Phenotypic Correlation Analysis in F2 Segregating Populations of Gossypiumhirsutum and Gossypiumarboreum for Boll-Related Traits
Abstract
:1. Introduction
2. Materials and Methods
2.1. Parental Plant Material and Hybridization
2.2. Planting and Phenotyping
2.3. Sample Preparation for Hybridizations
2.4. Measurement of Traits
2.5. Statistical Analysis
3. Results
3.1. Variation in Phenotypic Traits of Four F2 Populations
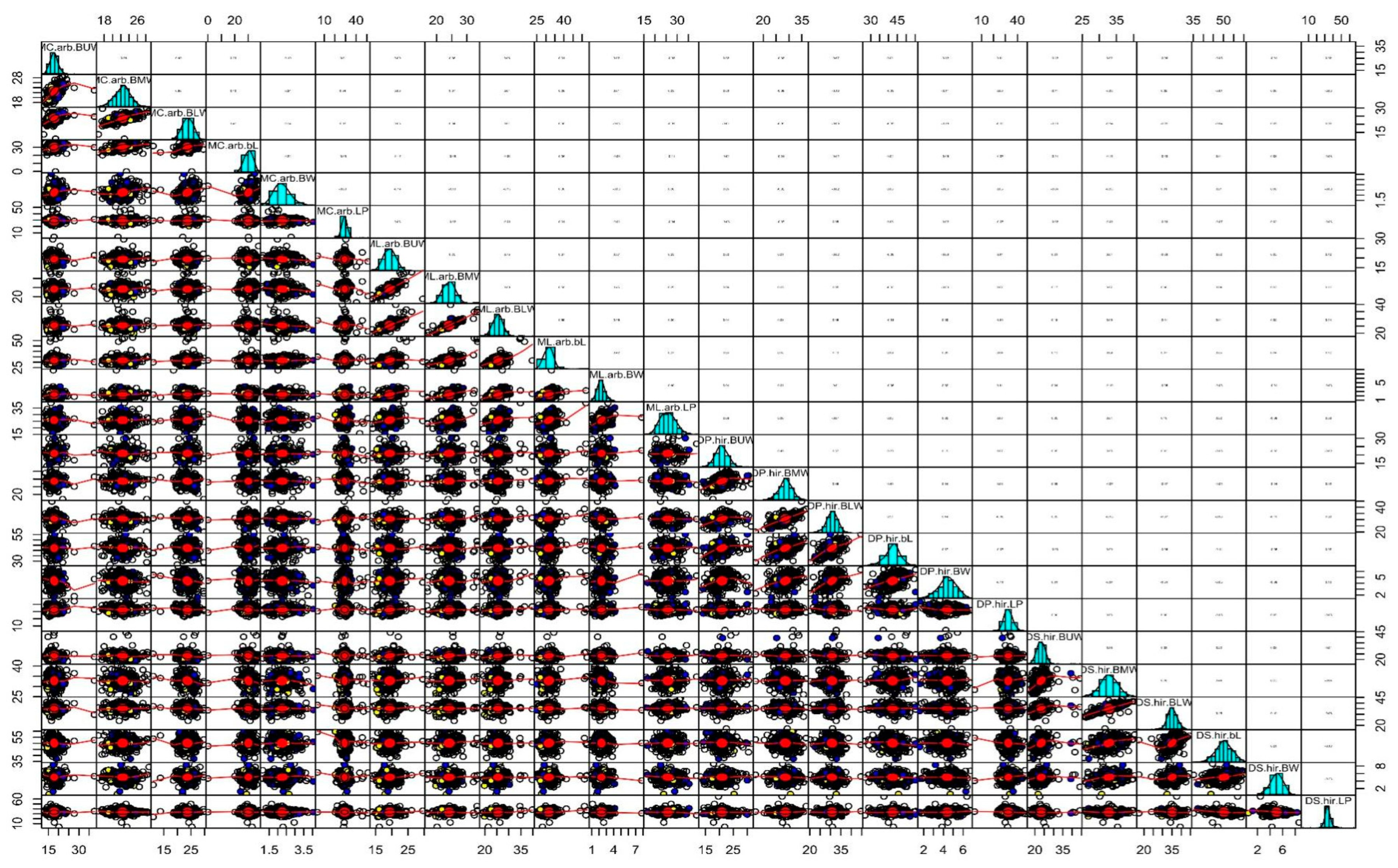
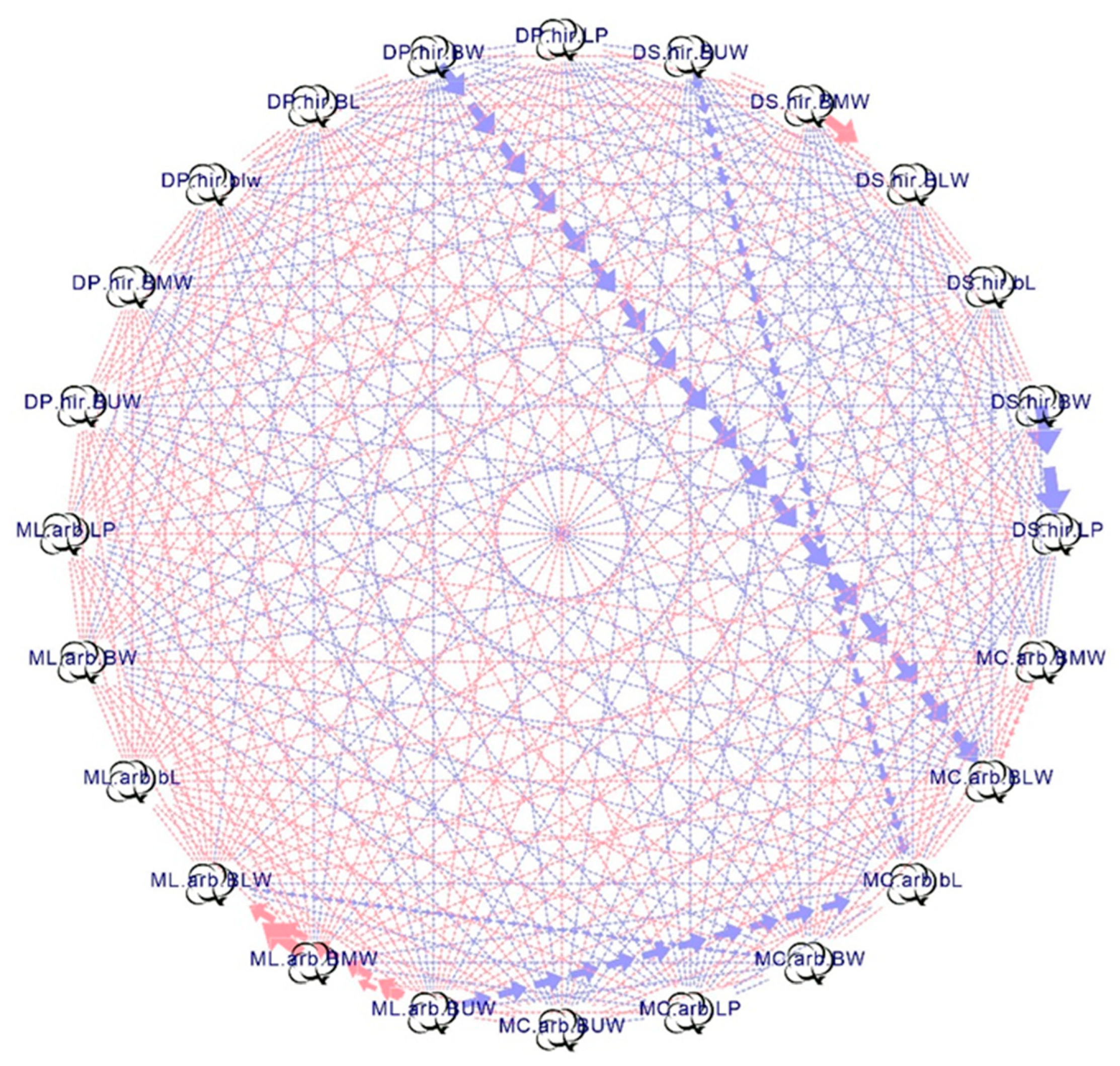
3.2. Correlation Analysis
3.3. Estimation of Heritability
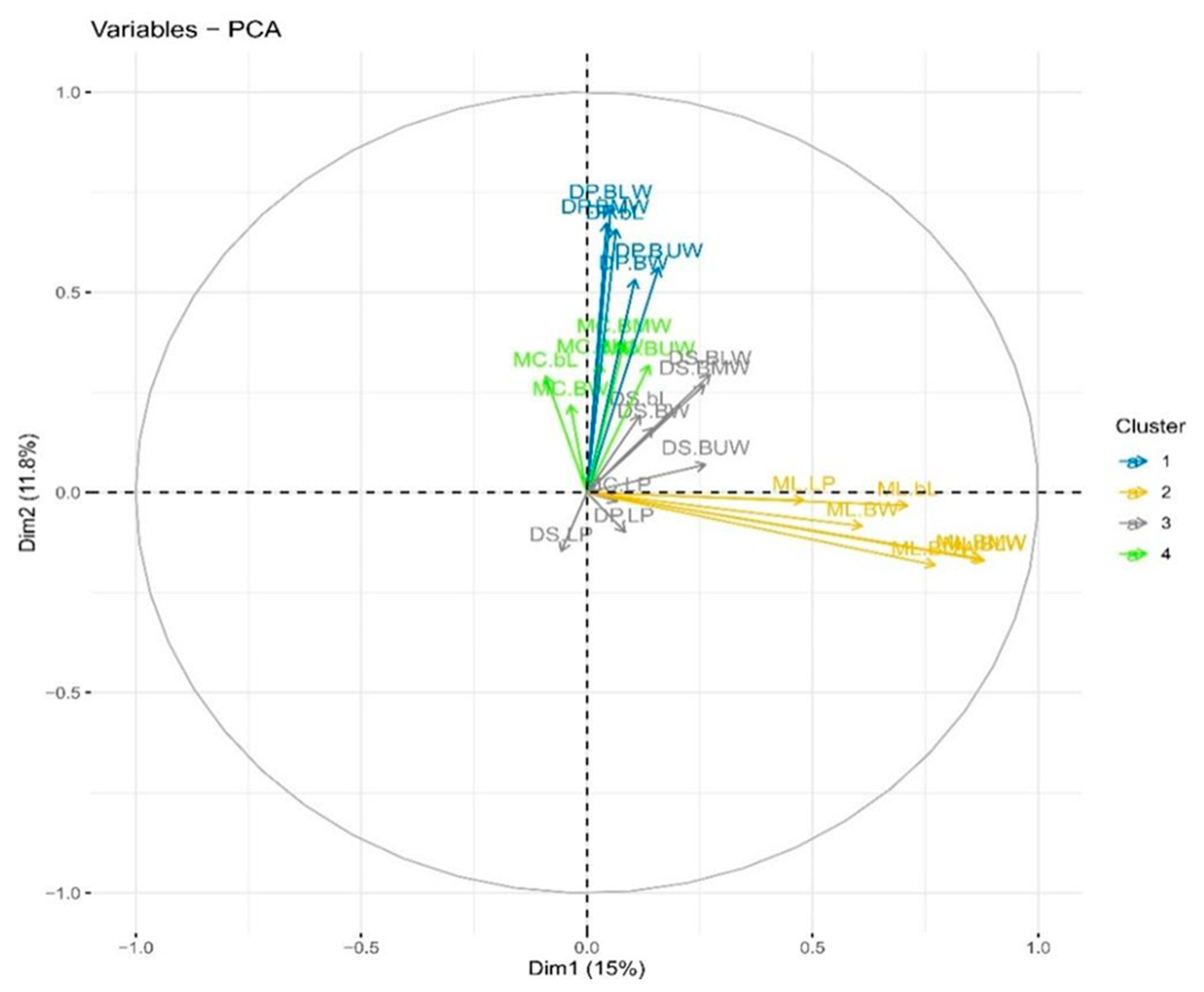
3.4. Principal Component Analysis (PCA)
3.5. Biplot Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Huang, G.; Wu, Z.; Percy, R.G.; Bai, M.; Li, Y.; Frelichowski, J.E.; Hu, J.; Wang, K.; Yu, J.Z.; Zhu, Y. Genome sequence of Gossypium herbaceum and genome updates of Gossypium arboreum and Gossypium hirsutum provide insights into cotton A-genome evolution. Nat. Genet. 2020, 52, 516–524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, K.; Wendel, J.F.; Hua, J. Designations for individual genomes and chromosomes in Gossypium. J. Cotton Res. 2018, 1, 3. [Google Scholar] [CrossRef] [Green Version]
- Gallagher, J.P.; Grover, C.; Rex, K.; Moran, M.; Wendel, J.F. A New Species of Cotton from Wake Atoll, Gossypium stephensii (Malvaceae). Syst. Bot. 2017, 42, 115–123. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.; Chen, J.; Fang, L.; Zhang, Z.; Ma, W.; Niu, Y.; Ju, L.; Deng, J.; Zhao, T.; Lian, J.; et al. Gossypium barbadense and Gossypium hirsutum genomes provide insights into the origin and evolution of allotetraploid cotton. Nat. Genet. 2019, 51, 739–748. [Google Scholar] [CrossRef] [Green Version]
- Chao, F.; Ma, Y.; Wu, S.; Liu, Z.; Wang, Z.; Yang, R.; Hu, G.; Zhou, Z.; Yu, H.; Zhang, M.; et al. Genome-wide association studies dissect the genetic networks underlying agronomical traits in soybean. Genome Biol. 2017, 18, 161. [Google Scholar] [CrossRef]
- Chen, Z.J.; Scheffler, B.; Dennis, E.; Triplett, B.A.; Zhang, T.; Guo, W.; Chen, X.; Stelly, D.; Rabinowicz, P.D.; Town, C.D.; et al. Toward Sequencing Cotton (Gossypium) Genomes: Figure. Plant Physiol. 2007, 145, 1303–1310. [Google Scholar] [CrossRef] [Green Version]
- Wendel, J.; Brubaker, C.; Seelanan, T. The Origin and Evolution of Gossypium. In Physiology of Cotton; Stewart, J., Oosterhuis, D., Heitholt, J., Mauney, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Ma, C.; Mamat, S.; Yao, J.; Isak, G. Spatio-temporal changes of cotton production in China from 1950 to 2015. Acta Geogr. Sin. 2020, 75, 1699–1710. [Google Scholar]
- Mehetre, S.; Mehetre, S.S.; Aher, A.R.; Gawande, V.L.; Patil, V.R.; Mokate, A.S. Induced polyploidy in Gossypium: A tool to overcome interspecific incompatibility of cultivated tetraploid and diploid cottons. Curr. Sci. 2003, 84, 1510–1512. [Google Scholar]
- Liu, D.; Guo, X.; Lin, Z.; Nie, Y.; Zhang, X. Genetic Diversity of Asian Cotton (Gossypium arboreum L.) in China Evaluated by Microsatellite Analysis. Genet. Resour. Crop. Evol. 2006, 53, 1145–1152. [Google Scholar] [CrossRef]
- Campbell, B.T.; Williams, V.E.; Park, W. Using molecular markers and field performance data to characterize the Pee Dee cotton germplasm resources. Euphytica 2009, 169, 285–301. [Google Scholar] [CrossRef]
- Tyagi, P.; Gore, M.A.; Bowman, D.T.; Campbell, B.T.; Udall, J.A.; Kuraparthy, V. Genetic diversity and population structure in the US Upland cotton (Gossypium hirsutum L.). Theor. Appl. Genet. 2014, 127, 283–295. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Ge, X.; Yang, Z.; Qin, W.; Sun, G.; Wang, Z.; Li, Z.; Liu, J.; Wu, J.; Wang, Y.; et al. Extensive intraspecific gene order and gene structural variations in upland cotton cultivars. Nat. Commun. 2019, 10, 2989. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; He, S.; Wang, X.; Sun, J.; Zhang, Y.; Zhang, G.; Wu, L.; Li, Z.; Liu, Z.; Sun, G.; et al. Resequencing a core collection of upland cotton identifies genomic variation and loci influencing fiber quality and yield. Nat. Genet. 2018, 50, 803–813. [Google Scholar] [CrossRef] [PubMed]
- Nie, J.-J.; Yuan, Y.-C.; Qin, D.-L.; Liu, Y.-H.; Wang, S.-L.; Li, J.-P.; Zhang, M.-L.; Zhao, N.; Guo, W.-J.; Qi, J.; et al. Spatial distribution of bolls affects yield formation in different genotypes of Bt cotton varieties. J. Integr. Agric. 2019, 18, 2492–2504. [Google Scholar] [CrossRef]
- Culp, T.W.; Harrell, D.C. Influence of Lint Percentage, Boll Size, and Seed Size on Lint Yield of Upland Cotton with High Fiber Strength. Crop Sci. 1975, 15, 741–746. [Google Scholar] [CrossRef]
- Zeng, L.; Meredith, W.R., Jr. Associations among lint yield, yield components, and fiber properties in an introgressed pop-ulation of cotton. Crop Sci. 2009, 49, 1647–1654. [Google Scholar] [CrossRef] [Green Version]
- Worley, S.; Culp, T.W.; Harrell, D.C. The relative contributions of yield components to lint yield of upland cotton, Gossypium hirsutum L. Euphytica 1974, 23, 399–403. [Google Scholar] [CrossRef]
- Sun, S.; Li, C.; Chee, P.W.; Paterson, A.H.; Jiang, Y.; Xu, R.; Robertson, J.S.; Adhikari, J.; Shehzad, T. Three-dimensional photogrammetric mapping of cotton bolls in situ based on point cloud segmentation and clustering. ISPRS J. Photogramm. Remote Sens. 2020, 160, 195–207. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, T.; Liu, Q.; Gao, X.; Zhu, X.; Zhang, T.; Zhou, B. Quantitative trait locus analysis of boll-related traits in an intraspecific population of Gossypium hirsutum. Euphytica 2014, 203, 121–144. [Google Scholar] [CrossRef]
- Majeedano, M.S.; Ahsaan, M.Z.; Somroo, A.W.; Channa, A.R. Heritability and correlation estimates for some yield traits of Gossypium hirsutum. Am. Res. Thoughts 2014, 1, 781–790. [Google Scholar]
- Fruchterman, T.M.J.; Reingold, E.M. Graph drawing by force-directed placement. Softw. Pract. Exp. 1991, 21, 1129–1164. [Google Scholar] [CrossRef]
- Benildo, G. Genomic and epigenomic bases of transgressive segregation–New breeding paradigm for novel plant phenotypes. Plant Sci. 2019, 288, 110213. [Google Scholar]
- Iqbal, M.A.; Ammad, A.; Zafar, Y. Characterization of indigenous Gossypium Arboreum, L. Genotypes for various fiber quality traits. Pak. J. Bot. 2015, 47, 2347–2354. [Google Scholar]
- Rezai, A.; Frey, K.J. Multivariate analysis of variation among wild oat accessions—Seed traits. Euphytica 1990, 49, 111–119. [Google Scholar] [CrossRef]
- Pezzotti, M.; Falcinelli, M.; Tomassini, C.; Veronesi, F. Evaluation of an Italian germplasm collection of Dactylis glomerata L. using a multivariate approach. J. Genet. Breed. 1994, 48, 17–23. [Google Scholar]
- Panthee, D.R.; Kc, R.B.; Regmi, H.N.; Subedi, P.P.; Bhattarai, S.; Dhakal, J. Diversity Analysis of Garlic (Allium sativum L.) Germplasms Available in Nepal Based on Morphological Characters. Genet. Resour. Crop. Evol. 2006, 53, 205–212. [Google Scholar] [CrossRef]
- Flores, F.; Gutierrez, J.; Lopez, J.; Moreno, M.; Cubero, J. Multivariate analysis approach to evaluate a germplasm collection of Hedysarum coronarium L. Genet. Resour. Crop. Evol. 1997, 44, 545–555. [Google Scholar] [CrossRef]
- Wu, J.; Jenkins, J.N.; McCarty, J.J., Jr.; Zhu, J. Genetic association of yield with its component traits in a recombinant inbred line population of cotton. Euphytica 2004, 140, 171–179. [Google Scholar] [CrossRef]
- McCarty, J.C.; Wu, J.; Jenkins, J.N. Genetic association of cotton yield with its component traits in derived primitive accessions crossed by elite upland cultivars using the conditional ADAA genetic model. Euphytica 2008, 161, 337–352. [Google Scholar] [CrossRef]
- McCarty, J.C.; Wu, J.; Jenkins, J.N. Primitive accession derived germplasm by cultivar crosses as sources for cotton improvement: I. Phenotypic values and variance components. Crop Sci. 2004, 44, 1226–1230. [Google Scholar] [CrossRef]
- Krishnarao, K.; Mary, T. Variability, correlation and path analysis of yield and fibre traits in upland cotton. Madras Agric. J. 1990, 77, 146–151. [Google Scholar]
- Miller, P.A.; Rawlings, J.O. Selection for Increased Lint Yield and Correlated Responses in Upland Cotton, Gossypium hirsutum L. Crop Sci. 1967, 7, 637–640. [Google Scholar] [CrossRef]
- Bridge, R.R.; Meredith, W.R.; Chism, J.F. Comparative Performance of Obsolete Varieties and Current Varieties of Upload Cotton. Crop Sci. 1971, 11, 29–32. [Google Scholar] [CrossRef]
- Ahuja, S.L.; Dhayal, L.S.; Monga, D. Performance of upland coloured cotton germplasm lines in line × tester crosses. Euphytica 2009, 169, 303–312. [Google Scholar] [CrossRef]
- Zeng, L.; Meredith, W.R.; Boykin, D.L.; Taliercio, E. Evaluation of an exotic germplasm population derived from multiple crosses among Gossypium tetraploid species. J. Cotton Sci. 2007, 11, 118–127. [Google Scholar]
- Xie, Z.-X.; Li, C.D.; Sun, H.C.; Zhu, J.J. Relationship between yield formation and source-sink of cotton cultivars with different boll weight. Cotton Sci. 2007, 3, 23–29. [Google Scholar]
- Tang, F.; Xiao, W. Genetic association of within-boll yield components and boll morphological traits with fibre properties in upland cotton (Gossypium hirsutum L.). Plant Breed. 2014, 133, 521–529. [Google Scholar]
- Souza, E.; Sorrells, M.E. Relationships among 70 North Amerian Oat Germplasms: II. Cluster Analysis Using Qualitative Characters. Crop Sci. 1991, 31, 605–612. [Google Scholar] [CrossRef]
- Firincioglu, H.; Erbektas, E.; Dogruyol, L.; Mutlu, Z.; Unal, S.; Karakurt, E.; Fırıncıoğlu, H. Phenotypic variation of Autumn and Spring-sown vetch (Vicia sativa ssp.) populations in central Turkey. Span. J. Agric. Res. 2009, 7, 596. [Google Scholar] [CrossRef] [Green Version]
| Genotypes | Parents | Species | BW (g) |
|---|---|---|---|
| Mei Zhongmian | Female | G. arboreum | 3.76 |
| Chimu Heizi | Female | G. arboreum | 1.28 |
| L-02292-3 | Male | G. arboreum | 1.33 |
| Dixie king | Female | G. hirsutum | 6.71 |
| Suyuan 04-44 | Male | G. hirsutum | 3.71 |
| Pamuk | Male | G. hirsutum | 3.75 |
| Genotypes | ID | Crosses | Species | BW (g) |
|---|---|---|---|---|
| Mei Zhongmian× Chimu Heizi | MC | Big boll × Small boll | G. arboreum | 2.1 |
| Mei Zhongmian × L-02292-3 | ML | Big boll × Small boll | G. arboreum | 3.0 |
| Dixie king × Suyuan 04-44 | DS | Big boll × Small boll | G. hirsutum | 6.2 |
| Dixie king × Pamuk | DP | Big boll × Small boll | G. hirsutum | 5.0 |
| Population | Traits | P1 | P2 | F2 Populations | ||||
|---|---|---|---|---|---|---|---|---|
| Min. | Max. | Mean | SD | CV% | ||||
| ML | BLW | 29.72 | 22.76 | 20.10 | 36.52 | 25.30 | 2.19 | 8.67 |
| BMW | 29.11 | 22.61 | 17.49 | 33.47 | 24.02 | 2.17 | 9.07 | |
| BUW | 27.35 | 17.03 | 13.97 | 29.55 | 19.28 | 1.81 | 9.41 | |
| BW | 3.93 | 1.63 | 0.99 | 5.98 | 2.29 | 0.48 | 21.16 | |
| LP | 32.68 | 18.13 | 16.67 | 35.32 | 26.15 | 2.92 | 11.18 | |
| BL | 37.66 | 29.26 | 24.74 | 52.77 | 31.73 | 3.041 | 9.58 | |
| MC | BLW | 29.72 | 22.52 | 17.99 | 29.48 | 23.68 | 1.94 | 8.22 |
| BMW | 29.11 | 22.4 | 17.48 | 28.82 | 22.38 | 1.91 | 8.56 | |
| BUW | 27.35 | 17.52 | 12.94 | 24.51 | 17.68 | 1.93 | 10.91 | |
| BW | 3.93 | 1.7 | 1.38 | 4.05 | 2.26 | 0.38 | 17.18 | |
| LP | 32.68 | 23.87 | 20.47 | 40.29 | 28.81 | 2.26 | 7.84 | |
| BL | 37.66 | 27.01 | 20.12 | 37.66 | 30.03 | 3.01 | 10.03 | |
| DS | BLW | 34.48 | 32.31 | 24.08 | 44.42 | 35.26 | 2.39 | 6.78 |
| BMW | 34.03 | 31.13 | 26.09 | 39.43 | 32.96 | 2.24 | 6.79 | |
| BUW | 26.74 | 23.69 | 17.45 | 30.10 | 23.20 | 2.15 | 9.27 | |
| BW | 5.79 | 3.93 | 1.25 | 7.45 | 5.02 | 0.83 | 16.54 | |
| LP | 35.77 | 35.37 | 13.57 | 46.4 | 33.38 | 3.01 | 9.02 | |
| BL | 45.52 | 50.51 | 38.74 | 60.27 | 50.03 | 3.78 | 7.55 | |
| DP | BLW | 34.48 | 26.35 | 24.02 | 37.97 | 30.78 | 2.39 | 7.79 |
| BMW | 34.03 | 26 | 22.81 | 35.65 | 28.67 | 2.33 | 8.14 | |
| BUW | 26.74 | 21.34 | 15.25 | 31.33 | 20.95 | 2.12 | 10.12 | |
| BW | 5.79 | 3.65 | 1.6 | 6.39 | 4.40 | 0.67 | 15.24 | |
| LP | 35.77 | 31.81 | 13.57 | 46.46 | 32.39 | 3.01 | 9.29 | |
| BL | 45.52 | 36.81 | 30.14 | 54.26 | 42.14 | 3.69 | 8.77 | |
| Populaiton | Traits | GCV | PCV | h2 | GA |
|---|---|---|---|---|---|
| MC | BUW | 6.45 | 16.57 | 15.13 | 0.91 |
| BMW | 5.87 | 12.29 | 22.80 | 1.29 | |
| BLW | 5.44 | 11.99 | 20.62 | 1.21 | |
| BL | 7.66 | 13.60 | 31.73 | 2.67 | |
| BW | 16.38 | 18.69 | 76.78 | 0.67 | |
| LP | 7.56 | 8.40 | 80.86 | 4.03 | |
| ML | BUW | 7.70 | 12.13 | 40.27 | 1.94 |
| BMW | 7.84 | 11.15 | 49.44 | 2.73 | |
| BLW | 7.49 | 10.65 | 49.46 | 2.75 | |
| BL | 8.66 | 11.21 | 59.67 | 4.37 | |
| BW | 19.63 | 23.96 | 67.12 | 0.76 | |
| LP | 10.56 | 12.35 | 73.09 | 4.86 | |
| DP | BUW | 6.18 | 15.21 | 16.53 | 1.08 |
| BMW | 4.80 | 12.36 | 15.05 | 1.10 | |
| BLW | 4.95 | 11.55 | 18.33 | 1.34 | |
| BL | 6.67 | 11.92 | 31.33 | 3.24 | |
| BW | 14.15 | 17.24 | 67.34 | 1.05 | |
| LP | 8.66 | 10.46 | 68.48 | 4.78 | |
| DS | BUW | 6.70 | 12.96 | 26.71 | 1.66 |
| BMW | 4.71 | 9.71 | 23.46 | 1.55 | |
| BLW | 4.40 | 9.97 | 19.49 | 1.41 | |
| BL | 5.66 | 10.36 | 29.84 | 3.19 | |
| BW | 15.41 | 18.61 | 68.58 | 1.32 | |
| LP | 8.36 | 10.22 | 66.86 | 4.70 |
| Population | Variable | Principal Components | ||||||
|---|---|---|---|---|---|---|---|---|
| PC1 | PC2 | PC3 | PC4 | PC5 | PC6 | PC7 | ||
| ML | BUW | 0.39 | 0.13 | −0.07 | 0.08 | −0.04 | −0.27 | −0.11 |
| BMW | 0.45 | 0.13 | −0.08 | 0.07 | −0.08 | −0.16 | −0.11 | |
| BLW | 0.45 | 0.13 | −0.07 | 0.09 | −0.07 | −0.16 | −0.08 | |
| BL | 0.36 | 0.04 | −0.08 | 0.01 | −0.21 | 0.16 | 0.03 | |
| BW | 0.31 | 0.07 | −0.04 | 0.09 | 0.21 | 0.31 | 0.13 | |
| LP | 0.25 | 0.02 | −0.03 | 0.05 | 0.13 | 0.57 | 0.09 | |
| MC | BUW | 0.07 | −0.16 | −0.35 | −0.18 | 0.22 | −0.16 | 0.05 |
| BMW | 0.03 | −0.20 | −0.45 | −0.18 | 0.06 | −0.01 | 0.13 | |
| BLW | 0.01 | −0.17 | −0.46 | −0.18 | 0.08 | 0.01 | 0.08 | |
| BL | −0.04 | −0.15 | −0.28 | −0.19 | −0.22 | 0.22 | −0.10 | |
| BW | −0.02 | −0.11 | −0.22 | −0.11 | −0.17 | −0.07 | −0.19 | |
| LP | 0.03 | 0.01 | 0.02 | −0.02 | −0.13 | −0.33 | 0.79 | |
| DP | BUW | 0.10 | −0.32 | 0.07 | 0.16 | 0.30 | 0.08 | 0.07 |
| BMW | 0.04 | −0.39 | 0.05 | 0.31 | 0.12 | −0.09 | −0.042 | |
| BLW | 0.04 | −0.40 | 0.04 | 0.29 | −0.04 | −0.09 | 0.03 | |
| BL | 0.05 | −0.39 | 0.08 | 0.206 | −0.13 | 0.02 | 0.05 | |
| BW | 0.07 | −0.30 | 0.03 | 0.18 | −0.25 | 0.05 | −0.13 | |
| LP | 0.05 | 0.06 | 0.07 | 0.005 | 0.65 | −0.09 | −0.05 | |
| DS | BUW | 0.15 | −0.04 | 0.22 | −0.177 | −0.03 | −0.02 | 0.30 |
| BMW | 0.16 | −0.18 | 0.28 | −0.369 | −0.06 | 0.05 | 0.001 | |
| BLW | 0.17 | −0.19 | 0.28 | −0.367 | −0.04 | 0.06 | 0.01 | |
| BL | 0.08 | −0.14 | 0.19 | −0.38 | 0.03 | 0.13 | −0.06 | |
| BW | 0.10 | −0.11 | 0.12 | −0.224 | −0.01 | −0.34 | −0.31 | |
| LP | −0.04 | 0.10 | −0.01 | 0.157 | −0.30 | 0.21 | 0.07 | |
| Eigenvalue | 3.62 | 2.92 | 2.68 | 2.306 | 1.23 | 1.13 | 1.04 | |
| Variability (%) | 15.10 | 12.20 | 11.20 | 9.60 | 5.10 | 4.70 | 4.30 | |
| Cumulative percentage (%) | 15.10 | 27.30 | 38.50 | 48.10 | 53.20 | 57.90 | 62.20 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iqbal, Z.; Hu, D.; Nazeer, W.; Ge, H.; Nazir, T.; Fiaz, S.; Gul, A.; Iqbal, M.S.; El-Sabrout, A.M.; Maryum, Z.; et al. Phenotypic Correlation Analysis in F2 Segregating Populations of Gossypiumhirsutum and Gossypiumarboreum for Boll-Related Traits. Agronomy 2022, 12, 330. https://doi.org/10.3390/agronomy12020330
Iqbal Z, Hu D, Nazeer W, Ge H, Nazir T, Fiaz S, Gul A, Iqbal MS, El-Sabrout AM, Maryum Z, et al. Phenotypic Correlation Analysis in F2 Segregating Populations of Gossypiumhirsutum and Gossypiumarboreum for Boll-Related Traits. Agronomy. 2022; 12(2):330. https://doi.org/10.3390/agronomy12020330
Chicago/Turabian StyleIqbal, Zubair, Daowu Hu, Wajad Nazeer, Hao Ge, Talha Nazir, Sajid Fiaz, Alia Gul, Muhammad Shahid Iqbal, Ahmed M. El-Sabrout, Zahra Maryum, and et al. 2022. "Phenotypic Correlation Analysis in F2 Segregating Populations of Gossypiumhirsutum and Gossypiumarboreum for Boll-Related Traits" Agronomy 12, no. 2: 330. https://doi.org/10.3390/agronomy12020330
APA StyleIqbal, Z., Hu, D., Nazeer, W., Ge, H., Nazir, T., Fiaz, S., Gul, A., Iqbal, M. S., El-Sabrout, A. M., Maryum, Z., Pan, Z., & Du, X. (2022). Phenotypic Correlation Analysis in F2 Segregating Populations of Gossypiumhirsutum and Gossypiumarboreum for Boll-Related Traits. Agronomy, 12(2), 330. https://doi.org/10.3390/agronomy12020330







