Using the Nematode, Steinernema carpocapsae, to Control Peachtree Borer (Synanthedon exitiosa): Optimization of Application Rates and Secondary Benefits in Control of Root-Feeding Weevils
Abstract
1. Introduction
2. Materials and Methods
2.1. Nematodes
2.2. Variations on the Rate of Nematodes Used
2.3. Variations in the Rate of Barricade® Gel Used
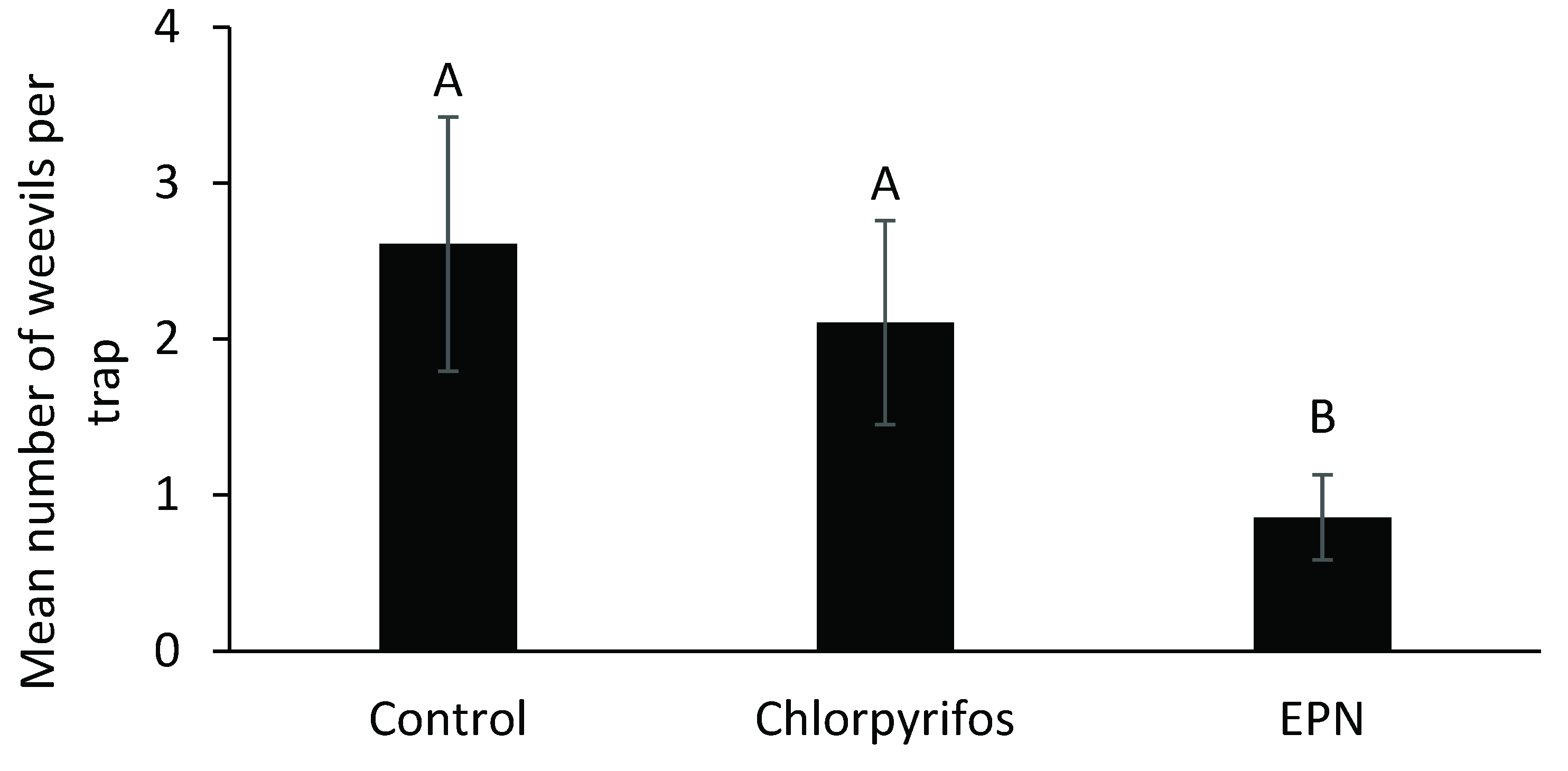
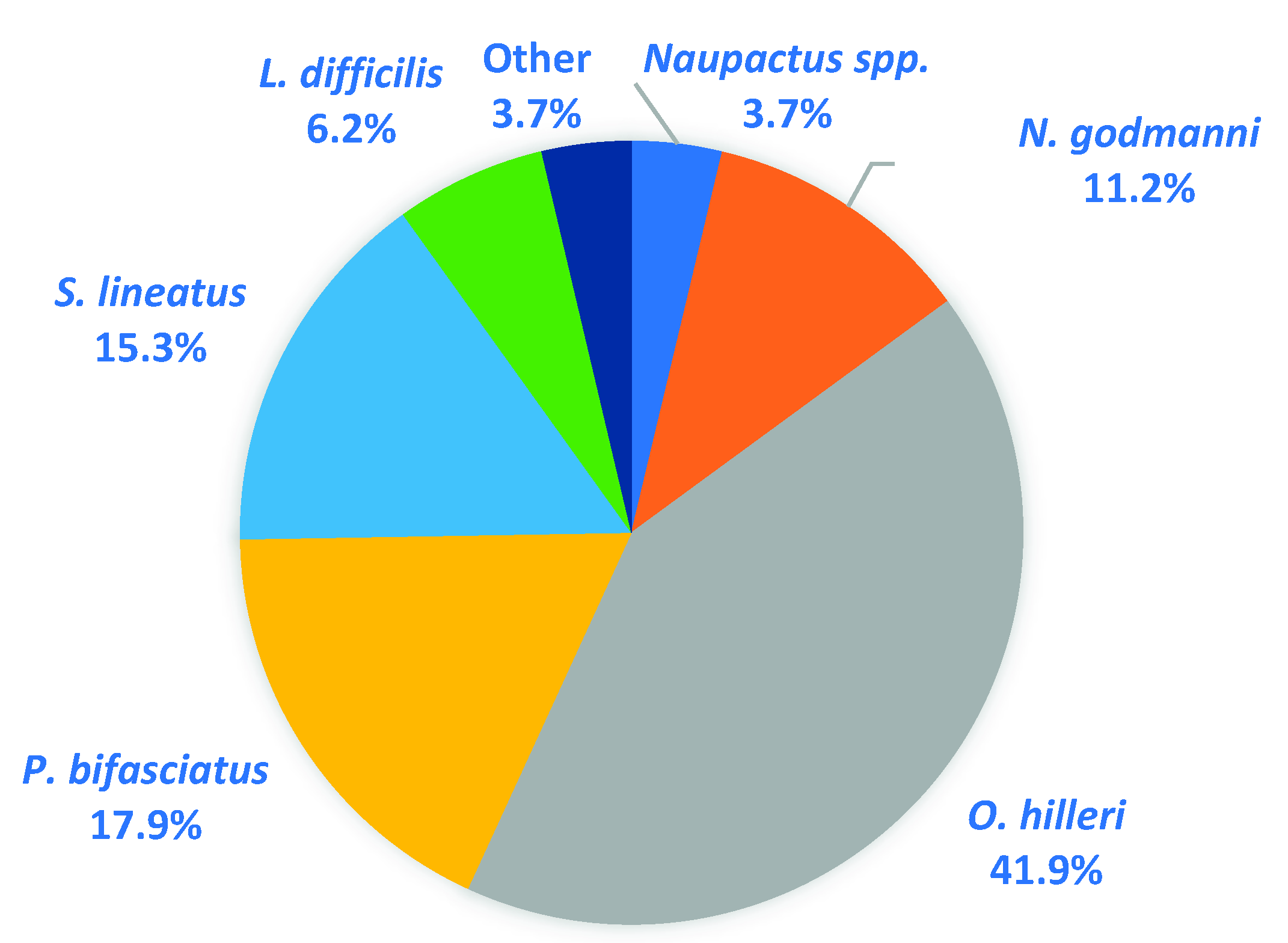
2.4. Effects of Treatments on Weevil Secondary Pests
2.5. Statistics
3. Results
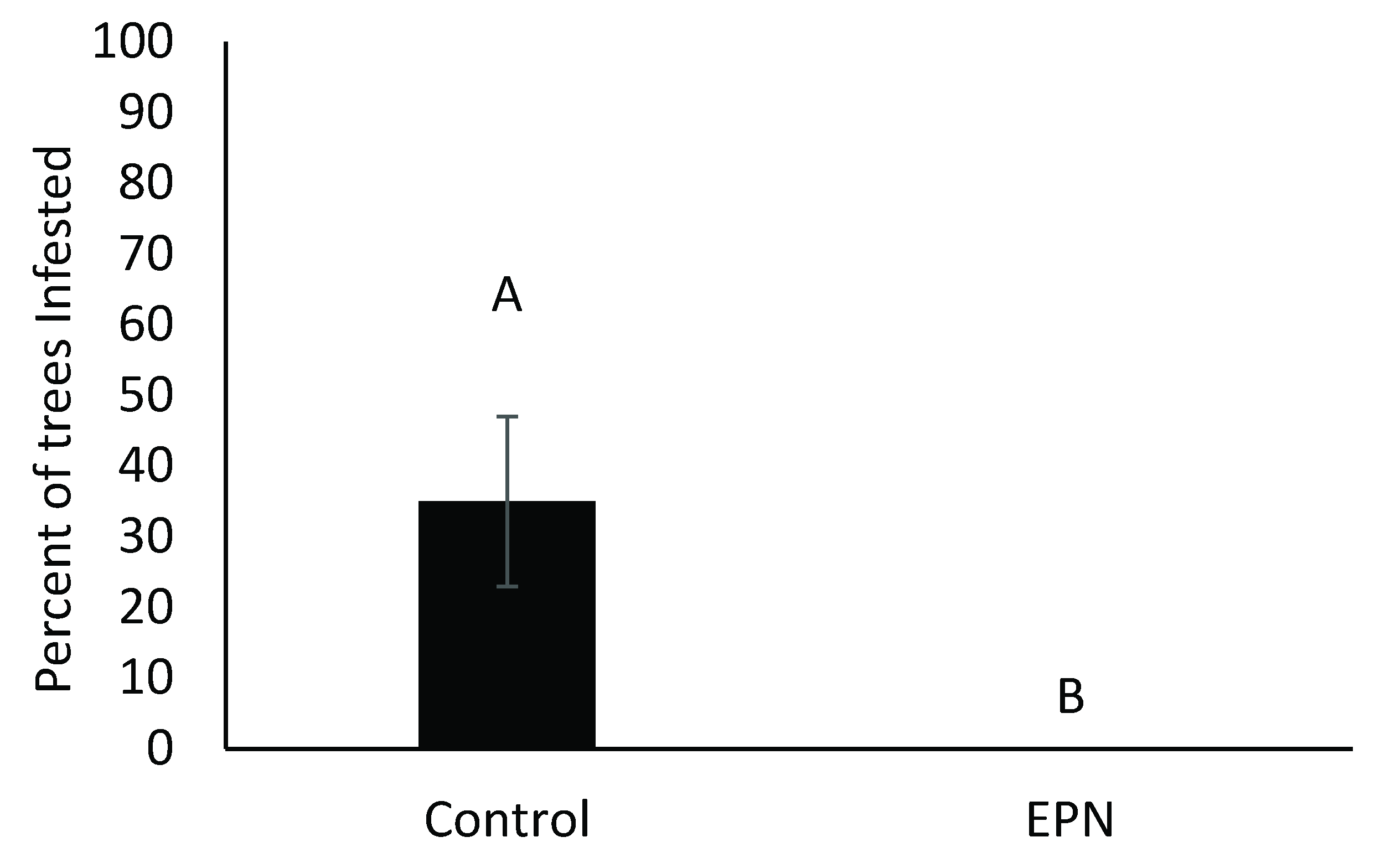
3.1. Entomopathogenic Nematode-Only Treatments
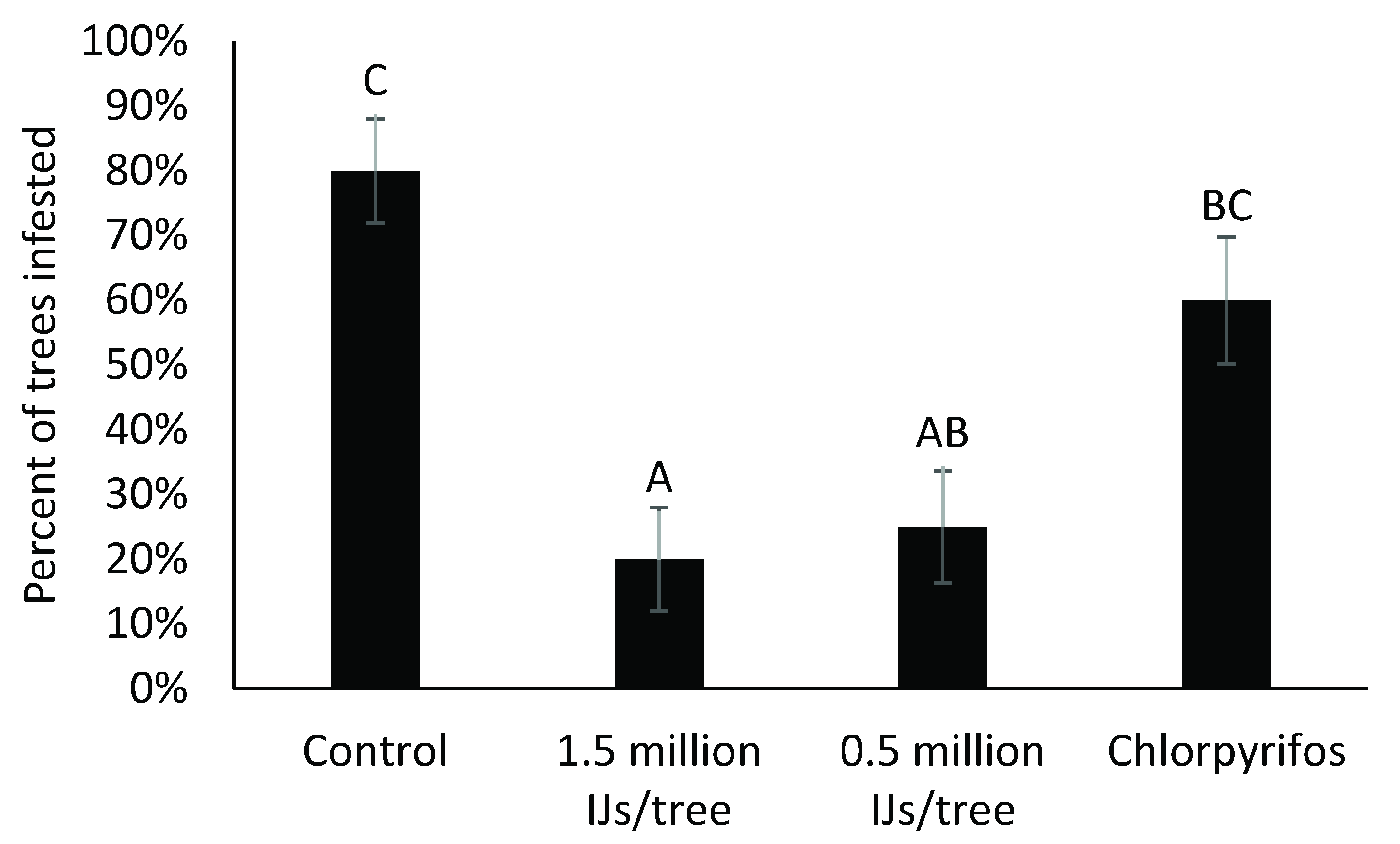
3.2. Variations on the Rate of Nematodes Used
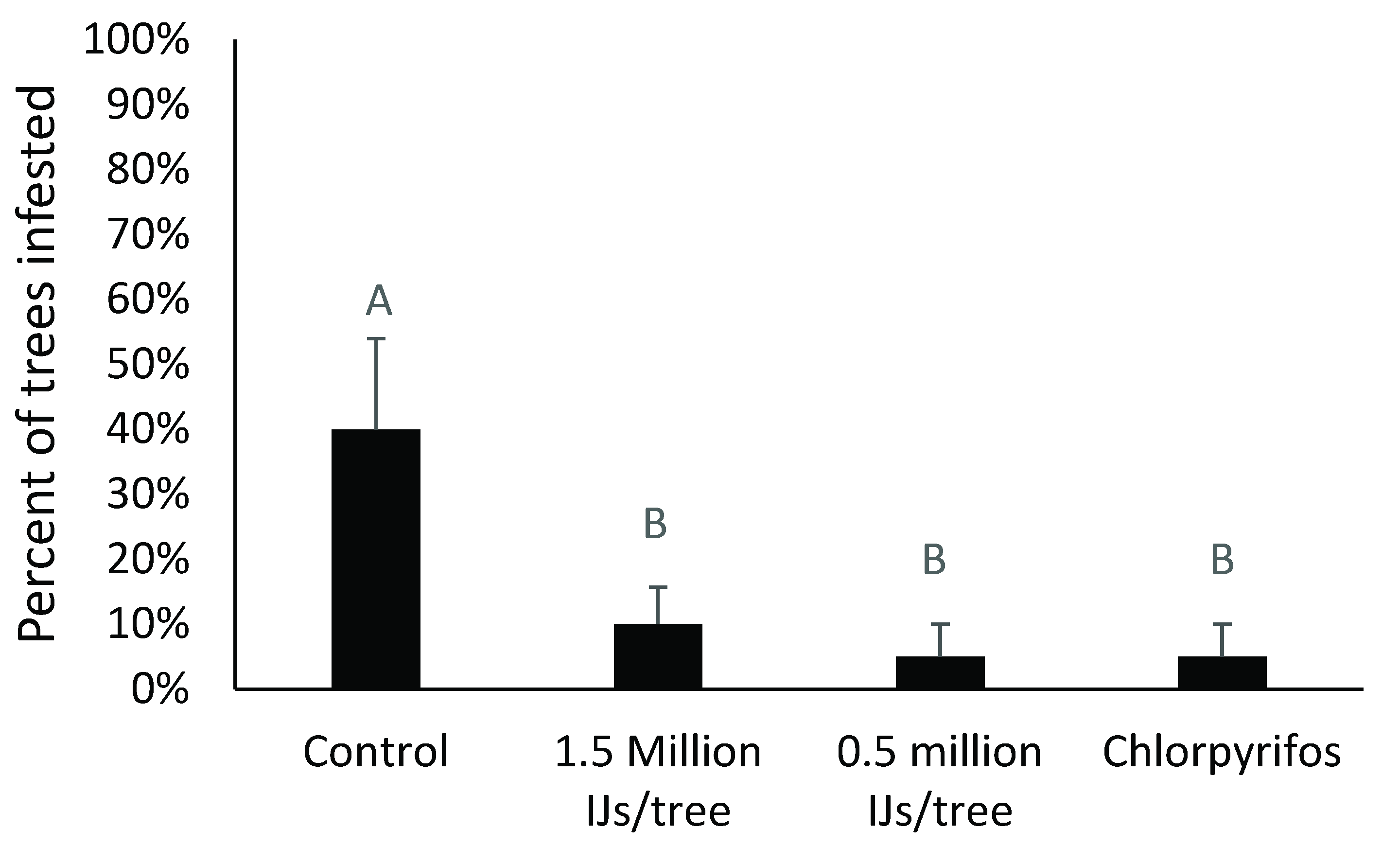
3.3. Variations in the Rate of Barricade® Gel Used
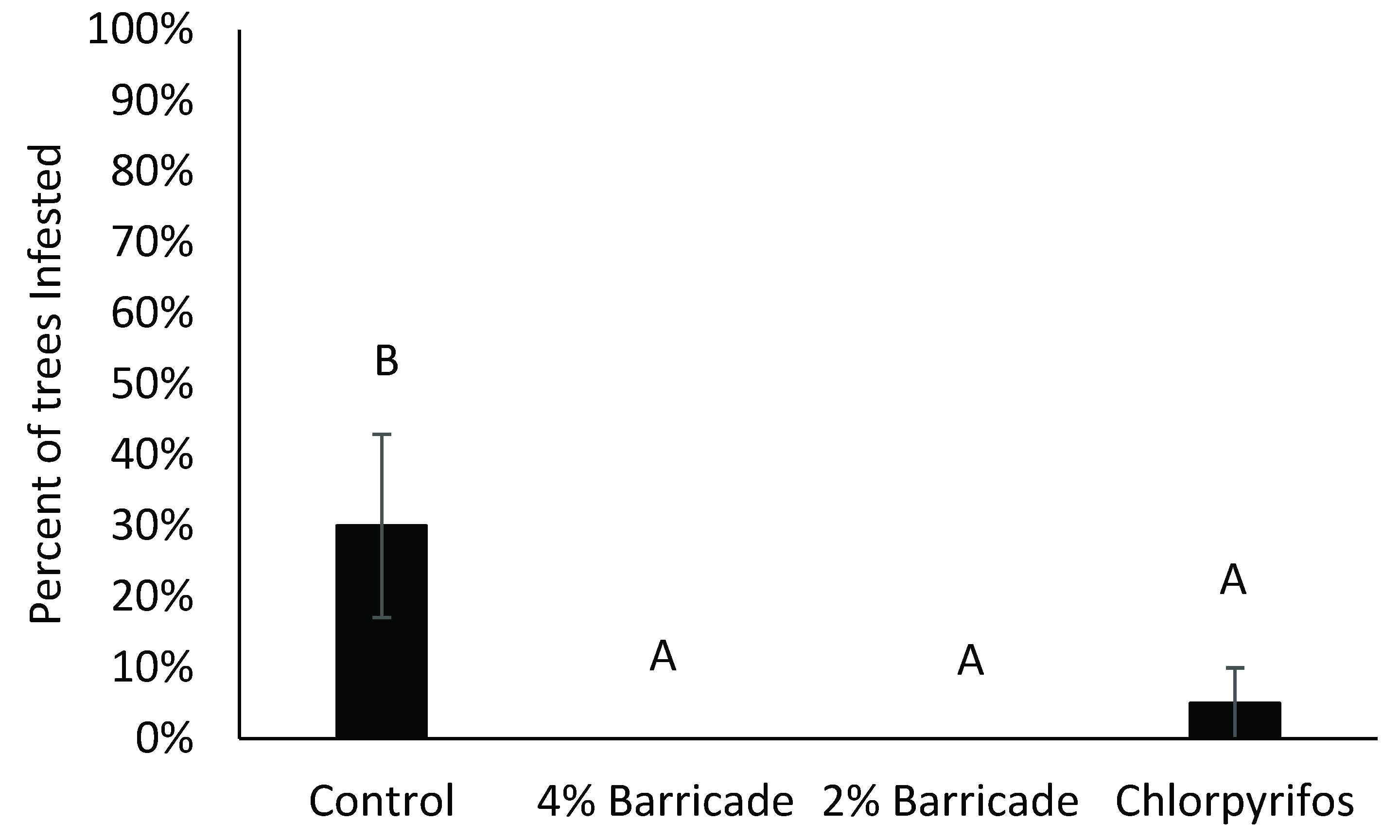
3.4. Effects of Treatments on Weevil Secondary Pests
4. Discussion and Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Johnson, D.; Cottrell, T.; Horton, D. Peachtree borer. In Southeastern Peach Grower’s Handbook; Horton, D., Johnson, D., Eds.; University of Georgia, Cooperative Extension Service: Athens, GA, USA, 2005; pp. 266–269. [Google Scholar]
- Frank, D.L.; Chandran, R.S. Orchard floor weed cover does not influence infestation of peach trees by the peachtree borer, Synanthedon exitiosa. In Proceedings of the Presented at the 1st International Electronic Conference on Entomology (IECE 2021), Online, 1–15 July 2021; p. 15. [Google Scholar]
- Yonce, C. Effectiveness of chlorpyrifos for control of Synanthedon pictipes and S. exitiosa in peach orchard tests of young trees with emphasis on timing applications. J. Econ. Entomol. 1980, 73, 827–828. [Google Scholar] [CrossRef]
- Blaauw, B.; Brannen, P.; Lockwood, D.; Schhnabel, G.; Ritchie, D. 2020 Southeastern Peach, Nectarine, and Plum Pest Management and Culture Guide, Bulletin 1171; University of Georgia: Athens, GA, USA, 2020. [Google Scholar]
- Shapiro-Ilan, D.I.; Cottrell, T.E.; Mizell, R.F., III; Horton, D.L.; Davis, J. A novel approach to biological control with entomopathogenic nematodes: Prophylactic control of the peachtree borer, Synanthedon exitiosa. Biol. Control 2009, 48, 259–263. [Google Scholar] [CrossRef]
- Shapiro-Ilan, D.I.; Cottrell, T.E.; Mizell, R.F., III; Horton, D.L.; Zaid, A. Field suppression of the peachtree borer, Synanthedon exitiosa, using Steinernema carpocapsae: Effects of irrigation, a sprayable gel and application method. Biol. Control 2015, 82, 7–12. [Google Scholar] [CrossRef]
- Shapiro-Ilan, D.I.; Cottrell, T.E.; Mizell, R.F., III; Horton, D.L. Curative control of the peachtree borer using entomopathogenic nematodes. J. Nematol. 2016, 48, 170. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.; Mathews, C.R. Biology of Oedophrys hilleri (Faust) (Coleoptera: Curculionidae): A potential new pest of peach in the eastern United States. Entomol. News 2009, 120, 185–193. [Google Scholar] [CrossRef]
- Wheeler, A., Jr.; Boyd, D.W., Jr. Distribution of an invasive weevil, Pseudocneorhinus bifasciatus Roelofs, in the southeastern United States. J. Entomol. Sci. 2005, 40, 25–30. [Google Scholar] [CrossRef]
- Cottrell, T.E.; Horton, D.L. Emergence of root-feeding weevils (Coleoptera: Curculionidae) in Central Georgia peach orchards. J. Entomol. Sci. 2013, 48, 184–194. [Google Scholar] [CrossRef]
- Piñero, J.C.; Shapiro-Ilan, D.; Cooley, D.R.; Tuttle, A.F.; Eaton, A.; Drohan, P.; Leahy, K.; Zhang, A.; Hancock, T.; Wallingford, A.K. Toward the integration of an attract-and-kill approach with entomopathogenic nematodes to control multiple life stages of plum curculio (Coleoptera: Curculionidae). Insects 2020, 11, 375. [Google Scholar] [CrossRef]
- Shapiro-Ilan, D.I.; Gouge, D.H.; Piggott, S.J.; Fife, J.P. Application technology and environmental considerations for use of entomopathogenic nematodes in biological control. Biol. Control 2006, 38, 124–133. [Google Scholar] [CrossRef]
- Dito, D.F.; Shapiro-Ilan, D.I.; Dunlap, C.A.; Behle, R.W.; Lewis, E.E. Enhanced biological control potential of the entomopathogenic nematode, Steinernema carpocapsae, applied with a protective gel formulation. Biocontrol. Sci. Technol. 2016, 26, 835–848. [Google Scholar] [CrossRef]
- Shapiro-Ilan, D.I.; Goolsby, J.A. Evaluation of Barricade® to enhance survival of entomopathogenic nematodes on cowhide. J. Invertebr. Pathol. 2021, 184, 107592. [Google Scholar] [CrossRef] [PubMed]
- Shapiro-Ilan, D.I.; Cottrell, T.E.; Mizell, R.F., III; Horton, D.L. Efficacy of Steinernema carpocapsae plus fire gel applied as a single spray for control of the lesser peachtree borer, Synanthedon pictipes. Biol. Control 2016, 94, 33–36. [Google Scholar] [CrossRef]
- Toledo, J.; Sánchez, J.E.; Williams, T.; Gómez, A.; Montoya, P.; Ibarra, J.E. Effect of soil moisture on the persistence and efficacy of Heterorhabditis bacteriophora (Rhabditida: Heterorhabditidae) against Anastrepha ludens (Diptera: Tephritidae) larvae. Fla. Entomol. 2014, 528–533. [Google Scholar] [CrossRef]
- Salame, L.; Glazer, I. Stress avoidance: Vertical movement of entomopathogenic nematodes in response to soil moisture gradient. Phytoparasitica 2015, 43, 647–655. [Google Scholar] [CrossRef]
- Shapiro-Ilan, D.I.; Hazir, S.; Lete, L. Viability and virulence of entomopathogenic nematodes exposed to ultraviolet radiation. J. Nematol. 2015, 47, 184. [Google Scholar]
- Devi, G.; George, J. Formulation of insecticidal nematode. Annu. Res. Rev. Biol. 2018, 1–10. [Google Scholar] [CrossRef]
- Hazir, S.; KAYA, H.; Touray, M.; Cimen, H.; Shapiro-Ilan, D.I. Basic laboratory and field manual for conducting research with the entomopathogenic nematodes, Steinernema and Heterorhabditis, and their bacterial symbionts. Turk. J. Zool. 2022, 46, 305–350. [Google Scholar] [CrossRef]
- Shapiro-Ilan, D.I.; Cottrell, T.E. Susceptibility of the lesser peachtree borer (Lepidoptera: Sesiidae) to entomopathogenic nematodes under laboratory conditions. Environ. Entomol. 2006, 35, 358–365. [Google Scholar] [CrossRef]
- Blaauw, B.; Brannen, P.; Lockwood, D.; Schhnabel, G.; Ritchie, D. 2022 Southeastern Peach, Nectarine, and Plum Pest Management and Culture Guide, Bulletin 1171; University of Georgia: Athens, GA, USA, 2022. [Google Scholar]
- McKern, J.A.; Johnson, D.T.; Lewis, B.A. Biology and control of the raspberry crown borer (Lepidoptera: Sesiidae). J. Econ. Entomol. 2014, 100, 398–404. [Google Scholar] [CrossRef]
- Gumus, A.; Karagoz, M.; Shapiro-Ilan, D.; Hazir, S. A novel approach to biocontrol: Release of live insect hosts pre-infected with entomopathogenic nematodes. J. Invertebr. Pathol. 2015, 130, 56–60. [Google Scholar] [CrossRef]
- Belien, T. Entomopathogenic nematodes as biocontrol agents of insect pests in orchards. CAB Rev. 2018, 13, 1–11. [Google Scholar] [CrossRef]
- Frank, D.L.; Starcher, S.; Chandran, R.S. Comparison of Mating Disruption and Insecticide Application for Control of Peachtree Borer and Lesser Peachtree Borer (Lepidoptera: Sesiidae) in Peach. Insects 2020, 11, 658. [Google Scholar] [CrossRef] [PubMed]
- Carde, R.T.; Minks, A.K. Control of moth pests by mating disruption: Successes and constraints. Annu. Rev. Entomol. 1995, 40, 559–585. [Google Scholar] [CrossRef]
- Georgis, R.; Manweiler, S. Entomopathogenic nematodes: A developing biological control technology. Agric. Zool. Rev. 1994, 6, 63–94. [Google Scholar]
- Kim, J.; Hiltpold, I.; Jaffuel, G.; Sbaiti, I.; Hibbard, B.E.; Turlings, T.C. Calcium-alginate beads as a formulation for the application of entomopathogenic nematodes to control rootworms. J. Pest Sci. 2021, 94, 1197–1208. [Google Scholar] [CrossRef]
- Fallon, D.J.; Kaya, H.K.; Gaugler, R.; Sipes, B.S. Effects of etomopathiogenic nematodes on Meloidogyne javanica on tomatoes and soybeans. J. Nematol. 2002, 34, 239. [Google Scholar] [PubMed]
- Sayedain, F.S.; Ahmadzadeh, M.; Fattah-Hosseini, S.; Bode, H.B. Soil application of entomopathogenic nematodes suppresses the root-knot nematode Meloidogyne javanica in cucumber. J. Plant Dis. Prot. 2021, 128, 215–223. [Google Scholar] [CrossRef]
- Hazir, S.; Shapiro-Ilan, D.I.; Bock, C.H.; Hazir, C.; Leite, L.G.; Hotchkiss, M.W. Relative potency of culture supernatants of Xenorhabdus and Photorhabdus spp. on growth of some fungal phytopathogens. Eur. J. Plant Pathol. 2016, 146, 369–381. [Google Scholar] [CrossRef]
- Shields, E.J. Utilizing persistent entomopathogenic nematodes in a conservation or a more classical biological control approach. In Nematode Pathogenesis of Insects and Other Pests; Springer: Berlin/Heidelberg, Germany, 2015; pp. 165–184. [Google Scholar]

| Location | Conditions | Treatment Year (s) | Million IJs/Tree | Barricade® Gel Rate | Target |
|---|---|---|---|---|---|
| Fayetteville | Organic | 2018 | 1.5 | - | S. exitiosa |
| Fort Valley Area A | Conventional | 2018, 2019 | 0.5, 1.5 | 2% | S. exitiosa |
| Fort Valley Area B | Conventional | 2018, 2019 | 1.0 | 2%, 4% | S. exitiosa |
| Byron | Conventional | 2018 | 1.5 | 4% | Weevils |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wong, C.; Oliveira-Hofman, C.; Blaauw, B.R.; Chavez, D.; Jagdale, G.; Mizell, R.F., III; Shapiro-Ilan, D. Using the Nematode, Steinernema carpocapsae, to Control Peachtree Borer (Synanthedon exitiosa): Optimization of Application Rates and Secondary Benefits in Control of Root-Feeding Weevils. Agronomy 2022, 12, 2689. https://doi.org/10.3390/agronomy12112689
Wong C, Oliveira-Hofman C, Blaauw BR, Chavez D, Jagdale G, Mizell RF III, Shapiro-Ilan D. Using the Nematode, Steinernema carpocapsae, to Control Peachtree Borer (Synanthedon exitiosa): Optimization of Application Rates and Secondary Benefits in Control of Root-Feeding Weevils. Agronomy. 2022; 12(11):2689. https://doi.org/10.3390/agronomy12112689
Chicago/Turabian StyleWong, Colin, Camila Oliveira-Hofman, Brett R. Blaauw, Dario Chavez, Ganpati Jagdale, Russell F. Mizell, III, and David Shapiro-Ilan. 2022. "Using the Nematode, Steinernema carpocapsae, to Control Peachtree Borer (Synanthedon exitiosa): Optimization of Application Rates and Secondary Benefits in Control of Root-Feeding Weevils" Agronomy 12, no. 11: 2689. https://doi.org/10.3390/agronomy12112689
APA StyleWong, C., Oliveira-Hofman, C., Blaauw, B. R., Chavez, D., Jagdale, G., Mizell, R. F., III, & Shapiro-Ilan, D. (2022). Using the Nematode, Steinernema carpocapsae, to Control Peachtree Borer (Synanthedon exitiosa): Optimization of Application Rates and Secondary Benefits in Control of Root-Feeding Weevils. Agronomy, 12(11), 2689. https://doi.org/10.3390/agronomy12112689










