Abstract
In the subtropical climate of Taiwan, the cool season (January–June) is most productive for rice cultivation. However, the cool season also sees a large variability and weather impact on the crop. To assess the effect of winter monsoon variability and the warming climate, a common ORYZA(v3) model was used to derive the potential growth and yield of the japonica rice variety in different agro-climatological areas of Taiwan. The simulation was constructed for three planting dates (15 January, 30 January, and 14 February) in three time periods (1986–2005, 2006–2025, and 2026–2045) under a high-emission (RCP8.5) scenario, using a dynamically downscaled regional climate simulation data set (CORDEX). The result indicates that increased temperature during the early season significantly shortens the rice vegetative phase in all planting dates. Compared to the 1986 condition, rice maturation is projected to be 6–9 days and 7–11 days earlier by 2045 for the central-west and the north-east regions, respectively. In the future, decreased duration of crop growth will lead to a lowered yield, while increased CO2 can enhance rice yield by 8.5–18%. Rice yield is projected to decline by 3.3-to-10% during 2026–2045, offsetting the fertilizing effect of increasing CO2. Meanwhile, yield variability will increase in the future, due to more exposure to extremely low- and high-yield conditions. As such, a large yield reduction resulting from the increased variability (down to 34%) can offset the increased mean yield.
1. Introduction
Rice (Oryza sativa) is sensitive to low temperature because its growth and development can be severely damaged at temperatures below 15 °C [1]. Situated in the East Asia winter monsoon and as one of the major rice growers, Taiwan’s first crop of rice is cultivated during January–June, in which the seedling stage is exposed to chilling stress [2]. The reproductive phases, especially anthesis, flowering and grain filling periods, are most susceptible to either low temperature (<18 °C) or high temperature (>32 °C), among other unfavorable environments [1,3,4]. Since the 1990s, Taiwan’s climate has seen persistent changes in both the mean and variability of precipitation, temperature, and atmospheric CO2 concentration [5,6]. The mean air temperature in Taiwan has increased at an average pace of 0.3 °C per decade since 1980 and winter in the northern region has seen the most warming [5]. This trend is accompanied by the increasing number of hot days (>30 °C) and decreasing cold days (<13 °C), accompanied by reduced winter precipitation [5,6]. Not all such changes are bad for rice, for the increase in winter temperature may reduce cold injury while the warmer spring may increase spikelet sterility.
The impacts of climate change on Taiwan’s rice yield have been studied using both crop models [7] and statistical models [8,9,10,11], with a focus on the mean yield change for the whole island of Taiwan. However, the growth and yield variability with respect to rice variety in different climatological areas have not yet been satisfactorily understood. While a warmer climate arguably is beneficial to rice growth, crop models predicted a reduction in rice yield by approximately 7.4% for each 1 °C increase in air temperature [7]. Statistical models suggested different impacts of climate change on rice yield with various magnitudes and adaptation approaches [8]. With a focus on mid- to high-latitude regions, past studies have suggested an additional benefit from rising CO2 in future rice yield [12,13,14,15]. In Asia, a simulated increment of CO2 up to 600 ppm can increase rice yield by 24–36%, but the advantage of CO2 will reduce with the increasing temperature [7]. As such, Taiwan’s rice yield was projected to increase from 2 to 28% depending on the crop models and warming scenarios selected [7].
This study aims to evaluate the influence of climate change during the winter-spring season on the variability of rice growth and yield in different regions. The study areas, data collection, and simulation framework are described in Section 2, including the common rice model ORYZA(v3) that validates Taiwan’s unique climate and rice production practices. Section 3 presents simulation outputs that consist of model calibration and validation (Section 3.1), responses of rice to temperature (Section 3.2 and Section 3.3), and potential rice yield with/without CO2 effects (Section 3.4 and Section 3.5). Section 4 presents a discussion, and Section 5 provides some conclusions.
2. Materials and Methods
2.1. Study Areas
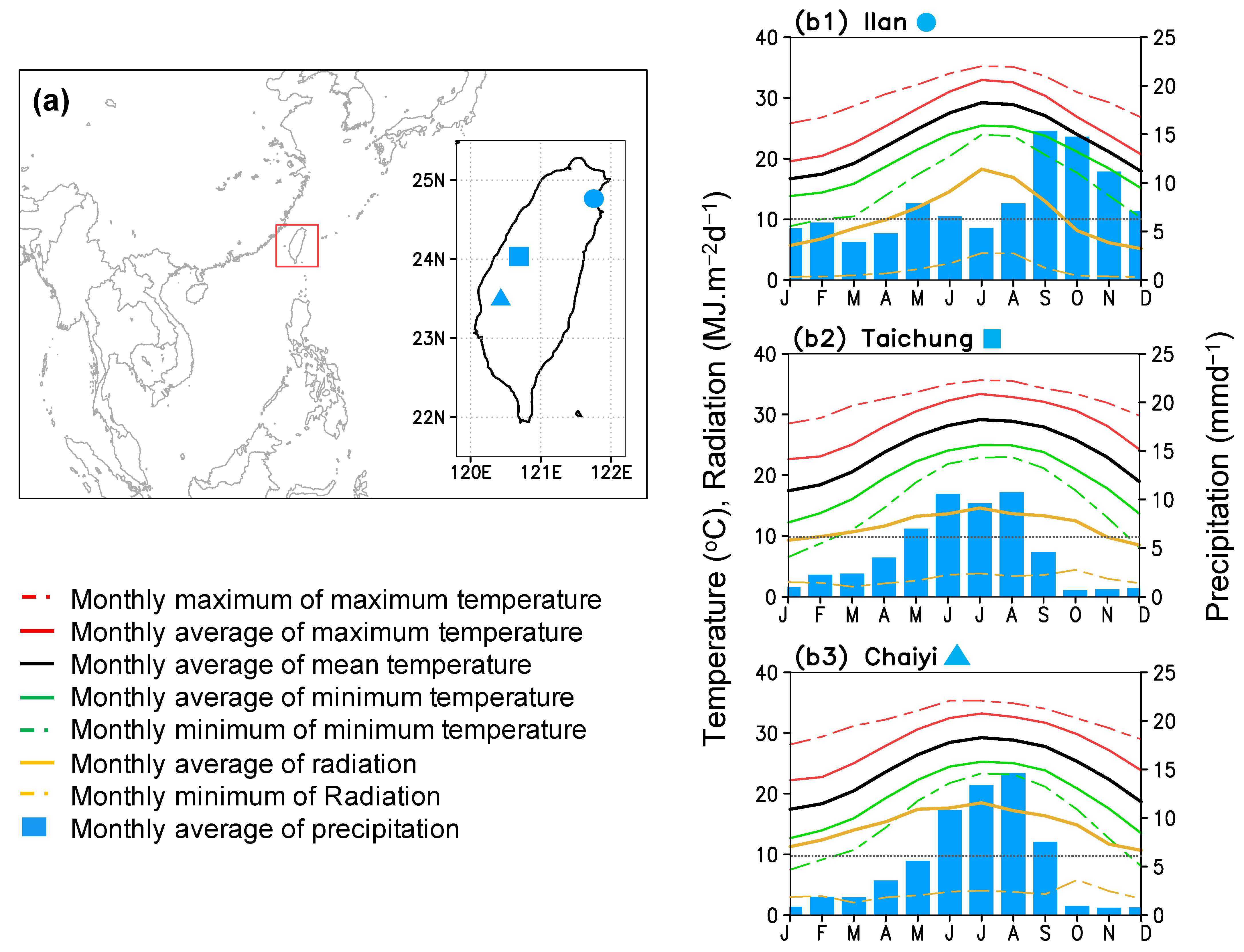
The study was conducted for three main cultivated areas in Taiwan: Taichung, Chaiyi, and Illan (Figure 1a), in which the dry season (January–June) comprises the first rice cultivation. Illan is in the north-east, while Taichung and Chaiyi are in the central-western island (Figure 1a inset). Climatology patterns of these three locations are different (Figure 1b1–b3). During the early to middle period of rice cultivation (January–March), the climate in Illan is wet-cold with low radiation due to cloud cover. In Taichung, the climate is dry-cold with moderate radiation, and in Chaiyi, it is dry-cold with high radiation. Peak rainy season occurs in late spring for Taichung, in summer for Chaiyi, and in fall-winter for Ilan (Figure 1b1–b3) [16]. During the growing period, Chaiyi, Taichung, and Ilan receive radiation of approximately 14.7, 11.5, and 9.6 MJ m−2 day−1, respectively. Rice cultivation in these three locations requires irrigation and rice growth takes place within average temperatures of 16–30 °C. The annual mean temperature of Illan (21.30 °C) is slightly lower than that of Taichung (22.51 °C) and Chaiyi (22.47 °C), making them ideal for rice cultivation. Because of the winter monsoon and cold surge, which can cause the minimum temperature to drop below 10 °C occasionally, the rice crop can be at risk for cold injury in January-February.
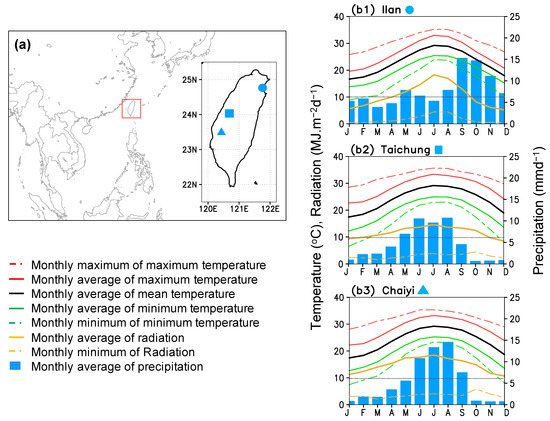
Figure 1.
Panel (a) shows location of Taiwan (red box) and study areas; Ilan (circle), Taichung (square), and Chaiyi (triangular), and panels (b1–b3) present climatology patterns of Ilan (1987–2016), Taichung (1990–2016), and Chaiyi (1987–2016).
2.2. Data Collection
2.2.1. Observed Rice Data
Growth and yield data of rice variety Tainung 71 (TN71) (Oryza sativa subsp. japonica) was obtained from field experiments conducted in the dry season during 2009 to 2016 at the Taiwan Agricultural Research Institute in Taichung (120.688 °E, 24.031 °N). Sufficient plant nutrients with 120 kg nitrogen ha−1, irrigation, and pest management were supplied to the rice fields. Major phenological stages were recorded and plant samples were collected weekly to measure leaf area index (LAI) and biomass of different plant parts (green leaf, dead leaf, stem, panicle, and total). Grain yield at harvest was reported at a moisture content of 14%.
2.2.2. Observed Weather Data
We obtained the observed daily precipitation (P), maximum temperature (Tmax), minimum temperature (Tmin), mean temperature (T), shortwave radiation (RAD), average wind speed (WS), and relative humidity (RH) from weather stations located in the three locations of Taichung (120.688° E, 24.031° N), Chaiyi (120.433° E, 23.496° N), and Illan (121.757° E, 24.764° N), for the period of 1987–2016 for Chaiyi and Illan and 1990–2016 for Taichung. We calculated saturated vapor pressure (Es, kPa) using T and actual vapor pressure (Ea, kPa) using Es and RH (Equations (S2) and (S3) in Table S1). Observed CO2 concentration in Taiwan was obtained from the NOAA website [17].
2.2.3. Simulated and Projected Climate Data
We used high-resolution regional climate model data from the historical experiment (1986–2005) and future climate projection (2006–2045) from the RCP 8.5 emission scenario. The datasets were outputs from three regional climate models downscaling the CMIP5 global simulations, which are archived by the Coordinated Regional Climate Downscaling Experiment (CORDEX) [18] with a 0.44° (~50 km) spatial resolution for East-Asia domain (EAS-44) (see model names in Table S1). Six variables of daily Tmax, Tmin, P-flux, surface downwelling RAD flux, near-surface RH, and near-surface WS were extracted on the grid points nearest to the weather stations in Taichung, Chaiyi, and Illan. We used the calculated Ea as described in (2). These modeled datasets were statistically bias-corrected (explained next in Section 2.3.2) before incorporating into the rice model. The data on annual CO2 concentrations were outputs of RCP 8.5 (high-emission) experiment from CMIP5.
2.3. Simulation Framework
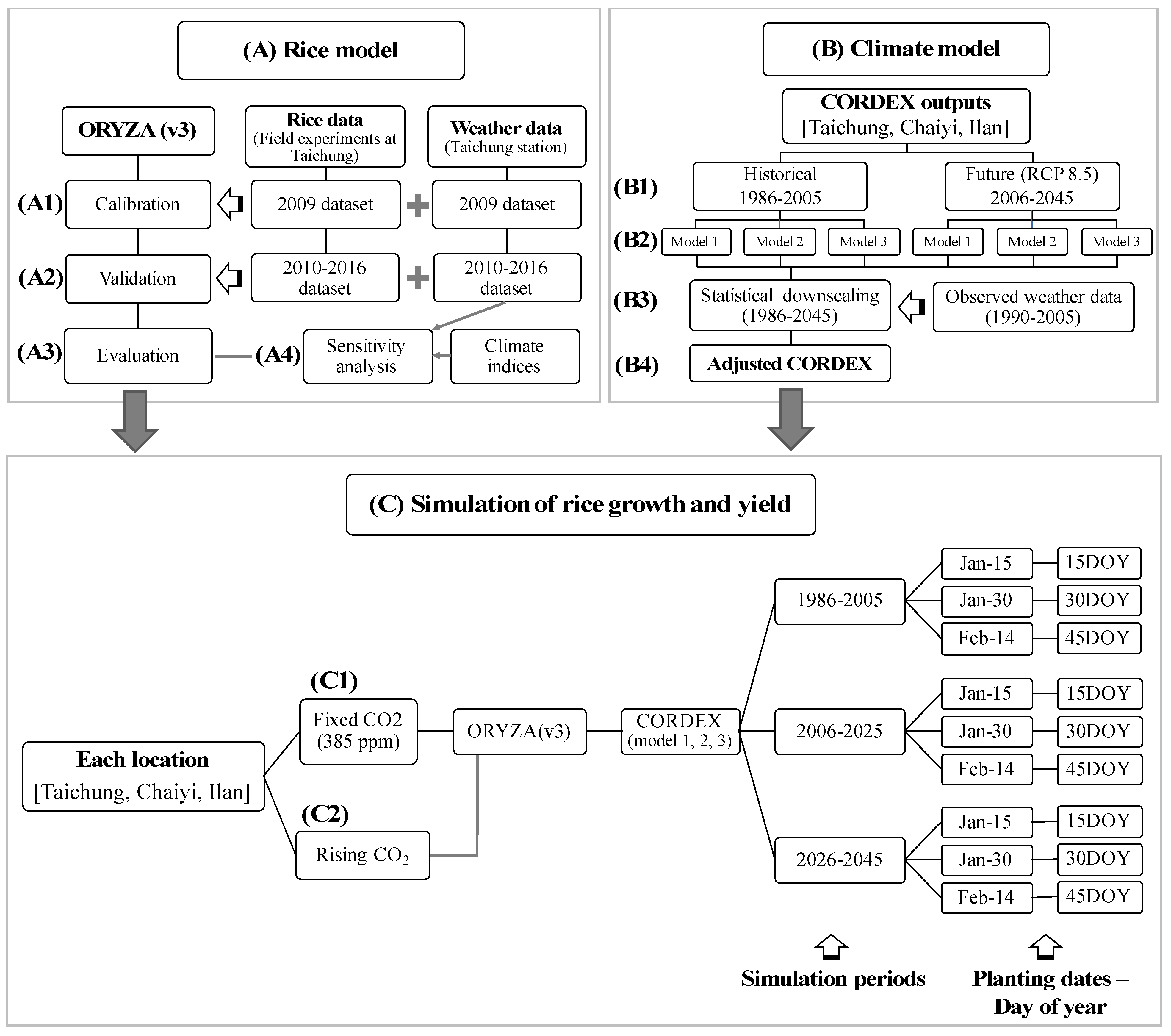
We used the ORYZA(v3) [19], the updated version of ORYZA2000 rice model [20], that can predict rice growth and yield in an irrigated lowland ecosystem with input variables of weather data, crop parameters, soil data, and cultural practices such as fertilizer and irrigation application [19,21,22]. The simulation of yield potential, obtained under no stress from water, nutrients, pests, disease, and weeds [23] can depict the impacts of temperature, solar radiation, and CO2. Since rice cultivation in the dry season in Taiwan is under irrigation, water supply from precipitation is not included. We conducted the simulation following three steps as described in Figure 2.
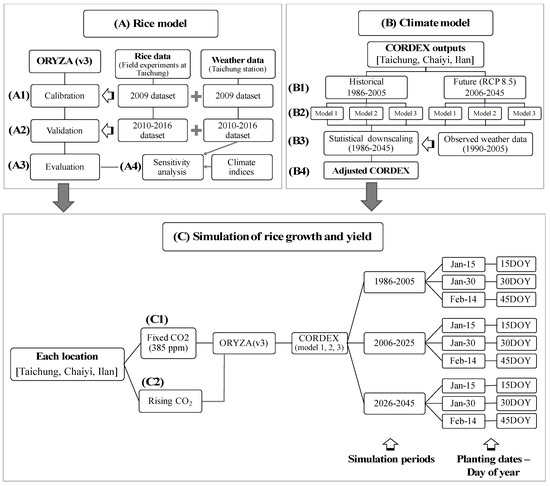
Figure 2.
Diagrams of methodological approaches to simulate rice growth and yield by using ORYZA(v3) rice model and outputs from CORDEX. Step A describes datasets and computational process in ORYZA (v3) for (A1) model calibration, (A2) model validation, (A3) model evaluation, and (A4) sensitivity analysis. Step B describes datasets and downscaling process of outputs from climate model: (B1) historical (1986–2005) and future (2006–2045) datasets, (B2) CORDEX models used in this study, (B3) downscaling process, and (B4) adjusted CORDEX dataset. Step C describes the components of rice growth and yield simulation for two scenarios of CO2 input: (C1) fixed CO2 at 385 ppm and (C2) rising CO2 concentration.
2.3.1. ORYZA(v3) Calibration, Validation, and Evaluation
We used a crop dataset from field experiments in 2009 for calibration and later datasets of 2010–2016 for validation (Figure 2, Step A). Calibration is done for important crop-parameters under potential production by using observed weather parameters and observed rice data (Figure 2, Step A1). We parameterized the development rates of four development stages (DVS) and used these rates (Table S2) for further calibration of the maximum value of relative leaf growth rate, partitioning factors for crop organs, rate of leaf death, and fraction of stem reserves. The calibrated values provide a good relation between observation and simulation in biomass and phenological dates, and were used for validation (Figure 2, Step A2).
Performance of the model after calibration was evaluated by comparing the simulated outputs from validation with observation (Figure 2, Step A3). We determined the slope (α), intercept (β), coefficient of determination (R2), and significance of the linear regression model using Student’s t-test [P(t)1]. Additionally, Student’s paired t-test of means with unequal variance [P(t)2] and absolute root of mean square error (RMSE) were measured (Equation (S3) in Table S1). The model provides the best simulation when α is 1, β is 0, P(t)1 is smaller than 0.05 (significance of regression), P(t)2 is larger than 0.05 (simulated and observed means are not different), and the RMSE is similar or lower to the standard deviation (SD) of observation.
2.3.2. Downscaling of Outputs from Climate Model
Figure 2 step B describes the CORDEX datasets (steps B1 and B2, Section 2.2 (3)) and downscaling process (steps B3 and B4). Each weather parameter of each model was adjusted to have its mean and variation close to the observed by using bias correction [24,25]. The adjusted CORDEX using monthly mean and RMSE of 1990–2005 was applied to the daily data (Equation (S4) in Table S1).
2.3.3. Simulation of Potential Rice Growth and Yield
The ORYZA model simulated rice growth and yield from 1986 to 2045 by using the adjusted-CORDEX data and two scenarios of CO2 input: with and without rising-CO2 concentration (Figure 2, Step C). We used fixed CO2 at 385 ppm for the simulation without a rising-CO2 effect (Figure 2, Step C1), which was measured at the end of historical period (2005) for rice growing season. The simulation with CO2 effects was run using projected CO2 data of 1986–2045 (Figure 2, Step C2). The simulation for both with and without rising CO2 was done for three planting dates based on day of year (DOY); 15 DOY (15 January), 30 DOY (30 January), and 45 DOY (14 February) and computed for three periods (1986–2005, 2006–2025, and 2026–2045). Percentage change of mean yields in the future were calculated and compared to historical yield (1986–2005). To illustrate yield distribution and variation, probability density function was calculated by following [26,27] with a bin size of 0.5 tons ha−1 for the three periods.
3. Results
3.1. ORYZA(v3) Performance
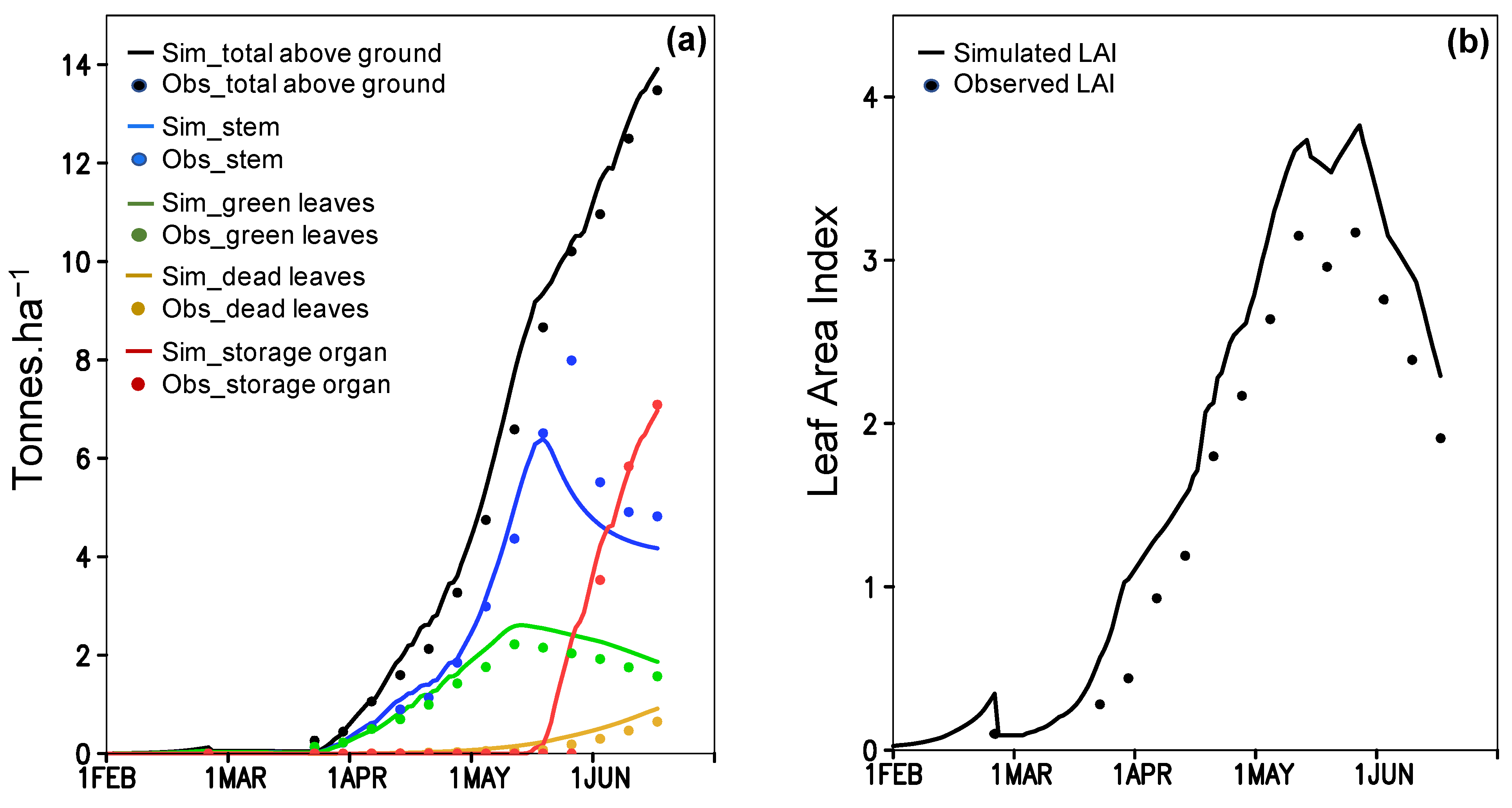
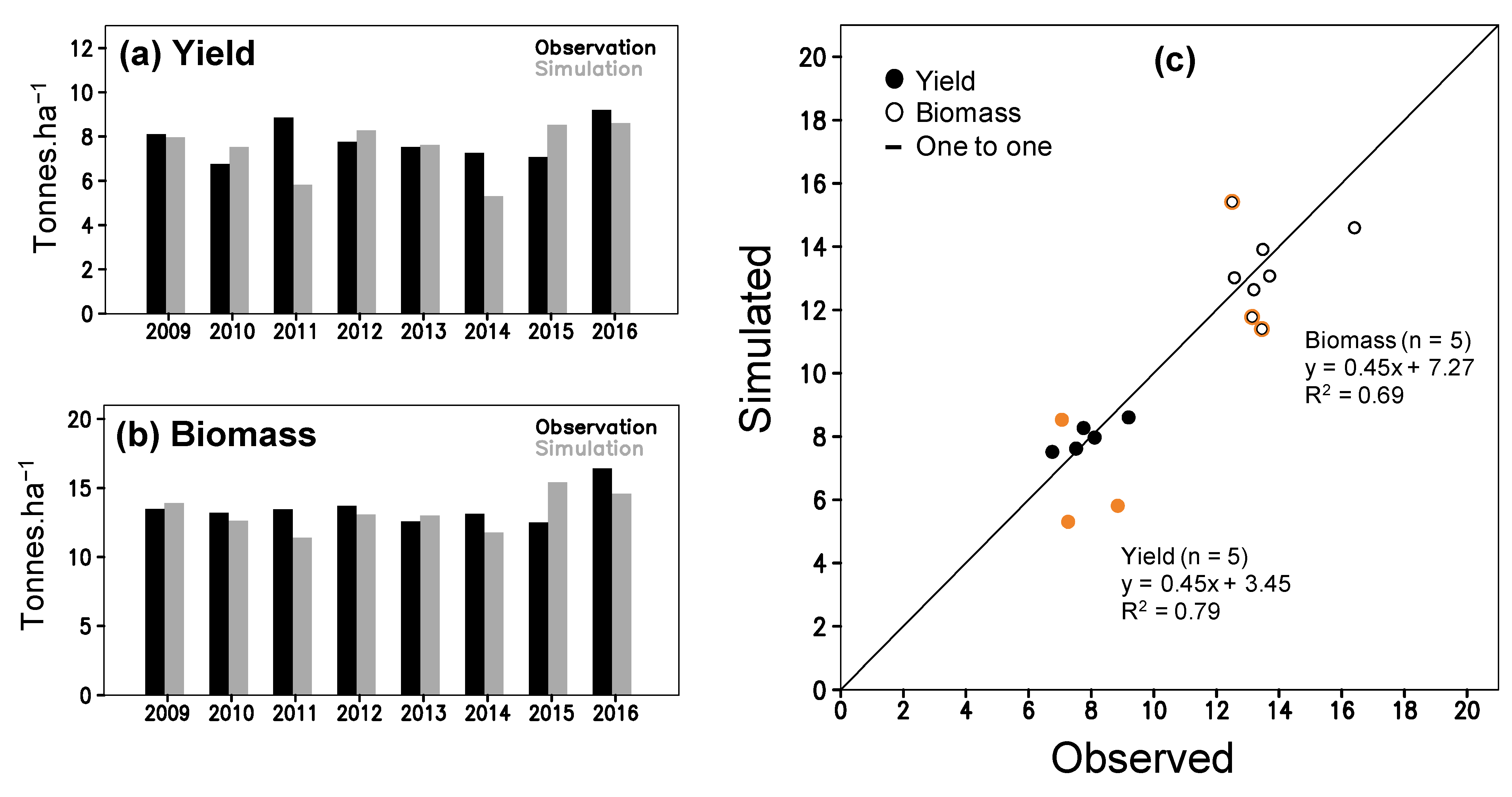
The parameterization between simulation and observation for the above ground biomass (Figure 3a) and LAI (Figure 3b) obtains a good fit (R2 > 0.93; Table S3). The simulation of phenological dates is validated by significant R2 (0.98 and 0.79; P(t)1 < 0.01) of linear regression (Table S4). During the validation, P(t)2 values for flowering (0.91) and maturity (0.93) dates do not indicate any statistical difference between the simulation and observation (Table S4). Simulated yield and total above ground biomass (TAGB) are also indifferent to the observation during 2009–2016, in which the P(t)2 of yield and TAGB are 0.51 and 0.59 (Table S4). The simulation provided RMSE of 1.433 tons ha−1 for yield and 1.538 tons ha−1 for TAGB that are higher than standard deviation (SD) of the observation (1.240 and 1.362 tons ha−1). The high RMSE results from large bias in simulated 2011, 2014 and 2015 data (Figure 4a,b) (see 4 for further discussion). If those three-year outliers are excluded, the correlation between simulation and observation became closer to the one-one line with a significant R2 (Figure 4c; Table S4). The new RMSE of yield (0.551 tons ha−1) and TAGB (1.043 tons ha−1) positively compares to the observed SD (0.892 and 1.479 tones ha−1) (Table S4). All results suggest acceptability of ORYZA(v3) performance to simulate rice growth and yield for the TN67 rice variety.
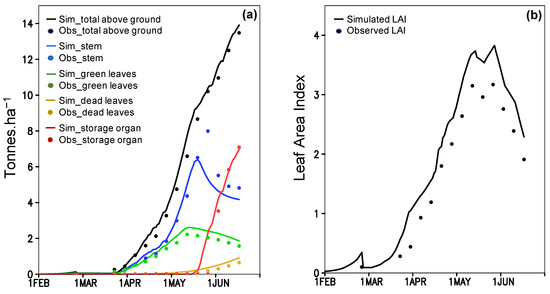
Figure 3.
ORYZA(v3) model calibration by using experimental data from 2009; observed (Obs) and simulated (Sim) (a) biomass, and (b) Leaf Area Index (LAI).
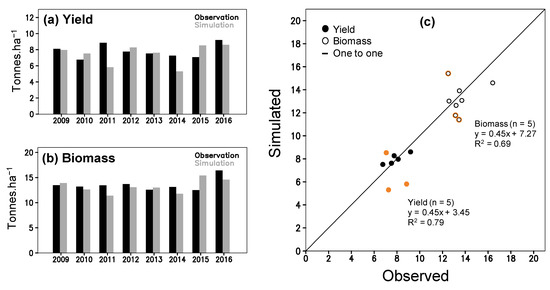
Figure 4.
ORYZA(v3) model validation by using experiment data from 2009–2016; observed and simulated (a) yield, and (b) total above ground biomass; panel (c) shows scatter plots for yield and biomass (orange-circle marks are 2011, 2014 and 2015).
3.2. Response of Rice Phenology on Warming Trend
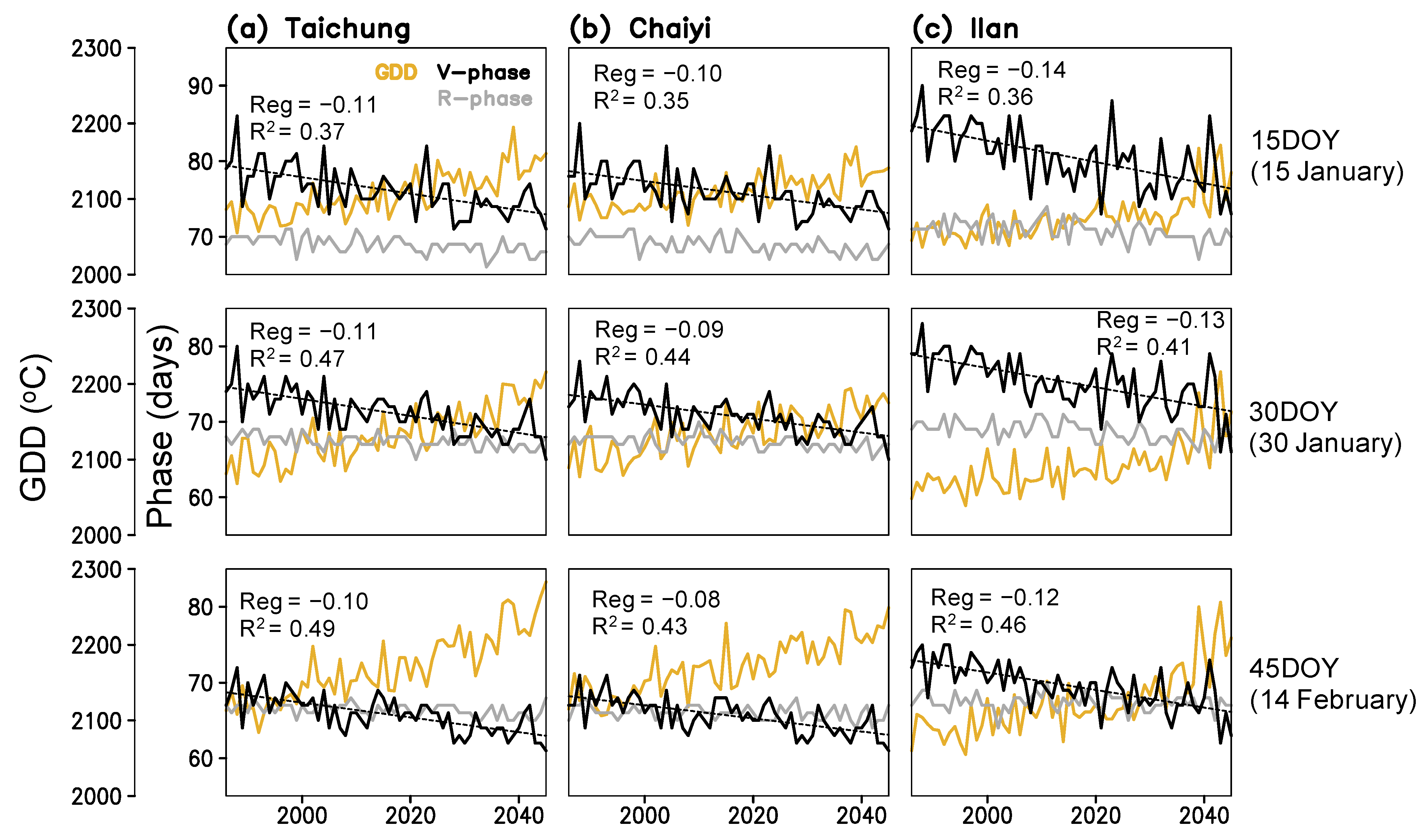
The mean temperature as projected by CORDEX illustrates a warming trend during the rice growing season at an increasing rate of 0.030, 0.025, and 0.031 °C year−1 at Taichung, Chaiyi, and Ilan, respectively (Figure S1). The change is from 22 ± 0.4 °C in the historical period (1986–2005) to 23 ± 0.4 °C by 2045 for Taichung and Chaiyi. The higher increase of mean temperature occurs at Ilan, ranging from 21 ± 0.3 °C to 23 ± 0.6 °C.
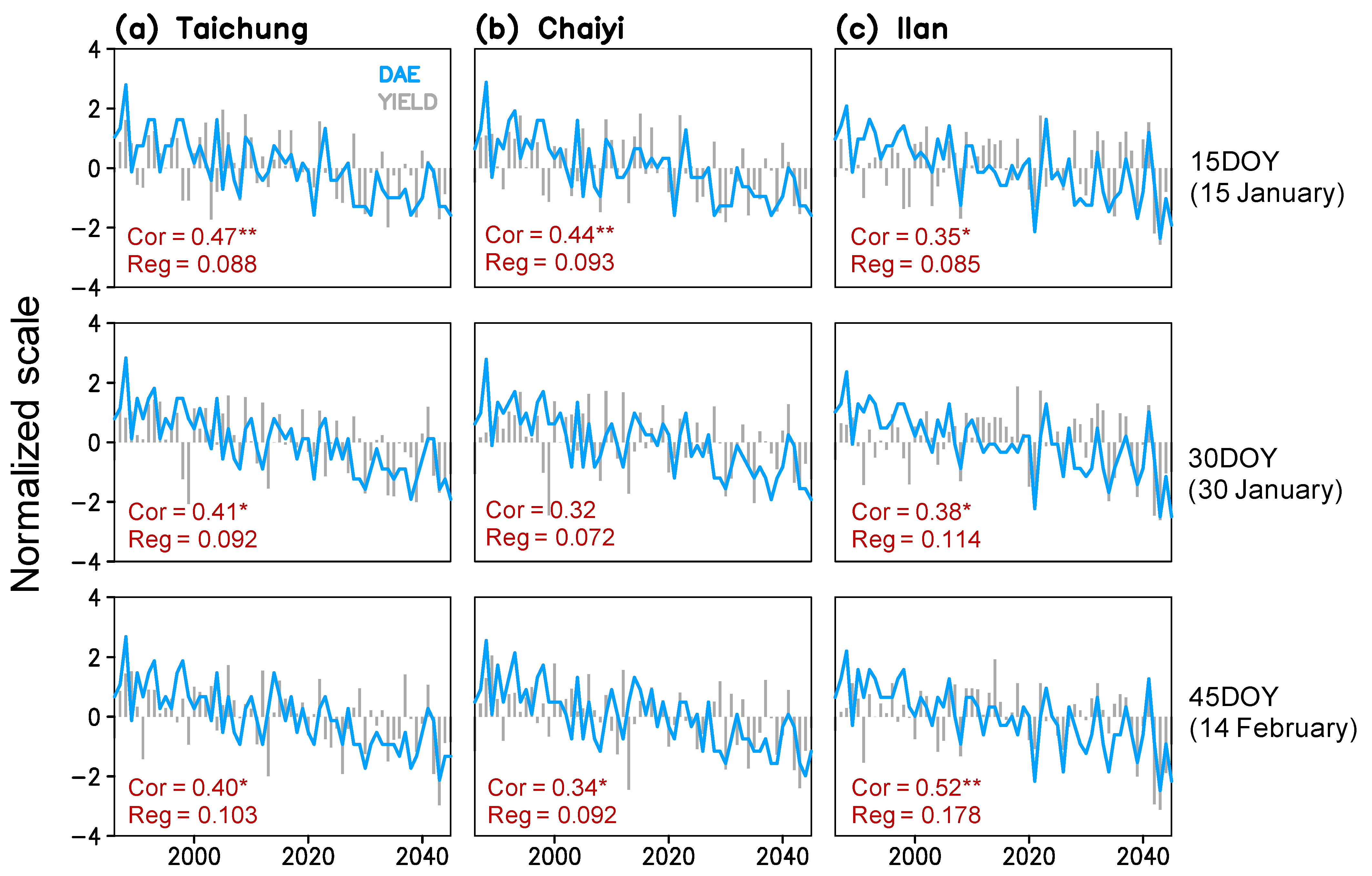
Figure 5 shows that rice phenological stages respond to the rising temperature with a simulated shortening. By 2045, rice maturation at Taichung, Chaiyi, and Illan will be faster by 7–9, 6–8, and 7–11 days compared to the 1980s. The change rates of the vegetative phase and maturity dates are greater in Ilan (1.2–1.4 and 1.2–1.8 day decade−1) than in Taichung (1.0–1.1 and 1.1–1.5 day decade−1) and Chaiyi (0.8–1.0 and 1.1–1.4 day decade−1). The earlier planting date results in the shorter duration of crop growth. On the other hand, the vegetative phase is more responsive to increased GDD than the reproductive stage (panicle initiation to maturation), regardless of with or without CO2 effects. This simulation suggests that the significant change in winter temperature (January-February) can lead to a decreasing trend of maturity date and, in turn, cause the yield reduction in all three locations throughout 1986–2045 (Figure 6).
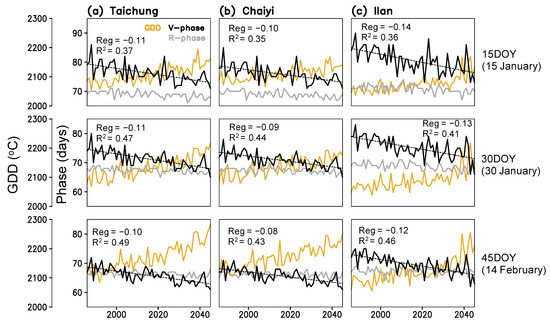
Figure 5.
Time series of simulated growing degree days (GDD), lengths of vegetative (V-phase) and reproductive (R-phase) stages for three planting dates (15, 30, 45 day of year) constructed for Taichung, Chaiyi, and Ilan; linear regression coefficients (Reg) and coefficient of determination (R2) between year and V-phase present in each panel.
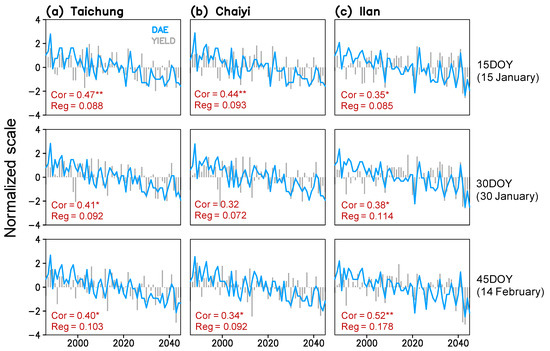
Figure 6.
Time series of simulated maturity date (day after emergence; DAE) and yield for three planting dates (15, 30, 45 day of year) constructed for Taichung, Chaiyi, and Ilan; correlation coefficients (Cor) and linear regression coefficients (Reg) between maturity date and yield present in each panel (*, ** indicate significance of the coefficients exceeding 99% and 99.9% confidence interval).
3.3. Spikelet Sterility Because of Temperature
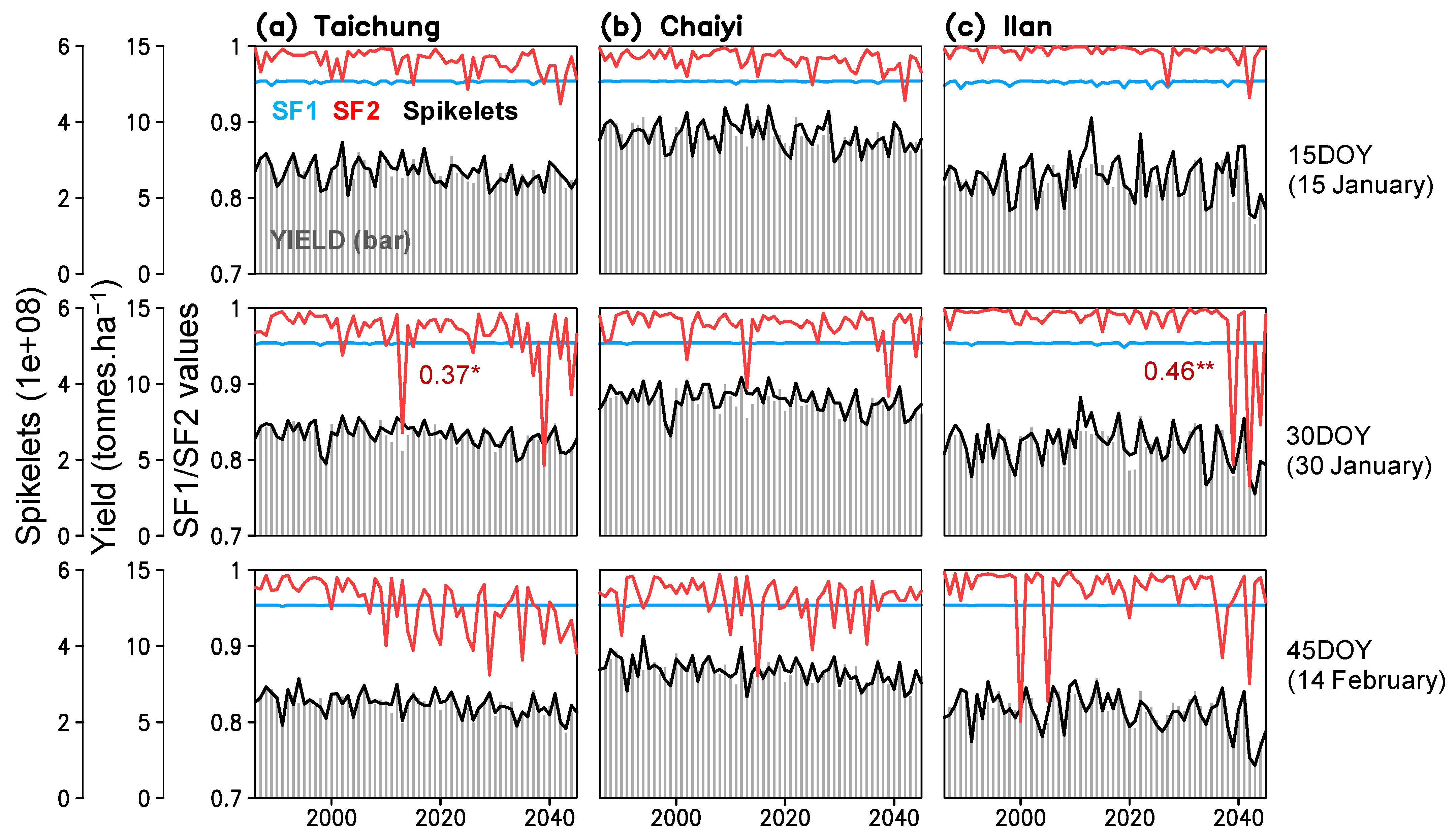
High or low temperature during the flowering stage causes spikelet sterility, which reduces pollen germination on the stigma during anthesis [28,29]. Spikelet sterility was simulated to reach critical low and high temperatures of 21 °C and 36.5 °C, respectively (Equations (S5)–(S7) in Table S1). Thus, we analyzed the spikelet sterility factors with respect to the low temperature (SF1; 1 = low sterility, 0 = high sterility) and high temperature (SF2; 1 = high fertility, 0 = low fertility). The result shows that rice growing in the three locations have low risk (SF1 > 0.95) from cold-related sterility (hereafter “cold sterility”), whereas heat-related sterility (hereafter “heat sterility”) tends to increase both with and without the rising CO2 (Figure 7). While heat sterility is low (SF2 > 0.95) for early planted rice, we note an increasing trend of heat sterility (SF2 < 0.95) for the later planting dates (30DOY and 45DOY). We also found that increased heat sterility for planting on 30DOY in Taichung and Ilan can cause low yield in the future (Figure 7a,c).
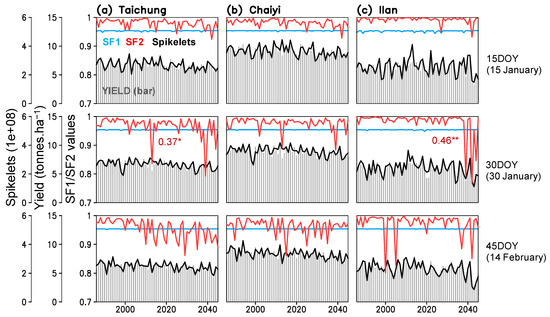
Figure 7.
Time series of simulated yield, number of spikelets, spikelet sterility factor because of low temperature (SF1), and spikelet fertility factor because of high temperature (SF2) for three planting dates (15, 30, 45 day of year) constructed for Taichung, Chaiyi, and Ilan; numbers are correlation coefficients of SF2 and yield (*, ** indicate significant of the coefficients exceeding 99% and 99.9% confidence interval).
3.4. Future Change of Rice Yield with Warming but without Rising CO2
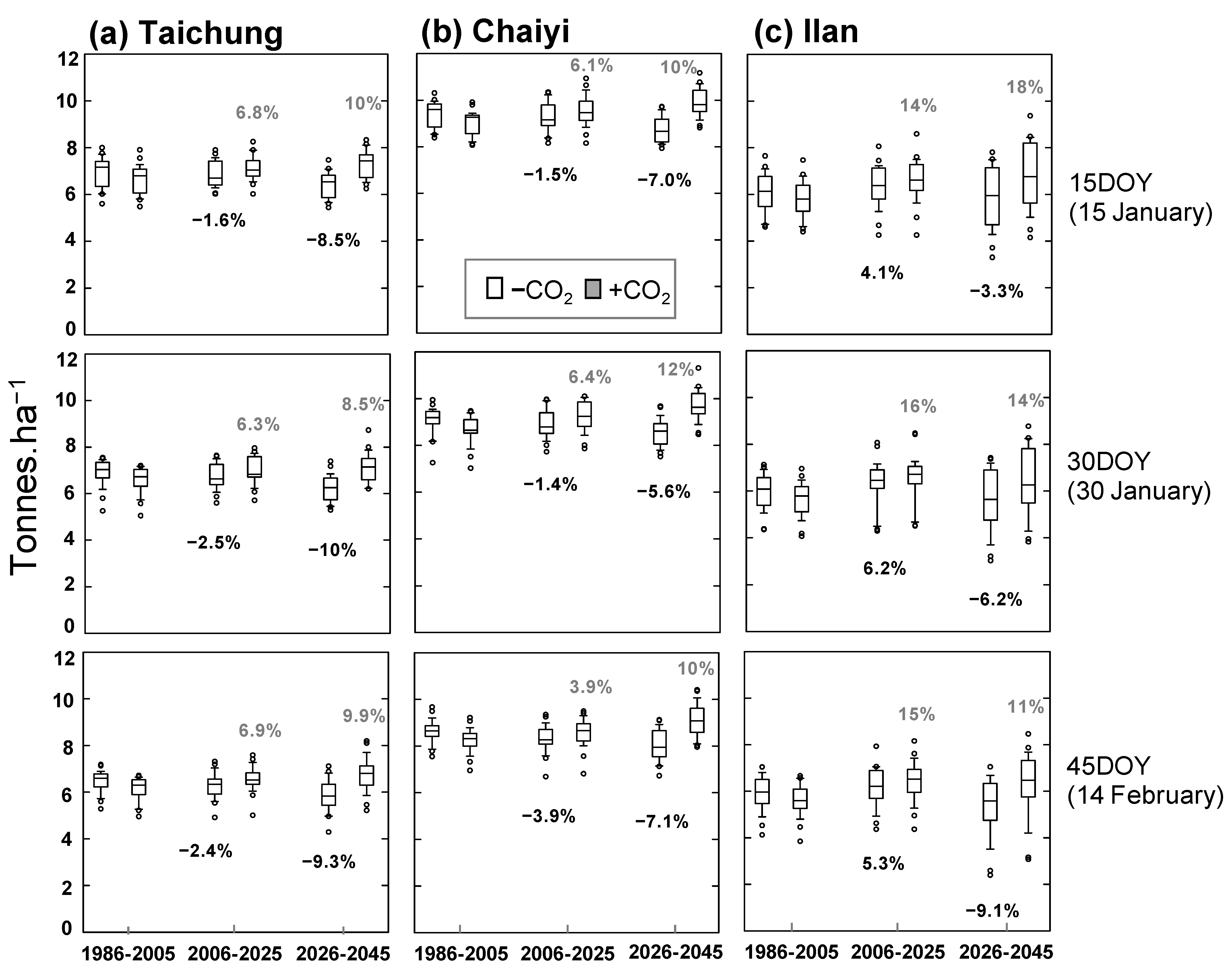
Reduction of average yield in the future is predicted for all locations, in which rice with later planting date is more vulnerable to the warming climate. The simulated yields at Taichung are predicted to reduce by 1.6–2.5% and 8.5–10% for 2006–2025 and 2026–2045, respectively (Figure 8a). Among the three cities, Chaiyi provides the highest simulated yield that corresponds to its suitable climate pattern for rice growing (see Section 2.1). Its future yields reduce to 1.4–3.9% by 2025 and 5.6–7.1% by 2045 from the historical yields (8.63–9.40 tons ha−1) (Figure 8b). By comparison, Ilan has the lowest rice productivity; its historical yields are 5.89–6.10 tons ha−1. The reason for the simulated yield in Ilan to be different from the other two locations (Figure 8c) is that increased temperature during 2006–2025 may provide fewer cold days or cause a mean-temperature shift from the optimum temperature for rice growth. Later in the season, the temperature may be too high above the critical threshold. Increased GDD (Figure S3) also shortens the growth length, while low radiation (Figure S4) may influence the yield reduction.
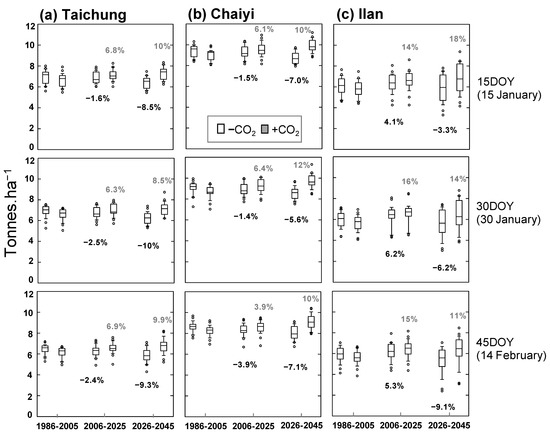
Figure 8.
Simulated yield (with and without projected CO2) for three planting dates (15, 30, 45 day of year) and three periods (1986–2005, 2006–2025, 2026–2045) constructed for Taichung, Chaiyi, and Ilan; numbers indicate percentage of averaged-yield change compared to historical period (1986–2005).
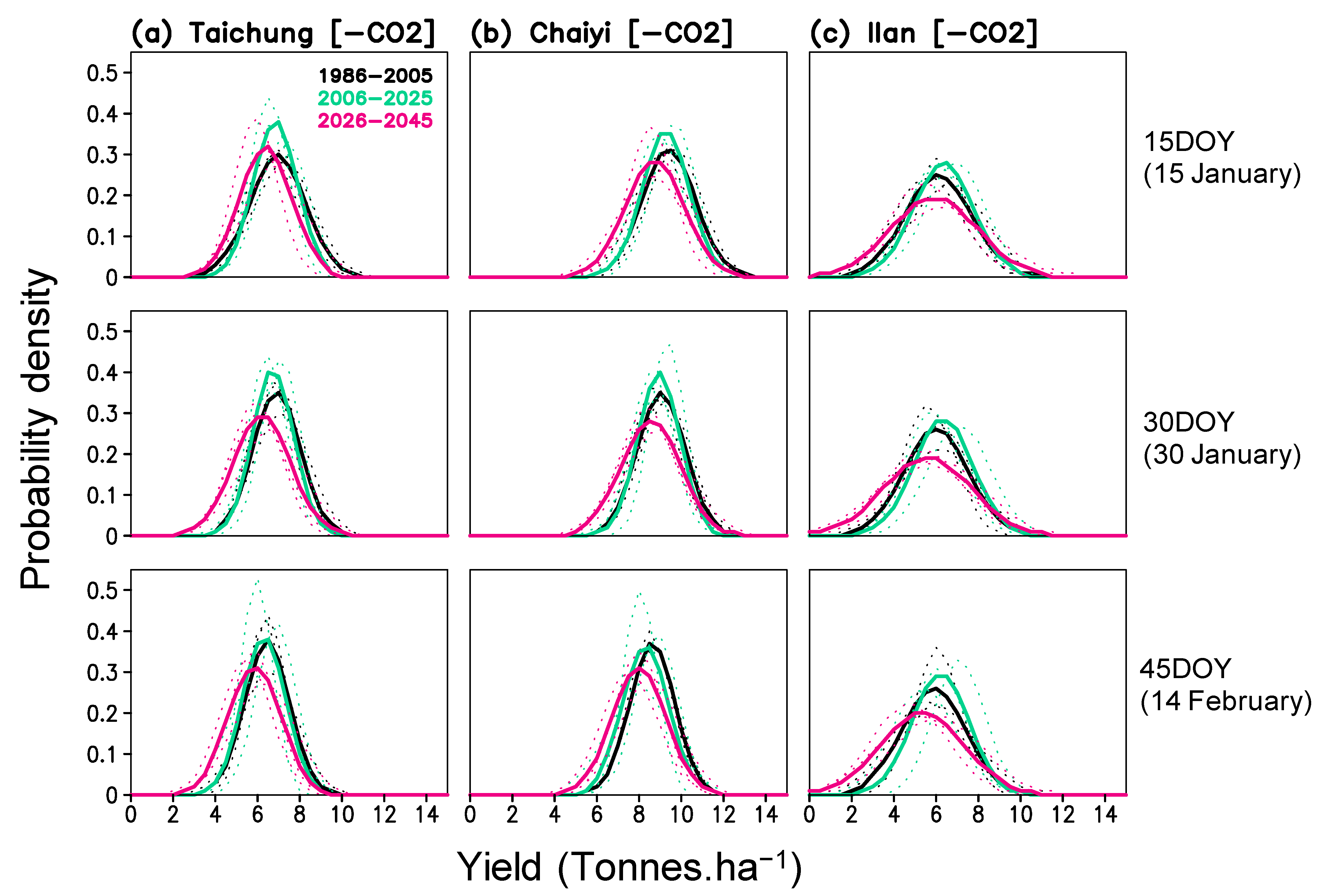
The simulated yields in the three locations also show an increase in their variability. To illustrate this result, we present the probability density of yield in Figure 9. The distribution curves show a shifted mean yield to the lower class in all locations and for all planting dates. The largest increase occurs during 2026–2045, particularly in Ilan. Increased yield variability also expands the probability of low yield to 2–4, 4–6, and 0–2 tons ha−1 for Taichung, Chaiyi, and Ilan, respectively; these are comparable to a 40–70%, 34–56%, and 67–100% reduction from the historical average yield. Therefore, it is arguable that the increase in variability has stronger impacts on rice production than the change in average yield. According to Figures S3 and S4, increased yield variability may be caused by increased GDD and radiation variability during the growing period.
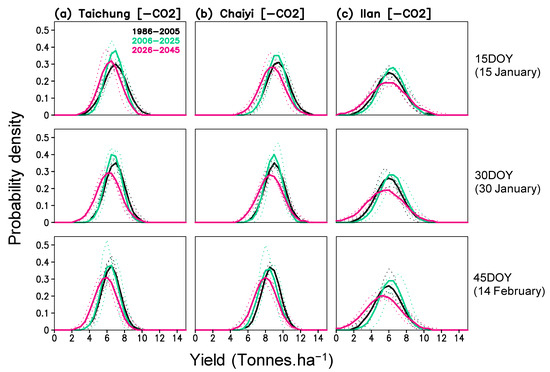
Figure 9.
Probability density function of simulated yield (without projected CO2) for three planting dates (15, 30, 45 day of year) and three periods (1986–2005, 2006–2025, 2026–2045) constructed for Taichung, Chaiyi, and Ilan; solid lines are averaged values from 3 models and dotted lines are values of each model.
3.5. Effects of Rising CO2 on Rice Yield
Concentration of CO2 since 1986 is projected to increase by 2.75 ppm year−1 and reach 513 ppm by 2045 (Figure S2). Accordingly, we next used the ORYZA(v3) to quantify CO2 effects on plant growth and yield through photosynthesis (Equation (S8) in Table S1). The simulation suggests rising CO2 concentration enhances rice yield in all locations and planting dates (Figure 8, shaded box). The simulated yield at Taichung would increase by 6.3–6.9% during 2006–2025 and 8.5–10% during 2026–2045 (Figure 8a). The yield can increase further if it is planted on 15 DOY rather than a later date. Similarly, the simulated yield in Chaiyi increases by 3.9–6.4% during 2006–2025 and 10–12% during 2026–2045, suggesting the best planting date to be 30 DOY (Figure 8b). The highest benefit of rising CO2 occurs in Ilan because future yields are projected to increase by 14–16% during 2006–2025 and 11–18% during 2026–2045. The earlier the crop is planted, the better it adapts to the increased temperature and CO2 (Figure 8c).
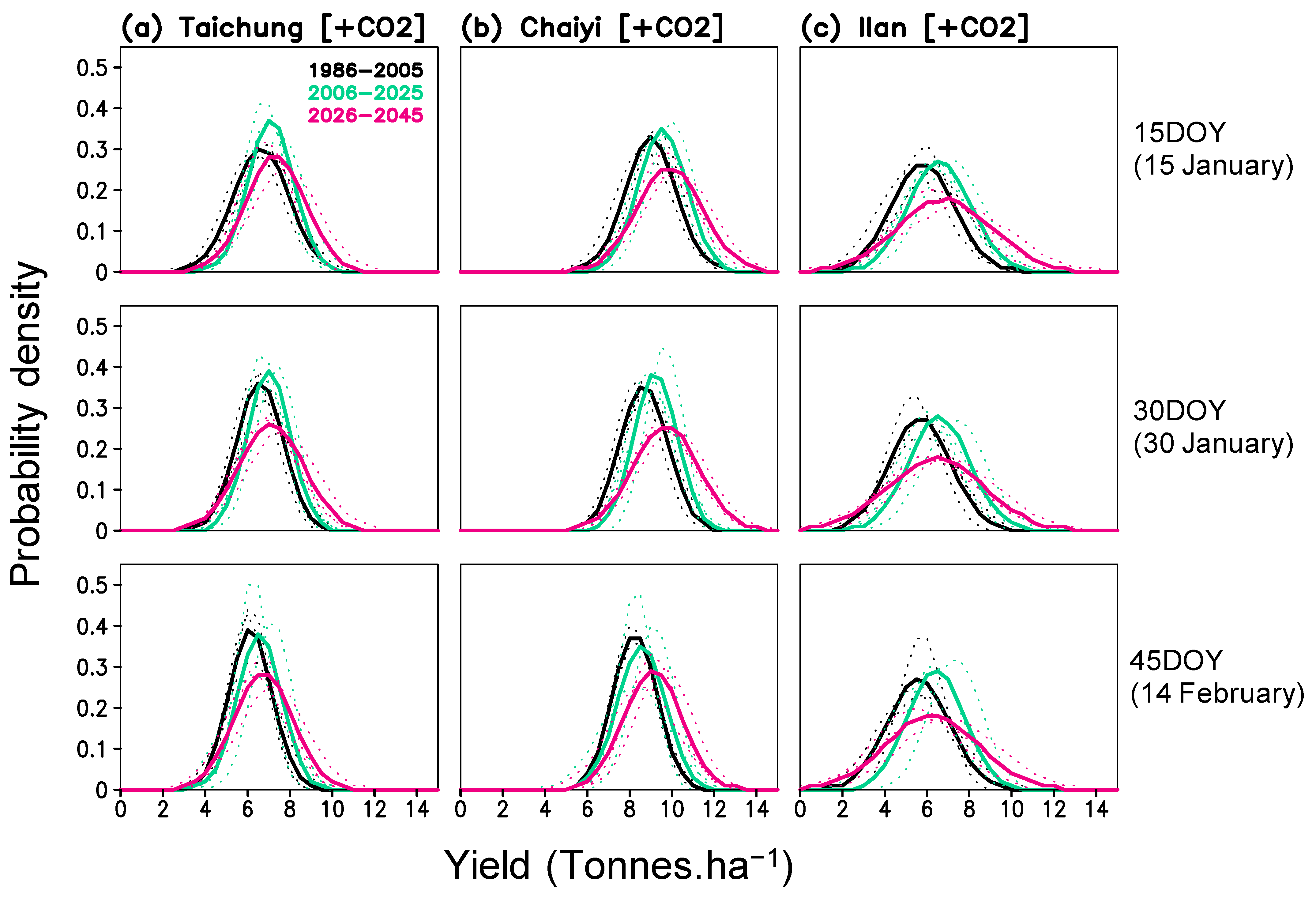
Rice yield potential under a CO2-rich climate also suggests a higher variability—i.e., an amplifying effect. The probability density of yield depicts the change in both mean and variability for the three locations (Figure 10). The highest yield in the future could reach 10–11 tons ha−1 for Taichung and 12–14 tons ha−1 in Chaiyi, whereas the lowest yield density may remain unchanged (Figure 10a,b). In Ilan, yield variability is higher than the other locations and may experience a pronounced shifting in both the lowest (0–2 tons ha−1) and highest yields (10–12 tons ha−1) (Figure 10c).
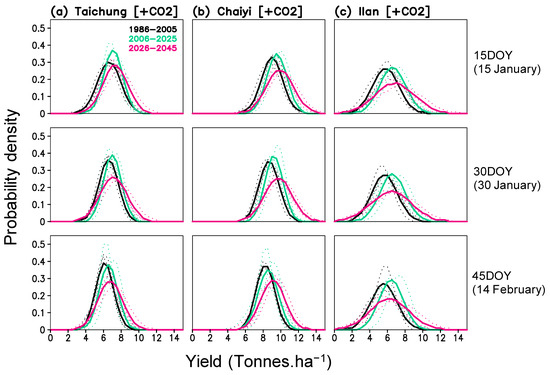
Figure 10.
Probability density function of simulated yield (with projected CO2) for three planting dates (15, 30, 45 day of year) and three periods (1986–2005, 2006–2025, 2026–2045) constructed for Taichung, Chaiyi, and Ilan; solid lines are averaged values from 3 models and dotted lines are values of each model.
4. Discussion
The presented analysis indicates that a decrease in the growing period stimulates the development rate, which limits biomass production and results in low yield [30]. Global warming tends to shorten the phenological stages, and this result obtained in Taiwan echoes previous studies [14,22,31,32,33]. Nonetheless, despite the fact that the ORYZA model has its limit performing for low temperatures [34,35,36], we did not find that the shortened vegetative growth can mitigate potential cold damage, neither can it increase cold sterility. This result presents a contrasting case from what was observed in Japan, a higher-latitude rice growing country, in that earlier rice phenological stage is susceptible to cold damage during the heading stage [31]. Moreover, the simulation without the CO2 effects produced a low correlation between cold/heat spikelet sterility and yield, suggesting either (1) a low risk from extreme temperature during flowering stage, (2) a bias of the rice model in estimating the sterility factors (SF1 and SF2), or (3) low sensitivity of the rice model to quantify temperature effects on yield.
Model deficiency does exist. For example, we note that SF1 sterility factor can be underestimated because the model calculates cooling degree days from daily T, not Tmin or cooling degree hours. Inaccurate SF2 could result from using too high a critical heat threshold (36.5 °C) for the japonica rice, which could be 33 °C [37,38]. Additionally, SF2 can be affected by the model ignoring flowering time and other factors, such as wind speed and relative humidity [28]. The actual sterility might be different from the calculation because a mere 1-h exposure to low/high temperature of rice anthesis can cause spikelet sterility [37,38,39], yet the model being used here only considers the daily average. Additionally, the simulated yield is more responsive to radiation (Table S6, Figure S5a) than to temperature via GDD (Table S5, Figure S5b) or sterility factors, further causing the biases.
The uncertainty of crop models to evaluate the effects of CO2-fertilization may vary upon model structure, CO2 concentration, and its interaction with other factors such as temperature [13]. Nonetheless, we found a disparity between actual and simulated CO2 effects on production due to certain growth factors, such as nitrogen (N) fertilizer [12], high temperature [13], and water supply [33,40]. In a normal growth condition, the elevated CO2 may enhance photosynthesis and water use efficiency, but the plants may be at risk to water deficit and high canopy temperature anyway because of stomata control [41]. Under high-CO2 conditions and low N supply, the photosynthetic nitrogen use efficiency during photosynthesis can be reduced [42]. Likewise, CO2 diffusing into leaves will decrease under high temperature and/or low water supply because of stomatal closure to avoid excessive water loss [42,43]. The increase of simulated rice yield due to a higher CO2 concentration as found here also echoes previous studies [12,13,15,33,44].
Finally, it is worthwhile to discuss the pronounced difference between simulated and observed yield in 2011, 2014, and 2015 (Figure 4a). The simulated yields are responsive to radiation and GDD, but the observed yields in the three years likely relate to seasonally low precipitation during the vegetative phase (Figure 4a, Figure S5a–c). This weather condition is different from the other years which may influence either field experiment outputs or the simulation performance. A dry-warm, high-vapor pressure deficit condition in 2015 may cause lower observed yield than the simulation (Figure S5c). Responding to high transpiration, leaf stomata will be closed to control its conductance, reducing CO2 diffusion and growth, even under non-limited soil water [45]. A dry-cold winter in 2011 and 2014 may be suitable for young plants compared to a wet-cold environment. Rice leaf has a thin layer of epicuticular wax [46] and high wettability [47] that may be sensitive to stomatal blockage by water film and reduce CO2 assimilation rate such as occurs in bean (Phaselous vulgaris) [48]. Thus, the negative effects of leaf wetness on photosynthesis in the dry-cold years is too low to be measured by the rice model. The simulation bias in the three years may be also associated with climate phenomena (Figure S5d).
5. Conclusions
We found that the ORYZA(v3) model is capable of simulating the rice growth and yield changes in the sub-tropical climate of Taiwan under global warming. A statistical bias correction method was adopted to downscale outputs from the CODEX regional climate models that provide the acceptability of future climate projection under a high emission scenario (RCP 8.5). Focusing on three study locations in Taiwan, the projected temperature suggests a warming trend in winter-spring season (January–June), which coincides with the rice growing period in Taiwan. The model indicates that the northern region of Ilan will experience a higher increase in temperature (~2 °C by 2045) than those in lower latitudes of the country.
The projected increase in winter-spring temperature at different climatological locations in Taiwan can impact rice growth and yield by 2045, regardless of having or not having the effect of increasing CO2 concentration. The significant increase in winter temperature can shorten the duration of rice growth by 6–11 days, in which the vegetative phase is more responsive to temperature than the reproductive phase. Heat sterility of spikelet is projected to increase for late planting dates. Together, these two changes shorten the time of biomass accumulation and decrease the seed set that leads to yield reduction. The simulated yields for different planting dates (15 January, 30 January, 14 February) suggest that rice grown in the later planting date is more vulnerable to the warming climate because of the accelerated maturity causing the mean yield to decrease by 3.3–10%. Such a yield reduction offsets the positive effect of rising CO2 concentrations, particularly for the early planting date in northeast Taiwan. These results suggest that one can use the early cultivation to adapt to the increased temperature and CO2. Meanwhile, as the yield variability increases owing to increased climate variability, the resultant yield reduction is projected to worse (by 67%). In other words, future rice production may be more vulnerable in terms of the yield variability than the mean yield change. The implication is that farmers will likely face more extreme weather events that cause abrupt yet significant reductions of yield, hampering their farming income. Future study should be devoted to predicting the yield anomaly caused by extreme climate events analyzed with longer records. Future research should also adopt multiple climate models and crop models to quantify, if not reduce, the uncertainty of simulations [13,22].
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agronomy12112625/s1, Figure S1: Seasonal (January–June) observed-temperature (OBS) and projected- temperature from CORDEX constructed for (a) Taichung, (b) Chaiyi, and (c) Ilan; linear regression coefficients (Reg) and coefficient of determination (R2) between year and temperature (Tmax, Tmin, Tmean) are presented in each panel. Figure S2: Annual observed CO2 and projected CO2 from RCP 8.5 Scenarios. Figure S3: Probability density function of total growing degree days (GDD) from rice emergence to maturation for three-planting dates (15, 30, 45 day of year) and three periods (1986–2005, 2006–2025, 2026–2045) constructed for (a) Taichung, (b) Chaiyi and (c) Ilan. The probability density distributions are derived by using 10 (°C of GDD) range of each bin. Figure S4: Probability density function of total radiation from rice emergence to maturation for three-planting dates (15, 30, 45 day of year) and three periods (1986–2005, 2006–2025, 2026–2045) constructed for (a) Taichung, (b) Chaiyi and (c) Ilan. The probability density distributions are derived by using 20 (Mj m-2) range of each bin. Figure S5: Time series of yield, biomass and number of spikelets superimposed on (a) total radiation from panicle initiation to flowering stages (DVS0.65 to DVS1.0), (b) growing degree day (GDD) during vegetative phase (DVS0.4), and (c) precipitation and vapor pressure deficit (VPD) during DVS0.4; Panel (d) presents time series of yield bias (simulated–observed yields) and climate indices (WNP, PMM-SST, Niño 3.4, WNP*PMM-SST); number in each panel indicates significant correlation coefficients (r) exceeding 99% (r > 0.83) and 90% (r > 0.62) confidence interval. [Methodologies to obtain climate indices are followed [49]; WNP is the western North Pacific, PMM-SST is the Pacific Meridional Mode–sea surface temperature]. Table S1: Lists of equations and names of CORDEX models used in this study. Table S2: Phenological development parameters used with TNG67 rice variety for parameterization of ORYZA(v3) model. Table S3: Statistics for observed and simulated outputs for TNG67 rice variety from calibration of ORYZA(v3) model. Table S4: Statistics for observed and simulated outputs for TNG67 rice variety from validation of ORYZA(v3) model. Table S5: Correlation coefficients between accumulated growing degree days and simulated rice yield for 1986–2045. Table S6: Correlation coefficients between accumulated radiation and simulated rice yield for 1986–2045.
Author Contributions
Conceptualization, P.P.; Methodology, P.P. and S.-Y.S.W.; Software, P.P., S.-Y.S.W. and J.-H.Y.; Formal Analysis, P.P.; Investigation, P.P. and M.-H.Y.; Data Curation, P.P.; Writing—Original Draft Preparation, P.P.; Writing—Review & Editing, P.P., S.-Y.S.W., J.-H.Y., P.G.J., E.C., Y.S. and M.-H.Y.; Visualization, P.P.; Supervision, S.-Y.S.W., P.G.J., E.C. and Y.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the Office of Ministry of Higher Education, Science, Research and Innovation; and the Thailand Science Research and Innovation through the Kasetsart University Reinventing University Program 2021. Partial support from the Utah Agriculture Experimental Station under #9618 is appreciated. J.-H.Y. is supported by the National Research Foundation of Korea (NRF-2021R1A2C1011827).
Data Availability Statement
The data supporting reported results in this study are available upon request from the corresponding author.
Acknowledgments
The authors thank the Center for Advanced Studies for Agriculture and Food, Kasetsart University Institute for Advanced Studies.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Krishnan, P.B.; Ramakrishnan, K.R.; Reddy, V.R. High temperature effects on rice growth, yield and grain quality. In Advances in Agronomy, 1st ed.; Sparks, D.L., Ed.; Elsevier: Amsterdam, The Netherlands, 2011; Volume 111, pp. 87–206. [Google Scholar]
- Council of Agriculture. Yearly Report of Taiwan’s Agriculture: Agricultural Production. Available online: https://eng.coa.gov.tw (accessed on 26 January 2019).
- Lee, M.H. Low temperature tolerance in rice: The Korean experience increased lowland rice production in the Mekong Region. In Proceedings of the ACIAR Proceedings No 101, Vientiane, Laos, 30 October–2 November 2000; Fukai, S., Basnayake, J.W.M., Eds.; Australian Centre for International Agricultural Research: Canberra, Australia, 2001. [Google Scholar]
- Ghadirnezhad, R.; Fallah, A. Temperature effect on yield and yield components of different rice cultivars in flowering stage. Int. J. Agron. 2014, 2014, 846707. [Google Scholar] [CrossRef]
- Hsu, H.-H.; Chen, C.T. Observed and projected climate change in Taiwan. Meteorol. Atmos. Phys. 2002, 79, 87–104. [Google Scholar] [CrossRef]
- Hsu, H.-H.; Chou, C.; Wu, Y.-C.; Lu, M.-M.; Chen, C.-T.; Chen, Y.M. Climate Change in Taiwan: Scientific Report 2011 (Summary); National Science Council: Taipei, Taiwan, 2011; p. 67. [Google Scholar]
- Matthews, R.B.; Kropff, M.J.; Horie, T.; Bachelet, D. Simulating the impact of climate change on rice production in Asia and evaluating options for adaptation. Agri. Syst. 1997, 54, 399–425. [Google Scholar] [CrossRef]
- Chang, C.-C. The potential impact of climate change on Taiwan’s agriculture. Agric. Econ. 2002, 27, 51–64. [Google Scholar] [CrossRef]
- Chen, C.-C.; Chang, C.-C. The impact of weather on crop yield distribution in Taiwan: Some new evidence from panel data models and implications for crop insurance. Agric. Econ. 2005, 33, 503–511. [Google Scholar] [CrossRef]
- Chiuen, Y.-W.; Huang, C.C.; Tan, C.H. Modeling the rice-climate indices in Taiwan. Clim. Chang. Econ. 2013, 4, 1350012. [Google Scholar] [CrossRef]
- Wu, S.J.; Chiueh, Y.-W.; Lien, H.-C.; Hsu, C.T. Modeling risk analysis for rice production due to agro-climate change in Taiwan. Paddy Water Environ. 2015, 13, 391–404. [Google Scholar] [CrossRef]
- Rosenzweig, C.; Elliott, J.; Deryng, D.; Ruane, A.C.; Müller, C.; Arneth, A.; Boote, K.J.; Folberth, C.; Blotter, M.; Khabarov, N.; et al. Assessing agricultural risks of climate change in the 21st century in a global gridded crop model intercomparison. Proc. Natl. Acad. Sci. USA 2014, 111, 3268–3273. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Hasegawa, T.; Yin, X.; Zhu, Y.; Boote, K.; Adam, M.; Bregaglio, S.; Buis, S.; Confalonieri, R.; Fumoto, T.; et al. Uncertainties in predicting rice yield by current crop models under a wide range of climate conditions. Glob. Chang. Biol. 2015, 21, 1328–1341. [Google Scholar] [CrossRef]
- Zhang, J.; Feng, L.; Zou, H.; Liu, D.L. Using ORYZA2000 to model cold rice yield response to climate change in the Heilongjiang province, China. Crop J. 2015, 3, 317–327. [Google Scholar] [CrossRef]
- Pugh, T.A.M.; Müller, C.; Elliott, J.; Deryng, D.; Folberth, C.; Olin, S.; Schmid, E.; Arneth, A. Climate change analogues suggest limited potential for intensification of production on current croplands under climate change. Nat. Commun. 2016, 7, 12608. [Google Scholar] [CrossRef] [PubMed]
- Yen, M.-C.; Chen, T.C. Short communication seasonal variation of the rainfall over Taiwan. Int. J. Climatol. 2000, 20, 803–809. [Google Scholar] [CrossRef]
- NOAA Website. Available online: Ftp://aftp.cmdl.noaa.gov/data/trace_gases/ (accessed on 29 September 2018).
- ESGF. Portal at CEDA. Available online: https://esgf-index1.ceda.ac.uk/search/cordex-ceda/ (accessed on 5 October 2018).
- Li, T.; Angeles, O.; Marcaida III, M.; Manalo, E.; Manalili, M.P.; Radanielson, A.; Mohanty, S. From ORYZA2000 to ORYZA (v3): An improved simulation model for rice in drought and nitrogen-deficient environments. Agric. For. Meteorol. 2017, 237–238, 246–256. [Google Scholar] [CrossRef]
- Bouman, B.A.M.; Kropff, M.J.; Tuong, T.P.; Wopereis, M.C.S.; ten Berge, H.F.M.; van Larr, H.H. ORYZA2000: Modeling Lowland Rice. International Rice; Research Institute: Metro Manila, Philippines, 2001; p. 235. [Google Scholar]
- Li, T.; Raman, A.K.; Marcaida III, M.; Kumar, A.; Angeles, O.; Radanielson, A.M. Simulation of genotype performances across a larger number of environments for rice breeding using ORYZA2000. Field Crops Res. 2013, 149, 312–321. [Google Scholar] [CrossRef]
- Zhang, S.; Tao, F. Modeling the response of rice phenology to climate change and variability in different climatic zones: Comparisons of five models. Eur. J. Agron. 2013, 45, 165–176. [Google Scholar] [CrossRef]
- Evans, L.T.; Fisher, R.A. Yield potential: Its definition, measurement and significance. Crop Sci. 1999, 39, 1544–1551. [Google Scholar] [CrossRef]
- Hawkins, E.; Osborne, T.M.; Ho, C.K.; Challinor, A.J. Calibration and bias correction of climate projections for crop modelling: An idealized case study over Europe. Agric. For. Meteorol. 2013, 170, 19–31. [Google Scholar] [CrossRef]
- Navarro-Racines, C.E.; Tarapues-Montenero, J.E. Bias-Correction in the CCAFS-Climate Portal: A Description of Methodologies. Available online: http://ccafs-climate.org/downloads/docs/BC_methods_explaining_v2_jrv.pdf (accessed on 16 August 2018).
- Parzen, E. On Estimation of a Probability Density Function and Mode; Technical Report No.40; Applied Mathematics and Statistics Laboratories, Stanford University: Stanford, CA, USA, 1961. [Google Scholar]
- Cimbala, J.M. Probability Density Function. Available online: https://www.mne.psu.edu/cimbala/me345/Lectures/Probability_density_functions.pdf (accessed on 14 January 2019).
- Matsui, T.M.; Omasa, K.; Horie, T. High temperature-induced spikelet sterility of Japonica rice at flowering in relation to air temperature, humidity and wind velocity conditions. Jpn. J. Crop Sci. 1997, 66, 449–455. [Google Scholar] [CrossRef]
- Zeng, Y.; Zhang, Y.; Xiang, J.; Uphoff, N.T.; Pan, X.; Zhu, D. Effects of low temperature stress on spikelet-related parameters during anthesis in Indica-Japonica hybrid rice. Plant Sci. 2017, 8, 1350. [Google Scholar] [CrossRef]
- Van Heemst, H.D.J. Crop phenology and dry matter distribution. In Modelling of Agricultural Production: Soil, Weather and Crops, 1st ed.; van Keulen, H., Wolf, J., Eds.; PUDOC: Wageningen, The Netherlands, 1986; pp. 13–60. [Google Scholar]
- Shimono, H. Earlier rice phenology as a result of climate change can increase the risk of cold damage during reproductive growth in northern Japan. Agric. Ecosyst. Environ. 2011, 144, 201–207. [Google Scholar] [CrossRef]
- Wang, X.; Ciais, P.; Li, L.; Ruget, F.; Vuichard, N.; Viovy, N.; Zhou, F.; Chang, J.; Wu, X.; Zhao, H.; et al. Management outweighs climate change on affecting length of rice growing period for early rice and single rice in China during 1991–2012. Agric. For. Meteorol. 2017, 233, 1–11. [Google Scholar] [CrossRef]
- Van Oort, P.A.J.; Zwart, S.J. Impacts of climate change on rice production in Africa and causes of simulated yield changes. Glob. Chang. Biol. 2018, 24, 1029–1045. [Google Scholar] [CrossRef] [PubMed]
- Devkota, K.P.; Manschadi, A.M.; Devkota, M.; Lamers, J.P.A.; Ruzibaev, E.; Egamberdiev, O.; Amiri, E.; Vlek, P.L.G. Simulating the impact of climate change on rice phenology and grain yield in irrigated drylands of central Asia. J. Appl. Meteorol. Climatol. 2013, 52, 2033–2050. [Google Scholar] [CrossRef]
- Van Oort, P.A.J.; de Vries, M.E.; Yoshida, H.; Saito, K. Improved climate risk simulations for rice in arid environments. PLoS ONE 2015, 10, e0118114. [Google Scholar] [CrossRef]
- Espe, M.B.; Yang, H.; Cassman, K.G.; Guilpart, N.; Sharifi, H.; Linquist, B.A. Estimating yield potential in temperate high-yielding, direct-seeded US rice production systems. Field Crops Res. 2016, 193, 123–132. [Google Scholar] [CrossRef]
- Jagadish, S.V.K.; Craufurd, P.Q.; Wheeler, T.R. High temperature stress and spikelet fertility in rice (Oryza sativa L.). J. Exp. Bot. 2007, 58, 1627–1635. [Google Scholar] [CrossRef] [PubMed]
- Bheemanahalli, R.; Sathishraj, R.; Tack, J.; Nalley, L.L.; Muthurajan, R.; Jagadish, K.S.V. Temperature thresholds for spikelets sterility and associated warming impacts for sub-tropical rice. Agri. For. Meteorol. 2016, 221, 122–130. [Google Scholar] [CrossRef]
- Nguyen, D.-N.; Lee, K.J.; Kim, D.-I.; Anh, N.T.; Lee, B.W. Modeling and validation of high-temperature induced spikelet sterility in rice. Field Crops Res. 2014, 156, 293–302. [Google Scholar] [CrossRef]
- Yang, M.; Xiao, W.; Zhao, Y.; Li, X.; Huang, Y.; Lu, F.; Hao, B.; Li, B. Assessment of potential climate change effects on the rice yield and water footprint in the Nanliujiang catchment, China. Sustainability 2018, 10, 242. [Google Scholar] [CrossRef]
- Haworth, M.; Killi, D.; Materassi, A.; Raschi, A.; Centritto, M. Imparied stomatal control is associated with reduced photosynthetic physiology in crop species grown at elevated [CO2]. Front. Plant Sci. 2016, 7, 1568. [Google Scholar] [CrossRef]
- Leakey, A.D.B.; Ainsworth, E.A.; Bernacchi, C.J.; Rogers, A.; Long, S.P.; Ort, D.R. Elevated CO2 effects on plant carbon, nitrogen, and water relations: Six important lessons from FACE. J. Exp. Bot. 2009, 60, 2859–2876. [Google Scholar] [CrossRef] [PubMed]
- DaMatta, F.M.; Grandis, A.; Arenque, B.C.; Buckeridge, M.S. Impacts of climate change on crop physiology and food quality. Food Res. Int. 2010, 43, 1814–1823. [Google Scholar] [CrossRef]
- White, J.W.; Hoogenboom, G.; Kimball, B.A.; Wall, G.W. Methodologies for simulating impacts of climate change on crop production. Field Crops Res. 2011, 124, 357–368. [Google Scholar] [CrossRef]
- Parent, B.; Suard, B.; Serraj, R.; Tardieu, F. Rice leaf growth and water potential are resilient to evaporative demand and soil water deficit once the effects of root system are neutralized. Plant Cell and Environ. 2010, 33, 1256–1267. [Google Scholar] [CrossRef] [PubMed]
- O’Toole, J.C.; Cruz, R.T.; Seiber, J.N. Epicuticular wax and cuticular resistance in rice. Physiol. Plant. 1979, 47, 239–244. [Google Scholar] [CrossRef]
- Wang, H.; Shi, H.; Wang, Y. The wetting of leaf surfaces and its ecological significances, Chapter 1. In Wetting and Wettability; IntechOpen: London, UK, 2015; pp. 295–321. [Google Scholar] [CrossRef]
- Hanba, Y.T.; Moriya, A.; Kimura, K. Effect of leaf surface wetness and wettability on photosynthesis in bean and pea. Plant Cell Environ. 2015, 27, 413–421. [Google Scholar] [CrossRef]
- Promchote, P.; Wang, S.-Y.S.; Shen, Y.; Johnson, P.G.; Yao, M.-H. A seasonal prediction for the wet-cold spells leading to winter crop damage in northwestern Taiwan with a combined empirical-dynamical approach. Int. J. Climatol. 2017, 28, 571–583. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).