Abstract
Determining how intermittently applied heat treatment during the preparation of vermicompost changes the effectiveness of the fertilizer is a challenge. In this study, organic Aloe vera was grown using heat-treated cattle manure vermicompost (IVC) and unheated cattle manure vermicompost (VC). Additionally, these two vermicomposts were combined with vermiwash (LV) and applied to the soil. Thus, the cumulative effect of vermicompost on soil biological properties (number of bacteria, dehydrogenase, urease, alkaline phosphatase, β-glycosidase) and plant growth (plant height, number of leaves, leaf biomass yield, number of suckers, fresh gel weight) was investigated. According to the results obtained, it was understood that HVC-30+LV, HVC-60+LV, and VC-60+LV applications were more effective on soil biological properties. On the other hand, HVC-30+LV and HVC-60+LV applications were found to be effective on plant growth. In addition, increases of 140% in soil bacterial number, 170% in dehydrogenase activity, 125% in urease activity, 122% in alkaline phosphatase activity, 123% in β-glycosidase activity, 65% in plant height, and 45% in leaf biomass yield and wet gel weight were observed. Accordingly, it can be stated that heat-treated cattle manure vermicompost applied to the soil at a rate of 30 t ha−1 together with vermiwash is beneficial for improving the biological properties of calcareous soil and for organic Aloe vera cultivation.
1. Introduction
The use of secondary metabolites from medicinal plants as a natural raw material source in the cosmetic and pharmaceutical industries is becoming increasingly common. One of the medicinal plants from which valuable phytochemicals are obtained is Aloe vera, a plant species in the Asphodelaceae (lily) family. It is known that the plant has much in common with cacti in terms of its affinity for dry climates, small spines on the edges of its leaves, and water storage properties of its leaves [1]. This plant, which thrives in hot and dry regions, is included in many products that serve both the beauty and health sectors due to the gel-like substance within its leaves [2,3,4,5,6].
Medicinal plants have significant commercial value in Turkey as well as in many countries with a Mediterranean climate. These plants are collected from nature, obtained through farming, and sold as raw materials to the relevant industry sectors. It is essential that the production of plants such as A. vera, whose phytochemicals are evaluated in the cosmetic and pharmaceutical industries, should be carried out within a specific plan. The industrial facilities that will process these plant materials want to source the raw material every year in the amounts they need [7]. Only in this way can standardized high-quality products can be produced. For this reason, growing these plants in controlled conditions away from toxic substances, such as pesticide residues, chemical accumulations, and heavy metals, is of great importance [8]. A. vera cultivation, which is common in countries such as Spain, India, the USA, South Africa, Brazil, and Mexico, is a newly developing sector in Turkey. The Aloe barbadensis Miller variety is mainly produced in greenhouse conditions in Turkey [9].
It was reported that ecological factors—such as slope exposure, altitude, and seasonal climate changes—and factors such as plant collection time, storage conditions, drying times, and drying temperatures are effective in obtaining optimum secondary metabolites from medicinal plants [10]. In A. vera cultivation, climate, soil characteristics, agronomic factors (plant sowing frequency, fertilization, irrigation, etc.), and harvest time are among the most important factors affecting plant biomass yield and fresh gel quality [11]. In addition, fertilization has an important place among the agronomic practices that most affect the yield and phytochemical quality in a vegetation period in the cultivation of these plants [12]. The use of organic fertilizers and/or soil conditioners rather than chemical fertilizers in A. vera cultivation is recommended because although chemical fertilizers promote the production of primary metabolites in the plant, they are known to have the effect of diluting secondary metabolites [13].
Biomass emerging from various organic wastes is transformed into a unique material called “vermicompost” via composting without a thermophilic phase using earthworms, which can be used as organic fertilizer for plant production or as a soil conditioner for the improvement of degraded soils [14]. The most important feature that distinguishes this material from other organic fertilizers is its high microbiological activity [15]. During vermicomposting, unique microorganisms in the worm gut enrich the vermicompost microbially by means of “coelomic fluid”. When this material is applied to the soil, the mentioned microorganisms colonize the fresh roots of the plant. They secrete enzymes, plant growth regulators, and antibiotics into the rhizosphere. Thus, they protect the roots against soil pathogens (fungi and nematodes, etc.) and they help the roots to develop and the plant to be nourished [16]. On the other hand, vermicompost is a porous material with low density, high water-holding and anion–cation exchange capacities. Due to these properties, it can retain nutrients, such as carbon, nitrogen, phosphate, calcium, iron, and zinc, by increasing the anionic and cationic activities in the soil [17]. Due to the chelating feature of coelomic liquid, the nutrients it contains are less affected by adverse soil conditions and are also rich in humic substances that increase capillary rooting [18]. For these reasons, vermicompost not only promises an effective/cheap method for waste management in agricultural production but also finds use as a soil conditioner and/or organic fertilizer. On the other hand, vermiwash is a liquid extract obtained from vermicomposting beds and used as liquid fertilizer, foliar spray, and disease control agent [19]. In this study, vermicomposts with different characteristics were applied to the soil alone and in combination with vermiwash under greenhouse conditions in a Mediterranean climate. Thus, the aim was to determine the cumulative effect of vermicompost on the biological properties of calcareous soil and the growth of A. vera.
2. Materials and Methods
2.1. Experimental Site
The study was carried out in a plastic greenhouse located in Alanya, Turkey (36°21′3″ N; 32°15′47″ E, altitude 185 m). The experiment covers September–August in two consecutive production seasons (2019–2020 and 2020–2021), and indoor climate data were recorded between these dates. The highest temperature in the greenhouse in the first year was 49.7 °C, the lowest temperature was 4.3 °C, and the average temperature was 27.0 °C. The highest relative humidity was 77%, the lowest relative humidity was 41%, and the average relative humidity was 61%. The average solar radiation was measured as 6.82 kWh/m2/day. In the second year, the highest temperature was 48.3 °C, the lowest temperature was 2.7 °C, and the average temperature was measured as 25.5 °C. The highest relative humidity was 79%, the lowest relative humidity was 48%, and the average relative humidity was 63.5%. The average solar radiation was measured as 7.02 kWh/m2/day.
2.2. Treatments and Experimental Design
Vermicomposts used as organic fertilizing material in the experiment were obtained from the Turkish company Ekosol INC.; heat-treated vermicompost (hereinafter referred to as HVC) and unheated vermicompost (hereafter VC) were used in the experiment. The heat treatment was carried out by the fertilizer company in a special oven at 70 °C. This process is routinely performed to protect the vermicompost from microbial contamination under factory conditions until it enters the bag. The experiment also aimed to support the HVC and VC throughout the plant cultivation seasons by applying vermiwash (LV) to the soil. The analysis results of the vermicomposts used in the study and the soil on which the experiment was established are shown in Table 1. The application rates of vermicompost to the soil were determined as recommended at 30–60 t ha−1 [20]. In addition, during the growing season, vermiwash was applied to some parcels of the soil via drip fertigation at the rate recommended by the fertilizer company. The study was carried out as a factorial according to the randomized plot design with three replications. Accordingly, vermicompost applications were as follows: Control (vermiwash–LV), HVC 10 t ha−1 (HVC-10), VC 10 t ha−1 (VC-10), HVC 30 t ha−1 (HVC-30), VC 30 t ha−1 (VC-30), HVC 60 t ha−1 (HVC-60), VC 60 t ha−1 (VC-60), HVC 10 t ha−1 + LV (HVC-10+LV), VC 10 t ha−1 + LV (VC-10+LV), HVC 30 t ha−1 + LV (HVC-30+LV), VC 30 t ha−1 + LV (VC-30+LV ), HVC 60 t ha−1 + LV (HVC-60+LV), VC 60 t ha−1 + LV (VC-60+LV).

Table 1.
Characteristics of experiment soil and organic fertilizers.
2.3. Experimental Setup
In the experiment, the suckers of the Aloe barbadensis Miller plant, which were previously obtained, were used as seedling material. To create a seedling-growing medium, a mixture of peat, perlite, and vermiculite was prepared, and the resulting medium was moistened to around 80%. This prepared moist mixture was transferred to viols with 128 holes, and it was ensured that all holes were filled with the mixture. Then, A. vera suckers were placed in viols, one per hole, and watered abundantly. In the following days, the seedlings’ irrigation, fertilization, and other maintenance processes were carried out. In this context, it was ensured that the seedlings were ready in approximately 25 days.
Before the greenhouse experiment was established, soil cultivation and weeding were performed and seedlings were made ready for planting. Then, plots of 6 m2 (1 m width, 6 m length, and approximately 30 cm height) were created by the experiment plan. In order to minimize the interaction of the experiment subjects with each other, approximately 1.5 m gaps were left between the plots. Then, the vermicomposts (according to dry matter calculation) were weighed by adhering to the applications detailed above. Afterwards, the fertilizers were mixed homogeneously on the plots. For two-season cultivation, A. vera seedlings were planted with at least ten plants (60 cm on rows, 60 cm between rows) in each plot, and the experiment period was started by providing water through the drip irrigation system. Plant maintenance procedures (irrigation, disease, pest monitoring, etc.) were carried out from seedling planting to harvest during the vegetation period. In this period, weeds were removed from the plots. During the growing period, drip irrigation was applied to the plants equally in each plot. Since the plant is sensitive to root rot with excessive watering, water was given carefully. For this, irrigation was performed by monitoring the moisture status of the soil, plant appearance (withering signs on the leaves), and solar radiation data (once every 7–10 days in the cold period and once every 2–3 days in the hot period).
2.4. Harvesting
Plant leaves were harvested for the first time 90 days after planting, and a total of 4 harvests were performed per season. Harvest times were determined based on the number of plant leaves and leaf weight. In the harvests, five randomly selected plants were harvested from each replication plot. Harvesting was performed by cutting only the mature leaves that have completed their development 3–5 cm above the soil surface. In each harvest period, soil and plant samples were taken and measurements and analyses were made. After all harvests, sampling, and measurements were completed, the greenhouse experiment was terminated for both seasons. The foreign materials on the plant were removed by pre-cleaning the plant materials obtained during the harvest periods. Then, measurement, weighing, and calculations were performed on the plant samples, and the results were given as the average of the five plants sampled. Accordingly, parameters such as plant height, the number of leaves, leaf biomass yield, the number of suckers, and fresh gel weight were determined.
2.5. Preparation of Analysis
Analyses of the details of the vermicomposts used in the experiment were carried out. Moisture, amount of organic matter, and amount of organic carbon were determined according to the mass lost after burning in the muffle furnace (ILD-MFL) [21]. pH and EC values were determined by measurements made with a pH-EC meter (Consort C1010) in a 1:10 ratio of vermicompost–pure water suspension [22]. Total nitrogen (N) determination was made according to the modified Kjeldahl method (Gerhardt Vapodest 300) [23]. Before the experiment was established, analyses of the details of the soil sample taken from 0–30 cm depth were performed. The air-dried soil was sieved through a 2 mm sieve (10 mesh) and made ready for analysis. The texture of the soil was determined via the hydrometer method (Hamilton Beach-CS) [24]. The lime content of the soil was determined by the calcimeter method (Eijkelkamp C-08.53) [25]. The pH and EC values of the soil were determined with 1/2.5 soil: distilled water mixture (Consort C1010) [22]. The organic matter and organic carbon (C) content of the soil were determined by the wet oxidation method [26]. Total N analysis was performed for vermicompost as described above.
Biological analyses were performed on soil samples from 0–15 cm depth in each harvest period. Soil samples taken for the aforementioned analyses were brought to the laboratory quickly and kept at +4 °C to minimize moisture loss. The biological properties of the soils were determined in moist soil samples, but the results were given for dry soil. Accordingly, the total number of aerobic mesophilic heterotrophic bacteria was determined by the dilution plate method, which is a cultural counting method [27]. Dehydrogenase activity was determined at 546 nm according to the triphenyl tetrazolium chloride (TTC) method [28]. Urease activity was determined at 578 nm according to the ammonium colorimetric method [29]. Alkaline phosphatase activity was determined at 410 nm according to the p-nitrophenyl phosphate (PNP) method [30]. β-glucosidase activity was determined at 410 nm according to the p-nitrophenyl-β-D-glucoside (PNG) method [31]. Numerical data for all enzyme activities were obtained via spectrophotometer (Perkin Elmer Lambda 25 UV-VIS).
2.6. Statistical Analysis
Data obtained from measurements and analyses for soil and plant samples were taken into statistical evaluation using SPSS 21.0 package program. In this context, the importance of numerical data (at the level of 5%) was determined through repeated measurement analysis (rANOVA). Subsequently, the results found to be significant were graded using Duncan’s multiple comparison test. In addition, the relationships between soil biological properties and plant growth parameters were determined using the Pearson correlation test [32].
3. Results
3.1. Number of Bacteria
It was observed that the number of soil bacteria followed a fluctuating course in both growing seasons with vermicompost applications (Figure 1). On the other hand, it was determined that the number of soil bacteria was higher in the second season than in the first. Changes in the number of bacteria values between harvest periods in the first and second seasons were also statistically significant (both p < 0.01 levels).
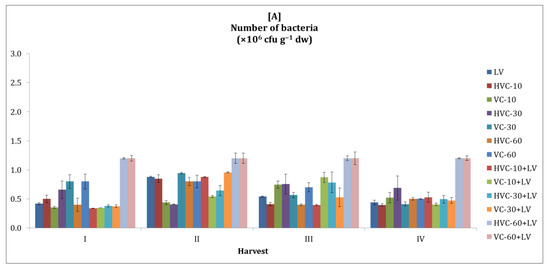
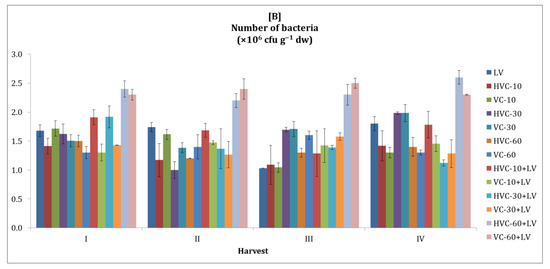
Figure 1.
The effects of applications on the number of bacteria in the soil ((A) first growth season, (B) second growth season). Error bars represent standard errors based on three replicates.
According to the statistical analysis, the effects of the interaction between fertilizer applications and temporal changes on the number of bacteria in the soil in both seasons were found to be statistically significant at the p < 0.05 level (Table 2). In addition, it was determined that the effects of the treatments on the number of bacteria were statistically significant (p < 0.05, p < 0.01, respectively) in both growing seasons. It was determined that HVC-60+LV and VC-60+LV applications (for both seasons, 140% increases were recorded according to the lowest applications) produced the highest values.

Table 2.
Effects of applications on soil biological properties.
3.2. Dehydrogenase
The changes in soil dehydrogenase activity with organic fertilization during the growing seasons are shown in Figure 2. In this context, it was observed that dehydrogenase activity was higher in the second season compared to the first season. According to the results obtained from the statistical analysis, it was determined that the interaction between fertilizer applications and the temporal change was insignificant in both seasons (Table 2). However, it was determined that the effect of fertilizer application was statistically significant and that HVC-30+LV (p < 0.05) in the first season (170% increase was recorded according to the lowest application) and VC-60+LV (p < 0.05) in the second season (59% increase was recorded according to the lowest application) were the applications that increased the dehydrogenase activity the most. In addition, it was determined that the change in enzyme activity between harvest periods in both seasons was statistically significant at the p < 0.01 level.
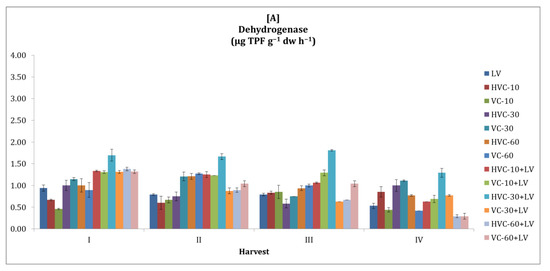
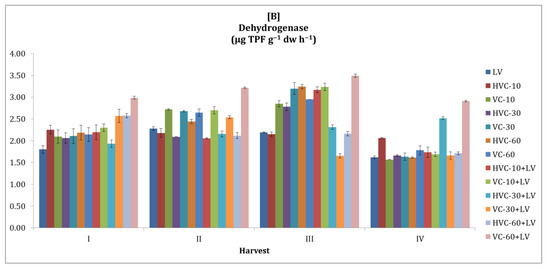
Figure 2.
The effects of the applications on soil dehydrogenase activity ((A) first growth season, (B) second growth season). Error bars represent standard errors based on three replicates.
3.3. Urease
It was observed that urease activity tended to fluctuate in both seasons (Figure 3). In general, it was observed that urease activity was higher in the second season compared to the first season. Statistically, it was determined that the interaction between fertilizer applications and the temporal change in the urease activity of the soil was insignificant. However, the effect of the applications was significant at the level of p < 0.01 (VC-60+LV) in the first season (a 125% increase was recorded according to the lowest application) and at the level of p < 0.001 (HVC-30+LV) in the second season (61% increase was recorded according to the lowest application) (Table 2). In addition, it was determined that the changes in urease activity between sampling periods within the season were statistically significant (p < 0.05 in both seasons).
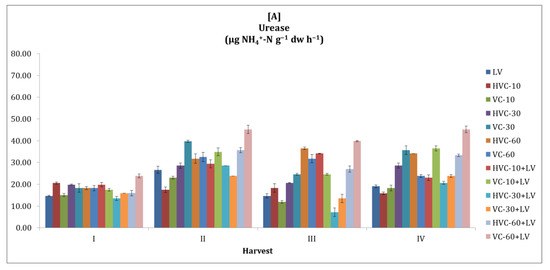
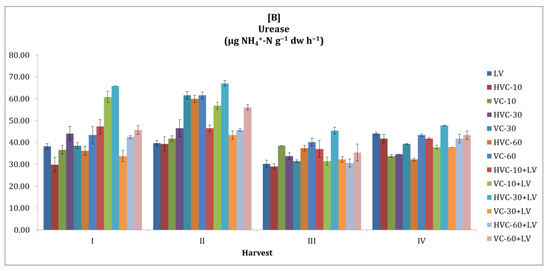
Figure 3.
The effects of the applications on soil urease activity ((A) first growth season, (B) second growth season). Error bars represent standard errors based on three replicates.
3.4. Alkaline Phosphatase
Soil alkaline phosphatase activity was higher in the second season compared to the first season (Figure 4). The effect of the interaction between fertilizer applications and temporal variation on alkaline phosphatase activity of the soil was found to be statistically significant at the p < 0.01 level only in the second season. In terms of the effect of the applications, it was determined that the changes in the second season were significant at the p < 0.05 level and that the HVC-30+LV and VC-60+LV applications were the applications that increased the alkaline phosphatase activity the most (Table 2). In addition, it was determined that the differences between the values obtained during the sampling periods in the second season (a 122% increase was recorded according to the lowest application) were statistically significant (at the levels of p < 0.05 and p < 0.01, respectively).
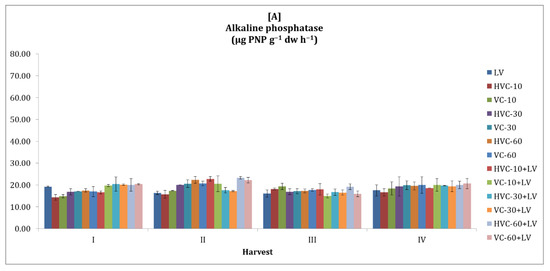
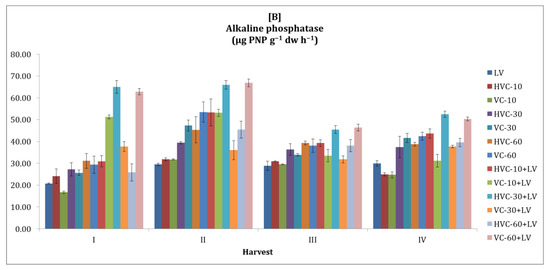
Figure 4.
The effects of the applications on the soil alkaline phosphatase activity ((A) first growth season, (B) second growth season). Error bars represent standard errors based on three replicates.
3.5. β-glycosidase
Similar to other enzyme activities, it was striking that the β-glycosidase activity of the soil was higher in the second season compared to the first season and fluctuated between sampling periods. (Figure 5). However, the effect of the interaction between fertilizer applications and temporal variation on β-glycosidase activity was statistically significant at the p < 0.01 level in both seasons. Considering the average values of the harvest periods of the applications, it was determined that VC-60+LV applications in the first season (a 123% increase was recorded according to the lowest application) and HVC-30+LV applications in the second season (a 97% increase was recorded according to the lowest application) increased the β-glycosidase activity of the soil more than other applications (Table 2). In addition, the effect of temporal change on the β-glycosidase activity of the soil was statistically significant at the level of p < 0.05 in the first season and at the level of p < 0.01 in the second season.
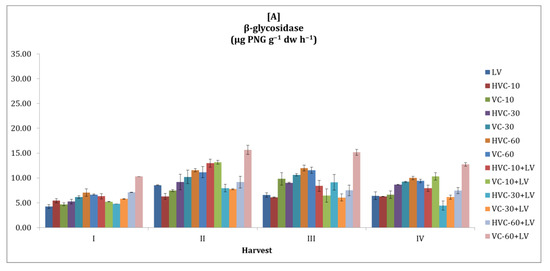
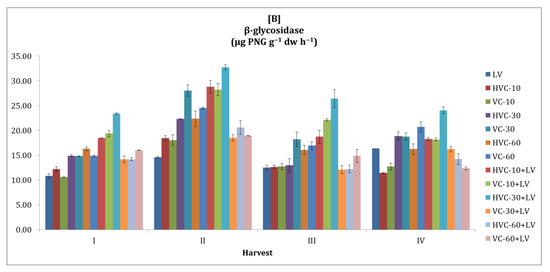
Figure 5.
The effects of the applications on soil β-glycosidase activity ((A) first growth season, (B) second growth season). Error bars represent standard errors based on three replicates.
3.6. Soil Reaction (pH)
The effect of vermicompost applications on soil reaction (pH) was statistically insignificant in both growing seasons. However, a slight increase in soil pH was detected in the first season compared to the beginning. A decreasing trend was detected in the second season (over a 5% decrease was recorded between seasons) (Table 3). On the other hand, temporal changes in pH values were found to be statistically significant at p < 0.05 in both periods.

Table 3.
The effects of the applications on the soil chemical properties.
3.7. Soil Electrical Conductivity (EC)
When the effects of the applications on the electrical conductivity (EC) of the soil were examined, it was determined that the changes in the values obtained were statistically insignificant in both seasons (Table 3). However, in terms of temporal variation, the differences in EC values were found to be statistically significant at the level of p < 0.05 in the first season and at the level of p < 0.01 in the second season. Moreover, it was determined that there was a slight increase in the EC of the soil in the second season compared to the first season (over a 65% increase was recorded between seasons).
3.8. Soil Organic C
It was determined that the effect of vermicompost applications on the organic C content of the soil was statistically significant at the p < 0.001 level in the second season (Table 3). Accordingly, in the second season, the highest organic C values were obtained with HVC-30+LV and VC-60+LV applications (over a 20% increase was recorded according to the lowest application). In addition, it was determined that the temporal changes of organic C values were statistically significant at the p < 0.01 level in both seasons.
3.9. Plant Growth
The effects of vermicompost applications on plant height, number of leaves, leaf biomass yield, number of suckers, and fresh gel weight of A. vera plant due to temporal variation are given in Table 4. As observed in all parameters examined, it was determined that higher plant values were obtained in the second season in direct proportion to the soil parameters. According to the results of the statistical analysis, the effect of the interaction between the treatments and the temporal changes on the plant height was found to be significant at the p < 0.01 level in both seasons (Table 4). However, the effect of applications on plant height was found to be statistically significant at the p < 0.01 level only in the second season. At this point, the highest value was determined in HVC-30+LV and HVC-60+LV applications (a 65% increase was recorded according to the lowest application). It was determined that the effects of applications on leaf biomass yield and gel weight were similarly significant at the level of p < 0.01 in the second season. The highest values for leaf biomass yield and gel weight parameters were obtained in HVC-30+LV application in the second season (45% increases were recorded according to the lowest applications). On the other hand, the effects of applications on the number of leaves and number of suckers were found to be statistically insignificant in both seasons. However, the changes between the values obtained between the sampling periods, valid for both parameters, were found to be statistically significant at the p < 0.05 level in both seasons.

Table 4.
Effects of the applications on the A. vera growth.
The correlations between the biological properties of the soil and the parameters related to the growth of A. vera were analyzed. According to this, positive correlations were found between soil urease activity and plant leaf biomass yield and soil urease activity and plant height (r = 0.531 and r = 0.456, respectively; p < 0.001). In addition, positive correlations were determined between the number of bacteria in the soil and the gel yield of the plant and between the soil’s β-glycosidase activity and the plant’s gel yield (r = 0.542 and r = 0.466, p < 0.001, respectively).
4. Discussion
Soil microorganisms play an active role in many chemical and physical changes that occur in the soil. They are essential elements of soil fertility, especially since they are involved in the cycle of nutrients such as carbon, nitrogen, phosphorus, and sulfur [33]. Organic matter is the leading food and energy source of these heterotrophic microorganisms, which play a critical role in nutrient cycles. Organic fertilization is known to increase the microbial number and activity in the soil [34]. Accordingly, the experiment soil is insufficient for optimal microbial diversity, number and activity since the fact that the humus is poor (1.23%). However, the vermicompost used is a good source of organic matter (HVC: 30%, VC: 35%) and the vermiwash has a high number of bacteria (4.9 × 107 cfu g−1 dw), the bacteria in the soil may have increased/been stimulated. The number of bacteria present in the soil is an indicator that can be used the state of soil fertility. Adding organic matter to the soil affects this parameter very positively. It is reported that the soil’s microbial number, biomass, and diversity increase with applying organic fertilizing materials such as vermicompost [35,36].
By measuring dehydrogenase activity, it is possible to obtain important data concerning agricultural soil quality. Enzyme activity is considered a pollution indicator since it is increased by the presence of toxic mineral nutrient enrichment in the soil [37]. It was reported that minerals (nitrate, nitrite, iron, zinc, manganese, and copper) whose concentrations increase rapidly in the soil solution and reach toxic levels for microorganisms inhibit dehydrogenase [28]. In the experiment, it was observed that vermicompost applications increased dehydrogenase activity similarly in both seasons. According to the results of the analysis, it can be seen that the experimental soil is insufficient in terms of many chemical and biological properties (Table 1). In this context, it is thought that the HVC (17%) and VC (20%) applied to the soil are good sources of carbon and that the high dehydrogenase activity of the LV (96 µg TPF g−1 dw h−1) combined with them are closely related to the result obtained.
Soil organic matter and nitrogen content are among the most critical factors affecting urease activity [38]. Organic fertilization is a primary agricultural practice that increases soil organic matter and nitrogen content. Since urease is an enzyme secreted out of the cell by microorganisms and hydrolyzes organic nitrogen, it is directly affected by increased organic matter in the soil [39]. The experimental soil’s organic matter content (1.23%) and total N (0.079%) content were relatively low. Accordingly, it may be reasonable to attribute the increase in urease activity to the nitrogen content of the fertilizers (VC: 1.2%, HVC: 1.5%, LV: 1%) used. It was reported that the increase in urease activity with the addition of organic fertilizer to the soil is mainly related to the fertilizer’s organic matter content and nitrogen content and that the nitrogen in the fertilizer acts as a substrate for the urease enzyme [40,41].
Phosphatase is an enzyme closely related to the soil’s organic-bound phosphorus content [42,43]. Although the amount of organic matter (1.23%) in the experimental soil is low, the amount of lime (16%) is high. The pH (7.3) is neutral–slightly alkaline; regular vermicompost applications have a positive effect on soil organic matter and are responsible for the increase in alkaline phosphatase activity that is thought to be. It was determined that vermicomposts used in this study were suitable materials in terms of organic matter content (HVC: 30%, VC: 30%) and that the alkaline phosphatase activity (283 µg PNP dw h−1) of vermiwash was high. In addition, it can be stated that the partial alkalinization that occurs depending on the pH (HVC: 7.4, VC: 7.6, LV: 8.5) of the fertilizers applied to the soil is indirectly responsible for the increase in enzyme activity. Similarly, it was reported that increased organic phosphorus content in calcareous soil with vermicompost application has a stimulating effect on microorganisms and, accordingly, increases alkaline phosphatase activity [44].
Similarly to other soil enzymes, the activity of the β-glycosidase enzyme is in close relationship with soil properties such as organic matter content, pH, temperature, humidity, microbial activity, and especially the composition of the organic matter source given to the soil [45]. Since β-glucosidase is an extracellular secretion synthesized by soil microorganisms to convert a complex organic compound such as cellulose into glucose, it is highly affected by organic matter added to the soil [46]. In the experiment, it was understood that the vermicomposts applied to carbon-poor soil (0.71%) were at reasonable levels in terms of carbon content (HVC: 17%, VC: 20%) and C:N ratios (HVC: 12:1, VC: 16:1). It is thought that β-glycosidase activity is increased because factors such as the amount of organic carbon and the C:N ratio are known to directly affect the stimulation of microorganisms and the determination of the microbial decomposition rate [47]. In addition, the high β-glycosidase activity (134 µg PNG dw h−1) of vermiwash applied to the soil can be considered a secondary factor affecting this situation’s emergence. On the other hand, it is thought that carbon sequestration in the soil increased with the regular application of vermicompost in both growing seasons. Therefore, there was a general increase in enzyme activity in the second season compared to the first. It was reported that in the case of regular application of organic fertilizers and/or soil conditioners to the soil, carbon sequestration increases in the medium and long term, and accordingly, soil fertility and quality are positively affected [48,49].
According to the widely known view, the respiration of microorganisms and, thus, the carbon dioxide concentration, increases in soil enriched with carbon with the application of organic fertilizers. Afterwards, the amount of carbonic acid increases depending on the amount of carbon dioxide that reacts with hydrogen in the soil solution, and thus, the soil pH decreases [50]. However, it is known that soil pH may increase contrary to expectations, depending on parameters such as the pH of the organic fertilizer added to the soil’s alkaline and whether the carbon content is high and in a composition resistant to decomposition. It was reported that some farm manures, such as cattle manure, increase soil pH in the short term, depending on the feed and litter (hay, etc.) fed to the animal [51].
Since farm manures are resistant to microbial decomposition, they do not cause rapid nutrient enrichment and salinity in the soil [52]. Although the soil EC value increased in the second season compared to the first, this increase did not create salinity in the soil to threaten plant growth (1437 µS cm−1). The most important factor affecting the decomposition rate of the vermicompost used in the experiment is the C:N ratio. Accordingly, rapid nutrient enrichment and salinization in the soil may have been suppressed due to the C:N ratios (HVC: 12:1, VC: 16:1) of vermicompost obtained from cattle manure not being suitable. In addition, the fact that these fertilizers probably contain carbon components (hemicellulose, cellulose, etc.) that are resistant to microbial decomposition is thought to be a factor that slows down the rate of decomposition and prevents the emergence of salinity in the soil. On the other hand, it can be stated that the increased nutritional need due to the development of the plant in the second season and the increase in the number of mineral nutrients that the plant exploits from the soil is also a suppressive factor on salinity.
When the growing seasons were compared, it was thought that the organic C values obtained in the second season were significantly higher than in the first season. When the cumulative effects of vermicompost applications were considered, this fertilizer supported carbon sequestration in the soil in regular use. It is known that farm manures and many other organic fertilizers have a long-term effect on the soil and increase carbon sequestration in the soil in regular use [53,54]. In this way, it is stated that the amount of stable organic matter in the soil increases, the stability of the aggregate increases, the structure improves, the microbial activity increases, the colloidal surface area increases, the amount of available water increases, and the nutrient availability increases so that soil degradation in agricultural areas can be minimized.
It is widely known that solid and liquid organic fertilizers can be used successfully in plant growth in organic agriculture. However, the performance of vermicompost in A. vera cultivation is much less known. The data obtained showed that the applications were not only effective on the biological and chemical properties of the soil but also had significant effects on the yield and quality of the plant. Two issues are noteworthy at this point. The first is that vermicompost applications combined with liquid form are more effective than applications in solid form alone. Despite the slow mineralization of vermicomposts, vermiwash enriched with mineral nutrients seems to have a faster effect on soil and plants. In this way, it is understood that the effectiveness of fertilizers supplemented with the liquid form on soil biological properties and plant growth increases more. On the other hand, the second noteworthy issue is that HVC provides better results on plant growth than VC. At this point, it is thought that the heat treatment application causes partial carbonization and increases the dry matter content of the fertilizer. Thus, the material becomes more chemically stable and is somewhat enriched with mineral nutrients.
5. Conclusions
Although it is more critical in the organic farming model, no matter which production model is applied in medicinal plant cultivation, production without artificial chemicals is required. Because the increased vegetative development of the plant with the intensive application of chemicals affects the secondary metabolites negatively, the market value of the plant decreases considerably. For this reason, the use of organic fertilizers in the cultivation of medicinal plants within the scope of organic agriculture comes to the fore. However, it is essential that the organic fertilizer is chosen to regulate its effects on the soil where the plant will be grown and that it supports plant growth at a sufficient level. In the study carried out with this approach, two vermicomposts of different characteristics (heat-treated and unheated) were applied to the soil in increasing proportions alone and combined with vermiwash to cultivate A. vera in two consecutive seasons. Thus, the cumulative effect of vermicompost on soil number of bacteria, dehydrogenase, urease, alkaline phosphatase, β-glycosidase and plant height, number of leaves, leaf biomass yield, number of suckers, and fresh gel weight was investigated. According to the results obtained, it was understood that HVC-30+LV, HVC-60+LV, and VC-60+LV applications were more effective on soil biological properties On the other hand, HVC-30+LV and HVC-60+LV applications effectively affect plant growth. In addition, it was determined that there was a 140% increase in soil bacterial number, 170% in dehydrogenase activity, 125% in urease activity, 122% in alkaline phosphatase activity, 123% in β-glycosidase activity, 65% in plant height, and 45% in leaf biomass yield and wet gel weight. Accordingly, it can be stated that heat-treated cattle manure vermicompost applied to the soil at a rate of 30 t ha−1 together with vermiwash is beneficial for improving the biological properties of calcareous soil and for organic Aloe vera cultivation. At this point, it is thought that there is a need for longer-term studies in which factors such as climate, soil, and cultivation model vary to increase the reliability of the results and to determine the effectiveness of vermicomposts with different properties in medicinal plant cultivation.
Author Contributions
Conceptualization, I.E.T. and H.O.; methodology, I.E.T. and H.O.; software, I.E.T. and H.O.; validation, I.E.T. and H.O.; formal analysis, I.E.T. and H.O.; investigation, I.E.T. and H.O.; resources, I.E.T. and H.O.; data curation, I.E.T. and H.O.; writing—original draft preparation, I.E.T. and H.O.; writing—review and editing, I.E.T. and H.O.; visualization, I.E.T. and H.O.; supervision, I.E.T. and H.O.; project administration, I.E.T. and H.O.; funding acquisition, I.E.T. and H.O. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Scientific Research Projects Coordination Unit of Alanya Alaaddin Keykubat University (grant number 2020-13-02-AAP01).
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Griffiths, H.; Males, J. Succulent plants. Curr. Biol. 2017, 27, R890–R896. [Google Scholar] [CrossRef]
- Ahlawat, K.S.; Khatkar, B.S. Processing, food applications and safety of aloe vera products: A review. J. Food Sci. Technol. 2011, 48, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Hekmatpou, D.; Mehrabi, F.; Rahzani, K.; Aminiyan, A. The effect of Aloe vera clinical trials on prevention and healing of skin wound: A Systematic Review. Iran. J. Med. Sci. 2019, 44, 1–9. [Google Scholar] [PubMed]
- Surjushe, A.; Vasani, R.; Saple, D.G. Aloe vera: A short review. Indian J. Dermatol. 2008, 53, 163–166. [Google Scholar] [CrossRef] [PubMed]
- Radha, M.H.; Laxmipriya, N.P. Evaluation of biological properties and clinical effectiveness of Aloe vera: A Systematic Review. J. Tradit. Complement. Med. 2015, 5, 21–26. [Google Scholar] [CrossRef]
- Hendrawati, T.R.; Ambarwati, H.; Susanty, R.A.N.; Hasyim, U.H. The effects of Aloe Vera gel addition on the effectiveness of sunscreen lotion. J. Rek. Pros. 2020, 14, 101–107. [Google Scholar] [CrossRef]
- Ramachandra, C.T.; Srinivasa, R.P. Processing of Aloe Vera leaf gel: A review. Am. J. Agric. Biol. Sci. 2008, 3, 502–510. [Google Scholar] [CrossRef]
- Eshun, K.; Qian, H. Aloe vera: A valuable ingredient for the food, pharmaceutical and cosmetic industries—A review. Crit. Rev. Food Sci. Nutr. 2004, 44, 91–96. [Google Scholar] [CrossRef]
- Baydar, H. Tıbbi ve Aromatik Bitkiler Bilimi ve Teknolojisi. In Medical and Aromatic Plants Science and Technology, 9th ed.; Nobel Akademik Yayıncılık: Ankara, Turkey, 2021; pp. 125–133. [Google Scholar]
- Li, Y.; Dexin Konga, D.; Michael, Y.F.; HongWu, R.S. The effect of developmental and environmental factors on secondary metabolites in medicinal plants. Plant Physiol. Biochem. 2020, 148, 80–89. [Google Scholar] [CrossRef]
- Cristiano, G.; Murillo-Amador, B.; De Lucia, B. Propagation techniques and agronomic requirements for the cultivation of Barbados Aloe (Aloe vera (L.) Burm. F.)—A review. Front. Plant Sci. 2016, 23, 1410. [Google Scholar] [CrossRef]
- Cardarelli, M.; Rouphael, Y.; Rea, E.; Lucini, L.; Pellizzoni, M.; Colla, G. Effects of fertilization, arbuscular mycorrhiza, and salinity on growth, yield, and bioactive compounds of two Aloe species. HortScience 2013, 48, 568–575. [Google Scholar] [CrossRef]
- Ibrahim, M.H.; Jaafar, H.Z.; Karimi, E.; Ghasemzadeh, A. Primary, secondary metabolites, photosynthetic capacity and antioxidant activity of the Malaysian Herb Kacip Fatimah (Labisia Pumila Benth) exposed to potassium fertilization under greenhouse conditions. Int. J. Mol. Sci. 2012, 13, 15321–15342. [Google Scholar] [CrossRef] [PubMed]
- Torun Kayabaşı, E.; Yılmaz, O. The importance of vermicompost in agricultural production and economy. Eurasian J. Agric. Res. 2021, 5, 146–159. [Google Scholar]
- Pathma, J.; Sakthivel, N. Microbial diversity of vermicompost bacteria that exhibit useful agricultural traits and waste management potential. Springerplus 2012, 4, 26. [Google Scholar] [CrossRef] [PubMed]
- Gudeta, K.; Julka, J.M.; Kumar, A.; Bhagatd, A.; Kumari, A. Vermiwash: An agent of disease and pest control in soil, a review. Heliyon 2021, 7, e06434. [Google Scholar] [CrossRef]
- Hoque, T.S.; Hasan, A.K.; Hasan, M.A.; Nahar, N.; Dey, D.K.; Mia, S.; Solaiman, Z.M.; Kader, M.A. Nutrient release from vermicompost under anaerobic conditions in two contrasting soils of Bangladesh and its effect on wetland rice crop. Agriculture 2022, 12, 376. [Google Scholar] [CrossRef]
- Hemati, A.; Alikhani, H.A.; Ajdanian, L.; Babaei, M.; Asgari Lajayer, B.; van Hullebusch, E.D. Effect of different enriched vermicomposts, humic acid extract and indole-3-acetic acid amendments on the growth of Brassica napus. Plants 2022, 11, 227. [Google Scholar] [CrossRef]
- Blouin, M.; Barrere, J.; Meyer, N.; Lartigue, S.; Barot, S.; Mathieu, J. Vermicompost significantly affects plant growth. A meta-analysis. Agron. Sustain. Dev. 2019, 39, 34. [Google Scholar] [CrossRef]
- Murillo-Amador, B.; Luna-Cisneros, M.J.; Nieto-Garibay, A.; Aguilar-García, M.; Troyo-Diéguez, E.; García-Hernández, J.L. Manual Parala Producciónde Sábilaenel Valledel Carrizal; Centro de Investigaciones Biológicas del Noroeste: La Paz, Mexico, 2007; pp. 52–62. [Google Scholar]
- Kacar, B. Gübre Analizleri [Analyzes of Fertilizer]; Ankara Üniversitesi Ziraat Fakültesi Eğitim, Araştırma ve Geliştirme Vakfı Yayınları: Ankara, Turkey, 1990; pp. 53–64. [Google Scholar]
- Jackson, M.L. Soil Chemical Analysis; Prentice Hall of India Private Limited: New Delhi, India, 1967; p. 498. [Google Scholar]
- Kacar, B. Bitki ve Toprağın Kimyasal Analizleri [Chemical Analyses of Plant and Soil]; Ankara Üniversitesi Ziraat Fakültesi Eğitim, Araştırma ve Geliştirme Vakfı Yayınları: Ankara, Turkey, 1995; p. 466. [Google Scholar]
- Bouyoucos, G.J. A recalibration of hydrometer method for making mechanical analysis of soils. Agron. J. 1951, 43, 434–438. [Google Scholar] [CrossRef]
- Çağlar, K.O. Toprak Bilgisi [Soil Science]; Ankara Üniversitesi Ziraat Fakültesi Yayınları: Ankara, Turkey, 1949; p. 268. [Google Scholar]
- Black, C.A. Methods of Soil Analysis Part 2: Chemical and Microbiological Properties; Soil Science Society of America Inc.: Madison, WI, USA, 1965; pp. 961–983. [Google Scholar]
- Parkinson, D.; Gray, T.R.C.; Williams, S.T. International Biological Programme Handbook 19: Methods for Studying the Ecology of Soil Microorganisms, 1st ed.; Blackwell Scientific Publications: Oxford, UK, 1971; p. 116. [Google Scholar]
- Thalmann, A. Dehydrogenase activity in soil. In Methods in Applied Soil Microbiology and Biochemistry, 1st ed.; Alef, K., Nannipieri, P., Eds.; Academic Press: San Diego, CA, USA, 1995; pp. 321–325. [Google Scholar]
- Hoffman, G.; Teicher, K. Ein kolorimetrisches verfabren zur bestimmung der urease aktivitat in boden [A colorimetric method to determine urease activity in soil]. Z Pflanzenernährung Düngung Bodenkunde 1961, 95, 55–63. [Google Scholar]
- Tabatabai, M.A.; Bremmer, J.M. Use of p-nitrophely phosphate for assay of soil phosphatase activity. Soil Biol. Biochem. 1969, 1, 301–307. [Google Scholar] [CrossRef]
- Eivazi, F.; Tabatabi, M.A. Assay of the β-glucosidase activity. In Methods in Applied Soil Microbiology and Biochemistry, 1st ed.; Alef, K., Nannipieri, P., Eds.; Academic Press: San Diego, CA, USA, 1995; pp. 350–351. [Google Scholar]
- SPSS, version 21.0; IBM: Chicago, IL, USA, 2008.
- Jacoby, R.; Peukert, M.; Succurro, A.; Koprivova, A.; Kopriva, S. The role of soil microorganisms in plant mineral nutrition-current knowledge and future directions. Front. Plant Sci. 2017, 19, 1617. [Google Scholar] [CrossRef] [PubMed]
- Lazcano, C.; Zhu-Barker, X.; Decock, C. Effects of organic fertilizers on the soil microorganisms responsible for N2O emissions: A Review. Microorganisms 2021, 9, 983. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.L.; Wu, T.Y.; Lima, P.N.; Shak, K.P.Y. The use of vermicompost in organic farming: Overview, effects on soil and economics. J. Sci. Food Agric. 2015, 95, 1143–1156. [Google Scholar] [CrossRef] [PubMed]
- Lv, M.; Li, J.; Zhang, W.; Zhou, B.; Dai, J.; Zhang, C. Microbial activity was greater in soils added with herb residue vermicompost than chemical fertilizer. Soil Ecol. Lett. 2020, 2, 209–219. [Google Scholar] [CrossRef]
- Kaczyńska, G.; Borowik, A.; Wyszkowska, J. Soil dehydrogenases as an indicator of contamination of the environment with petroleum products. Water Air Soil Pollut. 2015, 226, 372. [Google Scholar] [CrossRef]
- Liu, C.; Song, Y.; Dong, X.; Wang, X.; Ma, X.; Zhao, G.; Zang, S. Soil enzyme activities and their relationships with soil C, N, and P in peatlands from different types of permafrost regions, Northeast China. Front. Environ. Sci. 2021, 9, 670769. [Google Scholar] [CrossRef]
- Wu, J.; Wang, H.; Li, G.; Ma, W.; Wu, J.; Gong, Y.; Xu, G. Vegetation degradation impacts soil nutrients and enzyme activities in wet meadow on the Qinghai-Tibet Plateau. Sci. Rep. 2020, 4, 21271. [Google Scholar] [CrossRef]
- Roscoe, R.; Vasconcellos, C.; Furtini-Neto, A.G.; Guedes, A.A.; Fernandes, L.A. Urease activity and its relation to soil organic matter, microbial biomass nitrogen and urea-nitrogen assimilation by maize in a Brazilian Oxisol under no-tillage and tillage systems. Biol. Fertil. Soils 2000, 32, 52–59. [Google Scholar] [CrossRef]
- Shang, L.; Wan, L.; Zhou, X.; Li, S.; Li, X. Effects of organic fertilizer on soil nutrient status, enzyme activity, and bacterial community diversity in Leymus chinensis steppe in Inner Mongolia, China. PLoS ONE 2020, 15, e0240559. [Google Scholar] [CrossRef]
- Lemanowicz, J.; Bartkowiak, A.; Breza-Boruta, B. Changes in phosphorus content, phosphatase activity and some physicochemical and microbiological parameters of soil within the range of impact of illegal dumping sites in Bydgoszcz (Poland). Environ. Earth Sci. 2016, 75, 510. [Google Scholar] [CrossRef]
- Touhami, D.; McDowell, R.W.; Condron, L.M. Role of organic anions and phosphatase enzymes in phosphorus acquisition in the rhizospheres of legumes and grasses grown in a low phosphorus pasture soil. Plants 2020, 9, 1185. [Google Scholar] [CrossRef] [PubMed]
- Vinotha, S.P.; Parthasarathi, K.; Ranganathan, L.S. Enhanced phosphatase activity in earthworm casts is more of microbial origin. Curr. Sci. 2000, 79, 1158–1159. [Google Scholar]
- Tiwari, R.; Dwivedi, B.S.; Sharma, Y.M.; Sharma, A.; Dwivedi, A.K. Activities of β-glucosidase, phosphatase and dehydrogenase as soil quality indicators: A review. Int. J. Curr. Microbiol. Appl. Sci. 2019, 8, 834–846. [Google Scholar] [CrossRef]
- Srivastava, S.; Love-Nichols, J.A.; Dies, K.A.; Ledbetter, D.H.; Martin, C.L.; Chung, W.K.; Firth, H.V.; Frazier, T.; Hansen, R.L.; Prock, L.; et al. Meta-analysis and multidisciplinary consensus statement: Exome sequencing is a first-tier clinical diagnostic test for individuals with neurodevelopmental disorders. Genet. Med. 2019, 21, 2413–2421. [Google Scholar] [CrossRef]
- Liang, X.; Yuan, J.; Yang, E.; Meng, J. Responses of soil organic carbon decomposition and microbial community to the addition of plant residues with different C:N ratio. Eur. J. Soil Biol. 2017, 82, 50–55. [Google Scholar] [CrossRef]
- Gross, A.; Bromm, T.; Glaser, B. Soil organic carbon sequestration after biochar application: A Global Meta-Analysis. Agronomy 2021, 11, 2474. [Google Scholar] [CrossRef]
- Allam, M.; Radicetti, E.; Quintarelli, V.; Petroselli, V.; Marinari, S.; Mancinelli, R. Influence of organic and mineral fertilizers on soil organic carbon and crop productivity under different tillage systems: A Meta-Analysis. Agriculture 2022, 12, 464. [Google Scholar] [CrossRef]
- Goulding, K.W. Soil acidification and the importance of liming agricultural soils with particular reference to the United Kingdom. Soil Use Manag. 2016, 32, 390–399. [Google Scholar] [CrossRef]
- Whalen, J.K.; Chang, C.; Clayton, G.W.; Carefoot, J.P. Cattle manure amendments can increase the pH of acid soils. Soil Sci. Soc. Am. J. 2000, 64, 962–966. [Google Scholar] [CrossRef]
- Diacono, M.; Montemurro, F. Long-term effects of organic amendments on soil fertility: A review. Agron. Sustain. Dev. 2010, 30, 401–422. [Google Scholar] [CrossRef]
- Mandal, B.; Majumder, B.; Bandyopadhyay, P.K.; Hazra, G.C.; Gangopadhyay, A.; Samantaray, R.N.; Mishra, A.K.; Chaudhury, J.; Saha, M.N.; Kundu, S. The potential of cropping systems and soil amendments for carbon sequestration in soils under long-term experiments in subtropical India. Glob. Change Biol. 2007, 13, 357–369. [Google Scholar] [CrossRef]
- Liu, E.; Yan, C.; Mei, X.; Zhang, Y.; Fan, T. Long-term effect of manure and fertilizer on soil organic carbon pools in dryland farming in northwest China. PLoS ONE 2013, 8, e56536. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).