The Potassium-Dependent Transcriptome Analysis of Maize Provides Novel Insights into the Rescue Role of Auxin in Responses to Potassium Deficiency
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Experimental Design
2.2. Root Morphological and Seedling Dry Matter Accumulation
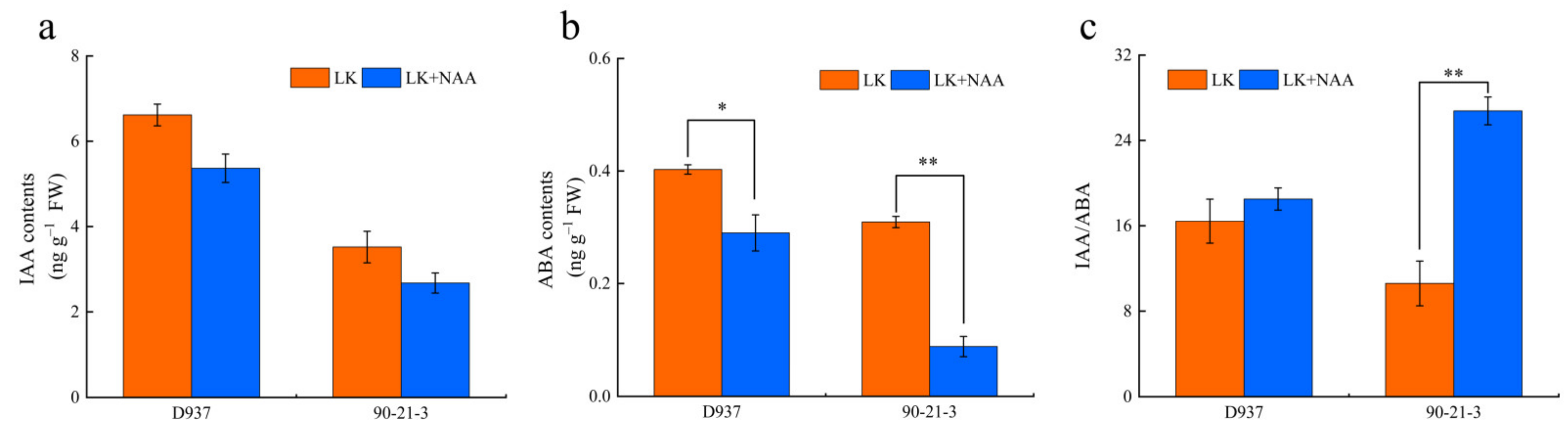
2.3. Hormone Determination
2.4. Determination of ROS and MDA
2.5. Antioxidant Enzyme Activity Assay
2.6. RNA-seq Analysis
2.7. qPCR
2.8. Statistical Analysis
3. Results
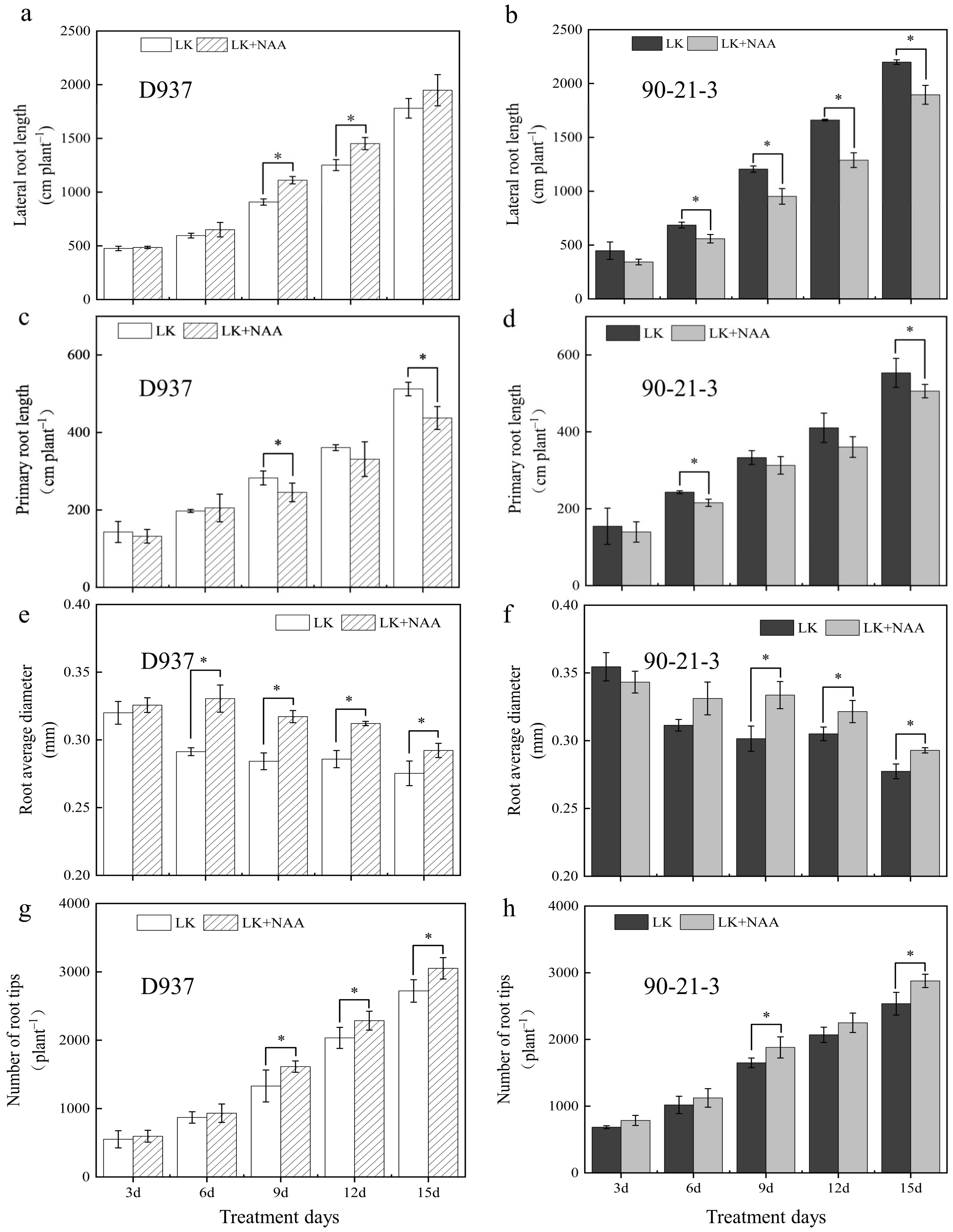
3.1. Changes in Root Morphology
3.2. Dry Biomass Accumulation in Seedlings
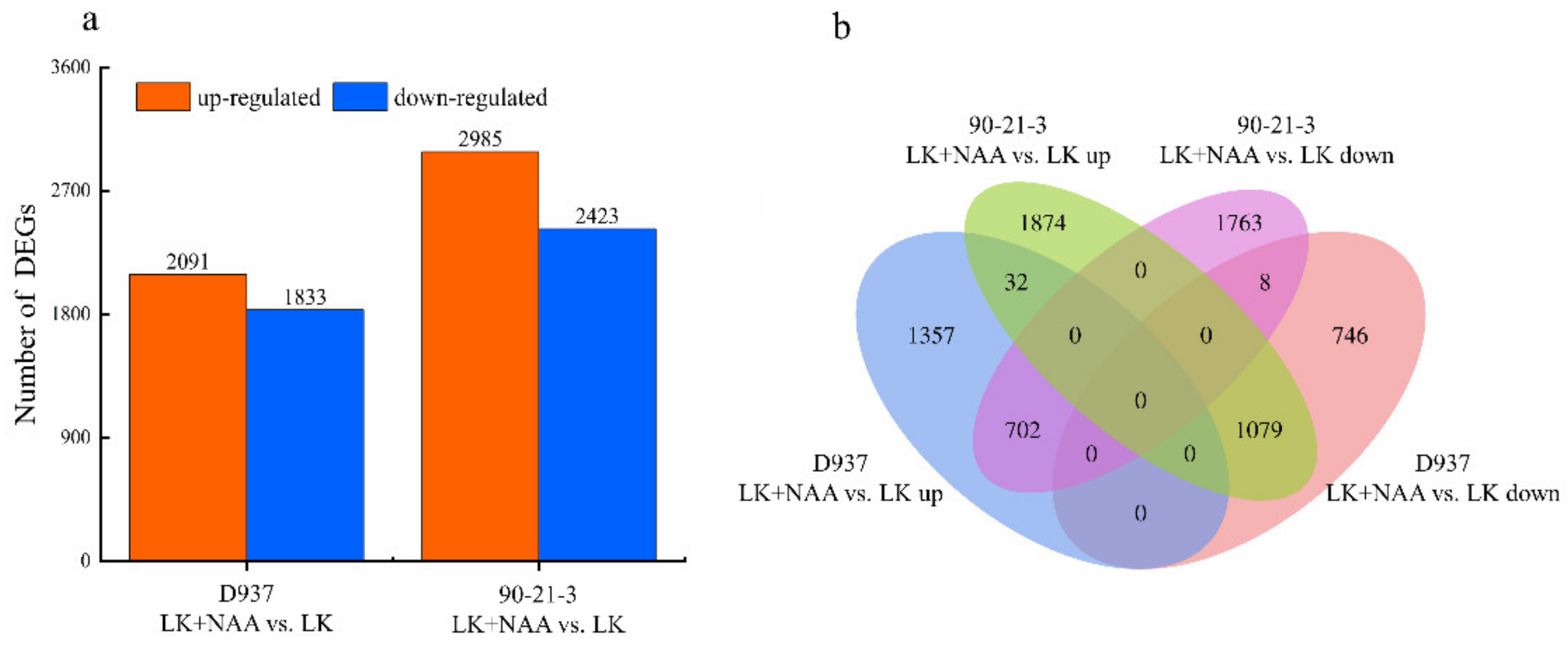
3.3. Transcriptome Sequencing Results and Sequence Assembly
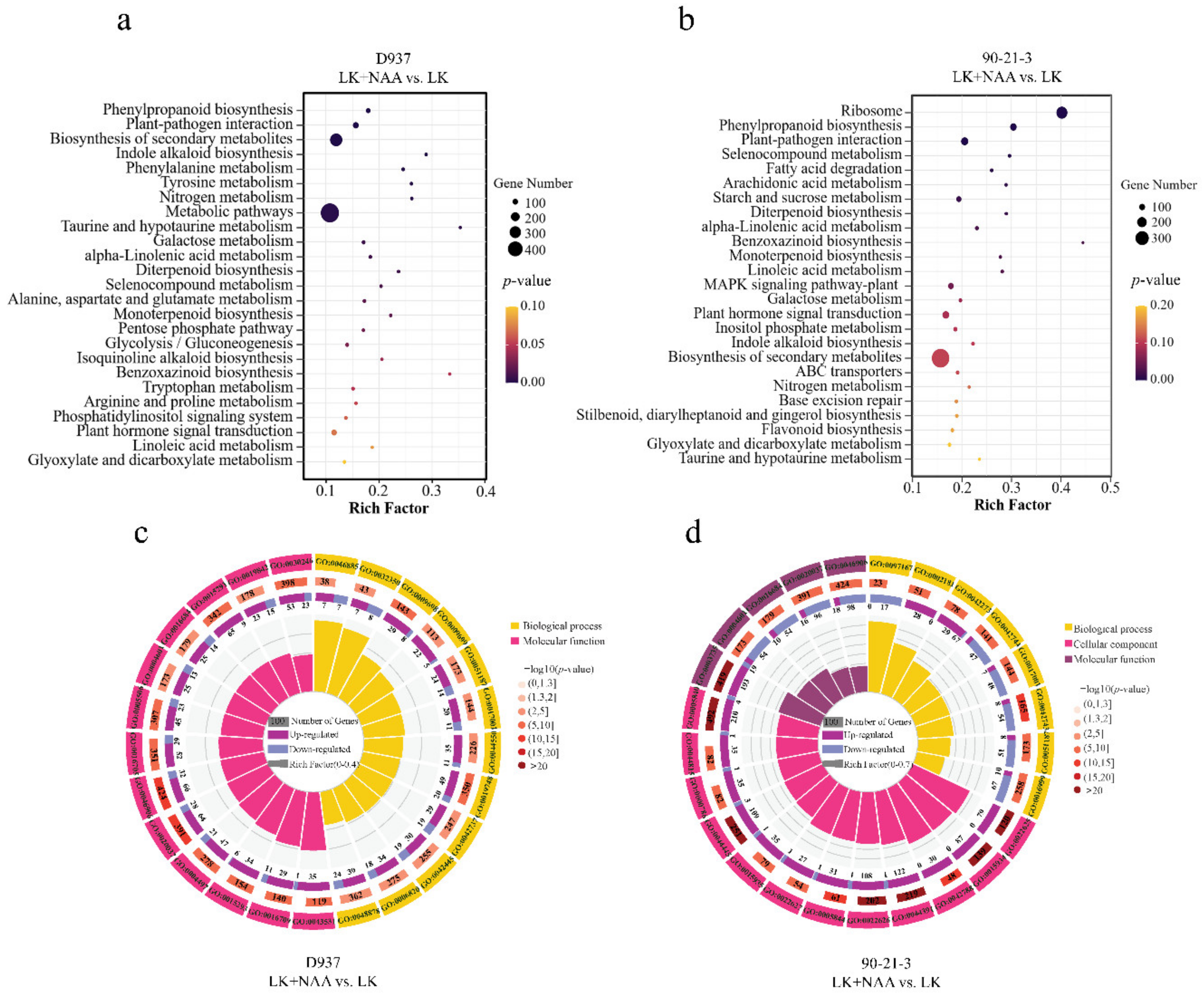
3.4. Enrichment Analysis of Differentially Expressed Genes
3.5. DEGs Related to K+ Acquisition and Transport
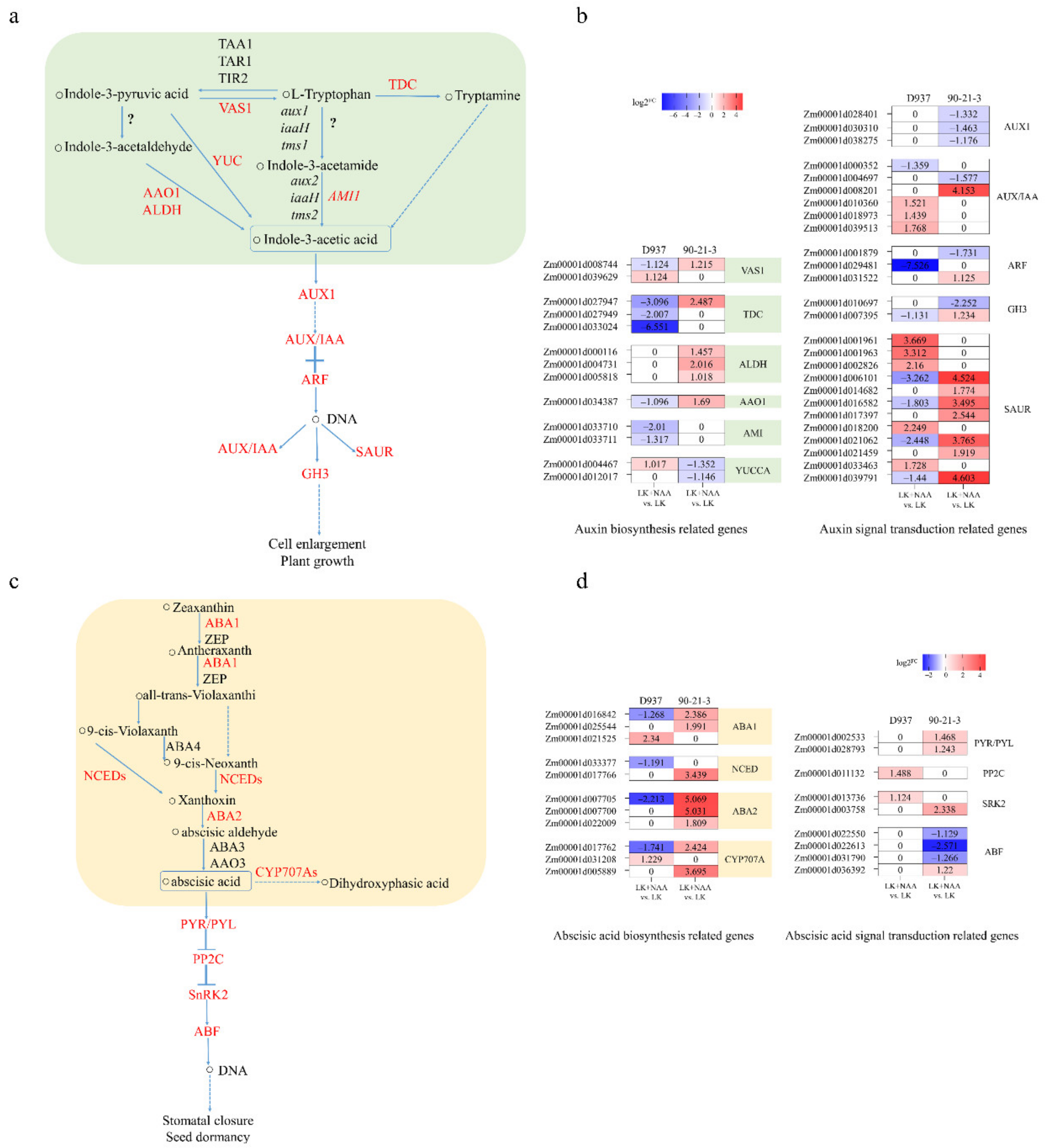
3.6. DEGs Related to Phytohormone Synthesis and Signaling
3.7. Oxidative Stress
3.8. Validation of Gene Expression by qPCR
4. Discussion
4.1. Regulatory Effect of Exogenous NAA on Low-K Tolerance of Maize
4.2. Regulation of Exogenous NAA on K+ Channels and Transporter Genes under Low-K Stress
4.3. Regulation of Exogenous NAA on Hormone Synthesis and the Signal Transduction Pathway under Low-K Stress
4.4. Exogenous NAA and Regulation of the Antioxidant System under Low-K Stress
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pettigrew, W.T. Potassium influences on yield and quality production for maize, wheat, soybean and cotton. Physiol. Plant 2008, 133, 670–681. [Google Scholar] [CrossRef] [PubMed]
- Clarkson, D.T.; Hanson, J.B. The Mineral Nutrition of Higher Plants. Annu. Rev. Plant Physiol. 1980, 31, 239–298. [Google Scholar] [CrossRef]
- Tang, R.J.; Zhao, F.G.; Yang, Y.; Wang, C.; Li, K.; Kleist, T.J.; Lemaux, P.G.; Luan, S. A calcium signalling network activates vacuolar K (+) remobilization to enable plant adaptation to low- K environments. Nat. Plants 2020, 6, 384–393. [Google Scholar] [CrossRef]
- Maathuis, F.J.M.; Sanders, D. Mechanisms of potassium absorption by higher plant roots. Physiol. Plant. 1996, 96, 158–168. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Y.-F.; Wu, W.-H. Potassium and phosphorus transport and signaling in plants. J. Integr. Plant Biol. 2021, 63, 34–52. [Google Scholar] [CrossRef] [PubMed]
- Gurav, P.P.; Datta, S.C.; Ray, S.K.; Choudhari, P.L.; Ahmed, N. Assessment of potassium release threshold levels of Vertisols (shrink-swell soils) in different agro-ecological regions of India. Appl. Clay Sci. 2018, 165, 155–163. [Google Scholar] [CrossRef]
- Shirale, A.O.; Meena, B.P.; Gurav, P.P.; Srivastava, S.; Biswas, A.K.; Thakur, J.K.; Somasundaram, J.; Patra, A.K.; Rao, A.S. Prospects and challenges in utilization of indigenous rocks and minerals as source of potassium in farming. J. Plant Nutr. 2019, 42, 2682–2701. [Google Scholar] [CrossRef]
- Chen, X.; Li, T.; Lu, D.; Cheng, L.; Zhou, J.; Wang, H. Estimation of soil available potassium in Chinese agricultural fields using a modified sodium tetraphenyl boron method. Land Degrad. Dev. 2020, 31, 1737–1748. [Google Scholar] [CrossRef]
- Romheld, V.; Kirkby, E.A. Research on potassium in agriculture: Needs and prospects. Plant Soil 2010, 335, 155–180. [Google Scholar] [CrossRef]
- Zorb, C.; Senbayram, M.; Peiter, E. Potassium in agriculture—Status and perspectives. J. Plant Physiol. 2014, 171, 656–669. [Google Scholar] [CrossRef]
- Du, Q.; Zhao, X.-h.; Xia, L.; Jiang, C.-j.; Wang, X.-g.; Han, Y.; Wang, J.; Yu, H.-q. Effects of potassium deficiency on photosynthesis, chloroplast ultrastructure, ROS, and antioxidant activities in maize (Zea mays L.). J. Integr. Agric. 2019, 18, 395–406. [Google Scholar] [CrossRef] [Green Version]
- Minjian, C.; Haiqiu, Y.; Hongkui, Y.; Chunji, J. Difference in Tolerance to Potassium Deficiency Between Two Maize Inbred Lines. Plant Prod. Sci. 2007, 10, 42–46. [Google Scholar] [CrossRef]
- Zhao, X.-h.; Yu, H.-q.; Wen, J.; Wang, X.-g.; Du, Q.; Wang, J.; Wang, Q. Response of root morphology, physiology and endogenous hormones in maize (Zea mays L.) to potassium deficiency. J. Integr. Agric. 2016, 15, 785–794. [Google Scholar] [CrossRef]
- Zhu, D.-D.; Li, Z.-H.; Guo, L.-X.; Lu, J.-W.; Cong, R.-H.; Ren, T.; Li, X.-K. The main driving factors and responses to increase in soil available potassium in the Yangtze River basin over the past 30 years. Land Degrad. Dev. 2021, 32, 4484–4493. [Google Scholar] [CrossRef]
- Chen, F.; Wan, K.-y.; Liu, Y.; Tao, Y. China’s grain relies on foreign fertilizer. Nature 2011, 476, 33. [Google Scholar] [CrossRef]
- Kumar, p.; Singh, t.; Singh, S.; Tuteja, N.; Prasad, R.; Singh, J. Potassium: A Key Modulator for Cell Homeostasis. J. Biotechnol. 2020, 324, 198–210. [Google Scholar] [CrossRef] [PubMed]
- Oldroyd, G.; Leyser, O. A plant’s diet, surviving in a variable nutrient environment. Science 2020, 368, eaba0196. [Google Scholar] [CrossRef] [PubMed]
- Forde, B.; Lorenzo, H. The nutritional control of root development. In Interactions in the Root Environment: An Integrated Approach: Proceedings of the Millenium Conference on Rhizosphere Interactions; Springer: Dordrecht, The Netherlands, 2002; pp. 51–68. [Google Scholar]
- Armengaud, P.; Breitling, R.; Amtmann, A. The Potassium-Dependent Transcriptome of Arabidopsis Reveals a Prominent Role of Jasmonic Acid in Nutrient Signaling. Plant Physiol. 2004, 136, 2556–2576. [Google Scholar] [CrossRef] [Green Version]
- Shin, R.; Schachtman, D.P. Hydrogen peroxide mediates plant root cell response to nutrient deprivation. Proc. Natl. Acad. Sci. USA 2004, 101, 8827. [Google Scholar] [CrossRef] [Green Version]
- White, P.J.; Bell, M.J.; Djalovic, I.; Hinsinger, P.; Rengel, Z. Potassium Use Efficiency of Plants; Springer: Cham, Switzerland, 2021; pp. 119–145. [Google Scholar]
- Høgh-Jensen, H.; Pedersen, M.B. Morphological Plasticity by Crop Plants and Their Potassium Use Efficiency. J. Plant Nutr. 2003, 26, 969–984. [Google Scholar] [CrossRef]
- Kim, M.J.; Ruzicka, D.; Shin, R.; Schachtman, D. The Arabidopsis AP2/ERF Transcription Factor RAP2.11 Modulates Plant Response to Low-Potassium Conditions. Mol. Plant 2012, 5, 1042–1057. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kellermeier, F.; Chardon, F.; Amtmann, A. Natural variation of Arabidopsis root architecture reveals complementing adaptive strategies to potassium starvation. Plant Physiol. 2013, 161, 1421–1432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, M.; Zhang, A.-J.; Chen, X.-G.; Jin, R.; Li, H.-M.; Tang, Z.-H. Effects of potassium deficiency on root morphology, ultrastructure and antioxidant enzyme system in sweet potato (Ipomoea batatas [L.] Lam.) during early growth. Acta Physiol. Plant. 2017, 39, 211. [Google Scholar] [CrossRef]
- Rengel, Z.; Damon, P.M. Crops and genotypes differ in efficiency of potassium uptake and use. Physiol. Plant. 2008, 133, 624–636. [Google Scholar] [CrossRef] [PubMed]
- Overvoorde, P.; Fukaki, H.; Beeckman, T. Auxin control of root development. Cold Spring Harb. Perspect. Biol. 2010, 2, a001537. [Google Scholar] [CrossRef] [Green Version]
- Cavallari, N.; Artner, C.; Benkova, E. Auxin-Regulated Lateral Root Organogenesis. Cold Spring Harb. Perspect. Biol. 2021, 13, a039941. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, W.H. Regulation of potassium transport and signaling in plants. Curr. Opin. Plant Biol. 2017, 39, 123–128. [Google Scholar] [CrossRef]
- Cherel, I.; Lefoulon, C.; Boeglin, M.; Sentenac, H. Molecular mechanisms involved in plant adaptation to low K+ availability. J. Exp. Bot. 2014, 65, 833–848. [Google Scholar] [CrossRef]
- Shin, R.; Burch, A.Y.; Huppert, K.A.; Tiwari, S.B.; Murphy, A.S.; Guilfoyle, T.J.; Schachtman, D.P. The Arabidopsis transcription factor MYB77 modulates auxin signal transduction. Plant Cell 2007, 19, 2440–2453. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Wu, W.-H.; Wang, Y. Potassium channel AKT1 is involved in the auxin-mediated root growth inhibition in Arabidopsis response to low K+ stress: AKT1 regulates primary root growth under low K+ stress. J. Integr. Plant Biol. 2017, 59, 895–909. [Google Scholar] [CrossRef] [Green Version]
- Feng, C.-z.; Luo, Y.-x.; Wang, P.-d.; Gilliham, M.; Long, Y. MYB77 regulates high-affinity potassium uptake by promoting expression of HAK5. New Phytol. 2021, 232, 176–189. [Google Scholar] [CrossRef]
- Zhao, S.; Zhang, M.L.; Ma, T.L.; Wang, Y. Phosphorylation of ARF2 Relieves Its Repression of Transcription of the K+ Transporter Gene HAK5 in Response to Low Potassium Stress. Plant Cell 2016, 28, 3005–3019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, W.; Liu, S.; Meng, L.; Xue, R.; Wang, C.; Liu, G.; Dong, C.; Wang, S.; Dong, J.; Zhang, Y. Potassium deficiency inhibits lateral root development in tobacco seedlings by changing auxin distribution. Plant Soil 2015, 396, 163–173. [Google Scholar] [CrossRef]
- Gao, K.; Chen, F.-j.; Yuan, L.-x.; Mi, G.-h. Cell Production and Expansion in the Primary Root of Maize in Response to Low-Nitrogen Stress. J. Integr. Agric. 2014, 13, 2508–2517. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, B.; Zhao, Y.; Zhang, J.; Li, Z. Auxin and GA signaling play important roles in the maize response to phosphate deficiency. Plant Sci. 2019, 283, 177–188. [Google Scholar] [CrossRef]
- Zhang, Z.-Y.; Wang, Q.-L.; Li, Z.-H.; Duan, L.-S.; Tian, X.-L. Effects of Potassium Deficiency on Root Growth of Cotton Seedlings and Its Physiological Mechanisms. Acta Agron. Sin. 2009, 35, 718–723. [Google Scholar] [CrossRef]
- Wu, Z.-G.; Jiang, W.; Tao, Z.-M.; Pan, X.-J.; Yu, W.-H.; Huang, H.-L. Morphological and stage-specific transcriptome analyses reveal distinct regulatory programs underlying yam (Dioscorea alata L.) bulbil growth. J. Exp. Bot. 2020, 71, 1899–1914. [Google Scholar] [CrossRef]
- Gong, X.; Shi, S.; Dou, F.; Song, Y.; Ma, F. Exogenous Melatonin Alleviates Alkaline Stress in Malus hupehensis Rehd. by Regulating the Biosynthesis of Polyamines. Molecules 2017, 22, 1542. [Google Scholar] [CrossRef] [Green Version]
- Li, F.; Duan, T.; Li, Y. Effects of the Fungal Endophyte Epichloë festucae var. lolii on Growth and Physiological Responses of Perennial Ryegrass cv. Fairway to Combined Drought and Pathogen Stresses. Microorganisms 2020, 8, 1917. [Google Scholar] [CrossRef]
- Maehly, A.C. The Assay of Catalases and Peroxidases. In Methods of Biochemical Analysis; Glick, D., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 1954; pp. 357–424. [Google Scholar] [CrossRef]
- Afgan, E.; Baker, D.; Van den Beek, M.; Blankenberg, D.; Bouvier, D.; Čech, M.; Chilton, J.; Clements, D.; Coraor, N.; Eberhard, C.; et al. The Galaxy platform for accessible, reproducible and collaborative biomedical analyses: 2016 update. Nucleic Acids Res. 2016, 44, 3–10. [Google Scholar] [CrossRef] [Green Version]
- Wu, T. Evaluation of candidate reference genes for real time quantitative PCR normalization in pear fruit. Afr. J. Agric. Res. 2012, 7, 3701–3704. [Google Scholar] [CrossRef]
- Yu, P.; Hochholdinger, F.; Li, C. Plasticity of Lateral Root Branching in Maize. Front. Plant Sci. 2019, 10, 363. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Li, Y.; Jin, Y.; Kan, L.; Shen, C.; Malladi, A.; Nambeesan, S.; Xu, Y.; Dong, C. Transcriptome Analysis of Pyrus betulaefolia Seedling Root Responses to Short-Term Potassium Deficiency. Int. J. Mol. Sci. 2020, 21, 8857. [Google Scholar] [CrossRef] [PubMed]
- Hafsi, C.; Romero-Puertas, M.C.; Del Río, L.A.; Abdelly, C.; Sandalio, L.M. Antioxidative response of Hordeum maritimum L. to potassium deficiency. Acta Physiol. Plant. 2010, 33, 193–202. [Google Scholar] [CrossRef]
- Sustr, M.; Soukup, A.; Tylova, E. Potassium in Root Growth and Development. Plants 2019, 8, 435. [Google Scholar] [CrossRef] [Green Version]
- Zheng, L.; Xiao, Z.; Song, W. Effects of Substrate and Exogenous Auxin on the Adventitious Rooting of Dianthus caryophyllus L. HortScience 2020, 55, 170–173. [Google Scholar] [CrossRef] [Green Version]
- Alarcon, M.V.; Salguero, J.; Lloret, P.G. Auxin Modulated Initiation of Lateral Roots Is Linked to Pericycle Cell Length in Maize. Front. Plant Sci. 2019, 10, 11. [Google Scholar] [CrossRef] [Green Version]
- Srivastava, A.K.; Shankar, A.; Nalini Chandran, A.K.; Sharma, M.; Jung, K.-H.; Suprasanna, P.; Pandey, G.K.; Foyer, C. Emerging concepts of potassium homeostasis in plants. J. Exp. Bot. 2020, 71, 608–619. [Google Scholar] [CrossRef]
- Philippar, K.; Ivashikina, N.; Ache, P.; Christian, M.; Lüthen, H.; Palme, K.; Hedrich, R. Auxin activates KAT1 and KAT2, two K+-channel genes expressed in seedlings of Arabidopsis thaliana. Plant J. 2004, 37, 815–827. [Google Scholar] [CrossRef]
- Sun, X.; Chen, H.; Wang, P.; Chen, F.; Yuan, L.; Mi, G. Low nitrogen induces root elongation via auxin-induced acid growth and auxin-regulated target of rapamycin (TOR) pathway in maize. J. Plant Physiol. 2020, 254, 153281. [Google Scholar] [CrossRef]
- Ma, T.L.; Wu, W.H.; Wang, Y. Transcriptome analysis of rice root responses to potassium deficiency. BMC Plant Biol. 2012, 12, 161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Yin, Q.; Dai, B.; Wang, K.-l.; Lu, L.; Qaseem, M.F.; Wang, J.; Li, H.; Wu, A.-M. The key physiology and molecular responses to potassium deficiency in Neolamarckia cadamba. Ind. Crops Prod. 2021, 162, 113260. [Google Scholar] [CrossRef]
- Fukui, K.; Hayashi, K.-i. Manipulation and Sensing of Auxin Metabolism, Transport and Signaling. Plant Cell Physiol. 2018, 59, 1500–1510. [Google Scholar] [CrossRef] [PubMed]
- Chhun, T.; Taketa, S.; Tsurumi, S.; Ichii, M. The effects of auxin on lateral root initiation and root gravitropism in a lateral rootless mutant Lrt1 of rice (Oryza sativa L.). Plant Growth Regul. 2003, 39, 161–170. [Google Scholar] [CrossRef]
- Kong, Y.; Zhu, Y.; Gao, C.; She, W.; Lin, W.; Chen, Y.; Han, N.; Bian, H.; Zhu, M.; Wang, J. Tissue-specific expression of SMALL AUXIN UP RNA41 differentially regulates cell expansion and root meristem patterning in Arabidopsis. Plant Cell Physiol. 2013, 54, 609–621. [Google Scholar] [CrossRef] [Green Version]
- Kant, S.; Bi, Y.M.; Zhu, T.; Rothstein, S.J. SAUR39, a small auxin-up RNA gene, acts as a negative regulator of auxin synthesis and transport in rice. Plant Physiol. 2009, 151, 691–701. [Google Scholar] [CrossRef] [Green Version]
- Xing, L.; Zhao, Y.; Gao, J.; Xiang, C.; Zhu, J.K. The ABA receptor PYL9 together with PYL8 plays an important role in regulating lateral root growth. Sci. Rep. 2016, 6, 27177. [Google Scholar] [CrossRef]
- Seo, M.; Marion-Poll, A. Abscisic acid metabolism and transport. Adv. Bot. Res. 2019, 92, 1–49. [Google Scholar] [CrossRef]
- Guo, K.; Tu, L.; He, Y.; Deng, J.; Wang, M.; Huang, H.; Li, Z.; Zhang, X. Interaction between calcium and potassium modulates elongation rate in cotton fiber cells. J. Exp. Bot. 2017, 68, 5161–5175. [Google Scholar] [CrossRef]
- Choudhary, A.; Kumar, A.; Kaur, N. ROS and oxidative burst: Roots in plant development. Plant Divers. 2020, 42, 33–43. [Google Scholar] [CrossRef]
- Dubrovsky, J.G.; Fukaki, H.; Laplaze, L.; Laskowski, M. Editorial: Root Branching: From Lateral Root Primordium Initiation and Morphogenesis to Function. Front. Plant Sci. 2019, 10, 1462. [Google Scholar] [CrossRef] [PubMed]
- Manzano, C.; Pallero-Baena, M.; Casimiro, I.; De Rybel, B.; Orman-Ligeza, B.; Van Isterdael, G.; Beeckman, T.; Draye, X.; Casero, P.; Del Pozo, J.C. The Emerging Role of Reactive Oxygen Species Signaling during Lateral Root Development. Plant Physiol. 2014, 165, 1105–1119. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Gene ID | D937 | 90-21-3 | Annotation |
|---|---|---|---|
| (log2 LK+NAA/LK) | (log2 LK+NAA/LK) | ||
| Zm00001d003859 | −4.047 | - | potassium transporter 19-like, HAK20 [Zea mays] |
| Zm00001d019631 | −1.221 | 1.302 | high-affinity potassium transporter, HAK18 [Zea mays] |
| Zm00001d020325 | −1.236 | 1.529 | high-affinity potassium transporter isoform X1, HAK23 [Zea mays] |
| Zm00001d022485 | −1.957 | 2.922 | potassium transporter 7-like, HAK7 [Zea mays] |
| Zm00001d025303 | - | 1.092 | potassium transporter 1-like, HAK19 [Zea mays] |
| Zm00001d033068 | - | 3.563 | high-affinity potassium transporter [Zea mays] |
| Zm00001d012717 | 1.681 | - | Potassium channel KAT1 [Zea mays] |
| Zm00001d037289 | - | −1.475 | outward rectifying potassium channel 1, ZORK [Zea mays] |
| Zm00001d010209 | - | 1.81 | potassium channel protein ZMK2 [Zea mays] |
| Zm00001d011473 | −4.139 | 3.424 | potassium channel 5, ZMK1 [Zea mays] |
| Zm00001d011731 | 2.318 | - | metal ion binding protein [Zea mays] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, D.; Wang, K.; Zhang, H.; Du, Q.; Liu, Y.; Wang, J.; Wang, X.; Yu, H.; Zhao, X. The Potassium-Dependent Transcriptome Analysis of Maize Provides Novel Insights into the Rescue Role of Auxin in Responses to Potassium Deficiency. Agronomy 2022, 12, 1318. https://doi.org/10.3390/agronomy12061318
Zhou D, Wang K, Zhang H, Du Q, Liu Y, Wang J, Wang X, Yu H, Zhao X. The Potassium-Dependent Transcriptome Analysis of Maize Provides Novel Insights into the Rescue Role of Auxin in Responses to Potassium Deficiency. Agronomy. 2022; 12(6):1318. https://doi.org/10.3390/agronomy12061318
Chicago/Turabian StyleZhou, Dongying, Kai Wang, He Zhang, Qi Du, Yingyan Liu, Jing Wang, Xiaoguang Wang, Haiqiu Yu, and Xinhua Zhao. 2022. "The Potassium-Dependent Transcriptome Analysis of Maize Provides Novel Insights into the Rescue Role of Auxin in Responses to Potassium Deficiency" Agronomy 12, no. 6: 1318. https://doi.org/10.3390/agronomy12061318
APA StyleZhou, D., Wang, K., Zhang, H., Du, Q., Liu, Y., Wang, J., Wang, X., Yu, H., & Zhao, X. (2022). The Potassium-Dependent Transcriptome Analysis of Maize Provides Novel Insights into the Rescue Role of Auxin in Responses to Potassium Deficiency. Agronomy, 12(6), 1318. https://doi.org/10.3390/agronomy12061318





