Long-Term Multilocal Monitoring of Leaf Rust Resistance in the Spring Bread Wheat Genetic Resources from Institute of Plant Genetic Resources (VIR)
Abstract
:1. Introduction
2. Environments, Materials, and Methods
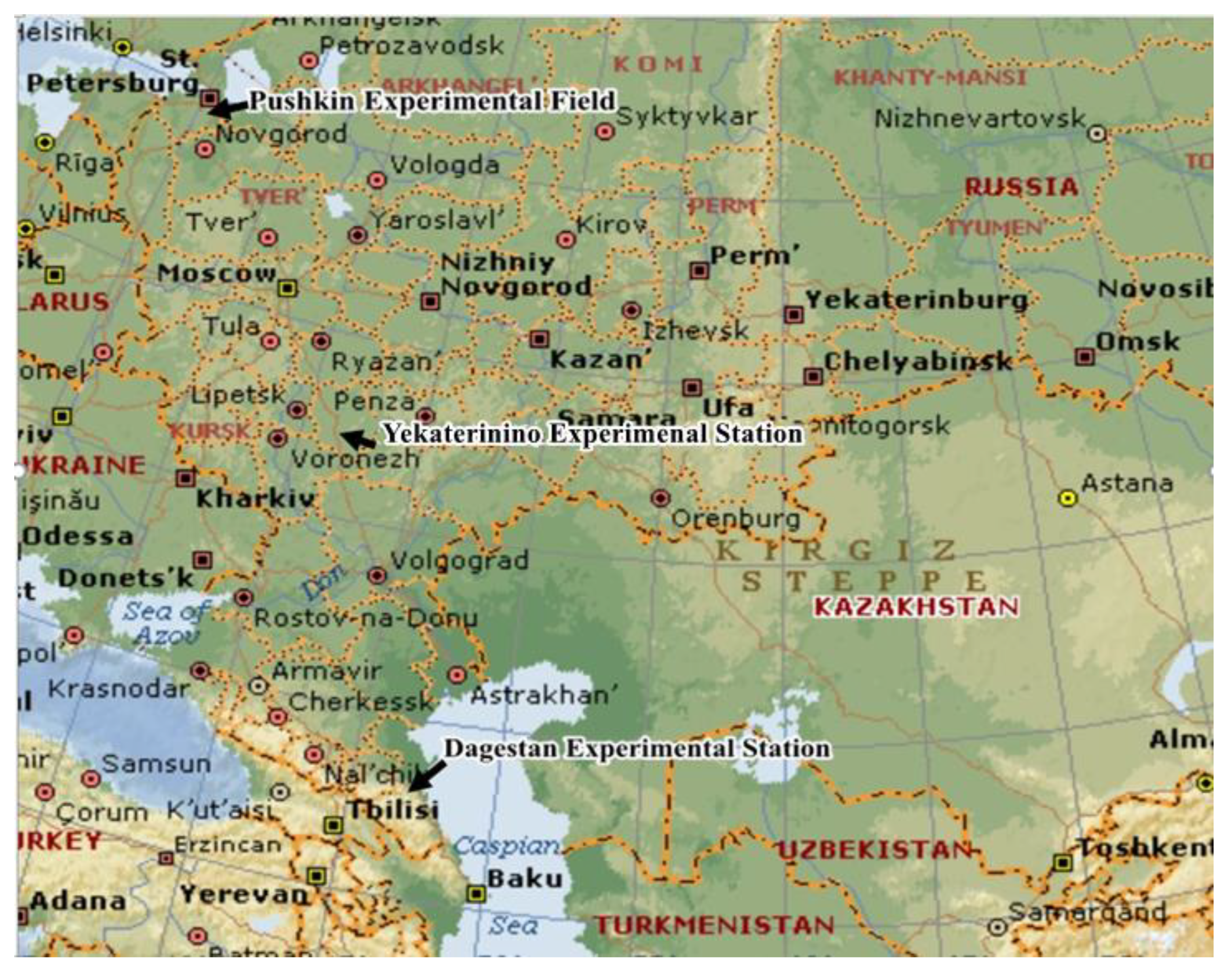
2.1. Regions of Wheat Assessment for Leaf Rust Resistance
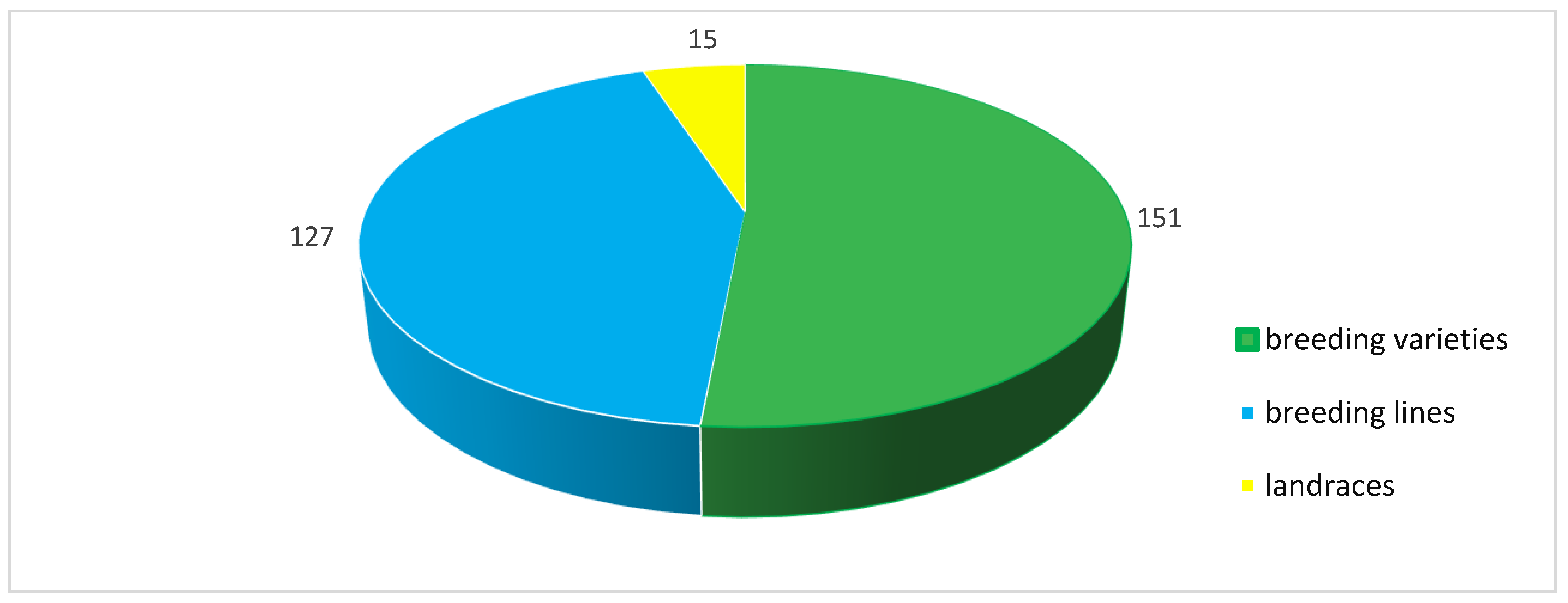
2.2. Plant Material
2.3. Methods
3. Results
3.1. Resistance of Wheat Samples under the Conditions of the Dagestan Experimental Station of VIR
3.2. Resistance of Wheat Samples under the Conditions of the Yekaterinino Experimental Station of VIR
3.3. Resistance of Wheat Samples under the Conditions of the VIR Pushkin Experimental Field
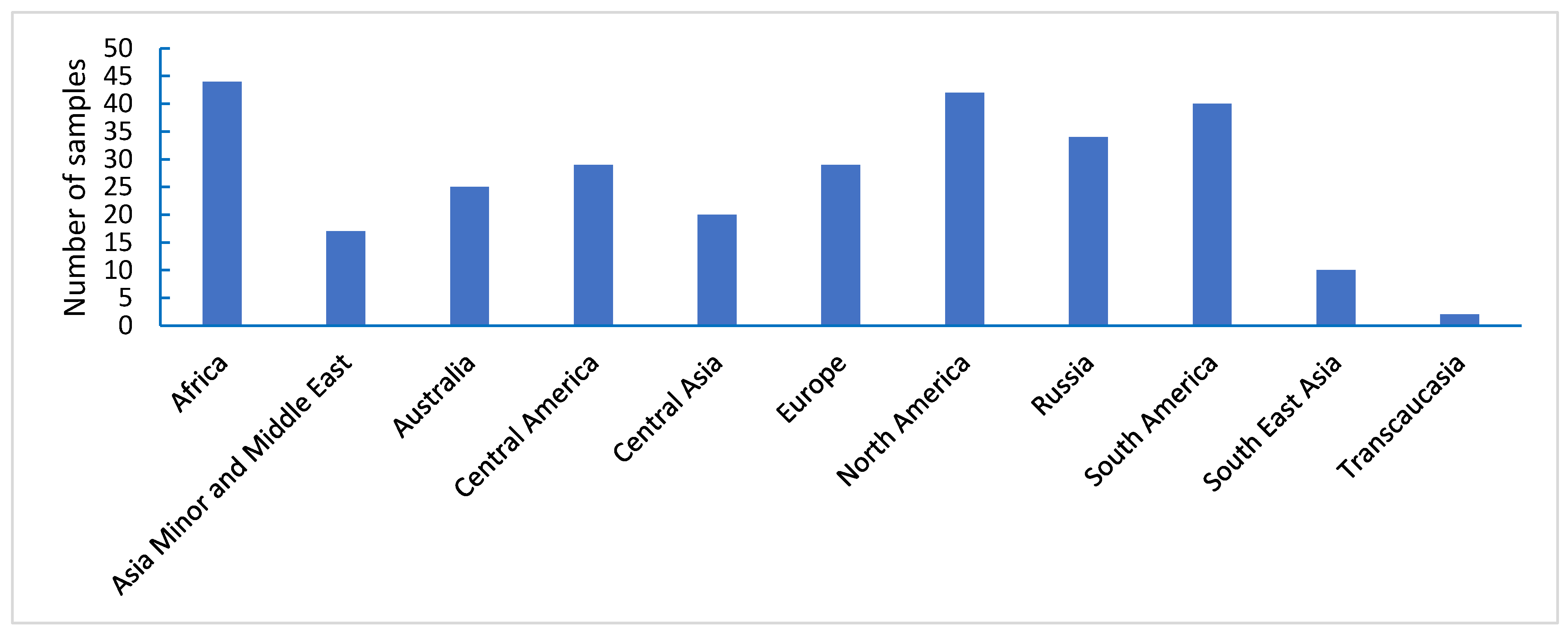
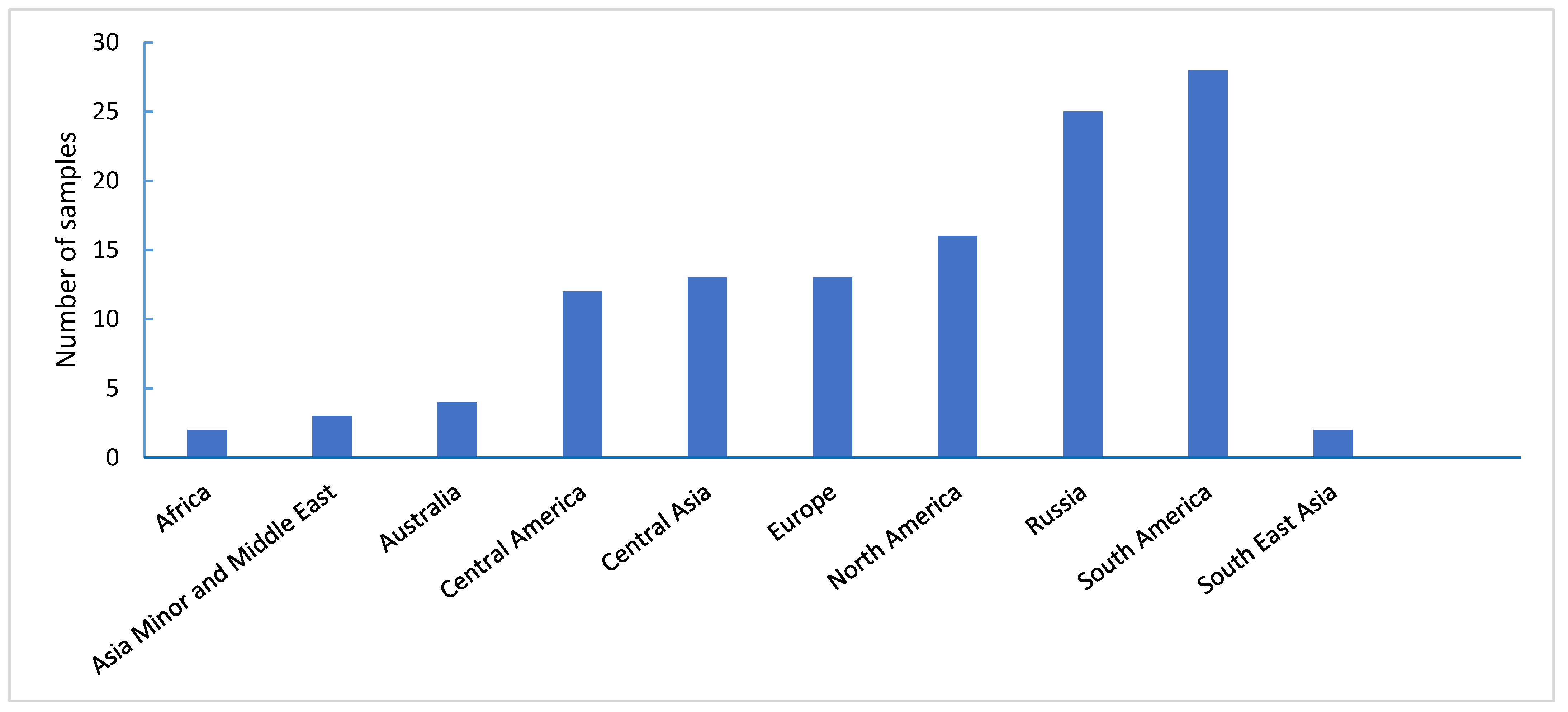
3.4. Juvenile Resistance of Wheat Samples to Leaf Rust
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Available online: https://ru.investing.com/news/economy/article-2073183.html (accessed on 7 October 2021).
- Available online: https://1prime.ru/Agriculture/20210924/834796778.html (accessed on 7 October 2021).
- Krupnov, V.A. Strategy for genetic protection of wheat from leaf rust in the Volga region. Vestn. Russ. Agric. Sci. 1997, 6, 12–15. (In Russian) [Google Scholar]
- Tyryshkin, L.G.; Syukov, V.V.; Zaharov, V.G.; Zuev, E.V.; Gashimov, M.E.; Kolesova, M.A.; Chikida, N.N.; Ershova, M.A.; Belousova, M.H. Sources of effective resistance to fungal diseases in wheat and its relatives—Search, creation and use in breeding. Proc. Appl. Bot. Genet. Breed. 2012, 170, 186–199. (In Russian) [Google Scholar]
- Zuev, E.V.; Shikhmuradov, A.Z.; Akhmedov, M.A.; Brykova, A.N.; Kudryavtseva, E.Y. Spring bread wheat. Sources of breeding traits for dagestan conditions. In Catalogue of the VIR World Collection; N.I. Vavilov All-Russian Institute of Plant Genetic Resources: Saint Petersburg, Russia, 2018; Volume 858, pp. 5–7, (In Russian). [Google Scholar] [CrossRef]
- Zuev, E.V.; Nikiforov, M.N.; Pankratov, N.N.; Platonov, V.N.; Loseva, V.A.; Brykova, A.N. Spring bread wheat. Sources of breeding traits for TAMBOV region conditions. In Catalogue of the VIR World Collection; N.I. Vavilov All-Russian Institute of Plant Genetic Resources: Saint Petersburg, Russia, 2017; Volume 840, p. 6. (In Russian) [Google Scholar]
- Zuev, E.V.; Brykova, A.N.; Kudryavtseva, E.Y. Spring bread wheat. The results of a long-term study of the collection under Leningrad region conditions. In Catalogue of the VIR World Collection; N.I. Vavilov All-Russian Institute of Plant Genetic Resources: Saint Petersburg, Russia, 2020; Volume 917, pp. 5–7, (In Russian). [Google Scholar] [CrossRef]
- Zuev, E.V. Spring Bread Wheat Landraces in the VIR World Collection; N.I. Vavilov All-Russian Institute of Plant Genetic Resources: Saint Petersburg, Russia, 2008; pp. 1–161. (In Russian) [Google Scholar]
- Rudenko, M.I.; Shitova, I.P.; Korneichuk, V.A. Guidelines to Study the World Wheat Collection; N.I. Vavilov All-Russian Institute of Plant Industry: Saint Petersburg, Russia, 1977; pp. 1–28. (In Russian) [Google Scholar]
- Gradchaninova, O.D.; Filatenko, A.A.; Rudenko, M.I. The Study of the Wheat Collection: Guidelines; N.I. Vavilov All-Russian Institute of Plant Industry: Saint Petersburg, Russia, 1985; pp. 1–27. (In Russian) [Google Scholar]
- Merezhko, A.F.; Udachin, R.A.; Zuev, E.V.; Filatenko, A.A.; Serbin, A.A.; Lyapunova, O.F.; Kosov, V.Y.; Kurkiev, U.K.; Okhotnikova, T.V.; Navruzbekov, N.A.; et al. Introduction, Conservation, and Study of the World Collection of Wheat, Aegilops and Triticale. Guidelines; N.I. Vavilov All-Russian Institute of Plant Industry: Saint Petersburg, Russia, 1999; pp. 1–81. (In Russian) [Google Scholar]
- Mains, E.B.; Jackson, H.S. Physiological specialization in leaf rust of wheat Puccinia triticina Erikss. Phytopathology 1926, 16, 89–120. [Google Scholar]
- Edwards, K.; Johnstone, C.; Thompson, C. A simple and rapid method for the preparation of plant genomic DNA for PCR analysis. Nucl. Acids Res. 1991, 19, 1349. [Google Scholar] [CrossRef] [PubMed]
- Dorokhov, D.B.; Klocke, E. A rapid and economic technique for RAPD-analysis of plant genomes. Russ. J. Genet. 1997, 33, 443–450. (In Russian) [Google Scholar]
- Gupta, S.K.; Charpe, A.; Koul, S.; Prabhu, K.V.; Haq, Q.M.R. Development and validation of molecular markers linked to an Aegilops umbellulata–derived leaf rust-resistance gene, Lr9, for marker-assisted selection in bread wheat. Genome 2005, 48, 823–830. [Google Scholar] [CrossRef]
- Gupta, S.K.; Charpe, A.; Prabhu, K.W.; Haque, O.M.R. Identification and validation of molecular markers linked to the leaf rust resistance gene Lr19 in wheat. Theor. Appl. Genet. 2006, 113, 1027–1036. [Google Scholar] [CrossRef]
- Mago, R.; Bariana, H.S.; Dundas, I.S.; Spielmeyer, W.; Lawrence, G.J.; Pryor, A.J.; Ellis, J.G. Development or PCR markers for the selection of wheat stem rust resistance genes Sr24 and Sr26 in diverse wheat germplasm. Theor. Appl. Genet. 2005, 111, 496–504. [Google Scholar] [CrossRef]
- McIntosh, R.A.; Yamazaki, Y.; Dubcovsky, J.; Rogers, J.; Morris, C.; Appels, R.; Xia, X.C. Catalogue of Gene Symbols for Wheat. 2013. Available online: https://shigen.nig.ac.jp/wheat/komugi/genes/macgene/2013/GeneSymbol.pdf (accessed on 3 September 2020).
- McIntosh, R.A.; Dubcovsky, J.; Rogers, W.J.; Morris, C.; Xia, X.C. Catalogue of Gene Symbols for Wheat: 2017 Supplement. Available online: https://shigen.nig.ac.jp/wheat/komugi/genes/macgene/supplement2017.pdf (accessed on 7 December 2020).
- Tyryshkin, L.G. Genetic Diversity of Wheat and Barley for Effective Diseases Resistance and the Possibility of Its Broadening. Doctoral Dissertation, N.I. Vavilov All-Russian Institute of Plant Industry, Saint Petersburg, Russia, 2007; pp. 1–254. (In Russian). [Google Scholar]
- Guiltiaeva, E.I. Genetic Structure of Puccinia triticina Populations in Russia and Its Variation under the Influence of Host Plants. Ph.D. Thesis, All-Russian Institute of Plant Protection, Saint Petersburg, Russia, 2018. (In Russian). [Google Scholar]
- Tyryshkin, L.G.; Volkova, G.V.; Kolomiets, T.M.; Brykova, A.N.; Zuev, E.V. Effective resistance to leaf rust in spring bread wheat accessions from new entries of VIR collection. Vavilovia 2019, 2, 35–43. (In Russian) [Google Scholar] [CrossRef]
- Vyuskov, A.A. Breeding of Spring Bread Wheat for Resistance to Leaf Rust in the Middle Volga Region. Ph.D. Thesis, N.I. Vavilov All-Russian Institute of Plant Industry, Saint Petersburg, Russia, 1988. (In Russian). [Google Scholar]
- Markelova, T.S. Study of the structure and variability of the wheat brown rust population in the Volga region. Agro XXI 2007, 4–6, 37–40. (In Russian) [Google Scholar]
- Meshkova, L.V.; Rosseeva, L.P.; Shreider, E.R.; Sidorov, A.V. Virulence of pathotypes of wheat leaf rust to TH Lr9 in the regions of Siberia and the Ural. In Proceedings of the II All-Russian Conference “Modern Problems of Plant Immunity to Harmful Organisms”, Saint Petersburg, Russia, 29 September–2 October 2008; pp. 70–73. (In Russian). [Google Scholar]
- Sayre, K.; Singh, R.P.; Huerta-Espino, J.; Rajaram, S. Genetic progress in reducing losses to leaf rust in CIMMYT derived Mexican spring wheat varieties. Crop. Sci. 1998, 38, 654–659. [Google Scholar] [CrossRef]
- Singh, R.P.; Huerta-Espino, J.; William, H.M. Genetics and breeding for durable resistance to leaf and stripe rusts in wheat. Turk. J. Agric. For. 2005, 29, 121–127. [Google Scholar]
- Pathan, A.K.; Park, R.F. Evaluation of seedling and adult plant resistance to leaf rust in European wheat varieties. Euphytica 2006, 149, 327–342. [Google Scholar] [CrossRef]
- Hovmoller, M.S. Sources of seedling and adult plant resistance to Puccinia striiformis f.sp. tritici in European wheats. Plant Breed. 2007, 126, 225–233. [Google Scholar] [CrossRef]
- Li, Z.F.; Xia, X.C.; He, Z.H.; Li, X.; Zhang, L.J.; Wang, H.Y.; Meng, Q.F.; Yang, W.X.; Li, G.Q.; Liu, D.Q. Seedling and slow rusting resistance to leaf rust in Chinese wheat varieties. Plant Dis. 2010, 94, 45–53. [Google Scholar] [CrossRef]
- Sun, Y.; Hu, Y.Y.; Yang, W.X.; Liu, D.Q. Evaluation of the resistance to leaf rust of 6 wheat lines. J. Triticeae Crops 2011, 31, 762–768. [Google Scholar]
- Vanzetti, L.S.; Campos, P.; Demichelis, M.; Lombardo, L.A.; Aurelia, P.R.; Vaschetto, L.M.; Bainotti, C.T.; Helguera, M. Identification of leaf rust resistance genes in selected Argentinean bread wheat varieties by gene postulation and molecular markers. Electron. J. Biotechnol. 2011, 14, 3. [Google Scholar] [CrossRef]
- Azzimonti, G.; Lannou, C.; Sache, I.; Goyeau, H. Components of quantitative resistance to leaf rust in wheat varieties: Diversity, variability and specificity. Plant Pathol. 2013, 62, 970–981. [Google Scholar] [CrossRef]
- Dakouri, A.; McCallum, B.D.; Radovanovic, N.; Cloutier, S. Molecular and phenotypic characterization of seedling and adult plant leaf rust resistance in a world wheat collection. Mol. Breed. 2013, 32, 663–677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Draz, I.S.; Abou-Elseoud, M.S.; Kamara, A.M.; Alaa-Eldein, O.A.; El-Bebany, A.F. Screening of wheat genotypes for leaf rust resistance along with grain yield. Ann. Agric. Sci. 2015, 60, 29–39. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Shi, L.; Wang, X.; Zhang, N.; Wei, X.; Zhang, L.; Yang, W.; Liu, D. Leaf rust resistance of 35 wheat varieties (lines). J. Plant Pathol. Microbiol. 2018, 9, 429. [Google Scholar] [CrossRef]
- Bershtein, E.M. Wheats of Australia. In Catalogue of the VIR World Collection; N.I. Vavilov All-Russian Institute of Plant Industry: Saint Petersburg, Russia, 1969; Volume 52, pp. 1–23. (In Russian) [Google Scholar]
- Yakubtsiner, M.M.; Krivchenko, V.I.; Myagkova, D.V.; Grigor’eva, O.G.; Trainina, S.I. Catalogue of Spring Wheat Accessions with Characteristics for Their Resistance to Leaf and Stem Rusts, Common Bunt, Loose Smut and Powdery Mildew; N.I. Vavilov All-Russian Institute of Plant Industry: Saint Petersburg, Russia, 1973; Volume 124, pp. 1–37. (In Russian) [Google Scholar]
- Myagkova, D.V.; Grigor’eva, O.G.; Vershinina, V.A.; Krivchenko, V.I.; Udachin, R.A.; Kudryavtseva, Z.V. Spring wheat accessions with characteristics for their resistance to leaf and stem rusts, common bunt, loose smut and powdery mildew. In Catalogue of the VIR World Collection; N.I. Vavilov All-Russian Institute of Plant Industry: Saint Petersburg, Russia, 1981; Volume 304, pp. 1–79. (In Russian) [Google Scholar]
- Udachin, R.A.; Myagkova, D.V.; Surganova, L.D.; Semenova, L.V. Spring bread wheat. Immunological and agrobiological evaluation of accessions under the conditions of the Non-Chernozem Zone of the RSFSR. In Catalogue of the VIR World Collection; N.I. Vavilov All-Russian Institute of Plant Industry: Saint Petersburg, Russia, 1990; Volume 567, pp. 1–38. (In Russian) [Google Scholar]
- Surganova, L.D.; Semenova, L.V.; Kuznetsova, O.I.; Naumova, V.I.; Brykova, A.N.; Lyapunova, O.A.; Starikova, I.A. Spring bread wheat. Agrobiological evaluation of accessions under the conditions of the Non-Chernozem Zone of Russia. In Catalogue of the VIR World Collection; N.I. Vavilov All-Russian Institute of Plant Industry: Saint Petersburg, Russia, 1992; Volume 623, pp. 1–35. (In Russian) [Google Scholar]
- Khakimova, A.G.; Zuev, E.V.; Brykova, A.N.; Klement’eva, N.F.; Chmeleva, Z.V.; Nikiforov, M.N. Complex evaluation of perspective spring bread wheat accessions for grain quality in the Central Chernozem region of Russia. In Catalogue of the VIR World Collection; N.I. Vavilov All-Russian Institute of Plant Industry: Saint Petersburg, Russia, 1999; Volume 698, pp. 1–47. (In Russian) [Google Scholar]
- Tyryshkin, L.G.; Lebedeva, T.V.; Zuev, E.V.; Loskutova, N.P.; Brykova, A.N.; Pyukkenen, V.P. Characterization of bread wheat accessions for resistance to obligate and facultative fungal pathogens. In Catalogue of the VIR World Collection; N.I. Vavilov All-Russian Institute of Plant Industry: Saint Petersburg, Russia, 2000; Volume 718, pp. 1–24. (In Russian) [Google Scholar]
- Tyryshkin, L.G.; Tyryshkina, N.A.; Zuev, E.V.; Brykova, A.N.; Kovaleva, M.M.; Ershova, M.A. Spring bread wheat. Characteristics of new VIR’s accessions for resistance to fungal diseases. In Catalogue of the VIR World Collection; N.I. Vavilov All-Russian Institute of Plant Industry: Saint Petersburg, Russia, 2004; Volume 749, pp. 1–19. (In Russian) [Google Scholar]
- Tyryshkin, L.G.; Lebedeva, T.V.; Kovaleva, M.M.; Zuev, E.V.; Brykova, A.N.; Stetsyuk, S.N. Spring bread wheat. Characteristics of new VIR’s accessions for resistance to leaf rust, dark-brown leaf spot blotch, Septoria nodorum blotch, powdery mildew, and scab. In Catalogue of the VIR World Collection; N.I. Vavilov All-Russian Institute of Plant Industry: Saint Petersburg, Russia, 2009; Volume 795, pp. 1–20. (In Russian) [Google Scholar]
- Tyryshkin, L.G.; Lebedeva, T.V.; Kovaleva, M.M.; Zuev, E.V.; Brykova, A.N.; Stetsyuk, S.N. Spring bread wheat. Characteristics of new VIR’s accessions for resistance to leaf rust, dark-brown leaf spot blotch, powdery mildew, and scab. In Catalogue of the VIR World Collection; N.I. Vavilov All-Russian Institute of Plant Genetic Resources: Saint Petersburg, Russia, 2015; Volume 823, pp. 1–19. (In Russian) [Google Scholar]
- Tyryshkin, L.G.; Lebedeva, T.V.; Kovaleva, M.M.; Zuev, E.V.; Brykova, A.N.; Kudryavtseva, E.Y. Spring bread wheat. Characteristics of new VIR’s accessions for resistance to leaf rust, dark-brown leaf spot blotch, powdery mildew and loose smut. In Catalogue of the VIR World Collection; N.I. Vavilov All-Russian Institute of Plant Genetic Resources: Saint Petersburg, Russia, 2020; Volume 913, pp. 1–23, (In Russian). [Google Scholar] [CrossRef]
- Kurbanova, P.M. Genetic Diversity of Spring Bread Wheat for Effective Adult Resistance to Leaf Rust. Ph.D. Thesis, N.I. Vavilov All-Russian Institute of Plant Genetic Resources, Saint Petersburg, Russia, 2009. (In Russian). [Google Scholar]
- Alderov, A.A. Theoretical and Applied Aspects of the Genus Triticum L. Genetic Resources Study in Dagestan; N.I. Vavilov All-Russian Institute of Plant Industry: Saint Petersburg, Russia, 2005; pp. 3–7. (In Russian) [Google Scholar]
- Official Website of Gossort Russia. Available online: http://gossortrf.ru (accessed on 15 November 2020).
- Vavilov, N.I. The Theory of Immunity of Plants to Infectious Diseases. The Theoretical Basis of Plant Breeding; Lenselkhozgis: Moscow-Leningrad, Russia, 1935; Volume 1, pp. 893–990. (In Russian) [Google Scholar]
- Czembor, J.H.; Czembor, H.J.; Attene, G.; Papa, R. Leaf rust resistance in selections from barley landraces collected in Sardinia. Plant Breed. Seed Sci. 2007, 56, 73–84. [Google Scholar]
- Abdullaev, R.A.; Lebedeva, T.V.; Alpatieva, N.V.; Yakovleva, O.V.; Kovaleva, O.N.; Radchenko, E.E.; Anisimova, I.N.; Batasheva, B.A.; Karabitsina, Y.I.; Kuznetsova, E.B. Genetic Diversity of Barley Accessions from Ethiopia for Powdery Mildew Resistance. Russ. Agric. Sci. 2019, 45, 232–235. [Google Scholar] [CrossRef]
- Tyryshkin, L.G. Genetic control of effective juvenile resistance to leaf rust in collection samples of Triticum aestivum L. Russ. J. Genet. 2006, 42, 294–300. [Google Scholar] [CrossRef]
- Sibikeev, S.N.; Badaeva, E.D.; Gultyaeva, E.I.; Druzhin, A.E.; Shishkina, A.A.; Dragovich, A.Y.; Kroupin, P.Y.; Karlov, G.I.; Khuat, T.M.; Divashuk, M.G. Comparative analysis of Agropyron intermedium (Host) Beauv 6Agi and 6Agi2 chromosomes in bread wheat varieties and lines with wheat–wheatgrass substitutions. Russ. J. Genet. 2017, 53, 298–309. [Google Scholar] [CrossRef]

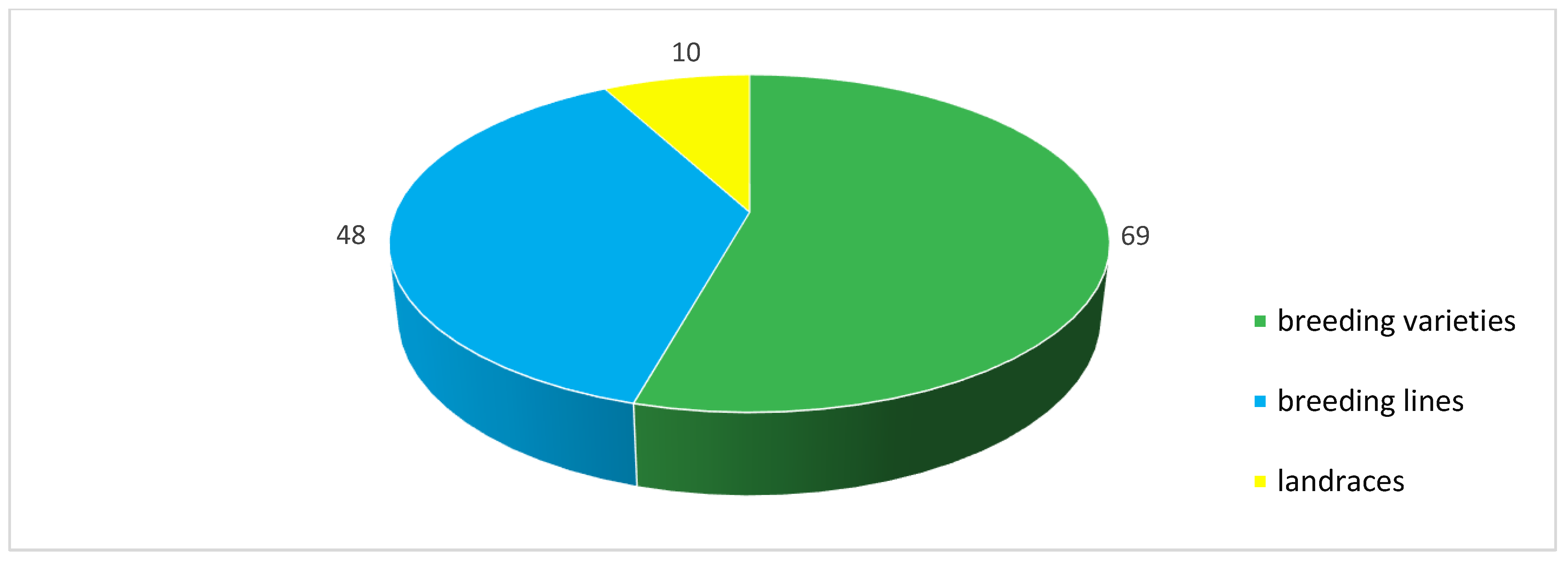
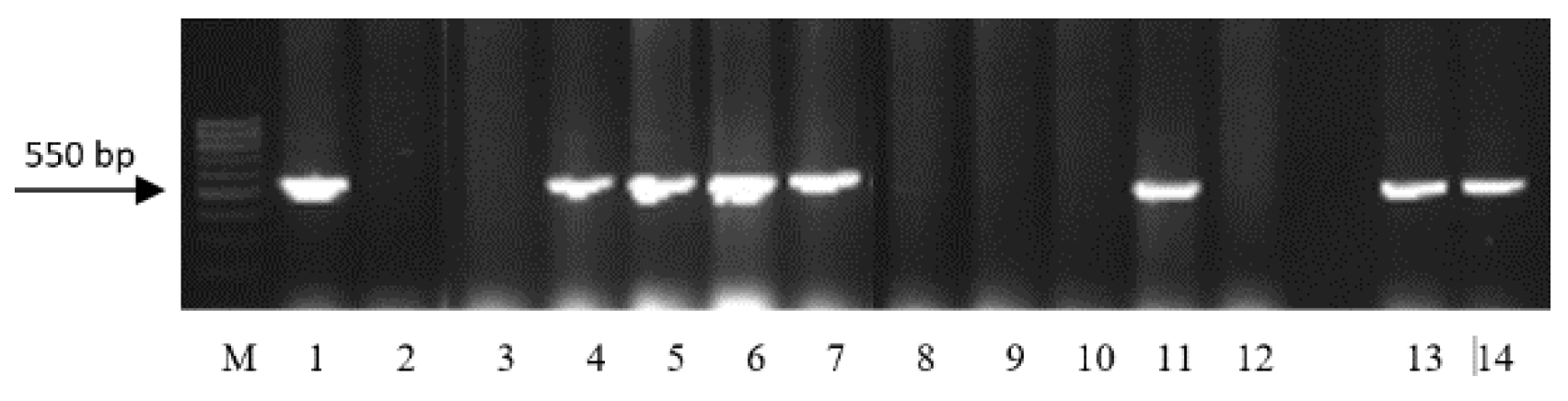
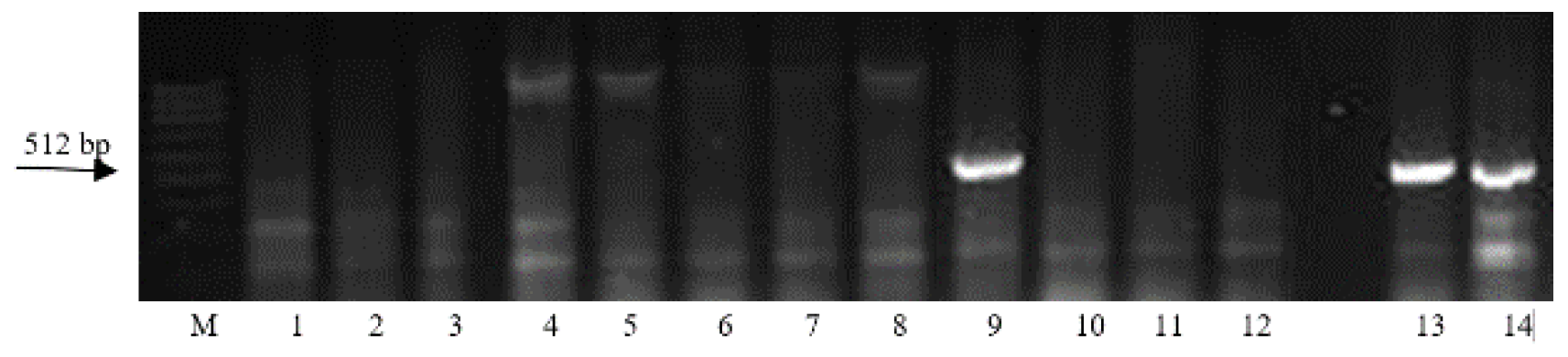
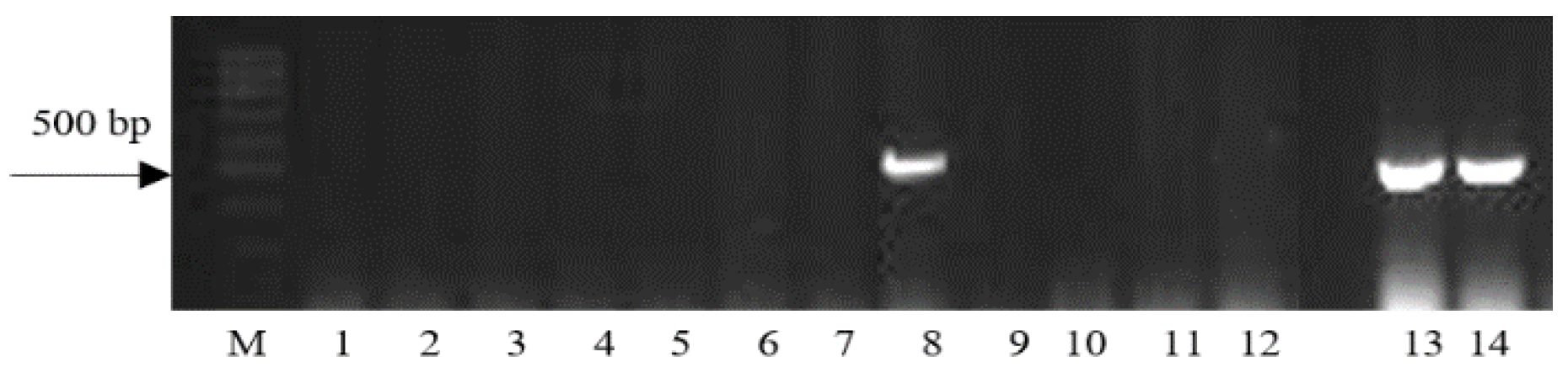
| Region | Number of Samples Evaluated at | ||
|---|---|---|---|
| DES | YES | PEF | |
| Russia | 1376 | 1217 | 2100 |
| Europe | 1226 | 802 | 1465 |
| Transcaucasia | 326 | 9 | 33 |
| Small Asia and the Middle East | 522 | 43 | 98 |
| Central Asia | 2811 | 683 | 884 |
| Southwest and East Asia | 571 | 112 | 413 |
| Africa | 635 | 129 | 227 |
| North and Central America | 1629 | 636 | 1604 |
| South America | 883 | 524 | 512 |
| Australia and New Zealand | 557 | 219 | 356 |
| Unknown | 13 | - | 12 |
| Total | 10,549 | 4384 | 7704 |
| % Whole collection | 70.1 | 29.1 | 51.2 |
| Disease Rating | Level of Resistance | Pustules | Leaf Surface Affected, % |
|---|---|---|---|
| 9 | Very high | Single, very small, surrounded with necrosis | 0–5 |
| 7 | High | Small, sometimes surrounded with chlorosis | <10 |
| 5 | Moderate | Small, without or with chlorosis | ~20 |
| 3 | Low | Large, sometimes merging, especially abundant at middle leaves | ~50 |
| 1 | Very low | Large, dense, merging at middle and sometimes upper leaves | >50 |
| Gene | Marker | Nucleotide Sequences of Primers | Size of DNA Amplification Product, bp | Reference |
|---|---|---|---|---|
| Lr9 | SCS5 | F: 5′-TGC GCC CTT CAA AGG AAG-3′ R: 5′-TGC GCC CTT CTG AAC TGT AT-3′ | 550 | [15] |
| Lr19 | SCS265 | F: 5′-GGC GGA TAA GCA GAG CAG AG-3′ R: 5′-GGC GGA TAA GTG GGT TAT GG-3′ | 512 | [16] |
| Lr24 | Sr24≠12 | F: 5′-CAC CCG TGA CAT GCT CGT A-3′ R: 5′-AAC AGG AAA TGA GCA ACG ATG T-3′ | 500 | [17] |
| Year | Disease Development | Year | Disease Development | Year | Disease Development | Year | Disease Development |
|---|---|---|---|---|---|---|---|
| 1962 | high | 1977 | high | 1992 | moderate | 2007 | high |
| 1963 | high | 1978 | high | 1993 | moderate | 2008 | high |
| 1964 | high | 1979 | moderate | 1994 | very low | 2009 | very low |
| 1965 | high | 1980 | high | 1995 | moderate | 2010 | very low |
| 1966 | moderate | 1981 | moderate | 1996 | low | 2011 | very low |
| 1967 | moderate | 1982 | moderate | 1997 | low | 2012 | moderate |
| 1968 | high | 1983 | moderate | 1998 | moderate | 2013 | very low |
| 1969 | high | 1984 | high | 1999 | low | 2014 | moderate |
| 1970 | moderate | 1985 | moderate | 2000 | very low | 2015 | very low |
| 1971 | very low | 1986 | moderate | 2001 | high | 2016 | moderate |
| 1972 | very low | 1987 | low | 2002 | very low | 2017 | moderate |
| 1973 | moderate | 1988 | moderate | 2003 | low | 2018 | very low |
| 1974 | high | 1989 | moderate | 2004 | moderate | 2019 | low |
| 1975 | high | 1990 | moderate | 2005 | moderate | 2020 | low |
| 1976 | moderate | 1991 | high | 2006 | moderate |
| Year | Disease Development | Year | Disease Development | Year | Disease Development | Year | Disease Development |
|---|---|---|---|---|---|---|---|
| 1970 | moderate | 1983 | high | 1996 | low | 2009 | high |
| 1971 | low | 1984 | high | 1997 | moderate | 2010 | low |
| 1972 | low | 1985 | high | 1998 | high | 2011 | high |
| 1973 | low | 1986 | low | 1999 | moderate | 2012 | moderate |
| 1974 | low | 1987 | high | 2000 | moderate | 2013 | high |
| 1975 | high | 1988 | low | 2001 | moderate | 2014 | moderate |
| 1976 | low | 1989 | high | 2002 | moderate | 2015 | low |
| 1977 | moderate | 1990 | high | 2003 | high | 2016 | moderate |
| 1978 | moderate | 1991 | high | 2004 | high | 2017 | high |
| 1979 | low | 1992 | high | 2005 | high | 2018 | low |
| 1980 | high | 1993 | high | 2006 | high | 2019 | low |
| 1981 | low | 1994 | high | 2007 | high | 2020 | moderate |
| 1982 | high | 1995 | high | 2008 | high |
| Year | Development of the Disease | Year | Development of the Disease | Year | Development of the Disease | Year | Development of the Disease |
|---|---|---|---|---|---|---|---|
| 1945 | moderate | 1968 | moderate | 1985 | moderate | 2001 | moderate |
| 1947 | moderate | 1969 | moderate | 1986 | high | 2002 | moderate |
| 1948 | moderate | 1970 | low | 1987 | low | 2004 | low |
| 1951 | moderate | 1971 | moderate | 1988 | moderate | 2006 | low |
| 1957 | high | 1972 | moderate | 1989 | high | 2007 | low |
| 1959 | moderate | 1973 | moderate | 1991 | moderate | 2009 | moderate |
| 1961 | high | 1974 | moderate | 1992 | low | 2010 | high |
| 1962 | moderate | 1975 | low | 1993 | low | 2013 | moderate |
| 1963 | high | 1980 | high | 1994 | moderate | 2014 | high |
| 1964 | high | 1981 | moderate | 1995 | moderate | 2017 | moderate |
| 1965 | moderate | 1982 | moderate | 1996 | low | 2019 | high |
| 1966 | high | 1983 | moderate | 1999 | moderate | 2020 | low |
| 1967 | moderate | 1984 | moderate | 2000 | high |
| Country/Region | Accessions |
|---|---|
| Australia | Skua (k-60613), Tasman (k-63180), Cunningham (k-64209), Sunstate (k-64218) |
| Brazil | OCEPAR 11—Juriti (k-62612), OCEPAR 8—Macuco (k-62614), BR 31—Miriti (k-62619), CEP 14—Tapes (k-60944), BR 34 (k-62185) |
| Canada | AC Barrie (k-64596) |
| Mexico | k-65603 |
| Netherlands | Tybalt (k-64897) |
| Poland | Nawra (k-64708) |
| South Africa | SST-23 (k-64138), SST-25 (k-64140) |
| Sweden | WW 17,283 (k-60997) |
| Switzerland | Toronit (k-66032) |
| United Kingdom | Sparrow (k-66090) |
| USA | Stoa (k-59033), Wampum (k-60588), MN 81,330 (k-60785), Russ (k-64595) |
| Unknown | PS 131 (k-64597), PS 133 (k-64598) |
| Russia, Altai | Altaiskaya 65 (k-64455), Altaiskaya 110 (k-65128) |
| Russia, Chelyabinsk | Quinta (k-63467), Duet (k-63500), Pamiaty Ruba (k-64378), Chelyaba 2 (to-64379), Chelyaba 75 (k-64871), Chelyaba Stepnaya (k-64872) |
| Russia, Kemerovo | Tuleevskaya k-63461) |
| Russia, Kurgan | Aria (k-64545) |
| Russia, Leningrad | LT 1 (k-65816) |
| Russia, Novosibirsk | ANK-4 (k-56395), Obskaya 14 (k-64363), Udacha (k-64372), Sibirskaya 24 (k-66442), Lubninka (k-64866), Novosibirskaya 44 (k-64867), Olga (k-65000), Novosibirskaya 18 (k-65820), Sibirskaya 17 (k-66017), Sibirskaya 21 (k-66269) |
| Russia, Omsk | Lavrusha (k-64984), Omskaya 41 (k-65253), OmGAU 100 (k-66387) |
| Russia, Penza | Yulia (k-63717); |
| Russia, Samara | Tulaikovskaya 10 (k-63714), Tulaikovskaya Zolotistaya (k-63715), Tulaikovskaya 100 (k-64643), Lutescens 30 (k-64647), Lutescens 101 (k-64648), Lutescens 13 (k-64649), Kinel’skaya Niva (k-64666), Tulaikovskaya 108 (k-65452), Ekada 113 (k-65453), Tulaikovskaya 110 (k-65454), Tulaikovskaya Nadezhda (k-65827), Kinel’skaya Yubileinaya (k-66270), Kinel’skaya Volna (k-66274), Tulaikovskaya 116 (k-66347); |
| Russia, Saratov | L 505 (k-62892), Dobrynya (k-64252), Voevoda (k-64997), Favorit (k-64998), Lebedushka (k-66410) |
| Russia, Tambov | Mertsana (k-65449) |
| Russia, Tyumen | Latona (k-64359), Tyumenka (k-66271); |
| Russia, Ulyanovsk | Ulyanovskaya 105 (k-66011) |
| Russia, Voronezh | Voronezhskaya 18 (k-65998), Voronezhskaya 20 (k-66257) |
| VIR Catalogue Number | Variety or Line | Origin | Resistant at Experimental Stations | Types of Reaction in Seedlings | Postulated Effective Lr Genes |
|---|---|---|---|---|---|
| 60980 | BR 16—Rio Verde | Brazil | YES, PEF | 3 | |
| 61523 | Henika | Poland | YES, PEF | 3 | |
| 62515 | Amidon | USA | YES, DES | 3 | |
| 62552 | HI 977 | India | YES, DES, PEF | 3 | |
| 62874 | Nordic | USA | YES, DES | 3 | |
| 62875 | Norm | USA | YES, DES | 3 | |
| 62878 | AC Minto | Canada | YES, DES | 3 | |
| 62893 | Ranniaya 93 | Ukraine | YES, DES | 3 | |
| 62898 | CDC Teal | Canada | YES, DES | 3 | |
| 63058 | Fjeld | USA | YES, DES | 3 | |
| 63194 | Swift | Australia | YES, DES | 3 | |
| 63215 | Long 82-2124-1 | China | YES, DES | 3 | |
| 64443 | Tepoca | Mexico | YES, PEF | 3 | |
| 64892 | McKenzie | Canada | YES, PEF | 3 | |
| 64643 | Tulaikovskaya 100 | RF, Samara region | DES, YES | 0 | Lr6Agi2 * |
| 64647 | Lutescens 30 | RF, Samara region | PEF, YES | 0 | Lr9 |
| 64666 | Kinelskaya Niva | RF, Samara region | DES, YES | 0 | Lr19 |
| 64866 | Lubninka | RF, Novosibirsk Region | DES, YES | 0 | Lr9 |
| 64867 | Novosibirskaya 44 | RF, Novosibirsk Region | DES, YES | 0 | Lr9 |
| 64872 | Cheliaba Stepnaya | RF, Chelyabinsk Region | DES, YES | 0 | Lr9 |
| 64997 | Voevoda | RF, Saratov Region | DES, YES | 0 | Lr6Agi1i * |
| 64998 | Favorit | RF, Saratov Region | DES, YES | 0 | Lr6Agi1 * |
| 65000 | Olga | RF, Novosibirsk Region | DES, YES | 0 | Lr9 |
| 56395 | ANK-4 | RF, Novosibirsk Region | YES, DES, PEF | 0 | Lr9 |
| 63180 | Tasman | Australia | YES, DES, PEF | 0 | Lr24 |
| 65816 | LT 1 | RF, Leningrad region | YES, DES, PEF | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tyryshkin, L.G.; Zeleneva, Y.V.; Brykova, A.N.; Kudryavtseva, E.Y.; Loseva, V.A.; Akhmedov, M.A.; Shikhmuradov, A.Z.; Zuev, E.V. Long-Term Multilocal Monitoring of Leaf Rust Resistance in the Spring Bread Wheat Genetic Resources from Institute of Plant Genetic Resources (VIR). Agronomy 2022, 12, 242. https://doi.org/10.3390/agronomy12020242
Tyryshkin LG, Zeleneva YV, Brykova AN, Kudryavtseva EY, Loseva VA, Akhmedov MA, Shikhmuradov AZ, Zuev EV. Long-Term Multilocal Monitoring of Leaf Rust Resistance in the Spring Bread Wheat Genetic Resources from Institute of Plant Genetic Resources (VIR). Agronomy. 2022; 12(2):242. https://doi.org/10.3390/agronomy12020242
Chicago/Turabian StyleTyryshkin, Lev Gennadievich, Yuliya Vital’evna Zeleneva, Alla Nikolaevna Brykova, Evgeniya Yurievna Kudryavtseva, Valentina Alekseevna Loseva, Magomed Alievich Akhmedov, Asef Zilfikarovich Shikhmuradov, and Evgeny Valerievich Zuev. 2022. "Long-Term Multilocal Monitoring of Leaf Rust Resistance in the Spring Bread Wheat Genetic Resources from Institute of Plant Genetic Resources (VIR)" Agronomy 12, no. 2: 242. https://doi.org/10.3390/agronomy12020242
APA StyleTyryshkin, L. G., Zeleneva, Y. V., Brykova, A. N., Kudryavtseva, E. Y., Loseva, V. A., Akhmedov, M. A., Shikhmuradov, A. Z., & Zuev, E. V. (2022). Long-Term Multilocal Monitoring of Leaf Rust Resistance in the Spring Bread Wheat Genetic Resources from Institute of Plant Genetic Resources (VIR). Agronomy, 12(2), 242. https://doi.org/10.3390/agronomy12020242






