Abstract
In the present scenario of a looming food crisis, improving per hectare rice productivity at a greater pace is among the topmost priorities of scientists and breeders. In the past decades, conventional, mutational, and marker-assisted breeding techniques have played a significant role in developing multiple desired rice varieties. However, due to certain limitations, these techniques cannot furnish the projected food security of the 2050 population’s aching stomachs. One of the possible options would be precise crop genome editing using various tools, viz., TALENs and CRISPR/Cas9 to resolve this multifaceted crisis. Initially, the potentiality of these technologies was tested only in the rice protoplasts. Later, the techniques were employed to edit calli with help of modified vectors, CRISPR variants, cassette cloning systems, and delivery methods. With the continuous technological advancements such as base editing, multiplexing, etc., the precision, rapidness, efficiency, reliability, potency, and range of applications of these platforms have increased and even been used for gene function studies. This leads to a revolution in the field of the rice improvement program, especially the stress tolerance against various pests and pathogens in which the susceptibility factors located within the rice genome are targeted through genome editing tools. Therefore, in this current article, we have summarized the advancements in the rice genome editing tools during the last decade concerning enhanced biotic stress tolerance. Additionally, we have focused on the regulatory aspects of genome editing with associated risks and limitations, and the prospects to reshape the rice genome for durable resistance to complex biotic stress.
1. Introduction
Over the last decade, the rapid pace of research on genome editing has revolutionized the field of applied biotechnology [1,2]. It enabled scientists to deploy these techniques in human health, crop improvement, etc. that has shown incredible potential to benefit human society. Interestingly, continuous crop improvement is compulsory to feed the mammoth 7.6 billion population, which will exceed the whopping mark of 9.7 billion by 2050 [3,4]. In the 20th century, crop improvement can be largely made through conventional breeding approaches, viz., mutagenesis and hybridization. Still, these approaches are now widely used in crop improvement. However, linkage drag of undesirable genes is a major drawback. Along with this, the labor-intensive, time-consuming, costly program makes it very complicated; thus, it becomes difficult to cope with the rapidly escalating demand for quality food to endure the world’s hunger and malnutrition challenges.
To resolve these issues of conventional breeding, modern approaches like marker-assisted breeding (MAS) and genome-wide association mapping (GWAS), aided with advanced genomic tools, were employed. Although they have great potential to speed up the breeding program, their efficiency and effectiveness were much less for the traits governed by the recessive genes/alleles, multiple genes (i.e., true polygenic traits), etc. Furthermore, the cost of genotyping is too high in the case of MAS and GWAS. To tackle these limitations, gene knock-in/gene knock-out strategies are adopted [5]. Of them, RNA Interference (RNAi) can be practiced to knock down the expression of a specific gene(s), but still has limited scope for wider application. Thus, the search for more powerful, precise, fast, and robust tools is always continuing for targeted crop genome improvement, which is of an urgent need to meet the global food demands.
Targeted genome modification is the best strategy to resolve all these problems permanently. This can be achieved through genetic engineering, which is a highly complicated and time-consuming venture. The genetic engineering tools lead to modifications in the genome via stable integration of foreign DNA elements but do not able to reach the end-users, mainly due to less social acceptance and higher bio-safety concerns [6]. Although it has a rich history in creating new crop genomes, it has been gradually outpaced by the emergence of genome editing tools. Genome editing mediates the targeted gene modification either through deletion or insertion. The genome editing tools make this targeted gene modification process simpler than the conventionally preferred methods, as well as genetic engineering. The genome editing (GE) tools, viz., clustered regularly interspaced short palindromic repeats (CRISPR) and transcription activator-like effector nucleases (TALEN), can be employed to make precise deletion and insertion of sequences that lead to the loss of gene function [7]. Nevertheless, these GE tools also have a gene regulatory property to upregulate or downregulate the gene expression. Thus, large-scale employment of genome editing technologies is evident in many crop plants, including rice (Oryza sativa L.; Family, Poaceae) [8,9].
Interestingly, genome editing technologies have been extensively exploited for rice genome modification to obtain numerous improved cultivars equipped with elite traits such as yield, quality, stress tolerance, etc. Yet, the biggest question still arises: ‘how much advancement in rice genome editing has been made in the last decade?’, especially concerning biotic stress tolerance. So far, many reviews are available that compile the pieces of evidence of rice genome editing for large-scale improvement [10,11,12,13]. However, the focus on the improvement of biotic stress tolerance is still limited. Thus, we are trying to collate all the information systematically in this article, so that various questions like the trait of interest to be edited, the strategy of genomic changes, steps to DNA modification, the scope of success, and the advent of different challenges, etc., could be addressed easily in the near future.
2. Advancements in Genome Editing Technologies
Genome editing is the technique of precise genome modifications that facilitate the targeted mutations within the genome [14] through the deletions, insertions, or substitution of single base or specific sequences [15,16]. The precursor of genome editing of plants dates back to the 1970s with the development of genetic engineering, in which the genome manipulation was carried out through the random introduction of specific gene sequences via homologous recombination (HR), and leads to the inactivation or ‘knock out’ of the targeted gene function. Further, the discovery of meganucleases during the 1980s improved the process of targeted genome engineering. All these discoveries led to the evolution of genome editing technologies which have been growing at a rapid pace over the past 10 years and have been established as an extraordinary genome engineering tool [17,18]. Genome editing can be performed both in vitro and in vivo [19] via in situ delivery of editing machinery, and the highly targeted genome alterations take place through the double-stranded DNA breaks (DSBs) by sequence-specific nucleases, followed by repairing either through non-homologous end-joining (NHEJ) or homologous recombination (HR)/homology-directed repair (HDR), depending on cellular types [20,21].
In the NHEJ mechanism, the broken ends are re-attached with the deletion or insertion of nucleotide sequences of varying lengths, which leads to the disruption of gene function [22], whereas, in the case of HDR, a homologous stretch of nucleotide sequences is introduced into the donor template that leads to more accurate repair with specific alterations of genomic sequences [23]. As the repairing in HDR is mediated with the help of a donor template, it is slower and less frequent than NHEJ [24], thus, the choice of HDR-mediated repair in plants is very difficult [25,26]. To create a gene knockout mutant via insertion/deletion or gene replacement, various sequence-specific nucleases, viz., zinc-finger nuclease (ZFN), TALENs, and CRISPR-associated proteins (Cas9, Cas12), can be employed. These nucleases are discovered through the groundbreaking work in bacteria, yeast, and mammalian systems, but are also applicable in a wide variety of crop plants for their trait improvement [27]. The details about these nucleases are highlighted in the below subsections.
2.1. Zinc Finger Nucleases (ZFNs)
ZFNs are an engineered protein consisting of a zinc finger domain at the N-terminal with an endonuclease domain at the C-terminal end [28,29]. The zinc finger domain is necessary for the specific recognition of the targeted DNA sequence, and the endonuclease domain of the FokI restriction enzyme (RE), isolated from the Flavobacterium okeanokoites, ensures the cleavage of the specific DNA sequences [30]. For its functionality, heterodimerization of FokI RE is indispensable; hence, two ZFNs must dimerize for binding both strands of DNA and to align FokI domains. ZFN contains a tandem array of three to six zinc fingers (Cys2His2), each recognizing approximately 3 bp of DNA [31]. The sequence-specific binding of the zinc-finger domain directs the nuclease to cleave a specific genomic site. This mechanism was exploited for designing ZFN mediated gene-editing tools that are extensively used for customized engineering of the genome in many organisms [32].
The breakthrough of ZFNs as programmable nuclease was initiated in mice to create gene knockout via DSBs of target sequences that rapidly disseminated in various laboratories [29]. Further, it was expanded in agriculture for crop improvement, but with restricted implications for genomic editing attempts in limited crops such as Arabidopsis, tobacco, and maize [33]. Further, the off-target binding of the ZF motifs, other than the target sequence, makes them inefficient as an editing tool [34]. Moreover, the designing of a ZFN molecule via protein engineering is very challenging and highly time-consuming; thus, it will not be cost-effective to create a particular mutation.
2.2. TALENs
For many years, ZFN was explored as the only programmable site-specific nuclease, but it has been out-paced with the discovery of a DNA binding effector protein, called transcriptional activator-like effector (TALE), isolated from plant-pathogenic bacteria, Xanthomonas [35,36]. It primarily acts as the transcriptional regulator of the disease susceptibility (S) genes in rice. This protein is characterized by the C-terminal activation domain (AD) and nuclear localization signal (NLS) required for transcriptional regulation, the central tandem repeat sequence acting as a DNA binding domain (DBD), and the N-terminal translocation signal sequence [37]. A series of 33–35 amino-acid long repeat sequences in the DBD is present; of them, two hypervariable amino acids at the 12th and 13th position, also known as the repeat-variable di-residues (RVDs), are responsible for the specific recognition of nucleotide bases [38]. The sequence-specific binding property of DBD is exploited for the further development of new gene-editing technology, i.e., transcription activator-like effector nucleases (TALEN).
Similar to ZFN, TALENs are customized by fusing the DBD of the transcriptional activator-like effector (TALE) with the FokI restriction enzyme [39]. However, unlike ZFN, the designing of TALEN is much easier, as the repeat sequence of the TALEs has specificity for targeting single sites in a genome. Further, the multimerization of the repeat sequence is not essential for the construction of a long array of DBD, as in ZFN; hence the engineering is quite easy and less time-consuming [40]. The identification of RVDs in repeat regions of TALEs helps in the recognition of their specificity for various binding targets, as each RVD has a single nucleotide target, thus allowing the flexibility for designing TALENs for a greater number of potential target sites than that of ZFNs. Therefore, the TALENs have been utilized for genome editing in a wide variety of plants. Additionally, the binding of TALEs with gene activators and receptors, apart from nuclease, leads to the formation of efficient artificial transcriptional regulators to achieve the desirable gene regulation. Despite the advantages of TALENs over ZFNs in terms of high target specificity and low off-target effect, the extensive repeat structure in the DBD of TALE protein becomes the major limiting factor for their use in target-specific editing of multiple genomes, and further, protein engineering is always tedious. To resolve these issues, genome editing using programmable RNA-guided DNA endonucleases has become more popular.
2.3. CRISPR/Cas System
The CRISPR, i.e., clustered regularly interspaced short palindromic repeats, is a mysterious DNA sequence found in the prokaryotic genome (including bacteria and archaea) which is often palindromic, consisting of 29 nucleotides (nt), long identical tandem repeats separated by a unique spacer (32 nt in length) [41]. It is a kind of locus consisting of several CRISPR-associated conserved protein-coding genes (Cas) exclusively involved in the adaptive immunity of prokaryotes against bacteriophages. This CRISPR-mediated immunity is functionally related to eukaryotic RNA interference (RNAi) [42], with the additional advantage of the development of genetic memory from past encounters. The CRISPR can recognize the small CRISPR RNAs (crRNAs) transcribed from the genetic memory (acquired in the CRISPR repeats) and use these small guide RNAs to cleave the virus genome [43,44]. This mechanism of CRISPR is explored and exploited further. The programmable nature of the CRISPR system led to the design of the RNA-guided DNA endonucleases-based genome editing tool, which is popularly known as CRISPR/Cas.
The CRISPR/Cas-based genome editing works based on the RNA:DNA base-pairing principle to target the host DNA and is used as a novel system for precise genome manipulation in many organisms, including plants [45]. This technique is more robust and simpler compared to ZFN and TALEN [46]. It is inexpensive, easy to apply, and has high versatility with great accuracy, even when deployed for multiplex genome editing, i.e., for the manipulation of multiple genes at the same time [47]. It has been showcased in various model plants (Arabidopsis, tobacco, etc.) and crop plants (rice, wheat, maize, tomato, potato, and soybean) as well as woody plants (apple, poplar, etc.) for durable trait improvements, from achieving higher yield and quality to alleviating biotic and abiotic stress troubles [48,49,50].
This CRISPR/Cas system involves the creation of dsDNA breaks at a desirable specific site in the genome with the help of a guide RNA (20–23 nt long), which is designed to be complementary to the target sequences and binds with the one strand of genomic DNA, using Watson–Crick base pairing to facilitate the Cas endonucleases’ mediated cleavage of dsDNA [51]. The DSBs are then mended by the cellular repair machinery, involving HDR or NHEJ mechanism, and generate genomic modifications such as mutation via deletion and insertion [52].
Interestingly, there are different Cas endonucleases (class 1 and class 2) that vary in their structure, composition, functional targets. Out of them, class 2 of Cas endonucleases is most commonly used in genome editing (Table 1), and these nucleases are optimized for their wide-scale application in genome editing. With great functional variation in terms of specificity and nucleic acid target, they are extended for precise editing of both DNA and RNA. Further, improvisation has led to the emergence of some innovative techniques such as base editing, prime editing, etc. See Table 1 for more precise editing of single/few nucleotides. The utility of these techniques for the improvement of plant biotic stress tolerance has opened up a new avenue in rice improvement (Figure 1).

Table 1.
Advancements in genome editing tools and their variants are available for rice improvement.
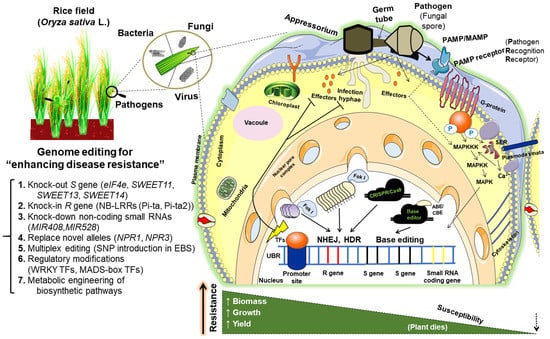
Figure 1.
Schematic illustration representing the genome editing strategies adopted for the enhancement of disease resistance in rice. Different approaches are functional knockout of the host S genes via either deletion/mutation/replace of the coding sequences, modification of single nucleotide polymorphisms in the recessive allele of R genes through base editing, knock-down of non-coding regulatory RNAs (miRNAs) by Cas13, replacement of central regulators like NPR1, engineering the promoter cis-elements by multiplex editing, modification of negative TFs of plant defense, metabolic engineering of secondary metabolite (lignin) synthesis to favor plant defense, etc. These strategies have been applied to intervene in the different steps of pathogenesis events of fungi, bacteria, and viruses.
3. Genome Editing Strategies for Biotic Stress Tolerance
The plant–pathogen interaction is a highly dynamic phenomenon that involves several events, including attachment, recognition, penetration, pathogen proliferation, etc. In each step, diverse molecular mechanisms interplay to regulate the gene function, which ultimately determines the outcome of the interaction, i.e., resistance or susceptibility. Elucidation of such molecules and their role in individual events is crucial to gain knowledge on the host and pathogen evolution. This information is also necessary to formulate a strategic approach for the management of pathogens. Now, in the era of genome editing and system biology, it becomes very important to search for the suitability of such technologies in formulating the plant–pathogen interaction desirably, so that broad-spectrum resistance can be achieved. For this, different genome editing-based strategies can be adopted that can be classified into three broad approaches [59]: (A.) Alteration of the pathogen’s targets in crops, (B.) Regulation of host immune response starting from recognition, and (C.) Enhancing plant immunity by in planta intervention of pathogens using genome editing tools.
Primarily, alteration of the pathogen’s targets could be possible through (1) Functional knockout of the host susceptibility (S) genes can lead to enhanced resistance or reduced compatibility in plants. This can be exemplified in rice, cucurbits, and tomato, where knock out of eIF4E and eIF4E (iso) results in enhanced resistance against plant viruses [60,61,62,63]. The complete disruption of genes having a pleiotropic effect may affect the yield and vigor. (2) Engineering the cis-elements in the promoter region of S genes can confer broad-spectrum defense. The deletion of effector binding elements (EBEs) in the promoter region of rice susceptibility genes, viz., Xa13 (OsSWEET11), Xa25 (OsSWEET13), and Xa41 (OsSWEET14) for the broad-spectrum resistance against Xanthomonas oryzae pv. oryzae (Xoo) races are evidenced [64]. (3) Modification of the coding sequences of the S genes via precise genome editing, or base editing. Base editors combined with the CRISPR/Cas9 system can be applied for the generation of single-base changes in the coding sequence of the gene. Thus, the introduction of single nucleotide polymorphism (SNP) in the pathogen’s effector binding site of the host protein without altering the catalytic domains is necessary to avoid the binding and subsequent reduction in pathogen growth. This may reduce the fitness costs of the plant. (4) Alteration of amino acids of surface receptor proteins in the plant may prevent the evasion of secreted pathogen effectors. For that, AtBAK1 [65] and RIN4 [66], etc. could be targeted without complete abolition of their natural function.
On the other hand, regulation of host immune response can also be possible through (1) Knock-out of negative regulators’ transcription factors (TFs) of plant defense responses may lead to enhanced disease resistance against pathogens. This can be evident from the deletion of OsERF922, a negative regulator of defense-related genes in rice which results in the reduced accumulation of abscisic acid and enhanced disease resistance to blast pathogen [67]. (2) Modification of central regulators of plant defense response can also reduce the infection level. From the extensive study, it is clear that the Non-expressor of Pathogenesis-Related proteins (NPR genes), such asNPR1, NPR3, etc., acts as the master regulator of the plant immune response. Of them, NPR1 plays a positive regulatory role in amplifying salicylic acid (SA)-mediated systemic acquired resistance (SAR) in the plant, like Arabidopsis, rice, etc., whereas NPR3 negatively regulates the NPR1 governed SAR pathways [68]. Thus, the CRISPR/Cas mediated mutation of OsNPR3 may enhance defense response in the plant, as exemplified in Cocco against Phytophthora tropicalis [69]. (3) Knockdown of non-coding RNAs, like miRNAs and siRNAs, by genome editing tools can also be possible to confer resistance in the rice plant. The promising applications of CRISPR/Cas9 for editing miRNA genes, viz., OsMIR408, and OsMIR528 in rice have already been demonstrated [70]. (4) Further, targeting plant defense via metabolic engineering by genome editing tools becomes another alternative [71]. Nowadays, manipulation of metabolic pathways in plants using the CRISPR/Cas tool is already well established in different plants like opium poppy [72] and tomato [73].
Enhancing plant immunity by in planta intervention of pathogens using genome editing tools is also well-practiced to manage the growth and development of pathogens within the plant system. This strategy is initially demonstrated to manage the infection of DNA viruses, including Tomato yellow leaf curl virus, Beet curly top virus, Merremia mosaic virus, Bean yellow dwarf virus, and Beet severe curly top virus, etc., as well as RNA viruses like Cucumber mosaic virus and Tobacco mosaic virus through in planta expression of CRISPR/Cas protein [74,75]. Nowadays, a similar strategy is employed to disrupt the bacterial genome by CRISPR nucleases [76], thus establishing a novel defense mechanism in the plant.
4. Applications of Genome Editing Technologies in Rice for Enhancing Biotic Stress Tolerance
4.1. Fungal Disease Resistance
Rice is affected by multiple fungal pathogens that result in extensive yield loss [77]. Of them, rice blast is a serious concern, as it is responsible for approximately 30% crop loss worldwide. The hemibiotrophic nature of the blast pathogen (Magnaporthe oryzae) makes them highly widespread and devastating, thus the emergence of new races is quite frequent, with a rapid breakdown of the host resistant (R) genes [78]. Therefore, it becomes very difficult to combat this pathogen only through the deployment of the newly identified R gene, which drives alterations in a pathogen’s population structure. Understandably, the search for an alternative option is in demand.
Thus far, various strategies are being adopted to generate blast resistance in rice. Of them, gene-editing is the most advanced and is applied for enhancing resistance against the different fungal pathogens of rice (Table 2). Initially, the host genes like mitogen-activated Protein Kinase 5 (OsMPK5) are targeted to illustrate the RNA-guided genome editing in rice. Three guide RNAs are designed to mutate OsMPK5 by CRISPR/Cas9 system, and the mutated protoplast (with 3–8% mutation frequency) showed broad-spectrum disease resistance in rice cultivar Nipponbare [79]. Later on, various disease susceptibility factors are knocked out using different gene-editing tools to achieve broad-spectrum defense. Like RNAi, an attempt is made to knock out the OsERF922TF, the negative regulator of defense in rice by CRISPR/Cas9 [67]. The binary vectors expressing Cas9/sgRNA cassette (pC-ERF922, pC-ERF922S1S2, and pC-ERF922S1S2S3) are delivered to create InDel mutations in the Japonica rice variety (Kuiku131) with 42% mutation frequency in the T0 generation. This targeted gene knockout results in the reduced accumulation of abscisic acid and subsequent increase in defense without alteration of other agronomic traits, indicating the accuracy of editing. Further, the genome editing of the OsERF922 locus at multiple sites via Cas9/multi-target sgRNA constructs leads to an increase in the mutation efficiency, with enhancement in defense against blast pathogen [67].

Table 2.
Application of genome editing tools for fungal disease resistance in rice.
Furthermore, blast resistance in rice is also demonstrated through the disruption of the OsSEC3A gene (encoding a subunit of the exocyst complex) by the CRISPR/Cas9 technology, in which the gene is targeted at multiple sites, i.e., 3rd and 10th exon of the OsSEC3A gene [81]. This mutation leads to the up-regulation of defense responsive genes, viz., PR proteins, salicylic acid, etc., resulting in enhanced resistance against blast pathogen, along with dwarfism and lesion-mimic phenotype [81]. A similar strategy is also applied to mutate the resistant gene Pi21, encoding a cytoplasmic proline-rich protein that acts as the susceptibility factor [90]. Knockout of Pi21 by the CRISPR/Cas9 tool enhances the resistance in rice to blast pathogen without affecting the major agronomic traits [82].
Another susceptibility factor, the BSR-D1 gene, was targeted for CRISPR/Cas9 mediated mutation to promote blast resistance in rice; the engineering of the promoter cis-element by single nucleotide substitution increases the binding of MYBS1 TF which suppresses the expression of peroxidase to enhance H2O2 production [84]. Similarly, a novel peroxidase synthesizing gene (Perox3) of rice is also knocked out by using the CRISPR/Cas9 tool to assess its functional significance in blast disease resistance [85]. Other than rice blast disease, sheath blight is another serious problem in rice cultivation. To confer broad-spectrum defense against this disease, the Phytochrome and Flowering Time 1 (OsPFT1) gene of Indica rice (var. ASD16) is successfully mutated by using CRISPR/Cas9 technology [86], but the functional assessment of the edited rice lines are yet to determine. Besides host factors (genes), various fungal genes are also targeted to decipher the gene functions in pathogen virulence. For example, deletion of USTA (Ustiloxin) and UvSLT2 (MAP kinase) genes of rice false smut (Ustilaginoidea virens) pathogen by CRISPR/Cas9 results in hampering ustiloxin biosynthesis (in the ustA mutant) and increased cell wall sensitivity (in uvslt2 mutant), which in turn reduces the virulence of fungal pathogen [88], thus successfully demonstrated an efficient gene replacement or genome editing strategy to impart resistance against rice false smut.
Further, disruption of the scytalone dehydratase (SDH) gene of M. oryzae by CRISPR/Cas9 affects the melanin biosynthesis and appressoria formation [89], thus reducing the infection in rice. However, the stable expression of the Cas9 plasmid construct in fungi is highly difficult. Thus, a novel genome-editing strategy is demonstrated using the transient application of purified CRISPR/Cas9 ribonucleoprotein (pre-complexed with gRNA) for the precise genetic manipulation in M. oryzae [91]. This plasmid-free approach can also be exploited for the genome editing of different fungal species. In this way, the targeted modification of the host genes via gene knock-in or knock-out and base editing, without off-target effect, is always demanding [92]. Thus, a continuous improvement in genome-editing technology is necessary for the fast and flexible genome manipulation of rice and its pathogens.
4.2. Bacterial Disease Resistance
Among bacterial diseases, bacterial leaf blight (BLB) of rice is one of the major stumbling blocks [93]. Due to the huge diversity in the pathogen (Xoo) population, it is quite difficult to manage. Utilization and exploration of host resistance is the best option to combat this problem. So far, 44 resistant (R) genes were identified [94]. Of them, one-third of R genes are recessive [95,96]. Although both dominant and recessive allelic forms are essential to confer resistance, ironically, some recessive alleles of genes such as xa1, xa4, and xa21 as well as dominant alleles of Xa5 and Xa13 act as the disease susceptibility factor and support the pathogen (Xoo) to induce disease [96]. The pathogen hijacks the host machinery by inducing the expression of S genes using its effectors, called transcription activator-like effectors (TALEs). OsSWEET family genes are one such kind of S genes that provide sucrose/sugar to the pathogen.
Up to now, more than 20 SWEET genes have been identified in rice and classified into five clades. Of them, Clade III SWEET genes, viz., OsSWEET11, OsSWEET12, OsSWEET13, OsSWEET14, and OsSWEET15, act as the major susceptibility factors for bacterial blight pathogen [97,98]. The disease induction takes place by specific recognition of EBEs located in the promoter region of the OsSWEET genes by pathogens’ effectors (TALEs) [64]. Thus, editing the promoter sequence of OsSWEET genes is very important for creating broad-spectrum resistance against Xoo.
Initially, the TALEN-based genome editing system is executed for the modification of rice susceptibility genes, viz., OsSWEET13 and OsSWEET14. Engineering of a promoter sequence carrying AvrXa7 EBE in the OsSWEET14 gene leads to enhanced resistance in rice to Xoo strains secreting the AvrXa7 [99]. Likewise, the editing of the promoter sequence of OsSWEET13 containing AvrXa7, Tal5, and TalC EBEs, conferring enhanced disease resistance in rice cultivars Kitaake or Nipponbare to Xoo strains carrying effector AvrXa7 or Tal5(F), but not against TalC [100].
In the meantime, the rapid development in CRISPR/Cas mediated genome editing led to the nascent application of CRISPR/Cas9 genome editing for the sequence-specific editing of the susceptibility genes (OsSWEET14 and OsSWEET11) promoters for host defense enhancement [101]. Thereafter, CRISPR/Cas9 is employed multiple times to target the promoter cis-elements of susceptibility genes, (Table 3) to protect rice from different strains of Xoo [64,102,103,104,105,106]. Further, the TALEN and CRISPR technology is also used to decipher the functionality of novel genes (like Xa10-Ni and Xa23-Ni) of rice against the BLB pathogen [107].

Table 3.
Targeted genome editing for disease resistance in rice against bacterial infection.
Besides BLB in rice, bacterial leaf streak (BLS) is another serious concern [112]. Similar to Xoo, the pathogen Xanthomonas oryzae pv. oryzicola (Xoc) also employs diverse effectors to regulate host susceptibility genes to cause disease. Tal7 is one of such effectors secreted by Xoc to suppress AvrXa7-Xa7 mediated defense via transcriptional activation of the rice gene Os09g29100. The engineering of the Tal7-binding site (EBEtal7) in the promoter of the Os09g29100 gene through TALEN results in the enhanced resistance to BLS pathogen (Xoc RS105) through the suppression of AvrXa7-Xa7 mediated defense [108]. Later on, promoter editing of other susceptibility genes was also performed to enhance resistance in rice. This can be exemplified from the disruption of the EBETal2g/Tal5d sequence in the promoter region of OsSULTR3;6 gene that confers enhanced resistance in rice cultivar IRBB10 to Xoc strains to contain either Tal2g or Tal5d effectors [109].
Further exercise is also done to achieve broad-spectrum resistance against Xoo and Xoc, both through CRISPR/Cas9 mediated genome editing [101]. In the majority of the experiments, the broad-spectrum resistance in the mutant lines is gained without the cost of normal agronomic traits, including yield [103]. These reports indicate the potential of advanced genome editing strategies in exploring and enhancing resistance in rice against bacterial pathogens (Table 3).
4.3. Virus Disease Resistance
Other than fungal and bacterial pathogens, the yield of rice is also severely affected by different viruses. Of them, Rice tungro disease is commonly serious in south-east Asia [113]. Due to the mixed infection of two different viruses, viz., Rice tungro spherical virus (RTSV) and Rice tungro bacilliform virus (RTBV), disease management is very difficult through common practices. The scope of utilizing resistant genotypes is also limited due to the lack of suitable resistance sources. Thus, genetic engineering becomes a suitable alternate option. The extensive research indicates the significance of some host factors, viz., eIF4G (translation initiation factor 4 gamma) andeIF(iso)4G, in the development of plant virus disease. During infection, RNA viruses use these host factors to translate protein from RNA and to regulate genome replication and colonization [114]. Thus, the genes encoding the translation initiation factors eIF4E and eIF(iso)4E in different plant species are mutated in multiple cases to combat different plant RNA viruses [61,115]. Therefore, eIF4G also supports the RTSV during infection, and the SNP in the 9th exon of the gene encoding eIF4G protein affects the Y1059V1060V1061 conserved amino acids and is responsible for the resistant phenotype [116]. An attempt is thus made to delete the coding sequences around this region by the CRISPR/Cas9 system to develop tungro disease-resistant mutants.
CRISPR/Cas9-mediated in-frame mutations of the flanking residues SVLFPNLAGKS, adjacent to the YVV conserved motif, leading to the enhancement in the resistance in RTSV-susceptible rice cultivar IR64 against tungro disease [60]. Similarly, deletion of the 79th proline in the N terminal domain of eIF4G protein by CRISPR/Cas9 mediated disruption of the coding sequence of eIF4G gene lends enhanced partial resistance to Rice black-streaked dwarf virus, but not against Rice stripe virus [117]. In this way, genome editing is considered the most suitable tool for the rapid generation of plant virus-resistant genotypes (Table 4). Considering all these modifications together, Figure 2 represents all of the key developments in genome editing-mediated rice improvement with the perspective of improved biotic stress tolerance in rice (Figure 2).

Table 4.
Targeted genome editing tools for disease resistance in rice against plant viruses.

Figure 2.
Timeline of key developments of genome editing technology in rice for enhanced disease resistance. All the entries are based on the data mining with eight keywords (rice, HR, NHEJ, CRISPR/Cas9, biotic stress, pathogens, genes, and editing) in PubMed (https://pubmed.ncbi.nlm.nih.gov/) (Accessed on 22 December 2021) and Google scholar (https://scholar.google.co.in/) (Accessed on 22 December 2021).
5. Other Potential Targets
So far, genome editing in rice has been successfully implemented against some fungal, bacterial, and viral pathogens. In the majority of cases, the susceptible genes are targeted through the engineering of either their coding sequences or promoter sequences. Based on the biological function of these plants S genes, they can be classified into three broad classes: (1) S genes act as the negative regulators of host defense like OsERF922 in rice, (2) S genes act as the potential target singled out by the pathogen to establish a compatible interaction, like eIF4G in rice, and (3) S genes facilitate metabolic/nutritional support to pathogens like OsSWEET11, OsSWEET13, and OsSWEET14 in rice [118,119,120]. Among them, mostly negative regulators and metabolic/nutritional facilitators are targeted for managing various pathogens in diverse crops. Impairment of the functionality of S genes leads to the restricted growth and development of pathogens within the plant system. Intriguingly, the choice of susceptible genes for editing very much depends upon the lifestyles of the pathogen(s). Sometimes, disruption of any S gene which leads to enhanced resistance to one pathogen may increase the host susceptibility to other pathogens that have contrasting lifestyles.
Hitherto, many genes were targeted for rice genome editing. However, few are exploited for inducing defense against pathogens. In rice, different negative regulators like TFs and other defense suppressors are targeted to induce host defense against blast pathogens, whereas the S genes providing metabolic/nutritional support to pathogens are targeted to suppress the development of bacterial pathogens. However, the susceptible genes of rice for insect pests and nematode protection have not been exploited yet [121], which may be due to the inaccessibility of suitable S gene(s). Therefore, some of the potential susceptible genes (S) of rice are listed (Table 5). Such genes can support the pathogenesis-related events of pathogens starting from host recognition to host entry, defense suppression, nutrient acquisition, translocation within the host system, colonization, and disease development. Thus, identification and functional characterization of such genes are important to modulate the host defense mechanism through genetic engineering. Nonetheless, the majority of the S genes are often involved in various host physiology.

Table 5.
List of unexplored susceptibility factors in rice as the possible target for genome editing.
Thus, the complete inactivation or disruption of S genes might lead to adverse pleiotropic effects other than host resistance [119]. Therefore, a one-by-one assessment of their functionality is crucial before targeted genome editing [141]. Nevertheless, disabling these S genes is suggested as the novel strategy to achieve durable and broad-spectrum resistance [142]. Recent advancements in genome editing technology provide the opportunity to precisely change a single nucleotide from the target site using base editing, without altering the physiological property. Furthermore, the CRISPR/Cas mediated gene replacement via HDR also opens up the scope of reversing the disease susceptibility into broad-spectrum resistance without affecting the other biological functionality.
6. Concerns Associated with Genome Editing in Rice
In the last decade, genome editing technologies have evolved rapidly. After so much technological development, the practical implication of these tools is quite challenging in different biological systems. It requires more scientific standardization depending on the type of organisms. Interestingly, genome editing evolved from the prokaryotic system and was later tested in eukaryotic cells. Initially, protoplast culture was used to demonstrate the genome editing in the plant, but this gradually shifted into the whole plant system. Scientists have contributed a lot to the application of genome editing tools in different plants in various ways. Of them, designing efficient vectors containing CRISPR/Cas and sgRNA constructs for plant transformation is the most significant.
Previously, both CRISPR/Cas and sgRNA constructs were delivered through separate vectors; but now, the entire cassette is inserted within one binary vector module to express the Cas protein and the guide RNA at the same magnitude. The higher expression of both is necessary for successful genome editing. In general, the Cas protein is expressed in plants under the control of various strong constitutive promoters, originated from either microbe (CaMV35S) or plant housekeeping genes (Ubiquitin1 and Actin 1); sometimes, tissue-specific promoters like meiocyte specific Zea maysdmc1 (Zmdmc1) are also necessary [143]. On the other hand, the small nuclear RNA (snRNA) promoters like A. thaliana U6 promoter (AtU6) [144], O. sativa U3 promoter (OsU3) [145], and Z. mays U3(ZmU3) promoter [146] are used for expression of the guide RNAs for genome editing in rice. The strong endogenous promoters like rice ubiquitin1 (OsUbi1) and actin1 (OsAct1) are commonly used for the expression of CRISPR/Cas9 protein, whereas the sgRNAs/crRNAs are driven under the control of the OsU3/OsU6 promoter [147]. This mixed dual promoter system may not always be effective due to the differential expression pattern of Cas protein (under control of the Pol II promoter) and gRNAs (under control of the Pol II promoter) [148]. Thus, in recent years, more advancements have been made in the direction of the development of either a dual promoter system (containing two separate Pol II promoter–terminator cassettes to express the Cas gene and guide RNA) or a single transcript unit (STU) system (utilizing only one bi-directional promoter to express the Cas gene and the guide RNAs) to ensure equimolar concentration of Cas protein and gRNA for successful multiplexed genome editing in plants [149].
Nevertheless, plant-based codon-optimized Cas protein-encoding genes are also introduced for efficient function in different crops. Furthermore, the stable expression of nucleases is achievable either through the Agrobacterium-mediated or biolistics approach, which guides the genome editing of meristematic calli with targeted DNA modifications. The selection of transformants containing the Cas-gRNA cassette becomes easier with the introduction of different selective markers, bialaphos resistance gene [150], kanamycin resistance gene [151], or hygromycin resistance gene [152], etc. Thereafter, successful regeneration of plants through tissue culture is achieved but is still an issue for some plant species. To resolve this issue, the development of non-tissue culture-based in planta transformation methods, like floral-dip transformation [153], germinating seed inoculation [154], and apical meristem-targeted in planta transformation [155], have been developed as alternatives to the regeneration-based approach. Similarly, in rice, non-tissue culture-based in planta transformation is also established through the floral-dip method [156], embryonic apical meristems technique of soaked seeds [157], and vacuum infiltration of soaked seeds [158] for successful, rapid regeneration. So far, in all these, the Cas-gRNA complex is delivered in the forms of plasmid DNA (pDNA) via AMT [159]. However, uniform delivery to each cell is a major drawback in the case of AMT. To avoid the problems associated with the delivery, an alternative approach, i.e., direct delivery of ribonucleoprotein complex (containing Cas protein complexed with sgRNA) is evolving gradually [160]. Exhaustive research is required to continue the development of gene-editing techniques to open new avenues in this direction.
Other than technical issues, some regulatory issues are also partnering with the commercial utilization of genome-edited plants. Although genome editing is a fundamentally different transgenic approach, the assessment of plants developed using genome editing technologies is a debatable issue. Usually, it is advocated that risk assessment of genome-edited crops would be according to the complexity of editing [161]. Depending on the extent of genome modification, genome-edited (GEd) organisms are categorized into three groups. In group 1, the GEd organisms containing single or few nucleotides with modification or small deletions are included. In group 2, GEd organisms containing targeted modification of several nucleotides are included, and in group 3, GEd organisms containing synthetic/foreign DNA showing a new trait attribute are included. The regulatory rules differ from group to group. According to the Draft Document on Genome Edited Organisms: Regulatory Framework and Guidelines for Risk Assessment released by the Department of Biotechnology (Ministry of Science and Technology, Government of India), the products/cells under the GEd group I should be assessed to check the targeted modification along with the off-target genomic changes; further, their phenotypic equivalence analysis should also be performed.
Similarly, the products/cells of group II should be assessed to check their targeted modification along with the off-target genomic changes; their assessment for trait efficacy through confined/contained field trails along with phenotypic equivalence analysis is necessary. However, the products/cells of group III containing large or foreign DNA in the genome may represent similar biosafety concerns as that of genetically engineered organisms (GMOs), thus the need to undertake biosafety regulations such as those of GMOs. The exception is also there for single nucleotide change conferring herbicide tolerance-related traits that will require additional biosafety studies. Usually, during genome editing, the nucleases–gRNAs cassette along with selective marker genes incorporated into the plant genome may remain in the plant up to T0 generation but get segregated in advanced generations [121]. Hence, precise guidelines should be formulated for biosafety/risk assessment of genome-edited plants.
7. Concluding Remarks
At the beginning of the 21st century, the genome editing technologies emerged as a part of biotechnological innovations meant for enhancing human’s understanding of biology. To date, these technologies are evolving at a rapid pace to make desirable genomic modifications for clarification of gene function as well as for practical/commercial utilization. The great potential of these tools makes them a good choice to beget huge advancements in agriculture, from trait improvement to diagnostics. In rice, the applications are not only restricted within gene knock-in and gene knock-out but also can be used for precise base editing. The single nucleotide polymorphism between the resistant and susceptible alleles of any gene can even be modified to attain the desirable trait.
Furthermore, gene-editing technology can also be used as a synthetic regulator of the genes that will help to engineer metabolic pathways in favor of plant defense against diverse insect pests and pathogens. Targeted mutagenesis using the TALENs and CRISPR tools is already implemented for the inactivation of susceptibility genes to achieve enhanced disease resistance against different rice pathogens [162]. However, their potential for insect-pest and nematode management in rice has not been tested, possibly due to the lack of available susceptibility genes for respective pests. Therefore, more exploration is required to decipher the susceptible genes which can be targeted for pest management. Above all, the negative side-effects for knocking out susceptible genes should also be addressed, as they not only modify the plant’s biology; sometimes, broad-spectrum resistance can be obtained at the cost of yield loss.
Nevertheless, biosafety issues are another concern, although genome-edited crops are scientifically distinct from genetically modified transgenics. Proper follow-up is necessary before field release. In addition, other environmental issues related to genome-edited disease-resistant crops should be noted, as they may be associated with the emergence of new races/strains of pathogens; maintaining the durability of the disease resistances will be a challenge. Thus, in the long run, the corroboration of genome editing technologies with other scientific fields is required for the efficient translation of information to gain long-term success in crop improvement. Moreover, with time, there will be an amalgamation of many techniques together; in that case, the most crucial point is that the target genes are more important for increasing crop resistance to pathogens, not the techniques.
Author Contributions
A.C., J.P., S.M., G.P., and V.M.M.A. together contributed to the conceptualization, overall design, and writing of the manuscript. H.P., S.K., A.R., A.B., and S.B. carried the review of literature for tables with A.C. and helped S.M. in drafting the figures. A.C., M.K.R., and S.M. critically reviewed and revised the manuscript. Lastly, A.C., S.M., G.P., V.M.M.A., and M.K.R. together finalized the manuscript for submission. All authors have read and agreed to the published version of the manuscript.
Funding
The work is financially supported by the Department of Biotechnology (DBT), India via Grant no. BT/PR32125/AGIII/103/1147/2019.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in this article only.
Acknowledgments
The author (S.M.) would like to acknowledge the Council of Scientific and Industrial Research (CSIR, India) for granting him the Junior Research Fellowship and Senior Research Fellowship (09/512(0233)/2017-EMR-I). Furthermore, all the authors would like to acknowledge the anonymous peer reviewers.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
TALENs: Transcription activator-like effector nucleases, CRISPR: Clustered regularly interspaced short palindromic repeats, ZFN: Zinc-finger nuclease, CRISPR/Cas: Clustered regularly interspaced short palindromic repeats/CRISPR-associated protein, MAS: Marker-assisted breeding, GWAS: Genome-wide association mapping, RNA: Ribonucleic acid, RNAi: RNA Interference, DNA: Deoxyribonucleic acid, GE: Genome editing, HR: Homologous recombination, DSBs: Double-stranded DNA breaks, NHEJ: Non-homologous end-joining, HDR: Homology-directed repair, RE: Restriction enzyme, ZF motif: Zinc-finger motif, TALE: Transcriptional activator-like effector, AD: Activation domain, NLS: Nuclear localization signal, S gene: susceptibility gene, DBD: DNA binding domain, RVDs: Repeat-variable di-residues, nt: Nucleotide, crRNAs: CRISPR RNAs, dsDNA: Double-stranded DNA, gRNA: guide RNA, sgRNA: Single guide RNA, G-C: Guanine to Cytosine, A-T: Adenine to Thymine, RT: Reverse transcriptase, pegRNA: Prime editing guide RNA, ssRNA: Single-stranded RNA, Cas9: CRISPR-associated protein9, Cas13: CRISPR-associated protein13, eIF4E: Eukaryotic Translation Initiation Factor 4E, i.e.: id est, R gene: Resistance gene, EBEs: Effector binding elements, EBS: Effector binding sites, Xoo: Xanthomonas oryzae pv. oryzae, Xoc: Xanthomonas oryzae pv. oryzicola, SNP: Single nucleotide polymorphism, TF: Transcription factor, NPR: Non-expressor of Pathogenesis-Related proteins, SAR: Systemic acquired resistance, miRNA: MicroRNA, siRNA: Small interfering RNA, GEd: Genome-edited, var.: Variety, cv.: Cultivar, cvs: Cultivars, OsPFT1: Phytochrome and Flowering Time 1, SDH: Scytalone dehydratase, SA: Salicylic acid, AMT: Agrobacterium-mediated transformation, USTA: Ustiloxin, BLB: Bacterial leaf blight, BLS: Bacterial leaf streak, PEG: Polyethylene glycol, RTSV: Rice tungro spherical virus, RTBV: Rice tungro bacilliform virus, RBSDV: Rice black-streaked dwarf virus, eIF4G: Translation initiation factor 4 gamma, BPH: Brown Planthopper, RDV: Rice dwarf virus, Mor: Magnaporthe oryzae, AO: L-ascorbate oxidase, RYMV: Rice yellow mottle virus, snRNA: Small nuclear RNA, Ubi1: Ubiquitin1, Act1: Actin1, Pol II: Polymerase II, STU: Single transcript unit, pDNA: plasmid DNA, GMOs: Genetically engineered organisms, NB-LRR: Nucleotide-binding site and leucine-rich repeats, PAMP: Pathogen-associated molecular patterns, MAMP: Microbe-associated molecular patterns.
References
- Li, H.; Yang, Y.; Hong, W.; Huang, M.; Wu, M.; Zhao, X. Applications of genome editing technology in the targeted therapy of human diseases: Mechanisms, advances, and prospects. Signal Transduct. Target. Ther. 2020, 5, 1. [Google Scholar] [CrossRef]
- Khalil, A.M. The genome editing revolution: Review. J. Genet. Eng. Biotechnol. 2020, 18, 68. [Google Scholar] [CrossRef] [PubMed]
- Valin, H.; Sands, R.D.; Van der Mensbrugghe, D.; Nelson, G.C.; Ahammad, H.; Blanc, E.; Bodirsky, B.; Fujimori, S.; Hasegawa, T.; Havlik, P.; et al. The future of food demand: Understanding differences in global economic models. Agri. Econ. 2014, 45, 51–67. [Google Scholar] [CrossRef]
- United Nations, Department of Economic and Social Affairs, Population Division, World Population Prospects 2019: Highlights (ST/ESA/SER.A/423). Available online: https://population.un.org/wpp/Publications/Files/WPP2019_Highlights.pdf (accessed on 9 January 2022).
- Ahmar, S.; Gill, R.A.; Jung, K.H.; Faheem, A.; Qasim, M.U.; Mubeen, M.; Zhou, W. Conventional and molecular techniques from simple breeding to speed breeding in crop plants: Recent advances and future outlook. Int. J. Mol. Sci. 2020, 21, 2590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Low, L.-Y.; Yang, S.-K.; Kok, D.-X.A.; Ong-Abdullah, J.; Tan, N.-P.; Lai, K.-S. Transgenic plants: Gene constructs, vector and transformation method. In New Visions in Plant Science; Çelik, Ö., Ed.; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef]
- Xu, K.; Segal, D.J.; Zhang, Z. Precise genome editing techniques and applications. Front. Genet. 2020, 11, 412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamburova, V.S.; Nikitina, E.V.; Shermatov, S.E.; Buriev, Z.T.; Kumpatla, S.P.; Emani, C.; Abdurakhmonov, I.Y. Genome editing in plants: An overview of tools and applications. Inter. J. Agron. 2017, 2017, 15. [Google Scholar] [CrossRef] [Green Version]
- Achary, V.M.M.; Reddy, M.K. CRISPR-Cas9 mediated mutation in GRAIN WIDTH and WEIGHT2 (GW2) locus improves aleurone layer and grain nutritional quality in rice. Sci. Rep. 2021, 11, 21941. [Google Scholar] [CrossRef]
- Mishra, R.; Joshi, R.K.; Zhao, K. Genome editing in rice: Recent advances, challenges, and future implications. Front. Plant. Sci. 2018, 9, 1361. [Google Scholar] [CrossRef]
- Zafar, K.; Sedeek, K.E.M.; Rao, G.S.; Khan, M.Z.; Amin, I.; Kamel, R.; Mukhtar, Z.; Zafar, M.; Mansoor, S.; Mahfouz, M.M. Genome editing technologies for rice improvement: Progress, prospects, and safety concerns. Front. Genome Ed. 2020, 2, 5. [Google Scholar] [CrossRef]
- Mehta, S.; Lal, S.K.; Sahu, K.P.; Venkatapuram, A.K.; Kumar, M.; Sheri, V.; Varakumar, P.; Vishwakarma, C.; Yadav, R.; Jameel, M.R.; et al. CRISPR/Cas9-Edited Rice: A New Frontier for Sustainable Agriculture. In New Frontiers in Stress Management for Durable Agriculture; Rakshit, A., Singh, H., Singh, A., Singh, U., Fraceto, L., Eds.; Springer: Singapore, 2020; pp. 427–458. [Google Scholar] [CrossRef]
- Tabassum, J.; Ahmad, S.; Hussain, B.; Mawia, A.M.; Zeb, A.; Ju, L. Applications and Potential of Genome-Editing Systems in Rice Improvement: Current and Future Perspectives. Agronomy 2021, 11, 1359. [Google Scholar] [CrossRef]
- Wada, N.; Ueta, R.; Osakabe, Y.; Osakabe, K. Precision genome editing in plants: State-of-the-art in CRISPR/Cas9-based genome engineering. BMC Plant Biol. 2020, 20, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ceasar, S.A.; Rajan, V.; Prykhozhij, S.V.; Berman, J.N.; Ignacimuthu, S. Insert, remove or replace: A highly advanced genome editing system using CRISPR/Cas9. Biochim. Biophys. Acta Bioenerg. 2016, 1863, 2333–2344. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, S.; Amaishi, Y.; Maki, I.; Enoki, T.; Mineno, J. Highly efficient genome editing for single-base substitutions using optimized ssODNs with Cas9-RNPs. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef]
- Hsu, P.D.; Lander, E.S.; Zhang, F. Development and applications of CRISPR-Cas9 for genome engineering. Cell 2014, 157, 1262–1278. [Google Scholar] [CrossRef] [Green Version]
- Chakraborty, C.; Teoh, S.L.; Das, S. The smart programmable CRISPR technology: A next-generation genome editing tool for investigators. Curr. Drug Targets 2017, 18, 1653–1663. [Google Scholar] [CrossRef] [PubMed]
- Bortesi, L.; Fischer, R. The CRISPR/Cas9 system for plant genome editing and beyond. Biotechnol. Adv. 2015, 33, 41–52. [Google Scholar] [CrossRef]
- Mao, Z.; Bozzella, M.; Seluanov, A.; Gorbunova, V. Comparison of nonhomologous end joining and homologous recombination in human cells. DNA Repair 2008, 7, 1765–1771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lieber, M.R. The mechanism of double-strand DNA break repair by the nonhomologous DNA end-joining pathway. Annu. Rev. Biochem. 2010, 79, 181–211. [Google Scholar] [CrossRef] [Green Version]
- Rouet, P.; Smih, F.; Jasin, M. Introduction of double-strand breaks into the genome of mouse cells by expression of a rare-cutting endonuclease. Mol. Cell. Biol. 1994, 14, 8096–8106. [Google Scholar] [CrossRef] [PubMed]
- Puchta, H. The repair of double-strand breaks in plants: Mechanisms and consequences for genome evolution. J. Exp. Bot. 2005, 56, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Miyaoka, Y.; Berman, J.R.; Cooper, S.B.; Mayerl, S.J.; Chan, A.H.; Zhang, B.; Karlin-Neumann, G.A.; Conklin, B.R. Systematic quantification of HDR and NHEJ reveals effects of locus, nuclease, and cell type on genome-editing. Sci. Rep. 2016, 6, 23549. [Google Scholar] [CrossRef]
- Mengiste, T.; Paszkowski, J. Prospects for the precise engineering of plant genomes by homologous recombination. Biol. Chem. 1999, 380, 749–758. [Google Scholar] [CrossRef]
- Vergunst, A.C.; Hooykaas, P.J.J. Recombination in the plant genome and its application in biotechnology. Crit. Rev. Plant. Sci. 1999, 18, 1–31. [Google Scholar] [CrossRef]
- Randhawa, S.; Sengar, S. The evolution and history of gene editing technologies. Prog. Mol. Biol. Transl. Sci. 2021, 178, 1–62. [Google Scholar] [CrossRef]
- Kim, Y.G.; Cha, J.; Chandrasegaran, S. Hybrid restriction enzymes: Zinc finger fusions to Fok I cleavage domain. Proc. Natl. Acad. Sci. USA 1996, 93, 1156–1160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Porteus, M.H.; Carroll, D. Gene targeting using zinc-finger nucleases. Nat. Biotechnol. 2005, 23, 967–973. [Google Scholar] [CrossRef] [PubMed]
- Bitinaite, J.; Wah, D.A.; Aggarwal, A.K.; Schildkraut, I. FokI dimerization is required for DNA cleavage. Proc. Natl. Acad. Sci. USA 1998, 95, 10570–10575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolfe, S.A.; Nekludova, L.; Pabo, C.O. DNA recognition by Cys2His2 Zinc finger proteins. Annu. Rev. Biophys. Biomol. Struct. 2000, 29, 183–212. [Google Scholar] [CrossRef]
- Urnov, F.D.; Rebar, E.J.; Holmes, M.C.; Zhang, H.S.; Gregory, P.D. Genome editing with engineered zinc finger nucleases. Nat. Rev. Genet. 2010, 11, 636–646. [Google Scholar] [CrossRef]
- Petolino, J.F. Genome editing in plants via designed zinc-finger nucleases. Vitr. Cell. Dev. Biol. Plant. 2015, 51, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Pattanayak, V.; Ramirez, C.L.; Joung, J.K.; Liu, D.R. Revealing off-target cleavage specificities of zinc-finger nucleases by in vitro selection. Nat. Methods 2011, 8, 765–770. [Google Scholar] [CrossRef] [Green Version]
- Boch, J.; Scholze, H.; Schornack, S.; Landgraf, A.; Hahn, S.; Kay, S.; Lahaye, T.; Nickstadt, A.; Bonas, U. Breaking the code of DNA binding specificity of TAL-type III effectors. Science 2009, 326, 1509–1512. [Google Scholar] [CrossRef] [PubMed]
- Moscou, M.J.; Bogdanove, A.J. A simple cipher governs DNA recognition by TAL effectors. Science 2009, 326, 1501. [Google Scholar] [CrossRef] [PubMed]
- Jankele, R.; Svoboda, P. TAL effectors: Tools for DNA targeting. Brief. Funct. Genom. 2014, 13, 409–419. [Google Scholar] [CrossRef] [PubMed]
- Mak, A.N.; Bradley, P.; Bogdanove, A.J.; Stoddard, B.L. TAL effectors: Function, structure, engineering and applications. Curr. Opin. Struct. Biol. 2013, 23, 93–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joung, J.K.; Sander, J.D. TALENs: A widely applicable technology for targeted genome editing. Nat. Rev. Mol. Cell Biol. 2013, 14, 49–55. [Google Scholar] [CrossRef] [Green Version]
- Gaj, T.; Gersbach, C.A.; Barbas, C.F., III. ZFN, TALEN, and CRISPR/Cas-based methods for genome engineering. Trends Biotechnol. 2013, 31, 397–405. [Google Scholar] [CrossRef] [Green Version]
- Chaudhary, K.; Chattopadhyay, A.; Pratap, D. The evolution of CRISPR/Cas9 and their cousins: Hope or hype? Biotechnol. Lett. 2018, 43, 2329. [Google Scholar] [CrossRef]
- Makarova, K.S.; Grishin, N.V.; Shabalina, S.A.; Wolf, Y.I.; Koonin, E.V. A putative RNA-interference-based immune system in prokaryotes: Computational analysis of the predicted enzymatic machinery, functional analogies with eukaryotic RNAi, and hypothetical mechanisms of action. Biol. Direct. 2006, 1, 7. [Google Scholar] [CrossRef] [Green Version]
- Barrangou, R.; Fremaux, C.; Deveau, H.; Richards, M.; Boyaval, P.; Moineau, S.; Romero, D.A.; Horvath, P. CRISPR provides acquired resistance against viruses in prokaryotes. Science 2007, 315, 1709–1712. [Google Scholar] [CrossRef]
- Brouns, S.J.; Jore, M.M.; Lundgren, M.; Westra, E.R.; Slijkhuis, R.J.; Snijders, A.P.; Dickman, M.J.; Makarova, K.S.; Koonin, E.V.; van der Oost, J. Small CRISPR RNAs guide antiviral defense in prokaryotes. Science 2008, 321, 960–964. [Google Scholar] [CrossRef] [Green Version]
- Mushtaq, M.; Ahmad Dar, A.; Skalicky, M.; Tyagi, A.; Bhagat, N.; Basu, U.; Bhat, B.A.; Zaid, A.; Ali, S.; Dar, T.-U.-H.; et al. CRISPR-based genome editing tools: Insights into technological breakthroughs and future challenges. Genes 2021, 12, 797. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, H.I.; Ahmad, M.J.; Asif, A.R.; Adnan, M.; Iqbal, M.K.; Mehmood, K.; Muhammad, S.A.; Bhuiyan, A.A.; Elokil, A.; Du, X.; et al. A review of CRISPR-based genome editing: Survival, evolution, and challenges. Curr. Issues Mol. Biol. 2018, 28, 47–68. [Google Scholar] [CrossRef] [Green Version]
- Mccarty, N.S.; Graham, A.E.; Studená, L.; Ledesma-Amaro, R. Multiplexed CRISPR technologies for gene editing and transcriptional regulation. Nat. Commun. 2020, 11, 1281. [Google Scholar] [CrossRef]
- Arora, L.; Narula, A. Gene editing and crop improvement using CRISPR-Cas9 system. Front. Plant Sci. 2017, 8, 1932. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaganathan, D.; Ramasamy, K.; Sellamuthu, G.; Jayabalan, S.; Venkataraman, G. CRISPR for crop improvement: An update review. Front. Plant Sci. 2018, 9, 985. [Google Scholar] [CrossRef]
- Montecillo, J.A.V.; Chu, L.L.; Bae, H. CRISPR-Cas9 system for plant genome editing: Current approaches and emerging developments. Agronomy 2020, 10, 1033. [Google Scholar] [CrossRef]
- Jiang, F.; Doudna, J.A. CRISPR-Cas9 structures and mechanisms. Annu. Rev. Biophys. 2017, 46, 505–529. [Google Scholar] [CrossRef] [Green Version]
- Shin, H.Y.; Wang, C.; Lee, H.K.; Yoo, K.H.; Zeng, X.; Kuhns, T.; Yang, C.M.; Mohr, T.; Liu, C.; Hennighausen, L. CRISPR/Cas9 targeting events cause complex deletions and insertions at 17 sites in the mouse genome. Nat. Commun. 2017, 8, 15464. [Google Scholar] [CrossRef]
- Carroll, D. Genome engineering with zinc-finger nucleases. Genetics 2011, 188, 773–782. [Google Scholar] [CrossRef] [Green Version]
- Wolf, S.; Wu, W.; Jones, C.; Perwitasari, O.; Mahalingam, S.; Tripp, R.A.; Chan, M.C. Structures of Cas9 endonucleases reveal RNA-mediated conformational activation. Science 2014, 343, 1247997. [Google Scholar] [CrossRef]
- Zetsche, B.; Gootenberg, J.S.; Abudayyeh, O.O.; Slaymaker, I.M.; Makarova, K.S.; Essletzbichler, P.; Volz, S.E.; Joung, J.; van der Oost, J.; Regev, A.; et al. Cpf1 is a single RNA-guided endonuclease of a class 2 CRISPR-Cas system. Cell 2015, 163, 759–771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahas, A.; Aman, R.; Mahfouz, M. CRISPR-Cas13d mediates robust RNA virus interference in plants. Genome Biol. 2019, 20, 263. [Google Scholar] [CrossRef] [Green Version]
- Rees, H.A.; Liu, D.R. Base editing: Precision chemistry on the genome and transcriptome of living cells. Nat. Rev. Genet. 2018, 19, 770–788. [Google Scholar] [CrossRef] [PubMed]
- Matsoukas, I.G. Prime editing: Genome editing for rare genetic diseases without double-strand breaks or donor DNA. Front. Genet. 2020, 11, 528. [Google Scholar] [CrossRef]
- Schenke, D.; Cai, D. Applications of CRISPR/Cas to improve crop disease resistance: Beyond inactivation of susceptibility factors. iScience 2020, 23, 101478. [Google Scholar] [CrossRef] [PubMed]
- Macovei, A.; Sevilla, N.R.; Cantos, C.; Jonson, G.B.; Slamet-Loedin, I.; Čermák, T.; Voytas, D.F.; Choi, I.; Chadha-Mohanty, P. Novel alleles of rice eIF4G generated by CRISPR/Cas9-targeted mutagenesis confer resistance to Rice tungro spherical virus. Plant Biotechnol. J. 2018, 16, 1918–1927. [Google Scholar] [CrossRef] [Green Version]
- Chandrasekaran, J.; Brumin, M.; Wolf, D.; Leibman, D.; Klap, C.; Pearlsman, M.; Sherman, A.; Arazi, T.; Gal-On, A. Development of broad virus resistance in non-transgenic cucumber using CRISPR/Cas9 technology. Mol. Plant Pathol. 2016, 17, 1140–1153. [Google Scholar] [CrossRef] [Green Version]
- Yoon, Y.-J.; Venkatesh, J.; Lee, J.-H.; Kim, J.; Lee, H.-E.; Kim, D.-S.; Kang, B.-C. Genome editing of eIF4E1 in tomato confers resistance to Pepper mottle virus. Front. Plant Sci. 2020, 11, 1098. [Google Scholar] [CrossRef] [PubMed]
- Moury, B.; Lebaron, C.; Szadkowski, M.; Ben Khalifa, M.; Girardot, G.; Bi, B.A.B.; Koné, D.; Nitiema, L.W.; Fakhfakh, H.; Gallois, J.-L. Knock-out mutation of eukaryotic initiation factor 4E2 (eIF4E2) confers resistance to Pepper veinal mottle virus in tomato. Virology 2020, 539, 11–17. [Google Scholar] [CrossRef]
- Oliva, R.; Ji, C.; Atienza-Grande, G.; Huguet-Tapia, J.C.; Perez-Quintero, A.; Li, T.; Eom, J.-S.; Li, C.; Nguyen, H.; Liu, B.; et al. Broad-spectrum resistance to bacterial blight in rice using genome editing. Nat. Biotechnol. 2019, 37, 1344–1350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, L.; Kim, P.; Yu, L.; Cai, G.; Chen, S.; Alfano, J.R.; Zhou, J.-M. Activation-dependent destruction of a co-receptor by a Pseudomonas syringae effector dampens plant immunity. Cell Host Microbe 2016, 20, 504–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodriguez, E.; El Ghoul, H.; Mundy, J.; Petersen, M. Making sense of plant autoimmunity and negative regulators. FEBS J. 2016, 283, 1385–1391. [Google Scholar] [CrossRef]
- Wang, F.; Wang, C.; Liu, P.; Lei, C.; Hao, W.; Gao, Y.; Liu, Y.-G.; Zhao, K. Enhanced rice blast resistance by CRISPR/Cas9-targeted mutagenesis of the ERF transcription factor gene OsERF922. PLoS ONE 2016, 11, e0154027. [Google Scholar] [CrossRef] [PubMed]
- Kuai, X.; MacLeod, B.J.; Després, C. Integrating data on the Arabidopsis NPR1/NPR3/NPR4 salicylic acid receptors; a differentiating argument. Front. Plant Sci. 2015, 6, 235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fister, A.S.; Landherr, L.; Maximova, S.N.; Guiltinan, M.J. Transient expression of CRISPR/Cas9 machinery targeting TcNPR3 enhances defense response in Theobroma cacao. Front. Plant Sci. 2018, 9, 268. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Deng, K.; Cheng, Y.; Zhong, Z.; Tian, L.; Tang, X.; Tang, A.; Zheng, X.; Zhang, T.; Qi, Y.; et al. CRISPR-Cas9 based genome editing reveals new insights into MicroRNA function and regulation in rice. Front. Plant Sci. 2017, 8, 1598. [Google Scholar] [CrossRef] [Green Version]
- Chattopadhyay, A.; Purohit, J.; Tiwari, K.K.; Deshmukh, R. Targeting transcription factors for plant disease resistance: Shifting paradigm. Curr. Sci. 2019, 117, 1598–1607. [Google Scholar] [CrossRef]
- Alagoz, Y.; Gurkok, T.; Zhang, B.; Unver, T. Manipulating the biosynthesis of bioactive compound alkaloids for next generation metabolic engineering in opium poppy using CRISPR-Cas 9 genome editing technology. Sci. Rep. 2016, 6, 30910. [Google Scholar] [CrossRef] [Green Version]
- Li, R.; Li, R.; Li, X.; Fu, D.; Zhu, B.; Tian, H.; Luo, Y.; Zhu, H. Multiplexed CRISPR/Cas9-mediated metabolic engineering of γ-aminobutyric acid levels in Solanum lycopersicum. Plant Biotechnol. J. 2018, 16, 415–427. [Google Scholar] [CrossRef] [Green Version]
- Cao, Y.; Zhou, H.; Zhou, X.; Li, F. Control of plant viruses by CRISPR/Cas system-mediated adaptive immunity. Front. Microbiol. 2020, 11, 593700. [Google Scholar] [CrossRef] [PubMed]
- Varanda, C.M.; Félix, M.; Campos, M.D.; Patanita, M.; Materatski, P. Plant viruses: From targets to tools for CRISPR. Viruses 2021, 3, 141. [Google Scholar] [CrossRef] [PubMed]
- Gil Lee, H.; Kim, D.H.; Choi, Y.-R.; Yu, J.; Hong, S.-A.; Seo, P.J.; Bae, S. Enhancing plant immunity by expression of pathogen-targeted CRISPR-Cas9 in plants. Gene Genome Ed. 2021, 1, 100001. [Google Scholar] [CrossRef]
- Singh, R.K.; Chattopadhyay, A.; Pundey, S.K. Status and Perspectives of Biological Control of Rice Diseases. In Microbial Empowerment in Agriculture; Sarma, B.K., Singh, A., Eds.; Biotech Books: New Delhi, India, 2016; Volume 1, pp. 335–372. [Google Scholar]
- Ning, X.; Yunyu, W.; Aihong, L. Strategy for use of rice blast resistance genes in rice molecular breeding. Rice Sci. 2020, 27, 263–277. [Google Scholar] [CrossRef]
- Xie, K.; Yang, Y. RNA-guided genome editing in plants using a CRISPR-Cas system. Mol. Plant. 2013, 6, 1975–1983. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Chen, X.; Liang, X.; Zhou, X.; Yang, F.; Liu, J.; He, S.Y.; Guo, Z. Alternative splicing of rice WRKY62 and WRKY76 transcription factor genes in pathogen defense. Plant Physiol. 2016, 171, 1427–1442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, J.; Chen, J.; Wang, M.; Ren, Y.; Wang, S.; Lei, C.; Cheng, Z.; Sodmergen. Disruption of OsSEC3A increases the content of salicylic acid and induces plant defense responses in rice. J. Exp. Bot. 2018, 69, 1051–1064. [Google Scholar] [CrossRef] [Green Version]
- Nawaz, G.; Usman, B.; Peng, H.; Zhao, N.; Yuan, R.; Liu, Y.; Li, R. Knockout of Pi21 by CRISPR/Cas9 and iTRAQ-based proteomic analysis of mutants revealed new insights into M. oryzae resistance in elite rice line. Genes 2020, 11, 735. [Google Scholar] [CrossRef]
- Li, S.; Shen, L.; Hu, P.; Liu, Q.; Zhu, X.; Qian, Q.; Wang, K.; Wang, Y. Developing disease-resistant thermosensitive male sterile rice by multiplex gene editing. J. Integr. Plant Biol. 2019, 61, 1201–1205. [Google Scholar] [CrossRef]
- Li, W.; Zhu, Z.; Chern, M.; Yin, J.; Yang, C.; Ran, L.; Cheng, M.; He, M.; Wang, K.; Wang, J.; et al. A natural allele of a transcription factor in rice confers broad-spectrum blast resistance. Cell 2017, 170, 114–126.e15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, Z.; Yin, J.; Chern, M.; Zhu, X.; Yang, C.; He, K.; Liu, Y.; He, M.; Wang, J.; Song, L.; et al. New insights into bsr-d1-mediated broad-spectrum resistance to rice blast. Mol. Plant Pathol. 2020, 21, 951–960. [Google Scholar] [CrossRef] [PubMed]
- Shah, P.R.; Varanavasiappan, S.; Kokiladevi, E.; Ramanathan, A.; Kumar, K.K. Genome editing of rice PFT1 gene to study its role in rice sheath blight disease resistance. Int. J. Curr. Microbiol. App. Sci. 2019, 8, 2356–2364. [Google Scholar] [CrossRef]
- Hong, Y.; Liu, Q.; Cao, Y.; Zhang, Y.; Chen, D.; Lou, X.; Cheng, S.; Cao, L. The OsMPK15 negatively regulates Magnaporthe oryza and Xoo disease resistance via SA and JA signaling pathway in rice. Front. Plant Sci. 2019, 10, 752. [Google Scholar] [CrossRef]
- Liang, Y.; Han, Y.; Wang, C.; Jiang, C.; Xu, J.-R. Targeted deletion of the USTA and UvSLT2 genes efficiently in Ustilaginoidea virens with the CRISPR-Cas9 system. Front. Plant Sci. 2018, 9, 699. [Google Scholar] [CrossRef]
- Yamato, T.; Handa, A.; Arazoe, T.; Kuroki, M.; Nozaka, A.; Kamakura, T.; Ohsato, S.; Arie, T.; Kuwata, S. Single crossover-mediated targeted nucleotide substitution and knock-in strategies with CRISPR/Cas9 system in the rice blast fungus. Sci. Rep. 2019, 9, 7427. [Google Scholar] [CrossRef]
- Fukuoka, S.; Saka, N.; Koga, H.; Ono, K.; Shimizu, T.; Ebana, K.; Hayashi, N.; Takahashi, A.; Hirochika, H.; Okuno, K.; et al. Loss of function of a proline-containing protein confers durable disease resistance in rice. Science 2009, 325, 998–1001. [Google Scholar] [CrossRef]
- Foster, A.J.; Martin-Urdiroz, M.; Yan, X.; Wright, H.S.; Soanes, D.M.; Talbot, N.J. CRISPR-Cas9 ribonucleoprotein-mediated co-editing and counterselection in the rice blast fungus. Sci. Rep. 2018, 8, 14355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arazoe, T. Genome editing using CRISPR/Cas9 system in the rice blast fungus. In Magnaporthe Oryzae, Methods in Molecular Biology; Jacob, S., Ed.; Humana Press: New York, NY, USA, 2021; p. 2356. [Google Scholar] [CrossRef]
- Chattopadhyay, A.; Nagaich, D.; Lima, J.M.; Verma, A.; Tiwari, K.K. Molecular Aspects of Bacterial Blight Resistance in Rice: Recent Advancement. In Biotic Stress Management in Rice; Shamim, M., Singh, K.N., Eds.; Apple Academic Press: Palm Bay, FL, USA, 2017; pp. 17–45. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, R.; Sengupta, D.; Das, S.N.; Pandey, M.K.; Bohra, A.; Sharma, N.K.; Sinha, P.; Sk, H.; Ghazi, I.A.; et al. Deployment of genetic and genomic tools toward gaining a better understanding of rice-Xanthomonas oryzae pv. oryzae interactions for development of durable bacterial blight resistant rice. Front. Plant Sci. 2020, 11, 1152. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, S. Rice versus Xanthomonas oryzae pv. oryzae: A unique pathosystem. Curr. Opin. Plant Biol. 2013, 16, 188–195. [Google Scholar] [CrossRef] [Green Version]
- Hu, K.; Cao, J.; Zhang, J.; Xia, F.; Ke, Y.; Zhang, H.; Xie, W.; Liu, H.; Cui, Y.; Cao, Y.; et al. Improvement of multiple agronomic traits by a disease resistance gene via cell wall reinforcement. Nat. Plants 2017, 3, 17009. [Google Scholar] [CrossRef] [PubMed]
- Streubel, J.; Pesce, C.; Hutin, M.; Koebnik, R.; Boch, J.; Szurek, B. Five phylogenetically close rice SWEET genes confer TAL effector-mediated susceptibility to Xanthomonas oryzae pv. oryzae. New Phytol. 2013, 200, 808–819. [Google Scholar] [CrossRef]
- Gupta, P.K.; Balyan, H.S.; Gautam, T. SWEET genes and TAL effectors for disease resistance in plants: Present status and future prospects. Mol. Plant Pathol. 2021, 22, 1014–1026. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Spalding, M.H.; Weeks, D.P.; Yang, B. High-efficiency TALEN-based gene editing produces disease-resistant rice. Nat. Biotechnol. 2012, 30, 390–392. [Google Scholar] [CrossRef] [PubMed]
- Blanvillain-Baufumé, S.; Reschke, M.; Solé, M.; Auguy, F.; Doucoure, H.; Szurek, B.; Meynard, D.; Portefaix, M.; Cunnac, S.; Guiderdoni, E.; et al. Targeted promoter editing for rice resistance to Xanthomonas oryzae pv. oryzae reveals differential activities for SWEET14-inducing TAL effectors. Plant Biotechnol. J. 2017, 15, 306–317. [Google Scholar] [CrossRef] [Green Version]
- Jiang, W.; Zhou, H.; Bi, H.; Fromm, M.; Yang, B.; Weeks, D.P. Demonstration of CRISPR/Cas9/sgRNA-mediated targeted gene modification in Arabidopsis, tobacco, sorghum and rice. Nucleic Acids Res. 2013, 41, e188. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Peng, Z.; Long, J.; Sosso, D.; Liu, B.; Eom, J.-S.; Huang, S.; Liu, S.; Cruz, C.V.; Frommer, W.; et al. Gene targeting by the TAL effector PthXo2 reveals cryptic resistance gene for bacterial blight of rice. Plant J. 2015, 82, 632–643. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.A.; Moon, H.; Park, C.J. CRISPR/Cas9-targeted mutagenesis of Os8N3 in rice to confer resistance to Xanthomonas oryzae pv. oryzae. Rice 2019, 12, 67. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Xu, X.; Gong, Q.; Li, Z.; Li, Y.; Wang, S.; Yang, Y.; Ma, W.; Liu, L.; Zhu, B.; et al. Engineering broad-spectrum bacterial blight resistance by simultaneously disrupting variable TALE-binding elements of multiple susceptibility genes in rice. Mol. Plant. 2019, 12, 1434–1446. [Google Scholar] [CrossRef] [Green Version]
- Zafar, K.; Khan, M.Z.; Amin, I.; Mukhtar, Z.; Yasmin, S.; Arif, M.; Ejaz, K.; Mansoor, S. Precise CRISPR-Cas9 mediated genome editing in super basmati rice for resistance against bacterial blight by targeting the major susceptibility gene. Front. Plant Sci. 2020, 11, 575. [Google Scholar] [CrossRef]
- Duy, P.N.; Lan, D.T.; Thu, H.P.; Thu, H.P.T.; Thanh, H.N.; Pham, N.P.; Auguy, F.; Manh, T.B.; Cunnac, S.; Pham, X.H. Improved bacterial leaf blight disease resistance in the major elite Vietnamese rice cultivar TBR225 via editing of the OsSWEET14 promoter. PLoS ONE 2021, 16, e0255470. [Google Scholar] [CrossRef]
- Wang, J.; Tian, D.; Gu, K.; Yang, X.; Wang, L.; Zeng, X.; Yin, Z. Induction of Xa10-like genes in rice cultivar Nipponbare confers disease resistance to rice bacterial blight. Mol. Plant Microbe Interact. 2017, 30, 466–477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, L.; Cao, Y.; Xu, Z.; Ma, W.; Zakria, M.; Zou, L.; Cheng, Z.; Chen, G. A transcription activator-like effector Tal7 of Xanthomonas oryzae pv. oryzicola activates rice gene Os09g29100 to suppress rice immunity. Sci. Rep. 2017, 7, 5089. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Xu, Z.; Li, Z.; Zakria, M.; Zou, L.; Chen, G. Increasing resistance to bacterial leaf streak in rice by editing the promoter of susceptibility gene OsSULRT3; 6. Plant Biotechnol. J. 2021, 19, 1101–1103. [Google Scholar] [CrossRef] [PubMed]
- Ni, Z.; Cao, Y.; Jin, X.; Fu, Z.; Li, J.; Mo, X.; He, Y.; Tang, J.; Huang, S. Engineering resistance to bacterial blight and bacterial leaf streak in rice. Rice 2021, 14, 38. [Google Scholar] [CrossRef]
- Yu, K.; Liu, Z.; Gui, H.; Geng, L.; Wei, J.; Liang, D.; Lv, J.; Xu, J.; Chen, X. Highly efficient generation of bacterial leaf blight-resistant and transgene-free rice using a genome editing and multiplexed selection system. BMC Plant Biol. 2021, 21, 197. [Google Scholar] [CrossRef] [PubMed]
- Niño-Liu, D.O.; Ronald, P.C.; Bogdanove, A.J. Xanthomonas oryzae pathovars: Model pathogens of a model crop. Mol. Plant Pathol. 2006, 7, 303–324. [Google Scholar] [CrossRef]
- Bunawan, H.; Dusik, L.; Bunawan, S.N.; Amin, N. Rice tungro disease: From identification to disease control. World Appl. Sci. J. 2014, 31, 1221–1226. [Google Scholar] [CrossRef]
- Hwang, J.; Oh, C.S.; Kang, B.C. Translation elongation factor 1B (eEF1B) is an essential host factor for Tobacco mosaic virus infection in plants. Virology 2013, 439, 105–114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pyott, D.E.; Sheehan, E.; Molnar, A. Engineering of CRISPR/Cas9-mediated potyvirus resistance in transgene-free Arabidopsis plants. Mol. Plant Pathol. 2016, 17, 1276–1288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.-H.; Muhsin, M.; Atienza, G.A.; Kwak, D.-Y.; Kim, S.-M.; De Leon, T.B.; Angeles, E.R.; Coloquio, E.; Kondoh, H.; Satoh, K.; et al. Single nucleotide polymorphisms in a gene for translation initiation factor (eIF4G) of rice (Oryza sativa) associated with resistance to Rice tungro spherical virus. Mol. Plant Microbe Interact. 2010, 23, 29–38. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Ma, S.; Hu, P.; Ji, Y.; Sun, F. Genome editing of rice eIF4G loci confers partial resistance to rice black-streaked dwarf virus. Viruses 2021, 13, 2100. [Google Scholar] [CrossRef]
- Engelhardt, S.; Stam, R.; Hückelhoven, R. Good riddance? breaking disease susceptibility in the era of new breeding technologies. Agronomy 2018, 8, 114. [Google Scholar] [CrossRef] [Green Version]
- Koseoglo, E.; van der Wolf, J.M.; Visser, R.G.F.; Bai, Y. Susceptibility reversed: Modified plant susceptibility genes for resistance to bacteria. Trends Plant Sci. 2021, 27, 69–79. [Google Scholar] [CrossRef]
- Kieu, N.P.; Lenman, M.; Wang, E.S.; Petersen, B.L.; Andreasson, E. Mutations introduced in susceptibility genes through CRISPR/Cas9 genome editing confer increased late blight resistance in potatoes. Sci. Rep. 2021, 11, 4487. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, S.; Kesiraju, K.; Saakre, M.; Rathinam, M.; Raman, V.; Pattanayak, D.; Sreevathsa, R. Genome editing for resistance to insect pests: An emerging tool for crop improvement. ACS Omega 2020, 5, 20674–20683. [Google Scholar] [CrossRef]
- Li, R.; Afsheen, S.; Xin, Z.; Han, X.; Lou, Y. OsNPR1 negatively regulates herbivore-induced JA and ethylene signaling and plant resistance to a chewing herbivore in rice. Physiol. Plant. 2013, 147, 340–351. [Google Scholar] [CrossRef] [PubMed]
- Chern, M.; Canlas, P.E.; Fitzgerald, H.A.; Ronald, P.C. Rice NRR, a negative regulator of disease resistance, interacts with Arabidopsis NPR1 and rice NH1. Plant J. 2005, 43, 623–635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grand, X.; Espinoza, R.; Michel, C.; Cros, S.; Chalvon, V.; Jacobs, J.; Morel, J.B. Identification of positive and negative regulators of disease resistance to rice blast fungus using constitutive gene expression patterns. Plant Biotechnol. J. 2012, 10, 840–850. [Google Scholar] [CrossRef]
- Ye, M.; Kuai, P.; Chen, S.; Lin, N.; Ye, M.; Hu, L.; Lou, Y. Silencing a Simple Extracellular Leucine-Rich Repeat Gene OsI-BAK1 Enhances the Resistance of Rice to Brown Planthopper Nilaparvata lugens. Int. J. Mol. Sci. 2021, 22, 12182. [Google Scholar] [CrossRef]
- Peng, Y.; Bartley, L.E.; Chen, X.; Dardick, C.; Chern, M.; Ruan, R.; Canlas, P.E.; Ronald, P.C. OsWRKY62 is a negative regulator of basal and Xa21-mediated defense against Xanthomonas oryzae pv. oryzae in rice. Mol. Plant. 2008, 1, 446–458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chujo, T.; Miyamoto, K.; Shimogawa, T.; Shimizu, T.; Otake, Y.; Yokotani, N.; Nishizawa, Y.; Shibuya, N.; Nojiri, H.; Yamane, H.; et al. OsWRKY28, a PAMP-responsive transrepressor, negatively regulates innate immune responses in rice against rice blast fungus. Plant Mol. Biol. 2013, 82, 23–37. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Ye, M.; Li, R.; Zhang, T.; Zhou, G.; Wang, Q.; Lu, J.; Lou, Y. The rice transcription factor WRKY53 suppresses herbivore-induced defenses by acting as a negative feedback modulator of Mitogen-Activated Protein Kinase activity. Plant Physiol. 2015, 169, 2907–2921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshii, M.; Shimizu, T.; Yamazaki, M.; Higashi, T.; Miyao, A.; Hirochika, H.; Omura, T. Disruption of a novel gene for a NAC-domain protein in rice confers resistance to Rice dwarf virus. Plant J. 2009, 57, 615–625. [Google Scholar] [CrossRef] [PubMed]
- Khong, G.N.; Pati, P.K.; Richaud, F.; Parizot, B.; Bidzinski, P.; Mai, C.D.; Bès, M.; Bourrié, I.; Meynard, D.; Beeckman, T.; et al. OsMADS26 negatively regulates resistance to pathogens and drought tolerance in rice. Plant Physiol. 2015, 169, 2935–2949. [Google Scholar] [CrossRef] [Green Version]
- Koo, S.C.; Choi, M.S.; Chun, H.J.; Shin, D.B.; Park, B.S.; Kim, Y.H.; Park, H.-M.; Seo, H.S.; Song, J.T.; Kang, K.Y.; et al. The calmodulin-binding transcription factor OsCBT suppresses defense responses to pathogens in rice. Mol. Cells 2009, 27, 563–570. [Google Scholar] [CrossRef]
- Wu, J.; Yang, R.; Yang, Z.; Yao, S.; Zhao, S.; Wang, Y.; Li, P.; Song, X.; Jin, L.; Zhou, T.; et al. ROS accumulation and antiviral defence control by microRNA528 in rice. Nat. Plants 2017, 3, 16203. [Google Scholar] [CrossRef]
- Shen, X.; Yuan, B.; Liu, H.; Li, X.; Xu, C.; Wang, S. Opposite functions of a rice mitogen-activated protein kinase during the process of resistance against Xanthomonas oryzae. Plant J. 2010, 64, 86–99. [Google Scholar] [CrossRef]
- Zhou, G.; Ren, N.; Qi, J.; Lu, J.; Xiang, C.; Ju, H.; Cheng, J.; Lou, Y. The 9-lipoxygenase Osr9-LOX1 interacts with the 13-lipoxygenase-mediated pathway to regulate resistance to chewing and piercing-sucking herbivores in rice. Physiol Plant. 2014, 152, 59–69. [Google Scholar] [CrossRef]
- Xiao, W.; Liu, H.; Li, Y.; Li, X.; Xu, C.; Long, M.; Wang, S. A rice gene of De Novo origin negatively regulates pathogen-induced defense response. PLoS ONE 2009, 4, e4603. [Google Scholar] [CrossRef] [Green Version]
- Shen, X.; Liu, H.; Yuan, B.; Li, X.; Xu, C.; Wang, S. OsEDR1 negatively regulates rice bacterial resistance via activation of ethylene biosynthesis. Plant Cell Environ. 2011, 34, 179–191. [Google Scholar] [CrossRef]
- Albar, L.; Bangratz-Reyser, M.; Hebrard, E.; Ndjiondjop, M.N.; Jones, M.; Ghesquiere, A. Mutations in the eIF(iso)4G translation initiation factor confer high resistance of rice to Rice yellow mottle virus. Plant J. 2006, 47, 417–426. [Google Scholar] [CrossRef]
- Zhang, H.; Li, L.; He, Y.; Qin, Q.; Chen, C.; Wei, Z.; Tan, X.; Xie, K.; Zhang, R.; Hong, G.; et al. Distinct modes of manipulation of rice auxin response factor OsARF17 by different plant RNA viruses for infection. Proc. Natl. Acad. Sci. USA 2020, 117, 9112–9121. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Zhang, L.; Mo, X.; Ji, H.; Bian, H.; Hu, Y.; Majid, T.; Long, J.; Pang, H.; Tao, Y.; et al. Rice aquaporin PIP1;3 and harpin Hpa1 of bacterial blight pathogen cooperate in a type III effector translocation. J. Exp. Bot. 2019, 70, 3057–3073. [Google Scholar] [CrossRef] [Green Version]
- Hui, S.; Shi, Y.; Tian, J.; Wang, L.; Li, Y.; Wang, S.; Yuan, M. TALE-carrying bacterial pathogens trap host nuclear import receptors for facilitation of infection of rice. Mol. Plant Pathol. 2019, 20, 519–532. [Google Scholar] [CrossRef] [PubMed]
- van Schie, C.C.; Takken, F.L. Susceptibility genes 101: How to be a good host. Annu. Rev. Phytopathol. 2014, 52, 551–581. [Google Scholar] [CrossRef] [PubMed]
- Pavan, S.; Jacobsen, E.; Visser, R.G.; Bai, Y. Loss of susceptibility as a novel breeding strategy for durable and broad-spectrum resistance. Mol. Breed. 2010, 25, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Feng, C.; Su, H.; Bai, H.; Wang, R.; Liu, Y.; Guo, X.; Liu, C.; Zhang, J.; Yuan, J.; Birchler, J.A.; et al. High-efficiency genome editing using a dmc1 promoter-controlled CRISPR/Cas9 system in maize. Plant Biotechnol. J. 2018, 16, 1848–1857. [Google Scholar] [CrossRef]
- Li, X.; Jiang, D.-H.; Yong, K.; Zhang, D.-B. Varied transcriptional efficiencies of multiple Arabidopsis U6 small nuclear RNA genes. J. Integrat. Plant Biol. 2007, 49, 222–229. [Google Scholar] [CrossRef]
- Hao, Y.; Zong, W.; Zeng, D.; Han, J.; Chen, S.; Tang, J.; Zhao, Z.; Li, X.; Ma, K.; Xie, X.; et al. Shortened snRNA promoters for efficient CRISPR/Cas-based multiplex genome editing in monocot plants. Sci. China Life Sci. 2020, 63, 933–935. [Google Scholar] [CrossRef]
- Qi, X.; Dong, L.; Liu, C.; Mao, L.; Liu, F.; Zhang, X.; Cheng, B.; Xie, C. Systematic identification of endogenous RNA polymerase III promoters for efficient RNA guide-based genome editing technologies in maize. Crop J. 2018, 6, 314–320. [Google Scholar] [CrossRef]
- Jun, R.; Xixun, H.; Kejian, W.; Chun, W. Development and application of CRISPR/Cas system in rice. Rice Sci. 2019, 26, 69–76. [Google Scholar] [CrossRef]
- Hassan, M.M.; Zhang, Y.; Yuan, G.; De, K.; Chen, J.G.; Muchero, W.; Tuskan, G.A.; Qi, Y.; Yang, X. Construct design for CRISPR/Cas-based genome editing in plants. Trends Plant Sci. 2021, 26, 1133–1152. [Google Scholar] [CrossRef]
- Ren, Q.; Zhong, Z.; Wang, Y.; You, Q.; Li, Q.; Yuan, M.; He, Y.; Qi, C.; Tang, X.; Zheng, X.; et al. Bidirectional promoter-based CRISPR-Cas9 systems for plant genome editing. Front. Plant Sci. 2019, 10, 1173. [Google Scholar] [CrossRef]
- Bao, A.; Chen, H.; Chen, L.; Chen, S.; Hao, Q.; Guo, W.; Qiu, D.; Shan, Z.; Yang, Z.; Yuan, S.; et al. CRISPR/Cas9-mediated targeted mutagenesis of GmSPL9 genes alters plant architecture in soybean. BMC Plant Biol. 2019, 19, 131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Danilo, B.; Perrot, L.; Mara, K.; Botton, E.; Nogué, F.; Mazier, M. Efficient and transgene-free gene targeting using Agrobacterium-mediated delivery of the CRISPR/Cas9 system in tomato. Plant Cell Rep. 2019, 38, 459–462. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Eggenberger, A.L.; Banakar, R.; McCaw, M.E.; Zhu, H.; Main, M.; Kang, M.; Gelvin, S.B.; Wang, K. CRISPR/Cas9-mediated targeted T-DNA integration in rice. Plant Mol. Biol. 2019, 99, 317–328. [Google Scholar] [CrossRef]
- Clough, S.J.; Bent, A.F. Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998, 16, 735–743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rohini, V.K.; Rao, K.S. Transformation of peanut (Arachis hypogaea L.): A non-tissue culture based approach for generating transgenic plants. Plant Sci. 2000, 150, 41–49. [Google Scholar] [CrossRef]
- Kesiraju, K.; Tyagi, S.; Mukherjee, S.; Rai, R.; Singh, N.K.; Sreevathsa, R.; Dash, P.K. An apical meristem-targeted in planta transformation method for the development of transgenics in flax (Linum usitatissimum): Optimization and validation. Front. Plant Sci. 2021, 11, 562056. [Google Scholar] [CrossRef] [PubMed]
- Ratanasut, K.; Rod-In, W.; Sujipuli, K. In planta Agrobacterium-Mediated Transformation of Rice. Rice Sci. 2017, 24, 181–186. [Google Scholar] [CrossRef]
- Supartana, P.; Shimizu, T.; Shioiri, H.; Nogawa, M.; Nozue, M.; Kojima, M. Development of simple and efficient in planta transformation method for rice (Oryza sativa L.) using Agrobacterium tumefaciens. J. Biosci. Bioeng. 2005, 100, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.Z.; Zhou, B.; Yang, Y.Z.; Mei, J.; Zhao, X.Y.; Guo, X.H.; Huang, X.Q.; Tang, D.Y.; Liu, X.M. Piercing and vacuum infiltration of the mature embryo: A simplified method for Agrobacterium-mediated transformation of indica rice. Plant Cell Rep. 2009, 28, 1065–1074. [Google Scholar] [CrossRef] [PubMed]
- Char, S.N.; Neelakandan, A.K.; Nahampun, H.; Frame, B.; Main, M.; Spalding, M.H.; Becraft, P.W.; Meyers, B.C.; Walbot, V.; Wang, K.; et al. An Agrobacterium-delivered CRISPR/Cas9 system for high-frequency targeted mutagenesis in maize. Plant Biotechnol. J. 2017, 15, 257–268. [Google Scholar] [CrossRef]
- Zhang, S.; Shen, J.; Li, D.; Cheng, Y. Strategies in the delivery of Cas9 ribonucleoprotein for CRISPR/Cas9 genome editing. Theranostics 2021, 11, 614–648. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, A.; Parkhi, V.; Char, B. Genome editing for crop improvement: A perspective from India. Vitr. Cell Dev. Biol. Plant. 2021, 57, 565–573. [Google Scholar] [CrossRef]
- Dilawari, R.; Kaur, N.; Priyadarshi, N.; Kumar, B.; Abdelmotelb, K.F.; Lal, S.K.; Singh, B.; Tripathi, A.; Aggarwal, S.K.; Jat, B.S.; et al. Genome Editing: A Tool from the Vault of Science for Engineering Climate-Resilient Cereals. In Harsh Environment and Plant Resilience: Molecular and Functional Aspects; Husen, A., Ed.; Springer: Cham, Switzerland, 2021; pp. 45–72. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).