Integrated Application of Inorganic and Organic Fertilizer Enhances Soil Organo-Mineral Associations and Nutrients in Tea Garden Soil
Abstract
:1. Introduction
2. Methods
2.1. Pot Experiment and Fertilizer Treatments
2.2. Soil Sampling
2.3. Chemical Analyses
2.4. SR-FTIR Spectrocopy
2.5. 2D correlation Spectra (2DCOS) Analysis
2.6. X-ray Powder Diffraction Analysis
2.7. Statistical Analysis
3. Results
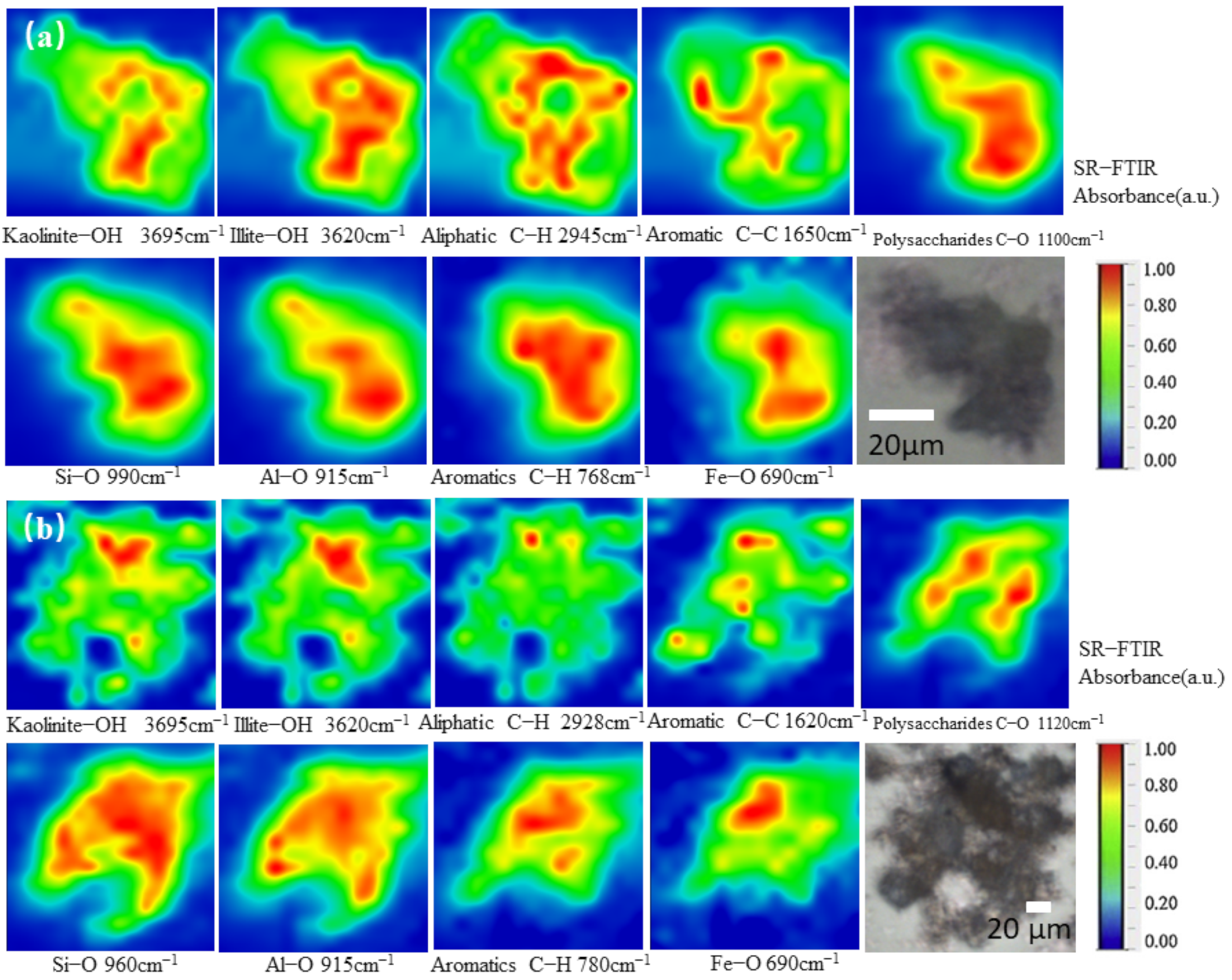
3.1. Spatial Distribution of Tea Plantation Soil by SR-FTIR
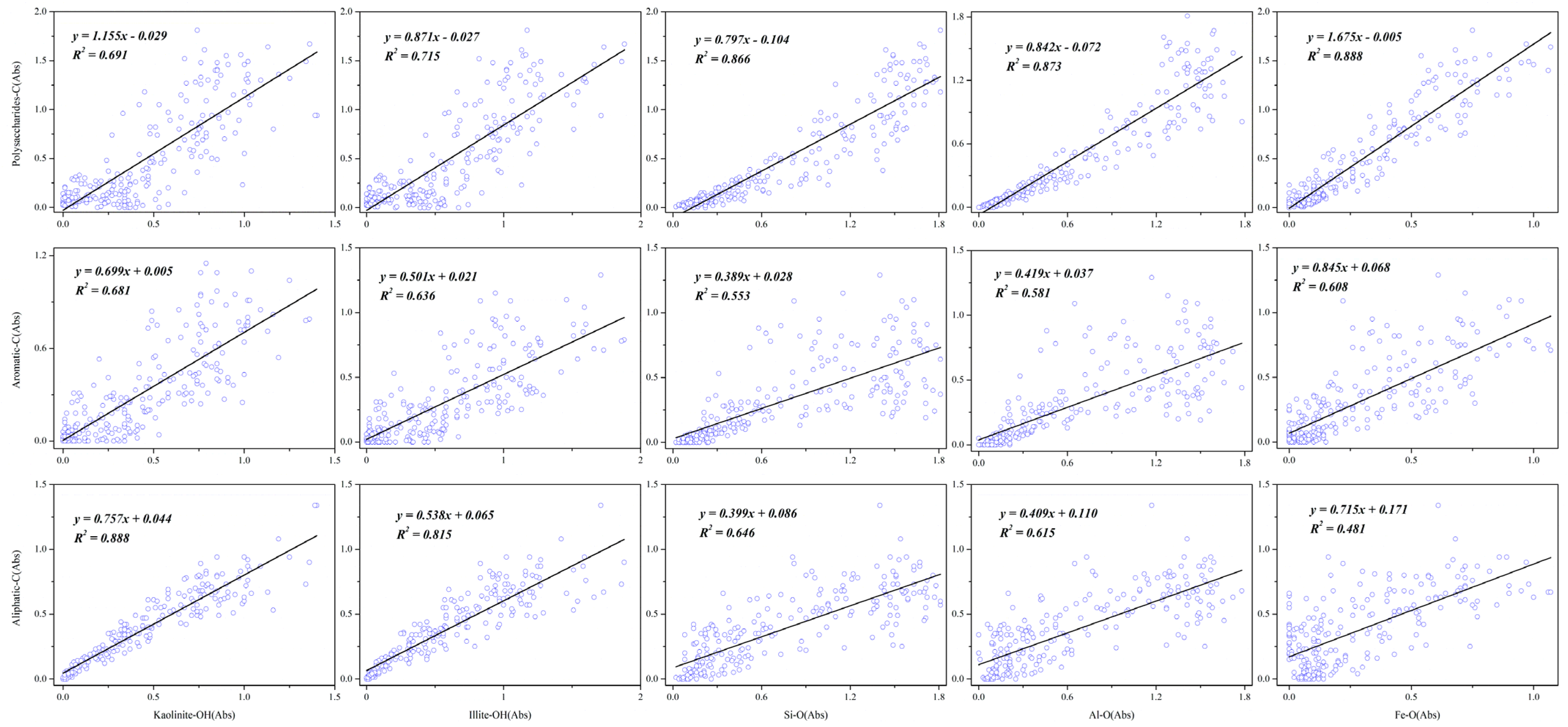
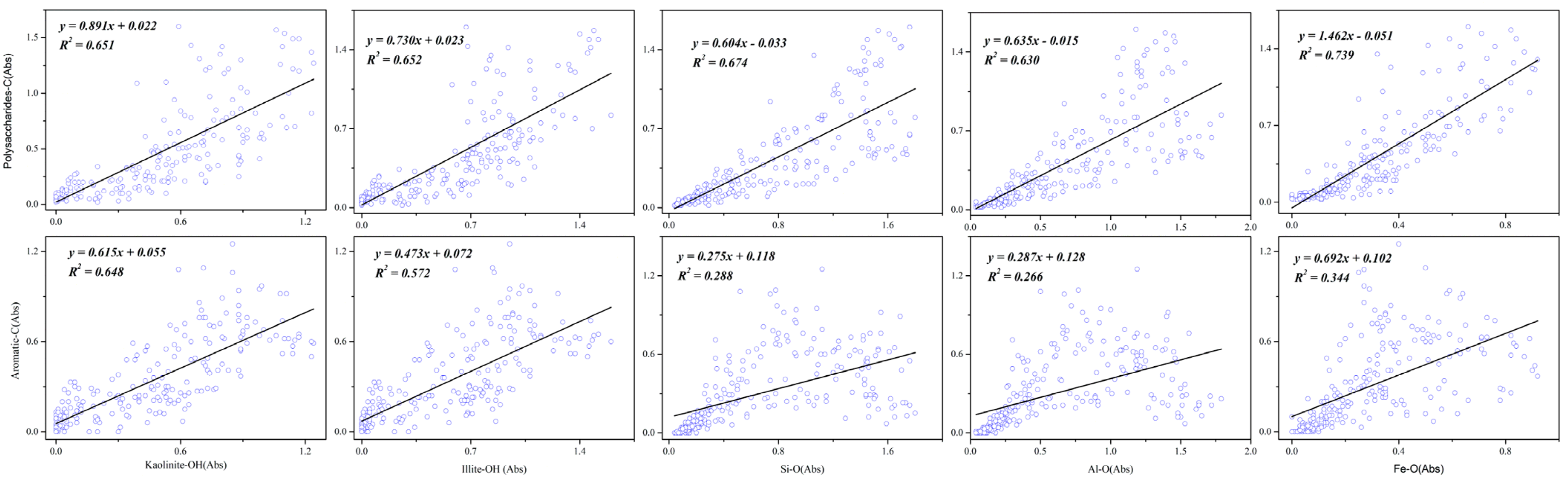
3.2. Spatial Correlation of Tea Plantation Soil by SR-FTIR
3.3. DCOS Analysis of Soil Microaggregates’ Stable Sequestration Reactivity
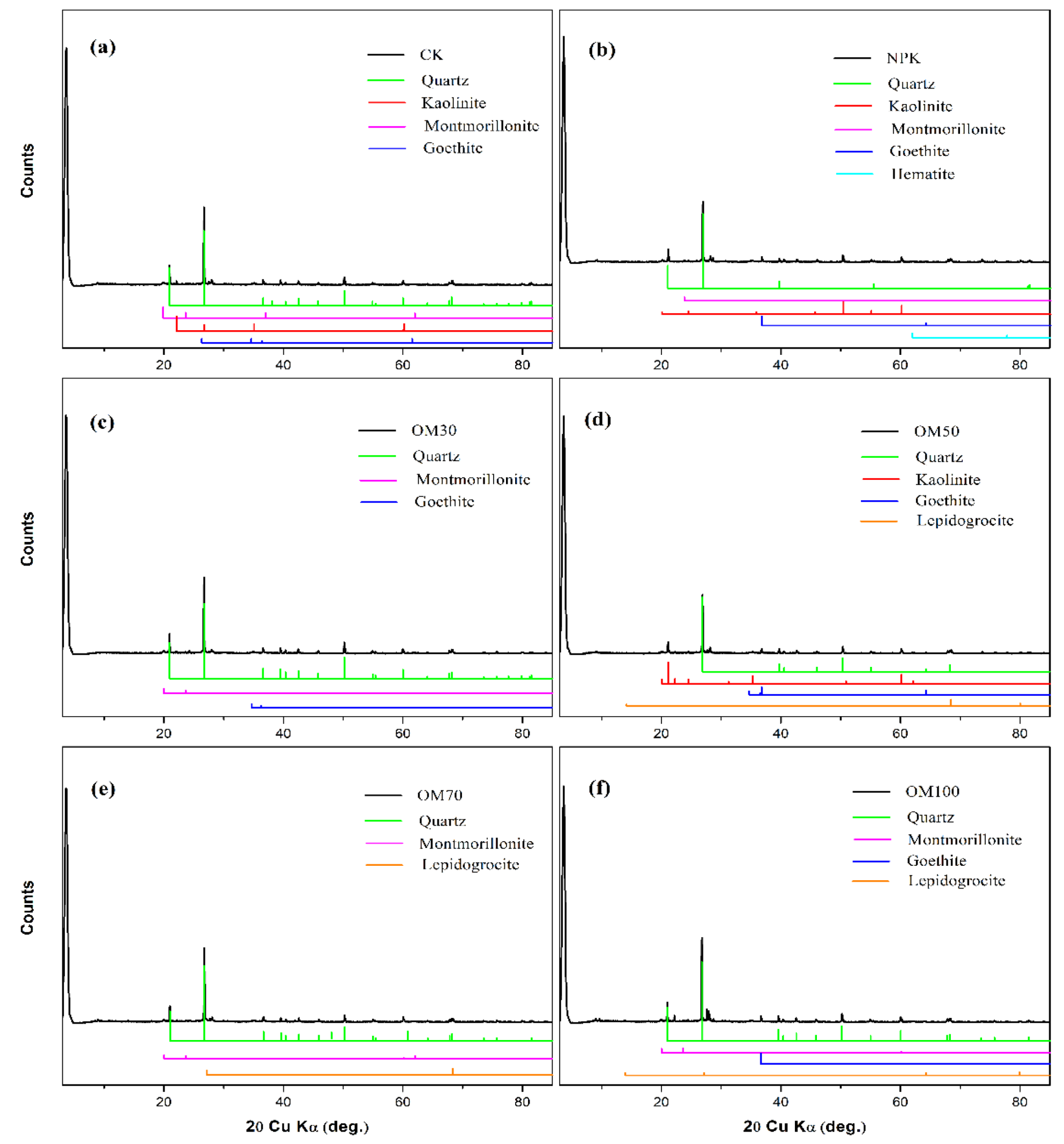
3.4. Mineral Composition of Soil Determined by XRD
3.5. Soil pH, TN, SOM, Available P and K
3.6. Total and Exchangeable Elemental Concentrations Content in Soils
4. Discussion
4.1. The Effect of Fertilizers on Organo-Mineral Association
4.2. The Effect of Fertilizers on Soil Nutrients
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yan, P.; Shen, C.; Fan, L.; Li, X.; Zhang, L.; Zhang, L.; Han, W. Tea planting affects soil acidification and nitrogen and phosphorus distribution in soil. Agric. Ecosyst. Environ. 2018, 254, 20–25. [Google Scholar] [CrossRef]
- Su, S.; Zhou, X.; Wan, C.; Li, Y.; Kong, W. Land use changes to cash crop plantations: Crop types: Multilevel determinants and policy implications. Land Use Policy 2015, 50, 379–389. [Google Scholar] [CrossRef]
- Kamau, D.M.; Spiertz, J.H.J.; Oenema, O. Carbon and nutrient stocks of tea plantations differing in age, genotype and plant population density. Plant Soil 2008, 307, 29–39. [Google Scholar] [CrossRef] [Green Version]
- Mudau, F.N.; Soundy, P.; du Toit, E.S. Nitrogen, phosphorus, and potassium nutrition increases growth and total poly-phenol concentrations of bush tea in a shaded nursery environment. Hort. Technol. 2007, 17, 107–110. [Google Scholar] [CrossRef]
- Ruan, J.; Haerdter, R.; Gerendas, J. Impact of nitrogen supply on carbon/nitrogen allocation: A case study on amino acids and catechins in green tea plants. Plant Biol. 2010, 12, 724–734. [Google Scholar] [CrossRef]
- Barak, P.; Jobe, B.O.; Krueger, A.R.; Peterson, L.A.; Laird, D.A. Effects of long-term soil acidification due to nitrogen fertilizer inputs in Wisconsin. Plant Soil 1997, 197, 61–69. [Google Scholar] [CrossRef]
- Li, S.Y.; Li, H.X.; Yang, C.L.; Wang, Y.D.; Xue, H.; Niu, Y.F. Rates of soil acidification in tea plantations and possible causes. Agric. Ecosyst. Environ. 2016, 233, 60–66. [Google Scholar] [CrossRef]
- Yang, X.D.; Ni, K.; Shi, Y.Z.; Yi, X.Y.; Zhang, Q.F.; Fang, L.; Ma, L.F.; Ruan, J.Y. Effects of long-term nitrogen application on soil acidification and solution chemistry of a tea plantation in China. Agric. Ecosyst. Environ. 2018, 252, 74–82. [Google Scholar] [CrossRef]
- Pagliai, M.; Vignozzi, N.; Pellegrini, S. Soil structure and the effect of management practices. Soil Tillage Res. 2004, 79, 131–143. [Google Scholar] [CrossRef]
- Rauber, L.P.; Andrade, A.P.; Friederichs, A.; Mafra, A.L.; Baretta, D.; da Rosa, M.G.; Heberle Mafra, M.S.; Correa, J.C. Soil physical indicators of management systems in traditional agricultural areas under manure application. Sci. Agric. 2018, 75, 354–359. [Google Scholar] [CrossRef] [Green Version]
- Mabaya, G.; Unami, K.; Fujihara, M. Stochastic optimal control of agrochemical pollutant loads in reservoirs for irrigation. J. Clean. Prod. 2017, 146, 37–46. [Google Scholar] [CrossRef]
- Wang, Z.Z.; Zhang, T.Q.; Tan, C.S.; Vadas, P.; Qi, Z.M.; Wellen, C. Modeling phosphorus losses from soils amended with cattle manures and chemical fertilizers. Sci. Total Environ. 2018, 639, 580–587. [Google Scholar] [CrossRef] [PubMed]
- Baffaut, C.; Ghidey, F.; Lerch, R.N.; Kitchen, N.R.; Sudduth, K.A.; Sadler, E.J. Long-term simulated runoff and water quality from grain cropping systems on restrictive layer soils. Agric. Water Manag. 2019, 213, 36–48. [Google Scholar] [CrossRef]
- Wang, P.P.; Zhang, W.D.; Li, M.H.; Han, Y.J. Does fertilizer education program increase the technical efficiency of chemical fertilizer use? Evidence from wheat production in China. Sustainability 2019, 11, 543. [Google Scholar] [CrossRef] [Green Version]
- Edmeades, D.C. The long-term effects of manures and fertilisers on soil productivity and quality: A review. Nutr. Cycl. Agroecosyst. 2003, 66, 165–180. [Google Scholar] [CrossRef]
- Diacono, M.; Montemurro, F. Long-Term Effects of Organic Amendments on Soil Fertility. A Review. Agron. Sustain. Dev. 2010, 30, 401–422. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.M.; Wang, M.K.; Zhuang, S.Y.; Chiang, P.N. Chemical and physical properties of rhizosphere and bulk soils of three tea plants cultivated in Ultisols. Geoderma 2006, 136, 378–387. [Google Scholar] [CrossRef]
- Huang, X.L.; Jiang, H.; Li, Y.; Ma, Y.C.; Tang, H.Y.; Ran, W.; Shen, Q.R. The role of poorly crystalline iron oxides in the stability of soil aggregate-associated organic carbon in a rice-wheat cropping system. Geoderma 2016, 279, 1–10. [Google Scholar] [CrossRef]
- Wissing, L.; Kölbl, A.; Häusler, W.; Schad, P.; Cao, Z.H.; Kögel-Knabner, I. Management-induced organic carbon accumulation in paddy soils: The role of organo-mineral associations. Soil Tillage Res. 2013, 126, 60–71. [Google Scholar] [CrossRef]
- Ji, L.; Ni, K.; Wu, Z.; Zhang, J.; Yi, X.; Yang, X.; Ling, N.; You, Z.; Guo, S.; Ruan, J. Effect of organic substitution rates on soil quality and fungal community composition in a tea plantation with long-term fertilization. Biol. Fertil. Soils 2020, 56, 633–646. [Google Scholar] [CrossRef]
- Ji, L.; Wu, Z.; You, Z.; Yi, X.; Ni, K.; Guo, S.; Ruan, J. Effects of organic substitution for synthetic N fertilizer on soil bacterial diversity and community composition: A 10-year field trial in a tea plantation. Agric. Ecosyst. Environ. 2018, 268, 124–132. [Google Scholar] [CrossRef]
- Six, J.; Elliott, E.T.; Paustian, K. Soil macroaggregate turnover and microaggregate formation: A mechanism for C sequestration under no-tillage agriculture. Soil Biol. Biochem. 2000, 32, 2099–2103. [Google Scholar] [CrossRef]
- Schmidt, M.W.I.; Torn, M.S.; Abiven, S.; Dittmar, T.; Guggenberger, G.; Janssens, I.A.; Kleber, M.; Kogel-Knabner, I.; Lehmann, J.; Manning, D.A.C.; et al. Persistence of soil organic matter as an ecosystem property. Nature 2011, 478, 49–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brar, B.S.; Singh, K.; Dheri, G.S.; Kumar, B. Carbon sequestration and soil carbon pools in a rice–wheat rotation system: Effect of long-term use of inorganic fertilizers and organic manure. Soil Tillage Res. 2013, 128, 30–36. [Google Scholar] [CrossRef]
- Mohanty, M.; Painuli, D.K.; Misra, A.K.; Ghosh, P.K. Soil quality effects of tillage and residue under rice-wheat rotation on a Vertisol in India. Soil Tillage Res. 2007, 92, 243–250. [Google Scholar] [CrossRef]
- Zhou, P.; Song, G.H.; Pan, G.X.; Li, L.Q.; Zhang, X.H. Role of chemical protection by binding to oxyhydrates in SOC sequestration in three typical paddy soils under long-term agro-ecosystem experiments from South China. Geoderma 2009, 153, 52–60. [Google Scholar] [CrossRef]
- Lehmann, J.; Solomon, D.; Kinyangi, J.; Dathe, L.; Wirick, S.; Jacobsen, C. Spatial complexity of soil organic matter forms at nanometre scales. Nat. Geosci. 2008, 1, 238–242. [Google Scholar] [CrossRef]
- Luo, L.; Lv, J.; Xu, C.; Zhang, S. Strategy for characterization of distribution and associations of organobromine compounds in soil using synchrotron radiation based spectromicroscopies. Anal. Chem. 2014, 86, 11002–11005. [Google Scholar] [CrossRef]
- Lehmann, J.; Kleber, M. The contentious nature of soil organic matter. Nature 2015, 528, 60–68. [Google Scholar] [CrossRef]
- Lehmann, J.; Kinyangi, J.; Solomon, D. Organic matter stabilization in soil microaggregates: Implications from spatial heterogeneity of organic carbon contents and carbon forms. Biogeochemistry 2007, 85, 45–57. [Google Scholar] [CrossRef]
- Yan, P.; Wu, L.; Wang, D.; Fu, J.; Shen, C.; Li, X.; Zhang, L.; Zhang, L.; Fan, L.; Wenyan, H. Soil acidification in Chinese tea plantations. Sci. Total Environ. 2020, 715, 136963. [Google Scholar] [CrossRef] [PubMed]
- Maillard, E.; Angers, D.A. Animal manure application and soil organic carbon stocks: A meta-analysis. Glob. Chang. Biol. 2014, 20, 666–679. [Google Scholar] [CrossRef] [PubMed]
- Mehlich, A. Mehlich 3 soil test extractant: A modification of Mehlich 2 extractant. Comm. Soil Sci. Plant Anal. 1984, 15, 1409–1416. [Google Scholar] [CrossRef]
- Zhao, F.; Mcgrath, S.P.; Crosland, A.R. Comparison of three wet digestion methods for the determination of plant sulfur by inductively coupled plasma atomic emission spectroscopy (ICP-AES). Comm. Soil Sci. Plant Anal. 1994, 25, 407–418. [Google Scholar] [CrossRef]
- Sun, F.; Li, Y.; Wang, X.; Chi, Z.; Yu, G. Using new hetero-spectral two-dimensional correlation analyses and synchrotron-radiation-based spectromicroscopy to characterize binding of Cu to soil dissolved organic matter. Environ. Pollut. 2017, 223, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Artz, R.R.E.; Chapman, S.J.; Jean Robertson, A.H.; Potts, J.M.; Laggoun-Défarge, F.; Gogo, S.; Comont, L.; Disnar, J.R.; Francez, A.J. FTIR spectroscopy can be used as a screening tool for organic matter quality in regenerating cutover peatlands. Soil Biol. Biochem. 2008, 40, 515–527. [Google Scholar] [CrossRef]
- Peltre, C.; Bruun, S.; Du, C.; Thomsen, I.K.; Jensen, L.S. Assessing soil constituents and labile soil organic carbon by mid-infrared photoacoustic spectroscopy. Soil Biol. Biochem. 2014, 77, 41–50. [Google Scholar] [CrossRef]
- Noda, I.; Ozaki, Y. Two-Dimensional Correlation Spectroscopy: Applications in Vibrational and Optical Spectroscopy; John Wiley & Sons, Ltd.: New York, NY, USA, 2005. [Google Scholar]
- Wen, Y.; Li, H.; Xiao, J.; Wang, C.; Shen, Q.; Ran, W.; He, X.; Zhou, Q.; Yu, G. Insights into complexation of dissolved organic matter and Al (III) and nano-minerals formation in soils under contrasting fertilizations using two-dimensional correlation spectroscopy and high resolution-transmission electron microscopy techniques. Chemosphere 2014, 111, 441–449. [Google Scholar] [CrossRef]
- Li, H.; Hu, S.; Polizzotto, M.L.; Chang, X.; Shen, Q.; Ran, W.; Yu, G. Fungal biomineralization of montmorillonite and goethite to short-range-ordered minerals. Geochim. Cosmochim. Acta 2016, 191, 17–31. [Google Scholar] [CrossRef]
- Yu, G.H.; Wu, M.J.; Wei, G.R.; Luo, Y.H.; Ran, W.; Wang, B.R.; Zhang, J.C.; Shen, Q.R. Binding of organic ligands with Al (III) in dissolved organic matter from soil: Implications for soil organic carbon storage. Environ. Sci. Technol. 2012, 46, 6102–6109. [Google Scholar] [CrossRef]
- Kleber, M.; Eusterhues, K.; Keiluweit, M.; Mikutta, C.; Mikutta, R.; Nico, P. Mineral–organic associations: Formation, properties, and relevance in soil environments. Adv. Agron. 2015, 130, 1–140. [Google Scholar]
- Xiao, J.; Wen, Y.L.; Li, H.; Hao, J.L.; Shen, Q.R.; Ran, W.; Mei, X.L.; He, X.H.; Yu, G.H. In situ visualization and characterization of the capacity of highly reactive minerals to preserve soil organic matter (SOM) in colloids at submicron scale. Chemosphere 2015, 138, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.H.; Xiao, J.; Hu, S.J.; Polizzotto, M.L.; Zhao, F.J.; Mcgrath, S.P.; Li, H.; Ran, W.; Shen, Q.R. Mineral availability as a key regulator of soil carbon storage. Environ. Sci. Technol. 2017, 51, 4960–4969. [Google Scholar] [CrossRef] [PubMed]
- Riedel, T.; Zak, D.; Biester, H.; Dittmar, T. Iron traps terrestrially derived dissolved organic matter at redox interfaces. Proc. Natl. Acad. Sci. USA 2013, 110, 10101–10105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lutzow, M.v.; Kögel-Knabner, I.; Ekschmitt, K.; Matzner, E.; Guggenberger, G.; Marschner, B.; Flessa, H. Stabilization of organic matter in temperate soils: Mechanisms and their relevance under different soil conditions-a review. Eur. J. Soil Sci. 2006, 57, 426–445. [Google Scholar] [CrossRef]
- Torres-Sallan, G.; Schulte, R.P.; Lanigan, G.J.; Byrne, K.A.; Reidy, B.; Simo, I.; Six, J.; Creamer, R.E. Clay illuviation provides a long-term sink for C sequestration in subsoils. Sci. Rep. 2017, 7, 45635. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wu, S.; Liu, Y.; Yi, Q.; You, F.; Ma, Y.; Thomsen, L.; Chan, T.S.; Lu, Y.R.; Hall, M.; et al. Arbuscular mycorrhizal symbiosis enhances water stable aggregate formation and organic matter stabilization in Fe ore tailings. Geoderma 2022, 406, 115528. [Google Scholar] [CrossRef]
- Ju, X.T.; Kou, C.L.; Christie, P.; Dou, Z.X.; Zhang, F.S. Changes in the soil environment from excessive application of fertilizers and manures to two contrasting intensive cropping systems on the North China Plain. Environ. Pollut. 2007, 145, 497e506. [Google Scholar] [CrossRef] [Green Version]
- Leita, L.; De Nobili, M.; Mondini, C.; Muhlbachova, G.; Marchiol, L.; Bragato, G.; Contin, M. Influence of inorganic and organic fertilization on soil microbial biomass, metabolic quotient and heavy metal bioavailability. Biol. Fert. Soils 1999, 28, 371–376. [Google Scholar] [CrossRef]
- Chaffron, S.; Rehrauer, H.; Pernthaler, J.; Von Mering, C. A global network of co-existing microbes from environmental and whole-genome sequence data. Genome Res. 2010, 20, 947–959. [Google Scholar] [CrossRef] [Green Version]
- Guo, J.H.; Liu, X.J.; Zhang, Y.; Shen, J.L.; Han, W.X.; Zhang, W.F.; Christie, P.; Goulding, K.W.T.; Vitousek, P.M.; Zhang, F.S. Significant acidification in major Chinese croplands. Science 2010, 327, 1008–1010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, Q.C.; Liu, X.J.; Hao, T.X.; Zeng, M.F.; Shen, J.B.; Zhang, F.S. Modeling soil acidification in typical Chinese cropping systems. Sci. Total Environ. 2018, 613–614, 1339–1348. [Google Scholar] [CrossRef] [Green Version]
- Shi, R.Y.; Liu, Z.D.; Li, Y.; Jiang, T.; Xu, M.; Li, J.Y.; Xu, R.K. Mechanisms for increasing soil resistance to acidification by long-term manure application. Soil Tillage Res. 2019, 185, 77–84. [Google Scholar] [CrossRef]
- Liu, Z.; Rong, Q.; Zhou, W.; Liang, G. Effects of inorganic and organic amendment on soil chemical properties, enzyme activities, microbial community and soil quality in yellow clayey soil. PLoS ONE 2017, 12, e0172767. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Gil, J.C.; Ceppi, S.B.; Velasco, M.I.; Polo, A.; Senesi, N. Long-term effects of amendment with municipal solid waste compost on the elemental and acidic functional group composition and pH-buffer capacity of soil humic acids. Geoderma 2004, 121, 135–142. [Google Scholar] [CrossRef]
- Wang, L.; Butterly, C.R.; Tian, W.; Herath, H.M.S.K.; Xi, Y.; Zhang, J.; Xiao, X. Effects of fertilization practices on aluminum fractions and species in a wheat soil. J. Soils Sediments 2016, 16, 1933–1943. [Google Scholar] [CrossRef]
- Xiao, K.; Xu, J.; Tang, C.; Zhang, J.; Brookes, P.C. Differences in carbon and nitrogen mineralization in soils of differing initial pH induced by electrokinesis and receiving crop residue amendments. Soil Biol. Biochem. 2013, 67, 70–84. [Google Scholar] [CrossRef]
- Cai, Z.; Wang, B.; Xu, M.; Zhang, H.; He, X.; Zhang, L.; Gao, S. Intensified soil acidification from chemical n fertilization and prevention bymanure in an 18-year field experiment in the red soil of southern China. J. Soils Sediments 2015, 15, 260–270. [Google Scholar] [CrossRef]

| Treatment | Total Nutrients of Fertilizers | Organic Fertilizer | ||||
|---|---|---|---|---|---|---|
| N | P2O5 | K2O | N | P2O5 | K2O | |
| CK | 0 | 0 | 0 | 0 | 0 | 0 |
| NPK | 350 | 85 | 55 | 0 | 0 | 0 |
| OM30 | 350 | 184 | 123 | 105 | 99 | 68 |
| OM50 | 350 | 260 | 169 | 175 | 165 | 114 |
| OM70 | 350 | 315 | 215 | 245 | 230 | 160 |
| OM100 | 350 | 329 | 228 | 350 | 329 | 228 |
| Peak Position (cm−1) | Vibration | Functional Group and References |
|---|---|---|
| 3695, 3620 | vO-H | Clay OH minerals (kaolinite-OH clay, illite-OH clay etc.) [28,30,35] |
| 2945 | vC-H | Aliphatic methyl and methylene groups [28,30,35] |
| 1650 | vC=C | Aromatics [35] |
| 1100 | vC-OH | Polysaccharides [36] |
| 981 | vSi-O | Silicates, clay minerals [37] |
| 915 | δAl-OH | Kaolinite and smectite [35,37] |
| 690 | vFe-O | Iron oxides [35] |
| Treatment | Number of Points | Kaolinite-OH 3695 cm−1 | Illite-OH 3620 cm−1 | Si-O 981 cm−1 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aliphatic-C-H 2945 cm−1 | Aromatic-C-C 1650 cm−1 | Polysaccharides C-OH 1100 cm−1 | Aliphatic-C-H 2945 cm−1 | Aromatic-C-C 1650 cm−1 | Polysaccharides C-OH 1100 cm−1 | Aliphatic-C 2945 cm−1 | Aromatic-C-C 1650 cm−1 | Polysaccharides C-OH 1100 cm−1 | |||||
| CK | 255 | y = 0.615x + 0.055R2 = 0.648 | y = 0.891x + 0.022R2 = 0.651 | y = 0.473x + 0.072R2= 0.572 | y = 0.730x + 0.023R2 = 0.652 | y = 0.275x + 0.118R2 = 0.288 | y = 0.604x − 0.033R2 = 0.674 | ||||||
| NPK | 195 | y = 0.734x + 0.117R2 = 0.920 | y = 0.497x + 0.153R2 = 0.553 | y = 0.982x − 0.084R2 = 0.800 | y = 0.545x + 0.177R2 = 0.839 | y = 0.377x + 0.188R2 = 0.526 | y = 0.796x − 0.055R2 = 0.870 | y = 0.571x + 0.242R2 = 0.684 | y = 0.419x + 0.217R2 = 0.483 | y = 0.965x − 0.043R2 = 0.952 | |||
| OM30 | 240 | y = 0.525x + 0.055R2 = 0.694 | y = 1.06x + 0.078R2 = 0.715 | y = 0.375x + 0.068R2 = 0.625 | y = 0.873x + 0.051R2 = 0.790 | y = 0.310x + 0.082R2 = 0.436 | y = 0.898x − 0.043R2 = 0.850 | ||||||
| OM50 | 195 | y = 0.774x + 0.051R2 = 0.905 | y = 0.570x + 0.165R2 = 0.643 | y = 0.816x + 0.057R2 = 0.758 | y = 0.566x + 0.096R2 = 0.806 | y = 0.414x + 0.200R2 = 0.563 | y = 0.648x + 0.069R2 = 0.799 | y = 0.709x + 0.077R2 = 0.639 | y = 0.507x + 0.192R2 = 0.427 | y = 0.999x − 0.059R2 = 0.959 | |||
| OM70 | 256 | y = 0.757x + 0.044R2 = 0.888 | y = 0.699x + 0.005R2 = 0.681 | y = 1.155x − 0.029R2 = 0.691 | y = 0.538x + 0.065R2 = 0.815 | y = 0.501x + 0.021R2 = 0.636 | y = 0.871x − 0.027R2 = 0.715 | y = 0.399x + 0.086R2 = 0.646 | y = 0.389x + 0.028R2 = 0.553 | y = 0.797x − 0.104R2 = 0.866 | |||
| OM100 | 255 | y = 0.841x + 0.023R2 = 0.934 | y = 0.620x + 0.052R2 = 0.677 | y = 0.967x + 0.086R2 = 0.712 | y = 0.670x + 0.033R2 = 0.885 | y = 0.480x + 0.066R2 = 0.605 | y = 0.815x + 0.076R2 = 0.756 | y = 0.477x + 0.053R2 = 0.577 | y = 0.320x + 0.094R2 = 0.346 | y = 0.780x − 0.024R2 = 0.892 | |||
| Treatment | Number of Points | Al-O 915 cm−1 | Fe-O 690 cm−1 | ||||||||||
| Aliphatic-C-H 2945 cm−1 | Aromatic-C-C 1650 cm−1 | Polysaccharides C-OH 1100 cm−1 | Aliphatic-C-H 2945 cm−1 | Aromatic-C-C 1650 cm−1 | Polysaccharides C-OH 1100 cm−1 | ||||||||
| CK | 255 | y = 0.287x + 0.128 R2 = 0.266 | y = 0.635x − 0.015 R2 = 0.630 | y = 1.462x − 0.051 R2= 0.739 | y = 0.692x + 0.102 R2 = 0.344 | ||||||||
| NPK | 195 | y = 0.559x + 0.269 R2 = 0.621 | y = 0.419x + 0.232 R2 = 0.458 | y = 0.998x − 0.030 R2 = 0.967 | y = 0.812x + 0.333 R2= 0.486 | y = 0.640x + 0.269 R2= 0.396 | y = 1.590x + 0.039 R2 = 0.911 | ||||||
| OM30 | 240 | y = 0.337x + 0.081 R2 = 0.430 | y = 1.020x − 0.075 R2 = 0.920 | y = 0.458x + 0.086 R2= 0.406 | y = 1.393x − 0.063 R2 = 0.878 | ||||||||
| OM50 | 195 | y = 0.829x + 0.058 R2 = 0.656 | y = 0.590x + 0.180 R2 = 0.434 | y = 1.151x − 0.078 R2 = 0.957 | y = 1.048x + 0.072 R2= 0.525 | y = 0.779x + 0.177 R2= 0.378 | y = 1.535x − 0.088 R2 = 0.852 | ||||||
| OM70 | 256 | y = 0.409x + 0.110 R2 = 0.615 | y = 0.419x + 0.037 R2 = 0.581 | y = 0.842x − 0.072 R2 = 0.873 | y = 0.715x + 0.171 R2= 0.481 | y = 0.845x + 0.068 R2= 0.608 | y = 1.675x − 0.005 R2 = 0.888 | ||||||
| OM100 | 255 | y = 0.468x + 0.075 R2 = 0.518 | y = 0.314x + 0.108 R2 = 0.310 | y = 0.773x +0.005R2 = 0.820 | y = 0.468x + 0.075 R2= 0.518 | y = 0.746x + 0.083 R2= 0.383 | y = 1.597x + 0.011 R2 = 0.764 | ||||||
| Treatment | pH | SOM | TN | C/N | AP | AK |
|---|---|---|---|---|---|---|
| g kg−1 | mg kg−1 | |||||
| CK | 6.53 ± 0.05 a | 18.02 ± 0.29 bc | 1.54 ± 0.07 ab | 6.82 ± 0.21 b | 196.17 ± 4.28 a | 208.55 ± 4.41 b |
| NPK | 5.76 ± 0.02 d | 10.31 ± 0.36 d | 1.07 ± 0.06 c | 5.58 ± 0.13 c | 44.94 ± 6.51 c | 176.75 ± 2.58 b |
| OM30 | 5.58 ± 0.03 e | 15.04 ± 2.65 cd | 1.33 ± 0.21 bc | 6.51 ± 0.14 b | 105.30 ± 43.50 bc | 225.38 ± 24.81 b |
| OM50 | 5.93 ± 0.07 c | 23.50 ± 1.35 ab | 1.92 ± 0.08 a | 7.09 ± 0.21 b | 226.57 ± 5.69 a | 313.60 ± 19.55 a |
| OM70 | 6.23 ± 0.05 b | 25.89 ± 2.01 a | 1.91 ± 0.12 a | 7.85 ± 0.13 a | 237.8 ± 0.85 a | 334.38 ± 19.63 a |
| OM100 | 6.16 ± 0.03 b | 20.13 ± 2.64 abc | 1.74 ± 0.17 ab | 6.68 ± 0.22 b | 151.17 ± 47.74 ab | 322.65 ± 25.12 a |
| Treatment | Ca | Mg | Fe | Al | Mn | Zn |
|---|---|---|---|---|---|---|
| CK | 4203.00 ± 239.56 a | 384.30 ± 2.72 c | 273.13 ± 2.99 ab | 1025.67 ± 11.40 c | 220.37 ± 4.65 b | 14.44 ± 0.72 bcd |
| NPK | 2822.67 ± 94.27 c | 483.00 ± 9.72 ab | 225.87 ± 2.53 c | 1111.67 ± 13.28 a | 303.40 ± 6.63 a | 7.91 ± 0.31 d |
| OM30 | 2887.67 ± 221.10 bc | 457.28 ± 38.70 abc | 253.93 ± 14.99 b | 1102.67 ± 17.67 a | 265.62 ± 32.68 ab | 11.88 ± 2.38 cd |
| OM50 | 3550.00 ± 232.33 ab | 410.45 ± 12.75 bc | 293.00 ± 8.16 a | 1087.17 ± 26.44 ab | 211.33 ± 4.30 b | 20.93 ± 3.39 ab |
| OM70 | 4216.83 ± 141.53 a | 415.42 ± 10.85 bc | 292.62 ± 4.01 a | 1051.33 ± 11.70 bc | 227.57 ± 4.83 b | 24.52 ± 2.12 a |
| OM100 | 3219.83 ± 269.70 bc | 505.67 ± 39.19 a | 287.77 ± 13.15 a | 1109.00 ± 6.21 a | 265.13 ± 25.34 ab | 17.27 ± 2.07 bc |
| Treatment | g kg−1 | mg kg−1 | ||||||
|---|---|---|---|---|---|---|---|---|
| K | Ca | Mg | Fe | P | Zn | Cu | Cr | |
| CK | 14.49 ± 0.12 a | 12.06 ± 0.30 a | 5.80 ± 0.06 a | 34.53 ± 0.09 a | 815.67 ± 11.57 ab | 104.08 ± 1.71 ab | 35.98 ± 1.04 ab | 105.62 ± 2.45 a |
| NPK | 12.93 ± 0.14 b | 9.95 ± 0.08 c | 4.94 ± 0.08 c | 33.61 ± 0.40 ab | 358.63 ± 17.07 d | 87.00 ± 2.41 b | 30.31 ± 0.24 b | 96.38 ± 0.38 b |
| OM30 | 13.42 ± 0.52 b | 10.23 ± 0.42 c | 5.17 ± 0.25 bc | 33.76 ± 0.42 ab | 533.71 ± 135.56 cd | 97.55 ± 10.31 b | 35.16 ± 4.61 ab | 98.38 ± 3.27 b |
| OM50 | 14.54 ± 0.01 a | 11.28 ± 0.32 ab | 5.82 ± 0.04 a | 34.58 ± 0.22 a | 861.71 ± 27.75 a | 120.08 ± 6.32 a | 40.52 ± 1.01 a | 98.62 ± 2.19 b |
| OM70 | 14.60 ± 0.08 a | 11.95 ± 0.06 a | 5.86 ± 0.05 a | 34.48 ± 0.38 a | 870.46 ± 25.54 a | 121.88 ± 4.56 a | 37.49 ± 2.73 ab | 96.34 ± 1.63 b |
| OM100 | 12.89 ± 0.12 b | 10.78 ± 0.34 bc | 5.57 ± 0.25 a b | 33.40 ± 0.20 b | 615.75 ± 117.46 bc | 106.69 ± 6.40 ab | 34.13 ± 2.95 ab | 92.98 ± 1.17 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Hu, Z.; Wan, Q.; Mu, B.; Li, G.; Yang, Y. Integrated Application of Inorganic and Organic Fertilizer Enhances Soil Organo-Mineral Associations and Nutrients in Tea Garden Soil. Agronomy 2022, 12, 1330. https://doi.org/10.3390/agronomy12061330
Li H, Hu Z, Wan Q, Mu B, Li G, Yang Y. Integrated Application of Inorganic and Organic Fertilizer Enhances Soil Organo-Mineral Associations and Nutrients in Tea Garden Soil. Agronomy. 2022; 12(6):1330. https://doi.org/10.3390/agronomy12061330
Chicago/Turabian StyleLi, Huan, Zhenmin Hu, Qing Wan, Bing Mu, Guifei Li, and Yiyang Yang. 2022. "Integrated Application of Inorganic and Organic Fertilizer Enhances Soil Organo-Mineral Associations and Nutrients in Tea Garden Soil" Agronomy 12, no. 6: 1330. https://doi.org/10.3390/agronomy12061330
APA StyleLi, H., Hu, Z., Wan, Q., Mu, B., Li, G., & Yang, Y. (2022). Integrated Application of Inorganic and Organic Fertilizer Enhances Soil Organo-Mineral Associations and Nutrients in Tea Garden Soil. Agronomy, 12(6), 1330. https://doi.org/10.3390/agronomy12061330






