Ant Diversity Is Enhanced by Ecological Infrastructures in Agroecosystems: A Case Study in Irrigated Mediterranean Farmland
Abstract
1. Introduction
2. Materials and Methods
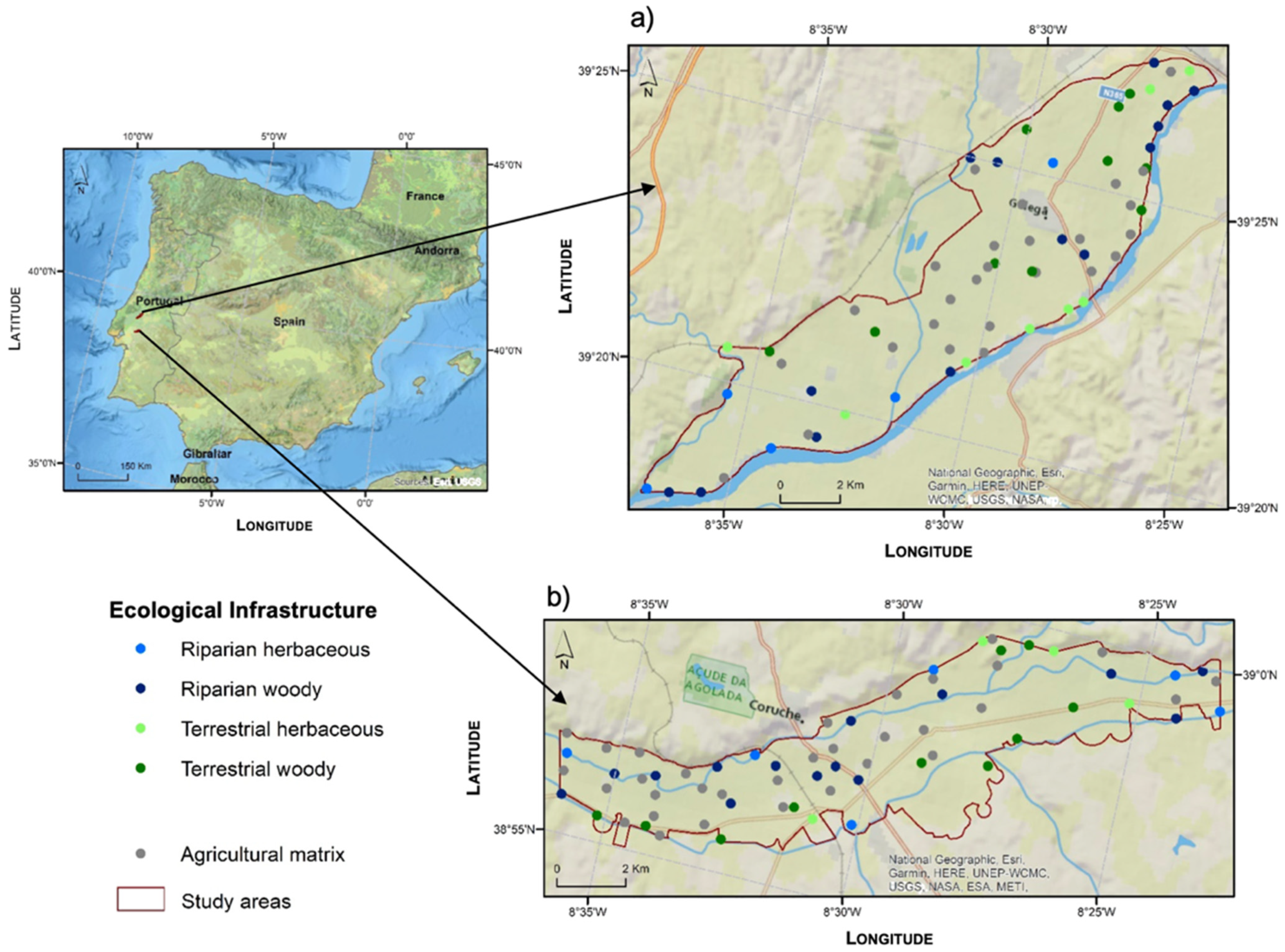
2.1. Study Area
2.2. Sampling Design
2.3. Ant Sampling and Identification
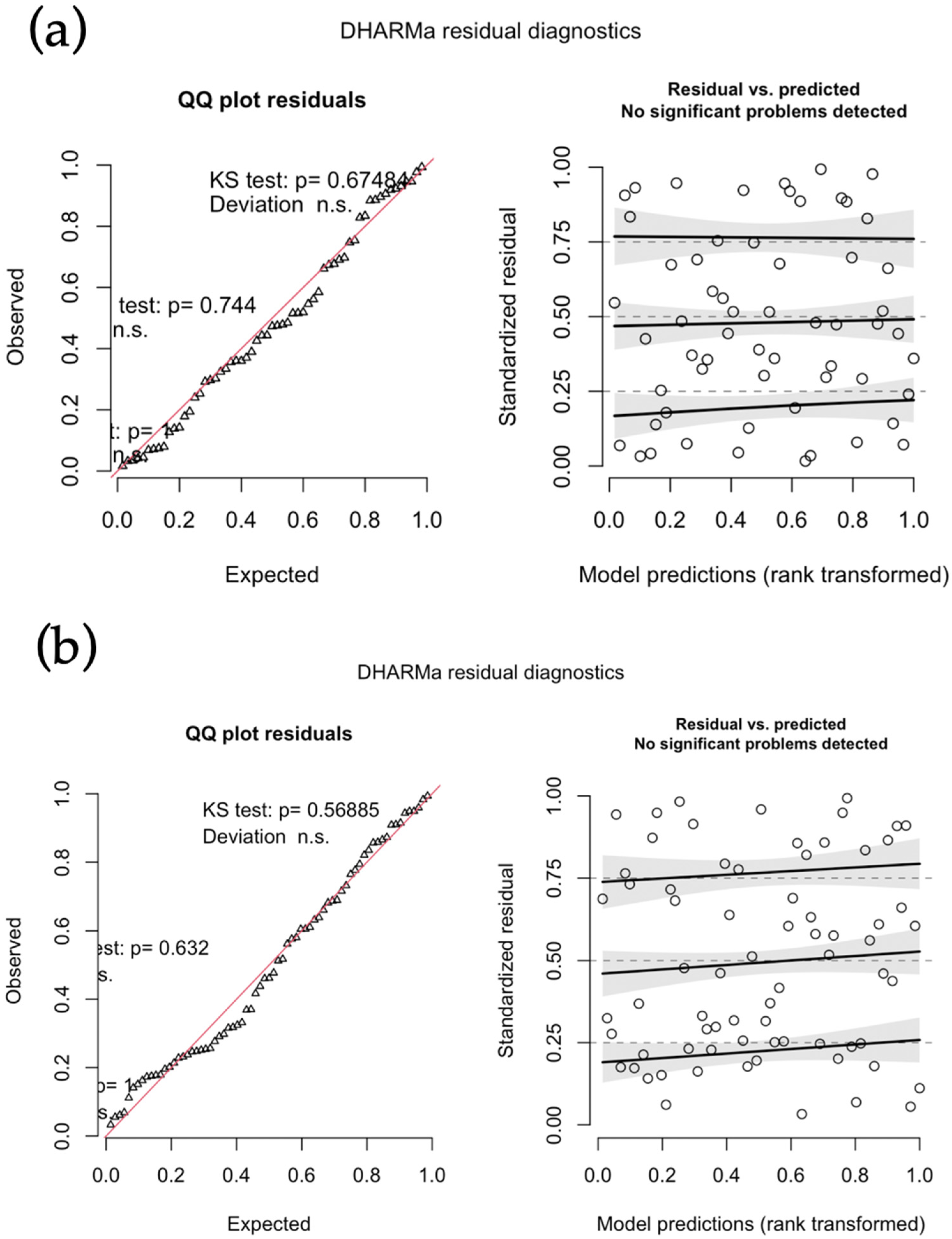
2.4. Statistical Analysis
3. Results
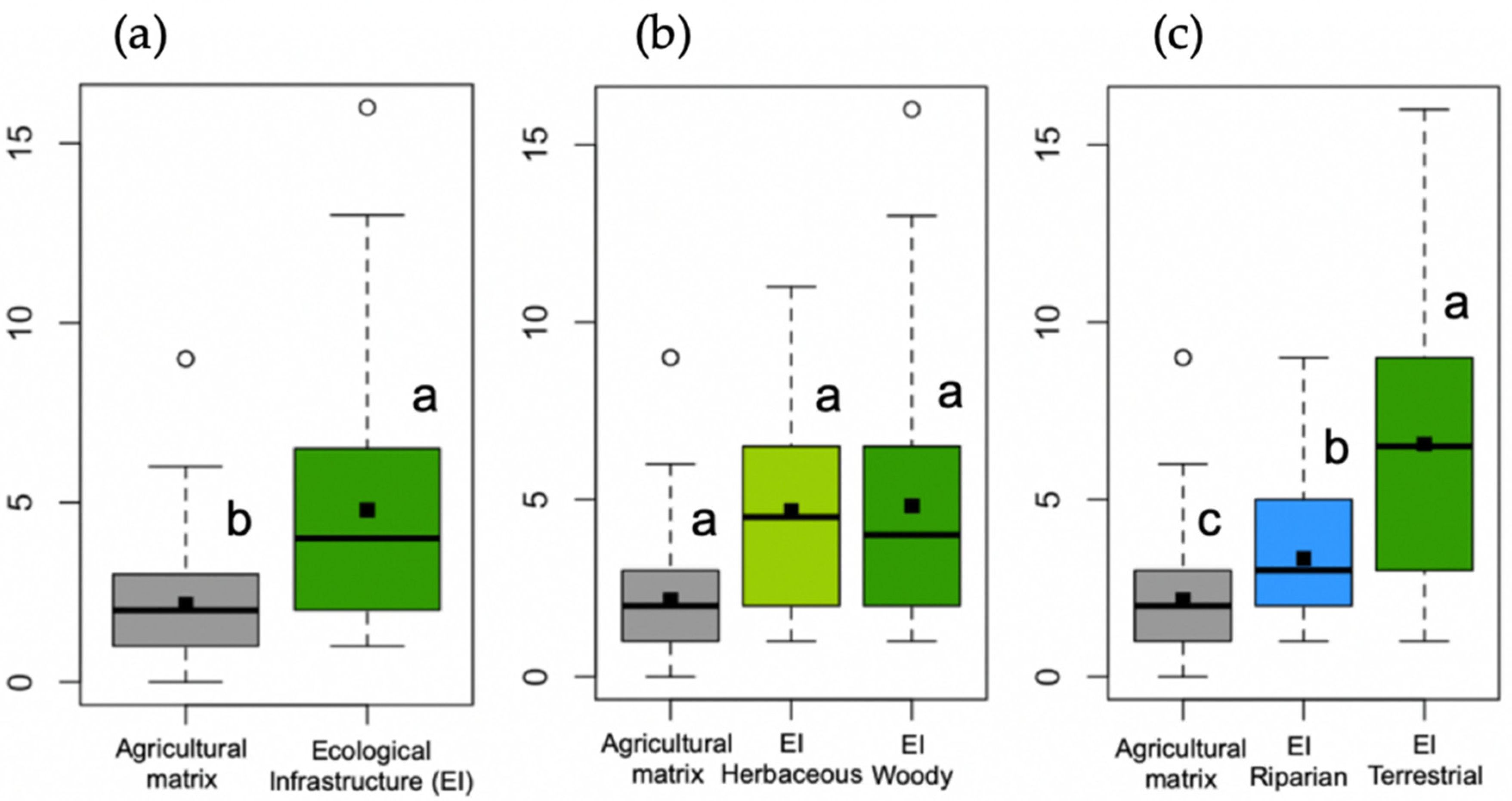
3.1. Agricultural Matrix Versus Ecological Infrastructures (Hypothesis 1)
3.1.1. Species Richness
3.1.2. Community Composition
3.2. Effect of the Ecological Infrastructure Typology (Hypothesis 2)
3.2.1. Species Richness
3.2.2. Community Composition
3.3. Drivers of Ant Richness in the Agricultural Matrix (Hypothesis 3)
3.4. Effect of Ecological Infrastructure Habitat Quality and Characteristics of the Surrounding Landscape (Hypothesis 4)
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Models | snd(Int) | dsp(Int) | Crop | Dist_EI | Dist_river | Dist_urban | K | logLik | AICc | ∆AICc | ωi |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Richness~Crop + scale(Dist_EI) | 1.17 | + | + | −0.39 | 5 | −105 | 222 | 0.0 | 0.518 | ||
| Richness~Crop + scale(Dist_EI) + scale(Dist_river) | 1.15 | + | + | −0.37 | −0.041 | 6 | −105 | 224 | 2.4 | 0.157 | |
| Richness~Crop + scale(Dist_EI) + scale(Dist_urban) | 1.17 | + | + | −0.39 | −0.035 | 6 | −105 | 224 | 2.4 | 0.156 | |
| Richness~Crop | 1.18 | + | + | 4 | −109 | 227 | 4.7 | 0.049 | |||
| Richness~Crop + scale(Dist_EI) + scale(Dist_river) + scale(Dist_urban) | 1.15 | + | + | −0.38 | −0.032 | −0.025 | 7 | −105 | 227 | 4.9 | 0.044 |
| Richness~Crop + scale(Dist_river) | 1.11 | + | + | −0.124 | 5 | −109 | 228 | 6.4 | 0.021 | ||
| Richness~Crop + scale(Dist_urban) | 1.18 | + | + | 0.036 | 5 | −109 | 229 | 7.0 | 0.015 | ||
| Richness~scale(Dist_EI) + scale(Dist_river) | 0.72 | + | −0.28 | −0.205 | 4 | −110 | 230 | 7.9 | 0.010 | ||
| Richness~scale(Dist_EI) | 0.74 | + | −0.31 | 3 | −112 | 230 | 8.2 | 0.008 | |||
| Richness~Crop + scale(Dist_river) + scale(Dist_urban) | 1.12 | + | + | −0.138 | 0.063 | 6 | −108 | 230 | 8.7 | 0.007 | |
| Richness~scale(Dist_EI) + scale(Dist_urban) | 0.73 | + | −0.33 | −0.116 | 4 | −111 | 231 | 9.6 | 0.004 | ||
| Richness~scale(Dist_river) | 0.75 | + | −0.257 | 3 | −113 | 232 | 9.7 | 0.004 | |||
| Richness~scale(Dist_EI) + scale(Dist_river) + scale(Dist_urban) | 0.71 | + | −0.29 | −0.186 | −0.048 | 5 | −110 | 232 | 10.2 | 0.003 | |
| Richness~1 (Null model) | 0.77 | + | 2 | −114 | 233 | 11.1 | 0.002 | ||||
| Richness~scale(Dist_river) + scale(Dist_urban) | 0.75 | + | −0.255 | −0.0036 | 4 | −114 | 234 | 12.0 | 0.001 | ||
| Richness~scale(Dist_urban) | 0.77 | + | −0.0814 | 3 | −114 | 235 | 12.9 | 0.001 |
| Models | snd(Int) | dsp(Int) | Arg_ant | Matrix_area | Riparian_area | Shrub_ richness | Terrestrial_area | HEIDI_index | K | logLik | AICc | ∆AICc | ωi |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Richness~Arg_ant + scale(Riparian_area) + scale(Terrestrial_area) | 1.3 | + | + | −0.14 | 0.16 | 5 | −165 | 340 | 0.00 | 0.192 | |||
| Richness~Arg_ant + scale(Terrestrial_area) | 1.3 | + | + | 0.20 | 4 | −166 | 341 | 0.11 | 0.182 | ||||
| Richness~Arg_ant + scale(Matrix_area) + scale(Terrestrial_area) | 1.3 | + | + | 0.06 | 0.21 | 5 | −166 | 342 | 1.79 | 0.079 | |||
| Richness~Arg_ant + scale(Terrestrial_area) + scale(HEIDI_index) | 1.3 | + | + | 0.21 | −0.038 | 5 | −166 | 343 | 2.13 | 0.066 | |||
| Richness~Arg_ant + scale(Matrix_area) + scale(Riparian_area) + scale(Terrestrial_area) | 1.4 | + | + | 0.03 | −0.13 | 0.17 | 6 | −165 | 343 | 2.19 | 0.064 | ||
| Richness~Arg_ant + scale(Shrub_richness) + scale(Terrestrial_area) | 1.3 | + | + | −0.03 | 0.21 | 5 | −166 | 343 | 2.21 | 0.064 | |||
| Richness~Arg_ant + scale(Riparian_area) + scale(Shrub_richness) + scale(Terrestrial_area) | 1.4 | + | + | −0.14 | −0.025 | 0.17 | 6 | −165 | 343 | 2.26 | 0.062 | ||
| Richness~Arg_ant + scale(Riparian_area) + scale(Terrestrial_area) + scale(HEIDI_index) | 1.3 | + | + | −0.14 | 0.16 | −0.23 | 6 | −165 | 343 | 2.27 | 0.062 | ||
| Richness~scale(Riparian_area) + scale(Terrestrial_area) | 1.5 | + | −0.20 | 0.16 | 4 | −168 | 344 | 3.21 | 0.039 | ||||
| Richness~Arg_ant + scale(Riparian_area) | 1.4 | + | + | −0.23 | 4 | −168 | 344 | 3.33 | 0.036 | ||||
| Richness~Arg_ant + scale(Matrix_area) + scale(Terrestrial_area) + scale(HEIDI_index) | 1.3 | + | + | 0.06 | 0.22 | −0.04 | 6 | −165 | 344 | 3.80 | 0.029 | ||
| Richness~Arg_ant + scale(Matrix_area) + scale(Shrub_richness)) + scale(Terrestrial_area) | 1.3 | + | + | 0.06 | −0.04 | 0.23 | 6 | −166 | 344 | 3.90 | 0.027 | ||
| Richness~Arg_ant + scale(Shrub_richness)) + scale(Terrestrial_area) + scale(HEIDI_index) | 1.3 | + | + | −0.02 | 0.21 | −0.03 | 6 | −166 | 345 | 4.43 | 0.021 | ||
| Richness~Arg_ant + scale(Matrix_area) + scale(Riparian_area) + scale(Shrub_richness) + scale(Terrestrial_area) | 1.4 | + | + | 0.04 | −0.13 | −0.03 | 0.18 | 7 | −165 | 345 | 4.49 | 0.020 | |
| Richness~Arg_ant + scale(Matrix_area) + scale(Riparian_area)) + scale(Terrestrial_area) + scale(HEIDI_index) | 1.4 | + | + | 0.04 | −0.12 | 0.17 | −0.03 | 7 | −165 | 345 | 4.50 | 0.020 | |
| Richness~Arg_ant + scale(Riparian_area) + scale(Shrub_richness)) + scale(Terrestrial_area) + scale(HEIDI_index) | 1.4 | + | + | −0.14 | −0.02 | 0.17 | −0.02 | 7 | −165 | 345 | 4.67 | 0.019 | |
| Richness~Arg_ant + scale(Matrix_area) + scale(Riparian_area)) + scale(Terrestrial_area) | 1.5 | + | + | 0.07 | −0.17 | 0.17 | 5 | −167 | 345 | 4.79 | 0.018 |
References
- Foley, J.A.; DeFries, R.; Asner, G.P.; Barford, C.; Bonan, G.; Carpenter, S.R.; Chapin, F.S.; Coe, M.T.; Daily, G.C.; Gibbs, H.K.; et al. Global Consequences of Land Use. Science 2005, 309, 570. [Google Scholar] [CrossRef] [PubMed]
- Landis, D.A. Designing agricultural landscapes for biodiversity-based ecosystem services. Basic Appl. Ecol. 2016, 18, 1–2. [Google Scholar] [CrossRef]
- Rusch, A.; Chaplin-Kramerc, R.; Gardinere, M.M.; Hawrof, V.; Holland, J.; Landis, D.; Thiesi, C.; Tscharntke, T.; Weisserj, W.W.; Winqvistk, C.; et al. Agricultural landscape simplification reduces natural pest control: A quantitative synthesis. Agric. Ecosyst. Environ. 2016, 221, 198–204. [Google Scholar] [CrossRef]
- Holland, J.M.; Bianchi, F.J.; Entling, M.H.; Moonen, A.-C.; Smith, B.M.; Jeanneret, P. Structure, function and management of semi-natural habitats for conservation biological control: A review of European studies. Pest Manag. Sci. 2017, 72, 1638–1651. [Google Scholar] [CrossRef]
- Boller, E.F.; Hani, F.; Poehling, H.M. Ecological infrastructures. In Ideabook on Functional Biodiversity at the Farm Level; Boller, E.F., Hani, F., Poehling, H.M., Eds.; Swiss Centre for Agricultural Extension and Rural Development (LBL): Lindau, Switzerland, 2004; Volume XIV, p. 212. [Google Scholar]
- Silva, J.M.C.; Wheeler, E. Ecosystems as infrastructure. Perspect. Ecol. Conserv. 2017, 15, 32–35. [Google Scholar] [CrossRef]
- European Commission. The Post-2020 Common Agricultural Policy: Environmental Benefits and Simplification. Agriculture and Rural Developmen; European Commission: Brussels, Belgium, 2019. [Google Scholar]
- European Commission. List of Potential Practices That Eco-Schemes Could Support; European Commission: Brussels, Belgium, 2021. [Google Scholar]
- Rotcheés-Ribalta, R.; Ruas, S.; Ahmed, K.D.; Gormally, M.; Moran, J.; Stout, J.; White, B.; ÓhUallacháin, D. Assessment of semi-natural habitats and landscape features on Irish farmland: New insights to inform EU Common Agricultural Policy implementation. Ambio 2021, 50, 346–359. [Google Scholar] [CrossRef]
- Brosi, B.J.; Armsworth, P.R.; Daily, G.C. Optimal design of agricultural landscapes for pollination services. Conserv. Lett. 2008, 1, 27–36. [Google Scholar] [CrossRef]
- Fahrig, L.; Baudry, J.; Brotons, L.; Burel, F.G.; Crist, T.O.; Fuller, R.J.; Sirami, C.; Siriwardena, G.M.; Martin, J.L. Functional landscape heterogeneity and animal biodiversity in agricultural landscapes. Ecol. Lett. 2011, 14, 101–112. [Google Scholar] [CrossRef]
- Duflot, R.; Ernoult, A.; Aviron, S.; Fahrig, L.; Burel, F. Relative effects of landscape composition and configuration on multi-habitat gamma diversity in agricultural landscapes. Agric. Ecosyst. Environ. 2017, 241, 62–69. [Google Scholar] [CrossRef]
- Larrieu, L.; Gonin, P. Ĺlndice de biodiversité potentielle (IBP): Une méthode simple et rapide pour évaluer la biodiversité 798 potentielle des peuplements forestiers. Rev. For. Fr. 2008, 60, 727–748. [Google Scholar] [CrossRef]
- Fonseca, A.; Zina, V.; Duarte, G.; Aguiar, F.C.; Rodríguez-González, P.M.; Ferreira, M.T.; Fernandes, M.R. Riparian Ecological Infrastructures: Potential for biodiversity-related ecosystem services in Mediterranean human-dominated landscapes. Sustainability 2021, 13, 10508. [Google Scholar] [CrossRef]
- Corbacho, C.; Sánchez, J.M.; Costillo, E. Patterns of structural complexity and human disturbance of riparian vegetation in agricultural landscapes of a Mediterranean area. Agric. Ecosyst. Environ. 2003, 95, 495–507. [Google Scholar] [CrossRef]
- Fernandes, M.R.; Aguiar, F.C.; Ferreira, M.T. Assessing riparian vegetation structure and the influence of land use using landscape metrics and geostatistical tools. Landsc. Urban. Plan. 2011, 99, 166–177. [Google Scholar] [CrossRef]
- Naiman, R.J.; Decamps, H.; Pollock, M. The role of riparian corridors in maintaining regional biodiversity. Ecol. Appl. 1993, 3, 209–212. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.; Fernandes, M.R.; Aguiar, F.C.; Branco, M.; Ferreira, M.T. Effects of riverine landscape changes on pollination services: A case study on the River Minho, Portugal. Ecol. Ind. 2018, 89, 656–666. [Google Scholar] [CrossRef]
- Riis, T.; Kelly-Quinn, M.; Aguiar, F.C.; Manolaki, P.; Bruno, D.; Bejarano, M.D.; Clerici, N.; Fernandes, M.R.; Franco, J.C.; Pettit, N.; et al. Global Overview of Ecosystem Services Provided by Riparian Vegetation. BioScience 2020, 70, 501–514. [Google Scholar] [CrossRef]
- Froidevaux, J.S.P.; Duarte, G.; Fonseca, A.; Zina, V.; Conde, S.; Ferreira, M.T.; Fernandes, M.R. The location and vegetation physiognomy of ecological infrastructures determine bat activity in Mediterranean floodplain landscapes. Agric. Ecosyst. Environ. 2022, 332, 107929. [Google Scholar] [CrossRef]
- Perfecto, I.; Armbrecht, I.; Philpott, S.M.; Soto-Pinto, L.; Dietsch, T.V. Shaded coffee and the stability of rainforest margins in northern Latin America. In Stability of Tropical Rainforest Margins. Environmental Science and Engineering; Tscharntke, T., Leuschner, C., Zeller, M., Guhardja, E., Bidin, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 227–263. [Google Scholar] [CrossRef]
- Wendt, C.F.; Nunes, A.; Verble, R.; Santini, G.; Boeiro, M.; Branquinho, C. Using a space-for-time approach to select the best biodiversity-based indicators to assess the effects of aridity on Mediterranean drylands. Ecol. Indic. 2020, 113, 106250. [Google Scholar] [CrossRef]
- Wendt, C.F.; Ceia-Hasse, A.; Nunes, A.; Verble, R.; Santini, G.; Boeiro, M.; Branquinho, C. Local environmental variables are key drivers of ant taxonomic and functional beta-diversity in a Mediterranean dryland. Sci. Rep. 2021, 11, 2292. [Google Scholar] [CrossRef] [PubMed]
- AntWeb. Version 8.78. California Academy of Science. Available online: https://www.antweb.org. (accessed on 11 August 2022).
- Hölldobler, B.; Wilson, E.O. The Ants; Harvard University Press: Cambridge, MA, USA, 1990; p. 746. [Google Scholar]
- Wilson, E.O.; Hölldobler, B. Eusociality: Origin and consequences. Proc. Natl. Acad. Sci. USA 2005, 102, 13367–13371. [Google Scholar] [CrossRef]
- Parr, C.L.; Bishop, T.R. The response of ants to climate change. Glob. Change Biol. 2022, 28, 3188–3205. [Google Scholar] [CrossRef] [PubMed]
- Sanders, D.; Van Veen, F.J.F. Ecosystem engineering and predation: The multi-trophic impact of two ant species. J. Anim. Ecol. 2011, 80, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Wills, B.D.; Landis, D.A. The role of ants in north temperate grasslands: A review. Oecologia 2018, 186, 323–338. [Google Scholar] [CrossRef]
- Parker, J.; Kronauer, D.J.C. How ants shape biodiversity. Curr. Biol. 2021, 31, R1141–R1224. [Google Scholar] [CrossRef]
- Folgarait, P.J. Ant biodiversity and its relationship to ecosystem functioning: A review. Biodivers. Conserv. 1998, 7, 1221–1244. [Google Scholar] [CrossRef]
- Del Toro, I.; Ribbons, R.R.; Pelini, S.L. The little things than run the world revisited: A review of the ant-mediated ecosystem services and disservices (Hymenoptera: Formicidae). Myrmecol. News 2012, 17, 133–146. [Google Scholar]
- Elizalde, L.; Arbetman, M.; Arnan, X.; Eggleton, P.; Leal, I.R.; Lescano, M.N.; Saez, A.; Werenkraut, V.; Pirk, G.I. The ecosystem services provided by social insects: Traits, management tools and knowledge gaps. Biol. Rev. 2020, 95, 1418–1441. [Google Scholar] [CrossRef]
- Peck, S.L.; Mcquaid, B.; Campbell, C.L. Using ant species (Hymenoptera: Formicidae) as a biological indicator of agroecosystem condition. Environ. Entomol. 1998, 27, 1102–1110. [Google Scholar] [CrossRef]
- Dauber, J.; Wolters, V. Edge effects on ant community structure and species richness in an agricultural landscape. Biodiv. Conserv. 2004, 13, 901–915. [Google Scholar] [CrossRef]
- Ng, K.; Nowrouzi, S.; Staunton, K.M.; Barton, P.; Driscoll, D.A. Ant community responses to farmland use and revegetation in a fragmented agricultural landscape. Agric. Ecosyst. Environ. 2021, 311, 107316. [Google Scholar] [CrossRef]
- Dauber, J.; Hirsch, M.; Simmering, D.; Waldhardt, R.; Otte, A.; Wolters, V. Landscape structure as an indicator of biodiversity: Matrix effects on species richness. Agric. Ecosyst. Environ. 2003, 98, 321–329. [Google Scholar] [CrossRef]
- García-Martínez, M.A.; Valenzuela-González, J.E.; Escobar-Sarria, F.; Castaño-Meneses, G. The surrounding landscape influences the diversity of leaf-litter ants in riparian cloud forest remnants. PLoS ONE 2017, 12, e0172464. [Google Scholar] [CrossRef] [PubMed]
- Frizzo, T.L.M.; Souza, L.M.; Sujii, E.R.; Togni, P.H.B. Ants provide biological control on tropical organic farms influenced by local and landscape factors. Biol. Control 2020, 151, 104378. [Google Scholar] [CrossRef]
- Kass, J.M.; Guénard, B.; Dudley, K.L.; Jenkins, C.N.; Azuma, F.; Fisher, B.L.; Parr, C.L.; Gibb, H.; Longino, J.T.; Ward, P.S.; et al. The global distribution of known and undiscovered ant biodiversity. Sci. Adv. 2022, 8, eabp9908. [Google Scholar] [CrossRef]
- Philpott, S.M.; Perfecto, I.; Armbrecht, I.; Parr, C.L. Ant diversity and function in disturbed and changing habitats. In Ant Ecology; Lach, L., Parr, C.L., Abbott, K., Eds.; Oxford University Press: Oxford, UK, 2010; pp. 137–157. [Google Scholar]
- European Commission; European Environmental Agency. The European Climate Adaptation Platform Climate—ADAPT. Available online: https://climate-adapt.eea.europa.eu/countries-regions/countries/portugal (accessed on 2 December 2021).
- Aguiar, F.C.; Ferreira, M.T.; Albuquerque, A.; Moreira, I. Alien and endemic flora at reference and non-reference sites in Mediterranean-type streams in Portugal. Aquat. Conserv. Mar. Freshw. Ecosyst. 2007, 17, 335–347. [Google Scholar] [CrossRef]
- Fonseca, A.; Duarte, G.; Zina, V.; Ferreira, M.T.; Fernandes, M.R. Ecological infrastructure Layers—Optimus Prime Project; Open Science Framework: Charlottesville, VA, USA, 2020. [Google Scholar] [CrossRef]
- Ferreira, M.T.; Aguiar, F.C.; Nogueira, C. Changes in riparian woods over space and time: Influence of environment and land 816 use. For. Ecol. Manag. 2005, 212, 145–159. [Google Scholar] [CrossRef]
- Wasser, L.; Chasmer, L.; Day, R.; Taylor, A. Quantifying land use effects on forested riparian buffer vegetation structure using 818 LiDAR data. Ecosphere 2015, 6, 1–17. [Google Scholar] [CrossRef]
- Gómez, C.; Espadaler, X. Seed dispersal curve of a Mediterranean myrmecochore: Influence of ant size and the distance to nests. Ecol. Res. 1998, 13, 347–354. [Google Scholar] [CrossRef]
- Gómez, C.; Espadaler, X. An update of the world survey of myrmecochorous dispersal distances. Ecography 1998, 36, 1193–1201. [Google Scholar] [CrossRef]
- Fonseca, A.; Duarte, G.; Zina, V.; Ferreira, M.T.; Fernandes, M.R. Explanatory Variables Database—Optimus Prime Project. Open Science Framework: Charlottesville, VA, USA, 2021. [Google Scholar] [CrossRef]
- Cros, S.; Cerdá, X.; Retana, J. Spatial and temporal variations in the activity patterns of Mediterranean ant communities. Ecoscience 1997, 4, 269–278. [Google Scholar] [CrossRef]
- Retana, J.; Cerdá, X. Patterns of diversity and composition of Mediterranean ground ant communities tracking spatial and temporal variability in the thermal environment. Oecologia 2000, 123, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Gómez, C.; Casellas, D.; Oliveras, J.; Bas, J.M. Structure of ground-foraging ant assemblages in relation to land-use change in the northwestern Mediterranean region. Biodivers. Conserv. 2003, 12, 2135–2146. [Google Scholar] [CrossRef]
- Angulo, E.; Boulay, R.; Ruano, F.; Tinaut, A.; Cerda, X. Anthropogenic impacts in protected areas: Assessing the efficiency of conservation efforts using Mediterranean ant communities. PeerJ 2016, 4, e2773. [Google Scholar] [CrossRef] [PubMed]
- Majer, J.D. The use of pitfall traps for sampling ants—A critique. Mem. Mus. Vic. 1997, 56, 323–329. [Google Scholar] [CrossRef]
- Parr, C.L.; Chown, S.L. Inventory and bioindicator sampling: Testing pitfall and Winkler methods with ants in a South African savanna. J. Insect Conserv. 2001, 5, 27–36. [Google Scholar] [CrossRef]
- Underwood, E.C.; Fisher, B.L. The role of ants in conservation monitoring: If, when, and how. Biol. Conserv. 2006, 132, 166–182. [Google Scholar] [CrossRef]
- Tista, M.; Fiedler, K. How to evaluate and reduce sampling effort for ants. J. Insect Conserv. 2011, 15, 547–559. [Google Scholar] [CrossRef]
- Samways, M.J. Community structure of ants (Hymenoptera: Formicidae) in a series of habitats associated with citrus. J. Appl. Ecol. 1983, 20, 833–847. [Google Scholar] [CrossRef]
- Vele, A.; Holusa, J.; Frouz, J. Sampling for ants in different-aged spruce forests: A comparison of methods. Eur. J. Soil Biol. 2009, 45, 301–305. [Google Scholar] [CrossRef]
- Schmidt, F.A.; Ribas, C.R.; Schoereder, J.H. How predictable is the response of ant assemblages to natural forest recovery? Implications for their use as bioindicators. Ecol. Indic. 2013, 24, 158–166. [Google Scholar] [CrossRef]
- Johnson, J.T.; Adkins, J.K. Canopy vegetation influences ant (Hymenoptera: Formicidae) communities in headwater stream riparian zones of central Appalachia. J. Insect Sci. 2014, 14, 237. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, A.H.; Ganaie, G.A.; Thomas, M.; Bhandari, R.; Rather, Y.A. Ant pitfall trap sampling: An overview. J. Soil Biol. 2018, 45, 301–305. [Google Scholar] [CrossRef]
- Collingwood, C.A.; Prince, A. A guide to ants of continental Portugal. Bol. Soc. Por. Entomol. 1998, 5, 1–49. [Google Scholar]
- Gómez, K.; Espadaler, X. Hormigas Ibéricas. Available online: http://www.hormigas.org (accessed on 3 April 2020).
- Lebas, C.; Galkowski, C.; Blatrix, R.; Wegnez, P. Guia de Campo de las Hormigas de Europa Ocidental; Omega: Barcelona, Spain, 2017; p. 416. [Google Scholar]
- R Core Team 2020. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.R-project.org/ (accessed on 1 February 2022).
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package. R Package Version 2.5–7. 2020. Available online: https://CRAN.R-project.org/package=vegan (accessed on 15 June 2022).
- Brooks, M.E.; Kristensen, K.; van Benthem, K.J.; Magnusson, A.; Berg, C.W.; Nielsen, A.; Skaug, H.J.; Maechler, M.; Bolker, B.M. glmmTMB Balances Speed and Flexibility Among Packages for Zero-inflated Generalized Linear Mixed Modeling. R J. 2017, 9, 378–400. [Google Scholar] [CrossRef]
- Burnham, K.P.; Anderson, D.R. Information and Likelihood Theory: A Basis for Model Selection and Inference. In Model Selection and Multimodel Inference, Burnham, K.P., Anderson, D.R., Eds.; Springer: New York, NY, USA, 2002; pp. 49–97. [Google Scholar]
- Hartig, F. DHARMa: Residual Diagnostics for Hierarchical (Multi-Level/Mixed) Regression Models. R Package Version 0.4.1. 2021. Available online: https://CRAN.R-project.org/package=DHARMa (accessed on 15 June 2022).
- Lüdecke, D.; Ben-Shachar, M.S.; Patil, I.; Waggoner, P.; Makowski, D. performance: An R Package for Assessment, Comparison and Testing of Statistical Models. J. Open Source Softw. 2021, 6, 3139. [Google Scholar] [CrossRef]
- Bartoń, K. MuMIn: Multi-Model Inference. R Package Version 1.43.17. 2020. Available online: https://CRAN.R-project.org/package=MuMIn (accessed on 15 June 2022).
- Burnham, K.P.; Anderson, D.R. Formal Inference from More Than One Model: Multimodel Inference (Mmi). In Model Selection and Multimodel Inference; Burnham, K.P., Anderson, D.R., Eds.; Springer: New York, NY, USA, 2002; pp. 149–205. [Google Scholar]
- Nooten, S.S.; Schultheiss, P.; Rowe, R.C.; Facey, S.L.; Cook, J.M. Habitat complexity affects functional traits and diversity of ant assemblages in urban green spaces (Hymenoptera: Formicidae). Myrmecol. News 2019, 29, 67–77. [Google Scholar]
- MacArthur, R.H.; MacArthur, J.W. On bird species diversity. Ecology 1961, 42, 594–598. [Google Scholar] [CrossRef]
- MacArthur, R.H.; Wilson, E.O. The Theory of Island Biogeography; Princeton University Press: Princeton, NJ, USA, 1967. [Google Scholar]
- Kreider, J.J.; Chen, T.-W.; Hartke, T.R.; Buchori, D.; Hidayat, P.; Nazarreta, R.; Scheu, S.; Drescher, J. Rainforest conversion to monocultures favors generalist ants with large colonies. Ecosphere 2012, 12, e03717. [Google Scholar] [CrossRef]
- Andersen, A.N. Responses of ant communities to disturbance: Five principles for understanding the disturbance dynamics of a globally dominant faunal group. J. Anim. Ecol. 2019, 88, 350–362. [Google Scholar] [CrossRef]
- Wetterer, J.K. Worldwide Spread of the Moorish sneaking Ant, Cardiocondyla mauritanica (Hymenoptera: Formicidae). Sociobiology 2012, 59, 3. [Google Scholar]
- Andersen, A.N. A Classification of Australian Ant Communities, Based on Functional Groups Which Parallel Plant Life-Forms in Relation to Stress and Disturbance. J. Biogeogr. 1995, 22, 15–29. [Google Scholar] [CrossRef]
- Hoffmann, B.D.; Andersen, A.N. Responses of ants to disturbance in Australia, with particular reference to functional groups. Austral. Ecol. 2003, 28, 444–464. [Google Scholar] [CrossRef]
- Morris, T.I.; Symondson, W.O.C.; Kidd, N.A.C.; Jervis, M.A.; Campos, M. Are ants significant predators of the olive moth Prays oleae? Crop Prot. 1998, 17, 365–366. [Google Scholar] [CrossRef]
- Campolo, O.; Palmeri, V.; Malacrinò, A.; Laudani, F.; Castracani, C.; Mori, A.; Grasso, D.A. Interaction between ants and the Mediterranean fruit fly: New insights for biological control. Biol. Control 2015, 90, 120–127. [Google Scholar] [CrossRef]
- Martínez-Núñez, C.; Rey, P.J.; Salido, T.; Manzaneda, A.J.; Camacho, F.M.; Isla, J. Ant community potential for pest control in olive groves: Management and landscape effects. Agric. Ecosyst. Environ. 2021, 305, 107185. [Google Scholar] [CrossRef]
- Cerdá, X.; Dejean, A. Predation by ants on arthropods and other Animals. In Predation in the Hymenoptera: An Evolutionary Perspective; Polidori, C., Ed.; Transworld Research Network: Kerala, India, 2011; pp. 39–78. [Google Scholar]
- Holway, D.A.; Cameron, E.K. The importance of scavenging in ant invasions. Curr. Opin. Insect Sci. 2021, 46, 39–42. [Google Scholar] [CrossRef]
- Zumeaga, H.; Azcárate, F.M.; Concepción, E.D.; Hevia, V.; Díaz, M. Landscape and agri-environmental scheme effects on ant communities in cereal croplands of central Spain. Agric. Ecosyst. Environ. 2021, 312, 107345. [Google Scholar] [CrossRef]
- Meissle, M.; Mouron, P.; Musa, T.; Bigler, F.; Pons, X.; Vasileiadis, V.P.; Otto, S.; Antichi, D.; Kiss, J.; Pálinkás, Z.; et al. Pests, pesticide use and alternative options in European maize production: Current status and future prospects. J. Appl. Entomol. 2010, 134, 357–375. [Google Scholar] [CrossRef]
- Wetterer, J.K.; Wild, A.L.; Suarez, A.V.; Roura-Pascual, N.; Espadaler, X. Worldwide spread of the Argentine ant, Linepithema humile (Hymenoptera: Formicidae). Myrmecol. News 2009, 12, 187–194. [Google Scholar]
- Lach, L. Argentine ants displace floral arthropods in a bio-diversity hotspot. Divers. Distrib. 2008, 14, 281–290. [Google Scholar] [CrossRef]
- Gómez, C.; Oliveras, J. Can the Argentine ant (Linepithema humile Mayr) replace native ants in myrmecochory? Acta Oecologica 2003, 24, 47–53. [Google Scholar] [CrossRef]
- Mgocheki, N.; Addison, P. Interference of ants (Hymenoptera: Formicidae) with biological control of the vine mealybug Planococcus ficus (Signoret) (Hemiptera: Pseudococcidae). Biol. Control 2009, 49, 180–185. [Google Scholar] [CrossRef]
- Oliveras, J.; Bas, J.M.; Casellas, D.; Gómez, C. Numerical dominance of the Argentine ant vs native ants and consequences on soil resource searching in Mediterranean cork-oak forests (Hymenoptera: Formicidae). Sociobiology 2005, 45, 1–16. [Google Scholar]
- Kolay, S.; Boulay, R.; D’Ettorre, P. Regulation of ants foraging: A review of the role of information use and personality. Front. Psychol. 2020, 11, 734. [Google Scholar] [CrossRef]
- Walters, A.C. Invasion of the Argentine ants (Hymenoptera, Formicidae) in South Australia: Impacts on community composition and abundance of invertebrates in urban parklands. Austral Ecol. 2006, 31, 367–376. [Google Scholar] [CrossRef]
- Roura-Pascual, N.; Bas, J.M.; Hui, C. The spread of the Argentine ant: Environmental determinants and impacts on native ant communities. Biol. Invasions 2010, 12, 2399–2412. [Google Scholar] [CrossRef]
- Rowles, A.D.; O’Dowd, D.J. Impacts of the invasive Argentine ant on native ants and other invertebrates in coastal scrub in south-eastern Australia. Austral Ecol. 2009, 34, 239–248. [Google Scholar] [CrossRef]
- Zina, V.; Branco, M.; Franco, J.C. Impact of the invasive Argentine ant in citrus agroecosystems: Effects on the diversity and frequency of native ant species foraging on tree canopy. Insects 2020, 11, 785. [Google Scholar] [CrossRef]
- Aguiar, F.C.; Ferreira, M.T. Plant invasions in the rivers of the Iberian Peninsula, south-western Europe: A review. Int. J. Plant Biol. 2013, 147, 4, 1107–1119. [Google Scholar] [CrossRef]
- Armbrecht, I.; Perfecto, I. Litter-twig dwelling ant species richness and predation potential within a forest fragment and neighboring coffee plantations of contrasting habitat quality in Mexico. Agric. Ecosyst. Environ. 2003, 97, 107–115. [Google Scholar] [CrossRef]
- Lee, J.C.; Menalled, F.D.; Landis, D.A. Refuge habitats modify impact of insecticide disturbance on carabid beetle communities. J. Appl. Ecol. 2001, 38, 472–483. [Google Scholar] [CrossRef]
- Crist, T.O. Biodiversity, species interactions, and functional roles of ants (Hymenoptera: Formicidae) in fragmented landscapes: A review. Myrmecol. News 2009, 12, 3–13. [Google Scholar]
- Santos, M.J.; Rosalino, L.M.; Matos, H.M.; Santos-Reis, M. Riparian ecosystem configuration influences mesocarnivores presence in Mediterranean landscapes. Eur. J. Wildl. Res. 2016, 62, 251–261. [Google Scholar] [CrossRef]
- Forio, M.A.E.; De Troyer, N.; Lock, K.; Witing, F.; Baert, L.; Saeyer, N.D.; Rîșnoveanu, G.; Popescu, C.; Burdon, F.J.; Kupilas, B.; et al. Small patches of riparian woody vegetation enhance biodiversity of invertebrates. Water 2020, 12, 3070. [Google Scholar] [CrossRef]
- Popescu, C.; Oprina-Pavelescu, M.; Dinu, V.; Cazacu, C.; Burdon, F.J.; Forio, M.A.E.; Kupilas, B.; Friberg, N.; Goethals, P.; McKie, B.G.; et al. Riparian Vegetation Structure Influences Terrestrial Invertebrate Communities in an Agricultural Landscape. Water 2021, 13, 188. [Google Scholar] [CrossRef]
- García-Martínez, M.A.; Escobar-Sarria, F.; López-Barrera, F.; Castaño-Meneses, G.; Valenzuela-González, J.E. Value of riparian vegetation remnants for leaf-litter ants (Hymenoptera: Formicidae) in a human-dominated landscape in Central Veracruz, Mexico. Environ. Entomol. 2015, 44, 1488–1497. [Google Scholar] [CrossRef]
- Buczkowski, G.; Richmond, D.S. The effect of urbanization on ant abundance and diversity: A temporal examination of factors affecting biodiversity. PLoS ONE 2012, 7, e41729. [Google Scholar] [CrossRef]
- Rocha, E.A.; Fellowes, M.D.E. Urbanization alters ecological interactions: Ant mutualists increase and specialist insect predators decrease on an urban gradient. Sci. Rep. 2020, 10, 6406. [Google Scholar] [CrossRef]
- Guénard, B.; Cardinal-De Casas, A.; Dunn, R.R. High diversity in an urban habitat: Are some animal assemblages resilient to long-term anthropogenic change? Urban Ecosyst. 2014, 18, 449–463. [Google Scholar] [CrossRef]
- Menke, S.B.; Guénard, B.; Sexton, J.O.; Weiser, M.D.; Dunn, R.R.; Silverman, J. Urban areas may serve as habitat and corridors for dry-adapted, heat tolerant species; an example from ants. Urban Ecosyst. 2011, 14, 135–163. [Google Scholar] [CrossRef]
- Crenna, E.; Sinkko, T.; Sala, S. Biodiversity impacts due to food consumption in Europe. J. Clean. Prod. 2019, 227, 378–391. [Google Scholar] [CrossRef] [PubMed]
- GPP—Gabinete de Planeamento, Políticas e Administração Geral. Análise Sectorial CEREAIS; GPP—Gabinete de Planeamento: Lisbon, Portugal, 2020. [Google Scholar]
- Bambaradeniya, C.N.B.; Amerasinghe, F.P. Biodiversity Associated with Rice Field Agroecosystem in Asian Countries: A Brief Review; Working Paper 63; International Water Management Institute: Colombo, Sri Lanka, 2003. [Google Scholar]
- Edirisinghe, J.P.; Bambaradeniya, C.N.B. Rice fields: An ecosystem rich in biodiversity. J. Natl. Sci. Found. Sri Lanka 2016, 34, 57–59. [Google Scholar] [CrossRef]
- De la Mora, A.; Murnen, C.J.; Philpott, S.M. Local and landscape drivers of biodiversity of four groups of ants in coffee landscapes. Biodivers Conserv. 2013, 22, 871–888. [Google Scholar] [CrossRef]
- Tscharntke, T.; Klein, A.M.; Kruess, A.; Steffan-Dewenter, I.; Thies, C. Landscape perspectives on agricultural intensification and biodiversity—Ecosystem service management. Ecol. Lett. 2005, 8, 857–874. [Google Scholar] [CrossRef]
- Ward, P.S. Distribution of the introduced Argentine ant (Iridomyrmex humilis) in natural habitats of the lower Sacramento Valley and its effects on the indigenous ant fauna. Hilgardia 1987, 55, 1–16. [Google Scholar] [CrossRef]
- Roura-Pascual, N.; Brotons, L.; Peterson, A.T.; Thuiller, W. Consensual predictions of potential distributional areas for invasive species: A case study of Argentine ants in the Iberian Peninsula. Biol. Invas. 2009, 11, 1017–1031. [Google Scholar] [CrossRef]
- Franco, J.C.; Branco, M.; Conde, S.; Garcia, A.; Fernandes, M.R.; Lima Santos, J.; Messina, T.; Duarte, G.; Fonseca, A.; Zina, V.; et al. Ecological Infrastructures May Enhance Lepidopterans Predation in Irrigated Mediterranean Farmland, Depending on Their Typology and the Predator Guild. Sustainability 2022, 14, 3874. [Google Scholar] [CrossRef]




| Applied To: | Variable Name | Abbreviation | Category | Type, Units, and Range | Description |
|---|---|---|---|---|---|
| Agricultural matrix data | Distance to the closest EI patch | Dist_EI | Proximity | Continuous: meters; [0, ∞] | Distance to the closest remnant habitat patch–EI edge |
| Distance to urban area | Dist_urban | Proximity | Continuous: meters; [0, ∞] | Distance to the closest urban area using Level 4 of COS 2018 layer (i.e., the Portuguese Land use and Occupancy Charter of 2018, www.dgterritorio.pt (accessed on 26 March 2021)) | |
| Distance to river | Dist_river | Proximity | Continuous: meters; [0, ∞] | Distance to the closest watercourse defined by the adapted HIDCOD layer (i.e., the Portuguese waterline layer based on the Digital Elevation Model of 25 m) | |
| Crop type | Crop_type | Habitat quality | Nominal: maize field, rice paddy, others (mixed types) | Crop type characterization within the agricultural matrix | |
| Ecological infrastructure (EI) data | Agricultural land | Agricultural_matrix | Area/density | Continuous: ha; [0, ∞] | Area of the agricultural matrix in a 200 m buffer contained within the study area |
| Area of riparian EI | Riparian_EI_area | Area/density | Continuous: ha; [0, ∞] | Sum of the areas of riparian EI; basic statistics of the spatial configuration | |
| Area of terrestrial EI | Terrestrial_EI_area | Area/density | Continuous: ha; [0, ∞] | Sum of the areas of terrestrial EI; basic statistics of the spatial configuration. | |
| Shrub richness | Shrub_richness | Habitat quality | Continuous: none; [0, ∞] | Number of shrub plant species in the EI patches | |
| HEIDI quality index | HEIDI_index | Habitat quality | Continuous: none; [0, ∞] | HEIDI value for short distance dispersers in the EI patches sensu Fonseca et al. [14] | |
| Argentine ant occurrence | Argentine_ant | Habitat quality | Nominal Bolean: presence, absence | Argentine ant occurrence in the EI patches |
| Null Model | Best-Fitted Model | Full Model | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Predictors | Incidence Rate Ratio (95% Confidence Interval) | Statistic | p-Value | Incidence Rate Ratio (95% Confidence Interval) | Statistic | p-Value | Incidence Rate Ratio (95% Confidence Interval) | Statistic | p-Value |
| (Intercept) | 2.17 (1.70–2.78) | 6.16 | <0.001 | 3.23 (2.35–4.44) | 7.23 | <0.001 | 3.16 (2.24–4.47) | 6.52 | <0.001 |
| Crop [Maize_field] | 0.39 (0.24–0.65) | –3.68 | <0.001 | 0.41 (0.23–0.71) | –3.17 | 0.002 | |||
| Crop [Other_mixed_types] | 0.69 (0.40–1.18) | –1.37 | 0.172 | 0.69 (0.40–1.20) | –1.31 | 0.191 | |||
| Dist_EI | 0.68 (0.50–0.92) | –2.47 | 0.013 | 0.68 (0.49–0.94) | –2.33 | 0.020 | |||
| Dist_urban | 0.98 (0.76–1.26) | –0.19 | 0.847 | ||||||
| Dist_river | 0.97 (0.73–1.29) | –0.22 | 0.828 | ||||||
| Null model | Best-fitted model | Full model | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Predictors | Incidence Rate Ratio (95% Confidence Interval) | Statistic | p-Value | Incidence Rate Ratio (95% Confidence Interval) | Statistic | p-Value | Incidence Rate Ratio (95% Confidence Interval) | Statistic | p-Value |
| (Intercept) | 4.79 (4.09–5.61) | 19.41 | <0.001 | 3.85 (3.13–4.74) | 12.72 | <0.001 | 3.88 (3.15–4.78) | 12.71 | <0.001 |
| Argentine ant [absence] | 1.41 (1.06–1.87) | 2.37 | 0.018 | 1.38 (1.03–1.85) | 2.18 | 0.030 | |||
| Riparian area | 0.87 (0.73–1.04) | –1.53 | 0.127 | 0.88 (0.73–1.07) | –1.29 | 0.196 | |||
| Terrestrial area | 1.17 (1.04–1.33) | 2.54 | 0.011 | 1.20 (1.04–1.38) | 2.52 | 0.012 | |||
| Matrix area | 1.04 (0.90–1.21) | 0.51 | 0.613 | ||||||
| Shrub richness | 0.98 (0.84–1.13) | –0.30 | 0.763 | ||||||
| HEIDI quality index | 0.98 (0.85–1.13) | –0.28 | 0.780 | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zina, V.; Fonseca, A.; Duarte, G.; Conde, S.; Fernandes, M.R.; Ferreira, M.T.; Franco, J.C. Ant Diversity Is Enhanced by Ecological Infrastructures in Agroecosystems: A Case Study in Irrigated Mediterranean Farmland. Agronomy 2022, 12, 2690. https://doi.org/10.3390/agronomy12112690
Zina V, Fonseca A, Duarte G, Conde S, Fernandes MR, Ferreira MT, Franco JC. Ant Diversity Is Enhanced by Ecological Infrastructures in Agroecosystems: A Case Study in Irrigated Mediterranean Farmland. Agronomy. 2022; 12(11):2690. https://doi.org/10.3390/agronomy12112690
Chicago/Turabian StyleZina, Vera, André Fonseca, Gonçalo Duarte, Sofia Conde, Maria Rosário Fernandes, Maria Teresa Ferreira, and José Carlos Franco. 2022. "Ant Diversity Is Enhanced by Ecological Infrastructures in Agroecosystems: A Case Study in Irrigated Mediterranean Farmland" Agronomy 12, no. 11: 2690. https://doi.org/10.3390/agronomy12112690
APA StyleZina, V., Fonseca, A., Duarte, G., Conde, S., Fernandes, M. R., Ferreira, M. T., & Franco, J. C. (2022). Ant Diversity Is Enhanced by Ecological Infrastructures in Agroecosystems: A Case Study in Irrigated Mediterranean Farmland. Agronomy, 12(11), 2690. https://doi.org/10.3390/agronomy12112690








