; O’Rourke, J.A.; Singh, A.; Sarkar, S.; Ganapathysubramanian, B.; Singh, A.K. Soybean root system architecture trait study through genotypic, phenotypic, and shape based clusters. Plant Phen. 2020, 2020, 1925495. [Google Scholar] [CrossRef] [PubMed]
- Fraudentali, I.; Rodrigues-Pousada, R.A.; Volpini, A.; Tavladoraki, P.; Angelini, R.; Cona, A. Stress triggered long distance communication leads to phenotypic plasticity: The case of the early root protoxylem maturation induced by leaf wounding in arabidopsis. Plants 2018, 7, 107. [Google Scholar] [CrossRef]
- Fang, C.; Zhang, H.; Wan, J.; Wu, Y.; Li, K.; Jin, C.; Chen, W.; Wang, S.; Wang, W.; Zhang, H.; et al. Control of leaf senescence by an MeOH-Jasmonates cascade that is epigenetically regulated by OsSRT1 in rice. Mol. Plant 2016, 9, 1366–1378. [Google Scholar] [CrossRef]
- Danakumara, T.; Kumari, J.; Singh, A.K.; Sinha, S.K.; Pradhan, A.K.; Sharma, S.; Jha, S.K.; Bansal, R.; Kumar, S.; Jha, G.K.; et al. Genetic dissection of seedling root system architectural traits in a diverse panel of hexaploid wheat through multi -locus genome wide association mapping for improving drought tolerance. Int. J. Mol. Sci. 2021, 22, 7188. [Google Scholar] [CrossRef] [PubMed]
- Ndour, P.M.S.; Barry, C.M.; Tine, D.; De la Fuente Cantó, C.; Gueye, M.; Barakat, M.; Ortet, P.; Achouak, W.; Ndoye, I.; Sine, B.; et al. Pearl millet genotype impacts microbial diversity and enzymatic activities in relation to root adhering soil aggregation. Plant Soil 2021, 464, 109–129. [Google Scholar] [CrossRef]
- Grzesiak, M.T.; Hordyńska, N.; Maksymowicz, A.; Grzesiak, S.; Szechyńska-Hebda, M. Variation among spring wheat (Triticum aestivum L.) genotypes in response to the drought stress. II—root system structure. Plants 2019, 8, 584. [Google Scholar] [CrossRef]
- Bhargava, S.; Sawant, K.; Tuberosa, R. Drought stress adaptation: Metabolic adjustment and regulation of gene expression. Plant Breed. 2013, 132, 21–32. [Google Scholar] [CrossRef]
- Li, C.; Li, L.; Reynolds, M.P.; Wang, J.; Chang, X.; Mao, X.; Jing, R. Recognizing the hidden half in wheat: Root system attributes associated with drought tolerance. J. Exp. Bot. 2021, 72, 5117–5133. [Google Scholar] [CrossRef]
- Fan, Y.; Miguez-Macho, G.; Jobbagy, E.G.; Jackson, R.B.; Otero-Casal, C. Hydrologic regulation of plant rooting depth. Proc. Natl. Acad. Sci. USA 2017, 114, 10572–10577. [Google Scholar] [CrossRef]
- Chen, L.; Phillips, A.L.; Condon, A.G.; Parry, M.A.; Hu, Y.G. GA-responsive dwarfing gene Rht12 affects the developmental and agronomic traits in common bread wheat. PLoS ONE 2013, 8, e62285. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.L.; Dunbabin, V.M.; Diggle, A.J.; Siddique, K.H.M.; Rengel, Z. Assessing variability in root traits of wild Lupinus angustifolius germplasm: Basis for modelling root system structure. Plant Soil 2012, 354, 141–155. [Google Scholar] [CrossRef]
- Watt, M.; Moosavi, S.; Cunningham, S.C.; Kirkegaard, J.A.; Rebetzke, G.J.; Richards, R.A. A rapid, controlled environment seedling root screen for wheat correlates well with rooting depths at vegetative, but not reproductive, stages at two field sites. Ann. Bot. 2013, 112, 447–455. [Google Scholar] [CrossRef]
- Wang, J.; Chen, Y.; Zhang, Y.; Zhang, Y.; Ai, Y.; Feng, Y.; Moody, D.; Diggle, A.; Damon, P.; Rengel, Z. Phenotyping and validation of root morphological traits in barley (Hordeum vulgare L.). Agronomy 2021, 11, 1583. [Google Scholar] [CrossRef]
- Chen, Y.; Ghanem, M.E.; Siddique, K.H. Characterising root trait variability in chickpea (Cicer arietinum L.) germplasm. J. Exp. Bot. 2017, 68, 1987–1999. [Google Scholar]
- Koevoets, I.T.; Venema, J.H.; Elzenga, J.T.M.; Testerink, C. Roots withstanding their environment: Exploiting root system architecture responses to abiotic stress to improve crop tolerance. Front. Plant Sci. 2016, 7, 1335. [Google Scholar] [CrossRef] [PubMed]
- Levin, M.; Resnick, N.; Rosianskey, Y.; Kolotilin, I.; Wininger, S.; Lemcoff, J.H.; Cohen, S.; Galili, G.; Koltai, H.; Kapulnik, Y. Transcriptional profiling of Arabidopsis thaliana plants’ response to low relative humidity suggests a shoot–root communication. Plant Sci. 2009, 177, 450–459. [Google Scholar] [CrossRef]
- Khan, M.R.; Imran, K.; Zahra, I.; Jens, L.; Naz, A.A. Drought responsive genes expressed predominantly in root tissues are enriched with homotypic cis regulatory clusters in promoters of major cereal crops. Crop. J. 2017, 5, 195–206. [Google Scholar] [CrossRef]
- Aidoo, M.K.; Bdolach, E.; Fait, A.; Lazarovitch, N.; Rachmilevitch, S. Tolerance to high soil temperature in foxtail millet (Setaria italica L.) is related to shoot and root growth and metabolism. Plant Physiol. Biochem. 2016, 106, 73–81. [Google Scholar] [CrossRef]
- York, L.M.; Galindo-Castaneda, T.; Schussler, J.R.; Lynch, J.P. Evolution of US maize (Zea mays L.) root architectural and anatomical phenes over the past 100 years corresponds to increased tolerance of nitrogen stress. J. Exp. Bot. 2015, 66, 2347–2358. [Google Scholar] [CrossRef]
- Talabi, A.O.; Badu-Apraku, B.; Fakorede, M.A.B. Genetic variances and relationship among traits of an early maturing maize population under drought stress and low nitrogen environments. Crop. Sci. 2017, 57, 681–692. [Google Scholar] [CrossRef]
- Galkovskyi, T.; Mileyko, Y.; Bucksch, A.; Moore, B.; Symonova, O.; Charles, A.P.; Topp, C.N.; Anjali, S.I.-P.; Zurek, P.R.; Fang, S.Q.; et al. GiA Roots: Software for the high throughput analysis of plant root system architecture. Bio. Med. Central 2012, 12, 116. [Google Scholar] [CrossRef]
- Chen, D.; Wang, S.; Cao, B.; Cao, D.; Leng, G.; Li, H.; Yin, L.; Shan, L.; Deng, X. Genotypic variation in growth and physiological response to drought stress and re-watering reveals the critical role of recovery in drought adaptation in maize seedlings. Front. Plant Sci. 2016, 6, 1241. [Google Scholar] [CrossRef]
- Mbinda, W.; Masaki, H. Breeding strategies and challenges in the improvement of blast disease resistance in finger millet. Front. Plant Sci. 2021, 11, 2151. [Google Scholar] [CrossRef] [PubMed]
- Stanik, N.; Lampei, C.; Rosenthal, G. Drought stress triggers differential survival and functional trait responses in the establishment of arnica montana seedlings. Plant Biol. 2021, 23, 1086–1096. [Google Scholar] [CrossRef] [PubMed]
- Passot, S.; Gnacko, F.; Moukouanga, D.; Lucas, M.; Guyomarc’h, S.; Ortega, B.M.; Atkinson, J.A.; Belko, M.N.; Bennett, M.J.; Gantet, P.; et al. Characterization of pearl millet root architecture and anatomy reveals three types of lateral roots. Front. Plant Sci. 2016, 7, 829. [Google Scholar] [CrossRef]
- Fujii, K.; Makita, N.; Kamara, M.; Küttim, M.; Sugita, S. Plasticity of pine tree roots to podzolization of boreal sandy soils. Plant Soil 2021, 464, 209–222. [Google Scholar] [CrossRef]
- Jonathan, P.L.; Tobias, W. Opportunities and challenges in the subsoil: Pathways to deeper rooted crops. J. Exp. Bot. 2015, 66, 2199–2210. [Google Scholar]
- Halder, T.; Liu, H.; Chen, Y.; Yan, G.; Siddique, K.H.M. Identification of candidate genes for root traits using genotype phenotype association analysis of near isogenic lines in hexaploid wheat (Triticum aestivum L.). Int. J. Mol. Sci. 2021, 22, 3579. [Google Scholar] [CrossRef]
- Zhang, B.; Liu, X.; Guo, Y.; Jia, X.; Yang, D.; Zhao, Y.; Dai, L.; Kou, S.; Zhang, X.; Hou, D.; et al. Investigation on agronomic characters of dwarf mutant 778 in broomcorn millet (Panicum miliaceum L.) and analysis of its sensitivity to GA. Biocatal. Agric. Biotechnol. 2020, 9, 7–11. [Google Scholar]
- Awad, W.; Byrne, P.F.; Reid, S.D.; Comas, L.H.; Haley, S.D. Great plains winter wheat varies for root length and diameter under drought stress. Agron J. 2018, 110, 226–235. [Google Scholar] [CrossRef]
- Lopes, M.S.; Reynolds, M.P. Partitioning of assimilates to deeper roots is associated with cooler canopies and increased yield under drought in wheat. Funct. Plant Biol. 2010, 37, 147–156. [Google Scholar] [CrossRef]
- Halli, H.M.; Angadi, S.; Kumar, A.; Govindasamy, P.; Madar, R.; El-Ansary, D.O.; Rashwan, M.A.; Abdelmohsen, S.A.M.; Abdelbacki, A.M.M.; Mahmoud, E.A.; et al. Influence of planting and irrigation levels as physical methods on maize root morphological traits, grain yield and water productivity in semi-arid region. Agronomy 2021, 11, 294. [Google Scholar] [CrossRef]
- Wasson, A.P.; Richards, R.A.; Chatrath, R.; Misra, S.C.; Prasad, S.V.S.; Rebetzke, G.J.; Watt, M. Traits and selection strategies to improve root systems and water uptake in water-limited wheat crops. J. Exp. Bot. 2012, 63, 3485–3498. [Google Scholar] [CrossRef]
- Wang, H.; Sun, H.; Xia, H.; Wu, T.; Li, P.; Xu, C.; Yang, Z. Natural variation and domestication selection of ZmCKX5 with root morphological traits at the seedling stage in maize. Plants 2020, 10, 1. [Google Scholar] [CrossRef]
- Huot, C.; Zhou, Y.; Philp, J.N.M.; Denton, M.D. Root depth development in tropical perennial forage grasses is related to root angle, root diameter and leaf area. Plant Soil 2020, 456, 145–158. [Google Scholar] [CrossRef]
- Voss-Fels, K.P.; Qian, L.; Parra-Londono, S.; Uptmoor, R.; Frisch, M.; Keeble-Gagnere, G.; Appels, R.; Snowdon, R.J. Linkage drag constrains the roots of modern wheat. Plant Cell Environ. 2017, 40, 717–725. [Google Scholar] [CrossRef]
- Lynch, J.P. Steep, cheap and deep: An ideotype to optimize water and N acquisition by maize root systems. Ann. Bot. 2013, 112, 347–357. [Google Scholar] [CrossRef]
- Garbowski, M.; Johnston, D.B.; Brown, C.S. Leaf and root traits, but not relationships among traits, vary with ontogeny in seedlings. Plant Soil 2021, 460, 247–261. [Google Scholar] [CrossRef]
- Paula, S.; Pausas, J.G. Root traits explain different foraging strategies between resprouting life histories. Oecologia 2011, 165, 321–331. [Google Scholar] [CrossRef][Green Version]
- Ostonen, I.; Puttsepp, U.; Biel, C.; Alberton, O.; Bakker, M.R.; Lohmus, K.; Majdi, H.; Metcalfe, D.; Olsthoorn, A.F.M.; Pronk, A.; et al. Specific root length as an indicator of environmental change. Plant Biosyst. 2007, 141, 426–442. [Google Scholar] [CrossRef]
- Atta, B.M.; Tariq, M.; Trethowan, R.M. Relationship between root morphology and grain yield of wheat in north-western NSW, Australia. Aust. J. Crop. Sci. 2013, 7, 2108–2115. [Google Scholar]
- Paola, E.C.; Claudia, K.; Michael, A.K.; Feike, A.D. Variation in specific root length among 23 wheat genotypes affects leaf δ13C and yield. Agric. Ecosyst Environ. 2017, 246, 21–29. [Google Scholar]
- Schneider, H.M.; Yang, J.T.; Brown, K.M.; Lynch, J.P. Nodal root diameter and node number in maize (Zea mays L.) interact to influence plant growth under nitrogen stress. Plant Direct 2021, 5, e00310. [Google Scholar] [CrossRef] [PubMed]
- Faye, A.; Sine, B.; Chopart, J.L.; Grondin, A.; Lucas, M.; Diedhiou, A.G.; Gantet, P.; Cournac, L.; Min, D.; Audebert, A.; et al. Development of a model estimating root length density from root impacts on a soil profile in pearl millet (Pennisetum glaucum (L.) R. Br). application to measure root system response to water stress in field conditions. PLoS ONE 2019, 14, e0214182. [Google Scholar] [CrossRef] [PubMed]
- Zegada-Lizarazu, W.; Iijima, M. Deep root water uptake ability and water use efficiency of pearl millet in comparison to other millet species. Plant Prod. Sci. 2015, 8, 454–460. [Google Scholar] [CrossRef]
- Chen, X.; Li, Y.; He, R.; Ding, Q. Phenotyping field state wheat root system architecture for root foraging traits in response to environment x management interactions. Sci. Rep. 2018, 8, 2642. [Google Scholar] [CrossRef]
- Polania, J.; Rao, I.M.; Cajiao, C.; Grajales, M.; Rivera, M.; Velasquez, F.; Raatz, B.; Beebe, S.E. Shoot and root traits contribute to drought resistance in recombinant inbred lines of MD 23–24 × SEA 5 of common bean. Front. Plant Sci. 2017, 8, 296. [Google Scholar] [CrossRef]



| Traits | Abbreviation | Description | Unit |
|---|---|---|---|
| Global traits | |||
| Root width | RW | Maximum distribution of roots in the horizontal direction | Cm |
| Maximal root depth | MRD | Maximum depth of the root system in the vertical direction | Cm |
| Maximal root length | MRL | Maximum length of the seminal or primary root | Cm |
| Root number | RN | Seminal and primary root number | Number |
| Root dry mass | RDM | Total root dry mass | Mg |
| Root length | RL | Total root length | Cm |
| Root diameter | RD | Average root diameter | Mm |
| Root area | RA | Total root surface area | mm2 |
| Root volume | RV | Total root volume | cm3 |
| Root length density | RLD | Average root length density | cm cm−3 |
| Root weight density | RWD | Average root t weight density | mg cm−3 |
| Specific root length | SRL | Total root length per unit root dry mass | cm mg−1 |
| Root to shoot dry mass ratio | RSM | Total root dry mass divided by the shoot dry mass | |
| Root growth rate | RGR | Average daily root growth (based on the longest seminal or primary root growth at 15 days after seed sowing) | cm d−1 |
| Root length ratio | RLR | Root length in Section 1 (top-root layer) over sub-root layer | |
| Root surface area ratio | RSAR | Root surface area in Section 1 (top-root layer) over sub-root layer | |
| Root volume ratio | RVR | Root volume in Section 1 (top-root layer) over sub-root layer | |
| Leaf number | LN | Total leaf number of each plant | |
| Shoot dry mass | SDM | Dry weight of the above-ground part | Mg |
| Total dry mass | TDM | Total dry mass (sum of root and shoot dry mass) | Mg |
| Local traits | |||
| Root dry mass s1 | RDM_s1 | Total root dry mass in Section 1 | Mg |
| Root dry mass s2 | RDM_s2 | Total root dry mass in Section 2 | Mg |
| Root dry mass s3 | RDM_s3 | Total root dry mass in Section 3 | Mg |
| Root dry mass in sub-root layer | RDM_sub | Combined root dry mass in sub-root layer (s2 and s3) | Mg |
| Root length s1 | RL_s1 | Total root length in Section 1 | Cm |
| Root length s2 | RL_s2 | Total root length in Section 2 | Cm |
| Root length s3 | RL_s3 | Total root length in Section 3 | Cm |
| Root length in sub-root layer | RL_sub | Combined root length in sub-root layer (s2 and s3) | Cm |
| Root diameter s1 | RD_s1 | Average root diameter in Section 1 | Mm |
| Root diameter s2 | RD_s2 | Average root diameter in Section 2 | Mm |
| Root diameter s3 | RD_s3 | Average root diameter in Section 3 | Mm |
| Root diameter in sub-root layer | RD_sub | Average root diameter in sub-root layer (s2 and s3) | Mm |
| Root area s1 | RA_s1 | Total root surface area in Section 1 | mm2 |
| Root area s2 | RA_s2 | Total root surface area in Section 2 | mm2 |
| Root area s3 | RA_s3 | Total root surface area in Section 3 | mm2 |
| Root area in sub-root layer | RA_sub | Combined root surface area in sub-root layer (s2 and s3) | mm2 |
| Root volume s1 | RV_s1 | Total root volume in Section 1 | cm3 |
| Root volume s2 | RV_s2 | Total root volume in Section 2 | cm3 |
| Root volume s3 | RV_s3 | Total root volume in Section 3 | cm3 |
| Root volume in sub-root layer | RV_sub | Combined root volume in sub-root layer (s2 and s3) | cm3 |
| Root length density s1 | RLD_s1 | Average root length density in Section 1 | cm cm−3 |
| Root length density s2 | RLD_s2 | Average root length density in Section 2 | cm cm−3 |
| Root length density s3 | RLD_s3 | Average root length density in Section 3 | cm cm−3 |
| Root length density in sub-root layer | RLD_sub | Combined root length density in sub-root layer (s2 and s3) | cm cm−3 |
| Root weight density s1 | RWD_s1 | Average root t weight density in Section 1 | mg cm−3 |
| Root weight density s2 | RWD_s2 | Average root t weight density in Section 2 | mg cm−3 |
| Root weight density s3 | RWD_s3 | Average root t weight density in Section 3 | mg cm−3 |
| Root weight density in sub-root layer | RWD_sub | Combined root weight density in sub-root layer (s2 and s3) | mg cm−3 |
| Traits | Minimum | Maximum | Median | Mean | SD | CV | p-Value |
|---|---|---|---|---|---|---|---|
| Global traits | |||||||
| RW | 1.5 | 14.5 | 8.50 | 7.36 | 3.65 | 0.50 | 0.000 |
| MRD | 0.6 | 11.1 | 3.15 | 4.70 | 3.07 | 0.65 | 0.000 |
| MRL | 4.0 | 13.4 | 9.83 | 9.61 | 2.16 | 0.22 | 0000 |
| RN | 5.1 | 16.4 | 11.8 | 11.43 | 2.60 | 0.23 | 0.000 |
| RDM | 0.10 | 32.0 | 7.85 | 9.83 | 6.01 | 0.61 | 0.000 |
| RL | 69.4 | 779 | 325 | 338.08 | 137.80 | 0.41 | 0.000 |
| RD | 0.25 | 0.66 | 0.42 | 0.42 | 0.09 | 0.21 | 0.000 |
| RA | 12.6 | 84.75 | 42.63 | 43.91 | 17.69 | 0.40 | 0.000 |
| RV | 0.10 | 1.12 | 0.44 | 0.48 | 0.23 | 0.48 | 0.000 |
| RLD | 383 | 1469 | 712 | 7706 | 265.67 | 0.34 | 0.000 |
| RWD | 0. 7 | 53.9 | 19.0 | 22.56 | 12.38 | 0.55 | 0.068 |
| SRL | 4.00 | 78.3 | 38.8 | 38.73 | 14.05 | 0.36 | 0.000 |
| RSM | 0.03 | 2.61 | 0.53 | 0.59 | 0.39 | 0.66 | 0.000 |
| RGR | 0.04 | 0.74 | 0.21 | 0.31 | 0.20 | 0.65 | 0.000 |
| LN | 3.00 | 19.00 | 11.3 | 11.14 | 2.69 | 0.24 | 0.000 |
| SDM | 3.50 | 69.1 | 16.0 | 18.49 | 10.68 | 0.58 | 0.000 |
| TDM | 3.50 | 80.9 | 25.6 | 28.32 | 14.13 | 0.50 | 0.000 |
| RLR | 0.31 | 1.95 | 0.66 | 0.80 | 0.37 | 0.46 | 0.001 |
| RSAR | 0.33 | 1.85 | 0.59 | 0.76 | 0.35 | 0.46 | 0.050 |
| RVR | 0.34 | 1.72 | 0.55 | 0.72 | 0.34 | 0.47 | 0.001 |
| Local traits | |||||||
| RDM_s1 | 0.001 | 16.6 | 5.45 | 6.00 | 2.62 | 0.44 | 0.000 |
| RDM_s2 | 0.00 | 23.8 | 2.33 | 3.14 | 3.70 | 1.18 | 0.196 |
| RDM_s3 | 0.00 | 7.33 | 0.30 | 0.72 | 1.17 | 1.63 | 0.000 |
| RDM_sub | 0.0 | 24.4 | 2.48 | 3.86 | 4.31 | 1.12 | 0.037 |
| RL_s1 | 69 | 202 | 135 | 135 | 30.9 | 0.23 | 0.000 |
| RL_s2 | 0.00 | 235 | 114.7 | 118 | 43.0 | 0.36 | 0.000 |
| RL_s3 | 0.00 | 449 | 95.7 | 85.7 | 85 | 0.99 | 0.006 |
| RL_sub | 0.00 | 660 | 197 | 203 | 116 | 0.57 | 0.000 |
| RD_s1 | 0.30 | 0.58 | 0.41 | 0.41 | 0.06 | 0.15 | 0.000 |
| RD_s2 | 0.29 | 0.58 | 0.42 | 0.42 | 0.07 | 0.17 | 0.000 |
| RD_s3 | 0.27 | 0.73 | 0.41 | 0.44 | 0.11 | 0.25 | 0.000 |
| RD_sub | 0.28 | 0.63 | 0.43 | 0.43 | 0.08 | 0.19 | 0.000 |
| RA_s1 | 8.35 | 26.92 | 17.2 | 17.2 | 4.82 | 0.28 | 0.000 |
| RA_s2 | 0.00 | 26.94 | 15.5 | 15.4 | 6.14 | 0.40 | 0.000 |
| RA_s3 | 0.00 | 42.5 | 12.7 | 11.3 | 9.85 | 0.87 | 0.000 |
| RA_sub | 0.00 | 63.56 | 26.04 | 26.7 | 14.1 | 0.53 | 0.000 |
| RV_s1 | 0.06 | 0.34 | 0.18 | 0.19 | 0.07 | 0.37 | 0.000 |
| RV_s2 | 0.00 | 0.36 | 0.17 | 0.17 | 0.08 | 0.47 | 0.000 |
| RV_s3 | 0.00 | 0.41 | 0.13 | 0.13 | 0.12 | 0.92 | 0.000 |
| RV_sub | 0.00 | 0.77 | 0.28 | 0.30 | 0.18 | 0.60 | 0.000 |
| RLD_s1 | 383 | 1503 | 823 | 885 | 288 | 0.33 | 0.000 |
| RLD_s2 | 0.00 | 1602 | 752 | 853 | 357 | 0.42 | 0.000 |
| RLD_s3 | 0.00 | 1746 | 480 | 579 | 519 | 0.90 | 0.004 |
| RLD_sub | 325 | 1635 | 691 | 761 | 299 | 0.39 | 0.000 |
| RWD_s1 | 0.007 | 79.56 | 30.63 | 36.3 | 18.0 | 0.50 | 0.000 |
| RWD_s2 | 2.42 | 97.1 | 15.1 | 18.7 | 14.6 | 0.78 | 0.000 |
| RWD_s3 | 0.25 | 27.6 | 4.82 | 6.53 | 6.72 | 1.03 | 0.095 |
| RWD_sub | 2.42 | 51.9 | 10.8 | 13.29 | 9.46 | 0.71 | 0.001 |
| Traits | Abbreviation | PC1 | PC2 | PC3 | PC4 |
|---|---|---|---|---|---|
| Leaf number | LN | 0.31 | 0.71 | −0.22 | −0.05 |
| Maximal root length (cm) | MRL | 0.81 | 0.36 | −0.07 | 0.18 |
| Root number | RN | 0.86 | 0.29 | 0.09 | −0.06 |
| Root dry mass (mg) | RDM | 0.75 | 0.24 | 0.53 | −0.07 |
| Shoot dry mass (mg) | SDM | 0.57 | 0.29 | −0.30 | 0.66 |
| Root length (cm) | RL | 0.86 | 0.02 | −0.13 | −0.09 |
| Root area (mm2) | RA | 0.87 | −0.35 | 0.05 | −0.03 |
| Root volume (cm3) | RV | 0.73 | −0.60 | 0.20 | −0.01 |
| Root diameter (mm) | RD | 0.13 | −0.91 | 0.25 | 0.13 |
| Root length density (cm cm−3) | RLD | −0.12 | 0.89 | −0.22 | −0.17 |
| Root weight density (mg cm−3) | RWD | 0.01 | 0.88 | 0.38 | −0.15 |
| Specific root length | SRL | −0.22 | −0.55 | −0.71 | −0.01 |
| Root to shoot dry mass ratio | RSM | 0.26 | −0.08 | 0.82 | −0.41 |
| Root growth rate (cm d−1) | RGR | 0.55 | −0.34 | 0.43 | 0.38 |
| Total dry mass (mg) | TDM | 0.77 | 0.33 | −0.01 | 0.48 |
| Root length ratio | RLR | −0.78 | 0.03 | 0.39 | 0.37 |
| Root surface area ratio | RSAR | −0.80 | 0.14 | 0.39 | 0.35 |
| Root volume ratio | RVR | −0.79 | 0.25 | 0.36 | 0.30 |
| Variation proportion | |||||
| Eigenvalue | 6.92 | 4.54 | 2.61 | 1.51 | |
| Variability (%) | 38.46 | 25.20 | 14.50 | 8.40 | |
| Cumulative (%) | 38.46 | 63.66 | 78.16 | 86.56 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, X.; Tian, Q.; Yan, J.; Chen, Y. Characterizing Root Morphological Traits in 65 Genotypes of Foxtail Millet (Setaria italica L.) from Four Different Ecological Regions in China. Agronomy 2022, 12, 1472. https://doi.org/10.3390/agronomy12061472
Yang X, Tian Q, Yan J, Chen Y. Characterizing Root Morphological Traits in 65 Genotypes of Foxtail Millet (Setaria italica L.) from Four Different Ecological Regions in China. Agronomy. 2022; 12(6):1472. https://doi.org/10.3390/agronomy12061472
Chicago/Turabian StyleYang, Xiaoxia, Qiaoyan Tian, Jiakun Yan, and Yinglong Chen. 2022. "Characterizing Root Morphological Traits in 65 Genotypes of Foxtail Millet (Setaria italica L.) from Four Different Ecological Regions in China" Agronomy 12, no. 6: 1472. https://doi.org/10.3390/agronomy12061472
APA StyleYang, X., Tian, Q., Yan, J., & Chen, Y. (2022). Characterizing Root Morphological Traits in 65 Genotypes of Foxtail Millet (Setaria italica L.) from Four Different Ecological Regions in China. Agronomy, 12(6), 1472. https://doi.org/10.3390/agronomy12061472







