Soil Seed Banks of Continental Grasslands with Different Water Regimes—A Comparative Study from the Aspect of Recovery Potential
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Area and Sampling Design
2.2. Data Collection
2.3. Data Analysis
3. Results
3.1. Checking the Separation of Grassland Types by Water Regime
3.2. Features of the Above-Ground Vegetation in the Grassland Types
3.3. Features of the Soil Seed Bank in the Grassland Types
3.4. Similarities between the Above-Ground Vegetation and the Soil Seed Banks from the Aspect of Recovery Potential in the Grassland Types
4. Discussion
4.1. The Above-Ground Vegetation in the Grassland Types
4.2. The Soil Seed Bank in the Grassland Types
4.3. Similarities between the Above-Ground Vegetation and the Soil Seed Bank from the Aspect of Recovery Potential in the Grassland Types
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| WB | Moisture Requirement Category |
|---|---|
| 1 | Plants of extremely dry habitats or bare rocks |
| 2 | Xero-indicators on habitats with long dry period |
| 3 | Xero-tolerants, but eventually occurring on fresh soils |
| 4 | Plants of semi-dry habitats |
| 5 | Plants of semi-humid habitats, under intermediate conditions |
| 6 | Plants of fresh soils |
| 7 | Plants of moist soils, not drying out and well aerated |
| 8 | Plants of moist soils tolerating short floods |
| 9 | Plants of wet, not well-aerated soils |
| 10 | Plants of frequently flooded soils |
| 11 | Water plants with floating or partly emergent leaves |
| 12 | Water plants, most wholly submerged in water |
| Introduced Data Combination | Linear Discriminant Analysis | Difference | |||||
|---|---|---|---|---|---|---|---|
| Dependent Var. | Independent Variables | LD1 | LD2 | LD | Target vs. LD | ||
| = Groups | (norm.) | (58.5%) | (41.5%) | Groups | Group | ||
| {G1 (6 quadrats) | ~ | Soil moisture content | −4.90 | −0.85 | G1 (6 quadrats) | ±0 quadrat | |
| G2 (18 quadrats) | + Carbonate content | −0.57 | −0.45 | G2 (18 quadrats) | ±0 quadrat | ||
| G3 (6 quadrats)} | + Organic matter content | −1.86 | 6.81 | G3 (6 quadrats) | ±0 quadrat | ||
| + Water-soluble salt content | −2.11 | −3.20 | |||||
| + Consistency acc. to Arany | 1.45 | −0.97 | |||||
| + pH determined in KCl | −0.63 | −0.80 | |||||
| + Vegetation cover | 3.06 | 3.22 | |||||
| + Species number | −2.51 | −4.63 | |||||
| Wet Grassland Type, N = 6 | Ecotone Grassland Type, N = 18 | Semi-Dry Grassland Type, N = 6 | |
|---|---|---|---|
| ± SD | ± SD | ± SD | |
| Soil moisture content (m/m%) | |||
| October 2012 | 43.73 ± 6.80 | 33.67 ± 3.79 | 26.05 ± 1.40 |
| November 2012 | 40.79 ± 8.26 | 33.24 ± 2.31 | 27.43 ± 1.53 |
| October 2013 | 41.17 ± 4.02 | 33.83 ± 2.43 | 23.16 ± 1.82 |
| Carbonate content (m/m%) | 0.10 ± 0.00 | 5.39 ± 4.48 | 0.10 ± 0.00 |
| Organic matter content (m/m%) | 7.86 ± 0.64 | 5.63 ± 0.63 | 5.24 ± 0.43 |
| Water-soluble salt content (m/m%) | 0.08 ± 0.01 | 0.07 ± 0.01 | 0.06 ± 0.02 |
| Consistency according to Arany | 83.83 ± 9.41 | 75.33 ± 4.83 | 69.33 ± 2.66 |
| pH determined in KCl | 6.46 ± 0.41 | 6.83 ± 0.34 | 6.04 ± 0.23 |
| WB | Presence in Vegetation | Presence in Seed Bank | ST | |||
|---|---|---|---|---|---|---|
| 0–5 cm | 5–10 cm | |||||
| Wet grassland type, N = 6 | ||||||
| Alopecurus pratensis | 6 | + | − | T | ||
| Anagallis arvensis ! | 4 | − | + | > | + | LP |
| Calystegia sepium | 9 | + | − | T | ||
| Carex acutiformis | 9 | + | + | > | − | T |
| Carex hirta | 7 | + | + | = | + | LP |
| Carex vulpine | 8 | + | − | T | ||
| Cirsium arvense ! | 4 | + | − | T | ||
| Cirsium canum ! | 8 | + | − | T | ||
| Colchicum autumnale ! | 6 | + | − | T | ||
| Erigeron annuus ! | 7 | + | + | > | + | SP |
| Filipendula ulmaria ! | 8 | + | − | T | ||
| Filipendula vulgaris ! | 4 | + | − | T | ||
| Galium boreale | 8 | + | − | T | ||
| Lathyrus pratensis | 7 | + | − | T | ||
| Lysimachia vulgaris | 8 | + | − | T | ||
| Lythrum salicaria | 9 | + | + | > | + | SP |
| Mentha aquatica | 9 | + | − | T | ||
| Ranunculus auricomus | 6 | + | + | > | − | T |
| Serratula tinctoria ! | 7 | + | − | T | ||
| Silene flos-cuculi ! | 7 | + | + | < | + | LP |
| Symphytum officinale | 8 | + | − | T | ||
| Thalictrum lucidum ! | 8 | + | + | > | + | SP |
| Veronica longifolia | 8 | + | + | > | + | SP |
| Ecotone grassland type, N = 18 | ||||||
| Achillea collina | 2 | + | + | > | + | SP |
| Alopecurus pratensis | 6 | + | − | T | ||
| Anagallis arvensis ! | 4 | − | + | = | + | LP |
| Arrhenatherum elatius | 5 | + | − | T | ||
| Calamagrostis epigejos ! | 5 | + | − | T | ||
| Calystegia sepium | 9 | + | − | T | ||
| Carex acutiformis | 9 | + | + | > | + | SP |
| Carex hirta | 7 | + | + | ≥ | + | LP |
| Carex muricata ssp. pairae | 5 | + | + | > | + | SP |
| Cirsium arvense ! | 4 | + | − | T | ||
| Colchicum autumnale ! | 6 | + | − | T | ||
| Crepis biennis ! | 5 | + | − | T | ||
| Dactylis glomerata | 6 | + | − | T | ||
| Erigeron annuus ! | 7 | + | + | > | + | SP |
| Erigeron canadensis ! | 4 | − | + | > | + | LP |
| Filipendula ulmaria ! | 8 | + | − | T | ||
| Filipendula vulgaris ! | 4 | + | − | T | ||
| Fragaria viridis | 3 | + | − | T | ||
| Galium verum | 4 | + | − | T | ||
| Inula salicina ! | 5 | + | − | T | ||
| Juncus articulatus ! | 8 | − | + | ≤ | + | LP |
| Lathyrus pratensis | 7 | + | − | T | ||
| Lathyrus tuberosus | 4 | + | − | T | ||
| Leucanthemum vulgare ! | 2 | + | + | ≥ | + | LP |
| Lysimachia vulgaris | 8 | + | − | T | ||
| Lythrum salicaria | 9 | + | + | > | + | SP |
| Medicago lupulina | 5 | − | + | > | + | LP |
| Peucedanum alsaticum | 3 | + | − | T | ||
| Picris hieracioides ! | 4 | + | + | > | − | T |
| Pimpinella saxifrage | 3 | + | − | T | ||
| Plantago major | 6 | + | + | > | + | SP |
| Plantago media | 5 | + | + | > | + | SP |
| Poa pratensis | 6 | + | + | < | + | LP |
| Pulmonaria mollis | 5 | + | − | T | ||
| Ranunculus auricomus | 6 | + | − | T | ||
| Ranunculus repens | 8 | + | + | < | + | LP |
| Salvia pratensis | 3 | + | − | T | ||
| Schedonorus pratensis | 6 | + | − | T | ||
| Serratula tinctoria ! | 7 | + | − | T | ||
| Silene flos-cuculi ! | 7 | + | + | < | + | LP |
| Solidago canadensis ! | 7 | + | + | > | − | T |
| Stellaria media | 5 | − | + | < | + | LP |
| Symphytum officinale | 8 | + | − | T | ||
| Taraxacum officinale ! | 5 | + | + | > | + | SP |
| Thalictrum lucidum ! | 8 | + | + | > | + | SP |
| Trifolium campestre ! | 4 | + | − | T | ||
| Trifolium pratense | 6 | + | − | T | ||
| Trisetum flavescens | 6 | + | − | T | ||
| Vicia hirsuta | 3 | + | − | T | ||
| Vicia sepium | 5 | + | − | T | ||
| Semi-dry grassland type, N = 6 | ||||||
| Achillea collina | 2 | + | − | T | ||
| Alopecurus pratensis | 6 | + | − | T | ||
| Brachypodium pinnatum | 4 | + | − | T | ||
| Carex hirta | 7 | + | + | ≤ | + | LP |
| Carex muricata ssp. pairae | 5 | − | + | > | + | SP |
| Carex praecox | 3 | + | − | T | ||
| Colchicum autumnale ! | 6 | + | − | T | ||
| Dactylis glomerata | 6 | + | − | T | ||
| Elytrigia repens | 5 | + | − | T | ||
| Erigeron annuus ! | 7 | − | + | > | + | SP |
| Festuca stricta ssp. sulcata | 3 | + | − | T | ||
| Filipendula vulgaris ! | 4 | + | − | T | ||
| Galium verum | 4 | + | − | T | ||
| Inula salicina ! | 5 | + | − | T | ||
| Lathyrus pratensis | 7 | + | − | T | ||
| Peucedanum alsaticum | 3 | + | − | T | ||
| Peucedanum cervaria | 2 | + | − | T | ||
| Poa pratensis | 6 | + | + | > | − | T |
| Ranunculus acris | 7 | + | − | T | ||
| Salvia pratensis | 3 | + | − | T | ||
| Serratula tinctoria ! | 7 | + | − | T | ||
| Silene latifolia | 4 | − | + | > | + | LP |
| Silene otites | 2 | + | + | > | − | T |
| Stellaria media ! | 5 | − | + | < | + | LP |
| Trifolium campestre ! | 4 | + | − | T | ||
| Trifolium montanum | 3 | + | − | T | ||
| Verbascum phoeniceum ! | 2 | + | + | > | − | T |
| Vicia cracca | 4 | + | − | T | ||
References
- Bossuyt, B.; Honnay, O. Can the seed bank be used for ecological restoration? An overview of seed bank characteristics in European communities. J. Veg. Sci. 2008, 19, 875–884. [Google Scholar] [CrossRef]
- Kiss, R. The role of soil seed bank in restoration and dynamics of Hungarian plant communities—A review of Hungarian seed bank research. Kitaibelia 2016, 21, 116–135. [Google Scholar] [CrossRef][Green Version]
- Thompson, K.; Bakker, J.P.; Bekker, R.M. Soil Seed Banks of North West Europe: Methodology, Density and Longevity; Cambridge University Press: Cambridge, UK, 1997. [Google Scholar]
- Abedi, M.; Bartelheimer, M.; Poschlod, P. Effects of substrate type, moisture and its interactions on soil seed survival of three Rumex species. Plant Soil 2014, 374, 485–495. [Google Scholar] [CrossRef]
- Bakker, J.P.; Poschlod, P.; Strykstra, R.J.; Bekker, R.M.; Thompson, K. Seed banks and seed dispersal: Important topics in restoration ecology. Acta Bot. Neerl. 1996, 45, 461–490. [Google Scholar] [CrossRef]
- Thompson, K.; Band, S.R.; Hodgson, J.G. Seed size and shape predict persistence in soil. Funct. Ecol. 1993, 7, 236–241. [Google Scholar] [CrossRef]
- Bekker, R.M.; Bakker, J.P. Seed traits: Essential for understanding seed longevity. Asp. Appl. Biol. 2003, 69, 1–9. [Google Scholar]
- Hopfensperger, K.N. A review of similarity between seed bank and standing vegetation across ecosystems. Oikos 2007, 116, 1438–1448. [Google Scholar] [CrossRef]
- Valkó, O.; Török, P.; Vida, E.; Arany, I.; Tóthmérész, B.; Matus, G. The role of soil seed banks in restoration of two hay meadows. Természetvédelmi Közlemények 2009, 15, 147–159, (In Hungarian with English summary). [Google Scholar]
- Ma, M.; Zhou, X.; Ma, Z.; Du, G. Composition of the soil seed bank and vegetation changes after wetland drying and soil salinization on the Tibetan Plateau. Ecol. Eng. 2012, 44, 18–24. [Google Scholar] [CrossRef]
- Simmering, D.; Waldhardt, R.; Otte, A. Quantifying determinants contributing to plant species richness in mosaic landscapes: A single- and multi-patch perspective. Landsc. Ecol. 2006, 21, 1233–1251. [Google Scholar] [CrossRef]
- Bakker, J.P. Nature Management by Grazing and Cutting: On the Ecological Significance of Grazing and Cutting Regimes Applied to Restore Former Species-rich Grassland Communities in the Netherlands (Geobotany 14); Kluwer Academic Publishers: Dordrecht, The Netherlands, 1989. [Google Scholar] [CrossRef]
- Wellstein, C.; Otte, A.; Waldhardt, R. Seed bank diversity in mesic grasslands in relation to vegetation type, management and site conditions. J. Veg. Sci. 2007, 18, 153–162. [Google Scholar] [CrossRef]
- Leck, M.A.; Schütz, W. Regeneration of Cyperaceae, with particular reference to seed ecology and seed banks. Perspect. Plant Ecol. Evol. Syst. 2005, 7, 95–133. [Google Scholar] [CrossRef]
- Ludewig, K.; Zelle, B.; Eckstein, R.L.; Mosner, E.; Otte, A.; Donath, T.W. Differential effects of reduced water potential on the germination of floodplain grassland species indicative of wet and dry habitats. Seed Sci. Res. 2014, 24, 49–61. [Google Scholar] [CrossRef]
- Thompson, K.; Bakker, J.P.; Bekker, R.M.; Hodgson, J.G. Ecological correlates of seed persistence in soil in the north-west European flora. J. Ecol. 1998, 86, 163–169. [Google Scholar] [CrossRef]
- Baker, H.G. Seed weight in relation to environmental conditions in California. Ecology 1972, 53, 997–1010. [Google Scholar] [CrossRef]
- Leishman, M.R.; Westoby, M. The role of seed size in seedling establishment in dry soil conditions—Experimental evidence from semi-arid species. J. Ecol. 1994, 82, 249–258. [Google Scholar] [CrossRef]
- Tautenhahn, S.; Heilmeier, H.; Götzenberger, L.; Klotz, S.; Wirth, C.; Kühn, I. On the biogeography of seed mass in Germany—Distribution patterns and environmental correlates. Ecography 2008, 31, 457–468. [Google Scholar] [CrossRef]
- Bekker, R.M.; Bakker, J.P.; Gradin, U.; Kalamees, P.; Milberg, P.; Poschlod, P.; Thompson, K.; Willems, H. Seed size, shape and vertical distribution in the soil: Indicators of seed longevity. Funct. Ecol. 1998, 12, 834–842. [Google Scholar] [CrossRef]
- Csontos, P. Methods for Studying the Natural Seed Bank (Synbiologia Hungarica 4); Scientia Kiadó: Budapest, Hungary, 2001. (In Hungarian) [Google Scholar]
- Csontos, P. Advances in studying soil seed bank and other seed-related ecological questions, concerning to the Hungarian flora. Kanitzia 2010, 17, 77–110, (In Hungarian with English summary). [Google Scholar]
- Csontos, P.; Kalapos, T.; Tamás, J. Seed trait databases—Ecological applications. J. Landsc. Ecol.—Tájökológiai Lapok 2013, 11, 335–339, (In Hungarian with English summary). [Google Scholar]
- Dövényi, Z. (Ed.) Inventory of Microregions in Hungary, 2nd ed.; MTA Geographical Research Institute: Budapest, Hungary, 2010. (In Hungarian) [Google Scholar]
- Schellenberger, J. Soil Seed Bank Examinations in Grassland Types with Different Water Regime. Ph.D. Thesis, Hungarian University of Agriculture and Life Sciences, Gödöllő, Hungary, 2021. (In Hungarian with English summary). [Google Scholar]
- Ter Heerdt, G.N.J.; Verweij, G.L.; Bekker, R.M.; Bakker, J.P. An improved method for seed bank analysis: Seedling emergence after removing the soil by sieving. Funct. Ecol. 1996, 10, 144–151. [Google Scholar] [CrossRef]
- Csontos, P. Seed bank ecology II. Techniques for estimation of seed banks in soil samples and comparison of methods. Acta Agronomica Óváriensis 2000, 42, 133–150, (In Hungarian with English summary). [Google Scholar]
- MSZ-08-0210:1977; Testing Organic Carbon Content in Soils. Hungarian Standards Institution: Budapest, Hungary, 1977. (In Hungarian)
- MSZ-08-0205:1978; Determination of Physical and Hydrophysical Properties of Soils. Hungarian Standards Institution: Budapest, Hungary, 1978. (In Hungarian)
- MSZ-08-0206-2:1978; Evaluation of Some Chemical Properties of the Soil: Laboratory Tests (pH Value, Phenolphtaleine Alkalinity Expressed in Soda, All Water Soluble Salts, Hydrolite (y1-Value) and Exchanging Acidity (y2-Value)). Hungarian Standards Institution: Budapest, Hungary, 1978. (In Hungarian)
- Borhidi, A. Social behaviour types, the naturalness and relative ecological indicator values of the higher plants in the Hungarian Flora. Acta Bot. Hung. 1995, 39, 97–181. [Google Scholar]
- Horváth, F.; Dobolyi, Z.K.; Morschhauser, T.; Lőkös, L.; Karas, L.; Szerdahelyi, T. Hungarian FLORA Database 1.2: List of Taxa and Relevant Attributes; FLÓRA Workgroup of MTA Institute of Ecology and Botany–MTM Department of Botany: Vácrátót, Hungary, 1995; (In Hungarian with English summary). [Google Scholar]
- Euro+Med (2006–): Euro+Med PlantBase—The Information Resource for Euro-Mediterranean Plant Diversity. Available online: http://ww2.bgbm.org/EuroPlusMed/ (accessed on 1 January 2022).
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 1–9. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2017. [Google Scholar]
- Maechler, M.; Rousseeuw, P.; Struyf, A.; Hubert, M.; Hornik, K. Cluster: Cluster Analysis Basics and Extensions. R Package Version 2.0.8; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Venables, W.N.; Ripley, B. Modern Applied Statistics with S, 4th ed.; Springer: New York, NY, USA, 2002. [Google Scholar] [CrossRef]
- Valkó, O.; Török, P.; Tóthmérész, B.; Matus, G. Restoration potential in seed banks of acidic fen and dry-mesophilous meadows: Can restoration be based on local seed banks? Restor. Ecol. 2011, 19, 9–15. [Google Scholar] [CrossRef]
- Jensen, K. Species composition of soil seed bank and seed rain of abandoned wet meadows and their relation to aboveground vegetation. Flora 1998, 193, 345–359. [Google Scholar] [CrossRef]
- Bölöni, J.; Molnár, Z.; Kun, A. (Eds.) Habitats of Hungary: Description and Determinant of Vegetation Types in Hungary: ÁNÉR 2011; MTA Institute of Ecology and Botany: Vácrátót, Hungary, 2011. (In Hungarian) [Google Scholar]
- Davies, C.E.; Moss, D.; Hill, M.O. EUNIS Habitat Classification Revised 2004. Report to the EEA European Topic Centre on Nature Protection and Biodiversity; European Economic Area: Copenhagen, Denmark, 2004. [Google Scholar]
- Zar, J.H. Biostatistical Analysis, 5th ed.; Prentice Hall: Upper Saddle River, NJ, USA, 2010. [Google Scholar]
- Thompson, K. Seed persistence in soil. In Methods in Comparative Plant Ecology: A Laboratory Manual; Hendry, G.A.F., Grime, J.P., Eds.; Chapman and Hall: London, UK, 1993; pp. 199–202. [Google Scholar]
- Csontos, P.; Tamás, J.; Tobisch, T. Presentation of seed dispersal database of the Hungarian Flora through analytical examples: Evaluation of Social Behaviour Types. In Botanical Researches in Hungary at the Millennium: Studies in Honour of Attila Borhidi’s 70th Birthday; Salamon-Albert, É., Ed.; PTE Department of Botany: Pécs, Hungary, 2002; pp. 557–569. (In Hungarian) [Google Scholar]
- Hungarian Ministry of Environmental Protection. 13/2001. (V. 9.) KöM decree on protected and specially protected plant and animal species, the range of specially protected caves and the publication of species of plant and animal species of conservation importance in the European Community. Magyar Közlöny 2001, 53, 3446–3511. (In Hungarian) [Google Scholar]
- Labrada, R. (Ed.) Weed Management for Developing Countries (FAO Plant Production and Protection Paper 120, Addendum 1); Food and Agriculture Organization of the United Nations: Rome, Italy, 2003. [Google Scholar]
- Wang, Y.; Jiang, D.; Toshio, O.; Zhou, Q. Recent advances in soil seed bank research. Contemp. Probl. Ecol. 2013, 6, 520–524. [Google Scholar] [CrossRef]
- Thompson, K. The functional ecology of seed banks. In Seeds: The Ecology of Regeneration in Plant Communities; Fenner, M., Ed.; CAB International: Wallingford, UK, 1992; pp. 231–258. [Google Scholar] [CrossRef]
- Thompson, K.; Grime, J.P. Seasonal variation in the seed banks of herbaceous species in ten contrasting habitats. J. Ecol. 1979, 67, 893–921. [Google Scholar] [CrossRef]
- Harper, J.L. Population Biology of Plants; Academic Press: London, UK, 1977. [Google Scholar]
- Brock, M.A.; Nielson, D.L.; Shiel, R.J.; Green, J.D.; Langley, J.D. Drought and aquatic community resilience: The role of eggs and seeds in sediments of temporary wetlands. Freshw. Biol. 2003, 48, 1207–1218. [Google Scholar] [CrossRef]
- Csontos, P. Fundamentals of Seed Bank Ecology, the Seed Ecological Study of the Hungarian Flora. D.Sc. Thesis, Hungarian Academy of Sciences, Budapest, Hungary, 2006. (In Hungarian). [Google Scholar]
- Csontos, P. Seed longevity studies on species of weedy places, dry grasslands and forests. Magy. Gyomkutatás Technol. 2006, 7, 101–112, (In Hungarian with English summary). [Google Scholar]
- Oomes, M.J.M.; Kuikman, P.J.; Jacobs, F.H.H. Nitrogen availability and uptake by grassland in mesocosms at two water levels and two water qualities. Plant Soil 1997, 192, 249–259. [Google Scholar] [CrossRef]
- Bekker, R.M.; Oomes, M.J.M.; Bakker, J.P. The impact of groundwater level on soil seed bank survival. Seed Sci. Res. 1998, 8, 399–404. [Google Scholar] [CrossRef]
- Albrecht, H.; Auerswald, K. Arable weed seedbanks and their relation to soil properties. Asp. Appl. Biol. 2003, 69, 11–20. [Google Scholar]
- Pakeman, R.J.; Small, J.L.; Torvell, L. Edaphic factors influence the longevity of seeds in the soil. Plant Ecol. 2012, 213, 57–65. [Google Scholar] [CrossRef]
- Kirkpatrick, B.L.; Bazzaz, F.A. Influence of certain fungi on seed germination and seedling survival of four colonizing annuals. J. Appl. Ecol. 1979, 16, 515–527. [Google Scholar] [CrossRef]
- Chambers, J.C.; MacMahon, J.A. A day in the life of a seed: Movements and fates of seeds and their implications for natural and managed systems. Annu. Rev. Ecol. Syst. 1994, 25, 263–292. [Google Scholar] [CrossRef]
- Kaiser, T.; Pirhofer-Walzl, K. Does the soil seed survival of fen-meadow species depend on the groundwater level? Plant Soil 2015, 387, 219–231. [Google Scholar] [CrossRef]
- Lv, Y.; Shen, M.; Meng, B.; Zhang, H.; Sun, Y.; Zhang, J.; Chang, L.; Li, J.; Yi, S. The similarity between vegetation and soil seed bank of grasslands in Inner Mongolia, China: Implications for the asymmetric response of productivity to precipitation. Plants 2021, 10, 1890. [Google Scholar] [CrossRef]
- Matus, G.; Verhagen, R.; Bekker, R.M.; Grootjans, A.P. Restoration of the Cirsio dissecti-Molinietum in The Netherlands: Can we rely on soil seed banks? Appl. Veg. Sci. 2003, 6, 73–84. [Google Scholar] [CrossRef]
- Kiss, R.; Deák, B.; Török, P.; Tóthmérész, B.; Valkó, O. Grassland seed bank and community resilience in a changing climate. Restor. Ecol. 2018, 26, S141–S150. [Google Scholar] [CrossRef]
- Sun, Y.; Yi, S.; Hou, F. Unmanned aerial vehicle methods makes species composition monitoring easier in grasslands. Ecol. Indic. 2018, 95, 825–830. [Google Scholar] [CrossRef]
- Fehérváry, I.; Kiss, T. Automatised identification of vegetation types on a floodplain area based on airborne LIDAR survey. J. Landsc. Ecol.—Tájökológiai Lapok 2020, 18, 127–140, (In Hungarian with English Summary). [Google Scholar]
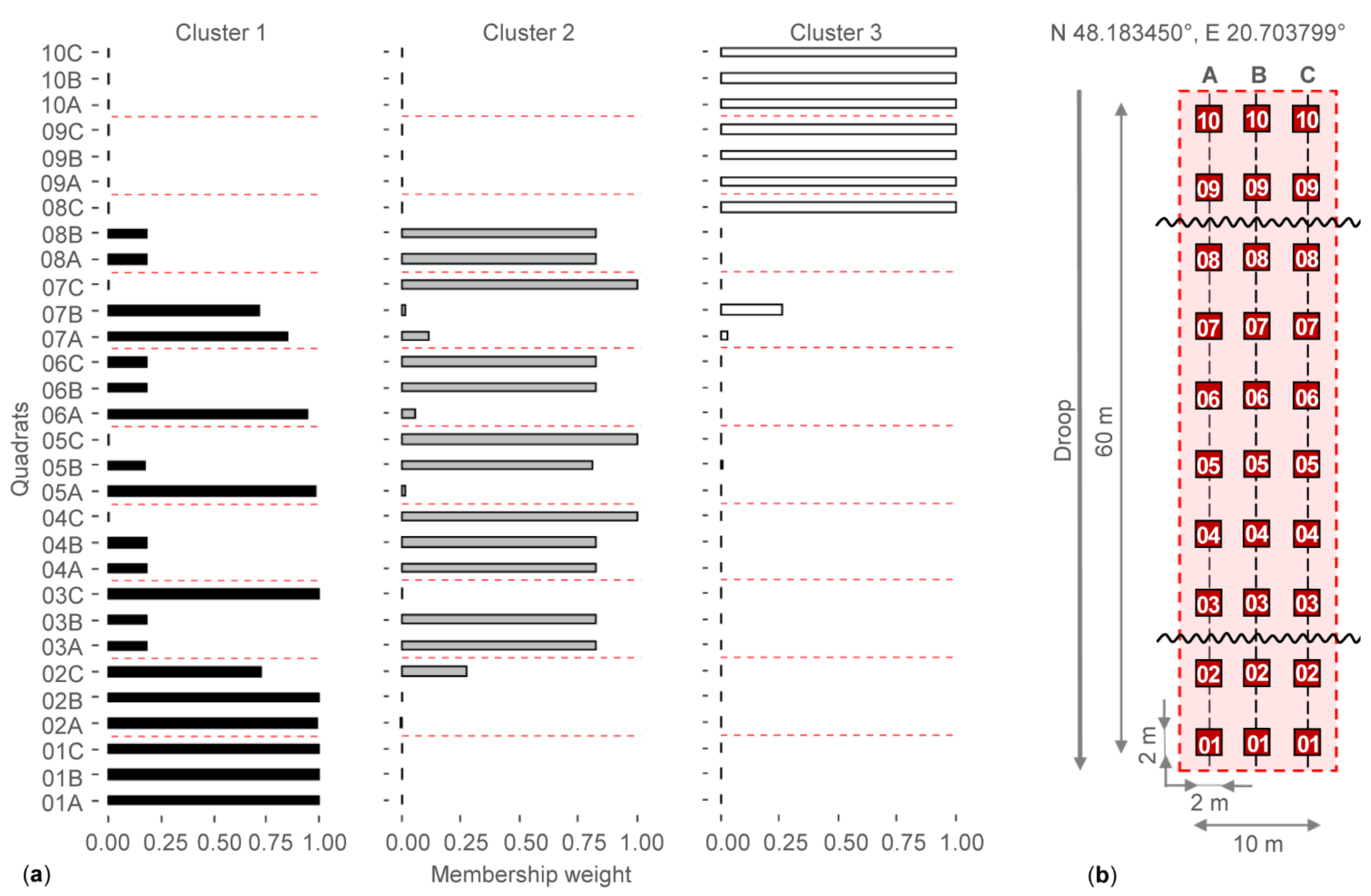
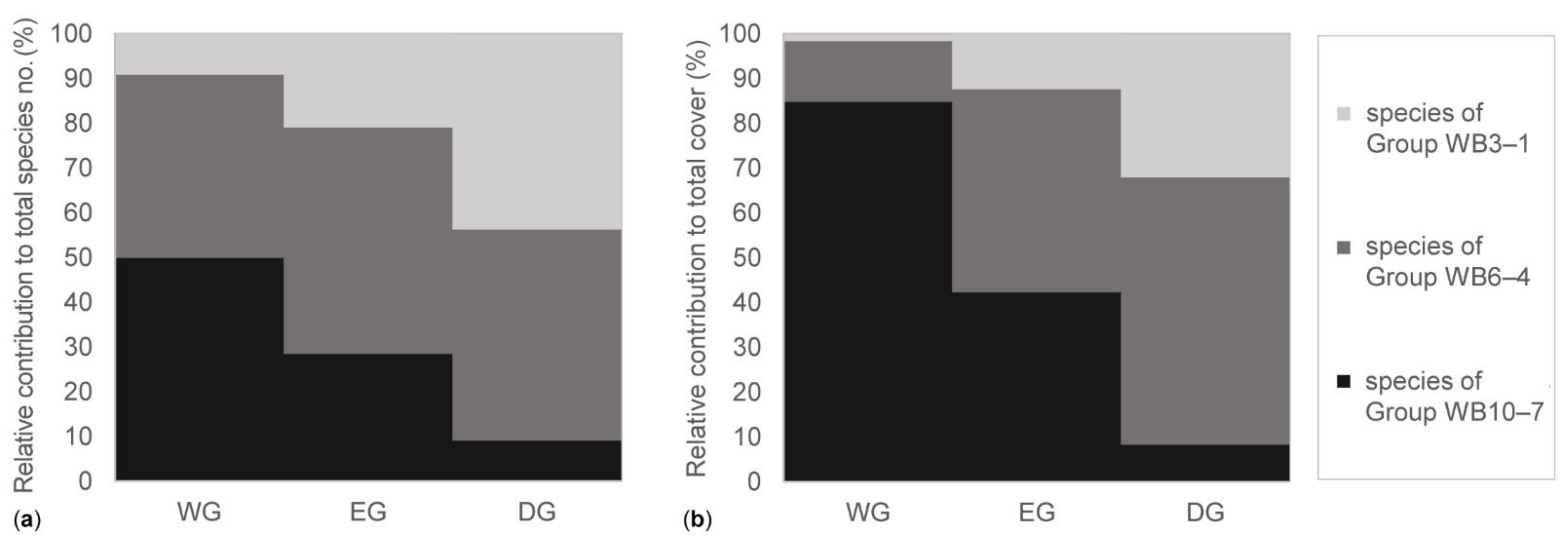

| Cover (%) | Species Number | Shannon Diversity | ||
|---|---|---|---|---|
| ± SD | ∑ | ± SD | ± SD | |
| Wet grassland type, N = 6 | 89.2 ± 4.9 a | 44.0 | 21.7 ± 5.3 a | 1.7 ± 0.7 |
| Ecotone grassland type, N = 18 | 104.7 ± 14.1 b | 91.0 | 27.9 ± 3.4 a | 2.4 ± 0.3 |
| Semi-dry grassland type, N = 6 | 126.7 ± 11.7 c | 55.0 | 27.5 ± 5.8 a | 2.2 ± 0.3 |
| Density (Seeds/m2) | Species Number | Shannon Diversity | ||
|---|---|---|---|---|
| ± SD | ∑ | ± SD | ± SD | |
| Wet grassland type, N = 6 | ||||
| 0–10 cm | 17,129.6 ± 10,372.7 a | 29.0 | 10.7 ± 2.0 a | 1.5 ± 0.4 |
| 0–5 cm | 14,240.9 ± 8666.9 | 26.0 | 9.5 ± 1.0 | 1.4 ± 0.3 |
| 5–10 cm | 2888.6 ± 1959.8 | 12.0 | 4.7 ± 1.5 | 1.2 ± 0.4 |
| Ecotone grassland type, N = 18 | ||||
| 0–10 cm | 14,423.9 ± 6659.3 a | 49.0 | 10.4 ± 3.8 a | 1.3 ± 0.6 |
| 0–5 cm | 10,591.6 ± 5131.1 | 40.0 | 8.1 ± 3.5 | 1.1 ± 0.6 |
| 5–10 cm | 3832.2 ± 2354.7 | 31.0 | 6.1 ± 2.2 | 1.3 ± 0.4 |
| Semi-dry grassland type, N = 6 | ||||
| 0–10 cm | 3697.4 ± 4130.2 b | 30.0 | 8.3 ± 5.0 a | 1.7 ± 0.4 |
| 0–5 cm | 3004.2 ± 3644.9 | 25.0 | 7.0 ± 4.5 | 1.5 ± 0.5 |
| 5–10 cm | 693.3 ± 701.9 | 12.0 | 2.5 ± 2.9 | 0.6 ± 0.8 |
| Veg. Cover (%) | Seed Bank Density (Seeds/m2) | ST | |||
|---|---|---|---|---|---|
| 0–10 cm | 0–5 cm | 5–10 cm | |||
| ± SD | ± SD | ± SD | ± SD | ||
| (a) The five most abundant species of vegetation by grassland type | |||||
| Wet grassland type, N = 6 | |||||
| Carex acutiformis | 50.1 ± 18.8 | 693.3 ± 580.0 | 693.3 ±580.0 | – | T |
| Colchicum autumnale ! | 4.9 ± 5.7 | – | – | – | T |
| Cirsium canum ! | 4.7 ± 5.2 | – | – | – | T |
| Lathyrus palustris | 3.3 ± 8.2 | – | – | – | ? |
| Lysimachia vulgaris | 3.3 ± 2.3 | – | – | – | T |
| Ecotone grassland type, N = 18 | |||||
| Carex muricata ssp. pairae | 14.6 ± 15.6 | 38.5 ± 74.1 | 28.9 ± 66.5 | 9.6 ± 40.9 | SP |
| Carex acutiformis | 7.9 ± 13.2 | 423.7 ± 468.9 | 240.7 ± 215.5 | 182.9 ± 322.7 | SP |
| Colchicum autumnale ! | 7.4 ± 12.8 | – | – | – | T |
| Cirsium canum ! | 7.4 ± 6.6 | 9.6 ± 40.9 | – | 9.6 ± 40.9 | ? |
| Dactylis glomerata | 5.8 ± 6.8 | – | – | – | T |
| Semi-dry grassland type, N = 6 | |||||
| Brachypodium pinnatum | 22.8 ± 11.8 | – | – | – | T |
| Elytrigia repens | 15.0 ± 23.0 | – | – | – | T |
| Peucedanum cervaria | 13.3 ± 13.1 | – | – | – | T |
| Filipendula vulgaris | 10.2 ± 6.0 | – | – | – | T |
| Vicia hirsuta | 8.7 ± 8.0 | 28.9 ± 70.8 | 28.9 ± 70.8 | – | ? |
| (b) The five most abundant species of seed bank by grassland type | |||||
| Wet grassland type, N = 6 | |||||
| Lythrum salicaria | 0.9 ± 0.5 | 9879.1 ± 7225.5 | 8492.6 ± 6180.4 | 1386.5 ± 1160.1 | SP |
| Erigeron annuus ! | 0.1 ± 0.3 | 2166.5 ± 991.1 | 1733.2 ± 701.9 | 433.3 ± 305.2 | SP |
| Veronica longifolia | 0.4 ± 0.5 | 1704.3 ± 2850.9 | 1299.9 ± 2303.9 | 404.4 ± 586.9 | SP |
| Carex acutiformis | 50.1 ± 18.8 | 693.3 ± 580.0 | 693.3 ± 580.0 | – | T |
| Anagallis arvensis ! | – | 635.5 ± 498.3 | 548.8 ± 519.0 | 86.7 ± 94.9 | LP |
| Ecotone grassland type, N = 18 | |||||
| Erigeron annuus ! | 3.7 ± 5.3 | 9397.7 ± 6266.4 | 7375.6 ± 5279.2 | 2022.0 ± 1454.9 | SP |
| Lythrum salicaria | 0.1 ± 0.2 | 1694.7 ± 1985.5 | 1357.7 ± 1698.4 | 337.0 ± 400.9 | SP |
| Silene flos-cuculi ! | 0.04 ± 0.06 | 606.6 ± 731.1 | 240.7 ± 320.9 | 365.9 ± 456.2 | LP |
| Juncus articulatus ! | – | 442.9 ± 876.2 | 211.8 ± 354.5 | 231.1 ± 582.5 | LP |
| Carex acutiformis | 7.9 ± 13.2 | 423.7 ± 468.9 | 240.7 ± 215.5 | 182.9 ± 322.7 | SP |
| Semi-dry grassland type, N = 6 | |||||
| Erigeron annuus ! | – | 1068.8 ± 2118.2 | 953.2 ± 1919.8 | 115.5 ± 209.9 | SP |
| Carex muricata ssp. pairae | – | 462.2 ± 597.0 | 433.3 ± 534.2 | 28.9 ± 70.8 | SP |
| Carex hirta | 4.0 ± 9.3 | 375.5 ± 614.4 | 173.3 ± 346.6 | 202.2 ± 318.0 | LP |
| Stellaria media ! | – | 173.3 ± 268.5 | 57.8 ± 89.5 | 115.5 ± 209.9 | LP |
| Lythrum salicaria | – | 144.4 ± 277.7 | 115.5 ± 209.9 | 28.9 ± 70.8 | ? |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schellenberger, J.; Csontos, P.; Csorba, Á.; Lengyel, A.; Málnási Csizmadia, G.; Szirmai, O.; Penksza, K.; Czóbel, S. Soil Seed Banks of Continental Grasslands with Different Water Regimes—A Comparative Study from the Aspect of Recovery Potential. Agronomy 2022, 12, 2830. https://doi.org/10.3390/agronomy12112830
Schellenberger J, Csontos P, Csorba Á, Lengyel A, Málnási Csizmadia G, Szirmai O, Penksza K, Czóbel S. Soil Seed Banks of Continental Grasslands with Different Water Regimes—A Comparative Study from the Aspect of Recovery Potential. Agronomy. 2022; 12(11):2830. https://doi.org/10.3390/agronomy12112830
Chicago/Turabian StyleSchellenberger, Judit, Péter Csontos, Ádám Csorba, Attila Lengyel, Gábor Málnási Csizmadia, Orsolya Szirmai, Károly Penksza, and Szilárd Czóbel. 2022. "Soil Seed Banks of Continental Grasslands with Different Water Regimes—A Comparative Study from the Aspect of Recovery Potential" Agronomy 12, no. 11: 2830. https://doi.org/10.3390/agronomy12112830
APA StyleSchellenberger, J., Csontos, P., Csorba, Á., Lengyel, A., Málnási Csizmadia, G., Szirmai, O., Penksza, K., & Czóbel, S. (2022). Soil Seed Banks of Continental Grasslands with Different Water Regimes—A Comparative Study from the Aspect of Recovery Potential. Agronomy, 12(11), 2830. https://doi.org/10.3390/agronomy12112830






