Bacillus velezensis Associated with Organomineral Fertilizer and Reduced Phosphate Doses Improves Soil Microbial—Chemical Properties and Biomass of Sugarcane
Abstract
1. Introduction
2. Materials and Methods
2.1. Cultivation Conditions, Experimental Design, Plant Material, and Treatments
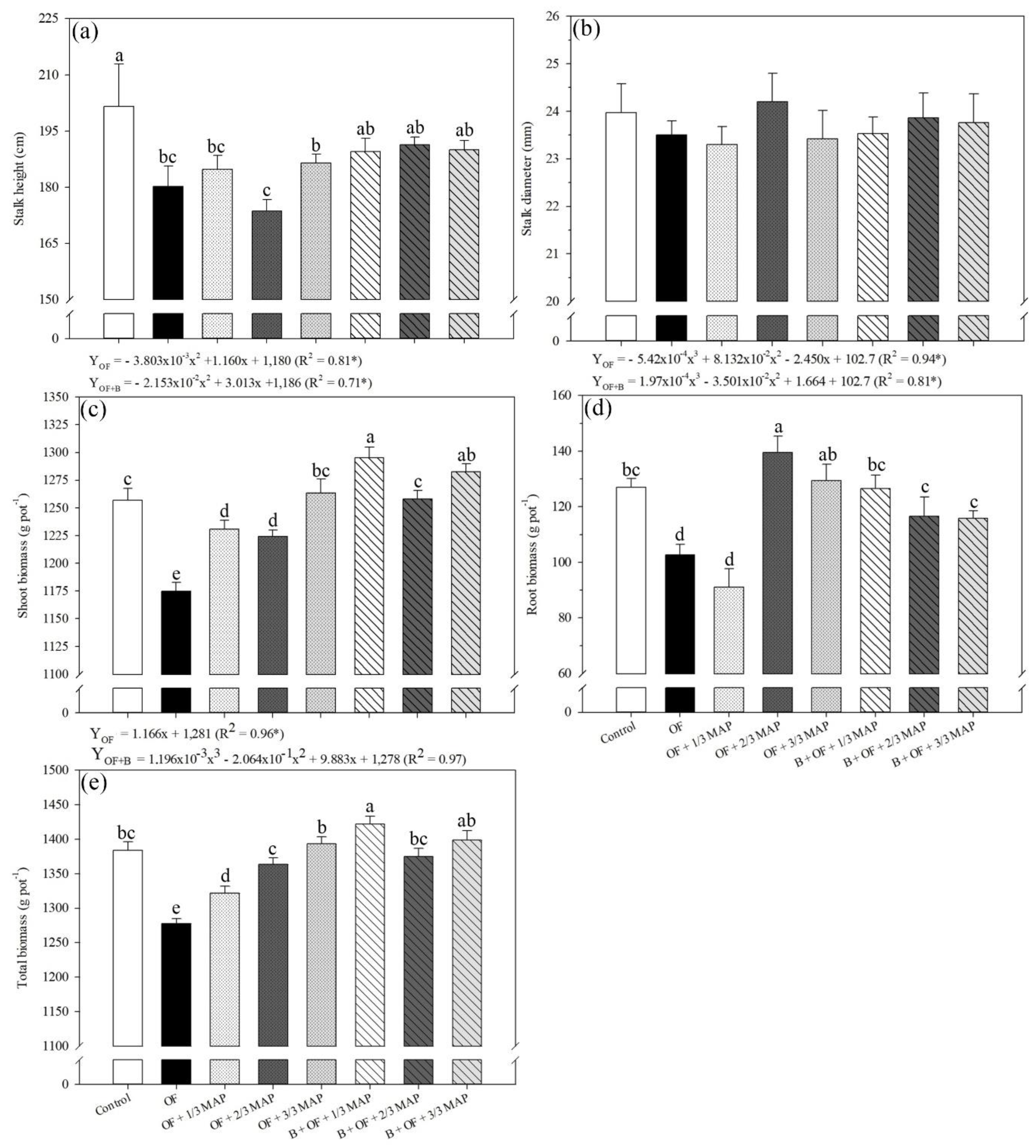
2.2. Morphological Variables
2.3. Foliar Biochemical Variables
2.4. Microbiological Variables
2.5. Soil Biochemical Variables
2.6. Harvest and Yield Variables
2.7. Statistical Analysis
3. Results
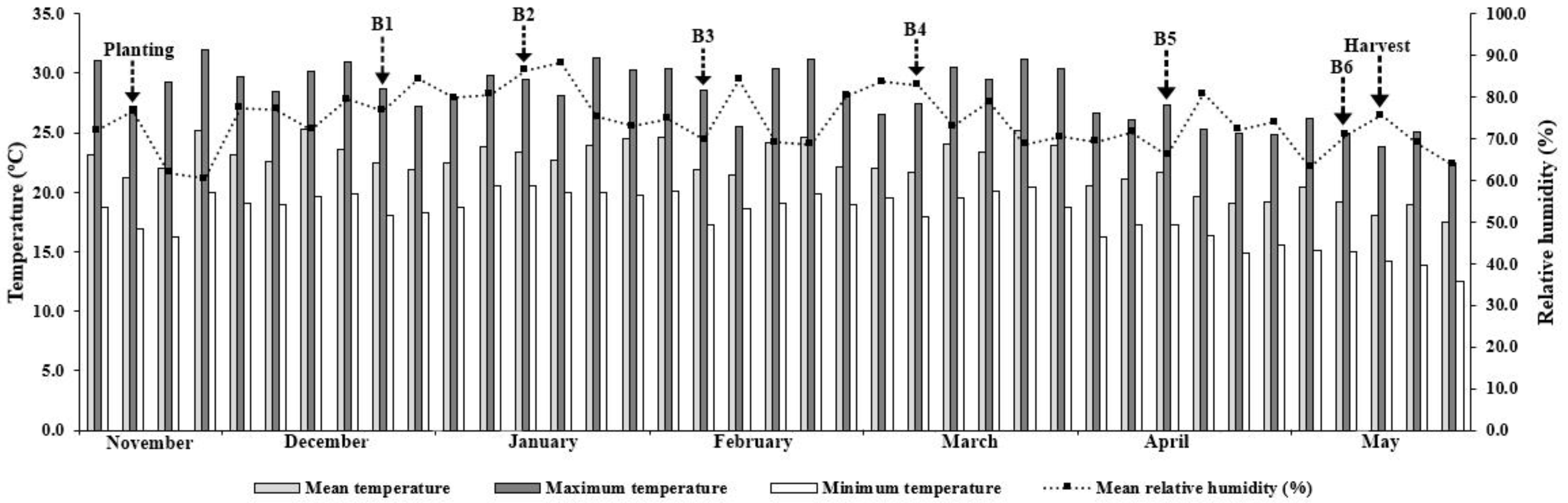
3.1. Environmental Conditions during the Experiment
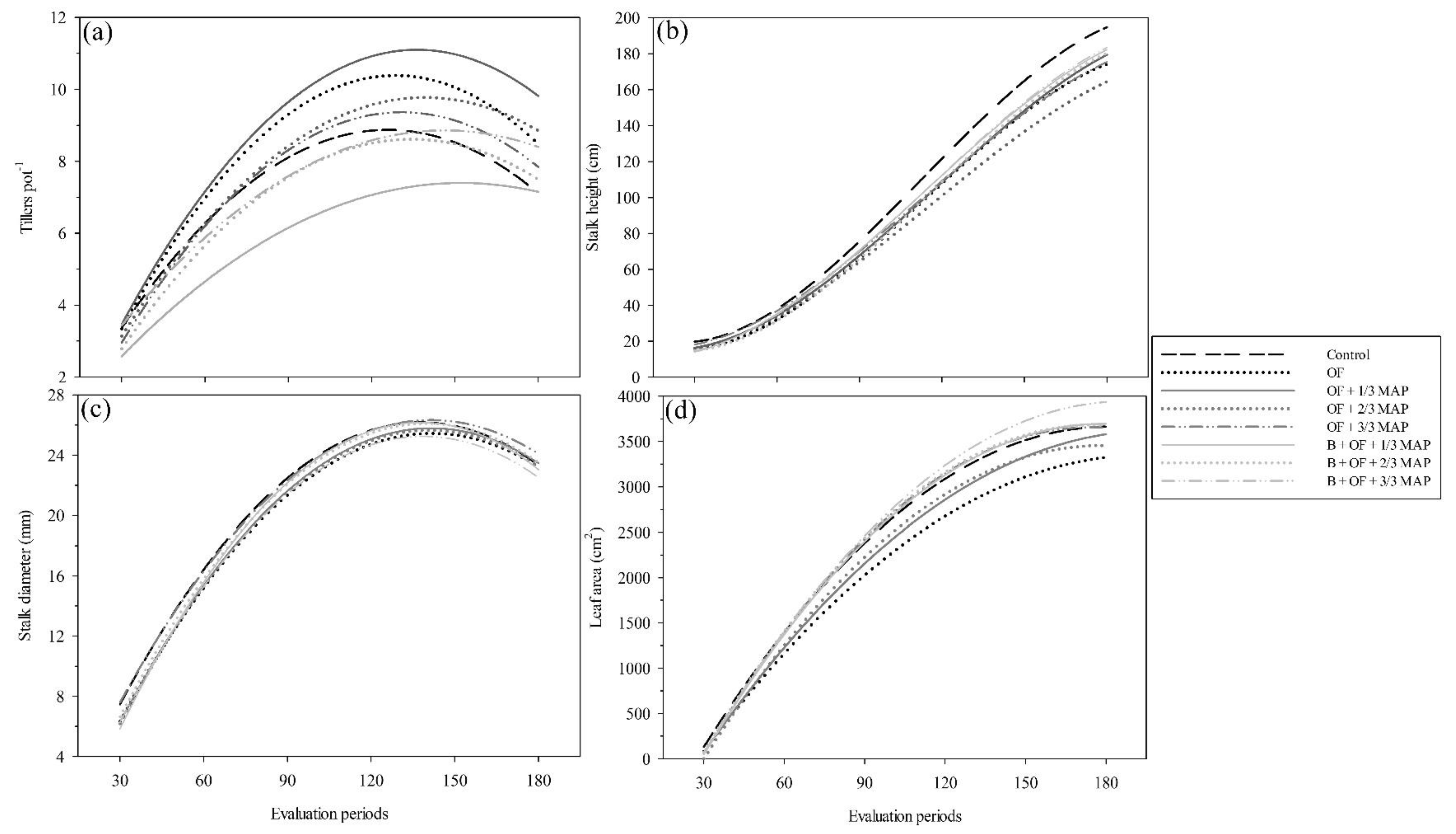
3.2. Morphological Analysis
3.3. Foliar Biochemical Variables
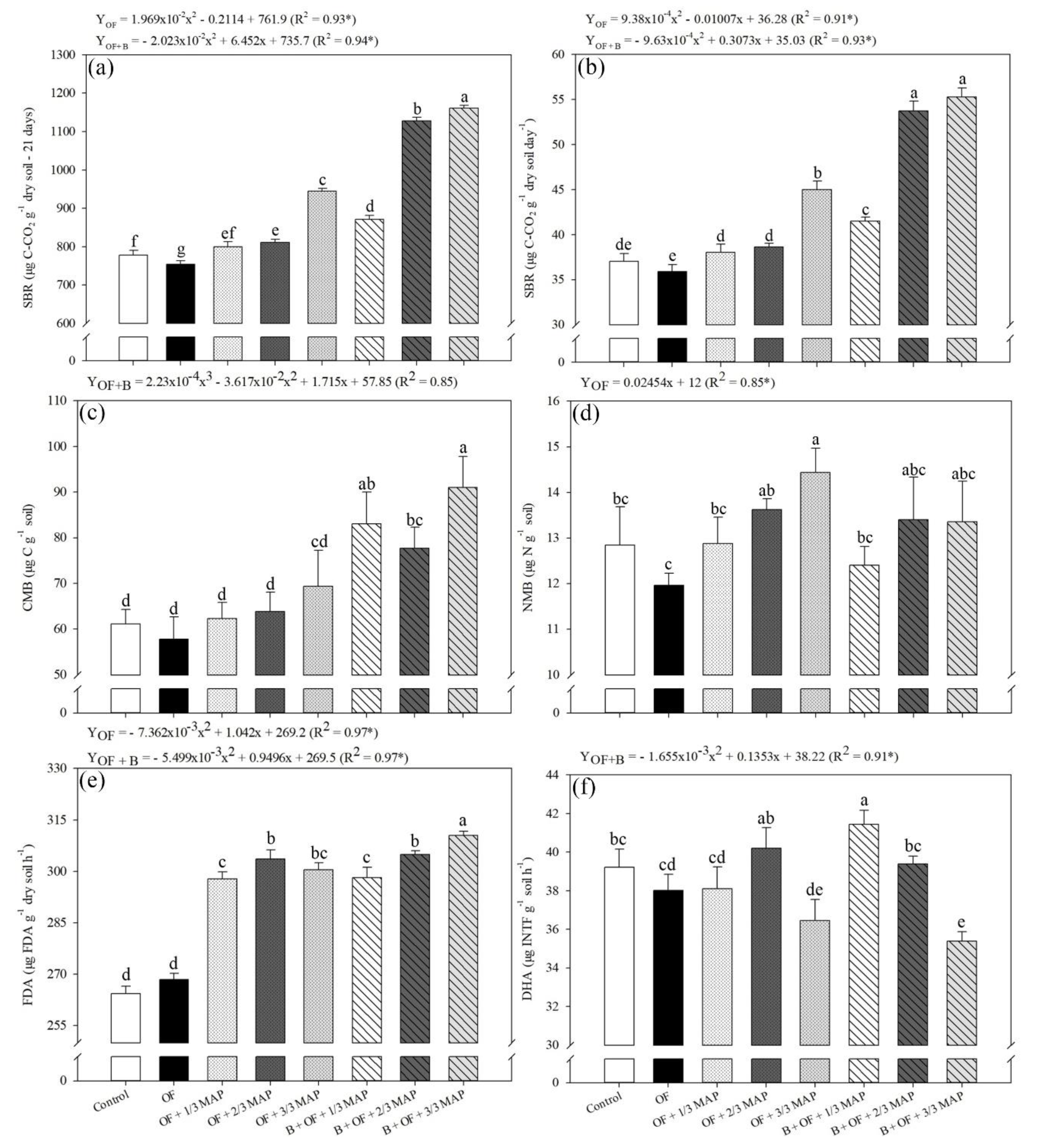
3.4. Microbiological Variables
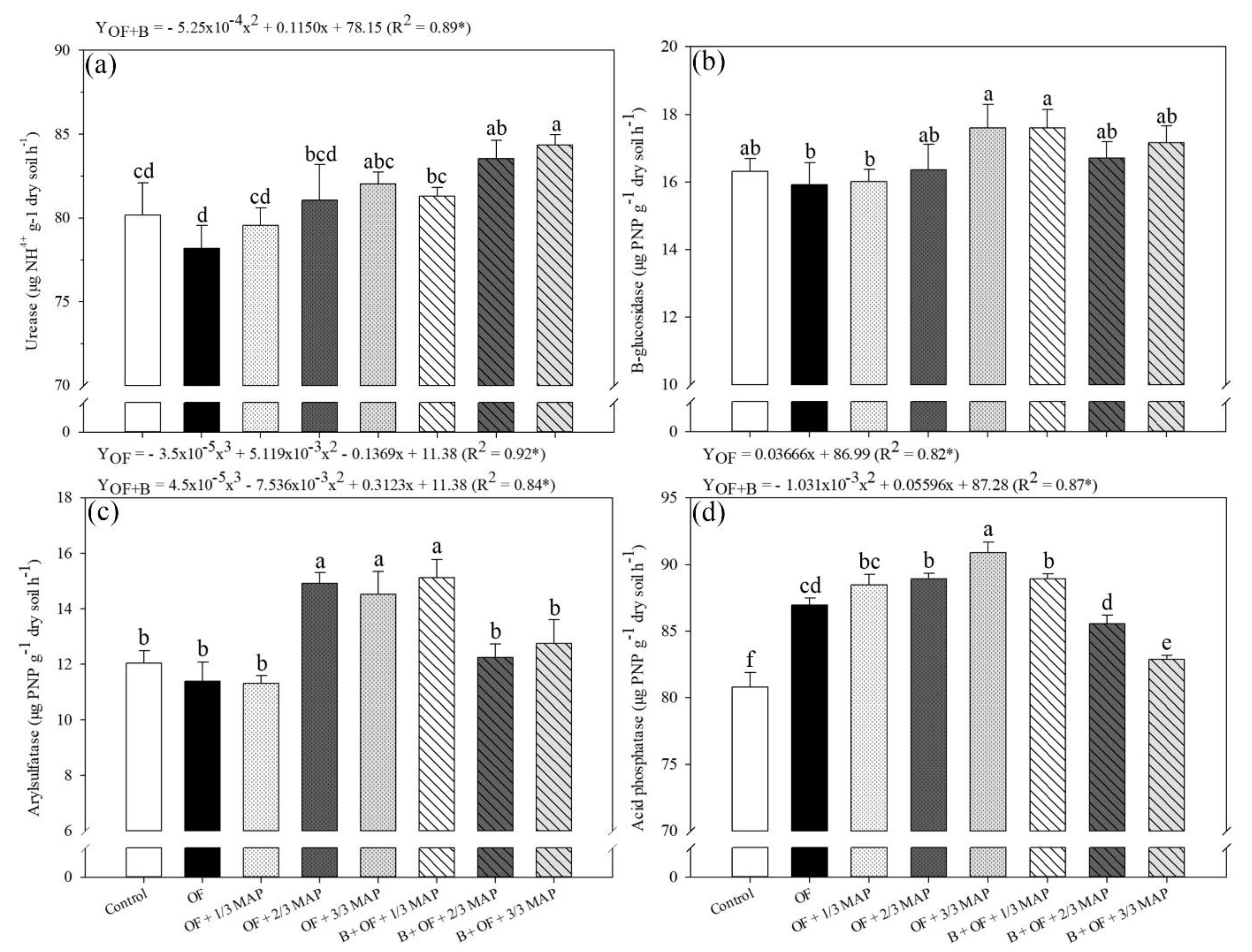
3.5. Soil Biochemical Variables
3.6. Harvest and Yield Variables
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Conab. Companhia Nacional de Abastecimento. Acompanhamento da Safra Brasileira—Cana-de-Açúcar; Primeiro levantamento, maio 2019—Safra 2019/20; Companhia Nacional de Abastecimento: Brasília, DF, Brazil, 2019.
- FAO—Food and Agriculture Organization. Database Faostat—Agriculture; Food and Agriculture Organization of the United Nations: Quebec, QC, Canada, 2020; Available online: http://www.fao.org/faostat/en/#rankings/countries_by_commodity (accessed on 1 September 2022).
- Conab. Companhia Nacional de Abastecimento. Acompanhamento da Safra Brasileira—Cana-de-Açúcar; Primeiro levantamento, abril 2022—Safra 2022/23; Companhia Nacional de Abastecimento: Brasília, DF, Brazil, 2022.
- Guignard, M.S.; Leitch, A.R.; Acquisti, C.; Eizaguirre, C.; Elser, J.J.; Hessen, D.O.; Jeyasingh, P.D.; Neiman, M.; Richardson, A.E.; Soltis, P.S.; et al. Impacts of Nitrogen and Phosphorus: From Genomes to Natural Ecosystems and Agriculture. Front. Ecol. Evol. 2017, 5, 70. [Google Scholar] [CrossRef]
- Marschner, H. Mineral Nutrition of Higher Plants; Elsevier: London, UK, 2012. [Google Scholar]
- Taiz, L.; Zeiger, E.; Moller, I.M.; Murphy, A. Fisiologia e Desenvolvimento Vegetal; Artmed: Porto Alegre, Brazil, 2017. [Google Scholar]
- Silva, A.M.S.; Oliveira, E.C.A.; Willadino, L.G.; Freire, F.J.; Rocha, A.T. Corrective phosphate application as a practice for reducing oxidative stress and increasing productivity in sugarcane. Cienc. Agron. 2019, 50, 188–196. [Google Scholar] [CrossRef]
- Caione, G.; Prado, R.D.M.; Campos, C.; Moda, L.R.; Vasconcelos, R.D.L.; Júnior, J.M.P. Response of Sugarcane in a Red Ultisol to Phosphorus Rates, Phosphorus Sources, and Filter Cake. Sci. World J. 2015, 2015, 405970. [Google Scholar] [CrossRef]
- Borges, B.M.M.N.; Abdala, D.B.; Souza, M.F.; Viglio, L.M.; Coelho, M.J.A.; Pavinato, P.S.; Franco, H.C.J. Organomineral phosphate fertilizer from sugarcane byproduct and its effects on soil phosphorus availability and sugarcane yield. Geoderma 2018, 339, 20–30. [Google Scholar] [CrossRef]
- Novais, R.F.; Smyth, T.J.; Nunes, F.N. Fósforo. In Fertilidade do Solo; Novais, R.F., Alvarez, V.V.H., Barros, N.F., Fontes, R.L.F., Cantarutti, R.B., Neves, J.C.L., Eds.; Sociedade Brasileira de Ciência do Solo: Viçosa, Minas Gerais, Brazil, 2007; pp. 471–550. [Google Scholar]
- Raij, B.V. Fertilidade do Solo e Manejo de Nutrientes; International Plant Nutrition Institute: Piracicaba, São Paulo, Brazil, 2011. [Google Scholar]
- Asomaning, S.K. Processes and factors affecting phosphorus sorption in soils. In Sorption in 2020s; Kyzas, G., Lazaridis, N., Eds.; IntechOpen: London, UK, 2020; pp. 1–15. [Google Scholar]
- Hanyabui, E.; Apori, S.O.; Frimpong, K.A.; Atiah, K.; Abindaw, T.; Ali, M.; Asiamah, J.Y.; Byalebeka, J. Phosphorus sorption in tropical soils. AIMS Agric. Food 2020, 5, 599–616. [Google Scholar] [CrossRef]
- Kumar, A.; Maurya, B.R.; Raghuwanshi, R.; Meena, V.S.; Islam, M.T. Co-inoculation with Enterobacter and Rhizobacteria on Yield and Nutrient Uptake by Wheat (Triticum aestivum L.) in the Alluvial Soil Under Indo-Gangetic Plain of India. J. Plant Growth Regul. 2017, 36, 608–617. [Google Scholar] [CrossRef]
- Meena, R.S.; Meena, V.S.; Meena, S.K.; Verma, J.P. The needs of healthy soils for a healthy world. J. Clean. Prod. 2015, 102, 560–561. [Google Scholar] [CrossRef]
- Rampazzo, P.E.; Marcos, F.C.C.; Cipriano, M.A.P.; Marchiori, P.E.R.; Freitas, S.S.; Machado, E.C.; Nascimento, L.C.; Brochi, M.; Ribeiro, R.V. Rhizobacteria improve sugarcane growth and photosynthesis under well-watered conditions. Ann. Appl. Biol. 2018, 172, 309–320. [Google Scholar] [CrossRef]
- Aye, P.P.; Pinjai, P.; Tawornpruek, S. Effect of Phosphorus Solubilizing Bacteria on Soil Available Phosphorus and Growth and Yield of Sugarcane. Walailak J. Sci. Technol. (WJST) 2021, 18, 10754. [Google Scholar] [CrossRef]
- Crusciol, C.A.C.; Campos, M.; Martello, J.M.; Alves, C.J.; Nascimento, C.A.C.; Pereira, J.C.R.; Cantarella, H. Organomineral Fertilizer as Source of P and K for Sugarcane. Sci. Rep. 2020, 10, 5398. [Google Scholar] [CrossRef]
- Shen, H.; He, X.; Liu, Y.; Chen, Y.; Tang, J.; Guo, T. A Complex Inoculant of N2-Fixing, P- and K-Solubilizing Bacteria from a Purple Soil Improves the Growth of Kiwifruit (Actinidia chinensis) plantlets. Front. Microbiol. 2016, 7, 841. [Google Scholar] [CrossRef]
- Saeid, A.; Prochownik, E.; Dobrowolska-Iwanek, J. Phosphorus Solubilization by Bacillus Species. Molecules 2018, 23, 2897. [Google Scholar] [CrossRef]
- Bald, D.R.; Rangel, C.P.; Vargas, A.; Girão, K.T.; Passaglia, L.M.P. Microbiota do solo: A diversidade invisível e a sua importância. Bio Diverso 2021, 1, 101–131. [Google Scholar]
- Sobucki, L.; Ramos, R.F.; Meireles, L.A.; Antoniolli, Z.I.; Jacques, R.J.S. Contribution of enzymes to soil quality and the evolution of research in Brazil. Rev. Bras. Cien. Solo 2021, 45, e0210109. [Google Scholar] [CrossRef]
- Cunha, A.R.; Martins, D. Classificação climática para os municípios de Botucatu e São Manuel, SP. Irriga 2009, 14, 1–11. [Google Scholar] [CrossRef]
- Santos, H.G.; Jacomine, P.K.T.; Anjos, L.; Oliveira, V.; Lumbreras, J.F.; Coelho, M.R.; Almeida, J.; Araujo Filho, J.; Oliveira, J.; Cunha, T.J.F. Sistema Brasileiro de Classificação de Solos; Embrapa: Brasília, Brazil, 2018. [Google Scholar]
- Braga Junior, R.L.C.; Landell, M.G.A.; Silva, D.N.; Bidóia, M.A.P.; Silva, T.N.; Silva, V.H.P.; Luz, A.M.; Anjos, I.A. Censo Varietal IAC; Região centro-sul—Safra 2019/20; Instituto Agronômico de Campinas: Campinas, São Paulo, Brazil, 2021; (Technical Bulletin, 225).
- Vitti, G.C.; Luz, P.H.C.; Altran, W.S. Nutrição e adubação. In Cana-de-açúcar: Do plantio à colheita; Santos, F., Bórem, A., Eds.; UFV: Viçosa/Minas Gerais, Brazil, 2013; pp. 49–79. [Google Scholar]
- Maguire, J.D. Speed of germination aid in selection and evaluation for seedling emergence and vigor. Crop Sci. 1962, 2, 176–177. [Google Scholar] [CrossRef]
- Hermann, E.R.; Câmara, G.M.S. Um método simples para estimar a área foliar de cana-de-açúcar. STAB Açúcar Álcool Subprodutos 1999, 17, 32–34. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Biochem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Yemm, E.W.; Cocking, E.C. The determination of amino acids with ninhydrin. Analyst 1955, 80, 209–213. [Google Scholar] [CrossRef]
- Ozawa, K.; Osaki, M.; Matisui, H.; Honma, M.; Tadano, T. Purification and properties of acidphosphatase secreted from lupin roots under phosphorus-deficiency conditions. J. Soil Sci. Plant Nutr. 1995, 41, 461–469. [Google Scholar] [CrossRef]
- Silva, E.E.; Azevedo, P.H.S.; De-Polli, H. Determinação da Respiração Basal (RBS) e Quociente Metabólico do Solo (qCO2); Embrapa Agrobiologia: Seropédica, Rio de Janeiro, Brazil, 2007; (Technical Bulletin, 225). [Google Scholar]
- Vance, E.D.; Brookes, P.C.; Jenkinson, D.S. An extraction method for measuring soil microbial biomass-C. Soil Biol. Biochem. 1987, 19, 703–707. [Google Scholar] [CrossRef]
- Ferreira, A.S.; Camargo, F.A.O.; Vidor, C. Utilização de microondas na avaliação da biomassa microbiana do solo. Rev. Bras. Cien. Solo 1999, 23, 991–996. [Google Scholar] [CrossRef]
- Green, V.S.; Stott, D.E.; Diack, M. Assay for fluorescein diacetate hydrolytic activity: Optimization for soil samples. Soil Biol. Biochem. 2006, 38, 693–701. [Google Scholar] [CrossRef]
- Mersi, W.; Schinner, F. An improved and accurate method for determining the dehydrogenase activity of soils with iodonitrotetrazolium chloride. Biol. Fertil. Soils 1991, 11, 216–220. [Google Scholar] [CrossRef]
- Kandeler, E.; Gerber, H. Short-term assay of soil urease activity using colorimetric determination of ammonium. Biol. Fertil. Soils 1988, 6, 68–72. [Google Scholar] [CrossRef]
- Eivazi, F.; Tabatabai, M.A. Glucosidases and galactosidases in soils. Soil Biol. Biochem. 1988, 20, 601–606. [Google Scholar] [CrossRef]
- Tabatabai, M.A.; Bremner, J.M. Arylsulfatase activity of soils. Soil Sci. Soc. Am. Proc. 1969, 34, 225–229. [Google Scholar] [CrossRef]
- Jaiphong, T.K.; Tominaga, J.; Watanabe, K.; Nakabaru, M.; Takaragawa, H.; Suwa, R.; Ueno, M.; Kawamitsu, Y. Effects of duration and combination of drought and flood conditions on leaf photosynthesis, growth and sugar content in sugarcane. Plant Prod. Sci. 2016, 19, 427–437. [Google Scholar] [CrossRef]
- Rodrigues, J.D.; Jadoski, C.J.; Fagan, E.B.; Ono, E.O.; Soares, L.H.; Dourado Neto, D. Fisiologia da Produção de Cana-de-Açúcar; ANDREI: São Paulo, Brazil, 2018. [Google Scholar]
- Alori, E.T.; Glick, B.R.; Babalola, O.O. Microbial phosphorus solubilization and its potential for use in sustainable agriculture. Front. Microbiol. 2017, 8, 971. [Google Scholar] [CrossRef]
- Sagar, A.; Yadav, S.S.; Sayyed, R.Z.; Sharma, S.; Ramteke, P.W. Bacillus subtilis: A multifarious plant growth promoter, biocontrol agent, and bioalleviator of abiotic stress. In Bacilli in Agrobiotechnology; Islam, M.T., Rahman, M., Pandey, P., Eds.; Springer: Cham, Switzerland, 2022; pp. 561–580. [Google Scholar]
- Richardson, A.E.; Simpson, R.J. Soil microorganisms mediating phosphorus availability update on microbial phosphorus. Plant Physiol. 2011, 156, 989–996. [Google Scholar] [CrossRef]
- Aboukila, E.F.; Nassar, I.N.; Rashad, M.; Hafez, M.; Norton, J.B. Reclamation of calcareous soil and improvement of squash growth using brewers’ spent grain and compost. J. Saudi Soc. Agric. Sci. 2018, 17, 390–397. [Google Scholar] [CrossRef]
- Deshev, M.G.; Desheva, G.N.; Stamatov, S.K. Germination and early seedling growth characteristics of Arachis hypogaea L. under salinity (NaCl) stress. Agric. Conspec. Sci. 2020, 85, 113–121. [Google Scholar]
- Hossain, M.A.; Burritt, D.J.; Fujita, M. Proline and Glycine Betainemodulate Cadmium-Induced Oxidative Stress Tolerance in Plants; John Wiley & Sons: Hoboken, NJ, USA, 2015. [Google Scholar]
- Less, H.; Galili, G. Principal transcriptional programs regulating plant amino acid metabolism in response to abiotic stresses. Plant Physiol. 2008, 147, 316–330. [Google Scholar] [CrossRef]
- Chiappero, J.; Cappellari, L.D.R.; Alderete, L.G.S.; Palermo, T.B.; Banchio, E. Plant growth-promoting rhizobacteria improve the antioxidant status in Mentha piperita grown under drought stress leading to anenhancement of plant growth and total phenolic content. Ind. Crops Prod. 2019, 139, 111–553. [Google Scholar] [CrossRef]
- Nunes, F.N.; Cantarutti, R.B.; Novais, R.F.; Silva, I.R.; Tótola, M.R.; Ribeiro, B.N. Atividade de fosfatases em gramíneas forrageiras em resposta à disponibilidade de fósforo no solo e à altura de corte das plantas. Rev. Bras. Cienc. Solo 2008, 32, 1899–1909. [Google Scholar] [CrossRef][Green Version]
- Bozzo, G.G.; Dunn, E.L.; Plaxton, W.C. Differential synthesis of phosphate-starvation inducible purple acid phosphatase isozymes in tomato (Lycopersicon esculentum) suspension cells and seedlings. Plant Cell Environ. 2006, 29, 303–313. [Google Scholar] [CrossRef]
- Ayinla, A.; Alagbe, I.A.; Olayinka, B.U.; Lawal, A.R.; Aboyeji, O.O.; Etejere, E.O. Effects of organic, inorganic, and organo-mineral fertilizer on the growth, yield and nutrient composition of Corchorus olitorious (L). Ceylon J. Sci. Biol. Sci. 2018, 47, 13–19. [Google Scholar] [CrossRef]
- Kominko, H.; Gorazda, K.; Wzorek, Z. The possibility of organo-mineral fertilizer production from sewage sludge. Waste Biomass Valorization 2017, 8, 1781–1791. [Google Scholar] [CrossRef]
- Zhen, Z.; Liu, H.; Wang, N.; Guo, L.; Meng, J.; Ding, N.; Wu, G.; Jiang, G. Effects of manure compost application on soil microbial community diversity and soil microenvironments in a temperate cropland in China. PLoS ONE 2014, 9, e108555. [Google Scholar] [CrossRef]
- Bond-Lamberty, B.; Bolton, H.; Fansler, S.; Heredia-Langner, A.; Liu, C.; McCue, L.A.; Smith, J.; Bailey, V. Soil respiration and bacterial structure and function after 17 years of a reciprocal soil transplant experiment. PLoS ONE 2016, 11, e0150599. [Google Scholar] [CrossRef]
- Siwik-Ziomek, A.; Szczepanek, M. Soil extracellular enzyme activities and uptake of N by oilseed rape depending on fertilization and seaweed bio-stimulant application. Agronomy 2019, 9, 480. [Google Scholar] [CrossRef]
- Hafez, M.; Popov, A.I.; Rashad, M. Influence of Agro-industrial wastes and Azospirillum on nutrients status and grain yield under corn plant growth in arid regions. Biosci. Res. 2019, 16, 2119–2130. [Google Scholar]
- Shi, S.; Tian, L.; Nasir, F.; Bahadur, A.; Batool, A.; Luo, S.; Yang, F.; Wang, Z.; Tian, C. Response of microbial communities and enzyme activities to amendments in saline-alkaline soils. Appl. Soil Ecol. 2019, 135, 16–24. [Google Scholar] [CrossRef]
- Sabaté, D.C.; Brandán, C.P. Bacillus amyloliquefaciens strain enhances rhizospheric microbial growth and reduces root and stem rot in a degraded agricultural system. Rhizosphere 2022, 22, 100544. [Google Scholar] [CrossRef]
- Ai, C.; Liang, G.; Sun, J.; He, P.; Tang, S.; Yang, S.; Zhou, W.; Wang, X. The alleviation of acid soil stress in rice by inorganic or organic ameliorants is associated with changes in soil enzyme activity and microbial community composition. Biol. Fertil. Soils 2015, 51, 465–477. [Google Scholar] [CrossRef]
- Achuba, F.; Peretiemo-Clarke, B. Effect of spent engine oil on soil catalase and dehydrogenase activities. Int. Agrophys. 2008, 22, 1–4. [Google Scholar]
- Ding, Z.; Kheir, A.M.S.; Ali, M.G.M.; Ali, O.A.M.; Abdelaal, A.I.N.; Lin, X.; Zhou, Z.; Wang, B.; Liu, B.; He, Z. The integrated effect of salinity, organic amendments, phosphorus fertilizers, and deficit irrigation on soil properties, phosphorus fractionation and wheat productivity. Sci. Rep. 2020, 10, 2736. [Google Scholar] [CrossRef]
- Schultz, P.; Urban, N.R. Effects of bacterial dynamics on organic matter decomposition and nutrient release from sediments: A modeling study. Ecol. Model. 2008, 210, 1–14. [Google Scholar] [CrossRef]
- Farrell, M.; Prendergast-Miller, M.; Jones, D.L.; Hill, P.W.; Condron, L.M. Soil microbial organic nitrogen uptake is regulated by carbon availability. Soil Biol. Biochem. 2014, 77, 261–267. [Google Scholar] [CrossRef]
- Fraser, F.C.; Todman, L.C.; Corstanje, R.; Deeks, L.K.; Harris, J.A.; Pawlett, M.; Whitmore, A.P.; Ritz, K. Distinct respiratory responses of soils to complex organic substrate are governed predominantly by soil architecture and its microbial community. Soil Biol. Biochem. 2016, 103, 493–501. [Google Scholar] [CrossRef]
- Egamberdieva, D.; Ma, H.; Shurigin, V.; Alimov, J.; Wirth, S.; Bellingrath-Kimura, S.D. Biochar additions alter the abundance of P-cycling-related bacteria in the rhizosphere soil of Portulaca oleracea L. under salt stess. Soil Syst. 2022, 6, 64. [Google Scholar] [CrossRef]
- Sabaté, D.C.; Petroselli, G.; Erra-Balsells, R.; Audisio, M.C.; Brandan, C.P. Beneficial effect of Bacillus sp. P12 on soil biological activities and pathogen control in common bean. Biol. Control 2020, 141, 104131. [Google Scholar] [CrossRef]
- Wu, K.J.; Dumat, C.; Li, H.Q.; Xia, H.P.; Li, Z.A.; Wu, J.T. Responses of soil microbial community and enzymes during plant-assisted biodegradation of di-(2-ethylhexyl) phthalate and pyrene. Int. J. Phytoremediat. 2019, 21, 683–692. [Google Scholar] [CrossRef]
- Duan, Y.; Zhou, Y.; Li, Z.; Chen, X.; Yin, C.; Mao, Z. Effects of Bacillus amyloliquefaciens QSB-6 on the growth of replanted apple tres and the soil microbial environment. Horticulturae 2022, 8, 83. [Google Scholar] [CrossRef]
- Saini, V.K.; Bhandari, S.C.; Tarafdar, J.C. Comparison of crop yield, soil microbial C.N. and P, N-fixation, nodulation and mycorrhizal infection in inoculated and non-inoculated sorghum and chickpea crops. Field Crops Res. 2004, 89, 39–47. [Google Scholar] [CrossRef]
- Ahmed, A.; Nasim, F.u.-H.; Batool, K.; Bibi, A. Microbial β-Glucosidase: Sources, production and applications. J. Appl. Environ. Microbiol. 2017, 5, 31–46. [Google Scholar] [CrossRef]
- Almeida, R.F.; Nave, E.R.; Mota, R.P. Soil quality: Enzymatic activity of soil β-glucosidase. GJARR—Glob. Sci. Res. J. 2015, 3, 146–150. [Google Scholar]
- Deng, S.P.; Tabatabai, M.A. Effect of tillage and residue management on enzyme activities in soils: III. Phosphatases and arylsulfatase. Biol. Fertil. Soils 1997, 24, 141–146. [Google Scholar] [CrossRef]
- Yan, X.; Wei, Z.; Hong, Q.; Lu, Z.; Wu, J. Phosphorus fractions and sorption characteristics in a subtropical paddy soil as influenced by fertilizer sources. Geoderma 2017, 295, 80–85. [Google Scholar] [CrossRef]
- Skujins, J. Dehydrogenase: An indicator of biological activities in arid soils. Bull. Ecol. Res. Commun. 1973, 17, 235–241. [Google Scholar]
- Montalvo, D.; Degryse, F.; Mclaughlin, M.J. Fluid fertilizers improve phosphorus diffusion but not lability in andisols and oxisols. Soil Sci. Soc. Am. J. 2014, 78, 214–224. [Google Scholar] [CrossRef]
- Pavinato, P.S.; Cherubin, M.R.; Soltangheisi, A.; Rocha, G.C.; Chadwick, D.R.; Jones, D.L. Revealing soil legacy phosphorus to promote sustainable agriculture in Brazil. Sci. Rep. 2020, 10, 15615. [Google Scholar] [CrossRef] [PubMed]
- Rosa, P.A.L.; Mortinho, E.S.; Jalal, A.; Galindo, F.S.; Buzetti, S.; Fernandes, G.C.; Barco Neto, M.; Pavinato, O.S.; Teixeira Filho, M.C.M. Inoculation with growth-promoting bacteria associated with the reduction of phosphate fertilization in sugarcane. Front. Environ. Sci. 2020, 8, 32. [Google Scholar] [CrossRef]
- Miljaković, D.; Marinković, J.; Balešević-Tubić, S. The significance of Bacillus spp. in disease suppression and growth promotion of field and vegetable crops. Microorganisms 2020, 8, 1037. [Google Scholar] [CrossRef]
- Alenezi, F.N.; Slama, H.B.; Bouket, A.C.; Cherif-Silini, H.; Silini, A.; Luptakova, L.; Nowakowska, J.A.; Oszako, T.; Belbahri, L. Bacillus velezensis: A treasure house of bioactive compoundsof medicinal, biocontrol and environmental importance. Forests 2021, 12, 1714. [Google Scholar] [CrossRef]


| pH | O.M. | Al3+ | H + Al | K | Ca | Mg | SB | CEC | V% | Presin | S | Cu | Fe | Mn | Zn | B |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CaCl2 | g dm−3 | mmolc dm−3 | mg dm−3 | |||||||||||||
| 6.0 | 40.1 | 0.7 | 20.8 | 0.6 | 75.2 | 26.4 | 102.2 | 123.0 | 83.0 | 61.8 | 34.1 | 0.3 | 22.7 | 0.7 | 1.2 | 0.2 |
| Planting fertilization | ||||||||||||||||
| Dose | N | P | K | |||||||||||||
| MAP recommended dose | 36.12 kg urea ha−1 (0.813 g urea pot−1) | 125 kg MAP ha−1 (2.8 g MAP pot−1) | 250 kg KCl ha−1 (5.635 g KCl pot−1) | |||||||||||||
| 2/3 of the MAP recommended dose | 46.3 kg urea ha−1 (1.04 g urea pot−1) | 83.33 kg MAP ha−1 (1.878 g MAP pot−1) | 250 kg KCl ha−1 (5.635 g KCl pot−1) | |||||||||||||
| 1/3 of the MAP recommended dose | 56.47 kg urea ha−1 (1.272 g urea pot−1) | 41.7 kg MAP ha−1 (0.939 g MAP pot−1) | 250 kg KCl ha−1 (5.635 g KCl pot−1) | |||||||||||||
| Without MAP | 66.6 kg urea ha−1 (1.5 g urea pot−1) | − | 250 kg KCl ha−1 (5.635 g KCl pot−1) | |||||||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, H.L.; Silva, G.F.d.; Carnietto, M.R.A.; Oliveira, L.C.; Nogueira, C.H.d.C.; Silva, M.d.A. Bacillus velezensis Associated with Organomineral Fertilizer and Reduced Phosphate Doses Improves Soil Microbial—Chemical Properties and Biomass of Sugarcane. Agronomy 2022, 12, 2701. https://doi.org/10.3390/agronomy12112701
Santos HL, Silva GFd, Carnietto MRA, Oliveira LC, Nogueira CHdC, Silva MdA. Bacillus velezensis Associated with Organomineral Fertilizer and Reduced Phosphate Doses Improves Soil Microbial—Chemical Properties and Biomass of Sugarcane. Agronomy. 2022; 12(11):2701. https://doi.org/10.3390/agronomy12112701
Chicago/Turabian StyleSantos, Hariane Luiz, Gustavo Ferreira da Silva, Melina Rodrigues Alves Carnietto, Laura Costa Oliveira, Carlos Henrique de Castro Nogueira, and Marcelo de Almeida Silva. 2022. "Bacillus velezensis Associated with Organomineral Fertilizer and Reduced Phosphate Doses Improves Soil Microbial—Chemical Properties and Biomass of Sugarcane" Agronomy 12, no. 11: 2701. https://doi.org/10.3390/agronomy12112701
APA StyleSantos, H. L., Silva, G. F. d., Carnietto, M. R. A., Oliveira, L. C., Nogueira, C. H. d. C., & Silva, M. d. A. (2022). Bacillus velezensis Associated with Organomineral Fertilizer and Reduced Phosphate Doses Improves Soil Microbial—Chemical Properties and Biomass of Sugarcane. Agronomy, 12(11), 2701. https://doi.org/10.3390/agronomy12112701






