Early Effects of No-Till Use on Durum Wheat (Triticum durum Desf.): Productivity and Soil Functioning Vary between Two Contrasting Mediterranean Soils
Abstract
1. Introduction
2. Materials and Methods
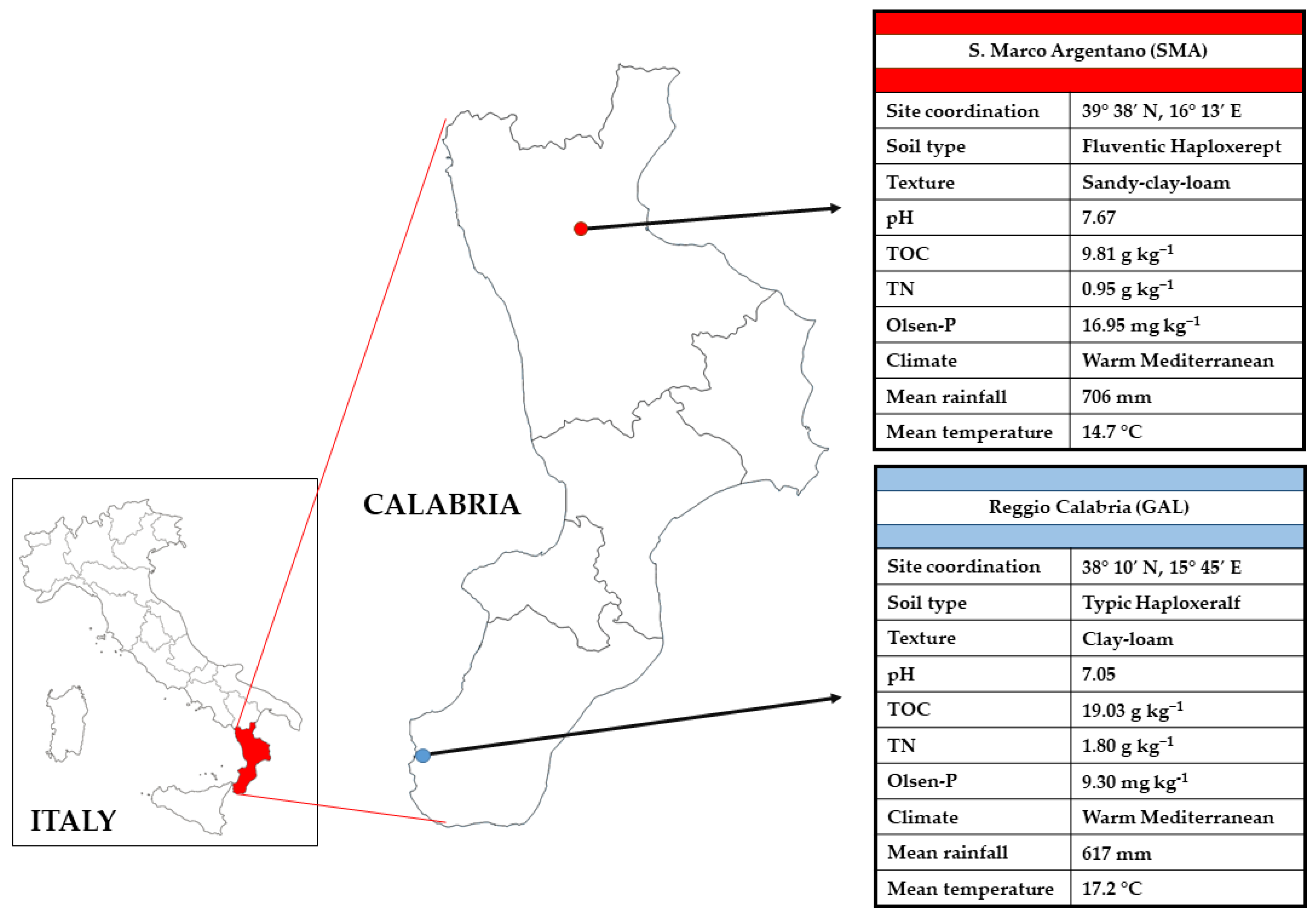
2.1. Experimental Sites Description
2.2. Experimental Design and Crop Management Sites Description
2.3. Plant Biomass Sampling and Analyses
2.4. Soil Sampling and Analyses
2.5. Statistical Analyses
3. Results
3.1. Weather Conditions
3.2. Wheat Growth, Yield and Grain Quality
3.3. Crop N and P Concentrations and Uptakes
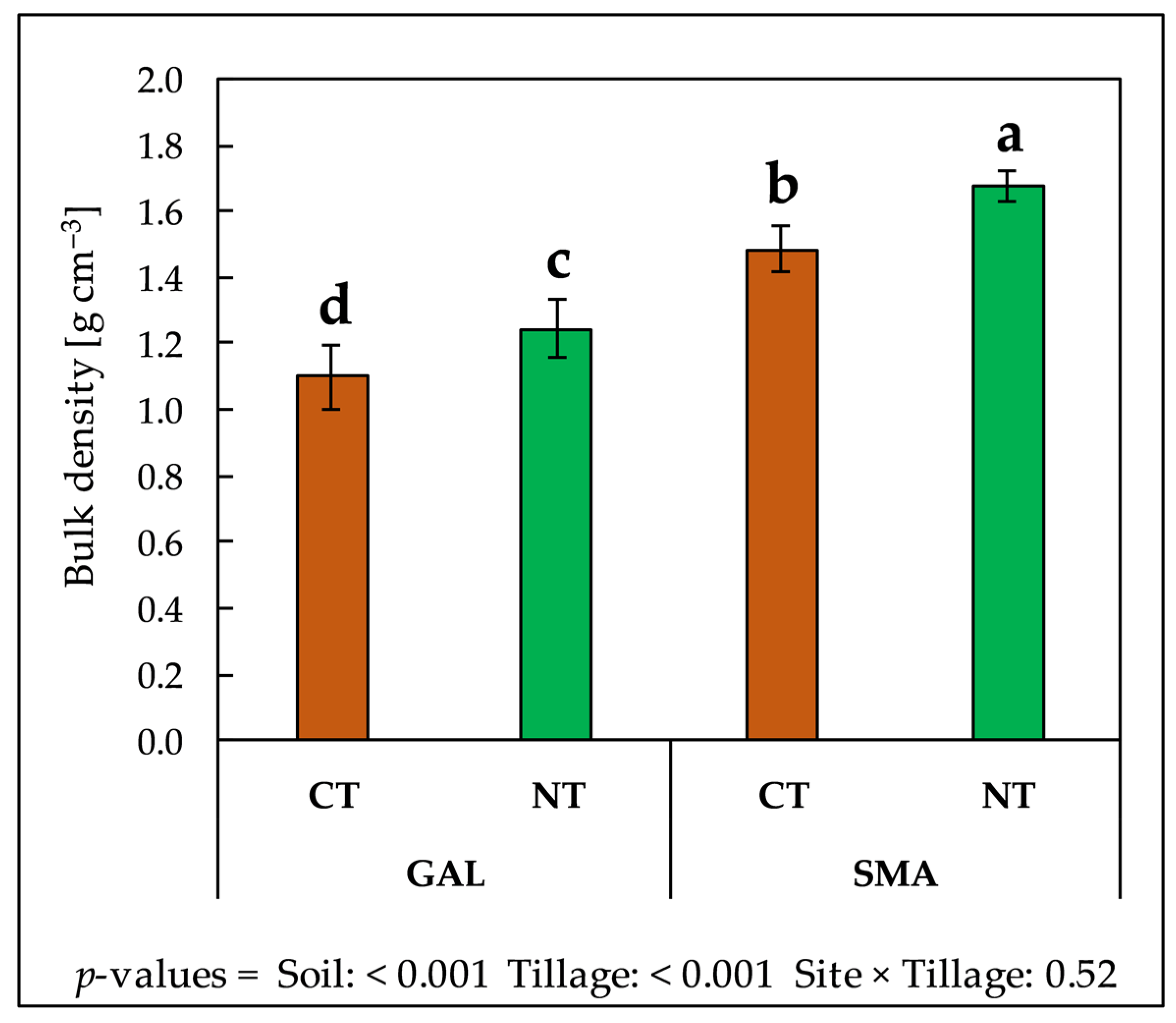
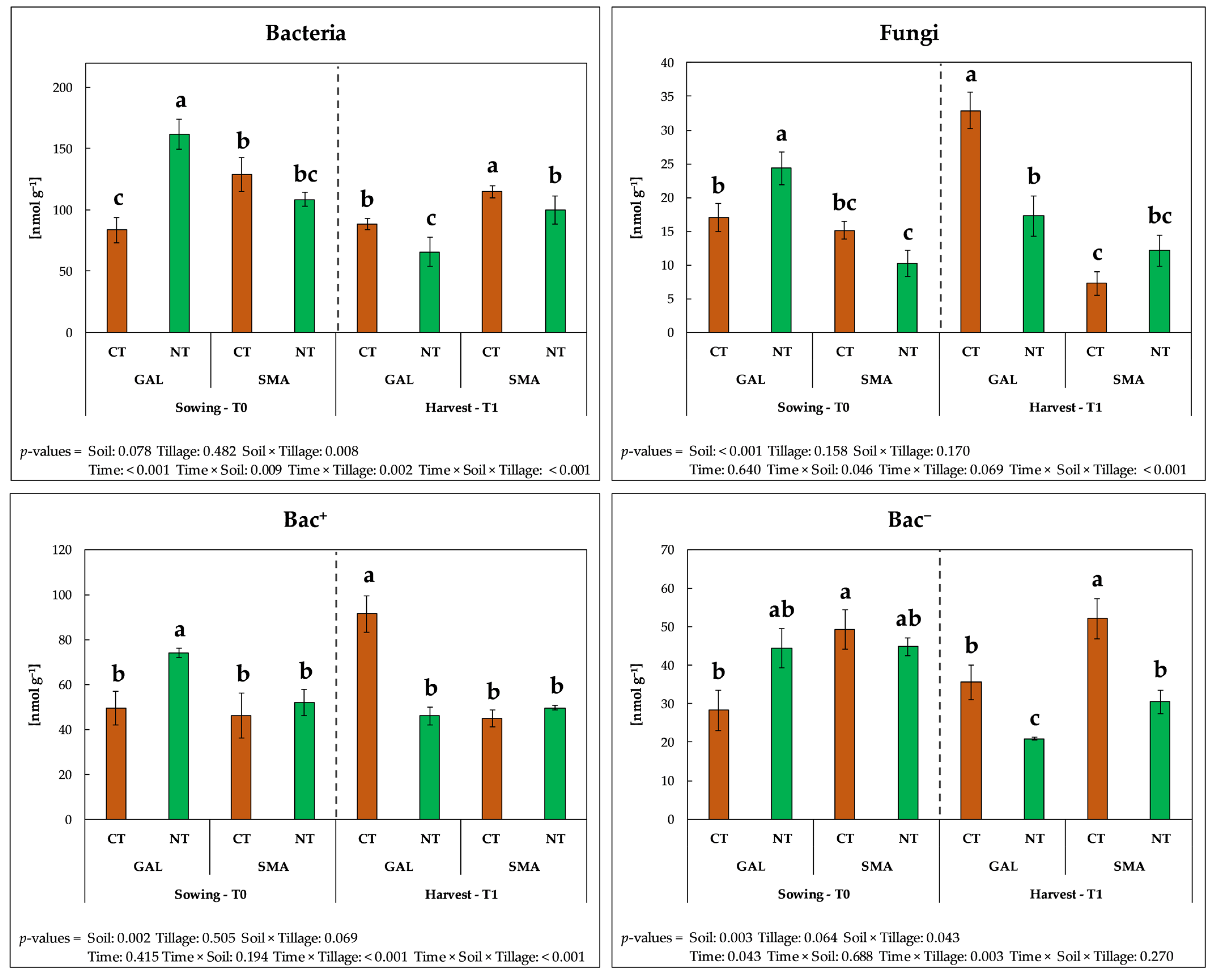
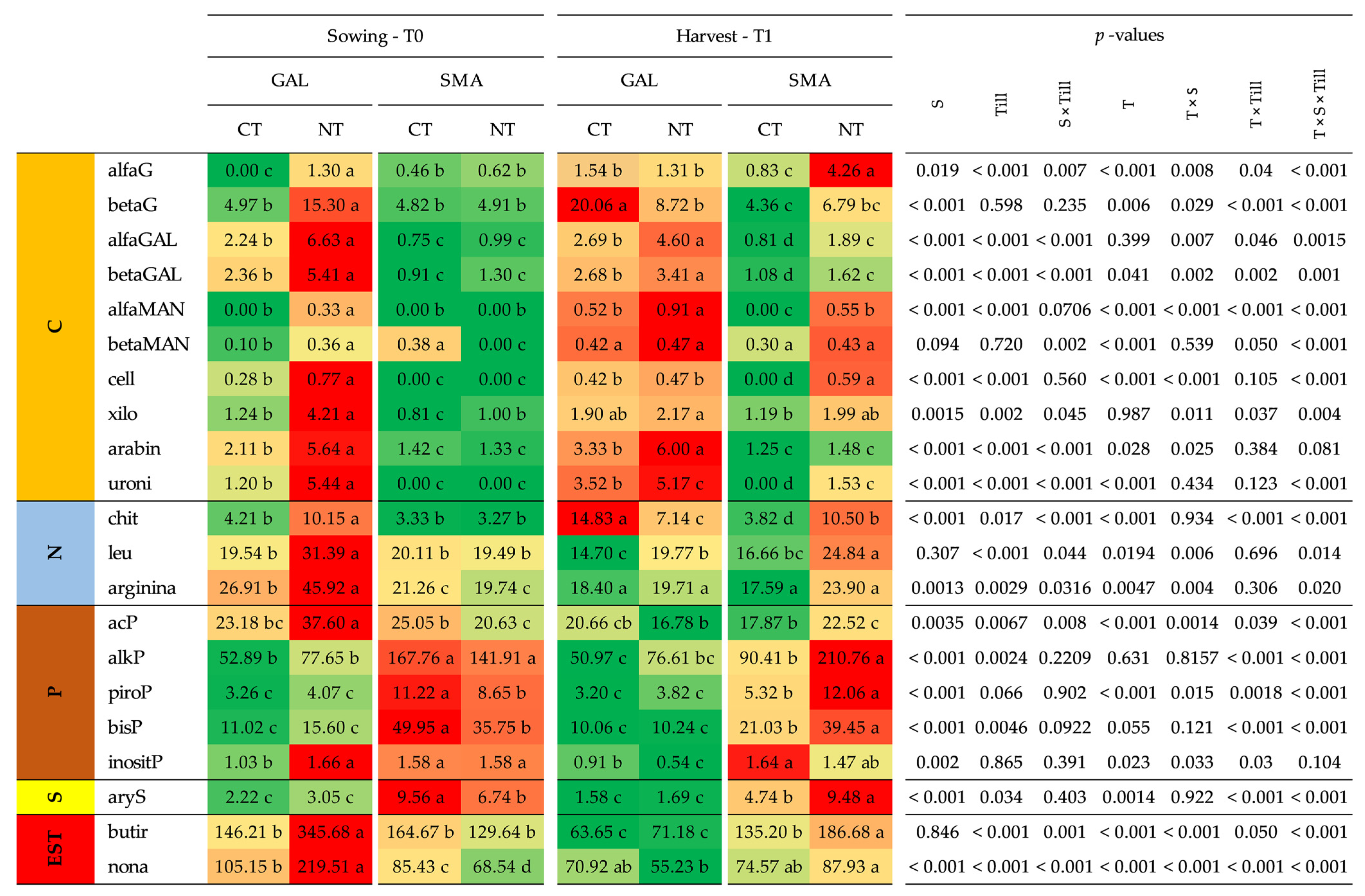
3.4. Soil Physical, Chemical and Biochemical Properties
3.5. Principal Component Analysis
3.6. Pearson’s Correlations Analysis
3.7. Multiple Linear Regressions
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Pittelkow, C.M.; Linquist, B.A.; Lundy, M.E.; Liang, X.; van Groenigen, K.J.; Lee, J.; van Gestel, N.; Six, J.; Venterea, R.T.; van Kessel, C. When does no-till yield more? A global meta-analysis. Field Crops Res. 2015, 183, 156–168. [Google Scholar] [CrossRef]
- Paustian, K.; Lehmann, J.; Ogle, S.; Reay, D.; Robertson, G.P.; Smith, P. Climate-smart soils. Nature 2016, 532, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Jordan, V.W.L.; Leake, A.R.; Ogilvy, S. Agronomic and environmental implications of soil management practices in integrated farming systems. Asp. Appl. Biol. 2000, 62, 61–66. [Google Scholar]
- Madari, B.; Machado, P.L.O.A.; Torres, E.; de Andrade, A.G.; Valencia, L.I.O. No tillage and crop rotation effects on soil aggregation and organic carbon in a Rhodic Ferralsol from southern Brazil. Soil Tillage Res. 2005, 80, 185–200. [Google Scholar] [CrossRef]
- Badagliacca, G.; Petrovičovà, B.; Pathan, S.I.; Roccotelli, A.; Romeo, M.; Monti, M.; Gelsomino, A. Use of solid anaerobic digestate and no-tillage practice for restoring the fertility status of two Mediterranean orchard soils with contrasting properties. Agric. Ecosyst. Environ. 2020, 300, 107010. [Google Scholar] [CrossRef]
- Shen, Y.; McLaughlin, N.; Zhang, X.; Xu, M.; Liang, A. Effect of tillage and crop residue on soil temperature following planting for a Black soil in Northeast China. Sci. Rep. 2018, 8, 4500. [Google Scholar] [CrossRef]
- Skaalsveen, K.; Ingram, J.; Clarke, L.E. The effect of no-till farming on the soil functions of water purification and retention in north-western Europe: A literature review. Soil Tillage Res. 2019, 189, 98–109. [Google Scholar] [CrossRef]
- Sekaran, U.; Sagar, K.L.; Denardin, L.G.D.O.; Singh, J.; Singh, N.; Abagandura, G.O.; Kumar, S.; Farmaha, B.S.; Bly, A.; Martins, A.P. Responses of soil biochemical properties and microbial community structure to short and long-term no-till systems. Eur. J. Soil Sci. 2020, 71, 1018–1033. [Google Scholar] [CrossRef]
- West, T.O.; Marland, G. A synthesis of carbon sequestration, carbon emissions, and net carbon flux in agriculture: Comparing tillage practices in the United States. Agric. Ecosyst. Environ. 2002, 91, 217–232. [Google Scholar] [CrossRef]
- Crotty, F.V.; Fychan, R.; Sanderson, R.; Rhymes, J.R.; Bourdin, F.; Scullion, J.; Marley, C.L. Understanding the legacy effect of previous forage crop and tillage management on soil biology, after conversion to an arable crop rotation. Soil Biol. Biochem. 2016, 103, 241–252. [Google Scholar] [CrossRef]
- Badagliacca, G.; Benítez, E.; Amato, G.; Badalucco, L.; Giambalvo, D.; Laudicina, V.A.; Ruisi, P. Long-term effects of contrasting tillage on soil organic carbon, nitrous oxide and ammonia emissions in a Mediterranean Vertisol under different crop sequences. Sci. Total Environ. 2018, 619–620, 18–27. [Google Scholar] [CrossRef]
- Kirkegaard, J. A review of trends in wheat yield responses to conservation cropping in Australia. Aust. J. Exp. Agric. 1995, 35, 835. [Google Scholar] [CrossRef]
- Kassam, A.; Friedrich, T.; Derpsch, R.; Lahmar, R.; Mrabet, R.; Basch, G.; González-Sánchez, E.J.; Serraj, R. Conservation agriculture in the dry Mediterranean climate. Field Crops Res. 2012, 132, 7–17. [Google Scholar] [CrossRef]
- Minasny, B.; Malone, B.P.; McBratney, A.B.; Angers, D.A.; Arrouays, D.; Chambers, A.; Chaplot, V.; Chen, Z.-S.S.; Cheng, K.; Das, B.S.; et al. Soil carbon 4 per mille. Geoderma 2017, 292, 59–86. [Google Scholar] [CrossRef]
- Escolano, J.J.; Pedreño, J.N.; Lucas, I.G.; Almendro Candel, M.B.; Zorpas, A.A. Decreased organic carbon associated with land management in Mediterranean environments. In Soil Management and Climate Change; Academic Press: Cambridge, MA, USA, 2018; pp. 1–13. [Google Scholar]
- Boselli, R.; Fiorini, A.; Santelli, S.; Ardenti, F.; Capra, F.; Maris, S.C.; Tabaglio, V. Cover crops during transition to no-till maintain yield and enhance soil fertility in intensive agro-ecosystems. Field Crops Res. 2020, 255, 107871. [Google Scholar] [CrossRef]
- Johnston, A.E.; Poulton, P.R. The importance of long-term experiments in agriculture: Their management to ensure continued crop production and soil fertility; the Rothamsted experience. Eur. J. Soil Sci. 2018, 69, 113–125. [Google Scholar] [CrossRef]
- Mbuthia, L.W.; Acosta-Martínez, V.; DeBruyn, J.; Schaeffer, S.; Tyler, D.; Odoi, E.; Mpheshea, M.; Walker, F.; Eash, N. Long term tillage, cover crop, and fertilization effects on microbial community structure, activity: Implications for soil quality. Soil Biol. Biochem. 2015, 89, 24–34. [Google Scholar] [CrossRef]
- Ruisi, P.; Giambalvo, D.; Saia, S.; Di Miceli, G.; Frenda, A.S.; Plaia, A.; Amato, G. Conservation tillage in a semiarid Mediterranean environment: Results of 20 years of research. Ital. J. Agron. 2014, 9, 1. [Google Scholar] [CrossRef]
- Schlegel, A.J.; Assefa, Y.; Haag, L.A.; Thompson, C.R.; Stone, L.R. Long-term tillage on yield and water use of grain sorghum and winter wheat. Agron. J. 2018, 110, 269–280. [Google Scholar] [CrossRef]
- Peng, Z.; Wang, L.; Xie, J.; Li, L.; Coulter, J.A.; Zhang, R.; Luo, Z.; Cai, L.; Carberry, P.; Whitbread, A. Conservation tillage increases yield and precipitation use efficiency of wheat on the semi-arid Loess Plateau of China. Agric. Water Manag. 2020, 231, 106024. [Google Scholar] [CrossRef]
- Ernst, O.R.; Dogliotti, S.; Cadenazzi, M.; Kemanian, A.R. Shifting crop-pasture rotations to no-till annual cropping reduces soil quality and wheat yield. Field Crops Res. 2018, 217, 180–187. [Google Scholar] [CrossRef]
- Woźniak, A.; Rachoń, L. Effect of tillage systems on the yield and quality of winter wheat grain and soil properties. Agriculture 2020, 10, 405. [Google Scholar] [CrossRef]
- Chen, S.; Yang, P.; Zhang, Y.; Dong, W.; Hu, C.; Oenema, O. Responses of cereal yields and soil carbon sequestration to four long-term tillage practices in the North China plain. Agronomy 2022, 12, 176. [Google Scholar] [CrossRef]
- Woźniak, A. The effect of tillage systems on yield and quality of durum wheat cultivars. Turk. J. Agric. For. 2013, 37, 133–138. [Google Scholar] [CrossRef]
- Kan, Z.R.; Liu, Q.Y.; He, C.; Jing, Z.H.; Virk, A.L.; Qi, J.Y.; Zhao, X.; Zhang, H.L. Responses of grain yield and water use efficiency of winter wheat to tillage in the North China Plain. Field Crops Res. 2020, 249, 107760. [Google Scholar] [CrossRef]
- López-Bellido, L.; Fuentes, M.; Castillo, J.E.; López-Garrido, F.J.; Fernández, E.J. Long-term tillage, crop rotation, and nitrogen fertilizer effects on wheat yield under rainfed Mediterranean conditions. Agron. J. 1996, 88, 783–791. [Google Scholar] [CrossRef]
- Amato, G.; Ruisi, P.; Frenda, A.S.; Di Miceli, G.; Saia, S.; Plaia, A.; Giambalvo, D. Long-term tillage and crop sequence effects on wheat grain yield and quality. Agron. J. 2013, 105, 1317–1327. [Google Scholar] [CrossRef]
- Six, J.; Ogle, S.M.; Jay Breidt, F.; Conant, R.T.; Mosier, A.R.; Paustian, K. The potential to mitigate global warming with no-tillage management is only realized when practised in the long term. Glob. Chang. Biol. 2004, 10, 155–160. [Google Scholar] [CrossRef]
- Helgason, B.L.; Walley, F.L.; Germida, J.J. Long-term no-till management affects microbial biomass but not community composition in Canadian prairie agroecosytems. Soil Biol. Biochem. 2010, 42, 2192–2202. [Google Scholar] [CrossRef]
- González-Sánchez, E.J.; Ordóñez-Fernández, R.; Carbonell-Bojollo, R.; Veroz-González, O.; Gil-Ribes, J.A. Meta-analysis on atmospheric carbon capture in Spain through the use of conservation agriculture. Soil Tillage Res. 2012, 122, 52–60. [Google Scholar] [CrossRef]
- Adetunji, A.T.; Lewu, F.B.; Mulidzi, R.; Ncube, B. The biological activities of β-glucosidase, phosphatase and urease as soil quality indicators: A review. J. Soil Sci. Plant Nutr. 2017, 17, 794–807. [Google Scholar] [CrossRef]
- Zuber, S.M.; Villamil, M.B. Soil Biology & Biochemistry Meta-analysis approach to assess effect of tillage on microbial biomass and enzyme activities. Soil Biol. Biochem. 2016, 97, 176–187. [Google Scholar]
- Stagnari, F.; Perpetuini, G.; Tofalo, R.; Campanelli, G.; Leteo, F.; Della Vella, U.; Schirone, M.; Suzzi, G.; Pisante, M. Long-term impact of farm management and crops on soil microorganisms assessed by combined DGGE and PLFA analyses. Front. Microbiol. 2014, 5, 644. [Google Scholar] [CrossRef]
- Giambalvo, D.; Amato, G.; Badagliacca, G.; Ingraffia, R.; Di Miceli, G.; Frenda, A.S.; Plaia, A.; Venezia, G.; Ruisi, P. Switching from conventional tillage to no-tillage: Soil N availability, N uptake, 15 N fertilizer recovery, and grain yield of durum wheat. Field Crops Res. 2018, 218, 171–181. [Google Scholar] [CrossRef]
- Melero, S.; López-Bellido, R.J.; López-Bellido, L.; Muñoz-Romero, V.; Moreno, F.; Murillo, J.M. Long-term effect of tillage, rotation and nitrogen fertiliser on soil quality in a Mediterranean Vertisol. Soil Tillage Res. 2011, 114, 97–107. [Google Scholar] [CrossRef]
- López-Bellido, L.; Muñoz-Romero, V.; Benítez-Vega, J.; Fernández-García, P.; Redondo, R.; López-Bellido, R.J. Wheat response to nitrogen splitting applied to a Vertisols in different tillage systems and cropping rotations under typical Mediterranean climatic conditions. Eur. J. Agron. 2012, 43, 24–32. [Google Scholar] [CrossRef]
- Badagliacca, G.; Laudicina, V.A.; Amato, G.; Badalucco, L.; Frenda, A.S.; Giambalvo, D.; Ingraffia, R.; Plaia, A.; Ruisi, P. Long-term effects of contrasting tillage systems on soil C and N pools and on main microbial groups differ by crop sequence. Soil Tillage Res. 2021, 211, 104995. [Google Scholar] [CrossRef]
- Soil Survey Staff. Keys to soil taxonomy. Soil Conserv. Serv. 2014, 12, 410. [Google Scholar]
- AOAC International. Official Methods of Analysis, 15th ed.; AOAC International: Arlington, VA, USA, 2016. [Google Scholar]
- Fujihara, S.; Sasaki, H.; Aoyagi, Y.; Sugahara, T. nitrogen-to-protein conversion factors for some cereal products in japan. J. Food Sci. 2008, 73, C204–C209. [Google Scholar] [CrossRef]
- List, D.; Ruwisch, I.; Langhans, P. Potential applications of flow injection analysis in fruit juice analysis. Fleuss. Obst. 1986, 53. [Google Scholar]
- Ruzicka, J.; Růžička, J.; Hansen, E.H. Flow Injection Analysis; John Wiley & Sons: Hoboken, NJ, USA, 1981; Volume 62, ISBN 0471081922. [Google Scholar]
- Weil, R.R.; Islam, K.R.; Stine, M.A.; Gruver, J.B.; Samson-Liebig, S.E. Estimating active carbon for soil quality assessment: A simplified method for laboratory and field use. Am. J. Altern. Agric. 2003, 18, 3–17. [Google Scholar]
- Culman, S.W.; Snapp, S.S.; Freeman, M.A.; Schipanski, M.E.; Beniston, J.; Lal, R.; Drinkwater, L.E.; Franzluebbers, A.J.; Glover, J.D.; Grandy, A.S.; et al. Permanganate oxidizable carbon reflects a processed soil fraction that is sensitive to management. Soil Sci. Soc. Am. J. 2012, 76, 494. [Google Scholar] [CrossRef]
- Olsen, S.R.; Cole, C.V.; Watandbe, F.; Dean, L. Estimation of Available Phosphorus in Soil by Extraction with sodium Bicarbonate. J. Chem. Inf. Model. 1954, 53, 1689–1699. [Google Scholar]
- Grossman, R.B.; Reinsch, T.G. Core method. In Methods of Soil Analysis. Part 4 Physical Methods; Dane, J.B.H.G., Topp, C., Eds.; Soil Science Society of America Inc.: Madison, WI, USA, 2002; Volume 4, pp. 207–210. [Google Scholar]
- White, D.C.; Davis, W.M.; Nickels, J.S.; King, J.D.; Bobbie, R.J. Determination of the sedimentary microbial biomass by extractible lipid phosphate. Oecologia 1979, 40, 51–62. [Google Scholar] [CrossRef]
- Wu, Y.; Ding, N.; Wang, G.; Xu, J.; Wu, J.; Brookes, P.C. Effects of different soil weights, storage times and extraction methods on soil phospholipid fatty acid analyses. Geoderma 2009, 150, 171–178. [Google Scholar] [CrossRef]
- Guckert, J.B.; Antworth, C.P.; Nichols, P.D.; White, D.C. Phospholipid, ester-linked fatty acid profiles as reproducible assays for changes in prokaryotic community structure of estuarine sediments. FEMS Microbiol. Lett. 1985, 31, 147–158. [Google Scholar] [CrossRef]
- Frostegard, A.; Baath, E. The use of phospholipid fatty acid analysis to estimate bacterial and fungal biomass in soil. Biol. Fertil. Soils 1996, 22, 59–65. [Google Scholar] [CrossRef]
- Zelles, L. Phospholipid fatty acid profiles in selected members of soil microbial communities. Chemosphere 1997, 35, 275–294. [Google Scholar] [CrossRef]
- Ferrarini, A.; Martani, E.; Fornasier, F.; Amaducci, S. High C input by perennial energy crops boosts belowground functioning and increases soil organic P content. Agric. Ecosyst. Environ. 2021, 308, 107247. [Google Scholar] [CrossRef]
- Bardelli, T.; Gómez-Brandón, M.; Ascher-Jenull, J.; Fornasier, F.; Arfaioli, P.; Francioli, D.; Egli, M.; Sartori, G.; Insam, H.; Pietramellara, G. Effects of slope exposure on soil physico-chemical and microbiological properties along an altitudinal climosequence in the Italian Alps. Sci. Total Environ. 2017, 575, 1041–1055. [Google Scholar] [CrossRef]
- Cowie, A.L.; Lonergan, V.E.; Rabbi, S.M.F.; Fornasier, F.; MacDonald, C.; Harden, S.; Kawasaki, A.; Singh, B.K. Impact of carbon farming practices on soil carbon in northern New South Wales. Arid Soil Res. Rehabil. 2013, 51, 707–718. [Google Scholar] [CrossRef]
- R CoreTeam. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Lê, S.; Josse, J.; Husson, F. FactoMineR: An R Package for Multivariate Analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef]
- Kassambara, A.; Mundt, F. Factoextra: Extract and visualize the results of multivariate data analyses. R Packag. Version 2017, 1, 337–354. [Google Scholar]
- Wei, T.; Simko, V. R Package “Corrplot”: Visualization of a Correlation Matrix. Version 0.84; 2017; Available online: https://github.com/taiyun/corrplot (accessed on 23 October 2022).
- Blanco-Canqui, H.; Stone, L.R.; Schlegel, A.J.; Lyon, D.J.; Vigil, M.F.; Mikha, M.M.; Stahlman, P.W.; Rice, C.W. No-till induced increase in organic carbon reduces maximum bulk density of soils. Soil Sci. Soc. Am. J. 2009, 73, 1871–1879. [Google Scholar] [CrossRef]
- Gozubuyuk, Z.; Sahin, U.; Ozturk, I.; Celik, A.; Adiguzel, M.C. Tillage effects on certain physical and hydraulic properties of a loamy soil under a crop rotation in a semi-arid region with a cool climate. Catena 2014, 118, 195–205. [Google Scholar] [CrossRef]
- Monti, M.; Badagliacca, G.; Romeo, M.; Gelsomino, A. No-Till and solid digestate amendment selectively affect the potential denitrification activity in two Mediterranean orchard soils. Soil Syst. 2021, 5, 31. [Google Scholar] [CrossRef]
- Keller, T.; Håkansson, I. Estimation of reference bulk density from soil particle size distribution and soil organic matter content. Geoderma 2010, 154, 398–406. [Google Scholar] [CrossRef]
- Reichert, J.M.; Mentges, M.I.; Rodrigues, M.F.; Cavalli, J.P.; Awe, G.O.; Mentges, L.R. Compressibility and elasticity of subtropical no-till soils varying in granulometry organic matter, bulk density and moisture. Catena 2018, 165, 345–357. [Google Scholar] [CrossRef]
- Badagliacca, G.; Romeo, M.; Lo Presti, E.; Gelsomino, A.; Monti, M. Factors governing total and permanganate oxidizable C pools in agricultural soils from southern Italy. Agriculture 2020, 10, 99. [Google Scholar] [CrossRef]
- López-Bellido, R.J.; Fontán, J.M.; López-Bellido, F.J.; López-Bellido, L. Carbon sequestration by tillage, rotation, and nitrogen fertilization in a Mediterranean Vertisol. Agron. J. 2010, 102, 310. [Google Scholar] [CrossRef]
- Liu, E.; Teclemariam, S.G.; Yan, C.; Yu, J.; Gu, R.; Liu, S.; He, W.; Liu, Q. Long-term effects of no-tillage management practice on soil organic carbon and its fractions in the northern China. Geoderma 2014, 213, 379–384. [Google Scholar] [CrossRef]
- Six, J.; Elliott, E.; Paustian, K. Soil macroaggregate turnover and microaggregate formation: A mechanism for C sequestration under no-tillage agriculture. Soil Biol. Biochem. 2000, 32, 2099–2103. [Google Scholar] [CrossRef]
- Sarker, J.R.; Singh, B.P.; Cowie, A.L.; Fang, Y.; Collins, D.; Dougherty, W.J.; Singh, B.K. Carbon and nutrient mineralisation dynamics in aggregate-size classes from different tillage systems after input of canola and wheat residues. Soil Biol. Biochem. 2018, 116, 22–38. [Google Scholar] [CrossRef]
- Majumder, B.; Kuzyakov, Y. Effect of fertilization on decomposition of 14C labelled plant residues and their incorporation into soil aggregates. Soil Tillage Res. 2010, 109, 94–102. [Google Scholar] [CrossRef]
- Shi, A.; Marschner, P. Drying and rewetting frequency influences cumulative respiration and its distribution over time in two soils with contrasting management. Soil Biol. Biochem. 2014, 72, 172–179. [Google Scholar] [CrossRef]
- Vázquez, E.; Teutscherova, N.; Dannenmann, M.; Töchterle, P.; Butterbach-Bahl, K.; Pulleman, M.; Arango, J. Gross nitrogen transformations in tropical pasture soils as affected by Urochloa genotypes differing in biological nitrification inhibition (BNI) capacity. Soil Biol. Biochem. 2020, 151, 108058. [Google Scholar] [CrossRef]
- Dai, Z.; Hu, J.; Fan, J.; Fu, W.; Wang, H.; Hao, M. No-tillage with mulching improves maize yield in dryland farming through regulating soil temperature, water and nitrate-N. Agric. Ecosyst. Environ. 2021, 309, 107288. [Google Scholar] [CrossRef]
- Badagliacca, G.; Benítez, E.; Amato, G.; Badalucco, L.; Giambalvo, D.; Laudicina, V.A.; Ruisi, P. Long-term no-tillage application increases soil organic carbon, nitrous oxide emissions and faba bean (Vicia faba L.) yields under rain-fed Mediterranean conditions. Sci. Total Environ. 2018, 639, 350–359. [Google Scholar] [CrossRef]
- Khan, A.R. Influence of tillage on soil aeration. J. Agron. Crop Sci. 1996, 177, 253–259. [Google Scholar] [CrossRef]
- Laudicina, V.A.; Palazzolo, E.; Piotrowska-Długosz, A.; Badalucco, L. Soil profile dismantlement by land levelling and deep tillage damages soil functioning but not quality. Appl. Soil Ecol. 2016, 107, 298–306. [Google Scholar] [CrossRef]
- Essington, M.E.; Howard, D.D. Phosphorus availability and speciation in long-term no-till and disk-till soil. Soil Sci. 2000, 165, 144–152. [Google Scholar] [CrossRef]
- Martinrueda, I.; Munozguerra, L.; Yunta, F.; Esteban, E.; Tenorio, J.; Lucena, J. Tillage and crop rotation effects on barley yield and soil nutrients on a Calciortidic Haploxeralf. Soil Tillage Res. 2007, 92, 1–9. [Google Scholar] [CrossRef]
- Obour, A.K.; Mikha, M.M.; Holman, J.D.; Stahlman, P.W. Changes in soil surface chemistry after fifty years of tillage and nitrogen fertilization. Geoderma 2017, 308, 46–53. [Google Scholar] [CrossRef]
- Cookson, W.R.; Murphy, D.V.; Roper, M.M. Characterizing the relationships between soil organic matter components and microbial function and composition along a tillage disturbance gradient. Soil Biol. Biochem. 2008, 40, 763–777. [Google Scholar] [CrossRef]
- Gil, S.V.; Meriles, J.; Conforto, C.; Basanta, M.; Radl, V.; Hagn, A.; Schloter, M.; March, G.J. Response of soil microbial communities to different management practices in surface soils of a soybean agroecosystem in Argentina. Eur. J. Soil Biol. 2011, 47, 55–60. [Google Scholar]
- García-Orenes, F.; Morugán-Coronado, A.; Zornoza, R.; Scow, K. Changes in Soil Microbial Community Structure Influenced by Agricultural Management Practices in a Mediterranean Agro-Ecosystem. PLoS ONE 2013, 8, e80522. [Google Scholar] [CrossRef]
- Stevenson, B.A.; Hunter, D.W.F.; Rhodes, P.L. Temporal and seasonal change in microbial community structure of an undisturbed, disturbed, and carbon-amended pasture soil. Soil Biol. Biochem. 2014, 75, 175–185. [Google Scholar] [CrossRef]
- Corbeels, M.; Scopel, E.; Cardoso, A.; Bernoux, M.; Douzet, J.-M.; Neto, M.S. Soil carbon storage potential of direct seeding mulch-based cropping systems in the Cerrados of Brazil. Glob. Chang. Biol. 2006, 12, 1773–1787. [Google Scholar] [CrossRef]
- Balota, E.L.; Colozzi Filho, A.; Andrade, D.S.; Dick, R.P. Long-term tillage and crop rotation effects on microbial biomass and C and N mineralization in a Brazilian Oxisol. Soil Tillage Res. 2004, 77, 137–145. [Google Scholar] [CrossRef]
- Jin, V.L.; Haney, R.L.; Fay, P.A.; Polley, H.W. Soil type and moisture regime control microbial C and N mineralization in grassland soils more than atmospheric CO2-induced changes in litter quality. Soil Biol. Biochem. 2013, 58, 172–180. [Google Scholar] [CrossRef]
- Kramer, C.; Gleixner, G. Soil organic matter in soil depth profiles: Distinct carbon preferences of microbial groups during carbon transformation. Soil Biol. Biochem. 2008, 40, 425–433. [Google Scholar] [CrossRef]
- Feng, X.; Simpson, M.J. Temperature and substrate controls on microbial phospholipid fatty acid composition during incubation of grassland soils contrasting in organic matter quality. Soil Biol. Biochem. 2009, 41, 804–812. [Google Scholar] [CrossRef]
- Fanin, N.; Kardol, P.; Farrell, M.; Nilsson, M.-C.; Gundale, M.J.; Wardle, D.A. The ratio of Gram-positive to Gram-negative bacterial PLFA markers as an indicator of carbon availability in organic soils. Soil Biol. Biochem. 2019, 128, 111–114. [Google Scholar] [CrossRef]
- López-Garrido, R.; Madejón, E.; León-Camacho, M.; Girón, I.; Moreno, F.; Murillo, J.M. Reduced tillage as an alternative to no-tillage under Mediterranean conditions: A case study. Soil Tillage Res. 2014, 140, 40–47. [Google Scholar] [CrossRef]
- Zhang, B.; Li, Y.; Ren, T.; Tian, Z.; Wang, G.; He, X.; Tian, C. Short-term effect of tillage and crop rotation on microbial community structure and enzyme activities of a clay loam soil. Biol. Fertil. Soils 2014, 50, 1077–1085. [Google Scholar] [CrossRef]
- Mooshammer, M.; Wanek, W.; Zechmeister-Boltenstern, S.; Richter, A. Stoichiometric imbalances between terrestrial decomposer communities and their resources: Mechanisms and implications of microbial adaptations to their resources. Front. Microbiol. 2014, 5, 22. [Google Scholar] [CrossRef]
- Zechmeister-Boltenstern, S.; Keiblinger, K.M.; Mooshammer, M.; Peñuelas, J.; Richter, A.; Sardans, J.; Wanek, W. The application of ecological stoichiometry to plant–microbial–soil organic matter transformations. Ecol. Monogr. 2015, 85, 133–155. [Google Scholar] [CrossRef]
- Alluvione, F.; Fiorentino, N.; Bertora, C.; Zavattaro, L.; Fagnano, M.; Chiarandà, F.Q.; Grignani, C. Short-term crop and soil response to C-friendly strategies in two contrasting environments. Eur. J. Agron. 2013, 45, 114–123. [Google Scholar] [CrossRef]
- Six, J.; Paustian, K. Aggregate-associated soil organic matter as an ecosystem property and a measurement tool. Soil Biol. Biochem. 2014, 68, A4–A9. [Google Scholar] [CrossRef]
- Han, Z.; Walter, M.T.; Drinkwater, L.E. N2O emissions from grain cropping systems: A meta-analysis of the impacts of fertilizer-based and ecologically-based nutrient management strategies. Nutr. Cycl. Agroecosyst. 2017, 107, 335–355. [Google Scholar] [CrossRef]
- Geisseler, D.; Horwath, W.R.; Scow, K.M. Soil moisture and plant residue addition interact in their effect on extracellular enzyme activity. Pedobiologia 2011, 54, 71–78. [Google Scholar] [CrossRef]
- Enggrob, K.L.; Larsen, T.; Peixoto, L.; Rasmussen, J. Gram-positive bacteria control the rapid anabolism of protein-sized soil organic nitrogen compounds questioning the present paradigm. Sci. Rep. 2020, 10, 15840. [Google Scholar] [CrossRef] [PubMed]
- Pittarello, M.; Ferro, N.D.; Chiarini, F.; Morari, F.; Carletti, P. Influence of tillage and crop rotations in organic and conventional farming systems on soil organic matter, bulk density and enzymatic activities in a short-term field experiment. Agronomy 2021, 11, 724. [Google Scholar] [CrossRef]
- Potthast, K.; Hamer, U.; Makeschin, F. Impact of litter quality on mineralization processes in managed and abandoned pasture soils in Southern Ecuador. Soil Biol. Biochem. 2010, 42, 56–64. [Google Scholar] [CrossRef]
- Mariano, E.; Jones, D.L.; Hill, P.W.; Trivelin, P.C.O. Mineralisation and sorption of dissolved organic nitrogen compounds in litter and soil from sugarcane fields. Soil Biol. Biochem. 2016, 103, 522–532. [Google Scholar] [CrossRef]
- Lagomarsino, A.; Grego, S.; Kandeler, E. Soil organic carbon distribution drives microbial activity and functional diversity in particle and aggregate-size fractions. Pedobiologia 2012, 55, 101–110. [Google Scholar] [CrossRef]
- Xu, Z.; Yu, G.; Zhang, X.; Ge, J.; He, N.; Wang, Q.; Wang, D. The variations in soil microbial communities, enzyme activities and their relationships with soil organic matter decomposition along the northern slope of Changbai Mountain. Appl. Soil Ecol. 2015, 86, 19–29. [Google Scholar] [CrossRef]
- Lal, R. Constraints to adopting no-till farming in developing countries. Soil Tillage Res. 2007, 94, 1–3. [Google Scholar] [CrossRef]
- Vakali, C.; Zaller, J.G.; Köpke, U. Reduced tillage effects on soil properties and growth of cereals and associated weeds under organic farming. Soil Tillage Res. 2011, 111, 133–141. [Google Scholar] [CrossRef]
- Mentges, M.I.; Reichert, J.M.; Rodrigues, M.F.; Awe, G.O.; Mentges, L.R. Capacity and intensity soil aeration properties affected by granulometry, moisture, and structure in no-tillage soils. Geoderma 2016, 263, 47–59. [Google Scholar] [CrossRef]
- Motzo, R.; Fois, S.; Giunta, F. Relationship between grain yield and quality of durum wheats from different eras of breeding. Euphytica 2004, 140, 147–154. [Google Scholar] [CrossRef]
- Giambalvo, D.; Ruisi, P.; Di Miceli, G.; Frenda, A.S.; Amato, G. Nitrogen use efficiency and nitrogen fertilizer recovery of durum wheat genotypes as affected by interspecific competition. Agron. J. 2010, 102, 707. [Google Scholar] [CrossRef]


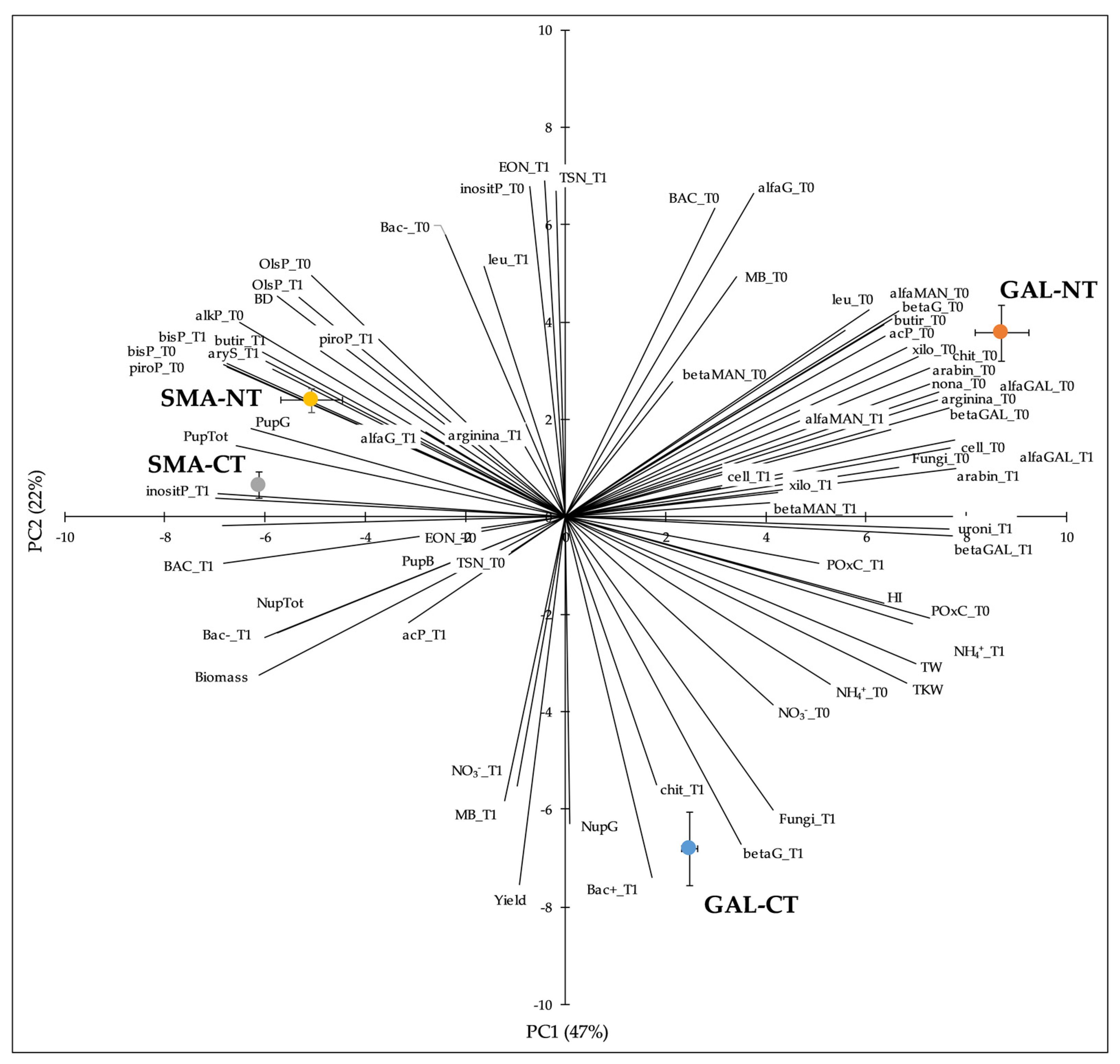
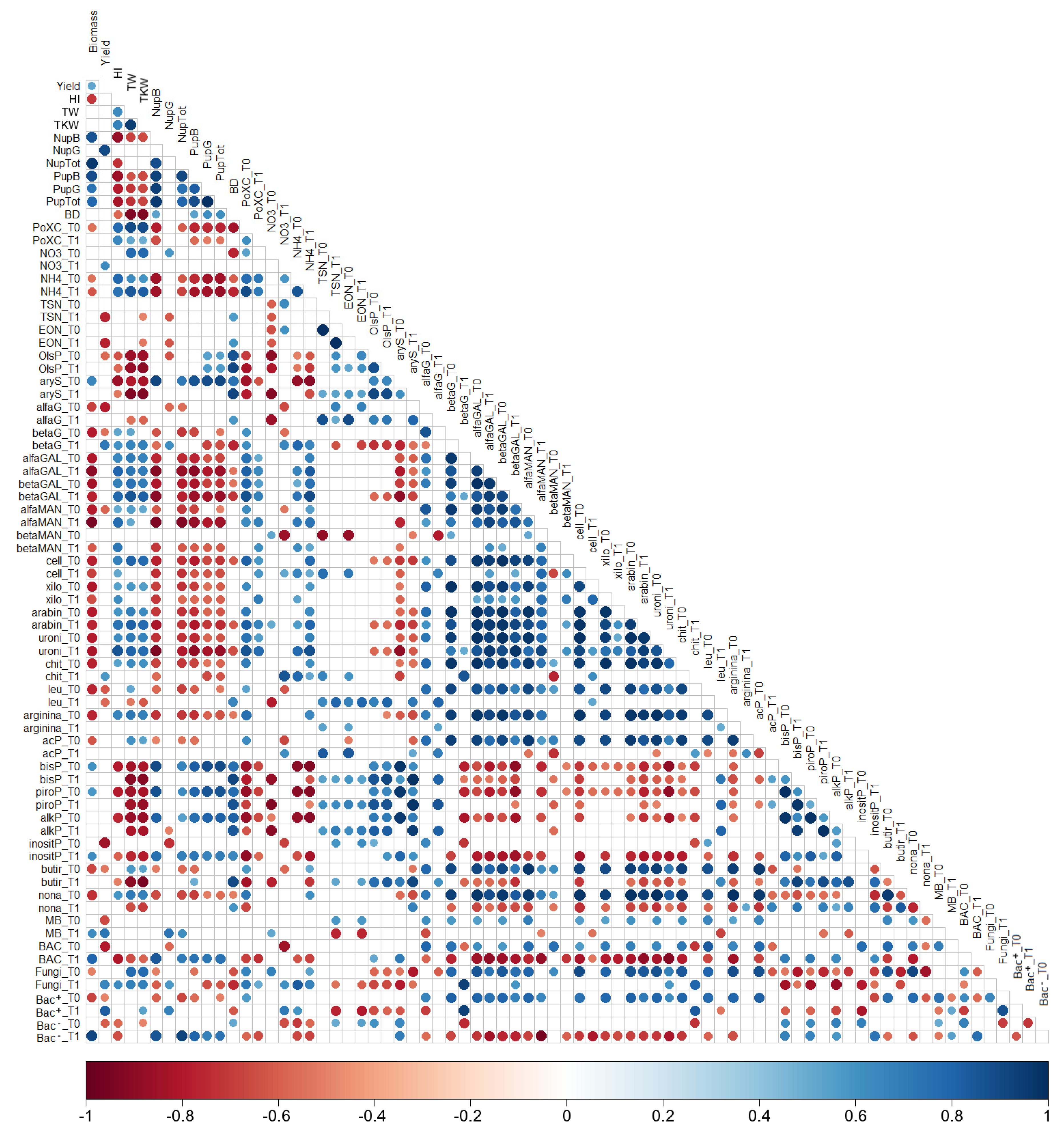
| Soil | Tillage | Biomass Yield | Grain Yield | HI | TW | TKW | N-Straw | N-Grain | P-Straw | P-Grain |
|---|---|---|---|---|---|---|---|---|---|---|
| t ha−1 | t ha−1 | % | kg hL−1 | g | % | % | ‰ | ‰ | ||
| GAL | CT | 12.8 b | 4.9 a | 0.39 a | 82.7 a | 47 a | 0.44 c | 2.0 a | 0.29 b | 2.1 c |
| NT | 9.5 c | 3.8 d | 0.39 a | 82.8 a | 46 a | 0.45 c | 2.1 a | 0.29 b | 2.8 a | |
| SMA | CT | 14.9 a | 4.3 b | 0.30 c | 79.7 b | 42 b | 0.68 a | 2.1 a | 0.33 a | 2.2 c |
| NT | 11.8 b | 4.1 c | 0.34 b | 78.5 c | 41 c | 0.60 b | 2.0 a | 0.35 a | 2.5 b | |
| p-values | ||||||||||
| Soil | <0.001 | 0.031 | <0.001 | <0.001 | <0.001 | <0.001 | 0.427 | 0.008 | 0.133 | |
| Tillage | <0.001 | <0.001 | 0.186 | 0.024 | 0.007 | 0.021 | 0.679 | 0.533 | <0.001 | |
| Soil × Tillage | 0.748 | <0.001 | 0.234 | 0.018 | 0.048 | 0.004 | 0.094 | 0.846 | 0.004 | |
| Soil | Tillage | N-Uptake | P-Uptake | ||||
|---|---|---|---|---|---|---|---|
| Straw | Grain | Total | Straw | Grain | Total | ||
| kg ha−1 | kg ha−1 | kg ha−1 | kg ha−1 | kg ha−1 | kg ha−1 | ||
| GAL | CT | 34.4 c | 99.9 a | 134.4 b | 2.3 bc | 16.0 c | 18.3 c |
| NT | 25.6 c | 82.1 b | 107.8 c | 1.7 c | 16.1 c | 17.8 c | |
| SMA | CT | 71.6 a | 89.5 ab | 161.1 a | 3.6 a | 23.0 a | 26.6 a |
| NT | 46.5 b | 81.2 b | 127.8 b | 2.7 b | 19.3 b | 22.0 b | |
| p-values | |||||||
| Soil | <0.001 | 0.107 | <0.001 | <0.001 | <0.001 | <0.001 | |
| Tillage | <0.001 | 0.002 | <0.001 | 0.002 | 0.028 | 0.007 | |
| Soil × Tillage | 0.003 | 0.171 | 0.418 | 0.501 | 0.023 | 0.025 | |
| Soil | Tillage | POxC | NO3−-N | NH4+-N | EON | TSN | OlsP | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| mg kg−1 | mg kg−1 | mg kg−1 | mg kg−1 | mg kg−1 | mg kg−1 | ||||||||
| T0 | T1 | T0 | T1 | T0 | T1 | T0 | T1 | T0 | T1 | T0 | T1 | ||
| GAL | CT | 423.3 a | 429.4 a | 1.86 a | 1.80 a | 3.94 a | 1.69 a | 48.6 b | 30.9 c | 54.4 b | 34.4 d | 6.7 c | 7.8 d |
| NT | 431.5 a | 439.4 a | 1.69 b | 0.45 c | 3.27 b | 1.76 a | 39.8 c | 57.5 b | 44.8 c | 59.7 b | 11.2 c | 10.8 c | |
| SMA | CT | 372.5 b | 387.6 b | 1.60 b | 0.48 c | 1.33 d | 0.59 b | 33.1 c | 46.2 b | 36.0 d | 47.3 c | 15.2 b | 14.3 b |
| NT | 369.3 b | 412.9 b | 0.80 c | 1.41 b | 2.74 c | 0.92 b | 63.6 a | 61.5 a | 67.2 a | 63.8 a | 21.3 a | 19.6 a | |
| p-values | |||||||||||||
| Soil | 0.001 | <0.001 | <0.001 | 0.002 | 0.012 | <0.001 | |||||||
| Tillage | 0.300 | <0.001 | 0.076 | <0.001 | <0.001 | <0.001 | |||||||
| Soil × Tillage | 0.919 | <0.001 | 0.002 | 0.002 | <0.001 | 0.191 | |||||||
| Time | 0.061 | <0.001 | <0.001 | 0.225 | 0.747 | 0.571 | |||||||
| Time × Soil | 0.218 | 0.020 | 0.006 | 0.225 | 0.158 | 0.284 | |||||||
| Time × Tillage | 0.391 | 0.076 | 0.035 | 0.037 | 0.036 | 0.441 | |||||||
| Time × Soil × Tillage | 0.447 | <0.001 | <0.001 | <0.001 | <0.001 | 0.843 | |||||||
| Global | GAL | SMA | ||||||
|---|---|---|---|---|---|---|---|---|
| Yield R2 = 0.9883 | Yield R2 = 0.9836 | Yield R2 = 0.9998 | ||||||
| Constant = 5.7119 | Constant = 6.6065 | Constant = 3.2668 | ||||||
| Variable | b | p | Variable | b | p | Variable | b | p |
| NH4+-N_T0 | 0.4573 | <0.001 | NH4+-N_T0 | 0.3089 | 0.0486 | aryS_T0 | 0.1346 | <0.001 |
| inositP_T0 | 1.0221 | <0.001 | BAC_T0 | −0.0136 | <0.001 | inositP_T0 | 0.0596 | <0.001 |
| nona_T1 | 0.0115 | <0.001 | bisP_T0 | 0.0037 | <0.001 | |||
| MB_T1 | 0.0033 | <0.001 | arabin_T1 | 0.0018 | <0.001 | |||
| Bac−_T1 | 0.0121 | <0.001 | NH4+-N_T1 | −0.1089 | <0.001 | |||
| OlsP_T1 | −0.0552 | <0.001 | Fungi_T0 | −0.0341 | <0.001 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Badagliacca, G.; Lo Presti, E.; Ferrarini, A.; Fornasier, F.; Laudicina, V.A.; Monti, M.; Preiti, G. Early Effects of No-Till Use on Durum Wheat (Triticum durum Desf.): Productivity and Soil Functioning Vary between Two Contrasting Mediterranean Soils. Agronomy 2022, 12, 3136. https://doi.org/10.3390/agronomy12123136
Badagliacca G, Lo Presti E, Ferrarini A, Fornasier F, Laudicina VA, Monti M, Preiti G. Early Effects of No-Till Use on Durum Wheat (Triticum durum Desf.): Productivity and Soil Functioning Vary between Two Contrasting Mediterranean Soils. Agronomy. 2022; 12(12):3136. https://doi.org/10.3390/agronomy12123136
Chicago/Turabian StyleBadagliacca, Giuseppe, Emilio Lo Presti, Andrea Ferrarini, Flavio Fornasier, Vito Armando Laudicina, Michele Monti, and Giovanni Preiti. 2022. "Early Effects of No-Till Use on Durum Wheat (Triticum durum Desf.): Productivity and Soil Functioning Vary between Two Contrasting Mediterranean Soils" Agronomy 12, no. 12: 3136. https://doi.org/10.3390/agronomy12123136
APA StyleBadagliacca, G., Lo Presti, E., Ferrarini, A., Fornasier, F., Laudicina, V. A., Monti, M., & Preiti, G. (2022). Early Effects of No-Till Use on Durum Wheat (Triticum durum Desf.): Productivity and Soil Functioning Vary between Two Contrasting Mediterranean Soils. Agronomy, 12(12), 3136. https://doi.org/10.3390/agronomy12123136








