Abstract
Mycorrhizae are symbiotic associations between fungi and plants and are primarily responsible for nutrient transfer and survival of both partners. The present study was conducted to explore the diversity of mycorrhizal fungi in the rhizospheric soil of perennial grass species (Saccharum spontaneum, Saccharum bengalense, Setaria verticillata, Cymbopogon jwarancusa, and Typha angustata) around the district Layyah. In the subsequent experiment, the rhizospheric soils were used as inoculants, and their impact on mycorrhizal colonization in the plant and soil, and growth and physiological attributes, of Cenchrus ciliaris were investigated. The maximum hyphal, vesicles, arbuscules, dark septate endophytic and ectomycorrhizal colonization, and spore percentage were observed in the case of R-S5, i.e., rhizospheric soil, collected from Saccharum bengalense. However, the maximum (0.9310) Simpson’s index of diversity was observed in the case of R-S4, i.e., rhizospheric soil collected from Setaria verticillata. Different mycorrhizal fungal morphotypes scattered over three genera, i.e., Acaulospora, Glomus, and Scutellospora, were recorded both from rhizosphere and trap cultures. The application of spores from rhizospheric soil collected from S. bengalense (R-S5) caused the maximum increase in plant height (19.5%), number of leaves plant−1 (17.6%), leaf area (108.0%), and chlorophyll contents (29.4%) of Cenchrus ciliaris, compared to other treatments. In conclusion, the inoculation of mycorrhizal fungi significantly improves the mycorrhizal characteristics of Cenchrus ciliaris and its rhizospheric soil and ultimately enhances the growth and physiological parameters of Cenchrus ciliaris.
1. Introduction
Mycorrhiza is a mutualistic relationship between soil-borne fungus and the roots of a wide range of plant species, including higher plants and perennial grass species [1]. Two types of mycorrhizal associations are known as ecto- and endo-mycorrhiza. The ecto-mycorrhiza is characterized by an extracellular fungal growth in the root cortex [2,3]. Boreal forest trees have more than 5000 species, mainly of the Basidiomycetes variety, and are more common in the temperate zone [4]. The arbuscular mycorrhizal fungi (AMF) belong to the taxonomic order called Glomales, which currently comprises six genera and are the most common underground symbiotic fungi with agricultural applications [5,6]. AMF have been observed in all ecosystems [7]. Extra-radical AMF acquire carbon from the plant, transform it into storage lipids that take up mineral nutrients from the soil, and then transfer them to the plant roots; in the opposite direction, carbon is exported to build spores and mycelium from inter radical mycelium [8,9,10].
Arbuscular mycorrhizae are the associations where a Glomeromycete fungus produces arbuscules, hyphae, and vesicles within root cortex cells [11]. These associations are due to the presence of arbuscules. Fungi in the roots are usually spread by linear hyphae or coiled hyphae [12]. An important feature of the AM fungi is the production of a large number of soil-borne spores having hundreds or thousands of nuclei, presumed asexuality, and multinucleate mycelium without true septa [1]. Over 200 species of fungi are capable of forming a mycorrhizal association with the majority of plants.
Symbioses among dark septate endophytes (DSE) and AMF in terrestrial environments are ubiquitous, giving tolerance against harsh soil conditions that may limit plant growth [13]. Symbioses with AMF are especially crucial for the uptake of slow-moving soil nutrients [1]. In nature, DSE and AMF colonize plant roots at the same time, which is frequent in plant ecosystems [14]. Despite the presence of DSE and AMF in plant roots, there has been little research into these symbioses as a whole, and existing information regarding DSE and AMF is uneven [15]. Although microscopic or molecular analyses of roots generally showed that DSE could be more abundant than AMF, little is known about the role of DSE symbiosis on plant fitness [16].
Mycorrhizal fungi colonize approximately 85% of the plants, which suggests that mycorrhizal symbiosis is the rule rather than the exception in the plant world [17]. Root colonization by mycorrhiza improved nutrient uptake; increased tolerance against pests and diseases, drought, and heavy metals; and had a significant impact on the development and health of host plants [18,19,20]. Most ectomycorrhizal fungi break down phenolic compounds in soil that may obstruct nutrient absorption [21]. Vesicular arbuscular mycorrhizal (VAM) and ectomycorrhizal (ECM fungi) can protect roots from nematodes and parasitic fungi [22]. Hyphal networks help in the seedling establishment or contribute to the growth via the provision of nutrients, especially when roots are inactive [1]. Mycorrhizal fungal hyphae are also an important food source for soil invertebrates [23]. The inoculation of AMF enhanced the growth, production, and phosphorus uptake in Setaria splendida [1,24].
Buffel grass (Cenchrus ciliaris L.) is valued for its production of excellent feed and intermittent grazing during drought periods in the tropics. It is also highly nutritious and regarded as appropriate for pasture in hot and dry environments [25]. It grows in dry and sandy locations with annual rainfalls of 250–750 mm (but it may survive much higher rainfall) between sea level and 2000 m, on marginally fertile shallow soils [25]. Such qualities boost its value as pasturage and broaden its spectrum of production. Some strains’ yield makes them suitable for foraging during the wet season. On a dry matter basis, buffel grass contains proteins (11.0%), fats (2.6%), total carbohydrates (73.2%), fibers (31.9%), and ash (13.2%) [26]. It is believed that feeding cattle green grass, and turning it into silage or hay, can boost their milk production, and give them a sleek, glossy appearance [26].
Mycorrhizae also influence soil microbial populations and exudates in the hyphosphere and mycorrhizosphere [27,28]. VAM fungal hyphae improve soil structure. Their importance in mechanical aggregation is due to the secretions of glomalin [29,30]. There is meager knowledge regarding the presence of mycorrhizal fungi in the desert ecosystem of Pakistan [31]. Nothing is known about the hidden potential for colonization status and AMF plant interaction, community formation, diversity, propagules, behavior, and spatial variations, in this region of Pakistan. Therefore, it is pertinent to explore the potential of different mycorrhiza in improving the growth and productivity of grasses, such as Cenchrus ciliaris, as these serve as a food source for different animals. Based on this hypothesis, the purpose of the present study is to investigate the diversity of mycorrhiza in the rhizospheric soils of perennial grass species in the Layyah district, and their subsequent impact on mycorrhizal colonization in soil and Cenchrus ciliaris, and its growth and physiological characteristics.
2. Materials and Methods
2.1. Study Area
In the present study, the diversity of mycorrhiza was studied in perennial grass species from District Layyah (Thind Kalan Nashab and Chak no 122/T.D.A.), Punjab, Pakistan between 2011–2013. The study area is located between longitude 30°58′0” N and latitude 70°56′0” E, with an altitude of 143 m above sea level [32]. Thind Kalan Nashab and Chak no 122/T.D.A. from District Layyah is situated about 280 km from The Islamia University, Bahawalpur. The study area is located along the bank of the Indus River. Layyah derives its name from a wild short stature shrub, commonly known as Layyah. The area of the district is naturally divided into the Nasheb area, Thal, and Sandy Thal desert. The climate of Layyah is arid, where summer is extremely hot while winter is cold. The weather is dry all year round, especially in Thal areas. The other parts of the district that are flooded from the Indus River or irrigated via inundation canals are comparatively less dry. Flood season and inundation by river results in abundant moisture on the ground, as well as in the air. The moisture reaches its maximum during the inundation period (August and September). The distribution and incidence of rainfall are quite regular and go along the seasons. The average rainfall does not exceed 18.25 cm, of which main downpours are experienced during the summer months. In summer, the temperature may rise to 51 °C. Dust storms are common during May, June, and July. The area is rich in diverse vegetation cover due to the local irrigation system. Shisham (Dalbergia sissoo L.) is the most common tree found in almost every part of the district. The arid climate of the area gives rise mainly to the xerophytic type of shrubs and herbs. The vegetation of the area is mostly of the perennial type, where plants usually blossom during the monsoon rains. The annual herbs and grasses have a shallow-branched root system.
2.2. Rhizospheric Soil Sampling
For the collection of rhizospheric soil of perennial grasses (Saccharum spontaneum L., Saccharum bengalense Retz., Setaria verticillata, Cymbopogon jwarancusa (Jones) Schult., and Typha angustata), the roots were gently taken out and shaken. The soil adhering to the roots was washed with sterile distilled water to remove the loosely bound soil with the roots. After that, the soil adhering to the roots was gently removed with a sterilized scissor. The wet sieving and decanting methods were used to extract spores from each collected rhizospheric soil sample [33]. The number of spores in each soil sample was immediately counted using a Zoom Stereomicroscope (DigiStar-2, Labomed, USA) and the number of spores per 100 g of soil was recorded.
Standard procedures were followed for soil physicochemical analysis. Due to its sandy texture, the soil was suspended in distilled water in a 1:2 (w/v) ratio for pH and electrical conductivity (EC), and pH and EC were measured using pH (HACH 8190) and EC meters (HACH-44600), respectively. For carbonates, bicarbonates, calcium, and magnesium, the titration method was used. For nutrient analysis, available nitrogen was determined through the Kjeldahl method, phosphorus through the bicarbonate method [34], and potassium by using a flame photometer. Organic matter was determined using the potassium dichromate method [35].
2.3. Pot Experiment
To investigate the impact of mycorrhizal inoculation on the growth and root colonization of Cenchrus ciliaris, a trap culture was conducted. The soil collected during the sampling was ground, sieved (2 mm), and autoclaved for 20 min at 121 °C. About 10 kg of soil was put in each plastic pot with a height and diameter of 30 × 20 cm. A large number of hyphae and spores collected from different rhizospheric soils were cultured on MS medium, and then the mycelium suspension containing 4000 spores mL−1 was prepared. For inoculation of soil with mycorrhiza, the spores (10 g or ± 50 AMF spores) [36] were placed immediately under the seedlings [37]. Six treatments were comprised of control and mycorrhizal inoculation with spores of different species collected from different rhizospheric soils (R-S) viz. control, R-S1 (Typha angustata), R-S2 (Cymbopogon jwarancusa, R-S3 (Saccharum spontaneum), R-S4 (Setaria verticillata), and, R-S5 (Saccharum bengalense). In the control treatment, only autoclaved soil was used as the growth medium for Cenchrus ciliaris. The treatments were arranged in a completely randomized design in triplicate. During the present investigation, Cenchrus ciliaris was selected as a test plant due to its nutritional value and adaptability under hot desert conditions. The seeds of Cenchrus ciliaris were surface sterilized with HgCl2 for 2–3 min and washed 3–4 times with sterilized distilled water. The sterilized seeds were pre-germinated in Petri dishes containing wet filter paper with sterilized distilled water and kept in dark. In each pot, four uniform seedlings were transplanted. The pots were irrigated with tap water daily. After three weeks of transplantation, the harvesting of Cenchrus ciliaris was completed.
For growth parameters, standard procedures were followed. For example, the plant height was measured by selecting the tiller with the maximum height from each pot. The selected plants were also used for measuring stem diameter with the help of the Vernier caliper. For the number of tillers plant−1, all the plants of each pot were explored and the average was calculated. The plant’s average number of leaves was computed similarly by dividing the total number of leaves by the number of tillers in each pot. The average leaf area was estimated using the following formula, which involved measuring the average leaf length and width in each pot.
For fresh biomass, the harvested plants from each pot were weighed using a digital balance (BL-320 H, Shimadzu Corporation, Tokyo, Japan). For dry weight, the plant samples were put into an oven at 65 °C for 48 h or until a constant weight was obtained. For chlorophyll contents, a portable absorbance-based dual-wavelength chlorophyll meter (SPAD-502; Minolta Corporation, Ltd., Osaka Japan) was used. Leaves from the canopy of each plant were randomly selected for the measurement of chlorophyll contents.
2.4. Root Colonization with Mycorrhiza
After harvesting the plants, the root samples in the pots were gently taken out of the soil. The root samples with intact epidermis were selected and washed carefully under running or tap water, avoiding serious damage to the epidermis, preserved in FAA (Formaldehyde: Acetic acid: Alcohol 5:5:90 v/v) solution, and kept at 4 °C until analyzed for mycorrhizal colonization. For mycorrhization, the roots were cleared, washed with KOH (10% w/v), and autoclaved at 121 °C for 20 min. After taking cooled samples from the autoclave, the root samples were treated with HCl (01 N) for five minutes and stained with trypan blue (0.05%) in lactophenol and left overnight [38].
The data regarding the frequency of mycorrhizal colonization were estimated via the glass slide method by randomly placing 300 stained root fragments (01 cm in length) on a glass slide with lactophenol. The glass slide was covered with a glass coverslip to avoid the formation of air bubbles. While quantifying mycorrhizal roots, a segment was considered mycorrhizal when any structures (such as hyphae, vesicles, or arbuscules) were observed. Biermann and Lindermann’s method [39] was used to calculate the infection percentage. Three hundred root segments of each plant per sample were examined under the compound microscope (Olympus Digi 2) at the magnification of 10× and 40×. The data for types of AMF structures, such as arbuscules, vesicles, intra, and extra-radical hyphae, were collected from each slide. The AMF colonization was estimated using the following parameters.
The percentage of hyphae/vesicles/spores/arbuscules in mycorrhizal parts of root fragments was calculated using the following formula:
For dark septate endophytic (DSE) and ectomycorrhizal colonization (ECM), the following formula was used:
For extraction of spores from the soil after harvesting Cenchrus ciliaris, the wet sieving and decanting method, as mentioned earlier, was adopted. The percentage, frequency, and relative frequency of spores were calculated using the following formula:
2.5. Identification of Spores
The taxonomic identification of spores was done using Morton’s method [40]. The color, size, and wall structure of the spores were used in their identification. The spores or species were identified using Pérez and Schenck’s [41] and Hall and Fish’s keys [42].
2.6. Simpson’s Diversity Index
Simpson’s diversity index was calculated using the equation below, as described by Simpson [43]:
where n is the total number of individuals of a particular mycorrhizal species and N is the total number of individuals of all species
2.7. Statistical Analysis
The data collected during the present research work were subjected to analysis of variance using Statistix 8.1 and treatment means were compared using the least significant difference (LSD) at p < 0.05 [44]. GraphPad Prism 8 (GraphPad Software, La Jolla, CA, USA) was used to calculate the mean values with standard deviation and for the graphical representation of data.
3. Results
3.1. Mycorrhizal Colonization
The root samples in the form of root fragments collected from five rhizospheric soils of different grasses [R-S1 (Saccharum spontaneum), R-S2 (Saccharum bengalense), R-S3 (Setaria verticillata), R-S4 (Cymbopogon jwarancusa) and R-S5 (Typha angustata)] were observed under a compound light microscope to observe the percent root colonization via mycorrhiza (Figure 1 and Figure 2a–f). Soil texture and plant species of the rhizospheric soils had a significant relation with the mycorrhizal colonization in different root fragments collected from different rhizospheric soils (Table 1 and Figure 2).
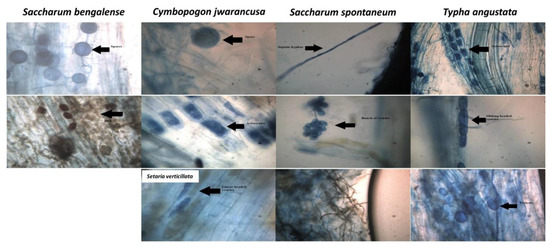
Figure 1.
Hyphae, vesicles, spores, arbuscules, DSE, and ECM observed in the soil collected from the rhizospheric samples of different grasses.
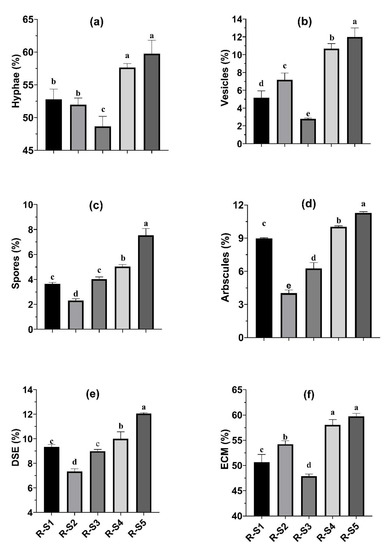
Figure 2.
Hyphal (a), vesicles (b), spores (c), arbuscules (d), DSE (e), and ECM (f) colonization in the roots of Cenchrus ciliaris extracted from trap culture experiment grown in various rhizospheric soils from selected plants. Each column with a different letter is significantly different at p < 0.05. Where R-S1 = rhizospheric soil of Typha angustata, R-S2 = rhizospheric soil of Cymbopogon jwarancusa, R-S3 = rhizospheric soil of Saccharum spontaneum, R-S4 = rhizospheric soil of Setaria verticillata, R-S5 = rhizospheric soil of Saccharum bengalense.

Table 1.
Physicochemical properties of collected rhizospheric soil samples.
As clear from Figure 2a, the maximum hyphal colonization (59.8%) was observed in the case of R-S5, followed by R-S4 (57.7%), R-S1 (52.8%), and R-S2 (52.0%). The minimum hyphal colonization was observed in the case of R-S3 (48.7%), i.e., the rhizospheric soil collected from the roots of Saccharum spontaneum. The maximum hyphal colonization in the case of R-S5 was 22.8% more than that observed in the case of R-S3. The hyphal colonization in the case of R-S5 was non-significantly (p < 0.05) different from R-S4. In the case of vesicle colonization, the R-S5 showed the maximum value, i.e., 12% and it was 4.3 times higher, as compared to that observed in the case of R-S3 (Figure 2b). The root fragments collected from the rhizospheric soil of Saccharum bengalense (R-S5) had the maximum spore percentage, compared to the other root fragments collected from the rhizospheric soils of different grasses, while that of the minimum was observed in the case of R-S2, i.e., 2.3% (Figure 2c). The percentage of arbuscules colonization was the highest (11.3%) in the case of root fragments collected from the rhizospheric soil (R-S5) of Saccharum bengalense, while the lowest was in the case of R-S2, i.e., 4.0% (Figure 2d). Regarding the percent colonization of dark septate endophytic (DSE) mycorrhiza, the root fragments from the rhizospheric soil of Saccharum bengalense showed the maximum (12.0%), while that of the minimum (7.3%) was recorded in the case of R-S2 (Figure 2e). In the case of ectomycorrhizal (ECM) colonization, the maximum colonization (59.8%) was observed in the case of R-S5, followed by R-S4 (58.0%) and R-S2 (54.2%) (Figure 2f).
The minimum ECM colonization (47.9%) was recorded in the case of R-S3 root fragments collected from the rhizospheric soil of Saccharum spontaneum and it was 24.8% less, in comparison to that observed in the case of root fragments of R-S5.
3.2. Growth Parameters and Chlorophyll Contents
The application of rhizospheric soils of different plants in Cenchrus ciliaris caused a significant increase in various growth parameters recorded (Figure 3a–f). In the case of plant height, the maximum (21.55 cm) was recorded with the application of rhizospheric soil from Saccharum bengalense (R-S5), while the minimum (18.03 cm) was observed with the application of rhizospheric soil from Cymbopogon jwarancusa (R-S2) and it was 19.5% more compared to that observed with R-S2 (Figure 3a). The number of tillers plant−1, the fresh weight of shoot and root, and the dry weight of shoot and root all showed a similar trend (Figure 3b–f). With the application of rhizospheric soil from Saccharum bengalense (R-S5), the maximum number of tillers plant−1 (8.24), fresh weight of shoot (22.96 g) and root (9.38 g), and dry weight of shoot (8.63 g) and root (2.56 g) in Cenchrus ciliaris were observed.
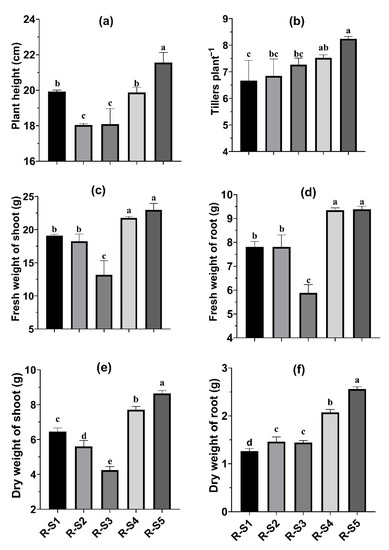
Figure 3.
Growth parameters, i.e., plant height (a), tillers plant−1 (b), fresh weight of shoot (c), fresh weight of root (d), dry weight of shoot (e), dry weight of root (f) of Cenchrus ciliaris, as affected by the application of rhizospheric soils from selected plants. Each column with a different letter is significantly different at p < 0.05. Where R-S1 = rhizospheric soil of Typha angustata, R-S2 = rhizospheric soil of Cymbopogon jwarancusa, R-S3 = rhizospheric soil of Saccharum spontaneum, R-S4 = rhizospheric soil of Setaria verticillata, R-S5 = rhizospheric soil of Saccharum bengalense.
The maximum values of the number of leaves plant−1 (13.21), leaf area (13.31 cm2), and chlorophyll contents (39.46 g plant−1) were observed with the application of rhizospheric soil obtained from Saccharum bengalense (R-S5), which were 17.6, 108, and 29.4% higher than those observed with the application of rhizospheric soil obtained from Saccharum spontaneum (R-S3) (Figure 4a–c).
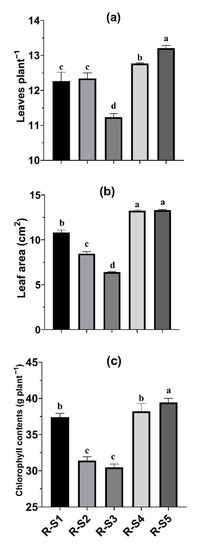
Figure 4.
Physiological parameters, i.e., number of leaves plant−1 (a), leaf area (b), and chlorophyll contents (c) of Cenchrus ciliaris, as affected by the application of rhizospheric soils from selected plants. Each column with a different letter is significantly different at p < 0.05. Where R-S1 = rhizospheric soil of Typha angustata, R-S2 = rhizospheric soil of Cymbopogon jwarancusa, R-S3 = rhizospheric soil of Saccharum spontaneum, R-S4 = rhizospheric soil of Setaria verticillata, R-S5 = rhizospheric soil of Saccharum bengalense.
3.3. Mycorrhizal Spore Diversity in Rhizospheric Soils
Mycorrhizal spore diversity in rhizospheric soil collected from different grasses is shown in Table 2. It is clear from the data presented in Table 2 that spores belonging to Glomus glomerculatum and Glomus pastulatum were the highest in number while that of the Acaulospora species were present in the lowest number in the case of the soil (R-S3) collected from the rhizosphere of Saccharum spontaneum. The maximum relative frequency (12.5) was recorded in the case of Glomus glomerculatum and Glomus pastulatum while the minimum (2.41) was observed in the case of Acaulospora species. The absolute frequency ranged from 0.33 to 0.83 (Table 2). In the case of R-S5, the maximum spores with maximum relative frequency (12.5) and species richness (n = 5) belonged to Glomus mosseae and Glomus tortuosum, while that of the minimum (2.23 and n = 2) in the case of Acaulospora bireticulata, respectively.

Table 2.
Diversity of mycorrhizal spores in rhizospheric soils of different grass species.
Similarly, the spores belonging to Glomus etunicatum and Acaculospora scrobiculata were present in the largest number in the case of the soil collected from the rhizosphere of Setaria verticillata (R-S4), while the lowest number of species belonged to Glomus fasiculatum, Glomus mosseae, Glomus aggregatum, and Glomus constrictum species. In R-S4, Acaculospora scorbiculata was observed with the maximum relative frequency, i.e., 12.5 while that of the minimum relative frequency, i.e., 2.41 was recorded in the case of Glomus constrictum. The maximum relative frequency (12.5) in the case of R-S2 was recorded in the case of Acaulospora scrobiculata, Glomus mosseae, Glomus aggregatum, Glomus pastulatum, and Glomus clarioidium, while that of the minimum (2.41) was observed in the case of Scutellospora nigra, Glomus glomerculatum, Glomus constrictum, Glomus etunicatum, and Glomus maculosum. Glomus clariodium and Glomus clarum spore species had the maximum relative frequency (12.5) in the soil collected from the rhizosphere of Typha angustata (R-S1), while that of the minimum (2.41) was recorded in the case of Glomus mosseae, Glomus constrictum, Glomus etunicatum, and Glomus aggregatum. The absolute frequency ranged from 0.33 to 0.83 (Table 2). In the case of Simpson’s index of diversity, the maximum (0.9310) was observed in the case of R-S5, followed by R-S4 (0.9138), R-S2 (0.9076), and R-S1 (0.8874), and the minimum was observed in the case of R-S3 (0.8083).
3.4. Mycorrhizal Spore Diversity in Rhizospheric Soil of Cenchrus ciliaris
After harvesting the Cenchrus ciliaris, the rhizospheric soil was analyzed regarding mycorrhizal spore diversity (Table 3). The maximum relative frequency of spores belonging to Acaulospora Scrobiculata was 9.96, while that of the minimum, i.e., 1.61 belonged to Acaulospora spp. and Scutellospora nigra. The absolute frequency ranged from 0.16 to 0.83. A Simpson’s index of diversity, i.e., 0.9586 was calculated from the spore profile of the soil collected from the rhizosphere of Cenchrus ciliaris.

Table 3.
Diversity of mycorrhizal spores in rhizospheric soil of trap cultured with Cenchrus ciliaris after harvesting.
3.5. Correlation among Growth Parameters and Diversity Characteristics of Mycorrhizal Fungi
Table 4 shows the correlation among growth parameters, such as dry weight of root, dry weight of shoot, fresh weight of root, fresh weight of shoot, leaf area, no. of leaves, plant height, and no. of tillers and diversity characteristics, i.e., Simpson’s diversity index and species richness of mycorrhizal fungi. Simpson’s diversity index had a significant relationship (p < 0.05) with growth parameters, such as the fresh weight of the root, the fresh weight of the shoot, and the no. of leaves. Similarly, the growth parameters, such as chlorophyll contents, dry weight of root and shoot, fresh weight of root and shoot, leaf area, no. of tillers, plant height, and no. of leaves, had a significant relationship (p < 0.05) among themselves.

Table 4.
Correlation among growth parameters and diversity characteristics of mycorrhizal fungi.
4. Discussion
The roots of higher plants live in symbiosis with mycorrhizal fungi [45]. AMF are obligate biotrophic fungi, found in the roots of plants with hyphae, vesicles, spores, arbuscules, dark septate endophytes (DSE), and ectomycorrhiza (ECM), and they can improve the mineral nutrition of host plants and, in turn, take carbohydrates from the host plant [46]. Earlier, a positive correlation between AMF spore density and root colonization was reported [47,48]. The density of viable AMF spores recovered from rhizosphere soil samples collected from the field and subsequent pot cultures ranged between 16 and 45 spores in 10 g−1 soil for the plants studied in this study (Table 2 and Table 3). The spore density was low, which is typical of arid and semi-arid environments [49]. Panwar and Tarafdar [50] attribute these differences to the length of the growing season and the type of tree root systems, which make the rhizosphere more conducive to spore propagation and AMF colonization [51]. Soil features have been shown to influence the structure and composition of AMF communities, and they have recently been identified as one of the essential variables in the formation of AMF communities [52]. Cofré et al. [53] reported variation in AMF species richness and relatively lower AMF colonization rates in different types of soil, which is consistent with our results.
Manoharan et al. [54] discovered that the genus Funneliformis had a higher mean relative abundance in agricultural soils, whereas Septoglomus was more abundant in permanent pasture grasslands, and Rhizophagus was more plentiful in permanent pastures and fields. Diversispora and Clareidoglomas were also more common in soils during organic farming, corroborating our findings. The richness and diversity of AMF communities have been shown to vary with environmental factors (climate and soil conditions) and spatial distance [55,56]. According to Zhu et al. [55], Ambispora, Archaeospora, Claroideoglomus, Gigaspora, Glomus, Paraglomus, and Scutellospora were dominant at the genus level and accounted for over 99% of the recovered sequence reads. AM fungal endophytes, i.e., DSE were found in all of the soil samples in our investigation, but the percentages varied. The brown fungus that was not stained by the stain represented the non-mycorrhizal infection.
AMF colonize plant roots differently, resulting in a variety of effects on plant growth, biomass allocation, and photosynthesis [8,16]. AMF colonization was reduced in soils with high phosphate concentrations in previous research. The available phosphorus contents in the soil through the application of chemical fertilizers were the major limiting factor influencing the colonization rates in the different soil types utilized in the current study [57].
Similarly, growth and physiological parameters were also enhanced with the application of rhizospheric soils containing AMF in the present study. According to Liang et al. [58], the diversity of different fungal species exerts a positive impact on the distribution of nitrogen and phosphorus and ultimately on plant growth and productivity. The increase in the availability of essential nutrients might have increased various growth and physiological parameters of Cenchrus ciliaris in the present study. Therefore, AMF diversity could be a significant contribution toward enhanced plant performance and sustainability in ecosystems [55]. Moreover, growth parameters are also affected by the fungal association in the root zone of plants fungal colonization, aiding the host plant in maintaining ionic balance by enhancing and/or selective uptake of nutrients [59]. Studies have demonstrated that arbuscules mycorrhizal connections are helpful for the plants growing under various Indian semi-arid settings [60].
Mycorrhizal symbiosis is the most common and ubiquitous plant–microbe interaction, and it is important for plant phosphorus supply and plant function in a variety of ways [1,18]. Improved nutrient uptake (mostly phosphorus), protection of roots against diseases, and easing of water stress are some of the immediate benefits that plants may reap [19,20]. Plants may improve their competitive ability because of these immediate benefits. Plant height, fresh and dry weight of shoot, and root of Cenchrus ciliaris increased the most in rhizospheric soils of Saccharum bengalense, compared to other treatments, possibly due to the favorable response of mycorrhizal inoculation under the moderate fertility state of the soil [2,3]. This is consistent with previous findings, which indicated that mycorrhizal inoculation raised the weight of plant roots and shoots substantially. These findings back up previous research that found that mycorrhizal inoculation benefits plants [18,21]. According to one interpretation of our findings, the soil treatments would have an impact on the indigenous AM community, which would then have a good impact on the plant community. Alloush et al. [61], Zaidi et al. [62], and Akhtar and Siddiqui [63] all reported on the impact of AMF on nutrient uptake in chickpeas. A. porrum had previously been shown to have a good influence on growth characteristics, such as root length, root number, and branching [10]. Similarly, Bago and Becard [8] and Huo et al. [16] found that inoculating mycorrhizal fungus enhanced branching quantity.
The leaf area of Cenchrus ciliaris was greatly enlarged in the present study after the application of rhizospheric soil from Saccharum bengalense. These findings are consistent with Chaudhry et al. [31], who discovered that when grasses were inoculated with VA endophytes, i.e., DSE, the biomass of the grasses rose considerably. Chaudhry et al. [64] studied morphological mycorrhizal diversity in two aromatic types of grasses (C. jawaruncusa and V. zizinoides). In results, no arbuscules were observed. Arbuscules are highly branched tree-like structures, which transfer phosphorus and other nutrients to plants [61,62,63]. However, in the present, arbuscules were observed, which might be due to the difference in the geographic location of the area from which the samples were collected. In the present study, AMF colonization was highly variable [65].
Diversity studies showed that only a few fungal spore morphotypes, i.e., A. bireticulata and Glomus species were consistently detected in all rhizospheric soils of the target plant species. These results are in line with other studies, which found similar species under arid and semi-arid environments [66,67]. The dominance of Glomus sp. might be attributed to its different temperature preferences [65]. Despite the rigorous research work presented, investigations in the future can elucidate the role of rhizospheric fungi in improving the growth and yield of plants under multiple abiotic stresses, such as drought and salinity, which was beyond the scope of the present study.
5. Conclusions
The present study confirmed that mycorrhizal fungi in the rhizospheric soils of different plants had a positive impact on the growth parameters of native grass (Cenchrus ciliaris) of district Layyah. From the analysis of rhizospheric soils from different plants, it was revealed that the maximum hyphal, vesicles, arbuscules, dark septate endophytic and ectomycorrhizal colonization, and spore percentage were observed in the case of R-S5, i.e., rhizospheric soil collected from Saccharum bengalense. However, the maximum (0.9310) Simpson’s index of diversity was observed in the case of R-S2, i.e., rhizospheric soil collected from Saccharum bengalense. The application of rhizospheric soil collected from Saccharum bengalense (R-S5) caused the maximum increase in growth and physiological parameters of Cenchrus ciliaris. In conclusion, the inoculation of mycorrhizal fungi significantly improved the mycorrhizal characteristics of Cenchrus ciliaris and its rhizospheric soil and ultimately enhanced the growth and physiological parameters of Cenchrus ciliaris. The rhizospheric fungi tested in the present study had great potential and, therefore, could be tested and validated under arid land conditions to enhance the growth and productivity of different crops facing simultaneous abiotic stresses of drought and salinity.
Author Contributions
Conceptualization, S.T. and M.S.C.; Data curation, S.T., A.D., I.H., N.U. and Q.M.; Formal analysis, A.D., I.H., A.P., N.U. and Q.M.; Funding, I.A.-A., A.E.-S.; Investigation, S.T.; Methodology, M.S.C., I.H., A.P. and Q.M.; Project administration, M.S.C., A.D., I.H. and Q.M.; Resources, S.T., M.S.C., A.D., I.H., A.P., N.U., I.A.-A., A.E.-S. and Q.M.; Software, A.D. and A.P.; Supervision, M.S.C.; Validation, S.T., M.S.C., A.D., I.H., A.P., N.U., I.A.-A., A.E.-S. and Q.M.; Visualization, S.T., M.S.C., A.D., I.H., A.P., N.U., I.A.-A., A.E.-S. and Q.M.; Writing—original draft, S.T. and N.U.; Writing—review and editing, S.T., M.S.C., A.D., I.H., A.P., N.U., I.A.-A., A.E.-S. and Q.M. All authors have read and agreed to the published version of the manuscript.
Funding
The authors extend their appreciation to the Researchers Supporting Project number (RSP-2021/298), King Saud University, Riyadh, Saudi Arabia.
Data Availability Statement
Not applicable.
Acknowledgments
The authors extend their appreciation to the Researchers Supporting Project number (RSP-2021/298), King Saud University, Riyadh, Saudi Arabia. The Department of Life Sciences, Cholistan Institute of Desert Studies, Islamia University of Bahawalpur Pakistan provided financial support for this research. The authors also acknowledge the Cholistan Institute of Desert Studies (CIDS) and all Department of Life Sciences teachers for their assistance with laboratory statistical analysis.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Corcoz, L.; Păcurar, F.; Pop-Moldovan, V.; Vaida, I.; Stoian, V.; Vidican, R. Mycorrhizal patterns in the roots of dominant Festuca rubra in a high-natural-value grassland. Plants 2021, 11, 112. [Google Scholar] [CrossRef] [PubMed]
- Jing, M.; Shi, Z.; Zhang, M.; Zhang, M.; Wang, X. Nitrogen and phosphorus of plants associated with arbuscular and ectomycorrhizas are differentially influenced by drought. Plants 2022, 11, 2429. [Google Scholar] [CrossRef] [PubMed]
- Da Costa, L.S.; Grazziotti, P.H.; Fonseca, A.J.; dos Santos Avelar, D.C.; Rossi, M.J.; de Barros Silva, E.; da Costa, E.C.S.; Grazziotti, D.C.F.S.; Ragonezi, C. Eucalyptus field growth, and colonization of clones pre-inoculated with ectomycorrhizal fungi. Agronomy 2022, 12, 1204. [Google Scholar] [CrossRef]
- Marupakula, S.; Mahmood, S.; Clemmensen, K.E.; Jacobson, S.; Högbom, L.; Finlay, R.D. Root-associated fungi respond more strongly than rhizosphere soil fungi to N fertilization in a boreal forest. Sci. Total Environ. 2021, 766, 142597. [Google Scholar] [CrossRef] [PubMed]
- Vidal, C.; González, F.; Santander, C.; Pérez, R.; Gallardo, V.; Santos, C.; Aponte, H.; Ruiz, A.; Cornejo, P. Management of rhizosphere microbiota and plant production under drought stress: A comprehensive review. Plants 2022, 11, 2437. [Google Scholar] [CrossRef]
- Khan, Y.; Shah, S.; Hui, T. The roles of arbuscular mycorrhizal fungi in influencing plant nutrients, photosynthesis, and metabolites of cereal crops—A review. Agronomy 2022, 12, 2191. [Google Scholar] [CrossRef]
- Ahmad, S.S.; Ahmad, T.; Rashid, A. The mediation of mycorrhizae in constituting association and distribution pattern of some plants in Murree Hills and Galliyats. Pak. J. Biol. Sci. 2004, 7, 1172–1176. [Google Scholar] [CrossRef][Green Version]
- Bago, B.; Bacrad, G. Bases of the obligate biotrophy of arbuscular mycorrhizal fungi. In Mycorrhizal Technology in Agriculture; Gianinazzi, S., Schuvpp, H., Barea, J.M., Haselwandter, K., Eds.; Birkhauser: Basel, Switzeland, 2002; pp. 33–48. [Google Scholar]
- Thapa, B.; Mowrer, J. Effects of carbon amendments, tillage and cover cropping on arbuscular mycorrhizal fungi association and root architecture in corn and cotton crop sequence. Agronomy 2022, 12, 2185. [Google Scholar] [CrossRef]
- An, X.; Liu, J.; Liu, X.; Ma, C.; Zhang, Q. Optimizing phosphorus application rate and the mixed inoculation of arbuscular mycorrhizal fungi and phosphate-solubilizing bacteria can improve the phosphatase activity and organic acid content in alfalfa soil. Sustainability 2022, 14, 11342. [Google Scholar] [CrossRef]
- Yurkov, A.P.; Puzanskiy, R.K.; Kryukov, A.A.; Gorbunova, A.O.; Kudriashova, T.R.; Jacobi, L.M.; Kozhemyakov, A.P.; Romanyuk, D.A.; Aronova, E.B.; Avdeeva, G.S.; et al. The role of Medicago lupulina interaction with rhizophagus irregularis in the determination of root metabolome at early stages of am symbiosis. Plants 2022, 11, 2338. [Google Scholar] [CrossRef]
- Brundrett, M.C.; Piche, Y.; Peterson, R.L. A developmental study of the early stages in vesicular-arbuscular mycorrhiza formation. Can. J. Bot. 1985, 63, 184–194. [Google Scholar] [CrossRef]
- Marquez, L.; Redman, R.; Rodriguez, R.; Roossinck, M. A virus in a fungus in a plant: Three-way symbiosis required for thermal tolerance. Science 2007, 315, 513–515. [Google Scholar] [CrossRef]
- Duell, E.B.; Cobb, A.B.; Wilson, G.W.T. Effects of commercial arbuscular mycorrhizal inoculants on plant productivity and intra-radical colonization in native grassland: Unintentional de-coupling of a symbiosis? Plants 2022, 11, 2276. [Google Scholar] [CrossRef]
- Munir, N.; Hanif, M.; Abideen, Z.; Sohail, M.; El-Keblawy, A.; Radicetti, E.; Mancinelli, R.; Haider, G. Mechanisms and strategies of plant microbiome interactions to mitigate abiotic stresses. Agronomy 2022, 12, 2069. [Google Scholar] [CrossRef]
- Huo, L.; Gao, R.; Hou, X.; Yu, X.; Yang, X. Arbuscular mycorrhizal and dark septate endophyte colonization in Artemisia roots respond differently to environmental gradients in eastern and central China. Sci. Total Environ. 2021, 795, 148808. [Google Scholar] [CrossRef]
- Jumrani, K.; Bhatia, V.S.; Kataria, S.; Alamri, S.A.; Siddiqui, M.H.; Rastogi, A. Inoculation with arbuscular mycorrhizal fungi alleviates the adverse effects of high temperature in soybean. Plants 2022, 11, 2210. [Google Scholar] [CrossRef]
- Mitra, D.; Djebaili, R.; Pellegrini, M.; Mahakur, B.; Sarker, A.; Chaudhary, P.; Khoshru, B.; Gallo, M.D.; Kitouni, M.; Barik, D.P.; et al. Arbuscular mycorrhizal symbiosis: Plant growth improvement and induction of resistance under stressful conditions. J. Plant Nutr. 2021, 44, 1993–2028. [Google Scholar] [CrossRef]
- Ai, Y.-J.; Li, F.-P.; Yang, J.-Q.; Lu, S.; Gu, H.-H. Research progress and potential functions of AMF and GRSP in the ecological remediation of metal tailings. Sustainability 2022, 14, 9611. [Google Scholar] [CrossRef]
- Säle, V.; Sieverding, E.; Oehl, F. Growth responses of three European weeds on different AMF species during early development. Plants 2022, 11, 2020. [Google Scholar] [CrossRef]
- Oliveira, J.S.D.; Ramos, N.P.; Júnior, J.L.; Xavier, L.P.; Andrade, E.H.; Mello, A.H.; Setzer, W.N.; Da Silva, J.K.R. Secondary metabolism and plant growth of Piper divaricatum (Piperaceae) inoculated with arbuscular mycorrhizal fungi and phosphorus supplementation. Agronomy 2022, 12, 596. [Google Scholar] [CrossRef]
- Nafady, N.A.; Sultan, R.; El-Zawahry, A.M.; Mostafa, Y.S.; Alamri, S.; Mostafa, R.G.; Hashem, M.; Hassan, E.A. Effective and promising strategy in management of tomato root-knot nematodes by Trichoderma harzianum and arbuscular mycorrhizae. Agronomy 2022, 12, 315. [Google Scholar] [CrossRef]
- Gujre, N.; Soni, A.; Rangan, L.; Tsang, D.C.; Mitra, S. Sustainable improvement of soil health utilizing biochar and arbuscular mycorrhizal fungi: A review. Environ. Pollut. 2021, 268, 115549. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.Z.; Imtiaz, M.; Ditta, A.; Rizwan, M.S.; Ashraf, M.; Mehmood, S.; Aziz, O.; Mubeen, F.; Ali, M.; Elahi, N.N.; et al. Response of growth, antioxidant enzymes and root exudates production towards As stress in Pteris vittata and Astragalus sinicus colonized by arbuscular mycorrhizal fungi. Environ. Sci. Pollut. Res. 2020, 27, 2340–2352. [Google Scholar]
- Quraishi, M.A.A.; Khan, K.G.; Yaqoob, M.S. Range Management in Pakistan; Qazi Publications: Lahore, Pakistan, 1993. [Google Scholar]
- Gohl, B. Tropical Feeds. Feed Information Summaries and Nutritive Values; FAO Animal Production and Health Series 12; FAO: Rome, Italy, 1981. [Google Scholar]
- Kheyri, Z.; Moghaddam, M.; Farhadi, N. Inoculation efficiency of different mycorrhizal species on growth, nutrient uptake, and antioxidant capacity of Calendula officinalis L.: A comparative study. J. Soil Sci. Plant Nutr. 2022, 22, 1160–1172. [Google Scholar] [CrossRef]
- Qin, Y.; Zhang, W.; Feng, Z.; Feng, G.; Zhu, H.; Yao, Q. Arbuscular mycorrhizal fungus differentially regulates P mobilizing bacterial community and abundance in rhizosphere and hyphosphere. Appl. Soil Ecol. 2022, 170, 104294. [Google Scholar] [CrossRef]
- Gerami, Z.; Lakzian, A.; Hemati, A.; Amirifar, A.; Asgari Lajayer, B.; Van Hullebusch, E.D. Effect of cadmium on sorghum root colonization by glomerular fungi and its impact on total and easily extractable glomalin production. Environ. Sci. Pollut. Res. 2021, 28, 34570–34583. [Google Scholar] [CrossRef]
- Heydari, L.; Bayat, H.; Gregory, A.S. Investigating the effect of inoculation of chickpea with Rhizobium and mycorrhizal fungi (Funneliformis mosseae) on soil mechanical and physical behavior. Geoderma 2021, 385, 114860. [Google Scholar] [CrossRef]
- Chaudhry, M.S.; Nasim, F.H.; Khan, A.G. Mycorrhizas in the perennial grasses of Cholistan desert, Pakistan. Int. J. Bot. 2006, 2, 210–218. [Google Scholar] [CrossRef][Green Version]
- Ullah, A.; Ahmad, I.; ur Rahman, M.H.; Waseem, M.; Waqas, M.M.; Bhatti, M.A.; Ahmad, A. Optimizing management options through empirical modeling to improve pearl millet production for semi-arid and arid regions of Punjab, Pakistan. Sustainability 2020, 12, 7715. [Google Scholar] [CrossRef]
- Gerdemann, J.W.; Nicolson, T.H. Spores of mycorrhizal Endogone species extracted from soil by wet sieving and decanting. Trans. Br. Mycol. Soc. 1963, 46, 235–244. [Google Scholar] [CrossRef]
- Olsen, S.R.; Cole, C.V.; Watanabe, F.S.; Dean, L.A. Estimation of Available Phosphorus in Soils by Extraction with Sodium Bicarbonate; Circular No.939; U.S. Department of Agriculture: Washington DC, USA, 1954. [Google Scholar]
- Walkley, A.; Black, I.A. An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Tahat, M.M.; Kamaruzaman, S.; Radziah, O.; Kadir, J.; Masdek, H.N. Response of Lycopersicum esculentum Mill. to Different arbuscular mycorrhizal fungi species. Asian J. Plant Sci. 2008, 7, 479–484. [Google Scholar] [CrossRef]
- Kazemi, R.; Ronaghi, A.; Yasrebi, J.; Ghasemi-Fasaei, R.; Zarei, M. Effect of shrimp waste–derived biochar and arbuscular mycorrhizal fungus on yield, antioxidant enzymes, and chemical composition of corn under salinity stress. J. Soil Sci. Plant Nutr. 2019, 19, 758–770. [Google Scholar] [CrossRef]
- Phillips, J.M.; Hayman, D.S. Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans. Br. Mycol. Soc. 1970, 55, 158–161. [Google Scholar] [CrossRef]
- Biermann, B.; Linderman, R.G. Use of vesicular-arbuscular mycorrhizal roots, intraradical vesicles, and extra radical vesicles as inoculum. New Phytol. 1983, 95, 97–105. [Google Scholar] [CrossRef]
- Morton, J.B.; Benny, L. Revised classification of arbuscular mycorrhizal fungi (Zygomycetes) a new order, Glomales, two new sub-orders Glomineae and Gaigasporineae, and two new families, Acaulosporaceae and Gigasporaceae with an emendation of Glomaceae. Mycotaxan 1990, 37, 471–491. [Google Scholar]
- Pérez, Y.; Schenck, N.C. A unique code for each species of VA mycorrhizal fungi. Mycologia 1990, 82, 256–260. [Google Scholar] [CrossRef]
- Hall, I.R.; Fish, B.J. A key to the Endogonaceae. Trans. Br. Mycol. Soc. 1979, 73, 261–270. [Google Scholar] [CrossRef]
- Simpson, E.H. Measurement of diversity. Nature 1949, 163, 168. [Google Scholar] [CrossRef]
- Steel, R.G.D.; Torrie, J.H.; Dicky, D.A. Principles and Procedures of Statistics—A Biometrical Approach; McGraw-Hill: Singapore, 1997; pp. 204–227. [Google Scholar]
- Yasmeen, T.; Tariq, M.; Iqbal, S.; Arif, M.S.; Riaz, M.; Shahzad, S.M.; Ali, S.; Noman, M.; Li, T. Ameliorative Capability of Plant Growth Promoting Rhizobacteria (PGPR) and Arbuscular Mycorrhizal Fungi (AMF) Against Salt Stress in the Plant. In Plant Abiotic Stress Tolerance; Springer: Berlin, Germany, 2019. [Google Scholar]
- Parihar, P.; Bora, M. Effect of mycorrhiza (Glomus mosseae) on morphological and biochemical properties of Ashwagandha (Withania somnifera) (L.) Dunal. J. Appl. Nat. Sci. 2018, 10, 1115–1123. [Google Scholar] [CrossRef]
- Sivakumar, N. Effect of edaphic factors and seasonal variation on spore density and root colonization of arbuscular mycorrhizal fungi in sugarcane fields. Ann. Microbiol. 2013, 63, 151–160. [Google Scholar] [CrossRef]
- Begum, N.; Qin, C.; Ahanger, M.A.; Raza, S.; Khan, M.I.; Ashraf, M.; Ahmed, N.; Zhang, L. Role of arbuscular mycorrhizal fungi in plant growth regulation: Implications in abiotic stress tolerance. Front. Plant Sci. 2019, 10, 1068. [Google Scholar] [CrossRef] [PubMed]
- Azcon, R.B.; Ferrol, N.; Azcón-Aguilar, C. Ecological and functional roles of mycorrhizas in semiarid ecosystems of Southeast Spain. J. Arid Environ. 2011, 75, 1292–1301. [Google Scholar]
- Panwar, J.; Tarafdar, J.C. Arbuscular mycorrhizal fungal dynamics under Mitragyna parvifolia (Roxb.) Korth. in the Thar Desert. Appl. Soil Ecol. 2006, 34, 200–208. [Google Scholar] [CrossRef]
- Mehboob; Vyas, A. Diversity of am fungi in the rhizosphere of Trigonellafoenum greacum in western Rajasthan. Int. J. Plant Anim. Environ. Sci. 2013, 3, 38–43. [Google Scholar]
- Hontoria, C.; García-González, I.; Quemada, M.; Roldán, A.; Alguacil, M.M. The cover crop determines the AMF community composition in the soil and roots of maize after a ten-year continuous crop rotation. Sci. Total Environ. 2019, 660, 913–922. [Google Scholar] [CrossRef]
- Cofré, N.; Becerra, A.G.; Marro, N.; Domínguez, L.; Urcelay, C. Soybean growth and foliar phosphorus concentration mediated by arbuscular mycorrhizal fungi from soils under different no-till cropping systems. Rhizosphere 2020, 16, 100254. [Google Scholar] [CrossRef]
- Manoharan, L.; Rosenstock, N.P.; Williams, A.; Hedlund, K. Agricultural management practices influence AMF diversity and community composition with cascading effects on plant productivity. Appl. Soil Ecol. 2017, 115, 53–59. [Google Scholar] [CrossRef]
- Zhu, X.; Yang, W.; Song, F.; Li, X. Diversity and composition of arbuscular mycorrhizal fungal communities in the cropland black soils of China. Glob. Ecol. Conserv. 2020, 22, e00964. [Google Scholar] [CrossRef]
- Shukla, A.; Kumar, A.; Prasad, R.; Kumar, N.; Dhyani, S.K.; Chaturvedi, O.P.; Arunachalam, A. Efficacy of arbuscular mycorrhizal fungi and bacterial inoculants in enhancing yield of Phaseolus mungo L. and Vigna radiata (L.) R. Wilczek under central Indian conditions. J. Soil Sci. Plant Nutr. 2022, 22, 1559–1571. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, D.; Liu, Y.; Cui, Z.; Chen, X.; Zou, C. Zinc uptake and accumulation in winter wheat relative to changes in root morphology and mycorrhizal colonization following varying phosphorus application on calcareous soil. Field Crop. Res. 2016, 197, 74–82. [Google Scholar] [CrossRef]
- Liang, J.-F.; An, J.; Gao, J.-Q.; Zhang, X.-Y.; Song, M.-H.; Yu, F.-H. Interactive effects of biochar and AMF on plant growth and greenhouse gas emissions from wetland microcosms. Geoderma 2019, 346, 11–17. [Google Scholar] [CrossRef]
- Evelin, H.; Kapoor, R.; Giri, B. Arbuscular mycorrhizal fungi in alleviation of salt stress: A review. Ann. Bot. 2009, 104, 1263–1280. [Google Scholar] [CrossRef]
- Mathur, N.; Singh, J.; Bohra, S.; Vyas, A. Arbuscular mycorrhizal status of medicinal halophytes in saline areas of Indian Thar desert. Int. J. Soil Sci. 2007, 2, 119–127. [Google Scholar]
- Alloush, G.A.; Zeto, S.K.; Clark, R.B. Phosphorus source, organic matter, and arbuscular mycorrhiza effects on growth and mineral acquisition of chickpea grown in acidic soil. J. Plant Nutr. 2000, 23, 1351–1369. [Google Scholar] [CrossRef]
- Zaidi, A.; Khan, M.S.; Amil, M. Interactive effect of rhizotrophic microorganisms on yield and nutrient uptake of chickpea (Cicer arietinum L.). Eur. J. Agron. 2003, 19, 15–21. [Google Scholar] [CrossRef]
- Akhtar, M.S.; Siddiqui, Z.A. Effects of Glomus fasciculatum and Rhizobium sp. on the growth and root-rot disease complex of chickpea. Arch. Phytopathol. Plant Protect. 2007, 40, 37–43. [Google Scholar] [CrossRef]
- Chaudhry, S.; Luhach, J.; Sharma, V.; Sharma, C. Assessment of diesel degrading potential of fungal isolates from sludge contaminated soil of petroleum refinery, Haryana. Res. J. Microbiol. 2012, 7, 182–190. [Google Scholar] [CrossRef]
- Bohrer, G.; Kagan-Zur, V.; Roth-Bejerano, N.; Ward, D. Effects of environmental variables on the vesicular-arbuscular mycorrhizal abundance in wild populations of Vangueria infausta. J. Veg. Sci. 2001, 12, 279–288. [Google Scholar] [CrossRef]
- Muthukumar, T.; Udaiyan, K. Seasonality of vesicular-arbuscular mycorrhizae in sedges in a semi-arid tropical grassland. Acta Oecol. 2002, 23, 337–347. [Google Scholar] [CrossRef]
- Elmostapha, O.; Hanane, D.; Rachid, B.; Lahcen, O. Application of arbuscular mycorrhizal fungi isolates from semi-arid Mediterranean ecosystems as biofertilizers in argan tree development. J. Soil Sci. Plant Nutr. 2022, 22, 944–955. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).