Foliar Application of GA3 Stimulates Seed Production in Cauliflower
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site
2.2. Planting Material
2.3. Raising of Seedling
2.4. Experimental Design, Layout, and Transplanting of Seedling Cultural Practices and Aftercare
2.5. Preparation and Application of GA3
2.6. Pest and Disease Control
2.7. Harvesting of Seed
2.8. Collection of Data
2.9. Seed Germination Test
2.10. Statistical Analysis
2.11. Economic Analysis
3. Results and Discussion
3.1. Plant Height
3.2. Length and Breadth of the Biggest Leaf
3.3. Number of Leaves at Curd Initiation
3.4. Days to Curd Initiation
3.5. Days to Flower Initiation
3.6. Length of Seed Stalk at Harvest
3.7. Number of Primary Flowering Branches
3.8. Number of Secondary Flowering Branches
3.9. Length of the Pod
3.10. Number of Pods per Plant
3.11. Number of Seeded Pods per Plant
3.12. Number of Empty Pods per Plant
3.13. Days to Seed Maturity
3.14. Number of Seeds per Pod
3.15. Weight of Seeds per Plant
3.16. Weight of 1000 Seeds
3.17. Seed Yield
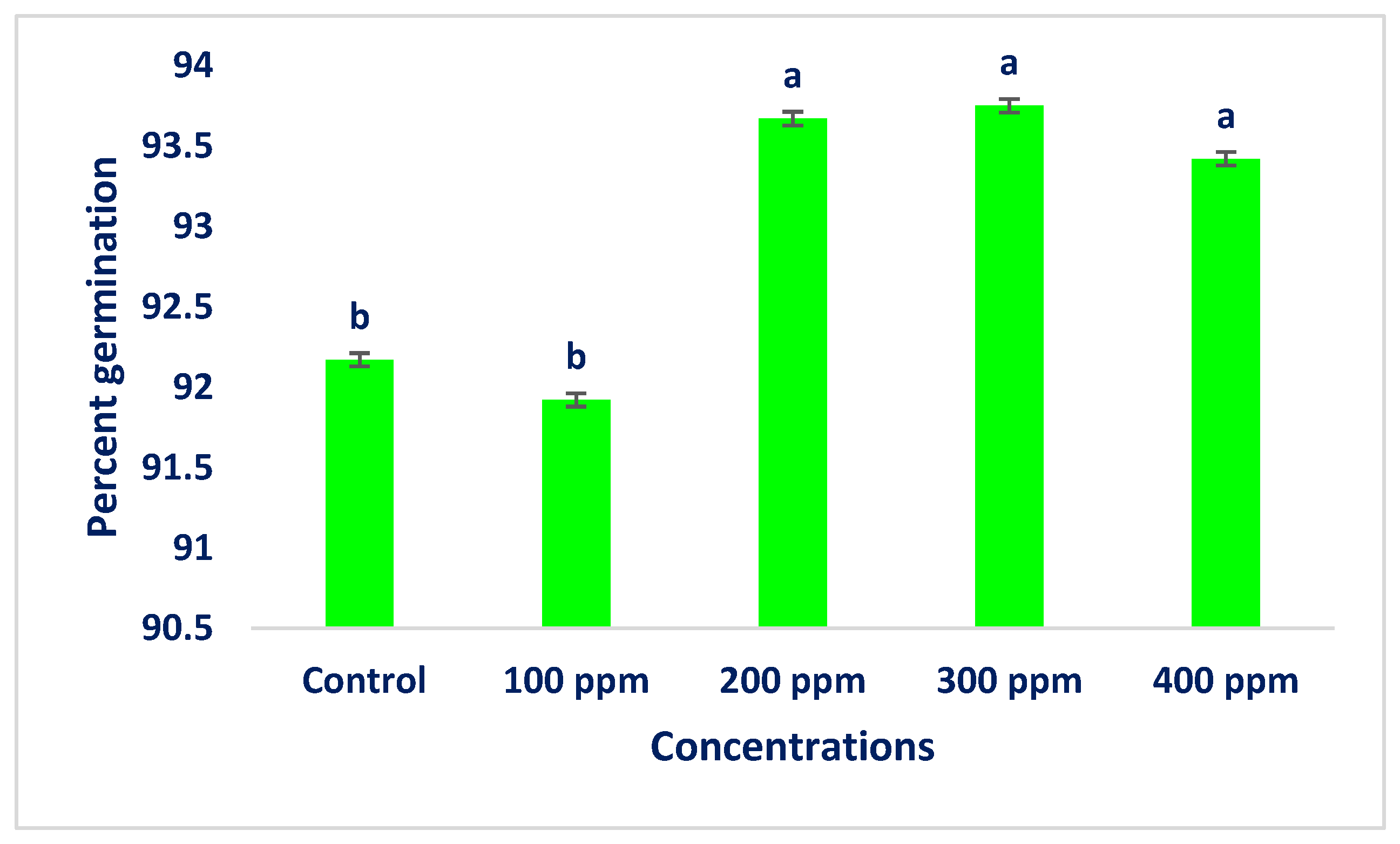
3.18. Seed Germination Test
3.19. Economic Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nimkar, S.A.; Korla, B.N. Studies on Comparison of Biparental and F4 Progenies in Late Cauliflower (Brassica oleracea L. var. botrytis). Int. J. Farm Sci. 2014, 4, 27–34. [Google Scholar]
- Bangladesh Bureau of Statistics (BBS). Yearbook of Agricultural Statistics-2020; Statistics and Informatics Division (SID) Ministry of Planning Government of the People’s Republic of Bangladesh: Dhaka, Bangladesh, 2021; pp. 332–333. [Google Scholar]
- Akter, S.; Islam, M.S.; Rahman, M.S. An Economic Analysis of Winter Vegetables Production in Some Selected Areas of Narsingdi District. J. Bangladesh Agric. Univ. 2011, 9, 241–246. [Google Scholar] [CrossRef]
- Bhandari, S.R.; Kwak, J. Chemical Composition and Antioxidant Activity in Different Tissue of Brassica Vegetables. Molecules 2015, 20, 1228–1243. [Google Scholar] [CrossRef]
- Podsedek, A. Natural Antioxidants and Antioxidant Capacity of Brassica Vegetables: A Review. LWT-Food Sci. Technol. 2007, 40, 1–11. [Google Scholar] [CrossRef]
- Kaur, P.; Mal, D. Effect of Foliar Spray of NAA and GA3 on The Growth, Curd Formation and Yield of Cauliflower (Brassica oleracea L. var. botrytis). J. Pharma. Phytochem. 2018, 7, 2805–2807. [Google Scholar]
- Ayub, A.; Mian, I.H.; Ahmed, H.U.; Alam, M.Z. Effect of Date of Planting on Altemaria Blight of Cauliflower Seed Crop. Bangladesh J. Plant Pathol. 2005, 21, 67–69. [Google Scholar]
- Emongor, V. Gibberellic Acid (GA3) Influence on Vegetative Growth, Nodulation, and Yield of Cowpea (Vigna unguiculata (L.) Walp. J. Agron. 2007, 6, 509–517. [Google Scholar]
- Ali, M.A.; Hossain, M.M.; Zakaria, M.; Islam, M.M. Effect of GA3 on quality seed production of onion in Bangladesh. Ecofriendly Agric. J. 2015, 8, 47–50. [Google Scholar]
- Richards, D.E.; Ait-Ali, K.E.T.; Harberd, N.P. How Gibberellin Regulates Growth and Development: A Molecular Genetic Analysis of Gibberellin Signaling. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2001, 52, 67–88. [Google Scholar] [CrossRef]
- Mohanta, H.C.; Hossain, M.M.; Islam, M.S.; Salam, M.A.; Saha, S.R. Effect of Plant Growth Regulators on Seed Yield of Carrot. Ann. Bangladesh Agric. 2015, 19, 23–31. [Google Scholar]
- Tiwari, D.K.; Pandey, P.; Giri, S.P.; Dwivedi, J.L. Effect of GA3 and Other Plant Growth Regulators on Hybrid Rice Seed Production. Asian J. Plant Sci. 2011, 10, 133–139. [Google Scholar] [CrossRef]
- Attallah, S.; Abbas, H.S. Effect of Gibberellic Acid on The Earliness of Cauliflower Curd Initiation Under Asuit Conditions. Assiut. J. Agric. Sci. 2016, 43, 48–56. [Google Scholar]
- Sarker, U.; Islam, M.T.; Rabbani, M.G.; Oba, S. Genotypic Variability for Nutrient, Antioxidant, Yield and Yield Contributing Traits in Vegetable Amaranth. J. Food Agric. Environ. 2014, 12, 168–174. Available online: https://www.wflpublisher.com/Abstract/5378 (accessed on 9 May 2022).
- Sarker, U.; Islam, M.T.; Rabbani, M.G.; Oba, S. Variability, heritability and genetic association in vegetable amaranth. Spanish J. Agric. Res. 2015, 13, 17. [Google Scholar] [CrossRef]
- Sarker, U.; Islam, M.T.; Oba, S. Salinity Stress Accelerates Nutrients, Dietary Fiber, Minerals, Phytochemicals and Antioxidant Activity in Amaranthus tricolor Leaves. PLoS ONE 2018, 13, e0206388. [Google Scholar] [CrossRef]
- Sarker, U.; Islam, M.T.; Rabbani, M.G.; Oba, S. Variability in Total Antioxidant Capacity, Antioxidant Leaf Pigments and Foliage Yield of Vegetable Amaranth. J. Integr. Agric. 2018, 17, 1145–1153. [Google Scholar] [CrossRef]
- Sarker, U.; Islam, M.T.; Rabbani, M.G.; Oba, S. Phenotypic Divergence in Amaranth for Total Antioxidant Capacity, Antioxidant Profile, Dietary Fiber, Nutritional and Agronomic Traits. Acta Agric. Scand. Sect. B-Soil Plant Sci. 2018, 68, 67–76. [Google Scholar] [CrossRef]
- Sarker, U.; Oba, S. Catalase, Superoxide Dismutase and Ascorbate-Glutathione Cycle Enzymes Confer Drought Tolerance of A. tricolor. Sci. Rep. 2018, 8, 16496. [Google Scholar] [CrossRef]
- Sarker, U.; Oba, S. Drought Stress Enhances Nutritional and Bioactive Compounds, Phenolic Acids and Antioxidant Capacity of Amaranthus Leafy Vegetable. BMC Plant Biol. 2018, 18, 258. [Google Scholar] [CrossRef]
- Sarker, U.; Oba, S. Drought Stress Effects on Growth, ROS Markers, Compatible Solutes, Phenolics, Flavonoids, and Antioxidant Activity in Amaranthus tricolor. Appl. Biochem. Biotechnol. 2018, 186, 999–1016. [Google Scholar] [CrossRef]
- Sarker, U.; Oba, S. Response of Nutrients, Minerals, Antioxidant Leaf Pigments, Vitamins, Polyphenol, Flavonoid and Antioxidant Activity in Selected Amaranth under Four Soil Water Content. Food Chem. 2018, 252, 72–83. [Google Scholar] [CrossRef] [PubMed]
- Sarker, U.; Oba, S. Augmentation of Leaf Color Parameters, Pigments, Vitamins, Phenolic Acids, Flavonoids and Antioxidant Activity in Selected Amaranthus tricolor under Salinity Stress. Sci. Rep. 2018, 8, 12349. [Google Scholar] [CrossRef] [PubMed]
- Azad, A.K.; Goshwami, B.K.; Rahman, M.L.; Malakar, P.K.; Hasan, S.; Rahman, H.H. (Eds.) Krishi Projukti Hatboi (In Bengali), 7th ed.; Bangladesh Agricultural Research Institute: Gazipur, Bangladesh, 2017; pp. 169–170. [Google Scholar]
- Sarker, U.; Oba, S.; Ercisli, S.; Assouguem, A.; Alotaibi, A.; Ullah, R. Bioactive Phytochemicals and Quenching Activity of Radicals in Selected Drought-Resistant Amaranthus tricolor Vegetable Amaranth. Antioxidants 2022, 11, 578. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.A.; Juraimi, A.S.; Rafii, M.Y.; Hamid, A.A.; Aslani, F.; Alam, M.Z. Effects of Salinity and Salinity-Induced Augmented Bioactive Compounds in Purslane (Portulaca oleracea L.) for Possible Economical Use. Food Chem. 2015, 169, 439–447. [Google Scholar] [CrossRef]
- Sarker, U.; Islam, M.T.; Rabbani, M.G.; Oba, S. Variability in Composition of Vitamins and Mineral Antioxidants in Vegetable Amaranth. Genetika 2015, 47, 85–96. [Google Scholar] [CrossRef]
- Sarker, U.; Islam, M.T.; Rabbani, M.G.; Oba, S. Genetic Variation and Interrelationships among Antioxidant, Quality, and Agronomic Traits in Vegetable Amaranth. Turk. J. Agric. For. 2016, 40, 526–535. [Google Scholar] [CrossRef]
- Sarker, U.; Islam, M.T.; Rabbani, M.G.; Oba, S. Antioxidant Leaf Pigments and Variability in Vegetable Amaranth. Genetika 2018, 50, 209–220. [Google Scholar] [CrossRef]
- Chakrabarty, T.; Sarker, U.; Hasan, M.; Rahman, M.M. Variability in Mineral Compositions, Yield and Yield Contributing Traits of Stem Amaranth (Amaranthus lividus). Genetika 2018, 50, 995–1010. [Google Scholar] [CrossRef]
- Sarker, U.; Oba, S. Salinity Stress Enhances Color Parameters, Bioactive Leaf Pigments, Vitamins, Polyphenols, Flavonoids and Antioxidant Activity in Selected Amaranthus Leafy Vegetables. J. Sci. Food Agric. 2019, 99, 2275–2284. [Google Scholar] [CrossRef]
- Sarker, U.; Oba, S. Protein, Dietary Fiber, Minerals, Antioxidant Pigments and Phytochemicals, and Antioxidant Activity in Selected Red Morph Amaranthus Leafy Vegetable. PLoS ONE 2019, 14, e0222517. [Google Scholar] [CrossRef]
- Sarker, U.; Oba, S. Nutraceuticals, Antioxidant Pigments, and Phytochemicals in the Leaves of Amaranthus spinosus and Amaranthus viridis Weedy Species. Sci Rep. 2019, 9, 20413. [Google Scholar] [CrossRef] [PubMed]
- Sarker, U.; Oba, S. Antioxidant Constituents of Three Selected Red and Green Color Amaranthus Leafy Vegetable. Sci. Rep. 2019, 9, 18233. [Google Scholar] [CrossRef] [PubMed]
- Rashad, M.M.I.; Sarker, U. Genetic Variations in Yield and Yield Contributing Traits of Green Amaranth. Genetika 2020, 52, 393–407. [Google Scholar] [CrossRef]
- Hasan-Ud-Daula, M.; Sarker, U. Variability, Heritability, Character Association, and Path Coefficient Analysis in Advanced Breeding Lines of Rice (Oryza sativa L.). Genetika 2020, 52, 711–726. [Google Scholar] [CrossRef]
- Azad, A.K.; Sarker, U.; Ercisli, S.; Assouguem, A.; Ullah, R.; Almeer, R.; Sayed, A.A.; Peluso, I. Evaluation of Combining Ability and Heterosis of Popular Restorer and Male Sterile Lines for the Development of Superior Rice Hybrids. Agronomy 2022, 12, 965. [Google Scholar] [CrossRef]
- Hasan, M.J.; Kulsum, M.U.; Majumder, R.R.; Sarker, U. Genotypic Variability for Grain Quality Attributes in Restorer Lines of Hybrid Rice. Genetika 2020, 52, 973–989. [Google Scholar] [CrossRef]
- Hossain, M.N.; Sarker, U.; Raihan, M.S.; Al-Huqail, A.A.; Siddiqui, M.H.; Oba, S. Influence of Salinity Stress on Color Parameters, Leaf Pigmentation, Polyphenol and Flavonoid Contents, and Antioxidant Activity of Amaranthus lividus Leafy Vegetables. Molecules 2022, 27, 1821. [Google Scholar] [CrossRef]
- Sarker, U.; Rabbani, M.G.; Oba, S.; Eldehna, W.M.; Al-Rashood, S.T.; Mostafa, N.M.; Eldahshan, O.A. Phytonutrients, Colorant Pigments, Phytochemicals, and Antioxidant Potential of Orphan Leafy Amaranthus Species. Molecules 2022, 27, 2899. [Google Scholar] [CrossRef]
- Sarker, U.; Azam, M.G.; Talukder, M.Z.A. Genetic Variation in Mineral Profiles, Yield Contributing Agronomic Traits, and Foliage Yield of Stem Amaranth. Genetika 2022, 54, 91–108. [Google Scholar] [CrossRef]
- Sarker, U.; Oba, S.; Alsanie, W.F.; Gaber, A. Characterization of Phytochemicals, Nutrients, and Antiradical Potential in Slim Amaranth. Antioxidants 2022, 11, 1089. [Google Scholar] [CrossRef]
- Sarker, U.; Oba, S. The Response of Salinity Stress-Induced A. tricolor to Growth, Anatomy, Physiology, Non-Enzymatic and Enzymatic Antioxidants. Front. Plant Sci. 2020, 11, 559876. [Google Scholar] [CrossRef] [PubMed]
- Sarker, U.; Oba, S. Nutritional and Bioactive Constituents and Scavenging Capacity of Radicals in Amaranthus hypochondriacus. Sci. Rep. 2020, 10, 19962. [Google Scholar] [CrossRef] [PubMed]
- Sarker, U.; Oba, S. Nutrients, Minerals, Pigments, Phytochemical, and Radical Scavenging Activity in Amaranthus blitum Leafy Vegetable. Sci. Rep. 2020, 10, 3868. [Google Scholar] [CrossRef] [PubMed]
- Sarker, U.; Oba, S. Leaf Pigmentation, Its Profiles and Radical Scavenging Activity in Selected Amaranthus tricolor Leafy Vegetables. Sci. Rep. 2020, 10, 18617. [Google Scholar] [CrossRef]
- Sarker, U.; Oba, S. Polyphenol and Flavonoid Profiles and Radical Scavenging Activity in Selected Leafy Vegetable Amaranthus gangeticus. BMC Plant Biol. 2020, 20, 499. [Google Scholar] [CrossRef] [PubMed]
- Sarker, U.; Oba, S. Phenolic Profiles and Antioxidant Activities in Selected Drought-Tolerant Leafy Vegetable Amaranth. Sci. Rep. 2020, 10, 18287. [Google Scholar] [CrossRef]
- Sarker, U.; Hossain, M.N.; Iqbal, M.A.; Oba, S. Bioactive Components and Radical Scavenging Activity in Selected Advance Lines of Salt-Tolerant Vegetable Amaranth. Front. Nutr. 2020, 7, 587257. [Google Scholar] [CrossRef]
- Sarker, U.; Oba, S. Nutraceuticals, Phytochemicals, and Radical Quenching Ability of Selected Drought-Tolerant Advance Lines of Vegetable Amaranth. BMC Plant Biol. 2020, 20, 564. [Google Scholar] [CrossRef]
- Sarker, U.; Hossain, M.M.; Oba, S. Nutritional and Antioxidant Components and Antioxidant Capacity in Green Morph Amaranthus Leafy Vegetable. Sci. Rep. 2020, 10, 1336. [Google Scholar] [CrossRef]
- Sarker, U.; Oba, S.; Daramy, M.A. Nutrients, Minerals, Antioxidant Pigments and Phytochemicals, and Antioxidant Capacity of the Leaves of Stem Amaranth. Sci. Rep. 2020, 10, 3892. [Google Scholar] [CrossRef]
- Sarker, U.; Islam, M.T.; Rabbani, M.G.; Oba, S. Genotypic Diversity in Amaranth for Antioxidant, Nutrient and Agronomic Traits. Indian J. Genet. Pl. Breed. 2017, 77, 173–176. [Google Scholar] [CrossRef]
- Sarker, U.; Oba, S. Color Attributes, Betacyanin, and Carotenoid Profiles, Bioactive Components, and Radical Quenching Capacity in Selected Amaranthus gangeticus Leafy Vegetables. Sci. Rep. 2021, 11, 11559. [Google Scholar] [CrossRef] [PubMed]
- Sarker, U.; Lin, Y.P.; Oba, S.; Yoshioka, Y.; Ken, H. Prospects and potentials of underutilized leafy Amaranths as vegetable use for health-promotion. Plant Physiol. Biochem. 2022, 182, 104–123. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.A.; Sarker, U.; MAK, M.; Islam, M.A.; Fatema-Tuj-Johora. Estimation of Genetic Divergence in Boro Rice (Oryza sativa L.). Int. J. Biol. Sci. 2014, 16, 28–36. [Google Scholar]
- Rai, P.K.; Sarker, U.K.; Roy, P.C.; Islam, A. Character Association in F4 Generation of Rice (Oryza sativa L.). Bangladesh J. Plant Breed. Genet. 2013, 26, 39–44. [Google Scholar] [CrossRef]
- Nath, J.K.; Sarker, U.; Mian, M.A.K.; Hossain, T. Genetic Divergence in T. Aman Rice. Ann. Bangladesh Agric. 2008, 12, 51–60. [Google Scholar]
- Siddique, M.N.A.; Sarker, U.; Mian, M.A.K. Genetic diversity in restorer line of rice. In Proceedings of the International Conference on Plant Breeding and Seed for Food Security; Bhuiyan, M.S.R., Rahman, L., Eds.; Plant Breeding and Genetics Society of Bangladesh: Dhaka, Bangladesh, 2009; pp. 137–142. [Google Scholar]
- Hasan, M.R.; Sarker, U.; Hossain, M.A.; Huda, K.M.K.; Mian, M.A.K.; Hossain, T.; Zahan, M.S.; Mahmud, M.N.H. Genetic Diversity in Micronutrient Dense Rice and Its Implication in Breeding Program. Ecofriendly Agric. J. 2012, 5, 168–174. [Google Scholar]
- Ganapati, R.K.; Rasul, M.G.; Mian, M.A.K.; Sarker, U. Genetic Variability and Character Association of T-Aman Rice (Oryza sativa L). Intl. J. Plant Biol. Res. 2014, 2, 1–4. [Google Scholar]
- Karim, D.; Sarker, U.; Siddique, M.N.A.; Miah, M.A.K.; Hasnat, M.Z. Variability and Genetic Parameter Analysis in Aromatic Rice. Int. J. Sustain. Crop Prod. 2007, 2, 15–18. [Google Scholar]
- Karim, D.; Siddique, M.N.A.; Sarker, U.; Hasnat, Z.; Sultana, J. Phenotypic and Genotypic Correlation Co-Efficient of Quantitative Characters and Character Association of Aromatic Rice. J. Biosci. Agric. Res. 2014, 1, 34–46. [Google Scholar] [CrossRef]
- Biswas, P.S.; Sarker, U.; Bhuiyan, M.A.R.; Khatun, S. Genetic Divergence in Cold Tolerant Irrigated Rice (Oryza sativa L.). Agric. 2006, 4, 15–20. [Google Scholar]
- Hasan, M.R.; Sarker, U.; Mian, M.A.K.; Hossain, T.; Mahmud, M.N.H. Genetic Variation in Micronutrient Dense Rice and Its Implication in Breeding for Higher Yield. Eco-friendly Agric. J. 2012, 5, 175–182. [Google Scholar]
- Rahman, M.H.; Sarker, U.; Main, M.A.K. Assessment of Variability of Floral and Yield Traits; I Restorer Lines of Rice. Ann. Bangladesh Agric. 2007, 11, 87–94. [Google Scholar]
- Rahman, M.H.; Sarker, U.; Main, M.A.K. Assessment of Variability of Floral and Yield Traits; II Maintainer Lines of Rice. Ann. Bangladesh Agric. 2007, 11, 95–102. [Google Scholar]
- Sarker, U.; Biswas, P.S.; Prasad, B.; Mian, M.A.K. Correlated Response, Relative Selection Efficiency and Path Analysis in Cold Tolerant Rice. Bangladesh J. Pl. Breed. Genet. 2001, 14, 33–36. [Google Scholar]
- Sarker, U.; Mian, M.A.K. Genetic Variability, Character Association and Path Analysis for Yield and Its Components in Rice. J. Asiat. Soc. Bangladesh Sci. 2003, 29, 47–54. [Google Scholar]
- Sarker, U.; Mian, M.A.K. Genetic Variations and Correlations between Floral Traits in Rice. Bangladesh J. Agric. Res 2004, 29, 553–558. [Google Scholar]
- Biswas, A.; Sarker, U.; Banik, B.R.; Rohman, M.M.; Mian, M.A.K. Genetic Divergence Study in Salinity Stress Tolerant Maize (Zea mays L.). Bangladesh J. Agric. Res. 2014, 39, 621–630. [Google Scholar] [CrossRef]
- Azam, M.G.A.; Sarker, U.; Mian, M.A.K.; Banik, B.R.; Talukder, M.Z.A. Genetic Divergence on Quantitative Characters of Exotic Maize Inbreds (Zea mays L.). Bangladesh J. Plant Breed. Genet. 2013, 26, 9–14. [Google Scholar] [CrossRef]
- Azam, M.G.; Sarker, U.; Maniruzzam; Banik, B.R. Genetic Variability of Yield and Its Contributing Characters on CIMMYT Maize Inbreds under Drought Stress. Bangladesh J. Agric. Res. 2014, 39, 419–426. [Google Scholar] [CrossRef]
- Talukder, M.Z.A.; Sarker, U.; Khan, A.B.M.M.M.; Moniruzzaman, M.; Zaman, M.M. Genetic Variability and Correlation Coefficient of Coconut (Cocos nucifera L.) in Barisal Region. Int. J. Biol. Sci. 2011, 11, 15–21. [Google Scholar]
- Talukder, M.Z.A.; Sarker, U.; Harun-Or-Rashid, M.; Zakaria, M. Genetic Diversity of Coconut (Cocos nucifera L.) in Barisal Region. Ann. Bangladesh Agric. 2015, 19, 13–21. [Google Scholar]
- Ashraf, A.T.M.; Rahman, M.M.; Hossain, M.M.; Sarker, U. Study of Correlation and Path Analysis in the Selected Okra Genotypes. Asian Res. J. Agric. 2020, 12, 1–11. [Google Scholar] [CrossRef]
- Ashraf, A.T.M.; Rahman, M.M.; Hossain, M.M.; Sarker, U. Study of the Genetic Analysis of Some Selected Okra Genotypes. Int. J. Adv. Res. 2020, 8, 549–556. [Google Scholar] [CrossRef]
- Ashraf, A.T.M.; Rahman, M.M.; Hossain, M.M.; Sarker, U. Performance Evaluation of Some Selected Okra Genotypes. Int. J. Plant Soil Sci. 2020, 32, 13–20. [Google Scholar] [CrossRef]
- Kayesh, E.; Sharker, M.S.; Roni, M.S.; Sarker, U. Integrated Nutrient Management for Growth, Yield and Profitability of Broccoli. Bangladesh J. Agric. Res. 2019, 44, 13–26. [Google Scholar] [CrossRef]
- Islam, M.T. The Effect of Some Growth Regulators on Yield and Biomass Production in Cabbage. Punjab Veg. Grower 1985, 20, 11–16. [Google Scholar]
- Patil, A.A.; Maniur, S.M.; Nalwadi, U.G. Effect of GA and NAA on Growth and Yield of Cabbage. South Indian Hortic. 1987, 35, 393–394. [Google Scholar]
- Akter, M.N. Effect of IAA and GA3 on Growth Curd Formation and Yield of Cauliflower. Master’s Thesis, Department of Horticulture and Postharvest Technology, Sher-e-Bangla Agricultural University, Dhaka, Bangladesh, 2007. [Google Scholar]
- Rahman, M.A.; Imran, M.; Ikrum, M.; Rahman, M.H.; Rabbani, M.G. Effects of Planting Date and Growth Hormone on Growth and Yield of Cauliflower. J. Environ. Sci. 2016, 9, 143–150. [Google Scholar] [CrossRef]
- Booij, R.; Struik, P.C. Effects of Temperature on Leaf and Curd Initiation in Relation to Juvenility of Cauliflower. Scientia Hortic. 1990, 44, 201–214. [Google Scholar] [CrossRef]
- Wurr, D.C.E.; Fellows, J.R. Temperature Influences on The Plant Development of Different Maturity Types of Cauliflower. Acta Hortic. 2000, 539, 69–74. [Google Scholar] [CrossRef]
- Hand, D.J.; Atherton, J.G. Curd initiation in the cauliflower. I. Juvenility. J. Exp. Bot. 1987, 38, 2050–2058. [Google Scholar] [CrossRef]
- Williams, C.A.; Atherton, J.G. A Role for Young Leaves in Vernalization of Cauliflower: I. Analysis of Leaf Development During Curd Induction. Physiol. Plant. 1990, 78, 61–66. [Google Scholar] [CrossRef]
- Haque, M.R. Effect of Fertilizer and Manure on Curd and Seed Yield of Cauliflower. Master’s Thesis, Department of Horticulture, Bangabandhu Sheikh Mujibur Rahman Agricultural University, Gazipur City, Bangladesh, 1999. [Google Scholar]
- Duclos, D.V.; Bjorkman, T. Gibberellin Control of Reproductive Transitions in Brassica oleracea Curd Development. J. Am. Soc. Hortic. Sci. 2015, 140, 57–67. [Google Scholar] [CrossRef]
- Lin, C.-Y.; Chen, K.-S.; Chen, H.-P.; Lee, H.-I. Curd Initiation and Transformation in Tropical Cauliflower Cultivars under Different Temperature Treatments. HortScience 2019, 54, 1351–1356. [Google Scholar] [CrossRef]
- Roy, R.; Nasiruddin, K.M. Effect of Different Levels of GA3 on Growth and Yield of Cabbage. J. Environ. Sci. Nat. Resour. 2011, 4, 79–82. [Google Scholar]
- Bhattacharya, A. Effect of High-Temperature Stress on the Metabolism of Plant Growth Regulators. In Effect of High Temperature on Crop Productivity and Metabolism of Macro Molecules; Bhattacharya, A., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 485–591. [Google Scholar]
- Guan, Y.; Xue, J.; Xue, Y.; Yang, R.; Wang, S.; Zhang, X. Effect of Exogenous GA3 on Flowering Quality, Endogenous Hormones, and Hormone- and Flowering-Associated Gene Expression in Forcing-Cultured Tree Peony (Paeonia suffruticosa). J. Integr. Agric. 2019, 18, 1295–1311. [Google Scholar] [CrossRef]
- Othman, Y.A.; Al-Ajlouni, M.G.; Asaf, T.S.; Sawalha, H.A.; Hani, M.B. Influence of gibberellic acid on the physiology and flower quality of gerbera and lily cut flowers. Int. J. Agric. Nat. Resour. 2021, 48, 21–33. [Google Scholar] [CrossRef]
- Sharifuzzaman, S.M.; Ara, K.A.; Rahman, M.H.; Kabir, K.; Talukdar, M.B. Effect of GA3, CCC and MH on Vegetative Growth, Flower Yield and Quality of Chrysanthemum. Int. J. Exp. Agric. 2011, 2, 17–20. [Google Scholar]
- Sitapara, H.H.; Vihol, N.J.; Patel, M.J.; Patel, J.S. Effect of Growth Regulators and Micronutrients on Growth and Yield of Cauliflower cv. ‘SNOWBALL-16’. Asian J. Hortic. 2011, 6, 348–351. [Google Scholar]
- Marth, P.C.; Audia, W.V.; Mitchell, J.W. Effects of Gibberellic Acid on Growth and Development of Plants of Various Genera and Species. Bot. Gaz. 1956, 118, 106–111. Available online: http://www.jstor.org/stable/2472972 (accessed on 12 January 2022). [CrossRef]
- Rastogi, A.; Siddiqui, A.; Mishra, B.K.; Srivastava, M.; Pandey, R. Effect of Auxin and Gibberellic Acid on Growth and Yield Components of Linseed (Linum usitatissimum L.). Crop Breed. Appl. Biotechnol. 2013, 13, 136–143. [Google Scholar] [CrossRef]
- Singh, R.D.; Kuksal, R.P.; Sem, J.N. Effect of Ethrel and CCC on Growth and Seed Production of Cauliflower Var. snowball-16. Indian J. Agric. Res. 1976, 10, 122–124. [Google Scholar]
- Ayyub, C.M.; Manan, A.; Pervez, M.A.; Ashraf, M.I.; Afzal, M.; Ahmed, S.; Jahangir, M.M.; Anwar, N.; Shaheen, M.R. Foliar Feeding with Gibberellic Acid (GA3): A Strategy for Enhanced Growth and Yield of Okra (Abelmoschus esculentus L. Moench.). Afr. J. Agric. Res. 2013, 8, 3299–3302. [Google Scholar]
- Hoque, M.M.; Haque, M.S. Effects of GA3 and its Mode of Application on Morphology and Yield Parameters of Mungbean Vigna radiata L.). Pakistan J. Biol. Sci. 2002, 5, 281–283. [Google Scholar] [CrossRef][Green Version]
- Zia, M.I.K. Effect of Plant Growth Regulators on Growth Curd Yield and Seed Production of Cauliflower. Master’s Thesis, Department of Horticulture, Sher-e-Bangla Agricultural University, Dhaka, Bangladesh, 2006. [Google Scholar]
- Mohammadi, G.; Khah, E.M.; Petropoulos, S.A.; Chachalis, B.D.; Akbari, F.; Yarsi, G. Effect of Gibberellic Acid and Harvesting Time on the Seed Quality of Four Okra Cultivars. J. Agric. Sci. 2014, 6, 200–211. [Google Scholar] [CrossRef][Green Version]
- Mangal, J.L.; Pandita, M.; Pandey, U.C. Role of Growth Regulators in Vegetable Seed Production—A Review. Haryana J. Hortic. Sci. 1980, 9, 77–81. [Google Scholar]


| GA3 Concentration | Plant Height (cm) at 80 DAT | Length of the Biggest (cm) Leaf at 80 DAT | Breadth of the Biggest Leaf at 80 DAT (cm) | Number of Leaves at Curd Initiation | Days to Curd Initiation | Days to Flower Initiation | Length of Seed Stalk (cm) at Harvest | Number of Primary Flowering Branches | Number of Secondary Flowering Branches | Length of Pod |
|---|---|---|---|---|---|---|---|---|---|---|
| G0 = Control | 42.72 ab | 37.95 NS | 17.45 b | 21.12 NS | 54.87 a | 87.70 a | 70.78 c | 10.18 ab | 26.90 b | 4.65 b |
| G1 = 100 ppm | 43.88 a | 38.47 NS | 18.05 a | 21.07 NS | 53.31 ab | 85.12 ab | 77.05 ab | 10.82 a | 31.42 a | 4.63 b |
| G2 = 200 ppm | 44.05 a | 37.95 NS | 17.75 ab | 20.72 NS | 51.02 b | 84.17 b | 79. 53 a | 10.88 a | 31.33 a | 4.98 a |
| G3 = 300 ppm | 43.83 a | 38.03 NS | 16.92 b | 20.95 NS | 52.97 ab | 86.24 ab | 78. 82 a | 10.23 ab | 31.20 a | 4.57 b |
| G4 = 400 ppm | 41.30 b | 39.01 NS | 17.73 ab | 20.69 NS | 53.47 ab | 86.57 ab | 75.25 b | 9.90 b | 28.53 ab | 4.71 b |
| Mean | 43.157 | 38.28 | 17.58 | 20.91 | 53.13 | 85.96 | 76.29 | 10.40 | 29.88 | 4.71 |
| CV% | 6.00 | 5.23 | 6.65 | 3.77 | 5.70 | 4.20 | 6.69% | 8.63 | 13.13 | 5.83 |
| Time of Application | Plant Height (cm) 80 DAT | Length of the Biggest (cm) Leaf at 80 DAT | Breadth of the Biggest Leaf (cm) at 80 DAT | Number of Leaves at Curd Initiation | Days to Curd Initiation | Days to Flower Initiation | Length of Seed Stalk (cm) at Harvest | Number of Primary Flowering Branches | Number of Secondary Flowering Branches | Length of Pod |
|---|---|---|---|---|---|---|---|---|---|---|
| T1 | 43.06 NS | 38.10 NS | 17.71 NS | 20.87 NS | 52.45 NS | 85.84 NS | 76.27 NS | 11.00 a | 31.96 a | 4.72 NS |
| T2 | 43.41 NS | 38.61 NS | 17.53 NS | 20.95 NS | 53.21 NS | 85.86 NS | 77.45 NS | 10.40 ab | 30.42 ab | 4.72 NS |
| T3 | 43.55 NS | 38.09 NS | 17.5 NS | 21.11 NS | 53.53 NS | 86.59 NS | 76.54 NS | 10.18 b | 28.99 b | 4.69 NS |
| T4 | 42.61 NS | 38.32 NS | 17.5 NS | 20.11 NS | 53.68 NS | 85.55 NS | 75.09 NS | 10.03 b | 28.13 b | 4.71 NS |
| Mean | 43.157 | 38.28 | 17.58 | 20.91 | 53.13 | 85.96 | 76.29 | 10.40 | 29.88 | 4.71 |
| CV% | 6.00 | 5.23 | 6.65 | 3.77 | 5.70 | 4.20 | 6.68 | 8.63 | 13.13 | 5.83 |
| Treatment Combination | Plant Height (cm) at 80 DAT | Length of the Biggest Leaf (cm) at 80 DAT | Breadth of the Biggest Leaf at 80 DAT (cm) | Number of Leaves at Curd Initiation | Days to Curd Initiation | Days to Flower Initiation | Length of Seed Stalk (cm) at Harvest | Number of Primary Flowering Branches | Number of Secondary Flowering Branches | Length of Pod |
|---|---|---|---|---|---|---|---|---|---|---|
| G0T1 | 42.53 bc | 38.40 ab | 17.33 ab | 21.47 ab | 54.93 abc | 87.27 abc | 67.8 0f | 10.67 a–d | 30.87 a–d | 4.76 a–e |
| G0T2 | 42.13 abc | 37.80 b | 17.07 ab | 20.73 ab | 52.80 a–d | 86.67 a–d | 72.40 def | 10.53 a–d | 27.80 cde | 4.76 a–e |
| G0T3 | 44.73 ab | 38.33 ab | 18.07 ab | 21.67 a | 56.20 a | 88.13 ab | 71.60 ef | 9.73 de | 25.87 de | 4.73 b–e |
| G0T4 | 41.47 abc | 37.27 ab | 17.33 ab | 20.6 0ab | 55.53 abc | 88.73 a | 71.33 ef | 9.80 cde | 23.07 e | 4.59 cde |
| G1T1 | 44.67 ab | 37.93 ab | 17.67 ab | 20.80 ab | 52.18 a–d | 83.17 bcd | 79.40 bc | 12.00 a | 32.47 abc | 4.69 b–e |
| G1T2 | 45.27 a | 39.07 ab | 18.33 ab | 21.40 ab | 53.47 a–d | 86.13 a–d | 76.33 b–e | 9.60 de | 30.67 a–d | 4.72 b–e |
| G1T3 | 42.93 abc | 37.60 ab | 18.07 ab | 21.33 ab | 53.20 a–d | 85.23 a–d | 78.27 bcd | 10.93 a–d | 32.40 abc | 4.49 de |
| G1T4 | 42.67 abc | 39.27 a | 18.13 ab | 20.73 ab | 54.40 a–d | 85.93 a-d | 74.20 b–f | 10.73 a–d | 30.13 a–d | 4.63 b–e |
| G2T1 | 39.97 c | 38.00 ab | 18.73 a | 20.27 b | 49.60 d | 84.00 a–d | 78.90 bcd | 11.27 abc | 34.87 a | 4.99 abc |
| G2T2 | 39.63 c | 37.53 ab | 18.00 ab | 20.73 ab | 50.53 cd | 81.77 d | 80.57 ab | 11.60 ab | 30.97 a–d | 5.21 a |
| G2T3 | 42.67 abc | 38.40ab | 17.53 ab | 20.73 ab | 51.73 a–d | 84.83 a–d | 79.93 ab | 10.63 a–d | 29.33 a–d | 4.64 b–e |
| G2T4 | 42.93 abc | 37.87 ab | 16.53ab | 21.13ab | 52.20a–d | 86.07a–d | 78.73bcd | 10.00cde | 30.13a-d | 5.06ab |
| G3T1 | 43.67 abc | 38.33 ab | 16.87 ab | 21.00 ab | 51.67 a–d | 86.23 a–d | 77.33 b–e | 9.80 cde | 31.07 a–d | 4.47 de |
| G3T2 | 44.67 ab | 39.20 a | 16.67 b | 21.07 ab | 53.53 a–d | 88.73 a | 72.87 c–f | 10.47 bcd | 34.33 ab | 4.43 e |
| G3T3 | 41.73 abc | 36.20 b | 17.00 ab | 20.87 ab | 51.53 a–d | 85.73 a–d | 79.03 bc | 9.80 cde | 28.73 a–e | 4.67 b–e |
| G3T4 | 45.27 a | 38.40 ab | 17.13 ab | 20.87 ab | 55.13 abc | 84.27 a–d | 86.07 a | 10.87 a–d | 30.67 a–d | 4.70 b–e |
| G4T1 | 44.47 ab | 37.83 ab | 17.93 ab | 20.83 ab | 53.87 a–d | 88.52 a | 77.93 b-e | 11.27 abc | 30.53 a–d | 4.67 b–e |
| G4T2 | 45.33 a | 39.47 a | 17.60 ab | 20.80 ab | 55.73 ab | 86.02 a–d | 73.27 c–f | 9.80 cde | 28.33 b–e | 4.71 b–e |
| G4T3 | 45.67 a | 39.93 a | 17.20 ab | 20.93 ab | 53.13 a–d | 89.00 a | 72.87 c–f | 9.80 cde | 28.60 a–e | 4.89 a–d |
| G4T4 | 40.73 bc | 38.80 ab | 18.20 ab | 20.20 b | 51.13 bcd | 82.73 cd | 76.93 b–e | 8.73 e | 26.67 cde | 4.55 cd |
| Mean | 43.16 | 38.28 | 17.58 | 20.91 | 53.13 | 85.96 | 76.29 | 10.40 | 29.88 | |
| CV% | 6.00 | 5.23 | 6.65 | 3.77 | 5.70 | 4.20 | 6.69 | 8.63 | 13.13 | 4.71 |
| GA3 Concentration | Number of Pods per Plant | Number of Seeded Pods per Plant | Number of Empty Pods per Plant | Days to Seed Maturity | Number of Seeds per Pod | Weight of Seeds per Plant (g) | Weight of 1000 Seeds (g) | Seed Yield (t/ha) |
|---|---|---|---|---|---|---|---|---|
| G0 = Control | 538.40 NS | 373.10 b | 165.40 ab | 149.60 NS | 8.86 b | 12.38 b | 4.75 ab | 0.18 b |
| G1 = 100 ppm | 560.20 NS | 414.50 ab | 145.70 bc | 146.40 NS | 9.32 b | 14.27 ab | 4.76 ab | 0.21 ab |
| G2 = 200 ppm | 600.90 NS | 465.00 a | 135.90 c | 147.20 NS | 10.87 a | 16.16 a | 4.83 a | 0.24 a |
| G3 = 300 ppm | 596.20 NS | 423.50 ab | 172.70 a | 148.10 NS | 8.75 b | 15.40 ab | 4.72 b | 0.22 ab |
| G4 = 400 ppm | 554.70 NS | 393.50 ab | 161.20 abc | 146.20 NS | 8.81 b | 14.38 ab | 4.72 b | 0.21 ab |
| Mean | 570.09 | 413.92 | 156.18 | 147.51 | 9.32 | 14.52 | 4.76 | 0.21 |
| CV% | 13.76 | 12.48 | 10.29 | 4.38 | 10.92 | 11.32 | 6.07 | 13.37 |
| Time of Application | Number of Pods per Plant | Number of Seeded Pods per Plant | Number of Empty Pods per Plant | Days to Seed Maturity | Number of Seeds per Pod | Weight of Seeds per Plant (g) | Weight of 1000 Seeds (g) | Seed Yield (t/ha) |
|---|---|---|---|---|---|---|---|---|
| T1 | 615.20 a | 460.10 a | 155.10 NS | 147.40 NS | 9.71 NS | 16.68 a | 4.80 a | 0.22 NS |
| T2 | 565.50 ab | 407.40 ab | 158.10 NS | 148.60 NS | 9.14 NS | 13.56 ab | 4.79 a | 0.21 NS |
| T3 | 569.10 ab | 416.90 ab | 152.30 NS | 147.10 NS | 9.16 NS | 14.72 ab | 4.84 a | 0.21 NS |
| T4 | 530.50 b | 371.30 b | 159.20 NS | 146.90 NS | 9.23 NS | 13.11 b | 4.61 b | 0.20 NS |
| Mean | 570.09 | 413.92 | 156.18 | 147.51 | 9.32 | 14.52 | 4.76 | 0.21 |
| CV% | 13.76 | 12.48 | 10.29 | 4.38 | 10.92 | 11.32 | 6.07 | 13.37 |
| Treatment Combination | Number of Pods per Plant | Number of Seeded Pods per Plant | Number of Empty Pods per Plant | Days to Seed Maturity | Number of Seeds per Pod | Weight of Seeds per Plant (g) | Weight of 1000 Seeds (g) | Seed Yield (t/ha) |
|---|---|---|---|---|---|---|---|---|
| G0T1 | 559.80 abc | 394.00 b–e | 165.80 a–d | 149.90 ab | 9.62 abc | 13.23 bc | 4.95 b–e | 0.19 abc |
| G0T2 | 546.10 abc | 356.70 cde | 189.50 ab | 148.30 abc | 7.47c | 12.17 bc | 4.55 j–m | 0.15 c |
| G0T3 | 516.40 bc | 360.30 cde | 156.10 a–d | 150.10 ab | 8.87 abc | 11.54 c | 4.37 m | 0.17 c |
| G0T4 | 531.30 abc | 381.30 b-e | 150.00 a–d | 149.90 ab | 9.50 abc | 12.60 bc | 5.14 ab | 0.19 abc |
| G1T1 | 634.40 ab | 462.00 a-e | 172.40 abc | 150.00 ab | 10.60 ab | 13.99 bc | 4.55 j–m | 0.22 abc |
| G1T2 | 435.00 c | 316.50 de | 118.50 d | 150.40 a | 8.05 bc | 12.67 bc | 4.77 e–h | 0.21 abc |
| G1T3 | 569.10 abc | 418.80 a–e | 150.30a–d | 138.70 c | 9.72 abc | 19.66 ab | 5.32 a | 0.24 abc |
| G1T4 | 602.40 abc | 460.80 a-e | 141.60 bcd | 146.70 abc | 8.83 abc | 10.76c | 4.39 lm | 0.17 c |
| G2T1 | 524.90 abc | 404.80 a–e | 120.10 d | 147.80 abc | 11.20 a | 22.75 a | 5.12 b | 0.27 a |
| G2T2 | 707.00 a | 570.00 a | 137.00 cd | 147.00 abc | 11.30 a | 16.78 abc | 4.75 f–i | 0.27 ab |
| G2T3 | 619.60 abc | 478.50 a-d | 141.10 bcd | 147.80 abc | 10.40 abc | 13.05 bc | 4.98 bcd | 0.23 abc |
| G2T4 | 552.30 abc | 406.60 a-e | 145.70 a–d | 146.40 abc | 10.40 abc | 12.05 c | 4.45 k–m | 0.21 abc |
| G3T1 | 705.50 a | 540.50 ab | 165.10 a–d | 149.70 ab | 8.93 abc | 16.87 abc | 4.68 g–j | 0.24 abc |
| G3T2 | 595.10 abc | 415.80 a–e | 179.30 abc | 148.00 abc | 9.10 abc | 11.47 c | 5.06 bc | 0.17 bc |
| G3T3 | 583.70 abc | 434.00 a-e | 149.70 a–d | 148.30 abc | 8.10 bc | 16.59 abc | 4.56 i–l | 0.24 abc |
| G3T4 | 500.30 bc | 303.70 e | 196.60 a | 146.40 abc | 8.87 abc | 16.68 abc | 4.59 h–k | 0.23 abc |
| G4T1 | 651.40 ab | 499.30 a–e | 152.10 a–d | 139.50 bc | 8.31 abc | 16.56 abc | 4.68 g–j | 0.19 abc |
| G4T2 | 544.20 abc | 377.90 b–e | 166.30 a–d | 149.50 ab | 9.77 abc | 14.68 bc | 4.80 d–g | 0.23 abc |
| G4T3 | 556.80 abc | 392.80 b–e | 164.10 a–d | 150.70 a | 8.63 abc | 12.78 bc | 4.91 c–f | 0.23 abc |
| G4T4 | 466.30 bc | 304.00 e | 162.30 a–d | 145.10 abc | 8.53 abc | 13.49 bc | 4.47 k–m | 0.22 abc |
| Mean | 570.09 | 413.92 | 156.17 | 147.51 | 9.32 | 14.52 | 4.76 | 0.21 |
| CV% | 13.76 | 12.48 | 10.29 | 4.38 | 10.92 | 11.32 | 6.07 | 13.37 |
| Treatment | Total Cost of Production (Tk/ha) | Yield (kg/ha) | Gross Return (Tk/ha) | Net Return (Tk/ha) | Benefit-Cost Ratio |
|---|---|---|---|---|---|
| G0 | 129,498 | 175.3 kg | 438,250 | 308,752 | 3.38 |
| G1 | 135,053 | 210.5 kg | 526,250 | 391,197 | 3.90 |
| G2 | 140,609 | 244.1 kg | 610,250 | 469,641 | 4.34 |
| G3 | 146,399 | 221.3 kg | 553,250 | 406,851 | 3.78 |
| G4 | 151,718 | 210.3 kg | 525,750 | 374,032 | 3.47 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prodhan, M.M.; Sarker, U.; Hoque, M.A.; Biswas, M.S.; Ercisli, S.; Assouguem, A.; Ullah, R.; Almutairi, M.H.; Mohamed, H.R.H.; Najda, A. Foliar Application of GA3 Stimulates Seed Production in Cauliflower. Agronomy 2022, 12, 1394. https://doi.org/10.3390/agronomy12061394
Prodhan MM, Sarker U, Hoque MA, Biswas MS, Ercisli S, Assouguem A, Ullah R, Almutairi MH, Mohamed HRH, Najda A. Foliar Application of GA3 Stimulates Seed Production in Cauliflower. Agronomy. 2022; 12(6):1394. https://doi.org/10.3390/agronomy12061394
Chicago/Turabian StyleProdhan, Md. Masud, Umakanta Sarker, Md. Azizul Hoque, Md. Sanaullah Biswas, Sezai Ercisli, Amine Assouguem, Riaz Ullah, Mikhlid H. Almutairi, Hanan R. H. Mohamed, and Agnieszka Najda. 2022. "Foliar Application of GA3 Stimulates Seed Production in Cauliflower" Agronomy 12, no. 6: 1394. https://doi.org/10.3390/agronomy12061394
APA StyleProdhan, M. M., Sarker, U., Hoque, M. A., Biswas, M. S., Ercisli, S., Assouguem, A., Ullah, R., Almutairi, M. H., Mohamed, H. R. H., & Najda, A. (2022). Foliar Application of GA3 Stimulates Seed Production in Cauliflower. Agronomy, 12(6), 1394. https://doi.org/10.3390/agronomy12061394










