Evaluation of the Effects of Returning Apple Shoots In Situ on Soil Quality in an Apple Orchard
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Study Materials and Research Design
2.3. Assay Method
2.3.1. Shoots Nutrient Content, Soil Fertility and the Content of Exchangeable Cations
2.3.2. Soil Neutral Sugar Contents
2.3.3. Soil Water-Soluble Total Free Amino Acid Contents
2.4. Data and Statistical Analysis
3. Results
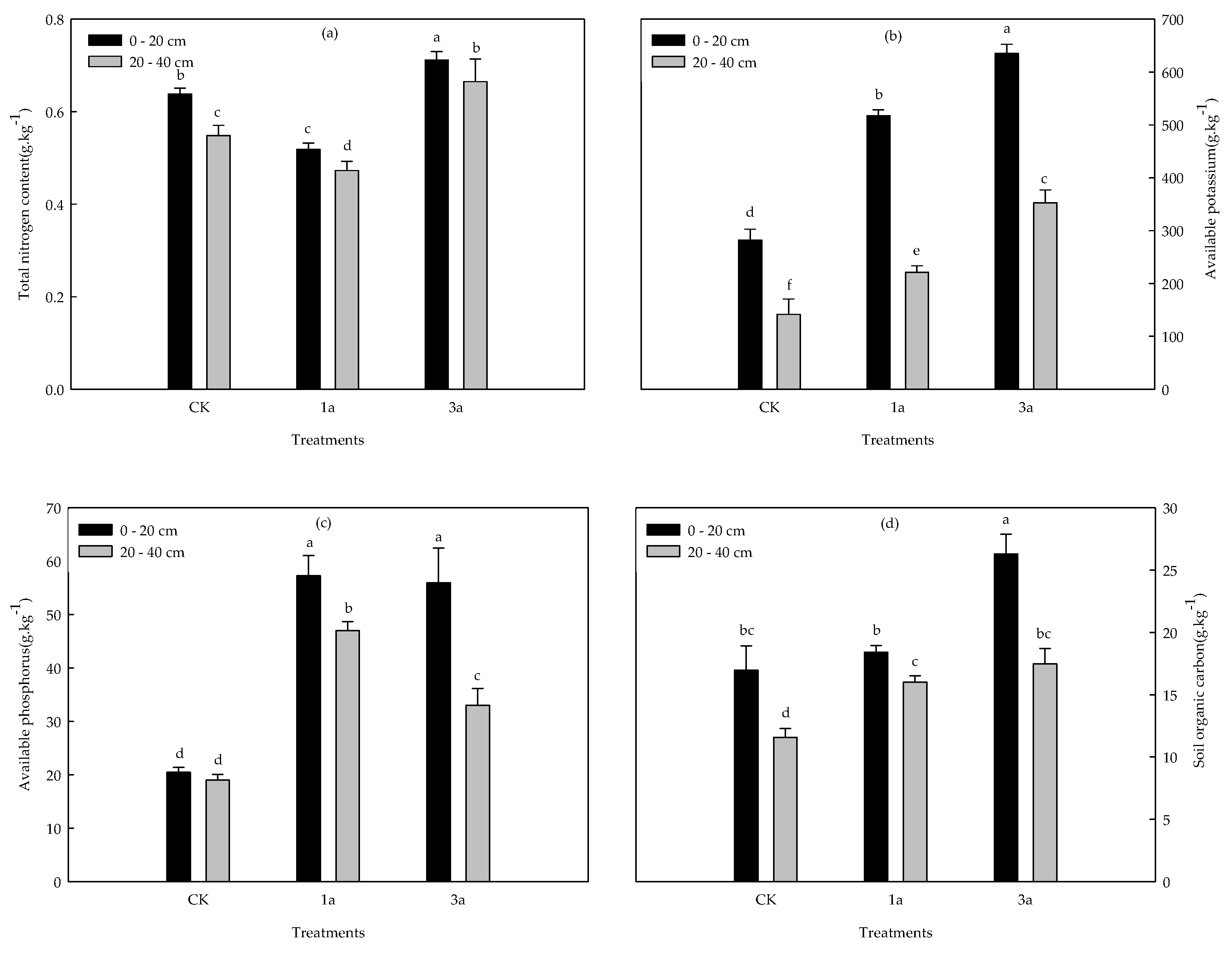
3.1. Variability of Soil Nutrient Properties
3.2. Variability of Soil Exchangeable Cations
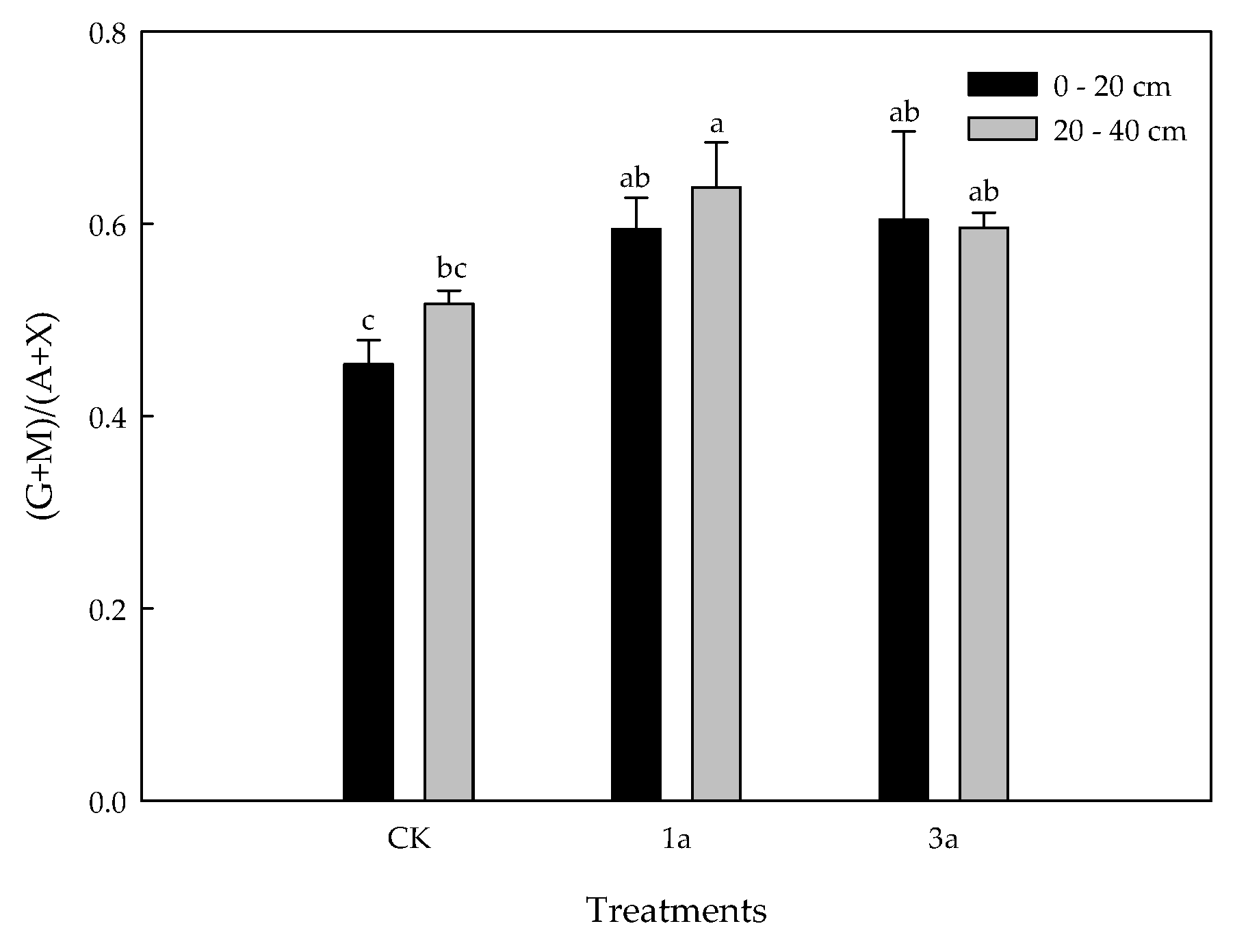
3.3. Variability of Soil Neutral Sugars
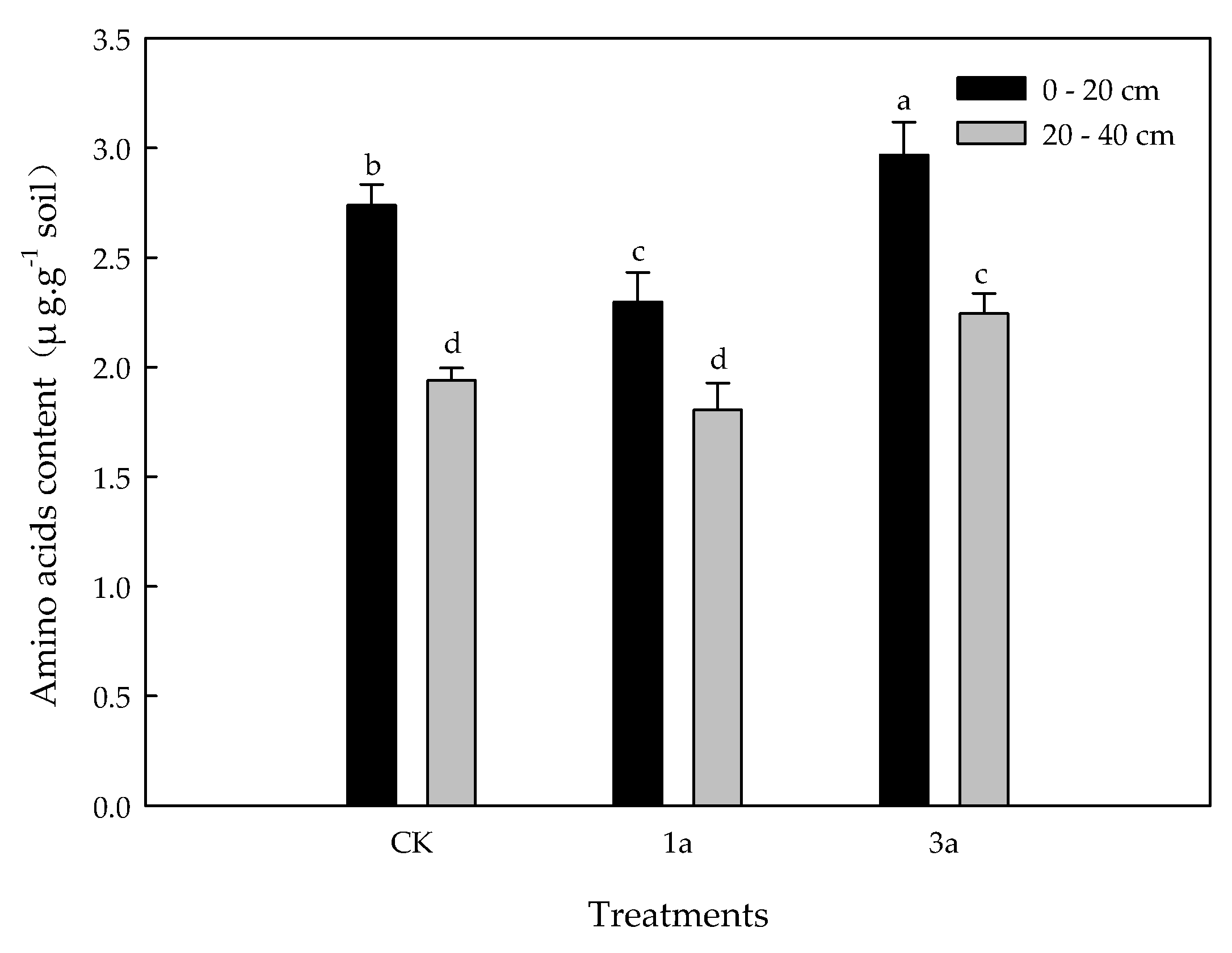
3.4. Variability of the Total Water-Soluble Free Amino Acids Content in the Orchard Soil
3.5. Correlation Coefficients between Various Soil Nutrient Indices
3.6. Soil Quality Index (SQI) Parameter
3.6.1. Weight, Eigenvalue and Contribution Rates of the Evaluation Index
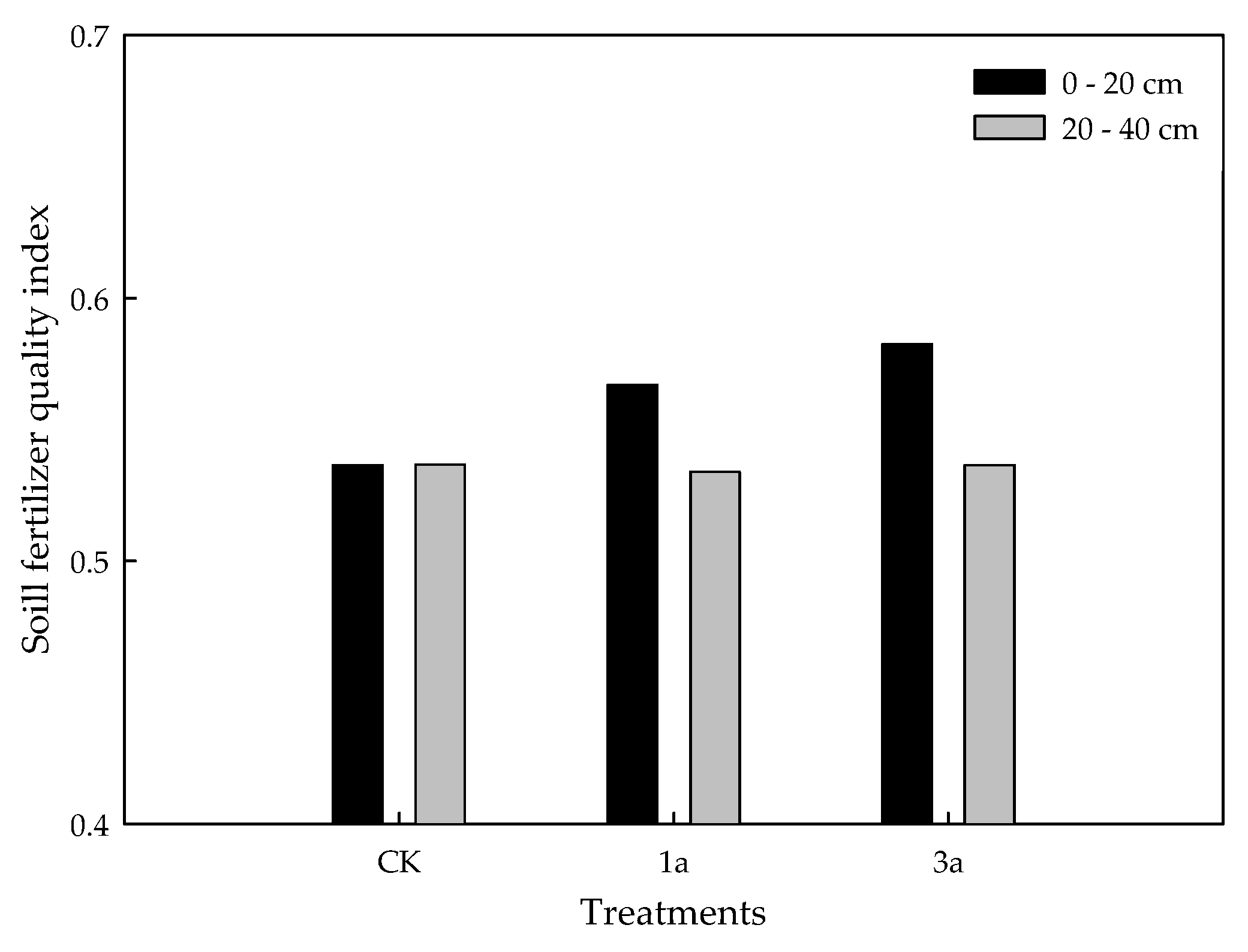
3.6.2. Soil Quality Comprehensive Index
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Doran, J.W.; Parkin, T.B. Defining and assessing soil quality. In Defining Soil Quality for A Sustainable Environment; Soil Science Society of America: Madison, WI, USA, 1994; Volume 35, pp. 1–21. [Google Scholar]
- Sharma, K.L.; Mandal, U.K.; Srinivas, K.; Vittal, K.P.R.; Mandal, B.; Grace, J.K.; Ramesh, V. Long-term soil management effects on crop yields and soil quality in a dryland Alfisol. Soil Tillage Res. 2005, 83, 246–259. [Google Scholar] [CrossRef]
- Carter, M.R.; Gregorich, E.G.; Anderson, D.W.; Doran, J.W.; Janzen, H.H.; Pierce, F.J. Concepts of soil quality and their significance. Dev. Soil Sci. 1997, 25, 1–19. [Google Scholar]
- Lal, R. Soil erosion and land degradation: The global risks. Adv. Soil Sci. 1990, 11, 129–172. [Google Scholar]
- Wang, Y.X.; Weng, B.Q.; Xing, S.H.; Huang, Y.B. Advance in soil organic carbon stock and the impact factors on orchard ecosystem research. Fujian J. Agric. Sci. 2011, 26, 1113–1122. [Google Scholar]
- Emmerling, C.; Schloter, M.; Hartmann, A.; Kandeler, E. Functional diversity of soil organisms—A review of recent research activities in Germany. J. Plant Nutr. Soil Sci. 2002, 165, 408–420. [Google Scholar] [CrossRef]
- Singh, G.; Jalota, S.K.; Singh, Y. Manuring and residue management effects on physical properties of a soil under the rice—Wheat system in Punjab, India. Soil Tillage Res. 2007, 94, 229–238. [Google Scholar] [CrossRef]
- Shao, J.; Li, Y.; Wei, C.; Xie, D. Effects of land management practices on labile organic carbon fractions in rice cultivation. Chin. Geogr. Sci. 2009, 19, 241–248. [Google Scholar] [CrossRef]
- Reich, P.B.; Oleksyn, J.; Modrzynski, J.; Mrozinski, P.; Hobbie, S.E.; Eissenstat, D.M.; Chorover, J.; Chadwick, O.A.; Hale, C.M.; Tjoelker, M.G. Linking litter calcium, earthworms and soil properties: A common garden test with 14 tree species. Ecol. Lett. 2005, 8, 811–818. [Google Scholar] [CrossRef]
- Uselman, S.M.; Qualls, R.G.; Lilienfein, J. Quality of soluble organic C, N, and P produced by different types and species of litter: Root litter versus leaf litter. Soil Biol. Biochem. 2012, 54, 57–67. [Google Scholar] [CrossRef]
- Mbuthia, L.W.; Acosta-Martínez, V.; DeBruyn, J.; Schaeffer, S.; Tyler, D.; Odoi, E.; Mpheshea, M.; Walker, F.; Eash, N. Long term tillage, cover crop, and fertilization effects on microbial community structure, activity: Implications for soil quality. Soil Biol. Biochem. 2015, 89, 24–34. [Google Scholar] [CrossRef]
- Li, Z.G.; Xie, Y.Z. Improving desertified soil properties by incorporating and mulching tree branch in Ningxia province. Trans. Chin. Soc. Agric. Eng. 2015, 10, 174–181. [Google Scholar]
- Ramos, M.E.; Benítez, E.; García, P.A.; Robles, A.B. Cover crops under different managements vs. frequent tillage in almond orchards in semiarid conditions: Effects on soil quality. Appl. Soil Ecol. 2010, 44, 6–14. [Google Scholar] [CrossRef]
- Jiménez, M.; de la Horra, A.; Pruzzo, L.; Palma, M. Soil quality: A new index based on microbiological and biochemical parameters. Biol. Fertil. Soils 2002, 35, 302–306. [Google Scholar] [CrossRef]
- Camposeo, S.; Vivaldi, G.A. Short-term effects of de-oiled olive pomace mulching application on a young super high-density olive orchard. Sci. Hortic. 2011, 129, 613–621. [Google Scholar] [CrossRef]
- Sánchez, E.E.; Giayetto, A.; Cichón, L.; Fernández, D.; Aruani, M.C.; Curetti, M. Cover crops influence soil properties and tree performance in an organic apple (Malus domestica Borkh) orchard in northern Patagonia. Plant Soil 2007, 292, 193–204. [Google Scholar] [CrossRef]
- Thomazini, A.; Mendonça, E.S.; Cardoso, I.M.; Garbin, M.L. SOC dynamics and soil quality index of agroforestry systems in the Atlantic rainforest of Brazil. Geoderma Reg. 2015, 5, 15–24. [Google Scholar] [CrossRef]
- Neilsen, G.; Forge, T.; Angers, D.; Neilsen, D.; Hogue, E. Suitable orchard floor management strategies in organic apple orchards that augment soil organic matter and maintain tree performance. Plant Soil 2014, 378, 325–335. [Google Scholar] [CrossRef]
- Zhang, B.; Du, J.F.; Xie, H.T.; Li, W.F.; Wang, L.F.; Zhang, X.D. Effects of long-term fertilization on features of neutral sugars in particulate organic matter. Chin. J. Soil Sci. 2010, 41, 617–621. [Google Scholar]
- He, G.X. Degeneration of soil fertility in pure Chinese fir succession. J. Zhejiang For. Coll. 2002, 19, 100–103. [Google Scholar]
- Zhang, N.W.; Dong, C.X.; Xu, Y.C. Nutrient amounts removed by the pruning branches and the fruit harvest from the pear tree. J. Nanjing Agric. Univ. 2013, 36, 37–42. [Google Scholar]
- He, D.J.; He, X.J.; Hong, W.; Liu, Y.S.; Bian, L.L.; Qi, D.H.; You, H.M. Research progress of coarse woody debris in forest ecosystems. For. Res. 2009, 22, 715–721. [Google Scholar]
- Hekkala, A.; Ahtikoski, A.; Päätalo, M.; Tarvainen, O.; Siipilehto, J.; Tolvanen, A. Restoring volume, diversity and continuity of deadwood in boreal forests. Biodivers. Conserv. 2016, 25, 1107–1132. [Google Scholar] [CrossRef]
- Bao, S.D. Soil Chemical Analysis; China Agricultural Press: Beijing, China, 2008. [Google Scholar]
- Olsen, S.R.; Cole, C.V.; Watanabe, F.S. Estimation of Available Phosphorus in Soils by Extraction with Sodium Bicarbonate; Circular/United States Department of Agriculture: Washington, DC, USA, 1954. [Google Scholar]
- Hanway, J.J.; Heidel, H. Soil Analysis Methods as Used in Iowa State College; Agricultural Bulletin No. 57; Soil Testing Laboratory, Iowa State College: Ames, IA, USA, 1952. [Google Scholar]
- Lanyon, L.E.; Heald, W.R. Magnesium, calcium, strontium, and barium. In Methods of Soil Analysis: Part 2 Chemical and Microbiological Properties, 2nd ed.; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; Agronomy Series, No. 9; American Society of Agronomy: Madison, WI, USA, 1982; pp. 247–262. [Google Scholar]
- Zhang, W.; He, H.; Zhang, X. Determination of neutral sugars in soil by capillary gas chromatography after derivatization to aldononitrile acetates. Soil Biol. Biochem. 2007, 39, 2665–2669. [Google Scholar] [CrossRef]
- Engel, M.H.; Macko, S.A.; Silfer, J.A. Carbon isotope composition of individual amino acids in the Murchison meteorite. Nature 1990, 348, 47–49. [Google Scholar] [CrossRef] [PubMed]
- Mairura, F.S.; Mugendi, D.N.; Mwanje, J.I.; Ramisch, J.J.; Mbugua, P.K.; Chianu, J.N. Integrating scientific and farmers’ evaluation of soil quality indicators in Central Kenya. Geoderma 2007, 139, 134–143. [Google Scholar] [CrossRef]
- Qi, Y.; Darilek, J.L.; Huang, B.; Zhao, Y.; Sun, W.; Gu, Z. Evaluating soil quality indices in an agricultural region of Jiangsu Province, China. Geoderma 2009, 149, 325–334. [Google Scholar] [CrossRef]
- Li, P.; Zhang, T.; Wang, X.; Yu, D. Development of biological soil quality indicator system for subtropical China. Soil Tillage Res. 2013, 126, 112–118. [Google Scholar] [CrossRef]
- Zhang, Q.F.; Song, Y.C. Relationship between plant community secondary succession and soil fertility in Tiantong, Zhejiang Province. Acta Ecol. Sin. 1999, 19, 174–178. [Google Scholar]
- Wang, J.G.; Yang, L.Z.; Shan, Y.H. Application of fuzzy mathematics to soil quality evaluation. Acta Pedol. Sin. 2001, 38, 76–183. [Google Scholar]
- Xu, M.X.; Liu, G.B.; Zhao, Y.G. Quality assessment of erosion soil on hilly Loess Plateau. Plant Nutr. Fertil. Sci. 2005, 11, 285–293. [Google Scholar]
- Andrews, S.S.; Karlen, D.L.; Mitchell, J.P. A comparison of soil quality indexing methods for vegetable production systems in Northern California. Agric. Ecosyst. Environ. 2002, 90, 25–45. [Google Scholar] [CrossRef]
- Andrews, S.S.; Mitchell, J.P.; Mancinelli, R.; Karlen, D.L.; Hartz, T.K.; Horwath, W.R.; Pettygrove, G.S.; Scow, K.M.; Munk, D.S. On-farm assessment of soil quality in California’s central valley. Agron. J. 2002, 94, 12–23. [Google Scholar]
- Andrews, S.S.; Flora, C.B.; Mitchell, J.P.; Karlen, D.L. Growers’ perceptions and acceptance of soil quality indices. Geoderma 2003, 114, 187–213. [Google Scholar] [CrossRef]
- Lü, C.H.; Zheng, F.L. Evaluation of soil quality during vegetation restoration in the Ziwu-ling area of Loess Platea. Sci. Soil Water Conserv. 2009, 7, 12–18. [Google Scholar]
- Doran, J.W.; Zeiss, M.R. Soil health and sustainability: Managing the biotic component of soil quality. Appl. Soil Ecol. 2000, 15, 3–11. [Google Scholar] [CrossRef]
- Zhu, L.; Hu, N.; Zhang, Z.; Xu, J.; Tao, B.; Meng, Y. Short-term responses of soil organic carbon and carbon pool management index to different annual straw return rates in a rice–wheat cropping system. Catena 2015, 135, 283–289. [Google Scholar] [CrossRef]
- Zhao, C.Z.; Lu, L.; Chen, B.H. Effect of field covering with plastic and straw on agricultural ecology of apple orchard in arid desert area. Chin. J. Eco-Agric. 2004, 1, 155–158. [Google Scholar]
- Nascente, A.S.; Li, Y.C.; Crusciol, C.A.C. Cover crops and no-till effects on physical fractions of soil organic matter. Soil Tillage Res. 2013, 130, 52–57. [Google Scholar] [CrossRef]
- Kuzyakov, Y. Priming effects: Interactions between living and dead organic matter. Soil Biol. Biochem. 2010, 42, 1363–1371. [Google Scholar] [CrossRef]
- Zhu, C.; Li, Z.; Wu, W.; Yang, P. Carbon and nitrogen mineralization of incubated sweet maize and white clover straw. Chin. J. Eco-Agric. 2009, 17, 423–428. [Google Scholar] [CrossRef]
- Heitkamp, F.; Wendland, M.; Offenberger, K.; Gerold, G. Implications of input estimation, residue quality and carbon saturation on the predictive power of the Rothamsted carbon model. Geoderma 2012, 170, 168–175. [Google Scholar] [CrossRef]
- Zhu, P.L.; Wang, Z.M.; Huang, D.M.; Yu, X.H.; Yan, S.H. Effect of inorganic nitrogen on mineralization of organic carbon in soil. Acta Pedol. Sin. 2001, 38, 457–463. [Google Scholar]
- Li, H.T.; Yu, G.R.; Li, J.Y.; Liang, T.; Chen, Y.R. Dynamics of litter decomposition and phosphorus and potassium release in Jinggang Mountain region of Jiangxi Province, China. Chin. J. Appl. Ecol. 2007, 18, 233–240. [Google Scholar]
- Hyvönen, R.; Olsson, B.; Lundkvist, H.; Staaf, H. Decomposition and nutrient release from Picea abies (L.) Karst. and Pinus sylvestris L. logging residues. For. Ecol. Manag. 2000, 126, 97–112. [Google Scholar] [CrossRef]
- Merilä, P.; Mustajärvi, K.; Helmisaari, H.S.; Hilli, S.; Lindroos, A.J.; Nieminen, T.M.; Nöjd, P.; Rautio, P.; Salemaa, M.; Ukonmaanaho, L. Above-and below-ground N stocks in coniferous boreal forests in Finland: Implications for sustainability of more intensive biomass utilization. For. Ecol. Manag. 2014, 311, 17–28. [Google Scholar] [CrossRef]
- Muvengwi, J.; Ndagurwa, H.G.T.; Nyenda, T. Enhanced soil nutrient concentrations beneath-canopy of savanna trees infected by mistletoes in a southern African savanna. J. Arid Environ. 2015, 116, 25–28. [Google Scholar] [CrossRef]
- Ndagurwa, H.G.T.; Dube, J.S.; Mlambo, D. The influence of mistletoes on nitrogen cycling in a semi-arid savanna, south-west Zimbabwe. J. Trop. Ecol. 2013, 29, 147–159. [Google Scholar] [CrossRef]
- Smolander, A.; Saarsalmi, A.; Tamminen, P. Response of soil nutrient content, organic matter characteristics and growth of pine and spruce seedlings to logging residues. For. Ecol. Manag. 2015, 357, 117–125. [Google Scholar] [CrossRef]
- Liu, Z.F.; Fu, B.J.; Liu, G.H.; Zhu, Y.G. Soil quality: Concept, indicators and its assessment. Acta Ecol. Sin. 2006, 26, 901–913. [Google Scholar]
- Ngo-Mbogba, M.; Yemefack, M.; Nyeck, B. Assessing soil quality under different land cover types within shifting agriculture in South Cameroon. Soil Tillage Res. 2015, 150, 124–131. [Google Scholar] [CrossRef]
| Total C (%) | Total N (g·kg−1) | Total P (g·kg−1) | Total K (g·kg−1) | Lignin (%) | Cellulose (%) | Hemicellulose (%) | |
|---|---|---|---|---|---|---|---|
| Annual shoot | 48.00 ± 2.21 | 4.32 ± 0.35 | 1.30 ± 0.11 | 5.92 ± 0.39 | 30.54 ± 1.22 | 39.04 ± 2.11 | 19.28 ± 1.68 |
| Treatment | Soil Layer (cm) | Ca2+ (mg·kg−1) | Na+ (mg·kg−1) | Mg2+ (mg·kg−1) |
|---|---|---|---|---|
| CK | 0–20 | 420.64 ± 6.15 b | 135.00 ± 10.14 c | 84.92 ± 3.52 b |
| 20–40 | 444.21 ± 17.22 a | 228.89 ± 17.82 b | 70.69 ± 3.21 cd | |
| 1 year | 0–20 | 394.03 ± 8.96 c | 248.89 ± 11.82 b | 66.99 ± 1.97 d |
| 20–40 | 407.14 ± 8.03 bc | 158.33 ± 10.00 c | 72.21 ± 1.49 c | |
| 3 years | 0–20 | 439.91 ± 3.75 a | 280.00 ± 8.33 a | 89.84 ± 1.12 a |
| 20–40 | 414.73 ± 6.57 b | 238.89 ± 10.00 b | 87.13 ± 1.10 ab |
| Treatment | Soil Depth (cm) | Concentrations of Neutral Sugars (mg·g−1) | |||||
|---|---|---|---|---|---|---|---|
| Glucose | Arabinose | Galactose | Mannose | Xylose | Rhamnose | ||
| CK | 0–20 | 0.44 ± 0.05 d | 0.82 ± 0.11 bc | 0.26 ± 0.03 e | 0.28 ± 0.09 c | 0.38 ± 0.07 d | 0.46 ± 0.11 b |
| 20–40 | 0.44 ± 0.07 d | 0.91 ± 0.06 b | 0.35 ± 0.02 d | 0.29 ± 0.05 c | 0.32 ± 0.05 d | 0.49 ± 0.07 b | |
| 1 year | 0–20 | 0.70 ± 0.11 c | 1.23 ± 0.14 a | 0.69 ± 0.09 bc | 0.44 ± 0.07 a | 0.67 ± 0.09 c | 0.71 ± 0.09 a |
| 20–40 | 1.36 ± 0.07 b | 0.68 ± 0.05 d | 0.73 ± 0.07 b | 0.38 ± 0.05 b | 1.07 ± 0.15 b | 0.32 ± 0.06 c | |
| 3 years | 0–20 | 1.38 ± 0.12 b | 0.74 ± 0.03 cd | 0.67 ± 0.07 c | 0.39 ± 0.03 ab | 1.02 ± 0.11 b | 0.39 ± 0.08 bc |
| 20–40 | 1.76 ± 0.25 a | 0.86 ± 0.01 bc | 0.90 ± 0.05 a | 0.35 ± 0.04 b | 1.25 ± 0.07 a | 0.33 ± 0.07 c | |
| SOC | TN | AP | AK | ACa | ANa | AMg | Rhamnose | Arabinose | Mannose | Glucose | Galactose | Xylose | Amino Acids | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SOC | 1 | |||||||||||||
| TN | 0.562 * | 1 | ||||||||||||
| AP | 0.605 ** | −0.074 | 1 | |||||||||||
| AK | 0.746 ** | 0.495 * | 0.759 ** | 1 | ||||||||||
| ACa | 0.126 | 0.448 | −0.376 | −0.094 | 1 | |||||||||
| ANa | 0.342 | 0.450 | 0.432 | 0.664 ** | 0.265 | 1 | ||||||||
| AMg | 0.608 ** | 0.880 ** | −0.093 | 0.354 | 0.361 | 0.188 | 1 | |||||||
| Rhamnose | −0.293 | −0.119 | −0.067 | 0.121 | −0.154 | −0.079 | −0.342 | 1 | ||||||
| Arabinose | −0.396 | −0.223 | 0.151 | 0.229 | −0.355 | 0.332 | −0.529 * | 0.694 ** | 1 | |||||
| Mannose | 0.411 | −0.154 | 0.877 ** | 0.663 ** | −0.422 | 0.413 | −0.207 | 0.029 | 0.317 | 1 | ||||
| Glucose | 0.533 * | 0.241 | 0.472 * | 0.326 | −0.103 | 0.344 | 0.383 | −0.760 ** | −0.360 | 0.440 | 1 | |||
| Galactose | 0.381 | 0.021 | 0.622 ** | 0.400 | −0.376 | 0.377 | 0.093 | −0.503 * | 0.009 | 0.681 ** | 0.906 ** | 1 | ||
| Xylose | 0.583 * | 0.221 | 0.542 * | 0.382 | −0.248 | 0.265 | 0.376 | −0.693 ** | −0.332 | 0.491 * | 0.975 ** | 0.914 ** | 1 | |
| Amino acids | 0.660 ** | 0.802 ** | 0.168 | 0.695 ** | 0.269 | 0.322 | 0.691 ** | 0.255 | −0.065 | 0.087 | −0.058 | −0.167 | −0.016 | 1 |
| Soil Property | Rotated Factor | Common Factor Variance | Norm Value | Weight | |||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | ||||
| SOC | 0.775 | 0.357 | 0.408 | −0.018 | 0.896 | 2.054 | 0.070 |
| TN | 0.875 | 0.120 | −0.208 | 0.275 | 0.900 | 2.117 | 0.070 |
| AP | 0.134 | 0.216 | 0.882 | 0.180 | 0.876 | 1.569 | 0.069 |
| AK | 0.620 | −0.008 | 0.664 | 0.390 | 0.978 | 1.874 | 0.077 |
| ACa | 0.378 | 0.034 | −0.695 | 0.340 | 0.743 | 1.505 | 0.058 |
| ANa | 0.280 | 0.144 | 0.192 | 0.905 | 0.955 | 1.215 | 0.075 |
| AMg | 0.855 | 0.338 | −0.224 | −0.048 | 0.898 | 2.138 | 0.070 |
| Rhamnose | −0.021 | −0.934 | 0.191 | 0.066 | 0.914 | 1.754 | 0.072 |
| Arabinose | −0.350 | −0.584 | 0.370 | 0.528 | 0.879 | 1.586 | 0.069 |
| Mannose | −0.021 | 0.163 | 0.887 | 0.265 | 0.885 | 1.537 | 0.069 |
| Glucose | 0.139 | 0.919 | 0.300 | 0.149 | 0.977 | 1.805 | 0.076 |
| Galactose | −0.082 | 0.735 | 0.572 | 0.245 | 0.934 | 1.689 | 0.073 |
| Xylose | 0.167 | 0.867 | 0.425 | 0.035 | 0.962 | 1.794 | 0.075 |
| Amino acids | 0.940 | −0.268 | 0.126 | 0.089 | 0.978 | 2.274 | 0.077 |
| Eigenvalue | 5.521 | 3.402 | 2.787 | 1.063 | |||
| Contribution rate of variance (%) | 39.44 | 24.30 | 19.91 | 7.59 | |||
| Cumulative (%) | 39.44 | 63.74 | 83.65 | 91.25 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, E.; Lyu, S.; Du, G.; Lyu, D. Evaluation of the Effects of Returning Apple Shoots In Situ on Soil Quality in an Apple Orchard. Agronomy 2022, 12, 2645. https://doi.org/10.3390/agronomy12112645
Zhou E, Lyu S, Du G, Lyu D. Evaluation of the Effects of Returning Apple Shoots In Situ on Soil Quality in an Apple Orchard. Agronomy. 2022; 12(11):2645. https://doi.org/10.3390/agronomy12112645
Chicago/Turabian StyleZhou, Enda, Sansan Lyu, Guodong Du, and Deguo Lyu. 2022. "Evaluation of the Effects of Returning Apple Shoots In Situ on Soil Quality in an Apple Orchard" Agronomy 12, no. 11: 2645. https://doi.org/10.3390/agronomy12112645
APA StyleZhou, E., Lyu, S., Du, G., & Lyu, D. (2022). Evaluation of the Effects of Returning Apple Shoots In Situ on Soil Quality in an Apple Orchard. Agronomy, 12(11), 2645. https://doi.org/10.3390/agronomy12112645




