Utilization of Biochar for Eliminating Residual Pharmaceuticals from Wastewater Used in Agricultural Irrigation: Application to Ryegrass
Abstract
1. Introduction
2. Materials and Methods
2.1. Wastewater and Biochar Sources and Characterization
2.2. Wastewater Treatment with Different Biochar Doses
2.3. Application of Wastewater for Irrigation
2.4. Pharmaceutical Concentrations in Soil and Ryegrass Samples
2.5. Fluorescein Diacetate Hydrolytic Activities (FDHA) Experiment
2.6. Statistical Analysis
3. Results
3.1. Wastewater Treatment with Biochar
3.2. Pharamceutical Concentrations in Soil and Plant Samples after Irrigation with Biochar-Treated Wastewater
3.3. Ryegrass Fresh Biomass after Irrigation with Biochar Treated Wastewater
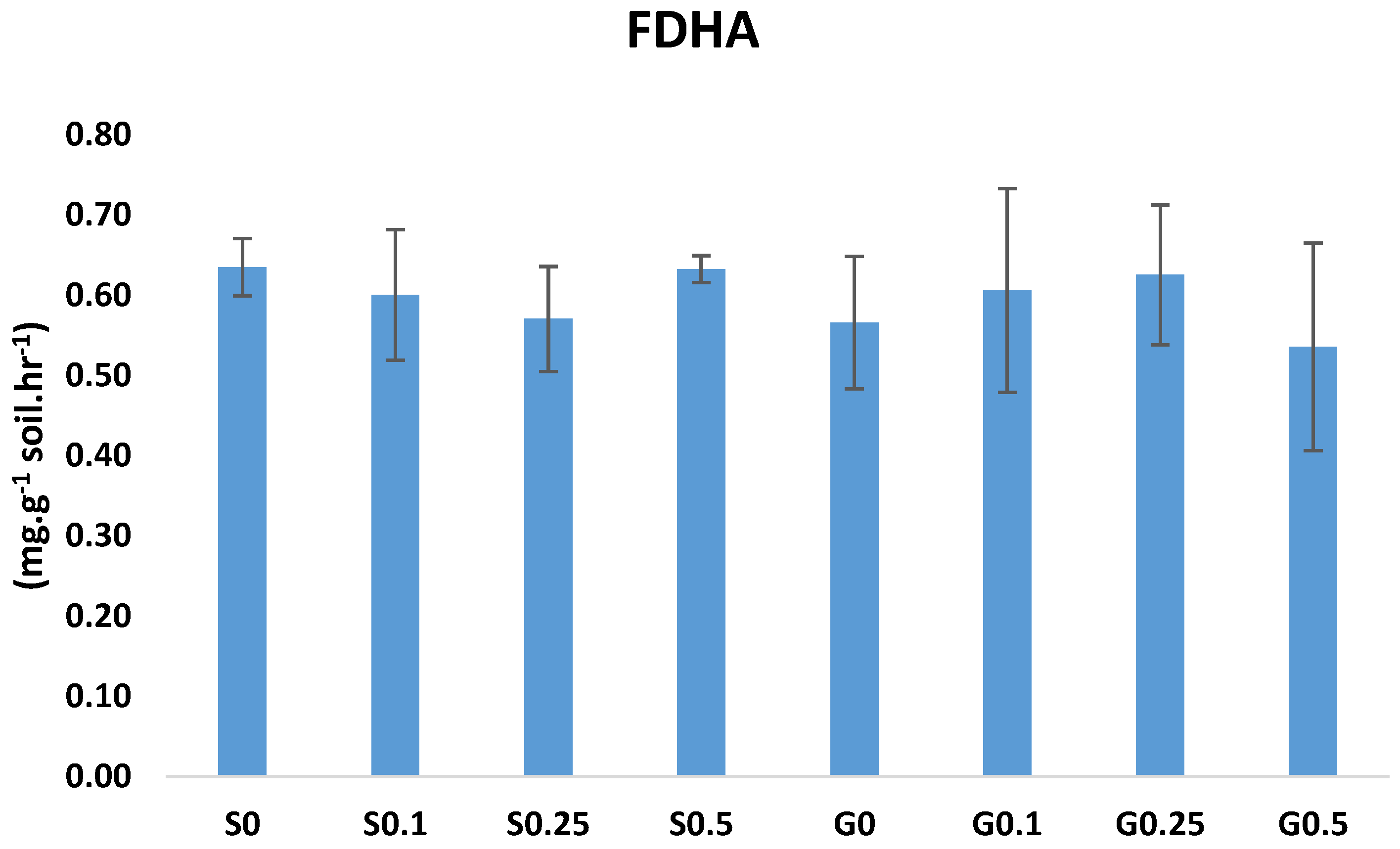
3.4. Fluorescein Diacetate Hydrolytic Activities (FDHA)
4. Discussion
4.1. Wastewater Treatments with Biochar
4.2. Pharmaceuticals Concentrations in Soil and Ryegrass
4.3. Impacts on Plant Biomass and Soil Microbial Activities
5. Conclusions and Perspectives
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lowder, S.K.; Skoet, J.; Raney, T. The Number, Size, and Distribution of Farms, Smallholder Farms, and Family Farms Worldwide. World Dev. 2016, 87, 16–29. [Google Scholar] [CrossRef]
- Godfray, H.C.J.; Beddington, J.R.; Crute, I.R.; Haddad, L.; Lawrence, D.; Muir, J.F.; Pretty, J.; Robinson, S.; Thomas, S.M.; Toulmin, C. Food Security: The Challenge of Feeding 9 Billion People. Science 2010, 327, 812–818. [Google Scholar] [CrossRef] [PubMed]
- Xiang, W.; Zhang, X.; Chen, J.; Zou, W.; He, F.; Hu, X.; Tsang, D.C.W.; Ok, Y.S.; Gao, B. Biochar Technology in Wastewater Treatment: A Critical Review. Chemosphere 2020, 252, 126539. [Google Scholar] [CrossRef] [PubMed]
- Wei, D.; Li, B.; Huang, H.; Luo, L.; Zhang, J.; Yang, Y.; Guo, J.; Tang, L.; Zeng, G.; Zhou, Y. Biochar-Based Functional Materials in the Purification of Agricultural Wastewater: Fabrication, Application and Future Research Needs. Chemosphere 2018, 197, 165–180. [Google Scholar] [CrossRef] [PubMed]
- Enaime, G.; Lübken, M. Agricultural Waste-based Biochar for Agronomic Applications. Appl. Sci. 2021, 11, 8914. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, A.; Ji, C.; Joseph, S.; Bian, R.; Li, L.; Pan, G.; Paz-Ferreiro, J. Biochar’s Effect on Crop Productivity and the Dependence on Experimental Conditions-a Meta-Analysis of Literature Data. Plant Soil 2013, 373, 583–594. [Google Scholar] [CrossRef]
- Huggins, T.M.; Haeger, A.; Biffinger, J.C.; Ren, Z.J. Granular Biochar Compared with Activated Carbon for Wastewater Treatment and Resource Recovery. Water Res. 2016, 94, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Ambaye, T.G.; Vaccari, M.; van Hullebusch, E.D.; Amrane, A.; Rtimi, S. Mechanisms and Adsorption Capacities of Biochar for the Removal of Organic and Inorganic Pollutants from Industrial Wastewater. Int. J. Environ. Sci. Technol. 2021, 18, 3273–3294. [Google Scholar] [CrossRef]
- Foereid, B. Biochar in Nutrient Recycling—The Effect and Its Use in Wastewater Treatment. Open J. Soil Sci. 2015, 5, 39–44. [Google Scholar] [CrossRef][Green Version]
- Enaime, G.; Baçaoui, A.; Yaacoubi, A.; Lübken, M. Biochar for Wastewater Treatment-Conversion Technologies and Applications. Appl. Sci. 2020, 10, 3492. [Google Scholar] [CrossRef]
- Deng, Y.; Zhang, T.; Wang, Q. Biochar Adsorption Treatment for Typical Pollutants Removal in Livestock Wastewater: A Review. In Engineering Applications of Biochar; Huang, J.-W., Ed.; IntechOpen: London, UK, 2017. [Google Scholar] [CrossRef]
- Ahmed, M.B.; Zhou, J.L.; Ngo, H.H.; Guo, W. Adsorptive Removal of Antibiotics from Water and Wastewater: Progress and Challenges. Sci. Total Environ. 2015, 532, 112–126. [Google Scholar] [CrossRef] [PubMed]
- Dalahmeh, S.; Ahrens, L.; Gros, M.; Wiberg, K.; Pell, M. Potential of Biochar Filters for Onsite Sewage Treatment: Adsorption and Biological Degradation of Pharmaceuticals in Laboratory Filters with Active, Inactive and No Biofilm. Sci. Total Environ. 2018, 612, 192–201. [Google Scholar] [CrossRef]
- Mojiri, A.; Baharlooeian, M.; Kazeroon, R.A.; Farraji, H.; Lou, Z. Removal of Pharmaceutical Micropollutants with Integrated Biochar and Marine Microalgae. Microorganisms 2021, 9, 4. [Google Scholar] [CrossRef] [PubMed]
- Tolaszová, J. Organic pollutants in sediments of selected Czech rivers. Ph.D. Thesis, University of Jan Evangelista in Ústí nad Labem, Ústí nad Labem, Czech Republic, 2019. [Google Scholar]
- Green, V.S.; Stott, D.E.; Diack, M. Assay for Fluorescein Diacetate Hydrolytic Activity: Optimization for Soil Samples. Soil Biol. Biochem. 2006, 38, 693–701. [Google Scholar] [CrossRef]
- Al Souki, K.S.; Burdová, H.; Mamirova, A.; Kuráň, P.; Kříženecká, S.; Oravová, L.; Tolaszová, J.; Nebeská, D.; Popelka, J.; Ust’ak, S.; et al. Evaluation of the Miscanthus × Giganteus Short Term Impacts on Enhancing the Quality of Agricultural Soils Affected by Single and/or Multiple Contaminants. Environ. Technol. Innov. 2021, 24, 101890. [Google Scholar] [CrossRef]
- Shraim, A.; Diab, A.; Alsuhaimi, A.; Niazy, E.; Metwally, M.; Amad, M.; Sioud, S.; Dawoud, A. Analysis of Some Pharmaceuticals in Municipal Wastewater of Almadinah Almunawarah. Arab. J. Chem. 2017, 10, S719–S729. [Google Scholar] [CrossRef]
- Ahmad, A.A.; Din, A.T.M.; Yahaya, N.K.E.M.; Karim, J.; Ahmad, M.A. Atenolol Sequestration Using Activated Carbon Derived from Gasified Glyricidia Sepium. Arab. J. Chem. 2020, 13, 7544–7557. [Google Scholar] [CrossRef]
- Venditti, S.; Brunhoferova, H.; Hansen, J. Behaviour of 27 Selected Emerging Contaminants in Vertical Flow Constructed Wetlands as Post-Treatment for Municipal Wastewater. Sci. Total Environ. 2022, 819, 153234. [Google Scholar] [CrossRef] [PubMed]
- Biel-Maeso, M.; Corada-Fernández, C.; Lara-Martín, P.A. Monitoring the Occurrence of Pharmaceuticals in Soils Irrigated with Reclaimed Wastewater. Environ. Pollut. 2018, 235, 312–321. [Google Scholar] [CrossRef]
- Ahmed, M.B.; Zhou, J.L.; Ngo, H.H.; Guo, W.; Chen, M. Progress in the Preparation and Application of Modified Biochar for Improved Contaminant Removal from Water and Wastewater. Bioresour. Technol. 2016, 214, 836–851. [Google Scholar] [CrossRef] [PubMed]
- Vom Eyser, C.; Palmu, K.; Schmidt, T.C.; Tuerk, J. Pharmaceutical Load in Sewage Sludge and Biochar Produced by Hydrothermal Carbonization. Sci. Total Environ. 2015, 537, 180–186. [Google Scholar] [CrossRef]
- Bahlmann, A.; Brack, W.; Schneider, R.J.; Krauss, M. Carbamazepine and Its Metabolites in Wastewater: Analytical Pitfalls and Occurrence in Germany and Portugal. Water Res. 2014, 57, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Yanala, S.R.; Pagilla, K.R. Use of Biochar to Produce Reclaimed Water for Irrigation Use. Chemosphere 2020, 251, 126403. [Google Scholar] [CrossRef] [PubMed]
- Grossberger, A.; Hadar, Y.; Borch, T.; Chefetz, B. Biodegradability of Pharmaceutical Compounds in Agricultural Soils Irrigated with Treated Wastewater. Environ. Pollut. 2014, 185, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Lin, A.Y.C.; Lin, C.A.; Tung, H.H.; Chary, N.S. Potential for Biodegradation and Sorption of Acetaminophen, Caffeine, Propranolol and Acebutolol in Lab-Scale Aqueous Environments. J. Hazard. Mater. 2010, 183, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Meyer, S.; Steinhart, H. Effects of Heterocyclic PAHs (N, S, O) on the Biodegradation of Typical Tar Oil PAHs in a Soil/Compost Mixture. Chemosphere 2000, 40, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Gworek, B.; Kijeńska, M.; Wrzosek, J.; Graniewska, M. Pharmaceuticals in the Soil and Plant Environment: A Review. Water Air Soil Pollut. 2021, 232, 145. [Google Scholar] [CrossRef]
- Thelusmond, J.R.; Kawka, E.; Strathmann, T.J.; Cupples, A.M. Diclofenac, carbamazepine and triclocarban biodegradation in agricultural soils and the microorganisms and metabolic pathways affected. Sci. Total Environ. 2018, 640, 1393–1410. [Google Scholar] [CrossRef] [PubMed]
- Biel-Maeso, M.; González-González, C.; Lara-Martín, P.A.; Corada-Fernández, C. Sorption and Degradation of Contaminants of Emerging Concern in Soils under Aerobic and Anaerobic Conditions. Sci. Total Environ. 2019, 666, 662–671. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Song, Y.; Bian, Y.; Wang, F.; Gu, C.; Yang, X.; Jiang, X. Effects of root exudates on the sorption of polycyclic aromatic hydrocarbons onto biochar. Env. Pollut. Bioavail. 2019, 31, 156–165. [Google Scholar] [CrossRef]
- Casas, M.E.; Larzabal, E.; Matamoros, V. Exploring the usage of artificial root exudates to enhance the removal of contaminants of emerging concern in slow sand filters: Synthetic vs. real wastewater conditions. Sci. Total Environ. 2022, 824, 153978. [Google Scholar] [CrossRef] [PubMed]
- Wardle, D.A.; Ghani, A.A. Critique of the Microbial Metabolic Quotient (Qc02) as a Bioindicator of Disturbance and Ecosystem Development. Soil Biol. Biochem. 1995, 27, 1601–1610. [Google Scholar] [CrossRef]
- Al Souki, K.S.; Liné, C.; Moravec, J.; Douay, F.; Pourrut, B. Response of Three Miscanthus × giganteus Cultivars to Toxic Elements Stress: Part 2, Comparison between Two Growing Seasons. Plants 2022, 11, 945. [Google Scholar] [CrossRef] [PubMed]
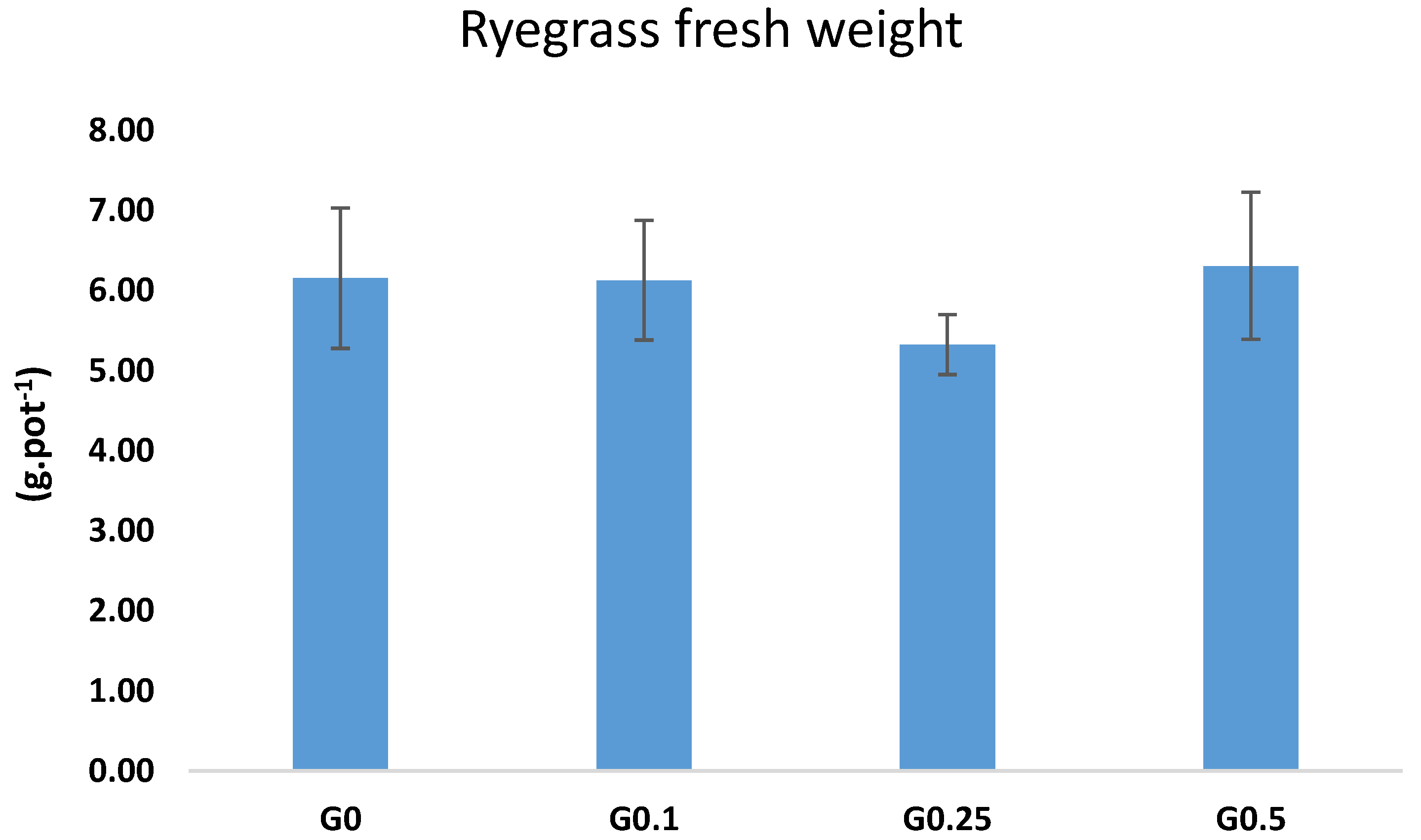
| Parameter | Standard Protocol | Concentration (mg L−1) |
|---|---|---|
| pH | ČSN ISO 10523, 757365 | 7.2 ± 0.1 |
| Biochemical Oxygen Demand (BOD) | ČSN EN ISO 5815-1, ČSN EN 1899-2 | 3.6 ± 0.1 |
| Chemical Oxygen Demand (COD) | ČSN ISO 15705 | 34.0 ± 0.6 |
| Total suspended solid (TSS) | ČSN EN 872, ČSN 75 7350 | 21.0 ± 1.0 |
| Total nitrogen | ČSN ISO 15705 | 16.0 ± 1 |
| Total phosphorus | ČSN EN ISO 6878 | 1.8 ± 0.1 |
| Total organic carbon | ČSN EN 1484 | 16.0 ± 0.7 |
| Parameter | Value |
|---|---|
| pH solution (H2O) | 10.0 ± 0.8 |
| Functional groups (-COOH, -OH) (mmol g−1) | 0.386 ± 0.02 |
| Area of micropores (m2 g−1) | 325.7 ± 30.3 |
| Brunauer–Emmett–Teller (BET) surface area (m2 g−1) | 571.6 ± 9.2 |
| Contaminant | Concentration at Treatment Level (ng L−1) | |||
|---|---|---|---|---|
| trt0 | trt0.1 | trt0.25 | trt0.5 | |
| 3-Hydroxycarbamazepine | 88.9 ± 8 a | 34.9 ± 3.3 b | <25 ± 5 c | <25 ± 5 c |
| Acebutolol | 184 ± 16.7 a | 84.9 ± 7 b | 60.7 ± 4.1 bc | 41.6 ± 4.3 c |
| Acridine | 255 ± 21 a | 230 ± 15.9 ab | 212 ± 22.4 abc | 203 ± 10.7 bc |
| Atenolol | 104 ± 10.8 a | 94.2 ± 8.8 ab | 78.3 ± 5.7 bc | 68.2 ± 6.6 c |
| Bezafibrate | 72.3 ± 4.5 a | 70.9 ± 4.2 a | 70.7 ± 4.1 a | 69.6 ± 3.9 a |
| Caffeine | 29,191 ± 838 a | 20,109 ± 422 b | 11,664 ± 333 c | 3247 ± 249 d |
| Carbamazepine | 543 ± 29 a | 209 ± 21.9 b | 109 ± 9.9 c | 53 ± 4.6 d |
| Clarithromycin | 1070 ± 114 a | 673 ± 35 b | 227 ± 15.4 c | 112 ± 10.3 c |
| Diclofenac | 2195 ± 139 a | 132 ± 9.5 b | 261 ± 22.2 b | 130 ± 9 b |
| Hydrochlorothiazide | 2972 ± 194 a | 1898 ± 80 b | 890 ± 78 c | 410 ± 22 d |
| Iopamidol | 25,667 ± 133 a | 24,920 ± 984 ab | 17,879 ± 576 c | 15,014 ± 301 d |
| Metoprolol | 346 ± 29.3 a | 135 ± 7 b | <25 ± 5 c | <25 ± 5 c |
| N-acetylsulfamethoxazole | 2514 ± 103 a | 2341 ± 168 a | 2263 ± 53 a | 1226 ± 45 b |
| Pentoxifylline | 172 ± 14.4 a | 129 ± 8.8 b | 94 ± 8.8 c | 66.9 ± 5.4 d |
| Phenazone | 1121 ± 24 a | 964 ± 62 b | 583 ± 27 c | 251 ± 20 d |
| Primidone | 180 ± 16.1 a | 157 ± 11.4 a | 124 ± 8.8 b | 100 ± 10 b |
| Sulfamethoxazole | 236 ± 17.2 a | 173 ± 14.5 b | 185 ± 17 bc | 147 ± 9.5 c |
| Tramadol | 1057 ± 81 a | 836 ± 67 b | 599 ± 20 c | 381 ± 16.1 d |
| Trimethoprim | 467 ± 33.3 a | 205 ± 11 b | 77.7 ± 5.5 c | 34.3 ± 2.9 c |
| Venlafaxine | 594 ± 29 a | 420 ± 23.9 b | 259 ± 11.7 c | 124 ± 4.7 d |
| Contaminant | Concentration in Soil (ng g−1) | |||||||
|---|---|---|---|---|---|---|---|---|
| S0 | S0.1 | S0.25 | S0.5 | G0 | G0.1 | G0.25 | G0.5 | |
| Acebutolol | 0.26 ± 0.01 a | 0.17 ± 0.01 abc | 0.24 ± 0.02 a | 0.18 ± 0.03 ab | 0.19 ± 0.00 ab | 0.11 ± 0.03 abc | <0.08 ± 0.00 c | <0.08 ± 0.00 c |
| Acridine | 1.24 ± 0.07 a | 1.45 ± 0.23 a | 1.45 ± 0.28 a | 1.77 ± 0.37 a | 1.87 ± 0.40 a | 1.78 ± 0.43 a | 1.83 ± 0.41 a | 1.84 ± 0.67 a |
| Caffeine | 7.82 ± 1.77 a | 7.56 ± 1.69 a | 7.59 ± 0.86 a | 7.59 ± 0.80 a | 7.99 ± 0.75 a | 8.65 ± 1.60 a | 7.53 ± 0.82 a | 7.35 ± 0.43 a |
| Carbamazepine | 0.56 ± 0.08 a | 0.41 ± 0.08 a | 0.35 ± 0.06 a | 0.29 ± 0.02 a | 0.55 ± 0.04 a | 0.37 ± 0.09 a | 0.32 ± 0.06 a | 0.25 ± 0.02 a |
| Clarithromycin | 1.76 ± 0.63 a | 1.20 ± 0.14 a | 1.25 ± 0.83 a | 0.93 ± 0.49 a | 2.25 ± 1.59 a | 0.93 ± 0.23 a | 0.61 ± 0.07 a | 0.62 ± 0.04 a |
| Hydrochlorothiazide | 1.63 ± 0.50 a | 0.90 ± 0.27 a | 0.70 ± 0.34 a | 0.43 ± 0.05 a | 1.99 ± 0.34 a | 0.97 ± 0.39 a | 0.58 ± 0.36 a | 0.23 ± 0.17 a |
| N-acetylsulfamethoxazole | 2.42 ± 0.19 a | 2.30 ± 0.37 a | 2.37 ± 0.47 a | 2.19 ± 0.27 a | 2.25 ± 0.20 a | 2.32 ± 0.31 a | 2.72 ± 0.33 a | 2.39 ± 0.28 a |
| Phenazone | 1.21 ± 0.30 ab | 0.76 ± 0.12 ab | 0.74 ± 0.23 ab | 0.74 ± 0.14 ab | 1.45 ± 0.69 a | 0.98 ± 0.47 ab | 0.87 ± 0.43 ab | 0.57 ± 0.16 b |
| Sulfamethoxazole | 0.51 ± 0.05 ab | 0.42 ± 0.02 ab | 0.32 ± 0.16 ab | 0.22 ± 0.03 ab | 0.65 ± 0.03 a | 0.43 ± 0.02 ab | 0.38 ± 0.02 ab | <0.08 ± 0.00 b |
| Tramadol | 1.03 ± 0.61 a | 0.57 ± 0.24 ab | 0.54 ± 0.33 ab | 0.21 ± 0.05 b | 0.36 ± 0.21 b | 0.49 ± 0.23 ab | 0.17 ± 0.05 b | 0.11 ± 0.05 b |
| Trimethoprim | 0.40 ± 0.01 a | 0.27 ± 0.04 a | 0.21 ± 0.09 a | 0.16 ± 0.09 a | 0.42 ± 0.32 a | 0.37 ± 0.32 a | 0.16 ± 0.09 a | <0.08 ± 0.00 a |
| Venlafaxine | 0.51 ± 0.09 a | 0.44 ± 0.05 a | 0.44 ± 0.17 a | 0.53 ± 0.40 a | 0.44 ± 0.35 a | 0.36 ± 0.07 a | 0.24 ± 0.04 a | 0.27 ± 0.09 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akintoroye, M.; Newton, R.A.; Kříženecká, S.; Hejda, S.; Krystyník, P.; Ahnert, M.; Trögl, J.; Krebs, P.; Al Souki, K.S. Utilization of Biochar for Eliminating Residual Pharmaceuticals from Wastewater Used in Agricultural Irrigation: Application to Ryegrass. Agronomy 2022, 12, 2987. https://doi.org/10.3390/agronomy12122987
Akintoroye M, Newton RA, Kříženecká S, Hejda S, Krystyník P, Ahnert M, Trögl J, Krebs P, Al Souki KS. Utilization of Biochar for Eliminating Residual Pharmaceuticals from Wastewater Used in Agricultural Irrigation: Application to Ryegrass. Agronomy. 2022; 12(12):2987. https://doi.org/10.3390/agronomy12122987
Chicago/Turabian StyleAkintoroye, Mayowa, Robert Ato Newton, Sylvie Kříženecká, Stanislav Hejda, Pavel Krystyník, Markus Ahnert, Josef Trögl, Peter Krebs, and Karim Suhail Al Souki. 2022. "Utilization of Biochar for Eliminating Residual Pharmaceuticals from Wastewater Used in Agricultural Irrigation: Application to Ryegrass" Agronomy 12, no. 12: 2987. https://doi.org/10.3390/agronomy12122987
APA StyleAkintoroye, M., Newton, R. A., Kříženecká, S., Hejda, S., Krystyník, P., Ahnert, M., Trögl, J., Krebs, P., & Al Souki, K. S. (2022). Utilization of Biochar for Eliminating Residual Pharmaceuticals from Wastewater Used in Agricultural Irrigation: Application to Ryegrass. Agronomy, 12(12), 2987. https://doi.org/10.3390/agronomy12122987








