Morphological and Biochemical Variation in Carrot Genetic Resources Grown under Open Field Conditions: The Selection of Functional Genotypes for a Breeding Program
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Plant Material and Cultivation of Carrot
2.3. Evaluation of Qualitative and Quantitative Morphological Parameters
2.4. Evaluation of Color Value
2.5. Extraction and Analysis of Carotenoids
2.6. Extraction and Analysis of Free Sugars and Total Sweetness Index (TSI) Analysis
2.7. Statistical Analyses
3. Results and Discussion
3.1. Variation in Qualitative and Quantitative Morphological Traits
3.2. Variation in Color Attributes
3.3. Variation in Biochemical Parameters
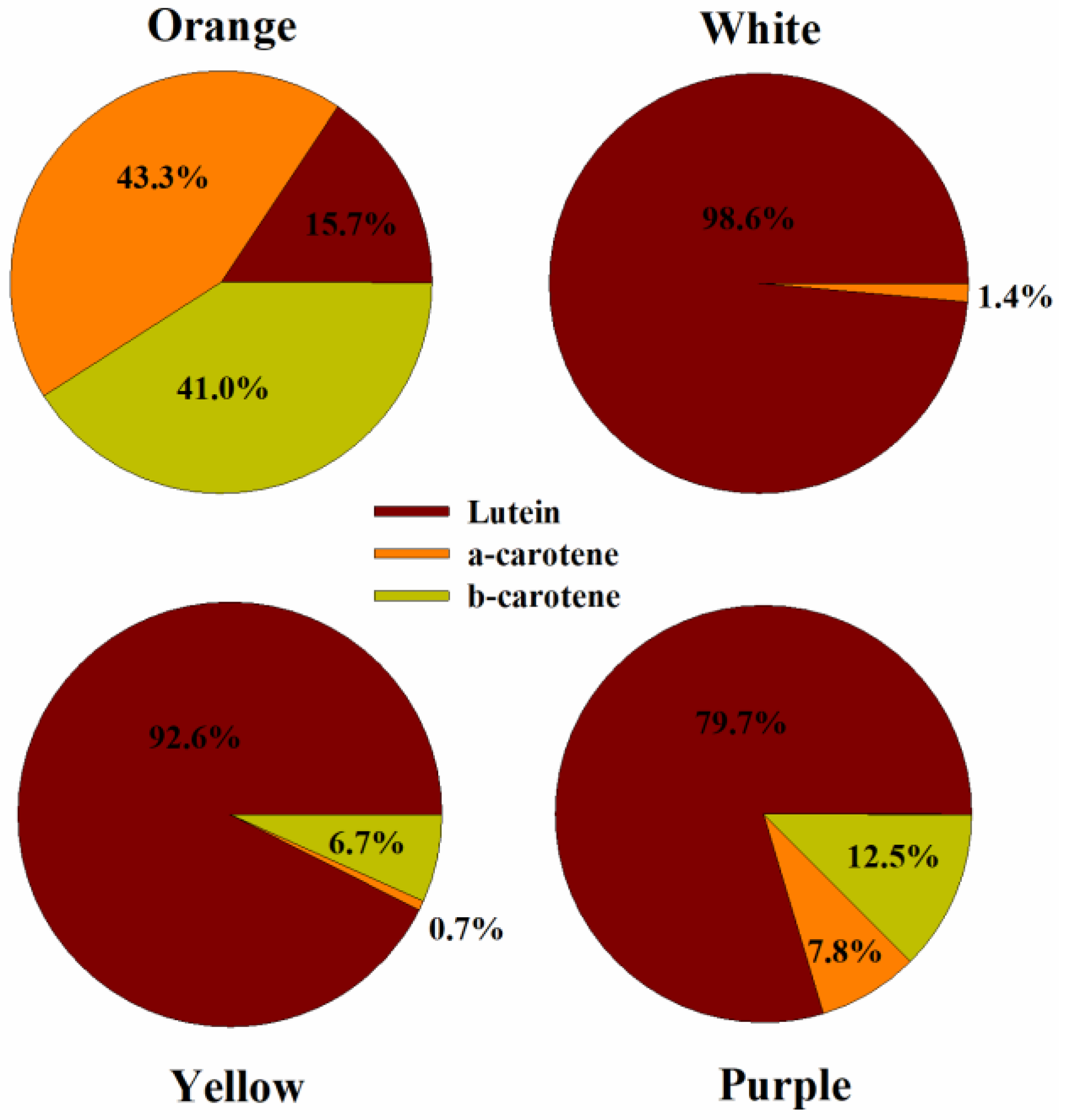
3.3.1. Variation in Carotenoids Profile and Content
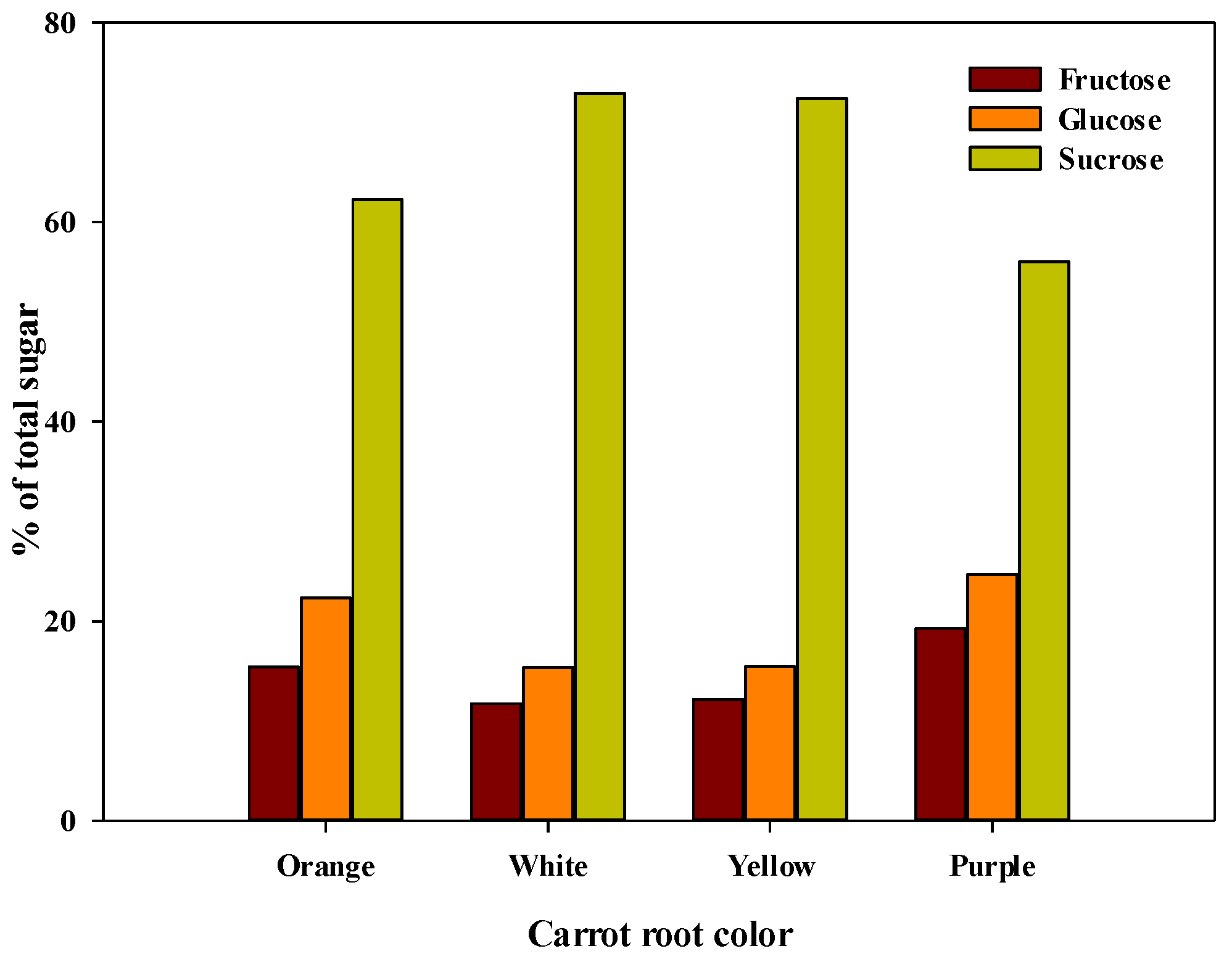
3.3.2. Variation in Sugar Content and Total Sweetness Index
3.4. Selection of Nutritionally Valuable Genetic Resources
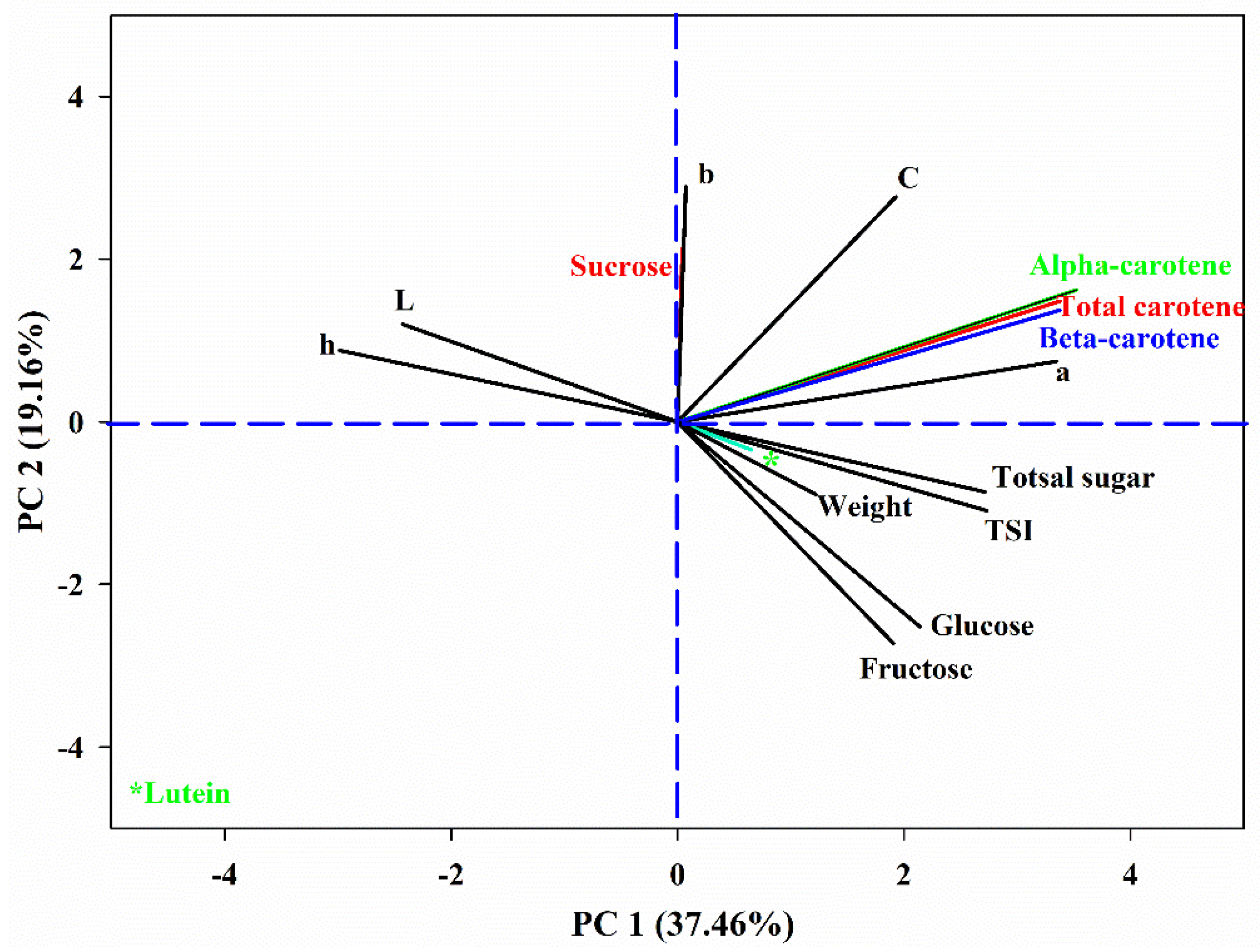
3.5. Correlation and Principal Component Analysis (PCA) and Hierarchical Cluster Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- FAO (Food and Agriculture Organization). Agricultural Statistical Database for 2019. Available online: http://www.fao.org/faostat/en/#data/QCL (accessed on 29 July 2021).
- Ahmad, T.; Cawood, M.; Iqbal, Q.; Arino, A.; Batool, A.; Tariq, R.M.S.; Azam, M.; Akhtar, S. Phytochemicals in Daucus carota and their health benefits-review article. Foods 2019, 8, 424. [Google Scholar] [CrossRef] [PubMed]
- Kulczynski, B.; Gramza-Michalowska, A.; Kobus-Cisowska, J.; Kmiecik, D. The role of carotenoids in the prevention and treatment of cardiovascular disease-current state of knowledge. J. Funct. Foods 2017, 38, 45–65. [Google Scholar] [CrossRef]
- Smolen, S.; Sady, W. The effect of various nitrogen fertilization and foliar nutrition regimes on the concentrations of sugar, carotenoids and phenolic compounds in carrot (Daucus carota L.). Sci. Hortic. 2009, 120, 315–324. [Google Scholar] [CrossRef]
- Soltoft, M.; Bysted, A.; Madsen, K.H.; Mark, A.B.; Bugel, S.G.; Nielsen, J.; Knuthsen, P. Effects of organic and conventional growth systems on the content of carotenoids in carrot roots, and on intake and plasma status of carotenoids in humans. J. Sci. Food Agric. 2011, 91, 767–775. [Google Scholar] [CrossRef]
- Jourdan, M.; Gagne, S.; Dubois-Laurent, C.; Maghraoui, M.; Huet, S.; Suel, A.; Hamama, L.; Briard, M.; Peltier, D.; Geoffriau, E. Carotenoid content and root color of cultivated carrot: A candidate-gene association study using an original broad unstructured population. PLoS ONE 2015, 10, e0116674. [Google Scholar] [CrossRef][Green Version]
- Clugston, R.D. Carotenoids and fatty liver disease: Current knowledge and research gaps. BBA-Mol. Cell Biol. Lipids 2020, 1865, 158597. [Google Scholar] [CrossRef] [PubMed]
- Eggersdorfer, M.; Wyss, A. Carotenoids in human nutrition and health. Arch. Biochem. Biophys. 2018, 652, 18–26. [Google Scholar] [CrossRef]
- Britton, G. Carotenoid research: History and new perspectives for chemistry in biological systems. BBA-Mol. Cell Biol. Lipids 2020, 1865, 158699. [Google Scholar] [CrossRef]
- Christensen, K.; Lawler, T. Dietary carotenoids and non-alcoholic fatty liver disease among US adults, NHANES 2003−2014. Nutrients 2019, 11, 1101. [Google Scholar] [CrossRef]
- Elvira-Torales, L.I.; García-Alonso, J.; Periago-Castón, M.J. Nutritional importance of carotenoids and their effect on liver health: A review. Antioxidants 2019, 8, 229. [Google Scholar] [CrossRef]
- Rowles III, J.L.; Erdman, J.W., Jr. Carotenoids and their role in cancer prevention. BBA-Mol. Cell Biol. Lipids 2020, 1865, 158613. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, H.; Yanai, H.; Ito, K.; Tomono, Y.; Koikeda, T.; Tsukahara, H.; Tada, N. Administration of natural astaxanthin increases serum HDL-cholesterol and adiponectin in subjects with mild hyperlipidemia. Atherosclerosis 2010, 209, 520–523. [Google Scholar] [CrossRef] [PubMed]
- Milani, A.; Basirnejad, M.; Shahbazi, S.; Bolhassani, A. Carotenoids: Biochemistry, pharmacology and treatment. Br. J. Pharmacol. 2017, 174, 1290–1324. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.J. A possible role for lutein and zeaxanthin in cognitive function in the elderly. Am. J. Clin. Nutr. 2012, 96, 1161S–1165S. [Google Scholar] [CrossRef]
- D’Odorico, A.; Martines, D.; Kiechl, S.; Egger, G.; Oberhollenzer, F.; Bonvicini, P.; Sturniolo, G.C.; Naccarato, R.; Willeit, J. High plasma levels of alpha- and beta-carotene are associated with a lower risk of atherosclerosis: Results from the Bruneck study. Atherosclerosis 2000, 153, 231–239. [Google Scholar] [CrossRef]
- Auerswald, H.; Schwarz, D.; Kornelson, C.; Krumbein, A.; Bruckner, B. Sensory analysis, sugar and acid content of tomato at different EC values of the nutrient solution. Sci. Hortic. 1999, 82, 227–242. [Google Scholar] [CrossRef]
- VandenLangenberg, K.M.; Bethke, P.C.; Nienhuis, J. Patterns of fructose, glucose, and sucrose accumulation in snap and dry bean (Phaseolus vulgaris) pods. HortScience 2012, 47, 874–878. [Google Scholar] [CrossRef]
- Li, B.W.; Andrews, K.W.; Pehrsson, P.R. Individual sugars, soluble, and insoluble dietary fiber contents of 70 high consumption foods. J. Food Compos. Anal. 2002, 15, 715–723. [Google Scholar] [CrossRef]
- Bonasia, A.; Conversa, G.; Lazzizera, C.; Gambacorta, G.; Elia, A. Morpho-biometrical, nutritional and phytochemical characterization of carrot landraces from Puglia region (Southern Italy). Sustainability 2021, 13, 3940. [Google Scholar] [CrossRef]
- Tsukakoshi, Y.; Naito, S.; Ishida, N.; Yasui, A. Variation in moisture, total sugar, and carotene content of Japanese carrots: Use in sample size determination. J. Food Compos. Anal. 2009, 22, 373–380. [Google Scholar] [CrossRef]
- Greene, S.L.; Gritsenko, M.; Vandewark, G. Relating morphologic and RAPD marker variation to collecting site environment in wild population of red clover (Trifolium pretense L.). Genet. Resour. Crop Evol. 2004, 51, 643–653. [Google Scholar] [CrossRef]
- Luitel, B.P.; Ro, N.-Y.; Ko, H.-C.; Sung, J.-S.; Rhee, J.-H.; Hur, O.-S. Phenotypic variation in a germplasm collection of pepper (Capsicum chinense Jacq.) from Korea. J. Crop Sci. Biotechnol. 2018, 21, 499–506. [Google Scholar] [CrossRef]
- Chen, C.; Ma, J.; Ma, J.; Ma, W.; Yang, J. Analysis of main agronomic traits in different varieties of carrots. J. Phys. 2020, 1549, 032053. [Google Scholar] [CrossRef]
- Tabor, G.; Yesuf, M.; Haile, M.; Kebede, G.; Tilahun, S. Performance of some Asian carrot (Daucus carota L. spp. sativa Hoffm.) cultivars under Ethiopian conditions: Carrot and seed yields. Sci. Hortic. 2016, 207, 176–182. [Google Scholar] [CrossRef]
- Baranski, R.; Allender, C.; Klimek-Chodacka, M. Towards better testing and more nutritious carrots: Carotenoid and sugar content variation in carrot genetic resources. Food Res. Int. 2012, 47, 182–187. [Google Scholar] [CrossRef]
- Bolton, A.; Simon, P. Variation for salinity tolerance during seed germination in diverse carrot [Daucus carota (L.)] germplasm. HortScience 2019, 54, 38–44. [Google Scholar] [CrossRef]
- Bozalan, N.K.; Karadeniz, F. Carotenoid profile, total phenolic content, and antioxidant activity of carrots. Int. J. Food Prop. 2011, 14, 1060–1068. [Google Scholar] [CrossRef]
- Kenny, O.; O’Beirne, D. Antioxidant phytochemicals in fresh-cut carrot disk as affected by peeling method. Postharvest Biol. Technol. 2010, 58, 247–253. [Google Scholar] [CrossRef]
- Philippe, D.; Dominique, D.; Sophie, B.-M.; Maxime, D.; Franck, V.; Jacques, D.; Francois, K. Spatial variation of root yield within cultivated carrot fields is strongly impacted by plant spacing. Sci. Hortic. 2018, 241, 29–40. [Google Scholar] [CrossRef]
- Que, F.; Hou, X.-L.; Wang, G.-L.; Xu, Z.-S.; Tan, G.-F.; Li, T.; Wang, Y.-H.; Khadr, A.; Xiong, A.-S. Advances in research on the carrot, an important root vegetable in the Apiaceae family. Hortic. Res. 2019, 6, 69. [Google Scholar] [CrossRef]
- Rodriguez-Concepcion, M.; Stange, C. Biosynthesis of carotenoids in carrot: An underground story comes to light. Arch. Biochem. Biophys. 2013, 539, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Stinco, C.M.; Szczepanska, J.; Marszalek, K.; Pinto, C.A.; Inacio, R.S.; Mapelli-Brahm, P.; Barba, F.J.; Lorenzo, J.M.; Saraiva, J.A.; Melendez-Martinez, A.J. Effect of high-pressure processing on carotenoid profile, color, microbial and enzymatic stability of cloudy carrot juice. Food Chem. 2019, 299, 125112. [Google Scholar] [CrossRef] [PubMed]
- Yoo, K.S.; Bang, H.; Pike, L.; Patil, B.S.; Lee, E.J. Comparing carotene, anthocyanins, and terpenoid concentrations in selected carrot lines of different colors. Hortic. Environ. Biotechnol. 2020, 61, 385–393. [Google Scholar] [CrossRef]
- Bhandari, S.R.; Lee, J.G. Ripening-dependent changes in antioxidants, color attributes, and antioxidant activities of seven tomato (Solanum lycopersisum L.) cultivars. J. Anal. Method Chem. 2016, 2016, 5498618. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, S.R.; Choi, C.S.; Rhee, J.; Jo, J.S.; Shin, Y.K.; Song, J.W.; Lee, J.G. Seasonal variation in agronomic characteristics and sugar content of cabbage genotypes. Chilean J. Agric. Res. 2021, 81, 80–91. [Google Scholar] [CrossRef]
- Magwaza, L.S.; Opara, U.L. Analytical methods for determination of sugars and sweetness of horticultural crops- A review. Sci. Hortic. 2015, 184, 179–192. [Google Scholar] [CrossRef]
- Cavagnaro, P.F.; Chung, S.-M.; Manin, S.; Yildiz, M.; Ali, A.; Alessandro, M.S.; Iorizzo, M.; Senalik, D.A.; Simon, P.W. Microsatellite isolation and marker development in carrot genomic distribution, linkage mapping, genetic diversity analysis and marker transferability across Apiaceae. BMC Genomics 2011, 12, 386. [Google Scholar] [CrossRef] [PubMed]
- Ellison, S.; Senalik, D.; Bostan, H.; Iorizzo, M.; Simon, P. Fine mapping, transcriptome analysis, and marker development for Y2, the gene that conditions b-carotene accumulation in carrot (Daucus carota L.). G3 2017, 7, 2665–2675. [Google Scholar] [CrossRef] [PubMed]
- Bannoud, F.; Ellison, S.; Paolinelli, M.; Horejsi, T.; Senalik, D.; Fanzone, M.; Iorizzo, M.; Simon, P.W.; Cavagnaro, P.F. Dissecting the genetic control of root and leaf tissue-specific anthocyanin pigmentation in carrot (Daucus carota L.). Theor. Appl. Genet. 2019, 132, 2485–2507. [Google Scholar] [CrossRef] [PubMed]
- Bannoud, F.; Carvajal, S.; Ellison, S.; Senalik, D.; Gomez Talquenca, S.; Iorizzo, M.; Simon, P.W.; Cavagnaro, P.F. Genetic and transcription profile analysis of tissue-specific anthocyanin pigmentation in carrot root phloem. Genes 2021, 12, 1464. [Google Scholar] [CrossRef]
- Hooks, T.; Niu, G.; Masabni, J.; Sun, Y.; Ganjegunte, G. Performance and phytochemical content of 22 pomegranate (Punica granatum) varieties. HortScience 2021, 56, 217–225. [Google Scholar] [CrossRef]
- Macko-Podgorni, A.; Stelmach, K.; Kwolek, K.; Machaj, G.; Ellison, S.; Senalik, D.A.; Simon, P.W.; Grezebelus, D. Mining for candidate genes controlling secondary growth of the carrot storage root. Int. J. Mol. Sci. 2020, 21, 4263. [Google Scholar] [CrossRef]
- Turner, S.D.; Ellison, S.L.; Senalik, D.A.; Simon, P.W.; Spalding, E.P.; Miller, N.D. An automated image analysis pipeline enables genetic studies of shoot and root morphology in carrot (Daucus carota L.). Front. Plant Sci. 2018, 9, 1703. [Google Scholar] [CrossRef] [PubMed]
- Pathare, P.B.; Opara, U.L.; Al-Said, F.A.J. Colour measurement and analysis in fresh and processed foods: A review. Food Bioprocess Technol. 2013, 6, 36–60. [Google Scholar] [CrossRef]
- Nisha, P.; Singhal, R.S.; Pandit, A.B. Kinetic modelling of colour degradation in tomato puree (Lycopersicon esculentum L.). Food Bioprocess Technol. 2011, 4, 781–787. [Google Scholar] [CrossRef]
- Ma, J.; Xu, Z.; Tan, G.; Wang, F.; Xiong, A. Distinct transcription profile of genes involved in carotenoid biosynthesis among six different color carrot (Daucus carota L.) cultivars. Acta Biochim. Biophys. Sin. 2017, 49, 817–826. [Google Scholar] [CrossRef]
- Arscott, S.A.; Tanumihardjo, S.A. Carrots of many colors provide basic nutrition and bioavailable phtyochemicals acting as a functional food. Compr. Rev. Food Sci. Food Saf. 2010, 9, 223–239. [Google Scholar] [CrossRef]
- Perrin, F.; Hartmann, L.; Dubois-Laurent, C.; Welsch, R.; Huet, S.; Hamama, L.; Briard, M.; Peltier, D.; Gange, S.; Geoffriau, E. Carotenoid gene expression explains the difference of carotenoid accumulation in carrot root tissues. Planta 2017, 245, 737–747. [Google Scholar] [CrossRef]
- Iorizzo, M.; Ellison, S.; Senalik, D.; Zeng, P.; Satapoomin, P.; Huang, J.; Bowman, M.; Iovene, M.; Sanseverino, W.; Cavagnaro, P.; et al. A high-quality carrot genome assembly provides new insights into carotenoid accumulation and asterid genome evolution. Nat. Genet. 2016, 48, 657–666. [Google Scholar] [CrossRef]
- Singh, D.P.; Beloy, J.; McInerney, J.K.; Day, L. Impact of boron, calcium and genetic factors on vitamin C, carotenoids, phenolic acids, anthocyanins and antioxidant capacity of carrot (Daucus carota). Food Chem. 2012, 132, 1161–1170. [Google Scholar] [CrossRef]
- Bufler, G. Accumulation and degradation of starch in carrot roots. Sci. Hortic. 2013, 150, 251–258. [Google Scholar] [CrossRef]
- Clausen, M.R.; Edelenbos, M.; Bertram, H.C. Mapping the variation of the carrot metabolome using 1H NMR spectroscopy and consensus PCA. J. Agric. Food Chem. 2014, 62, 4392–4398. [Google Scholar] [CrossRef] [PubMed]
- Benamor, J.; Mezghani, N.; Periago, M.J.; Navarro-Gonzalez, I.; Elvira-Torales, L.I.; Mezghani, N.; Ouakrim, Y.; Tarchoun, N. Variations in the sugars and antioxidant compounds related to root color in Tunisian carrot (Daucus carota subsp. sativus) landraces. Ital. J. Food Sci. 2020, 32, 654–673. [Google Scholar]
- Yusuf, E.; Tkacz, K.; Turkiewics, I.P.; Wojdylo, A.; Nowicka, P. Analysis of chemical compounds’ content in different varieties of carrots, including qualification and quantification of sugars, organic acids, minerals, and bioactive compounds by UPLC. Eur. Food Res. Technol. 2021, 247, 3053–3062. [Google Scholar] [CrossRef]
- Nookaraju, A.; Upadhyaya, C.P.; Pandey, S.K.; Young, K.E.; Hong, S.J.; Park, S.K.; Park, S.W. Molecular approaches for enhancing sweetness in fruits and vegetables. Sci. Hortic. 2010, 127, 1–15. [Google Scholar] [CrossRef]
- Bernstein, P.S.; Li, B.; Vachali, P.P.; Gorusupudi, A.; Shyam, R.; Henriksen, B.S.; Nolan, J.M. Lutein, zeaxanthin, and meso-zeaxanthin: The basic and clinical science underlying carotenoid-based nutritional interventions against ocular disease. Prog. Retin. Eye Res. 2016, 50, 34–66. [Google Scholar] [CrossRef]
- Wang, Y.L.; Cui, R.; Xiao, Y.Y.; Fang, J.M.; Xu, Q. Effect of carotene and lycopene on the risk of prostate cancer: A systematic review and dose-response meta-analysis of observational studies. PLoS ONE 2015, 10, e0137427. [Google Scholar] [CrossRef]
- Sommer, A.; Vyas, K.S. A global clinical view on vitamin A and carotenoids. Am. J. Clin. Nutr. 2012, 96, 1204S–1206S. [Google Scholar] [CrossRef]

| Parameters | Classification | Frequency | Percentage (%) |
|---|---|---|---|
| Leaf Posture | Standing upright | 73 | 40.6 |
| Standing slightly | 106 | 58.9 | |
| Lying down | 1 | 0.6 | |
| Leaf color | Dark green | 20 | 11.1 |
| Green | 109 | 60.6 | |
| Non-green | 51 | 28.3 | |
| Flowering | Weak | 16 | 8.9 |
| Medium | 37 | 20.6 | |
| Strong | 127 | 70.6 | |
| The leaf emerging area | Narrow | 51 | 28.3 |
| Medium | 83 | 46.1 | |
| Wide | 46 | 25.6 | |
| Petiole anthocyanin coloring | No | 121 | 67.2 |
| Yes | 59 | 32.8 | |
| Shoulder shape | Flat | 53 | 29.4 |
| Flat-round | 86 | 47.8 | |
| Round | 17 | 9.4 | |
| Round-flat | 20 | 11.1 | |
| Sharp | 4 | 2.2 | |
| Tip shape | Blunt | 73 | 40.6 |
| Little sharp | 74 | 41.1 | |
| Sharp | 33 | 18.3 | |
| External color (Internal color) | White | 16 (16) | 8.9 (8.9) |
| Yellow | 14 (18) | 7.8 (10.0) | |
| Orange | 142 (146) | 78.9 (81.1) | |
| Purple | 8 (0) | 4.4 (0.0) | |
| Carrot shape (lengthwise) | Doran type | 18 | 10.0 |
| Middle inverted triangle (1) | 57 | 31.7 | |
| Narrow inverted triangle (2) | 55 | 30.6 | |
| Middle of 1 and 2 | 42 | 23.3 | |
| Narrow long ellipse | 8 | 4.4 | |
| Muscle skin condition (Surface curvature) | None | 42 | 23.3 |
| Weak | 49 | 27.2 | |
| Moderate | 53 | 29.4 | |
| Severe | 16 | 8.9 | |
| Very severe | 20 | 11.1 | |
| Curvature depth | Missing or very small | 91 | 50.6 |
| Small | 45 | 25.0 | |
| Middle | 31 | 17.2 | |
| Many | 13 | 7.2 | |
| Shoulder epidermis anthocyanin pigmentation | No | 108 | 60.0 |
| Yes | 72 | 40.0 | |
| Green area in the shoulder epidermis | None | 71 | 39.4 |
| Small | 78 | 43.3 | |
| Middle | 28 | 15.6 | |
| Many | 3 | 1.7 | |
| Intensity of the outer color of the muscle | Light | 53 | 29.4 |
| Medium | 101 | 56.1 | |
| Dark green | 26 | 14.4 |
| Parameter | Descriptive | Root Color | Overall | ||||
|---|---|---|---|---|---|---|---|
| Orange (n = 142) | Purple (n = 8) | White (n = 16) | Yellow (n = 14) | ||||
| Leaf | Length (cm) | Range | 30.0–76.0 | 65.0–112.0 | 42.0–70.0 | 40.0–94.0 | 30.0–112.0 |
| Average | 53.3 a | 86.8 c | 57.3 ab | 63.0 b | 55.9 | ||
| Root | Weight (g) | Range | 24.2–223.0 | 63.2–223.1 | 22.6–128.8 | 43.3–133.9 | 22.6–223.1 |
| Average | 107.2 a | 140.0 b | 77.4 a | 88.7 a | 104.6 | ||
| Length (cm) | Range | 8.1–22.5 | 9.1–21.5 | 9.2–17.5 | 10.1–18.7 | 8.1–22.5 | |
| Average | 14.3 a | 13.0 a | 13.89 a | 13.64 a | 14.2 | ||
| Diameter (cm) | Range | 2.0–5.7 | 3.5–7.7 | 1.8–5.5 | 2.90–4.97 | 1.8–7.7 | |
| Average | 3.9 a | 5.5 b | 3.6 a | 3.84 a | 4.0 | ||
| Parameter | Descriptive | Root Color | |||
|---|---|---|---|---|---|
| Orange (n = 142) | Purple (n = 8) | White (n = 16) | Yellow (n = 14) | ||
| L | Range | 44.5(45.1)–59.1(70.0) | 26.3(60.6)–50.9(71.1) | 52.3(50.7)–68.6(78.3) | 55.9(61.2)–80.5(72.8) |
| Average | 52.8 B(53.4 a) | 40.6 A(65.4 b) | 63.2 C(68.7 b) | 62.4 C(67.0 b) | |
| a | Range | 13.4(0.6)–61.1(38.0) | 13.2(0.7)–24.2(6.5) | 2.3(0.6)–7.4(32.1) | 1.5(0.5)–8.9(6.4) |
| Average | 25.0 C(25.3 b) | 18.5 B(2.8 a) | 5.4 A(3.5 a) | 5.3 A(2.1 a) | |
| b | Range | 29.6(35.0)–44.6(64.0) | 2.6(43.4)–32.5(54.7) | 25.0(26.3)–42.2(52.2) | 33.7(42.2)–44.4(65.8) |
| Average | 36.9 B(48.0 b) | 14.7 A(47.9 b) | 35.5 B(38.4 a) | 40.3 C(54.7 c) | |
| C | Range | 37.1(36.2)–53.7(65.6) | 18.8(43.9)–35.6(54.1) | 25.1(26.4)–42.6(52.3) | 34.1(42.3)–44.8(65.8) |
| Average | 44.6 D(54.2 c) | 25.5 A(48.1 b) | 36.3 B(38.7 a) | 40.8 C(54.9 c) | |
| h | Range | 48.9(54.7)–69.0(89.6) | 6.0(49.1)–64.4(89.2) | 77.5(84.2)–85.8(90.8) | 66.4(83.0)–88.2(90.4) |
| Average | 56.4 B(62.7 a) | 38.6 A(81.7 b) | 81.5 C(87.3 c) | 81.2 C(87.9 c) | |
| Carotenoid (mg 100 g−1) | Descriptive | Root Color | Overall (n = 180) | |||
|---|---|---|---|---|---|---|
| Orange (n = 142) | Purple (n = 8) | White (n = 16) | Yellow (n = 14) | |||
| Lutein | Range | 4.87–23.48 | 12.10–17.65 | 0.91–12.79 | 4.31–23.47 | 0.91–23.48 |
| Average | 11.82 b | 14.81 c | 4.95 a | 13.04 bc | 11.44 | |
| α-carotene | Range | 12.40–73.32 | 0.00–8.93 | 0.00–1.13 | 0.00–0.98 | 0.00–73.23 |
| Average | 35.75 b | 2.40 a | 0.13 a | 0.07 a | 28.33 | |
| β-carotene | Range | 12.65–71.10 | 0.30–12.08 | 0.00–0.00 | 0.11–1.79 | 0.00–71.10 |
| Average | 33.35 b | 3.56 a | 0.00 a | 0.88 a | 26.54 | |
| Total carotenoid | Range | 42.29–142.42 | 12.41–38.22 | 0.91–13.18 | 5.00–25.26 | 0.91–142.42 |
| Average | 80.93 c | 20.77 b | 5.09 a | 13.99 ab | 66.31 | |
| Parameter | Descriptive | Root Color | Overall (n = 180) | |||
|---|---|---|---|---|---|---|
| Orange (n = 142) | Purple (n = 8) | White (n = 16) | Yellow (n = 14) | |||
| Fructose (mg g−1) | Range | 10.3–98.4 | 11.91–117.61 | 5.4–76.6 | 7.9–72.1 | 5.4–117.6 |
| Average | 47.7 b | 64.6 c | 31.2 a | 34.6 ab | 46.0 | |
| Glucose (mg g−1) | Range | 13.4–144.9 | 14.92–150.25 | 5.8–112.2 | 10.6–97.0 | 5.8–150.2 |
| Average | 69.2 b | 82.9 b | 40.9 a | 44.1 a | 65.4 | |
| Sucrose (mg g−1) | Range | 65.6–284.5 | 84.4–259.4 | 74.6–303.2 | 115.0–244.2 | 65.6–303.2 |
| Average | 191.7 a | 174.0 a | 193.3 a | 195.8 a | 191.4 | |
| Total sugar (mg g−1) | Range | 201.0–381.0 | 259.8–356.7 | 130.7–333.7 | 159.5–332.0 | 130.7–381.0 |
| Average | 308.7 b | 321.5 b | 265.4 a | 274.4 a | 302.7 | |
| TSI | Range | 206.0–388.0 | 262.2–379.4 | 132.3–345.9 | 164.4–344.8 | 132.3–388.0 |
| Average | 315.9 b | 333.9 b | 271.2 a | 281.1 a | 310.1 | |
| S.N. | IT No. | Name | Carotenoid Content (mg 100 g−1) | Free Sugar Content (mg g−1) | TSI | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Lu | α-C | β-C | Total | Fru | Glu | Suc | Total | ||||
| 1 | 100527 | Yeoleum 5 Chon | 12.7 ± 0.6 | 60.3 ± 7.4 | 44.8 ± 4.3 | 117.7 ± 11.5 | 51.3 ± 6.2 | 61.4 ± 5.6 | 221.5 ± 5.5 | 334.2 ± 6.9 | 345.1 ± 8.6 |
| 2 | 100541 | Yangchun 5 Chun | 8.2 ± 1.2 | 69.7 ± 4.2 | 55.8 ± 6.0 | 133.8 ± 8.6 | 51.4 ± 1.2 | 71.0 ± 5.3 | 243.6 ± 7.4 | 366.0 ± 6.7 | 374.6 ± 6.1 |
| 3 | 200315 | NPL-KIK-1996-5071 | 11.6 ± 0.7 | 49.6 ± 2.7 | 43.5 ± 1.7 | 104.7 ± 1.6 | 11.7 ± 1.4 | 18.4 ± 2.7 | 232.1 ± 6.2 | 262.2 ± 5.8 | 263.6 ± 6.2 |
| 4 | 203364 | Local-Bukhara | 21.6 ± 1.5 | 12.4 ± 0.9 | 18.3 ± 1.0 | 52.2 ± 3.4 | 35.0 ± 0.6 | 52.1 ± 5.0 | 114.0 ± 9.2 | 201.0 ± 14.3 | 206.0 ± 13.3 |
| 5 | 203368 | Mirzoi Krasnaya | 23.5 ± 0.8 | 15.5 ± 0.9 | 21.4 ± 3.1 | 60.4 ± 4.5 | 36.5 ± 3.2 | 58.8 ± 4.4 | 166.2 ± 14.7 | 261.5 ± 19.5 | 265.6 ± 18.9 |
| 6 | 204087 | A130-1-8-116 | 7.5 ± 1.4 | 46.7 ± 3.1 | 34.1 ± 4.4 | 88.4 ± 5.0 | 43.9 ± 5.3 | 58.2 ± 13.0 | 253.1 ± 7.4 | 355.2 ± 11.0 | 363.2 ± 10.7 |
| 7 | 204229 | Gonsenheimer Treib | 11.9 ± 0.4 | 55.0 ± 5.3 | 47.4 ± 0.7 | 114.3 ± 4.8 | 74.9 ± 1.5 | 89.4 ± 5.5 | 140.8 ± 1.4 | 305.1 ± 7.4 | 321.1 ± 7.0 |
| 8 | 210170 | Inari 5 Sun | 12.4 ± 1.3 | 51.7 ± 3.7 | 41.7 ± 2.0 | 105.9 ± 4.0 | 59.5 ± 3.7 | 101.1 ± 16.5 | 192.1 ± 10.7 | 352.7 ± 8.9 | 358.1 ± 6.5 |
| 9 | 210171 | Koushin 5 Sun | 10.0 ± 1.6 | 60.0 ± 1.0 | 33.7 ± 2.9 | 104.7 ± 3.8 | 68.8 ± 2.5 | 102.7 ± 7.8 | 179.0 ± 10.9 | 350.4 ± 0.9 | 360.2 ± 1.3 |
| 10 | 210175 | Youmei 5 Sun | 8.6 ± 1.5 | 73.3 ± 4.1 | 45.1 ± 0.4 | 127.1 ± 3.1 | 66.0 ± 11.2 | 87.7 ± 21.2 | 221.3 ± 27.5 | 375.0 ± 19.1 | 386.9 ± 19.7 |
| 11 | 220541 | NPL-GYS-2004-14 | 7.1 ± 0.6 | 60.5 ± 4.5 | 39.8 ± 2.3 | 107.3 ± 3.9 | 30.0 ± 6.0 | 46.8 ± 10.4 | 236.9 ± 7.9 | 313.7 ± 17.5 | 317.5 ± 17.9 |
| 12 | 261793 | Moskovskaya Zimnaya A-515 | 9.2 ± 0.9 | 53.1 ± 1.6 | 44.2 ± 3.3 | 106.5 ± 4.9 | 64.5 ± 6.2 | 92.5 ± 10.2 | 183.7 ± 17.5 | 340.7 ± 4.2 | 350.8 ± 5.3 |
| 13 | 274037 | WIR2313 | 18.6 ± 2.2 | 28.8 ± 1.2 | 31.9 ± 2.9 | 79.3 ± 2.8 | 40.4 ± 3.5 | 68.0 ± 7.4 | 262.7 ± 13.5 | 371.1 ± 6.9 | 374.9 ± 8.4 |
| 14 | 274184 | UZB-KJG-2006-61 | 14.5 ± 0.5 | 16.4 ± 0.8 | 13.9 ± 1.4 | 44.9 ± 2.4 | 36.0 ± 8.4 | 47.0 ± 1.9 | 284.5 ± 14.3 | 367.5 ± 4.7 | 374.2 ± 2.3 |
| 15 | 276366 | Shantane | 22.5 ± 0.3 | 13.8 ± 1.5 | 12.7 ± 2.3 | 49.0 ± 3.0 | 17.8 ± 2.4 | 20.6 ± 2.1 | 239.2 ± 1.6 | 277.6 ± 4.9 | 281.6 ± 5.8 |
| 16 | 283365 | Zavyalovskaya Mestnaya | 14.3 ± 2.2 | 46.2 ± 3.7 | 50.7 ± 3.5 | 111.3 ± 6.7 | 27.9 ± 2.4 | 48.9 ± 4.3 | 266.7 ± 10.0 | 343.5 ± 5.2 | 345.7 ± 4.9 |
| 17 | 288816 | NIIOKH 336 | 16.7 ± 0.9 | 52.2 ± 3.2 | 48.8 ± 2.8 | 117.6 ± 5.9 | 56.1 ± 5.3 | 78.7 ± 2.5 | 173.1 ± 12.2 | 307.9 ± 18.2 | 317.0 ± 20.7 |
| 18 | 288869 | Corozon De Buey | 14.7 ± 1.2 | 49.4 ± 1.0 | 46.5 ± 2.7 | 110.7 ± 3.3 | 45.0 ± 1.9 | 59.8 ± 4.3 | 180.2 ± 4.5 | 285.0 ± 1.5 | 293.1 ± 3.3 |
| 19 | 288909 | Nantskaya | 10.8 ± 1.1 | 34.7 ± 2.7 | 40.4 ± 0.6 | 85.9 ± 2.3 | 56.3 ± 4.0 | 90.3 ± 8.0 | 211.0 ± 13.3 | 357.6 ± 13.8 | 364.1 ± 11.0 |
| 20 | 300048 | Mirzoi krasniy | 20.4 ± 3.1 | 20.5 ± 2.3 | 26.2 ± 3.1 | 67.0 ± 3.5 | 21.9 ± 2.2 | 33.6 ± 2.9 | 208.4 ± 12.2 | 263.8 ± 17.1 | 266.8 ± 17.5 |
| 21 | 301847 | PI 288459 | 17.2 ± 0.8 | 8.9 ± 1.5 | 12.1 ± 2.2 | 38.2 ± 3.5 | 117.6 ± 8.5 | 150.2 ± 20.8 | 88.8 ± 18.7 | 356.7 ± 11.2 | 379.4 ± 9.7 |
| 22 | 301938 | Kuroda | 8.2 ± 0.8 | 68.4 ± 2.6 | 43.3 ± 2.5 | 119.9 ± 5.7 | 39.4 ± 4.2 | 69.6 ± 16.5 | 226.7 ± 20.6 | 335.8 ± 4.2 | 338.8 ± 6.4 |
| 23 | 301947 | Piliwsky Zbior | 13.4 ± 1.0 | 45.6 ± 0.9 | 50.2 ± 4.4 | 109.2 ± 4.9 | 83.4 ± 10.3 | 111.5 ± 10.1 | 149.7 ± 12.3 | 344.6 ± 7.9 | 359.6 ± 10.2 |
| 24 | 302276 | Local Nevinnomysskaya | 15.5 ± 0.5 | 59.5 ± 3.1 | 51.2 ± 4.2 | 126.2 ± 7.5 | 40.8 ± 7.4 | 57.5 ± 9.1 | 189.3 ± 11.1 | 287.6 ± 5.1 | 294.2 ± 6.8 |
| 25 | 325075 | PI 341208 | 10.4 ± 0.7 | 35.0 ± 4.7 | 35.3 ± 1.1 | 80.6 ± 5.5 | 81.4 ± 7.3 | 126.0 ± 4.8 | 169.6 ± 7.5 | 377.0 ± 3.6 | 387.5 ± 6.6 |
| 26 | 325079 | PI 502347 | 19.3 ± 1.4 | 35.4 ± 2.4 | 30.9 ± 2.0 | 85.6 ± 2.8 | 35.0 ± 3.7 | 63.4 ± 5.0 | 180.8 ± 10.2 | 279.2 ± 7.9 | 281.4 ± 7.7 |
| 27 | 325093 | PI 652216 | 11.6 ± 0.5 | 58.2 ± 5.0 | 36.2 ± 1.5 | 106.0 ± 3.7 | 42.6 ± 2.5 | 45.4 ± 3.6 | 252.3 ± 9.5 | 340.4 ± 12.1 | 350.8 ± 11.2 |
| 28 | 331120 | PI 515996 | 14.5 ± 0.9 | 56.8 ± 2.5 | 71.1 ± 3.1 | 142.4 ± 3.9 | 31.9 ± 3.8 | 54.6 ± 6.6 | 214.1 ± 9.3 | 300.5 ± 12.8 | 303.4 ± 12.8 |
| 29 | 331126 | Pien Kan Hung | 6.3 ± 1.0 | 50.1 ± 1.5 | 38.7 ± 2.8 | 95.1 ± 3.3 | 53.0 ± 6.9 | 81.2 ± 2.6 | 246.8 ± 15.4 | 381.0 ± 9.3 | 388.0 ± 5.6 |
| 30 | 910240 | Fertodi Voros | 9.1 ± 0.3 | 57.6 ± 2.6 | 48.6 ± 2.9 | 115.3 ± 1.1 | 15.3 ± 1.0 | 20.3 ± 2.0 | 242.5 ± 6.4 | 278.1 ± 8.0 | 280.9 ± 7.6 |
| Parameter | a | b | C | h | Lutein | α-Carotene | β-Carotene | T Carotenoid | Fructose | Glucose | Sucrose |
|---|---|---|---|---|---|---|---|---|---|---|---|
| L | −0.578 ** | 0.506 ** | 0.069 | 0.853 ** | −0.211 ** | −0.354 ** | −0.348 ** | −0.384 ** | −0.198 ** | −0.209 ** | −0.086 |
| a | 0.049 | 0.504 ** | −0.734 ** | 0.117 | 0.723 ** | 0.722 ** | 0.750 ** | 0.190 * | 0.245 ** | 0.091 | |
| b | 0.802 ** | 0.484 ** | −0.017 | 0.223 ** | 0.232 ** | 0.229 ** | −0.156 * | −0.126 | 0.099 | ||
| C | −0.046 | 0.064 | 0.598 ** | 0.592 ** | 0.614 ** | −0.02 | 0.029 | 0.131 | |||
| h | −0.193 ** | −0.543 ** | −0.545 ** | −0.578 ** | −0.234 ** | −0.278 ** | −0.034 | ||||
| Lutein | −0.046 | 0.108 | 0.153 * | 0.071 | 0.069 | −0.06 | |||||
| α-carotene | 0.886 ** | 0.958 ** | 0.168 * | 0.233 ** | 0.116 | ||||||
| β-carotene | 0.969 ** | 0.203 ** | 0.291 ** | 0.023 | |||||||
| T carotenoid | 0.197 ** | 0.273 ** | 0.067 | ||||||||
| Fructose | 0.953 ** | −0.682 ** | |||||||||
| Glucose | −0.660 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhandari, S.R.; Rhee, J.; Choi, C.S.; Jo, J.S.; Shin, Y.K.; Song, J.W.; Kim, S.-H.; Lee, J.G. Morphological and Biochemical Variation in Carrot Genetic Resources Grown under Open Field Conditions: The Selection of Functional Genotypes for a Breeding Program. Agronomy 2022, 12, 553. https://doi.org/10.3390/agronomy12030553
Bhandari SR, Rhee J, Choi CS, Jo JS, Shin YK, Song JW, Kim S-H, Lee JG. Morphological and Biochemical Variation in Carrot Genetic Resources Grown under Open Field Conditions: The Selection of Functional Genotypes for a Breeding Program. Agronomy. 2022; 12(3):553. https://doi.org/10.3390/agronomy12030553
Chicago/Turabian StyleBhandari, Shiva Ram, Juhee Rhee, Chang Sun Choi, Jung Su Jo, Yu Kyeong Shin, Jae Woo Song, Seong-Hoon Kim, and Jun Gu Lee. 2022. "Morphological and Biochemical Variation in Carrot Genetic Resources Grown under Open Field Conditions: The Selection of Functional Genotypes for a Breeding Program" Agronomy 12, no. 3: 553. https://doi.org/10.3390/agronomy12030553
APA StyleBhandari, S. R., Rhee, J., Choi, C. S., Jo, J. S., Shin, Y. K., Song, J. W., Kim, S.-H., & Lee, J. G. (2022). Morphological and Biochemical Variation in Carrot Genetic Resources Grown under Open Field Conditions: The Selection of Functional Genotypes for a Breeding Program. Agronomy, 12(3), 553. https://doi.org/10.3390/agronomy12030553








