The Effect of Various Foliar Treatments and Nitrogen Nutrition Levels on the Yield and Physicochemical Parameters of Flowering Chinese Cabbage
Abstract
1. Introduction
2. Materials and Methods
2.1. Experiment Design and Plant Cultivation
2.2. Analysis of Macro- and Microelements in Plants
2.3. Preparation of Samples for Physicochemical Analyses
2.4. Colour Measurements
2.5. Chlorophyll a (Chl a), Chlorophyll b (Chl b) and Carotenoid Content
2.6. TPC and TFC Measurements
2.7. Antioxidant Activity
2.8. Statistical Analysis
3. Results
3.1. Yield
3.2. Nutrient Content
3.3. Colour Measurement
3.4. Chl a, Chl b, and Carotenoid Content
3.5. Total Polyphenol Content and Total Flavonoid Content
3.6. Antioxidant Activity
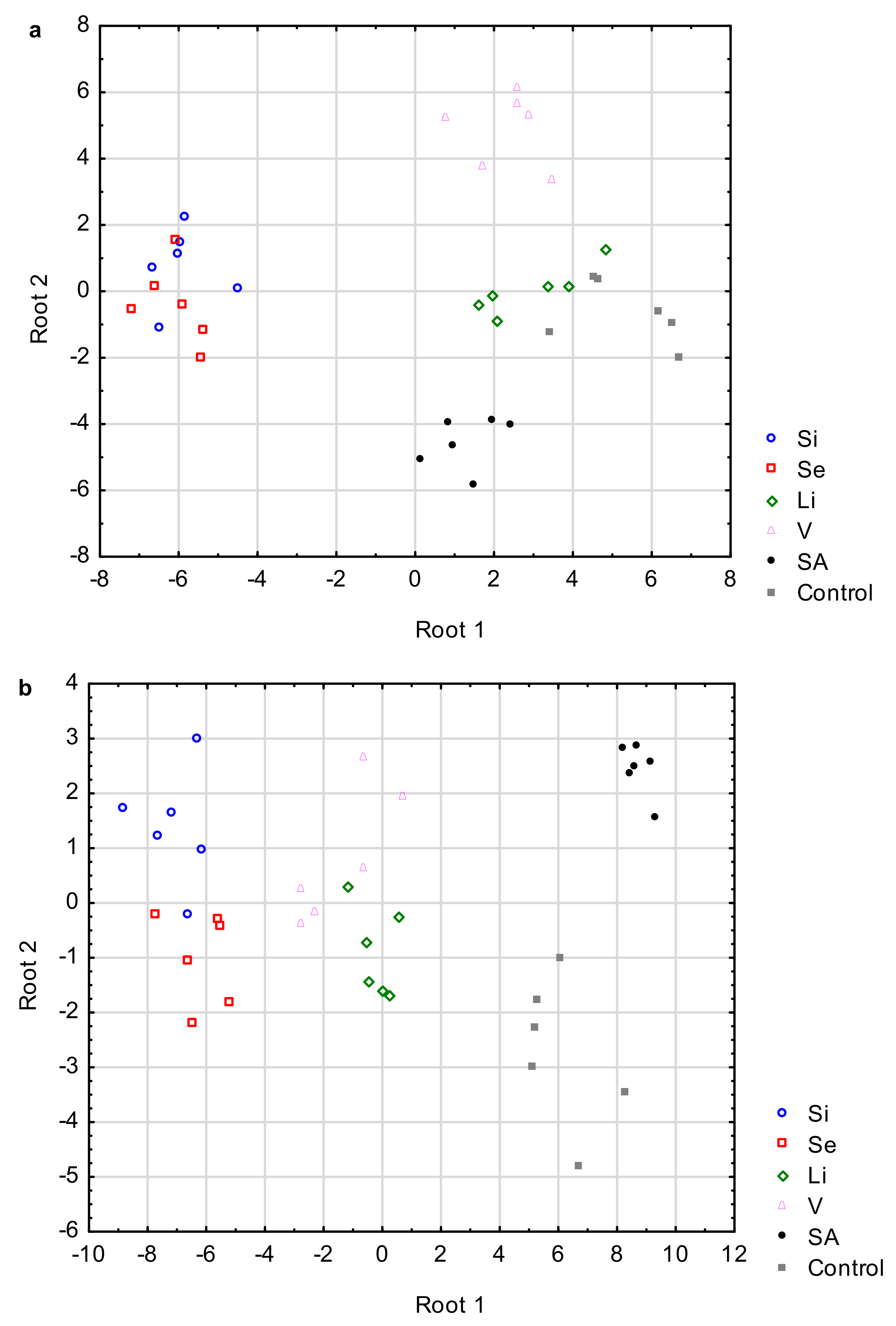
3.7. Multivariate Analysis
4. Discussion
4.1. Yielding
4.2. Plant Nutrient Status
4.3. Pigments
4.4. Antioxidant Activity
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Frink, C.R.; Waggoner, P.E.; Ausubel, J.H. Nitrogen fertilizer: Retrospect and prospect. Proc. Natl. Acad. Sci. USA 1999, 96, 1175–1180. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Liu, H.; Sun, G. The Effects of Different Controlled-release Nitrogen Fertilizers on Yield and Quality of Flowering Chinese Cabbage. China Veg. 2006, 8, 16–18. [Google Scholar]
- FAOSTAT. 2021. Available online: https://www.fao.org/3/ca6746en/ca6746en.pdf (accessed on 22 January 2022).
- Jensen, E.S.; Carlsson, G.; Hauggaard-Nielsen, H. Intercropping of grain legumes and cereals improves the use of soil N resources and reduces the requirement for synthetic fertilizer N: A global-scale analysis. Agron. Sustain. Dev. 2020, 40. [Google Scholar] [CrossRef]
- Niu, J.; Liu, C.; Huang, M.; Liu, K.; Yan, D. Effects of Foliar Fertilization: A Review of Current Status and Future Perspectives. J. Soil Sci. Plant Nutr. 2020, 21, 104–118. [Google Scholar] [CrossRef]
- Ling, F.; Silberbush, M. Response of maize to foliar vs. soil application of nitrogen–phosphorus–potassium fertilizers. J. Plant Nutr. 2002, 25, 2333–2342. [Google Scholar] [CrossRef]
- Huang, H.; Li, M.; Rizwan, M.; Dai, Z.; Yuan, Y.; Hossain, M.M.; Cao, M.; Xiong, S.; Tu, S. Synergistic effect of silicon and selenium on the alleviation of cadmium toxicity in rice plants. J. Hazard. Mater. 2021, 401, 123393. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, F.; Ahmad, R.; Ashraf, M.Y.; Waraich, E.A.; Khan, S.Z. Effect of selenium foliar spray on physiological and biochemical processes and chemical constituents of wheat under drought stress. Ecotoxicol. Environ. Saf. 2015, 113, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.Z.; Wang, Y.; Wang, S.H.; Yin, L.P.; Xu, G.J.; Zheng, C.; Lei, C.; Zhang, M.Z. Selenium increases chlorogenic acid, chlorophyll and carotenoids of Lycium chinense leaves. J. Sci. Food Agric. 2013, 93, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Saffaryazdi, A.; Lahouti, M.; Ganjeali, A.; Bayat, H. Impact of Selenium Supplementation on Growth and Selenium Accumulation on Spinach (Spinacia oleracea L.) Plants. Not. Sci. Biol. 2012, 4, 95–100. [Google Scholar] [CrossRef]
- Wu, Z.; Liu, S.; Zhao, J.; Fuhua, W.; Yingqiong, D.; Zou, S.; Li, H.; Wen, D.; Huang, Y. Comparative responses to silicon and selenium in relation to antioxidant enzyme system and the glutathione-ascorbate cycle in flowering Chinese cabbage (Brassica campestris L. ssp. chinensis var. utilis) under cadmium stress. Environ. Exp. Bot. 2017, 133, 1–11. [Google Scholar] [CrossRef]
- Wang, B.; Chu, C.; Wei, H.; Zhang, L.; Ahmad, Z.; Wu, S.; Xie, B. Ameliorative effects of silicon fertilizer on soil bacterial community and pakchoi (Brassica chinensis L.) grown on soil contaminated with multiple heavy metals. Environ. Pollut. 2020, 267, 115411. [Google Scholar] [CrossRef] [PubMed]
- D’Imperio, M.; Renna, M.; Cardinali, A.; Buttaro, D.; Santamaria, P.; Serio, F. Silicon biofortification of leafy vegetables and its bioaccessibility in the edible parts. J. Sci. Food Agric. 2016, 96, 751–756. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, L.; Tanveer, M.; Tian, C. Lithium biofortification of medicinal tea Apocynum venetum. Sci. Rep. 2019, 9, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Aihemaiti, A.; Gao, Y.; Meng, Y.; Chen, X.; Liu, J.; Xiang, H.; Xu, Y.; Jiang, J. Review of plant-vanadium physiological interactions, bioaccumulation, and bioremediation of vanadium-contaminated sites. Sci. Total Environ. 2020, 712, 135637. [Google Scholar] [CrossRef]
- Zhao, H.; Xie, X.; Read, P.; Loseke, B.; Gamet, S.; Li, W.; Xu, C. Biofortification with selenium and lithium improves nutraceutical properties of major winery grapes in the Midwestern United States. Int. J. Food Sci. Technol. 2020, 56, 1–13. [Google Scholar] [CrossRef]
- Shahzad, B.; Tanveer, M.; Hassan, W.; Shah, A.N.; Anjum, S.A.; Cheema, S.A.; Ali, I. Lithium toxicity in plants: Reasons, mechanisms and remediation possibilities–A review. Plant Physiol. Biochem. 2016, 107, 104–115. [Google Scholar] [CrossRef]
- Da Silva, R.R.; de Faria, A.J.G.; do Carmo Alexandrino, G.; Ribeiro, E.A.; dos Santos, A.C.M.; Deusdara, T.T.; do Nascimento, I.R.; Nascimento, V.L. Enrichment of lithium in lettuce plants through agronomic biofortification. J. Plant Nutr. 2019, 42, 2102–2113. [Google Scholar] [CrossRef]
- Sentíes-Herrera, H.E.; Trejo-Téllez, L.I.; Volke-Haller, V.H.; Cadena-Íñiguez, J.; Sánchez-García, P.; Gómez-Merino, F.C. Iodine, Silicon, and Vanadium Differentially Affect Growth, Flowering, and Quality Components of Stalks in Sugarcane. Sugar Tech 2018, 20, 518–533. [Google Scholar] [CrossRef]
- Wu, Z.; Yang, J.; Zhang, Y.; Wang, C.; Guo, S.; Yu, Y. Growth responses, accumulation, translocation and distribution of vanadium in tobacco and its potential in phytoremediation. Ecotoxicol. Environ. Saf. 2021, 207, 111297. [Google Scholar] [CrossRef]
- Hayat, Q.; Hayat, S.; Irfan, M.; Ahmad, A. Effect of exogenous salicylic acid under changing environment: A review. Environ. Exp. Bot. 2010, 68, 14–25. [Google Scholar] [CrossRef]
- Fariduddin, Q.; Hayat, S.; Ahmad, A. Salicylic acid influences net photosynthetic rate, carboxylation efficiency, nitrate reductase activity, and seed yield in Brassica juncea. Photosynthetica 2003, 41, 281–284. [Google Scholar] [CrossRef]
- Chen, X.; Zhu, Z.; Gerendás, J.; Zimmermann, N. Glucosinolates in Chinese Brassica campestris vegetables: Chinese cabbage, purple cai-tai, choysum, pakchoi, and turnip. HortScience 2008, 43, 571–574. [Google Scholar] [CrossRef]
- Yang, Y.; Guo, J.; Liu, Y. The nutritive composition and assessment of Brassica parachinensis. Food Technol. 2002, 9, 74–76. [Google Scholar]
- Mo, H.Z.; Zhu, Y.Y.; Zhang, M. Selenium enrichment pattern in flowering Chinese cabbage, cabbage and asparagus. Agro Food Ind. Hi-Tech 2006, 17, 39–42. [Google Scholar]
- IUNG. Analytical Methods in Agricultural Chemistry Stations Part II. Plant Analyses; Institute of Soil Science and Plant Cultivation: Puławy, Poland, 1972; pp. 25–83. [Google Scholar]
- Bosiacki, M.; Roszyk, J. The comparing methods of mineralization of plant material on the content of heavy metals. Res. Didact. Appar. 2010, 15, 37–41. [Google Scholar]
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and Carotenoids (2008): Measurement and Characterization by UV-VIS. Curr Protoc. Food Anal. Chem. 2001, 1, F4.3.1–F4.3.8. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Ozgen, M.; Reese, R.N.; Tulio, A.Z.; Scheerens, J.C.; Miller, A.R. Modified 2,2-azino-bis-3-ethylbenzothiazoline-6-sulfonic acid (ABTS) method to measure antioxidant capacity of selected small fruits and comparison to ferric reducing antioxidant power (FRAP) and 2,2′-diphenyl-1-picrylhydrazyl (DPPH) methods. J. Agric. Food Chem. 2006, 54, 1151–1157. [Google Scholar] [CrossRef]
- Sánchez-Moreno, C.; Larrauri, J.A.; Saura-Calixto, F. A procedure to measure the antiradical efficiency of polyphenols. J. Sci. Food Agric. 1998, 76, 270–276. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Nie, J.; Liao, Y.; Huang, S.; Tang, J. Effects of Different Nitrogen Fertilizer Rates on Yield and Quality of Brassica compestris L. var. purpurea Bailey. Hunan Agric. Sci. 2011, 239, 70–76. [Google Scholar]
- Krezel, J.; Kołota, E. The effect of nitrogen fertilization on yield and biological value of Chinese cabbage grown from seed growing for autumn harvest. Folia Univ. Agric. Stetin. Agric. 2004, 95, 197–200. [Google Scholar]
- Ekelund, N.G.A.; Danilov, R.A. The influence of selenium on photosynthesis and” light-enhanced dark respiration”(LEDR) in the flagellate Euglena gracilis after exposure to ultraviolet radiation. Aquat. Sci. 2001, 63, 457–465. [Google Scholar] [CrossRef]
- Shrift, A. Aspects of selenium metabolism in higher plants. Annu. Rev. Plant Physiol. 1969, 20, 475–494. [Google Scholar] [CrossRef]
- Aly, R.A.M.; Abdel-Halim, K.Y. Effect of bio-fertilizer and foliar spray of selenium of growth, yield and quality of potato plants. Acad. J. Life Sci. 2020, 6, 1–7. [Google Scholar]
- Hawrylak-Nowak, B.; Kalinowska, M.; Szymańska, M. A study on selected physiological parameters of plants grown under lithium supplementation. Biol. Trace Elem. Res. 2012, 149, 425–430. [Google Scholar] [CrossRef]
- Bakhat, H.F.; Rasul, K.; Farooq, A.B.U.; Zia, Z.; Natasha; Fahad, S.; Abbas, S.; Shah, G.M.; Rabbani, F.; Hammad, H.M. Growth and physiological response of spinach to various lithium concentrations in soil. Environ. Sci. Pollut. Res. 2020, 27, 39717–39725. [Google Scholar] [CrossRef]
- García-Jiménez, A.; Trejo-Téllez, L.I.; Guillén-Sánchez, D.; Gómez-Merino, F.C. Vanadium stimulates pepper plant growth and flowering, increases concentrations of amino acids, sugars and chlorophylls, and modifies nutrient concentrations. PLoS ONE 2018, 13, e0201908. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, M.A.; Attallah, S.Y.; Mark, C. Salicylic Acid Enhances Growth, Yield and Quality of Lettuce Plants (Lactuca sativa L.) under Drought Stress Conditions. J. Plant Prod. 2020, 11, 1581–1586. [Google Scholar] [CrossRef]
- Kabata-Pendias, A.; Mukherjee, A.B. Trace Elements from Soil to Human; Springer: Berlin, Germany, 2007; pp. 87–93. [Google Scholar]
- Pérez, P.; Martínez, M.C. Effect of vanadium on lettuce growth, cationic nutrition, and yield. J. Environ. Sci. Health Part A Environ. Sci. Eng. Toxicol. 1995, 30, 73–87. [Google Scholar] [CrossRef]
- Kawano, T.; Furuichi, T.; Muto, S. Controlled salicylic acid levels and corresponding signaling mechanisms in plants. Plant Biotechnol. 2004, 21, 319–335. [Google Scholar] [CrossRef][Green Version]
- Pennanen, A.; Xue, T.; Hartikainen, H. Protective role of selenium in plant subjected to severe UV irradiation stress. J. Appl. Bot. 2002, 76, 66–76. [Google Scholar]
- Singh, B.B. Effect of vanadium on the growth, yield and chemical composition of maize (Zea mays L.). Plant Soil 1971, 34, 209–213. [Google Scholar] [CrossRef]
- Basiouny, F.M. Distribution of vanadium and its influence on chlorophyll formation and iron metabolism in tomato plants. J. Plant Nutr. 1984, 7, 1059–1073. [Google Scholar] [CrossRef]
- Gunes, A.; Pilbeam, D.J.; Inal, A.; Coban, S. Influence of silicon on sunflower cultivars under drought stress, I: Growth, antioxidant mechanisms, and lipid peroxidation. Commun. Soil Sci. Plant Anal. 2008, 39, 1885–1903. [Google Scholar] [CrossRef]
- Shahid, M.; Niazi, N.K.; Khalid, S.; Murtaza, B.; Bibi, I.; Rashid, M.I. A critical review of selenium biogeochemical behavior in soil-plant system with an inference to human health. Environ. Pollut. 2018, 234, 915–934. [Google Scholar]
- Choudhury, S.; Panda, S.K. Role of salicylic acid in regulating cadmium induced oxidative stress in Oryza sativa L. roots. Bulg. J. Plant Physiol. 2004, 30, 95–110. [Google Scholar]


| Foliar Spray | Pot Cultivation | Hydroponic | ||||
|---|---|---|---|---|---|---|
| N-70 * | N-90 * | Mean ** | N-70 | N-90 | Mean | |
| Se | 118.9 fg | 150.0 bcd | 134.8 c | 163.8 bc | 190.1 abc | 176.9 b |
| Si | 126.9 efg | 146.9 cd | 136.9 c | 139.6 c | 187.3 abc | 163.4 b |
| Li | 160.1 bc | 182.0 a | 171.1 a | 157.9 bc | 189.9 abc | 173.9 b |
| V | 134.8 def | 168.3 ab | 151.6 b | 167.1 bc | 166.3 bc | 166.7 b |
| SA | 113.6 g | 143.3 cde | 128.5 c | 176.3 abc | 200.6 ab | 188.4 ab |
| Control | 152.0 bcd | 154.9 bc | 153.5 b | 200.6 ab | 222.3 a | 211.5 a |
| Mean ** | 134.4 b | 157.7 a | 167.5 b | 192.8 a | ||
| ±SD | ±16.8 | ±13.5 | ±18.5 | ±16.7 | ||
| N | P | K | Ca | Mg | Na | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Foliar Spray | N-70 * | N-90 * | Mean * | N-70 | N-90 | Mean | N-70 | N-90 | Mean | N-70 | N-90 | Mean | N-70 | N-90 | Mean | N-70 | N-90 | Mean |
| Pot cultivation | ||||||||||||||||||
| Se | 3.08 f | 3.76 abc | 3.42 bc | 0.50 b | 0.48 b | 0.49 b | 4.21 c | 4.77 abc | 4.49 a | 4.09 a | 3.97 ab | 4.03 a | 0.29 a | 0.28 ab | 0.28 a | 0.14 ab | 0.17 a | 0.15 a |
| Si | 3.20 ef | 3.73 abcd | 3.47 bc | 0.51 ab | 0.54 ab | 0.53 ab | 4.55 bc | 5.15 a | 4.85 a | 4.02 ab | 3.69 ab | 3.86 a | 0.27 ab | 0.28 ab | 0.28 a | 0.14 ab | 0.17 a | 0.16 a |
| Li | 3.29 ef | 3.43 cde | 3.36 bc | 0.51 ab | 0.54 ab | 0.52 ab | 4.87 abc | 5.13 ab | 5.00 a | 3.86 ab | 3.89 ab | 3.87 a | 0.26 abc | 0.27 abc | 0.27 ab | 0.13 ab | 0.16 ab | 0.15 ab |
| V | 3.00 f | 3.52 bcde | 3.29 c | 0.49 b | 0.50 b | 0.50 b | 4.72 abc | 4.67 abc | 4.70 a | 3.60 abc | 2.84 bc | 3.22 ab | 0.26 abc | 0.27 abc | 0.26 ab | 0.15 ab | 0.15 ab | 0.15 ab |
| SA | 3.83 ab | 3.94 a | 3.89 a | 0.61 a | 0.57 ab | 0.59 a | 4.96 abc | 4.68 abc | 4.82 a | 2.22 c | 3.03 abc | 2.63 b | 0.21 c | 0.25 abc | 0.23 b | 0.13 b | 0.13 ab | 0.13 b |
| Control | 3.41 ed | 3.71 abcd | 3.56 b | 0.53 ab | 0.50 ab | 0.52 ab | 4.81 abc | 4.82 abc | 4.82 a | 3.26 abc | 3.64 ab | 3.45 ab | 0.25 abc | 0.24 bc | 0.25 ab | 0.14 ab | 0.16 a | 0.15 ab |
| Mean ** | 3.31 b | 3.68 a | 0.53 a | 0.52 a | 4.69 a | 4.87 a | 3.51 a | 3.51 a | 0.26 a | 0.26 a | 0.14 b | 0.16 a | ||||||
| ±SD | ±0.33 | ±0.19 | ±0.05 | ±0.07 | ±0.51 | ±0.34 | ±0.85 | ±0.83 | ±0.03 | ±0.03 | ±0.02 | ±0.02 | ||||||
| Hydroponic | ||||||||||||||||||
| Se | 5.09 d | 5.67 ab | 5.38 ab | 0.42 e | 0.61 a | 0.51 ab | 6.66 a | 6.36 a | 6.51 a | 2.41 abc | 2.59 ab | 2.50 a | 0.78 cd | 0.85 ab | 0.81 ab | 0.18 ab | 0.17 abc | 0.18 ab |
| Si | 5.04 d | 5.65 abc | 5.34 ab | 0.55 abcde | 0.46 cde | 0.50 ab | 6.27 a | 6.57 a | 6.42 a | 2.30 abc | 2.59 a | 2.44 a | 0.75 bcd | 0.96 a | 0.85 a | 0.16 bc | 0.17 abc | 0.17 ab |
| Li | 5.25 bcd | 4.97 d | 5.11 b | 0.47 bcde | 0.44 de | 0.45 b | 6.45 a | 6.47 a | 6.46 a | 2.51 abc | 2.57 abc | 2.54 a | 0.85 ab | 0.81 bc | 0.83 ab | 0.19 ab | 0.18 ab | 0.19 a |
| V | 5.37 bcd | 5.60 abc | 5.48 a | 0.55 abcd | 0.58 abc | 0.57 a | 6.20 a | 6.66 a | 6.43 a | 2.25 abc | 2.19 bc | 2.22 a | 0.81 abc | 0.68 cd | 0.75 abc | 0.20 a | 0.14 c | 0.17 ab |
| SA | 5.11 d | 5.81 a | 5.46 a | 0.54 abcde | 0.59 ab | 0.57 a | 6.51 a | 6.67 a | 6.59 a | 2.12 c | 2.43 abc | 2.28 a | 0.74 bcd | 0.64 d | 0.69 c | 0.18 ab | 0.16 bc | 0.17 ab |
| Control | 4.39 e | 5.23 cd | 4.81 c | 0.50 abcde | 0.58 abc | 0.54 a | 6.61 a | 6.57 a | 6.59 a | 2.57 abc | 2.38 abc | 2.48 a | 0.73 bcd | 0.75 bcd | 0.74 bc | 0.17 abc | 0.14 c | 0.16 b |
| Mean | 5.04 b | 5.49 a | 0.51 a | 0.54 a | 6.45 a | 6.55 a | 2.36 a | 2.46 a | 0.78 a | 0.78 a | 0.18 a | 0.16 b | ||||||
| ±SD | ±0.45 | ±0.47 | ±0.07 | ±0.09 | ±0.37 | ±0.40 | ±0.32 | ±0.22 | ±0.10 | ±0.12 | ±0.02 | ±0.02 | ||||||
| Fe | Mn | Zn | Cu | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Foliar Spray | N-70 * | N-90 * | Mean ** | N-70 | N-90 | Mean | N-70 | N-90 | Mean | N-70 | N-90 | Mean |
| Pot cultivation | ||||||||||||
| Se | 178.4 bc | 173.5 bc | 175.9 abc | 45.9 bc | 49.0 bc | 47.5 bcd | 72.3 abc | 76.9 ab | 74.6 ab | 5.52 bc | 5.48 bc | 5.50 b |
| Si | 175.1 bc | 211.9 a | 193.5 a | 51.7 b | 56.5 b | 54.1 ab | 76.3 abc | 76.7 ab | 76.5 ab | 5.07 bc | 5.36 bc | 5.21 b |
| Li | 164.4 cd | 157.3 cd | 160.9 c | 40.0 cd | 49.9 bc | 45.0 cd | 73.5 abc | 81.1 a | 77.3 a | 5.68 bc | 4.69 c | 5.19 b |
| V | 173.2 bc | 165.5 cd | 169.4 bc | 47.0 bc | 49.4 bc | 48.2 bc | 71.2 abc | 69.9 abc | 70.6 ab | 5.18 bc | 8.42 a | 6.80 a |
| SA | 140.3 d | 185.5 abc | 162.9 c | 34.3 d | 47.1 bc | 40.7 d | 64.2 c | 73.4 abc | 68.8 b | 6.37 b | 5.65 bc | 6.01 ab |
| Control | 181.2 bc | 197.4 ab | 189.3 ab | 50.9 bc | 70.1 a | 60.5 a | 68.9 bc | 77.5 ab | 73.2 ab | 5.63 bc | 5.19 bc | 5.41 b |
| Mean ** | 168.8 b | 181.9 a | 45.00 b | 53.7 a | 71.1 b | 75.9 a | 5.58 a | 5.80 a | ||||
| ±SD | ±21.0 | ±22.5 | ±7.1 | ±10.1 | ±6.4 | ±6.5 | ±1.02 | ±1.45 | ||||
| Hydroponic | ||||||||||||
| Se | 162.7 b | 142.2 bcd | 152.5 b | 41.5 de | 59.8 bc | 50.6 bc | 61.4 ef | 95.6 ab | 78.5 ab | 4.22 cd | 6.31 a | 5.26 ab |
| Si | 148.0 cb | 193.1 a | 170.5 a | 34.5 ef | 63.8 b | 49.1 c | 70.2 cde | 99.0 a | 84.6 a | 3.74 d | 6.04 ab | 4.89 b |
| Li | 103.9 f | 114.2 ef | 109.1 c | 45.6 de | 69.0 b | 57.3 b | 76.5 c | 75.0 d | 75.7 b | 3.27 d | 3.70 d | 3.49 c |
| V | 111.4 ef | 124.0 cdef | 117.7 c | 45.1 de | 49.2 cd | 47.1 cd | 87.8 b | 66.3 def | 77.1 b | 4.21 cd | 3.59 d | 3.90 c |
| SA | 106.8 ef | 132.0 cde | 119.4 c | 52.1 cd | 28.9 f | 40.5 d | 90.6 ab | 60.4 f | 75.5 b | 5.82 ab | 6.13 a | 5.98 a |
| Control | 113.7 ef | 119.5 def | 116.6 c | 49.9 cd | 82.5 a | 66.2 a | 91.8 ab | 76.4 c | 84.1 a | 4.99 bc | 4.31 cd | 4.65 b |
| Mean | 124.4 b | 137.5 a | 44.8 b | 58.9 a | 79.7 a | 78.8 a | 4.38 b | 5.01 a | ||||
| ±SD | ±25.6 | ±30.3 | ±7.2 | ±8.2 | ±12.3 | ±15.3 | ±0.95 | ±1.01 | ||||
| L* (D65) | a* (D65) Green | b* (D65) Yellow | h* [°] (D65) | C* | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Foliar Spray | N-70 | N-90 | N-70 | N-90 | N-70 | N-90 | N-70 | N-90 | N-70 | N-90 |
| Pot cultivation | ||||||||||
| Se | 49.2 f | 48.5 j | −8.68 g | −8.50 c | 22.7 g | 22.0 j | 110.9 c | 111.1 b | 24.3 g | 23.6 j |
| Si | 49.1 i | 49.1 h | −8.52 d | −8.74 h | 21.4 l | 23.0 f | 111.7 a | 110.8 d | 23.0 l | 24.6 f |
| Li | 49.2 g | 49.6 d | −9.14 j | −8.55 e | 23.7 d | 22.5 i | 111.1 b | 110.8 d | 25.4 d | 24.1 i |
| V | 51.8 a | 49.5 e | −8.20 a | −8.64 f | 21.8 k | 22.6 h | 110.6 e | 111.0 c | 23.3 k | 24.2 h |
| SA | 47.4 l | 47.7 k | −8.35 b | −8.83 i | 24.1 c | 24.7 a | 109.1 i | 109.7 g | 25.5 c | 26.2 a |
| Control | 49.8 c | 50.0 b | −8.53 d | −8.63 f | 24.5 b | 23.3 e | 109.2 h | 110.4 f | 25.9 b | 24.8 e |
| Mean ±SD | 49.4 ±1.34 | 49.1 ±0.79 | −8.87 ±0.31 | −8.65 ±0.11 | 23.0 ±1.18 | 23.0 ±0.87 | 110.5 ±0.99 | 110.6 ±0.48 | 24.6 ±1.14 | 23.0 ±0.86 |
| Hydroponic | ||||||||||
| Se | 48.4 a | 47.1 g | −8.91 l | −8.37 c | 22.0 d | 20.45 i | 112.0 d | 112.3 b | 23.8 d | 22.1 j |
| Si | 47.9 c | 48.1 b | −8.31 b | −8.31 b | 19.9 j | 20.52 h | 112.7 a | 112.1 c | 21.5 k | 22.1 i |
| Li | 46.8 h | 47.3 f | −8.78 i | −8.56 e | 22.1 c | 20.47 i | 111.6 f | 112.7 a | 23.8 c | 22.2 h |
| V | 47.6 d | 46.6 i | −8.58 f | −8.44 d | 21.2 f | 20.90 g | 112.0 de | 112.0 e | 22.9 e | 22.5 g |
| SA | 45.3 l | 45.9 k | −8.83 k | −8.66 g | 23.5 a | 22.53 b | 110.6 j | 111.0 i | 25.1 a | 24.1 b |
| Control | 46.5 j | 47.4 e | −8.81 j | −8.28 a | 23.5 a | 22.03 e | 110.5 k | 111.3 h | 25.1 a | 22.8 f |
| Mean ±SD | 47.1 ±0.92 | 47.1 ±0.72 | −8.70 ±0.21 | −8.44 ±0.14 | 22.1 ±1.31 | 21.02 ±0.76 | 111.6 ±0.81 | 111.9 ±0.58 | 23.7 ±1.29 | 22.7 ±0.73 |
| Foliar Spray | Chl a (mg g−1 DW) | Chl b (mg g−1 DW) | Carotenoid (x + c) (mg g−1 DW) | Chl a + Chl b (mg g−1 DW) | Chl a/Chl b | (Chl a + Chl b)/ (x + c) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N-70 | N-90 | N-70 | N-90 | N-70 | N-90 | N-70 | N-90 | N-70 | N-90 | N-70 | N-90 | |
| Pot cultivation | ||||||||||||
| Se | 6.86 a | 6.92 a | 2.19 ab | 2.21 ab | 1.76 ab | 1.80 ab | 9.04 a | 9.13 a | 3.13 b | 3.14 b | 5.14 a | 5.08 a |
| Si | 6.85 a | 7.12 a | 2.20 ab | 2.26 a | 1.71 b | 1.84 a | 9.05 a | 9.38 a | 3.11 b | 3.15 b | 5.29 a | 5.11 a |
| Li | 5.91 c | 5.04 d | 1.84 cd | 1.65 de | 1.52 cd | 1.34 f | 7.75 c | 6.69 d | 3.21 b | 3.05 bc | 5.10 a | 4.98 ab |
| V | 4.62 e | 5.75 c | 1.32 f | 1.94 c | 1.33 f | 1.44 de | 5.94 e | 7.69 c | 3.53 a | 2.97 bc | 4.52 b | 5.34 a |
| SA | 5.81 c | 6.31 b | 2.07 abc | 2.01 bc | 1.49 cd | 1.58 c | 7.88 bc | 8.32 b | 2.83 c | 3.14 b | 5.27 a | 5.27 a |
| Control | 4.89 ed | 5.63 c | 1.62 e | 1.99 bc | 1.25 g | 1.38 ef | 6.50 d | 7.62 c | 3.02 bc | 2.84 c | 5.22 a | 5.52 a |
| Mean ±SD | 5.82 ±0.90 | 6.13 ±0.77 | 1.87 ±0.36 | 2.01 ±0.21 | 1.51 ±0.20 | 1.56 ±0.20 | 7.70 ±1.24 | 8.14 ±0.97 | 3.14 ±0.26 | 3.05 ±0.13 | 5.09 ±0.41 | 5.22 ±0.23 |
| Hydroponic | ||||||||||||
| Se | 8.24 b | 8.80 a | 1.67 ab | 1.82 a | 2.29 ef | 2.23 f | 9.91 d | 10.62 ab | 4.94 bc | 4.84 bc | 4.33 b | 4.77 a |
| Si | 9.02 a | 8.47 b | 1.85 a | 1.70 ab | 2.50 ab | 2.39 cde | 10.87 a | 10.17 cd | 4.89 bc | 4.97 bc | 4.35 b | 4.31 b |
| Li | 8.36 b | 8.91 a | 1.68 ab | 1.73 ab | 2.33 def | 2.55 a | 10.05 cd | 10.64 ab | 4.98 bc | 5.17 bc | 4.31 b | 4.18 bc |
| V | 8.25 b | 8.36 b | 1.79 a | 1.56 bc | 2.39 bcde | 2.50 ab | 10.04 cd | 9.92 d | 4.62 c | 5.37 b | 4.20 bc | 3.96 c |
| SA | 7.81 c | 8.43 b | 1.30 d | 1.76 a | 2.48 abc | 2.41 bcd | 9.10 e | 10.19 cd | 6.11 a | 4.81 bc | 3.68 d | 4.23 b |
| Control | 8.45 b | 6.91 d | 1.85 a | 1.48 c | 2.45 abcd | 2.05 g | 10.30 bc | 8.39 f | 4.58 c | 4.68 c | 4.21 bc | 4.09 bc |
| Mean ±SD | 8.35 ±0.38 | 8.31 ±0.69 | 1.69 ±0.21 | 1.67 ±0.15 | 2.41 ±0.10 | 2.35 ±0.18 | 10.04 ±0.56 | 9.99 ±0.80 | 5.02 ±0.63 | 4.98 ±0.34 | 4.18 ±0.27 | 4.26 ±0.28 |
| Foliar Spray | TPC | TFC | TEAC | DPPH | FRAP | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| (mg GAE g−1) | (mg QE g−1) | (μmol g−1) | (μmol TE g−1) | (mmol TE g−1) | ||||||
| N-70 | N-90 | N-70 | N-90 | N-70 | N-90 | N-70 | N-90 | N-70 | N-90 | |
| Pot cultivation | ||||||||||
| Se | 8.69 c | 8.87 bc | 2.68 a | 2.45 b | 615.4 d | 646.2 b | 184.5 bc | 177.8 ef | 38.7 b | 39.5 a |
| Si | 9.10 b | 6.21 g | 2.32 bc | 1.94 f | 589.7 f | 647.9 b | 179.0 de | 167.4 h | 38.5 b | 38.8 b |
| Li | 8.16 d | 8.60 c | 2.16 de | 2.13 de | 576.3 g | 593.1 f | 182.0 cd | 192.4 a | 34.2 e | 34.4 e |
| V | 6.87 f | 8.67 c | 2.13 de | 2.26 cd | 576.4 g | 606.0 de | 166.2 h | 187.3 b | 29.9 f | 36.3 d |
| SA | 9.46 a | 8.59 c | 2.35 bc | 1.78 g | 720.4 a | 602.1 ef | 166.2 h | 173.7 g | 36.1 d | 36.7 cd |
| Control | 7.49 e | 8.62 c | 2.09 e | 2.17 de | 629.8 c | 647.5 b | 175.7 fg | 186.1 b | 34.8 e | 37.1 c |
| Mean ±SD | 8.30 ±0.94 | 8.26 ±0.96 | 2.29 ±0.22 | 2.13 ±0.22 | 618.0 ±51.9 | 623.8 ±24.6 | 175.6 ±7.60 | 180.8 ±8.92 | 35.4 ±3.08 | 37.1 ±1.77 |
| Hydroponic | ||||||||||
| Se | 7.31 c | 6.66 cde | 1.86 b | 1.58 b | 630.6 a | 614.7 b | 201.3 b | 224.1 a | 44.8 a | 43.0 b |
| Si | 8.61 b | 6.19 e | 2.03 b | 3.69 a | 607.4 bc | 530.8 g | 195.2 c | 204.5 b | 44.3 a | 35.8 f |
| Li | 8.38 b | 6.10 e | 2.27 b | 1.66 b | 598.5 c | 552.1 f | 205.9 b | 177.0 e | 39.6 d | 39.3 d |
| V | 7.17 cd | 8.60 b | 2.57 b | 2.03 b | 607.7 bc | 609.8 bc | 186.0 d | 176.4 e | 41.8 c | 39.6 d |
| SA | 10.0 a | 6.51 de | 2.17 b | 1.68 b | 549.7 f | 517.6 h | 155.9 f | 108.1 g | 34.5 g | 33.7 h |
| Control | 8.56 b | 8.03 b | 2.03 b | 2.11 b | 578.7 d | 566.6 e | 183.3 d | 184.1 d | 38.7 e | 34.7 g |
| Mean ±SD | 8.34 ±0.99 | 7.01 ±1.06 | 2.15 ±0.24 | 2.12 ±1.05 | 595.4 ±26.78 | 565.3 ±38.3 | 188.0 ±17.2 | 179.1 ±37.0 | 40.6 ±3.63 | 37.7 ±3.45 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, W.; Muzolf-Panek, M.; Kleiber, T. The Effect of Various Foliar Treatments and Nitrogen Nutrition Levels on the Yield and Physicochemical Parameters of Flowering Chinese Cabbage. Agronomy 2022, 12, 737. https://doi.org/10.3390/agronomy12030737
Liu W, Muzolf-Panek M, Kleiber T. The Effect of Various Foliar Treatments and Nitrogen Nutrition Levels on the Yield and Physicochemical Parameters of Flowering Chinese Cabbage. Agronomy. 2022; 12(3):737. https://doi.org/10.3390/agronomy12030737
Chicago/Turabian StyleLiu, Wenping, Małgorzata Muzolf-Panek, and Tomasz Kleiber. 2022. "The Effect of Various Foliar Treatments and Nitrogen Nutrition Levels on the Yield and Physicochemical Parameters of Flowering Chinese Cabbage" Agronomy 12, no. 3: 737. https://doi.org/10.3390/agronomy12030737
APA StyleLiu, W., Muzolf-Panek, M., & Kleiber, T. (2022). The Effect of Various Foliar Treatments and Nitrogen Nutrition Levels on the Yield and Physicochemical Parameters of Flowering Chinese Cabbage. Agronomy, 12(3), 737. https://doi.org/10.3390/agronomy12030737






