Comparison of Heat-Treated and Unheated Vermicompost on Biological Properties of Calcareous Soil and Aloe Vera Growth under Greenhouse Conditions in a Mediterranean Climate
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site
2.2. Treatments and Experimental Design
2.3. Experimental Setup
2.4. Harvesting
2.5. Preparation of Analysis
2.6. Statistical Analysis
3. Results
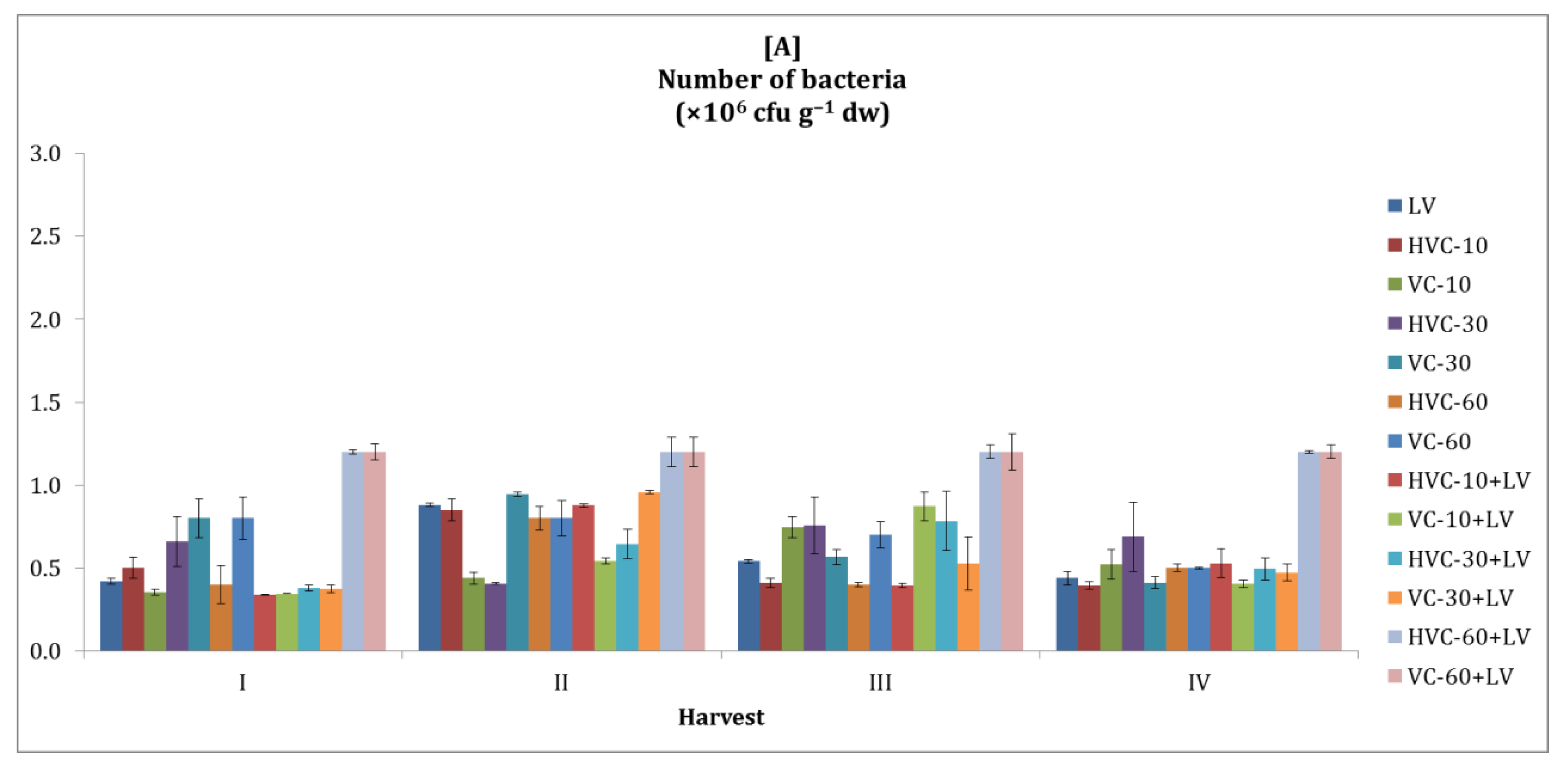
3.1. Number of Bacteria
3.2. Dehydrogenase
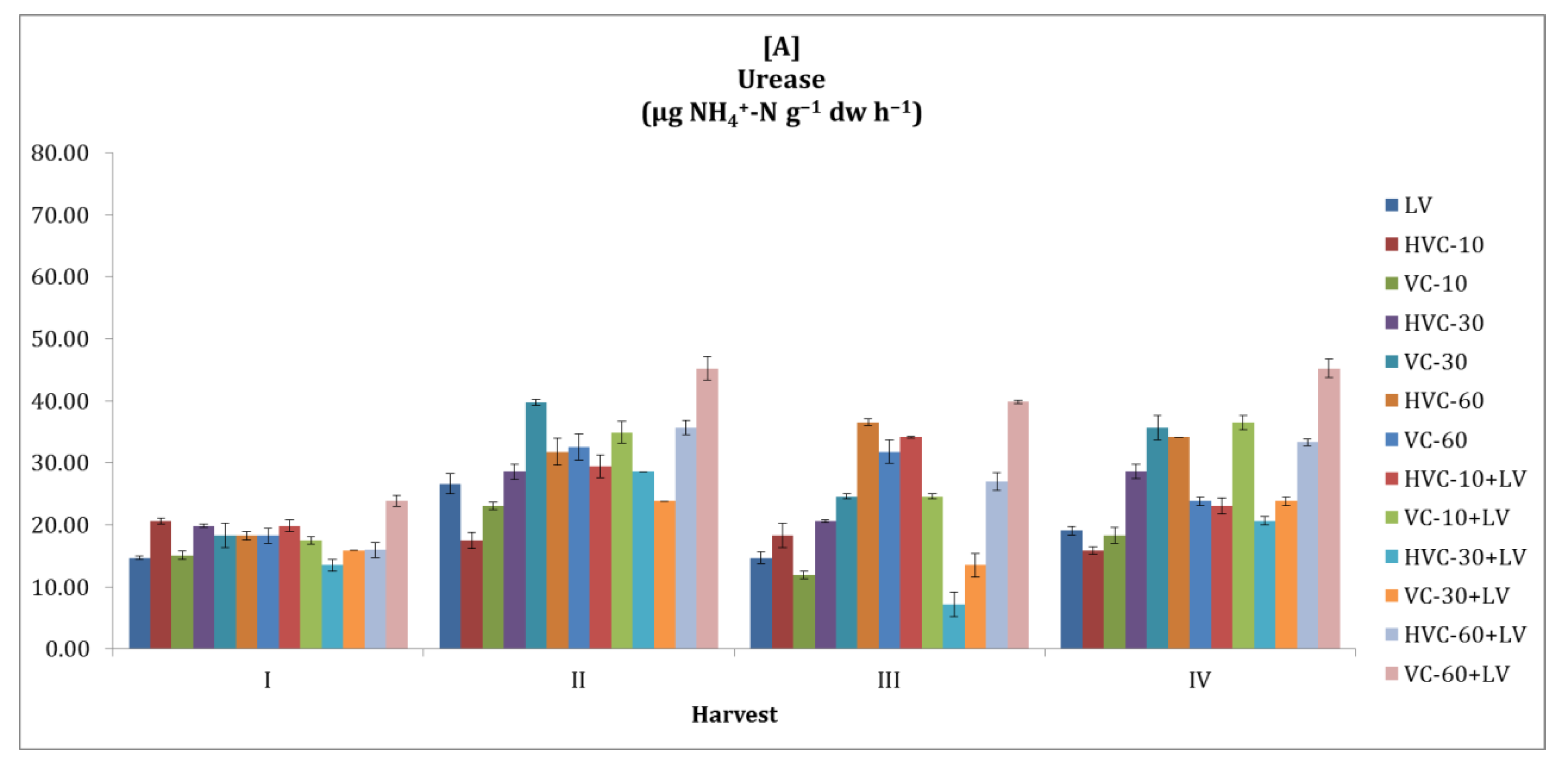
3.3. Urease
3.4. Alkaline Phosphatase
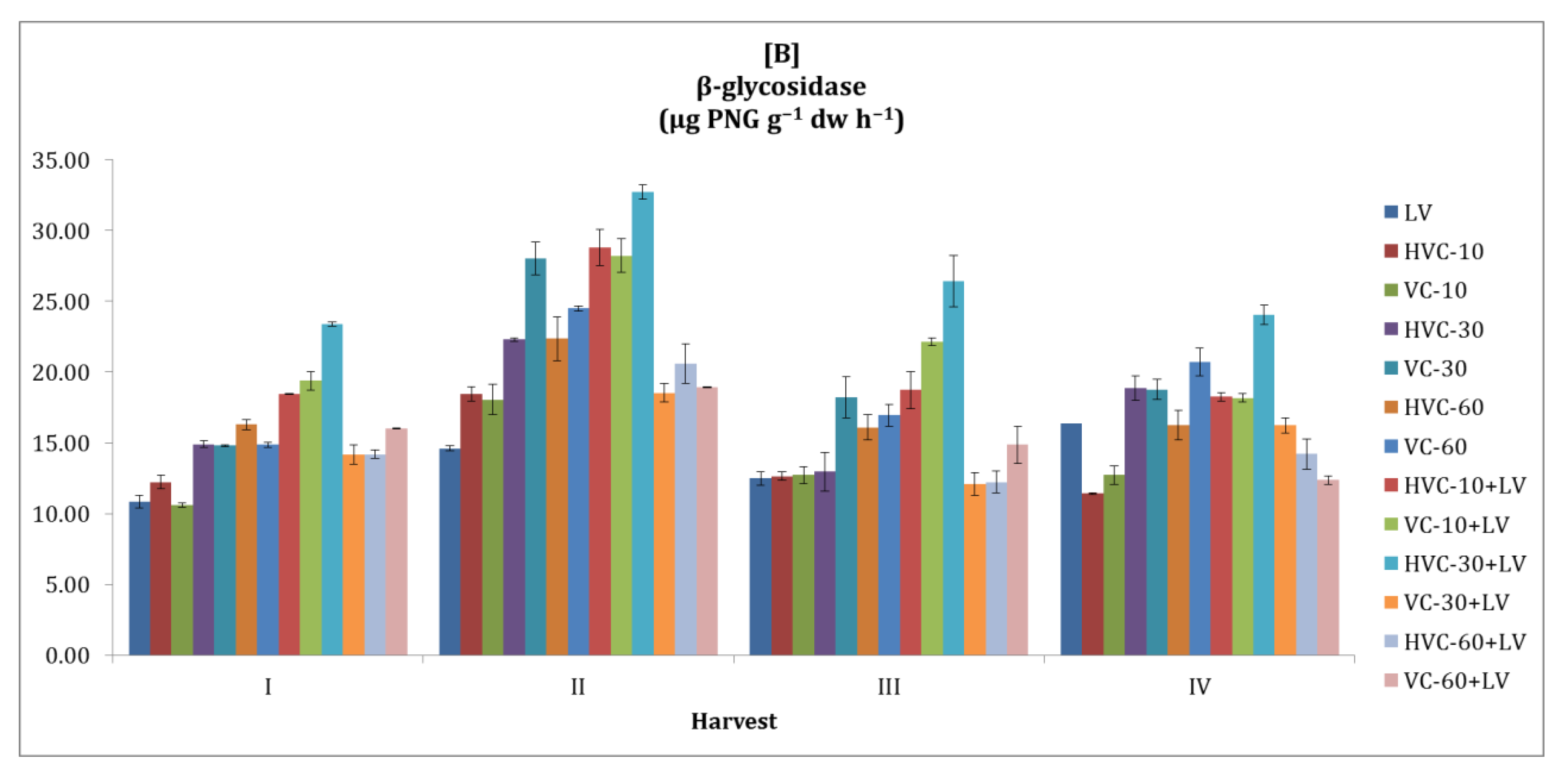
3.5. β-glycosidase
3.6. Soil Reaction (pH)
3.7. Soil Electrical Conductivity (EC)
3.8. Soil Organic C
3.9. Plant Growth
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Griffiths, H.; Males, J. Succulent plants. Curr. Biol. 2017, 27, R890–R896. [Google Scholar] [CrossRef]
- Ahlawat, K.S.; Khatkar, B.S. Processing, food applications and safety of aloe vera products: A review. J. Food Sci. Technol. 2011, 48, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Hekmatpou, D.; Mehrabi, F.; Rahzani, K.; Aminiyan, A. The effect of Aloe vera clinical trials on prevention and healing of skin wound: A Systematic Review. Iran. J. Med. Sci. 2019, 44, 1–9. [Google Scholar] [PubMed]
- Surjushe, A.; Vasani, R.; Saple, D.G. Aloe vera: A short review. Indian J. Dermatol. 2008, 53, 163–166. [Google Scholar] [CrossRef] [PubMed]
- Radha, M.H.; Laxmipriya, N.P. Evaluation of biological properties and clinical effectiveness of Aloe vera: A Systematic Review. J. Tradit. Complement. Med. 2015, 5, 21–26. [Google Scholar] [CrossRef]
- Hendrawati, T.R.; Ambarwati, H.; Susanty, R.A.N.; Hasyim, U.H. The effects of Aloe Vera gel addition on the effectiveness of sunscreen lotion. J. Rek. Pros. 2020, 14, 101–107. [Google Scholar] [CrossRef]
- Ramachandra, C.T.; Srinivasa, R.P. Processing of Aloe Vera leaf gel: A review. Am. J. Agric. Biol. Sci. 2008, 3, 502–510. [Google Scholar] [CrossRef]
- Eshun, K.; Qian, H. Aloe vera: A valuable ingredient for the food, pharmaceutical and cosmetic industries—A review. Crit. Rev. Food Sci. Nutr. 2004, 44, 91–96. [Google Scholar] [CrossRef]
- Baydar, H. Tıbbi ve Aromatik Bitkiler Bilimi ve Teknolojisi. In Medical and Aromatic Plants Science and Technology, 9th ed.; Nobel Akademik Yayıncılık: Ankara, Turkey, 2021; pp. 125–133. [Google Scholar]
- Li, Y.; Dexin Konga, D.; Michael, Y.F.; HongWu, R.S. The effect of developmental and environmental factors on secondary metabolites in medicinal plants. Plant Physiol. Biochem. 2020, 148, 80–89. [Google Scholar] [CrossRef]
- Cristiano, G.; Murillo-Amador, B.; De Lucia, B. Propagation techniques and agronomic requirements for the cultivation of Barbados Aloe (Aloe vera (L.) Burm. F.)—A review. Front. Plant Sci. 2016, 23, 1410. [Google Scholar] [CrossRef]
- Cardarelli, M.; Rouphael, Y.; Rea, E.; Lucini, L.; Pellizzoni, M.; Colla, G. Effects of fertilization, arbuscular mycorrhiza, and salinity on growth, yield, and bioactive compounds of two Aloe species. HortScience 2013, 48, 568–575. [Google Scholar] [CrossRef]
- Ibrahim, M.H.; Jaafar, H.Z.; Karimi, E.; Ghasemzadeh, A. Primary, secondary metabolites, photosynthetic capacity and antioxidant activity of the Malaysian Herb Kacip Fatimah (Labisia Pumila Benth) exposed to potassium fertilization under greenhouse conditions. Int. J. Mol. Sci. 2012, 13, 15321–15342. [Google Scholar] [CrossRef] [PubMed]
- Torun Kayabaşı, E.; Yılmaz, O. The importance of vermicompost in agricultural production and economy. Eurasian J. Agric. Res. 2021, 5, 146–159. [Google Scholar]
- Pathma, J.; Sakthivel, N. Microbial diversity of vermicompost bacteria that exhibit useful agricultural traits and waste management potential. Springerplus 2012, 4, 26. [Google Scholar] [CrossRef] [PubMed]
- Gudeta, K.; Julka, J.M.; Kumar, A.; Bhagatd, A.; Kumari, A. Vermiwash: An agent of disease and pest control in soil, a review. Heliyon 2021, 7, e06434. [Google Scholar] [CrossRef]
- Hoque, T.S.; Hasan, A.K.; Hasan, M.A.; Nahar, N.; Dey, D.K.; Mia, S.; Solaiman, Z.M.; Kader, M.A. Nutrient release from vermicompost under anaerobic conditions in two contrasting soils of Bangladesh and its effect on wetland rice crop. Agriculture 2022, 12, 376. [Google Scholar] [CrossRef]
- Hemati, A.; Alikhani, H.A.; Ajdanian, L.; Babaei, M.; Asgari Lajayer, B.; van Hullebusch, E.D. Effect of different enriched vermicomposts, humic acid extract and indole-3-acetic acid amendments on the growth of Brassica napus. Plants 2022, 11, 227. [Google Scholar] [CrossRef]
- Blouin, M.; Barrere, J.; Meyer, N.; Lartigue, S.; Barot, S.; Mathieu, J. Vermicompost significantly affects plant growth. A meta-analysis. Agron. Sustain. Dev. 2019, 39, 34. [Google Scholar] [CrossRef]
- Murillo-Amador, B.; Luna-Cisneros, M.J.; Nieto-Garibay, A.; Aguilar-García, M.; Troyo-Diéguez, E.; García-Hernández, J.L. Manual Parala Producciónde Sábilaenel Valledel Carrizal; Centro de Investigaciones Biológicas del Noroeste: La Paz, Mexico, 2007; pp. 52–62. [Google Scholar]
- Kacar, B. Gübre Analizleri [Analyzes of Fertilizer]; Ankara Üniversitesi Ziraat Fakültesi Eğitim, Araştırma ve Geliştirme Vakfı Yayınları: Ankara, Turkey, 1990; pp. 53–64. [Google Scholar]
- Jackson, M.L. Soil Chemical Analysis; Prentice Hall of India Private Limited: New Delhi, India, 1967; p. 498. [Google Scholar]
- Kacar, B. Bitki ve Toprağın Kimyasal Analizleri [Chemical Analyses of Plant and Soil]; Ankara Üniversitesi Ziraat Fakültesi Eğitim, Araştırma ve Geliştirme Vakfı Yayınları: Ankara, Turkey, 1995; p. 466. [Google Scholar]
- Bouyoucos, G.J. A recalibration of hydrometer method for making mechanical analysis of soils. Agron. J. 1951, 43, 434–438. [Google Scholar] [CrossRef]
- Çağlar, K.O. Toprak Bilgisi [Soil Science]; Ankara Üniversitesi Ziraat Fakültesi Yayınları: Ankara, Turkey, 1949; p. 268. [Google Scholar]
- Black, C.A. Methods of Soil Analysis Part 2: Chemical and Microbiological Properties; Soil Science Society of America Inc.: Madison, WI, USA, 1965; pp. 961–983. [Google Scholar]
- Parkinson, D.; Gray, T.R.C.; Williams, S.T. International Biological Programme Handbook 19: Methods for Studying the Ecology of Soil Microorganisms, 1st ed.; Blackwell Scientific Publications: Oxford, UK, 1971; p. 116. [Google Scholar]
- Thalmann, A. Dehydrogenase activity in soil. In Methods in Applied Soil Microbiology and Biochemistry, 1st ed.; Alef, K., Nannipieri, P., Eds.; Academic Press: San Diego, CA, USA, 1995; pp. 321–325. [Google Scholar]
- Hoffman, G.; Teicher, K. Ein kolorimetrisches verfabren zur bestimmung der urease aktivitat in boden [A colorimetric method to determine urease activity in soil]. Z Pflanzenernährung Düngung Bodenkunde 1961, 95, 55–63. [Google Scholar]
- Tabatabai, M.A.; Bremmer, J.M. Use of p-nitrophely phosphate for assay of soil phosphatase activity. Soil Biol. Biochem. 1969, 1, 301–307. [Google Scholar] [CrossRef]
- Eivazi, F.; Tabatabi, M.A. Assay of the β-glucosidase activity. In Methods in Applied Soil Microbiology and Biochemistry, 1st ed.; Alef, K., Nannipieri, P., Eds.; Academic Press: San Diego, CA, USA, 1995; pp. 350–351. [Google Scholar]
- SPSS, version 21.0; IBM: Chicago, IL, USA, 2008.
- Jacoby, R.; Peukert, M.; Succurro, A.; Koprivova, A.; Kopriva, S. The role of soil microorganisms in plant mineral nutrition-current knowledge and future directions. Front. Plant Sci. 2017, 19, 1617. [Google Scholar] [CrossRef] [PubMed]
- Lazcano, C.; Zhu-Barker, X.; Decock, C. Effects of organic fertilizers on the soil microorganisms responsible for N2O emissions: A Review. Microorganisms 2021, 9, 983. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.L.; Wu, T.Y.; Lima, P.N.; Shak, K.P.Y. The use of vermicompost in organic farming: Overview, effects on soil and economics. J. Sci. Food Agric. 2015, 95, 1143–1156. [Google Scholar] [CrossRef] [PubMed]
- Lv, M.; Li, J.; Zhang, W.; Zhou, B.; Dai, J.; Zhang, C. Microbial activity was greater in soils added with herb residue vermicompost than chemical fertilizer. Soil Ecol. Lett. 2020, 2, 209–219. [Google Scholar] [CrossRef]
- Kaczyńska, G.; Borowik, A.; Wyszkowska, J. Soil dehydrogenases as an indicator of contamination of the environment with petroleum products. Water Air Soil Pollut. 2015, 226, 372. [Google Scholar] [CrossRef]
- Liu, C.; Song, Y.; Dong, X.; Wang, X.; Ma, X.; Zhao, G.; Zang, S. Soil enzyme activities and their relationships with soil C, N, and P in peatlands from different types of permafrost regions, Northeast China. Front. Environ. Sci. 2021, 9, 670769. [Google Scholar] [CrossRef]
- Wu, J.; Wang, H.; Li, G.; Ma, W.; Wu, J.; Gong, Y.; Xu, G. Vegetation degradation impacts soil nutrients and enzyme activities in wet meadow on the Qinghai-Tibet Plateau. Sci. Rep. 2020, 4, 21271. [Google Scholar] [CrossRef]
- Roscoe, R.; Vasconcellos, C.; Furtini-Neto, A.G.; Guedes, A.A.; Fernandes, L.A. Urease activity and its relation to soil organic matter, microbial biomass nitrogen and urea-nitrogen assimilation by maize in a Brazilian Oxisol under no-tillage and tillage systems. Biol. Fertil. Soils 2000, 32, 52–59. [Google Scholar] [CrossRef]
- Shang, L.; Wan, L.; Zhou, X.; Li, S.; Li, X. Effects of organic fertilizer on soil nutrient status, enzyme activity, and bacterial community diversity in Leymus chinensis steppe in Inner Mongolia, China. PLoS ONE 2020, 15, e0240559. [Google Scholar] [CrossRef]
- Lemanowicz, J.; Bartkowiak, A.; Breza-Boruta, B. Changes in phosphorus content, phosphatase activity and some physicochemical and microbiological parameters of soil within the range of impact of illegal dumping sites in Bydgoszcz (Poland). Environ. Earth Sci. 2016, 75, 510. [Google Scholar] [CrossRef]
- Touhami, D.; McDowell, R.W.; Condron, L.M. Role of organic anions and phosphatase enzymes in phosphorus acquisition in the rhizospheres of legumes and grasses grown in a low phosphorus pasture soil. Plants 2020, 9, 1185. [Google Scholar] [CrossRef] [PubMed]
- Vinotha, S.P.; Parthasarathi, K.; Ranganathan, L.S. Enhanced phosphatase activity in earthworm casts is more of microbial origin. Curr. Sci. 2000, 79, 1158–1159. [Google Scholar]
- Tiwari, R.; Dwivedi, B.S.; Sharma, Y.M.; Sharma, A.; Dwivedi, A.K. Activities of β-glucosidase, phosphatase and dehydrogenase as soil quality indicators: A review. Int. J. Curr. Microbiol. Appl. Sci. 2019, 8, 834–846. [Google Scholar] [CrossRef]
- Srivastava, S.; Love-Nichols, J.A.; Dies, K.A.; Ledbetter, D.H.; Martin, C.L.; Chung, W.K.; Firth, H.V.; Frazier, T.; Hansen, R.L.; Prock, L.; et al. Meta-analysis and multidisciplinary consensus statement: Exome sequencing is a first-tier clinical diagnostic test for individuals with neurodevelopmental disorders. Genet. Med. 2019, 21, 2413–2421. [Google Scholar] [CrossRef]
- Liang, X.; Yuan, J.; Yang, E.; Meng, J. Responses of soil organic carbon decomposition and microbial community to the addition of plant residues with different C:N ratio. Eur. J. Soil Biol. 2017, 82, 50–55. [Google Scholar] [CrossRef]
- Gross, A.; Bromm, T.; Glaser, B. Soil organic carbon sequestration after biochar application: A Global Meta-Analysis. Agronomy 2021, 11, 2474. [Google Scholar] [CrossRef]
- Allam, M.; Radicetti, E.; Quintarelli, V.; Petroselli, V.; Marinari, S.; Mancinelli, R. Influence of organic and mineral fertilizers on soil organic carbon and crop productivity under different tillage systems: A Meta-Analysis. Agriculture 2022, 12, 464. [Google Scholar] [CrossRef]
- Goulding, K.W. Soil acidification and the importance of liming agricultural soils with particular reference to the United Kingdom. Soil Use Manag. 2016, 32, 390–399. [Google Scholar] [CrossRef]
- Whalen, J.K.; Chang, C.; Clayton, G.W.; Carefoot, J.P. Cattle manure amendments can increase the pH of acid soils. Soil Sci. Soc. Am. J. 2000, 64, 962–966. [Google Scholar] [CrossRef]
- Diacono, M.; Montemurro, F. Long-term effects of organic amendments on soil fertility: A review. Agron. Sustain. Dev. 2010, 30, 401–422. [Google Scholar] [CrossRef]
- Mandal, B.; Majumder, B.; Bandyopadhyay, P.K.; Hazra, G.C.; Gangopadhyay, A.; Samantaray, R.N.; Mishra, A.K.; Chaudhury, J.; Saha, M.N.; Kundu, S. The potential of cropping systems and soil amendments for carbon sequestration in soils under long-term experiments in subtropical India. Glob. Change Biol. 2007, 13, 357–369. [Google Scholar] [CrossRef]
- Liu, E.; Yan, C.; Mei, X.; Zhang, Y.; Fan, T. Long-term effect of manure and fertilizer on soil organic carbon pools in dryland farming in northwest China. PLoS ONE 2013, 8, e56536. [Google Scholar] [CrossRef] [PubMed]







| Parameter | Soil | Unheated Vermicompost (VC) | Heat-Treated Vermicompost (HVC) | Vermiwash (LV) |
|---|---|---|---|---|
| pH | 7.3 | 7.6 | 7.4 | 8.5 |
| EC (µS cm−1) | 218 | 3800 | 4500 | 9000 |
| Lime (%) | 16 | - | - | - |
| Sand (%) | 19.14 | - | - | - |
| Clay (%) | 55.82 | - | - | - |
| Loam (%) | 25.04 | - | - | - |
| Moisture (%) | - | 35 | 20 | - |
| Organic matter (%) | 1.23 | 35 | 30 | 7 |
| Organic C (%) | 0.71 | 20 | 17 | 4 |
| Total N (%) | 0.079 | 1.2 | 1.5 | 1 |
| C:N ratio | 9:1 | 16:1 | 12:1 | 4:1 |
| Urease (µg NH4+-N dw h−1) | 39 | 185 | 167 | 312 |
| Alkaline phosphatase (µg PNP dw h−1) | 26 | 173 | 161 | 283 |
| β-glycosidase (µg PNG dw h−1) | 2 | 69 | 55 | 134 |
| Dehydrogenase (µg TPF dw h−1) | 0.5 | 46 | 38 | 96 |
| Number of bacteria (cfu g−1 dw) | 1.1 × 105 | 2.7 × 105 | 2.8 × 103 | 4.9 × 107 |
| Treatments | Number of Bacteria (×106 cfu g−1 dw) | Dehydrogena se (µg TPF g−1 dw h−1) | Urease (µg NH4+-N g−1 dw h−1) | Alkaline Phosphatase (µg PNP g−1 dw h−1) | β-glycosidase (µg PNG g−1 dw h−1) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Growth Season | ||||||||||
| 1th | 2nd | 1th | 2nd | 1th | 2nd | 1th | 2nd | 1th | 2nd | |
| LV | 0.6 bc 1 | 1.6 b | 0.76 cd | 1.97 e | 18.75 c | 38.10 bc | 17.30 | 27.27 d | 6.44 d | 13.58 e |
| HVC-10 | 0.5 c | 1.3 c | 0.74 cd | 2.16 d | 18.06 c | 35.00 c | 16.23 | 27.93 d | 6.01 d | 13.68 e |
| VC-10 | 0.5 c | 1.4 bc | 0.60 d | 2.31 c | 17.06 d | 37.70 bc | 17.50 | 25.70 d | 7.15 c | 13.52 e |
| HVC-30 | 0.6 bc | 1.6 b | 0.83 c | 2.15 d | 24.40 bc | 39.76 bc | 18.33 | 35.07 c | 8.01 bc | 17.25 c |
| VC-30 | 0.7 bc | 1.6 b | 1.05 b | 2.40 bc | 29.56 b | 42.74 b | 18.68 | 37.11 c | 9.05 b | 19.95 b |
| HVC-60 | 0.5 c | 1.4 bc | 0.98 bc | 2.37 bc | 30.16 b | 41.47 b | 19.17 | 38.65 | 10.15 ab | 17.75 c |
| VC-60 | 0.7 bc | 1.4 bc | 0.90 bc | 2.38 bc | 26.59 bc | 47.10 b | 18.86 | 40.84 b | 9.70 b | 19.25 b |
| HVC-10+LV | 0.5 c | 1.7 b | 1.07 b | 2.29 c | 26.59 bc | 43.13 b | 19.01 | 41.81 b | 8.91 bc | 21.05 b |
| VC-10+LV | 0.5 c | 1.4 bc | 1.13 b | 2.48 b | 28.37 b | 46.71 b | 18.86 | 42.27 b | 8.79 bc | 21.97 b |
| HVC-30+LV | 0.9 b | 1.4 bc | 1.62 a | 2.23 cd | 17.46 d | 56.52 a | 18.61 | 57.21 a | 6.57 d | 26.64 a |
| VC-30+LV | 0.9 b | 1.4 bc | 0.90 bc | 2.10 d | 19.25 c | 36.79 bc | 18.33 | 35.79 c | 6.42 d | 15.26 d |
| HVC-60+LV | 1.2 a | 2.4 a | 0.81 c | 2.14 d | 28.00 b | 40.16 b | 20.65 | 37.23 c | 7.81 c | 15.31 d |
| VC-60+LV | 1.2 a | 2.4 a | 0.92 bc | 3.15 a | 38.53 a | 45.12 b | 19.80 | 56.60 a | 13.46 a | 15.54 d |
| rANOVA (LSD %5) | ||||||||||
| Harvest (H) | 9.2 **2 | 8.21 ** | 7.63 ** | 9.75 ** | 1.38 * | 2.46 * | 1.98 * | 7.28 ** | 1.89 * | 9.27 ** |
| Treatment (T) | 1.2 *3 | 8.36 ** | 2.87 * | 2.98 * | 3.42 ** | 52.13 *** | Ns | 2.66 * | 8.63 ** | 2.67 * |
| H × T | 1.3 * | 2.35 * | Ns 4 | Ns | Ns | Ns | Ns | 9.12 ** | 7.25 ** | 9.54 ** |
| Treatments | pH | EC (µS cm−1) | Organic C (%) | |||
|---|---|---|---|---|---|---|
| Growth Season | ||||||
| 1th | 2nd | 1th | 2nd | 1th | 2nd | |
| LV | 7.49 | 7.22 | 1053 | 1437 | 1.00 | 1.12 d 1 |
| HVC-10 | 7.46 | 7.23 | 869 | 1251 | 1.01 | 1.16 c |
| VC-10 | 7.48 | 7.19 | 923 | 1367 | 1.05 | 1.15 c |
| HVC-30 | 7.49 | 7.25 | 856 | 1359 | 1.04 | 1.24 b |
| VC-30 | 7.45 | 7.15 | 891 | 1276 | 1.09 | 1.26 b |
| HVC-60 | 7.43 | 7.17 | 927 | 1392 | 1.00 | 1.18 bc |
| VC-60 | 7.41 | 7.16 | 968 | 1265 | 1.06 | 1.19 bc |
| HVC-10+LV | 7.51 | 7.24 | 1026 | 1230 | 1.04 | 1.20 bc |
| VC-10+LV | 7.48 | 7.21 | 984 | 1328 | 1.06 | 1.26 b |
| HVC-30+LV | 7.49 | 7.14 | 991 | 1364 | 1.01 | 1.35 a |
| VC-30+LV | 7.47 | 7.18 | 1068 | 1408 | 1.03 | 1.22 b |
| HVC-60+LV | 7.42 | 7.19 | 1092 | 1396 | 1.02 | 1.24 b |
| VC-60+LV | 7.46 | 7.20 | 1027 | 1377 | 1.04 | 1.36 a |
| rANOVA (LSD %5) | ||||||
| Harvest (H) | 2.63 * | 3.02 * | 2.62 * | 8.93 ** | 7.62 ** | 9.24 **3 |
| Treatment (T) | Ns 5 | Ns | Ns | Ns | Ns | 24.20 ***2 |
| H × T | Ns | Ns | Ns | Ns | 2.98 * | 3.21 *4 |
| Treatments | Plant Height (cm) | Number of Leaves (cm) | Leaf Biomass Yield (g) | Number of Suckers (piece) | Fresh Gel Weight (g) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Growth Season | ||||||||||
| 1th | 2nd | 1th | 2nd | 1th | 2nd | 1th | 2nd | 1th | 2nd | |
| LV | 34.2 | 41.2 c 1 | 13.9 | 19.8 | 453 | 523 d | 3.92 | 4.99 | 385.05 | 444.55 d |
| HVC-10 | 36.1 | 44.3 c | 14.2 | 20.2 | 450 | 586 c | 4.10 | 5.10 | 382.5 | 498.1 c |
| VC-10 | 30.6 | 37.5 d | 13.8 | 21.0 | 439 | 567 cd | 3.85 | 5.03 | 373.15 | 481.95 cd |
| HVC-30 | 40.0 | 52.8 b | 13.6 | 20.3 | 468 | 518 d | 4.02 | 5.04 | 397.8 | 440.3 d |
| VC-30 | 33.2 | 44.2 c | 14.5 | 21.1 | 460 | 585 c | 4.06 | 5.00 | 391 | 497.25 c |
| HVC-60 | 34.1 | 46.6 c | 14.7 | 19.9 | 448 | 596 c | 3.98 | 5.11 | 380.8 | 506.6 c |
| VC-60 | 36.2 | 44.6 c | 15.0 | 20.0 | 449 | 587 c | 3.97 | 4.98 | 381.65 | 498.95 c |
| HVC-10+LV | 38.1 | 52.2 b | 14.2 | 20.5 | 452 | 717 ab | 4.07 | 4.97 | 384.2 | 609.45 ab |
| VC-10+LV | 39.1 | 51.3 b | 13.9 | 20.8 | 448 | 698 b | 3.99 | 5.02 | 380.8 | 593.3 b |
| HVC-30+LV | 37.6 | 62.1 a | 14.8 | 19.9 | 468 | 751 a | 4.00 | 5.01 | 397.8 | 638.35 a |
| VC-30+LV | 38.0 | 50.5 b | 14.4 | 20.4 | 472 | 587 c | 4.06 | 5.12 | 401.2 | 498.95 c |
| HVC-60+LV | 39.9 | 60.2 a | 14.5 | 20.9 | 459 | 568 cd | 4.11 | 4.99 | 390.15 | 482.8 cd |
| VC-60+LV | 34.9 | 49.5 b | 14.9 | 21.0 | 498 | 617 bc | 4.08 | 5.02 | 423.3 | 524.45 bc |
| rANOVA (LSD %5) | ||||||||||
| Harvest (H) | 2.67 * | NS | 1.12 *3 | 1.67 * | Ns | 1.58 * | 1.92 * | 1.37 * | Ns | 1.46 * |
| Treatment (T) | NS | 5.65 **2 | NS 4 | NS | Ns | 6.27 ** | NS | NS | Ns | 5.89 ** |
| H × T | 1.98 * | 6.62 ** | NS | NS | Ns | Ns | NS | NS | Ns | Ns |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tavali, I.E.; Ok, H. Comparison of Heat-Treated and Unheated Vermicompost on Biological Properties of Calcareous Soil and Aloe Vera Growth under Greenhouse Conditions in a Mediterranean Climate. Agronomy 2022, 12, 2649. https://doi.org/10.3390/agronomy12112649
Tavali IE, Ok H. Comparison of Heat-Treated and Unheated Vermicompost on Biological Properties of Calcareous Soil and Aloe Vera Growth under Greenhouse Conditions in a Mediterranean Climate. Agronomy. 2022; 12(11):2649. https://doi.org/10.3390/agronomy12112649
Chicago/Turabian StyleTavali, Ismail Emrah, and Huseyin Ok. 2022. "Comparison of Heat-Treated and Unheated Vermicompost on Biological Properties of Calcareous Soil and Aloe Vera Growth under Greenhouse Conditions in a Mediterranean Climate" Agronomy 12, no. 11: 2649. https://doi.org/10.3390/agronomy12112649
APA StyleTavali, I. E., & Ok, H. (2022). Comparison of Heat-Treated and Unheated Vermicompost on Biological Properties of Calcareous Soil and Aloe Vera Growth under Greenhouse Conditions in a Mediterranean Climate. Agronomy, 12(11), 2649. https://doi.org/10.3390/agronomy12112649






