Diversity of Tartary Buckwheat (Fagopyrum tataricum) Landraces from Liangshan, Southwest China: Evidence from Morphology and SSR Markers
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Phenotypic Data Analysis
2.3. DNA Isolation and PCR Amplification
2.4. Genetic Diversity and Structure Analysis
3. Results
3.1. Morphological Traits Diversity of Seeds
3.2. Polymorphism and Allelic Diversity
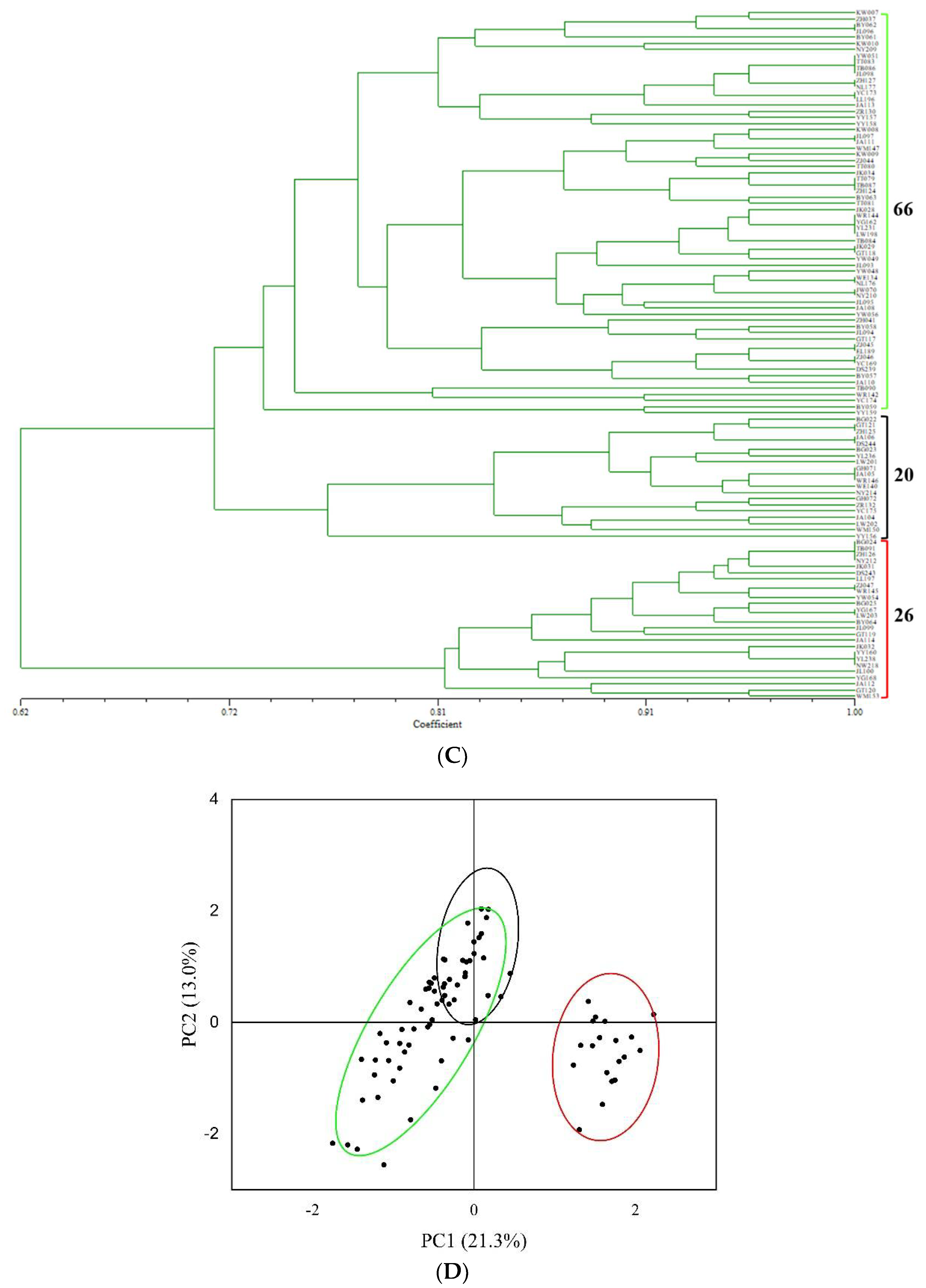
3.3. Genetic Diversity of Tartary Buckwheat’s 29 Populations (Villages) Based on SSR Analysis
3.4. The Relationship between Morphological Traits of Seeds and SSR Markers
4. Discussion
4.1. Genetic Diversity of Morphological Traits of Seed in Tartary Buckwheat
4.2. Molecular Characterization and Genetic Diversity of Tartary Buckwheat Landraces
4.3. Genetic Structure of Tartary Buckwheat Populations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ohsako, T.; Yamane, K.; Ohnishi, O. Two new Fagopyrum (Polygonaceae) species, F-gracilipedoides and F-jinshaense from Yunnan, China. Genes Genet. Syst. 2002, 77, 399–408. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Huda, M.N.; Lu, S.; Jahan, T.; Ding, M.Q.; Jha, R.; Zhang, K.X.; Zhang, W.; Georgiev, M.I.; Park, S.U.; Zhou, M.L. Treasure from garden: Bioactive compounds of buckwheat. Food Chem. 2021, 335, 127653. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Campbell, C.G. Tartary buckwheat breeding (Fagopyrum tataricum L. Gaertn.) through hybridization with its Rice-Tartary type. Euphytica 2007, 156, 399–405. [Google Scholar] [CrossRef]
- Hunt, H.V.; Shang, X.; Jones, M.K. Buckwheat: A crop from outside the major Chinese domestication centres? A review of the archaeobotanical, palynological and genetic evidence. Veg. Hist. Archaeobot. 2018, 27, 493–506. [Google Scholar] [CrossRef]
- Zhang, K.X.; He, M.; Fan, Y.; Zhao, H.; Gao, B.; Yang, K.L.; Li, F.L.; Tang, Y.; Gao, Q.; Lin, T.; et al. Resequencing of global Tartary buckwheat accessions reveals multiple domestication events and key loci associated with agronomic traits. Genome Biol. 2021, 22, 23. [Google Scholar] [CrossRef]
- Liu, C.-L.; Chen, Y.-S.; Yang, J.-H.; Chiang, B.-H. Antioxidant activity of tartary (Fagopyrum tataricum (L.) Gaertn.) and common (Fagopyrum esculentum moench) buckwheat sprouts. J. Agric. Food Chem. 2008, 56, 173–178. [Google Scholar] [CrossRef]
- Karki, R.; Park, C.-H.; Kim, D.-W. Extract of buckwheat sprouts scavenges oxidation and inhibits pro-inflammatory mediators in lipopolysaccharide-stimulated macrophages (RAW264.7). J. Integr. Med. 2013, 11, 246–252. [Google Scholar] [CrossRef]
- Matsui, T.; Kudo, A.; Tokuda, S.; Matsumoto, K.; Hosoyama, H. Identification of a New Natural Vasorelaxatant Compound, (+)-Osbeckic Acid, from Rutin-free Tartary Buckwheat Extract. J. Agr. Food Chem. 2010, 58, 10876–10879. [Google Scholar] [CrossRef]
- Jeon, J.; Kim, J.K.; Wu, Q.; Park, S.U. Effects of cold stress on transcripts and metabolites in tartary buckwheat (Fagopyrum tataricum). Environ. Exp. Bot. 2018, 155, 488–496. [Google Scholar] [CrossRef]
- Zhou, M.-L.; Bai, D.-Q.; Tang, Y.; Zhu, X.-M.; Shao, J.-R. Genetic diversity of four new species related to southwestern Sichuan buckwheats as revealed by karyotype, ISSR and allozyme characterization. Plant Syst. Evol. 2012, 298, 751–759. [Google Scholar] [CrossRef]
- Bonafaccia, G.; Marocchini, M.; Kreft, I. Composition and technological properties of the, flour and bran from common and tartary buckwheat. Food Chem. 2003, 80, 9–15. [Google Scholar] [CrossRef]
- Zheng, C.; Hu, C.; Ma, X.; Peng, C.; Zhang, H.; Qin, L. Cytotoxic phenylpropanoid glycosides from Fagopyrum tataricum (L.) Gaertn. Food Chem. 2012, 132, 433–438. [Google Scholar] [CrossRef] [PubMed]
- Bellon, M.R.; Gotor, E.; Caracciolo, F. Conserving landraces and improving livelihoods: How to assess the success of on-farm conservation projects? Int. J. Agric. Sustain. 2015, 13, 167–182. [Google Scholar] [CrossRef]
- Bajracharya, J.; Brown, A.H.D.; Joshi, B.K.; Panday, D.; Baniya, B.K.; Sthapit, B.R.; Jarvis, D.I. Traditional seed management and genetic diversity in barley varieties in high-hill agro-ecosystems of Nepal. Genet. Resour. Crop Evol. 2012, 59, 389–398. [Google Scholar] [CrossRef]
- Xu, F.; Tang, C.; Yu, T.; Dai, L.; Zhang, H. Diversity of paddy rice varieties from Yuanyang Hani’ s terraced fields in Yunnan, China. Acta Ecol. Sin. 2010, 30, 3346–3357. [Google Scholar]
- Xu, F.-R.; Yang, Y.-Y.; Zhang, E.-L.; A, X.-X.; Tang, C.-F.; Dong, C.; Zhang, F.-F.; Liu, X.; Dai, L.-Y. On-farm conservation and utilization of paddy rice, wheat and maize landrace varieties in 15 unique ethnic groups in Yunnan, China. Yi Chuan Hered. 2012, 34, 1466–1474. [Google Scholar] [CrossRef]
- Song, Y.; Dong, Y.; Wang, J.; Feng, J.; Long, C. Tartary buckwheat (Fagopyrum tataricum Gaertn.) landraces cultivated by Yi people in Liangshan, China. Genet. Resour. Crop Evol. 2020, 67, 745–761. [Google Scholar] [CrossRef]
- Song, Y.; Fang, Q.; Jarvis, D.; Bai, K.; Liu, D.; Feng, J.; Long, C. Network Analysis of Seed Flow, a Traditional Method for Conserving Tartary Buckwheat (Fagopyrum tataricum) Landraces in Liangshan, Southwest China. Sustainability 2019, 11, 4263. [Google Scholar] [CrossRef]
- Malysheva-Otto, L.; Ganal, M.W.; Law, J.R.; Reeves, J.C.; Roeder, M.S. Temporal trends of genetic diversity in European barley cultivars (Hordeum vulgare L.). Mol. Breed. 2007, 20, 309–322. [Google Scholar] [CrossRef]
- Bashir, E.M.A.; Ali, A.M.; Ali, A.M.; Mohamed, E.I.; Melchinger, A.E.; Parzies, H.K.; Haussmann, B.I.G. Genetic diversity of Sudanese pearl millet (Pennisetum glaucum (L.) R. Br.) landraces as revealed by SSR markers, and relationship between genetic and agro-morphological diversity. Genet. Resour. Crop Evol. 2015, 62, 579–591. [Google Scholar] [CrossRef]
- Govindaraj, M.; Vetriventhan, M.; Srinivasan, M. Importance of Genetic Diversity Assessment in Crop Plants and Its Recent Advances: An Overview of Its Analytical Perspectives. Genet. Res. Int. 2015, 2015, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Ohsawa, R.; Tsutsumi, T.; Uehara, H.; Namai, H.; Ninomiya, S. Quantitative evaluation of common buckwheat (Fagopyrum esculentum Moench) kernel shape by elliptic Fourier descriptor. Euphytica 1998, 101, 175–183. [Google Scholar] [CrossRef]
- Fang, X.M.; Huang, K.H.; Nie, J.; Zhang, Y.L.; Zhang, Y.K.; Li, Y.S.; Wang, W.W.; Xu, X.; Ruan, R.W.; Yuan, X.H.; et al. Genome-wide mining, characterization, and development of microsatellite markers in Tartary buckwheat (Fagopyrum tataricum Garetn.). Euphytica 2019, 215, 183. [Google Scholar] [CrossRef]
- Bulan, M.S.; Wu, J.C.; Emshwiller, E.; Berres, M.E.; Posner, J.L.; Peng, D.Y.; Wang, X.H.; Li, J.F.; Stoltenberg, D.E.; Zhang, Y.P. Social and environmental influences on tartary buckwheat (Fagopyrum tataricum Gaertn.) varietal diversity in Yunnan, China. Genet. Resour. Crop Evol. 2017, 64, 113–125. [Google Scholar] [CrossRef]
- Gupta, N.; Sharma, S.K.; Rana, J.C.; Chauhan, R.S. AFLP fingerprinting of tartary buckwheat accessions (Fagopyrum tataricum) displaying rutin content variation. Fitoterapia 2012, 83, 1131–1137. [Google Scholar] [CrossRef]
- Huang, W.; Jarvis, D.I.; Ahmed, S.; Long, C. Tartary Buckwheat Genetic Diversity in the Himalayas Associated with Farmer Landrace Diversity and Low Dietary Dependence. Sustainability 2017, 9, 1806. [Google Scholar] [CrossRef]
- Iwata, H.; Imon, K.; Tsumura, Y.; Ohsawa, R. Genetic diversity among Japanese indigenous common buckwheat (Fagopyrum esculentum) cultivars as determined from amplified fragment length polymorphism and simple sequence repeat markers and quantitative agronomic traits. Genome 2005, 48, 367–377. [Google Scholar] [CrossRef]
- Hou, S.; Sun, Z.; Bin, L.; Xu, D.; Wu, B.; Zhang, B.; Wang, X.; Han, Y.; Zhang, L.; Qiao, Z.; et al. Genetic Diversity of Buckwheat Cultivars (Fagopyrum tartaricum Gaertn.) Assessed with SSR Markers Developed from Genome Survey Sequences. Plant Mol. Biol. Rep. 2016, 34, 233–241. [Google Scholar] [CrossRef]
- Ma, K.-H.; Kim, N.-S.; Lee, G.-A.; Lee, S.-Y.; Lee, J.K.; Yi, J.Y.; Park, Y.-J.; Kim, T.-S.; Gwag, J.-G.; Kwon, S.-J. Development of SSR markers for studies of diversity in the genus Fagopyrum. Theor. Appl. Genet. 2009, 119, 1247–1254. [Google Scholar] [CrossRef]
- Li, R.; Shi, T.; Chen, Q.; Pan, F.; Chen, Q. Construction of EST-SSR fingerprinting and analysis of genetic diversity of thirty-five registered tartary buckwheat cultivars (Fagopyrum tataricum) in China. Plant Sci. J. 2017, 35, 267–275. [Google Scholar]
- Chambers, R. The origins and practice of participatory rural appraisal. World Dev. 1994, 22, 953–969. [Google Scholar] [CrossRef]
- Chambers, R. Participatory Rural Appraisal (PRA)—Challenges, Potentials and Paradigm. World Dev. 1994, 22, 1437–1454. [Google Scholar] [CrossRef]
- Zhang, Z.W.; Lin, R.F. Description Standard and Data Standard of Buckwheat Germplasm Resources; China Agriculture Press: Beijing, China, 2007. [Google Scholar]
- Lei, Q.Y.; Zhou, J.J.; Zhang, W.H.; Luo, J.; Wu, K.N.; Long, C.L. Morphological diversity of panicle traits in Kam fragrant glutinous rice (Oryza sativa). Genet. Resour. Crop Evol. 2018, 65, 775–786. [Google Scholar] [CrossRef]
- Gao, F.; Zhang, Z.; Wu, B. Construction and Application of SSR Molecular Markers System for Genetic Diversity Analysis of Chinese Tartary Buckwheat Germplasm Resources. Sci. Agric. Sin. 2012, 45, 1042–1053. [Google Scholar]
- Shi, T.; Li, R.; Chen, Q.; Li, Y.; Pan, F.; Chen, Q. De novo sequencing of seed transcriptome and development of genic-SSR markers in common buckwheat (Fagopyrum esculentum). Mol. Breed. 2017, 37, 147. [Google Scholar] [CrossRef]
- Peakall, R.; Smouse, P.E. GENALEX 6: Genetic analysis in Excel. Population genetic software for teaching and research. Mol. Ecol. Notes 2006, 6, 288–295. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Jombart, T. Adegenet: A R package for the multivariate analysis of genetic markers. Bioinformatics 2008, 24, 1403–1405. [Google Scholar] [CrossRef]
- Belalia, N.; Lupini, A.; Djemel, A.; Morsli, A.; Mauceri, A.; Lotti, C.; Khelifi-Slaoui, M.; Khelifi, L.; Sunseri, F. Analysis of genetic diversity and population structure in Saharan maize (Zea mays L.) populations using phenotypic traits and SSR markers. Genet. Resour. Crop Evol. 2019, 66, 243–257. [Google Scholar] [CrossRef]
- Senthilkumaran, R.; Bisht, I.S.; Bhat, K.V.; Rana, J.C. Diversity in buckwheat (Fagopyrum spp.) landrace populations from north-western Indian Himalayas. Genet. Resour. Crop Evol. 2008, 55, 287–302. [Google Scholar] [CrossRef]
- Berlin, B.; Breedlove, D.E.; Raven, P.H. General principles of classification and nomenclature in folk biology. Am. Anthropol. 1973, 75, 214–242. [Google Scholar] [CrossRef]
- Mekbib, F. Infra-specific folk taxonomy in sorghum (Sorghum bicolor (L.) Moench) in Ethiopia: Folk nomenclature, classification, and criteria. J. Ethnobiol. Ethnomed. 2007, 3, 38. [Google Scholar] [CrossRef] [PubMed]
- Loko, L.E.Y.; Toffa, J.; Adjatin, A.; Akpo, A.J.; Orobiyi, A.; Dansi, A. Folk taxonomy and traditional uses of common bean (Phaseolus vulgaris L.) landraces by the sociolinguistic groups in the central region of the Republic of Benin. J. Ethnobiol. Ethnomed. 2018, 14, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Dansi, A.; Adjatin, A.; Adoukonou-Sagbadja, H.; Falade, V.; Adomou, A.C.; Yedomonhan, H.; Akpagana, K.; de Foucault, B. Traditional leafy vegetables in Benin: Folk nomenclature, species under threat and domestication. Acta Bot. Gall. 2009, 156, 183–199. [Google Scholar] [CrossRef][Green Version]
- Ayenan, M.A.T.; Danquah, A.; Ahoton, L.E.; Ofori, K. Utilization and farmers’ knowledge on pigeonpea diversity in Benin, West Africa. J. Ethnobiol. Ethnomed. 2017, 13, 37. [Google Scholar] [CrossRef]
- Kishore, G.; Gupta, S.; Pandey, A. Assessment of population genetic diversity of Fagopyrum tataricum using SSR molecular marker. Biochem. Syst. Ecol. 2012, 43, 32–41. [Google Scholar] [CrossRef]
- Reed, D.H.; Frankham, R. Correlation between fitness and genetic diversity. Conserv. Biol. 2003, 17, 230–237. [Google Scholar] [CrossRef]
- Sgro, C.M.; Lowe, A.J.; Hoffmann, A.A. Building evolutionary resilience for conserving biodiversity under climate change. Evol. Appl. 2011, 4, 326–337. [Google Scholar] [CrossRef]
- Barnaud, A.; Deu, M.; Garine, E.; Chantereau, J.; Bolteu, J.; Koida, E.O.; McKey, D.; Joly, H.I. A weed-crop complex in sorghum: The dynamics of genetic diversity in a traditional farming system. Am. J. Bot. 2009, 96, 1869–1879. [Google Scholar] [CrossRef]
- Jarvis, D.I.; Hodgkin, T. Wild relatives and crop cultivars: Detecting natural introgression and farmer selection of new genetic combinations in agroecosystems. Mol. Ecol. 1999, 8, S159–S173. [Google Scholar] [CrossRef]
- Westengen, O.T.; Okongo, M.A.; Onek, L.; Berg, T.; Upadhyaya, H.; Birkeland, S.; Khalsa, S.D.K.; Ring, K.H.; Stenseth, N.C.; Brysting, A.K. Ethnolinguistic structuring of sorghum genetic diversity in Africa and the role of local seed systems. Proc. Natl. Acad. Sci. USA 2014, 111, 14100–14105. [Google Scholar] [CrossRef] [PubMed]
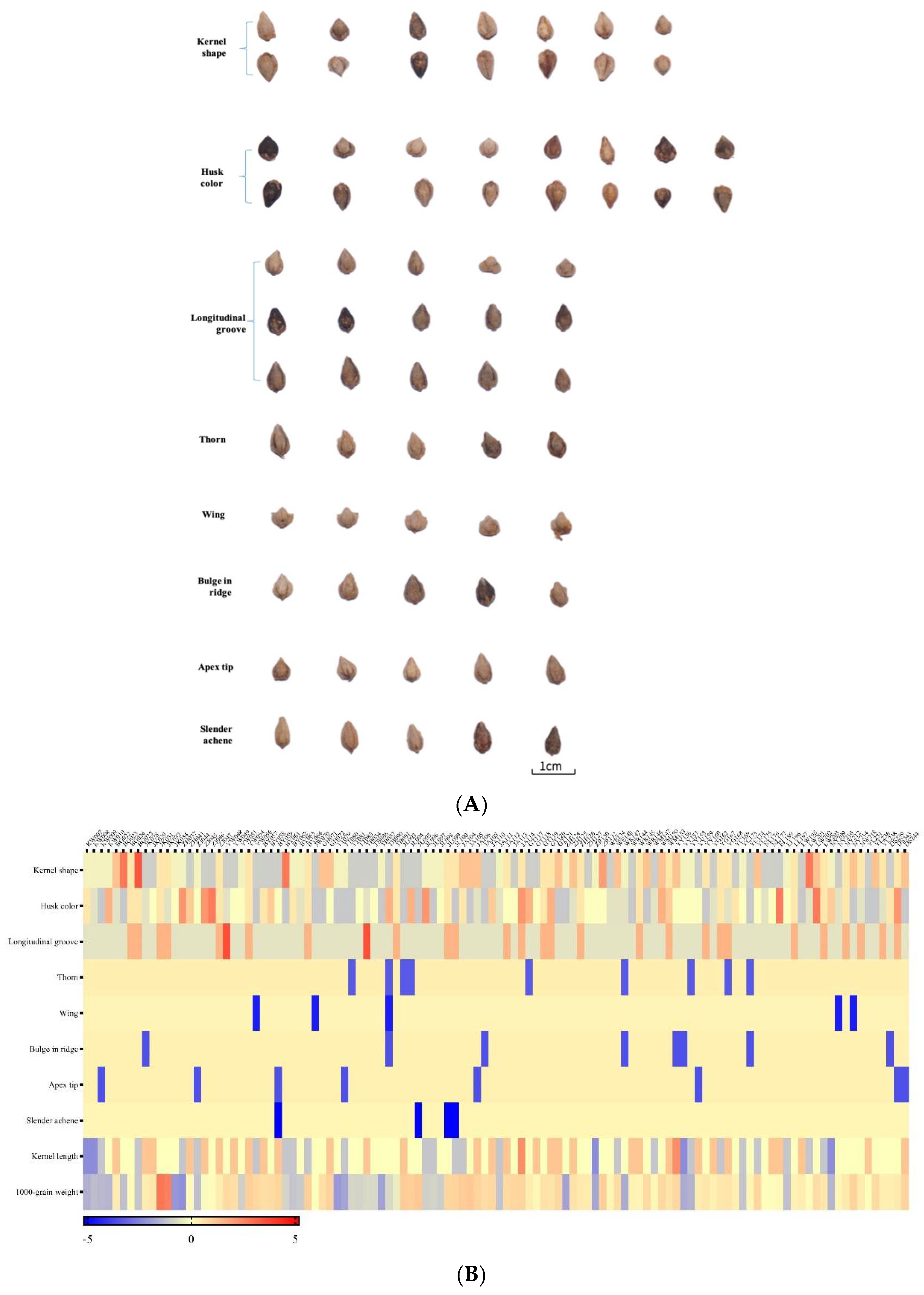


| Agronomic Traits | Given Valuations |
|---|---|
| Kernel shape | Triangular (1), ovate triangular (2), triple cone (3), quadrangular (4), polygonal (5), ovate quadrangular (6), ovate triple cone (7) |
| Husk color | Gray (1), dark gray (2), light gray (3), purple black (4), brown (5),light brown (6), purple (7), light purple (8) |
| Longitudinal groove | Yes (1), no (2), indistinct (3) |
| Thorn | Yes (1), no (2) |
| Wing | Yes (1), no (2) |
| Bulge in ridge | Yes (1), no (2) |
| Apex tip | Yes (1), no (2) |
| Slender achene | Yes (1), no (2) |
| Kernel length | The length of kernel (mm) |
| 1000-grain weight | The weight of 1000 pure seeds in the dry air (g) |
| No. | Trait | Minimum | Maximum | Range | Average | SD | CV (%) | Shannon-Index |
|---|---|---|---|---|---|---|---|---|
| 1 | Kernel shape | 1 | 7 | 6 | 2.35 | 1.35 | 57.45 | 0.32 |
| 2 | Husk color | 1 | 8 | 7 | 3.08 | 1.85 | 60.06 | 0.31 |
| 3 | Longitudinal groove | 1 | 3 | 2 | 1.27 | 0.48 | 37.80 | 0.27 |
| 4 | Thorn | 1 | 2 | 1 | 1.92 | 0.27 | 14.06 | 0.20 |
| 5 | Wing | 1 | 2 | 1 | 1.96 | 0.21 | 10.71 | 0.14 |
| 6 | Bulge in ridge | 1 | 2 | 1 | 1.93 | 0.26 | 13.47 | 0.19 |
| 7 | Apex tip | 1 | 2 | 1 | 1.93 | 0.26 | 13.47 | 0.19 |
| 8 | Slender achene | 1 | 2 | 1 | 1.96 | 0.19 | 9.69 | 0.12 |
| 9 | Kernel length | 3.00 | 7.00 | 4.00 | 5.02 | 0.86 | 17.13 | 0.17 |
| 10 | 1000-grain weight | 16.58 | 29.57 | 12.99 | 22.45 | 2.57 | 11.45 | 0.24 |
| Primers | Na | Ne | I | Ho | He | PIC |
|---|---|---|---|---|---|---|
| P1 | 5.000 | 2.873 | 1.116 | 0.345 | 0.655 | 0.581 |
| P3 | 3.000 | 1.394 | 0.488 | 0.716 | 0.284 | 0.248 |
| P6 | 3.000 | 2.912 | 1.083 | 0.340 | 0.660 | 0.582 |
| P12 | 4.000 | 2.784 | 1.200 | 0.356 | 0.644 | 0.596 |
| P22 | 2.000 | 1.987 | 0.690 | 0.501 | 0.500 | 0.373 |
| P58 | 5.000 | 2.686 | 1.117 | 0.370 | 0.631 | 0.565 |
| P59 | 9.000 | 2.357 | 1.366 | 0.422 | 0.578 | 0.559 |
| P60 | 5.000 | 1.368 | 0.577 | 0.730 | 0.271 | 0.256 |
| P64 | 6.000 | 1.826 | 0.902 | 0.546 | 0.454 | 0.414 |
| P70 | 3.000 | 2.106 | 0.797 | 0.473 | 0.527 | 0.412 |
| Total | 45.000 | |||||
| Average | 4.500 | 2.229 | 0.934 | 0.480 | 0.520 | 0.459 |
| Locus | Polygonal | Gray | Light Gray | Brown | Thorn | Bulge in Ridge | Apex Tip | Slender Achene | Kernel Length | 1000-Grain Weight |
|---|---|---|---|---|---|---|---|---|---|---|
| P1 | 0.190 | 0.232 | ||||||||
| P3 | 0.342 | −0.344 | ||||||||
| P6 | 0.209 | |||||||||
| P12 | 0.219 | 0.192 | ||||||||
| P22 | −0.200 | |||||||||
| P58 | −0.204 | −0.218 | −0.333 | 0.192 | 0.224 | −0.210 | −0.237 | |||
| P59 | 0.225 | 0.194 | 0.253 | 0.242 | 0.244 | |||||
| P60 | −0.193 | 0.278 | 0.321 | 0.192 | 0.222 | 0.244 | 0.263 | |||
| P64 | 0.337 | −0.500 | ||||||||
| P70 | 0.189 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, Y.; Cheng, Z.; Dong, Y.; Liu, D.; Bai, K.; Jarvis, D.; Feng, J.; Long, C. Diversity of Tartary Buckwheat (Fagopyrum tataricum) Landraces from Liangshan, Southwest China: Evidence from Morphology and SSR Markers. Agronomy 2022, 12, 1022. https://doi.org/10.3390/agronomy12051022
Song Y, Cheng Z, Dong Y, Liu D, Bai K, Jarvis D, Feng J, Long C. Diversity of Tartary Buckwheat (Fagopyrum tataricum) Landraces from Liangshan, Southwest China: Evidence from Morphology and SSR Markers. Agronomy. 2022; 12(5):1022. https://doi.org/10.3390/agronomy12051022
Chicago/Turabian StyleSong, Yingjie, Zhuo Cheng, Yumei Dong, Dongmei Liu, Keyu Bai, Devra Jarvis, Jinchao Feng, and Chunlin Long. 2022. "Diversity of Tartary Buckwheat (Fagopyrum tataricum) Landraces from Liangshan, Southwest China: Evidence from Morphology and SSR Markers" Agronomy 12, no. 5: 1022. https://doi.org/10.3390/agronomy12051022
APA StyleSong, Y., Cheng, Z., Dong, Y., Liu, D., Bai, K., Jarvis, D., Feng, J., & Long, C. (2022). Diversity of Tartary Buckwheat (Fagopyrum tataricum) Landraces from Liangshan, Southwest China: Evidence from Morphology and SSR Markers. Agronomy, 12(5), 1022. https://doi.org/10.3390/agronomy12051022







