Abstract
To clarify the regulatory effects of different N fertilizer treatments on the root morphology, physiological characteristics, and assimilate accumulation of drip-irrigated spring wheat under the northern border climate, we used strong wheat Xinchun 38 (cv. XC 38) and medium gluten wheat Xinchun 49 (cv. XC 49) as test materials in 2019 and 2020, and cultivated them in soil columns at Nck (300 kg·ha−1), A1 (240 kg·ha−1), A2 (210 kg·ha−1), and N0 (0 kg·ha−1). We also studied the effects of N application on root morphological characteristics, key enzymes of N metabolism, antioxidant enzymes, dry matter accumulation distribution, and yield of drip-irrigated wheat. The results showed that the root morphological characteristics, key enzymes of nitrogen metabolism, antioxidant enzymes, shoot dry matter accumulation, spike dry matter, and yield all showed an increasing and then decreasing trend with increasing nitrogen application. Among them, A1 treatment showed the best root length density (RLD), root volume density (RVD), root mass density (RMD), nitrate reductase (NR), glutamine synthetase (GS), glutamate synthetase (GOGAT), glutamic-pyruvic transaminase (GPT), superoxide dismutase (SOD), peroxidase (POD), root activity, shoot dry matter accumulation, spike dry matter, and yield, which were significantly higher than other treatments. The malondialdehyde (MDA) content decreased by 3.36–15.70% compared with other treatments. Correlation analysis showed that yields were positively correlated with RLD, RVD, RMD, GS, and GPT and negatively correlated with MDA. Nitrogen treatments and varietal intercropping had significant effects on RLD, root activity, NR, GS, GPT, POD, and yield. Therefore, moderate N reduction (240 kg·ha−1) under the drip irrigation pattern in Xinjiang can improve the morphological characteristics and physiological functions of wheat roots, promote the distribution and transport of dry matter to spikes, and facilitate yield formation.
1. Introduction
Nitrogen is a major limiting factor for plant growth and development, and a reasonable supply of nitrogen is a key step to ensure high crop yields. In Xinjiang, conventional irrigated wheat fields are usually planted with high nitrogen and yields. Still, diminishing returns occur as nitrogen fertilizer use continues to increase [1]. Excessive nitrogen residues can cause environmental problems such as groundwater pollution and the greenhouse effect, damaging the farmland environment and the sustainability of grain production [2]. In recent years, fertilizer inputs have tended to develop positively in Xinjiang, relying on drip irrigation technology for precise fertilizer control and implementing the Action Plan for Zero Growth in Chemical Fertilizer Application by 2020 [3]. However, problems such as excessive fertilizer application and unreasonable application methods exist. Therefore, it is essential to explore the appropriate amount of nitrogen application and promote the efficient production of drip-irrigated wheat for the sustainable development of the wheat industry in Xinjiang.
From the perspective of wheat itself, a developed and strong root system is the basic factor for nitrogen absorption for and utilization of wheat. At present, the research on nitrogen absorption capacity is mostly carried out from the perspective of the root system, and its morphological and physiological characteristics are closely related to canopy growth and yield formation [4,5]. Studies have shown that the level of nitrogen supply has a significant regulatory effect on root morphology, function, and yield formation [6]. Root length density (RLD), root bulk density (RVD), and root mass density (RMD) are essential indicators of root morphological characteristics. They are significantly and positively correlated with aboveground growth and yield formation [7,8]. Increased nitrogen fertilization can promote increases in RLD, RVD, and RMD, thus facilitating the transfer of the dry matter to the spike [9]. RLD and RVD of wheat roots increased with increasing N fertilization in the range of 0–275 kg·ha−1, and decreased beyond 275 kg·ha−1 [10]. Nitrogen metabolism is a crucial material metabolism in plants, and the level of activity of its key enzymes reflects the changes in the intensity of nitrogen metabolism in plants [11]. Nitrogen affects the physiological metabolism of wheat roots, and a proper reduction in nitrogen application can maintain high nitrogen metabolism enzymes activities, prolong the functional period of the root system, and promote assimilate formation [12]. The nitrogen absorbed by crops is metabolized to synthesize all the proteins, nucleic acids, and other nitrogen compounds needed in the body through a series of metabolism. NR, GS, GOGAT, and GPT are the main enzymes involved in this metabolism process and play an important role in nitrogen metabolism. Xu et al. [13] showed that the activities of nitrate reductase (NR), glutamine synthetase (GS), and glutamate synthetase (GOGAT) in roots tended to increase with the increase in nitrogen application. However, when the nitrogen application amount reached 360 kg·ha−1, the increase in NR, GS and GOGAT activities slowed down, and there was no significant difference between the three metabolic enzyme activities under the 240 kg·ha−1. Wang et al. [14] concluded that when the nitrogen application rate of corn was 0–450 kg·ha−1, the activity of the key enzymes of nitrogen metabolism in high-yield fields increased first and then decreased, and the activity of the above enzymes was the largest when the nitrogen application rate was 300 kg·ha−1, while the activity of the above enzymes in middle-yield fields increased with the increase in the nitrogen application rate.
Root activity reflects the strength of the nutrient absorption ability of crops to a certain extent. The higher the root activity, the stronger the nutrient absorption ability. Nitrogen fertilizer can significantly improve the root activity, and an appropriate amount of nitrogen fertilizer can improve the root activity, but excessive nitrogen application will inhibit the root activity [15]. Liao et al. [16] showed that soybean roots showed increased root activity at 50 kg·ha−1 of N fertilizer application compared to a 75 kg·ha−1 treatment. Adversity causes the accumulation of large amounts of reactive oxygen species (ROS) in the plant, and ROS regulates the plant’s response to adversity stress by activating the antioxidant system in the plant.
The main antioxidant enzymes in plants include superoxide dismutase (SOD) and peroxidase (POD), etc. These enzymes can scavenge ROS, reduce the damage to cell membranes caused by excessive accumulation of ROS, and then reduce the damage to plants caused by adversity stress [17,18]. Xu et al. [19] thought that in the range of 0–120 kg·ha−1 nitrogen application level, with the increase in the nitrogen fertilizer level, the SOD and POD activities of castor bean continued to increase, while the content of malondialdehyde (MDA) continued to decrease. When the nitrogen application level was increased to 180 kg·ha−1, the activity of antioxidant enzymes no longer increased, but decreased. A suitable amount of nitrogen application is beneficial to the transfer of assimilates for spike and yield improvement, while excessive nitrogen application will lead to excessive nutrient growth in the early stage and affect the transfer of dry matter accumulation and yield improvement [20]. Zheng et al. [21] found that spike dry matter and yields increased with increasing N application in the range of 0–180 kg/ha in the wheat region of the North China Plain, and yields increased by 14.23–23.24%, with no significant changes or a decreasing trend in each index when N application increased to 240 kg·ha−1.
Due to the complexity of the crop root–soil system and the constraints of research tools, studies on the high yield and efficiency of wheat in different N fertilization modes have mainly focused on the accumulation and translocation of aboveground assimilates, the relationship with yields [22], and the effects of N application on root morphology [23] and root activity [24,25], but there are few studies on the physiological and morphological characteristics of wheat roots under drip irrigation. Especially under reduced N conditions, the regulatory mechanism and pathway related to how to improve the activities of key enzymes and antioxidant enzymes in root nitrogen metabolism and promote the transport of assimilates to spike and improve yield are still unclear. According to the unique ecological environment of Xinjiang, this study selected two spring wheat varieties with different gluten types through soil column cultivation and studied the effects of different nitrogen changes on the root morphology and spatial distribution, root activity, changes in key enzyme activities of nitrogen metabolism, dry matter accumulation, and yield of spring wheat under drip irrigation conditions, which provided a scientific basis for the nitrogen-saving and high-yield cultivation of spring wheat under drip irrigation in Xinjiang.
2. Materials and Methods
2.1. Experiment Station and Experimental Design
The experiment was conducted from 2019 to 2020 at the experimental station of the College of Agriculture, Shihezi University, Shihezi, Xinjiang (44°18′ N, 85°59′ E). Annual precipitation ranged from 180–270 mm, and annual evapotranspiration from 1000 to 1500 mm. The average temperature and precipitation during the 2-year growing season (March to July) at the study site are shown in Figure S1.
This experiment adopts the method of PVC soil columns in the field. PVC pipes can control wheat roots’ vertical growth depth and nitrogen content. The PVC pipe with a wall thickness of 1 cm, an inner diameter of 10 cm, and a height of 60 cm was used. Table S1 shows some characteristics of the soil. Before sowing, place the PVC pipe in the square soil pit dug in advance according to the size, lay a layer of stones with a diameter of about 0.03–0.08 cm at the bottom of the pit, and then lay two layers of nylon mesh (100 mesh) on it to make the water drain freely. The soil comes from the test field and is air dried, sieved through 50 meshes, and then loaded into PVC pipes. The soil column shall be irrigated and compacted one month before sowing to make the soil column consistent with the field condition as much as possible.
The experiment was a split-zone design with variety as the main zone and nitrogen as a sub-zone. The secondary zone consisted of four nitrogen fertilizer treatments: Nck: 300 kg·ha−1; N0: 0 kg·ha−1; A1: 240 kg·ha−1; A2: 210 kg·ha−1. The wheat varieties tested were Xinchun 38 (XC 38, strong gluten, protein content is 15.04%) and Xinchun 49 (XC 49, medium gluten, protein content is 12.89%). A total of 120 kg·ha−1 P2O5 was applied as basal fertilizer, and 600 mm was irrigated during the reproductive period, with nine irrigation and seven fertilizer applications. The amount of irrigation in each period was precisely controlled by means of a water meter; 20% of the nitrogen fertilizer (urea) was used as basal fertilizer, and the remaining 80% was applied retrospectively with water (Table S2). The 10 seeds were sown in each soil column, and seedlings were thinned to 5 at the trefoil stage. Seeds were sown on 6 April 2019 and 28 March 2020 at a depth of 4 cm, in wide and narrow rows, with a row spacing of 12.5 + 20 + 12.5 + 15 cm (Figure S2), and drip irrigation tape (tube diameter 16 mm, drip head spacing 30 cm, flow rate 2.6 L·h−1) was placed in the middle of the soil column. Wheat was harvested on 12 July 2019, and 2 July 2020, and the other field management practices were the same as for field production.
2.2. Sampling and Measurement
At the jointing stage (12 May 2019 and 6 May 2020), booting stage (29 May 2019 and 23 May 2020), flowering stage (9 June 2019 and 2 June 2020), and milk ripening stage (21 June 2019 and 18 June 2020), roots in 0–20 cm, 20–40 cm, and 40–60 cm soil layers were taken to measure the growth and distribution of roots in soil columns. During each sampling, carefully take out each treated PVC pipe and cut the extracted root into sections according to the depth of different soil layers. According to the method described by Gwenzi et al. [26], weeds and dead roots, etc., are separated and removed from the living roots during cleaning. The living roots are hydrated in deionized water and stored in the refrigerator for standby.
Distribute the roots evenly in the tray, put them into the root box, scan the black and white TIF images with the scanner (Epson V500, Shanghai, China), and analyze them with the Win-Rhizo software (v2009, Regent Instrument, Quebec, QC, Canada) to obtain the root length, root volume and other related parameters of wheat. After scanning, the roots were sterilized in an oven at 105 °C for 30 min, dried at 75 °C, and weighed, and the root length density (RLD), root volume density (RVD), and root mass density (RMD) were calculated according to the method of Mehrabi et al. [27]
RLD (cm·cm3) = Root length/PVC pipe volume (20 cm was a layer, T = the same below)
RVD (mm·cm3) = Root volume/PVC pipe volume
RMD (mg·cm3) = Root mass/PVC pipe volume
The roots in the other part of the column were immediately frozen in liquid nitrogen and then stored in a −80 °C refrigerator to determine the physiological traits of the root.
(1) Glutamine synthetase (GS) (EC 6.3.1.2) activity: according to the method of Cruz M et al. [28].
(2) Determination of nitrate reductase (NR) (EC 1.6.6.1) activity: according to the method of Cruz M et al. [28].
(3) Glutamate synthase (GOGAT) (EC 1.4.7.1) activity was determined by the method of Singh R D et al. [29].
(4) Glutamic-pyruvic transaminase (GPT) (EC 2.6.1.2) activity: according to the method of Baars et al. [30].
(5) Malondialdehyde (MDA), superoxide dismutase (SOD) (EC 1.15.1.1), and peroxidase (POD) (EC 1.11.1.7) were determined according to the method of Guo et al. [31].
(6) Root activity: using the triphenyl tetrazolium chloride (TTC) method, according to Man et al. [32].
At maturity, three PVC tubes were taken from each treatment, the aboveground parts of the wheat were cut from the tiller nodes to distinguish between stems, leaves, and spikes, and the spikes were weighed after manual threshing to obtain the yield per plant. The PVC pipes were dug out, the roots were picked up from the 0–60 cm soil layer, each organ was put into a paper bag and immediately put in an oven at 105 °C for 30 min, dried at 75 °C to constant weight before weighing, and the spike mass fraction was calculated.
spike mass fraction = spike dry mass/shoot dry mass
2.3. Statistical Analysis
All statistical analyses were performed using SPSS 26.0 for Windows (IBM Corp., Armonk, NY, USA). Duncan’s new plural range test was used, and differences were statistically significant when p < 0.05. Pearson’s correlation analysis and linear regression analysis were used to analyze the correlation between indicators. Regression modeling techniques were used to investigate the relationship between wheat seed yield and the morphological and physiological characteristics of the root system at different soil depths, and multiple linear regression analysis (the “stepwise” method) was used to identify the main factors contributing to yield variation in each treatment. Wheat seed yield was the dependent variable, and the morphological and physiological characteristics of the root system at different soil depths were the independent variables.
3. Results and Analysis
3.1. Root Morphological Characteristics
The effects of nitrogen application on the morphological characteristics of the root are shown in Table 1 and Table 2. As the reproductive period progressed, root length density (RLD), root volume density (RVD), and root mass density (RMD) increased first and then decreased, reaching the maximum at the flowering stage in both years. Nitrogen application had a significant effect (p < 0.05) on root morphological indicators, with RLD, RVD, and RMD all decreasing in the order A1 > Nck > A2 > N0 as N application decreased. At the flowering stage, RLD, RVD, and RMD at A1 were significantly (17.44–138.83%, 14.33–139.15%, 15.09–168.94%) higher than other treatments, respectively. In terms of soil depth, RLD, RVD, and RMD decreased significantly with deeper soil depth. Moderately reduced N application increased root density in 20–60 cm soil, with A1 treatment being the best, followed by Nck. Under the A1 treatment, RLD, RVD, and RMD of cv. XC 38 were 8.35%, 5.11%, and 3.59% higher than those of cv. XC 49, respectively. In both years, the morphological parameters of wheat roots in 2019 were higher than in 2020. RLD, RVD, and RMD decreased significantly from the flowering to the milk ripening period, and the reduction range among treatments in 2019 was smaller than in 2020. Nitrogen application and variety intercropping had a significant effect on RLD.

Table 1.
Effect of different nitrogen application rates on the root morphology of drip-irrigated spring wheat in 2019.

Table 2.
Effect of different nitrogen application rates on the root morphology of drip-irrigated spring wheat in 2020.
3.2. Root Physiological Characteristics
The effect of nitrogen application on root activity is shown in Figure 1. Root activity gradually decreased with the advancement of the reproductive period under different treatments, with the most significant average decrease (62.74–65.72%) in the flowering–milk ripening period, with the lowest reduction in the A1 treatment. Nitrogen fertilization had a significant effect on root activity. A1 treatment was the highest in different soil layers, with significant differences among all treatments. A1 increased 21.04–79.93% on average compared with other treatments. From the depth of the soil layer, root activity in the 40–60 cm soil layer increased 3.34–49.97% and 1.33–16.32% on average compared with 0–20 and 20–40 cm. Between different years, the root activity of each treatment in 2019 was 6.09–6.37% higher than that in 2020 on average, and the decline of root activity in the flowering–milk ripening stage in 2019 (60.62–63.05%) was less than that in 2020 (64.96–68.53%). Under A1 treatment, root activity of cv. XC 38 was 3.17% higher than that of cv. XC 49 at the jointing stage. The interaction effect of the variety and nitrogen fertilizer treatment on root activity reached a significant level.
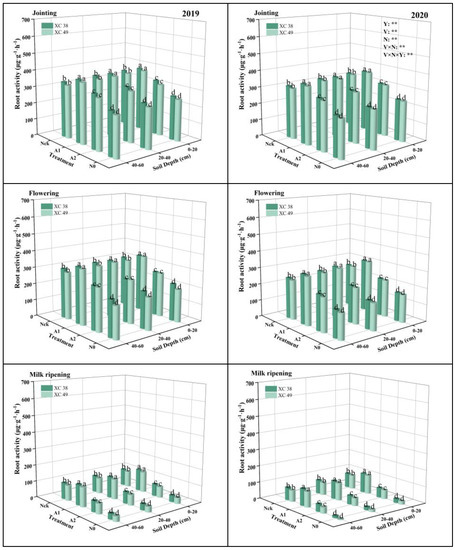
Figure 1.
Effect of nitrogen supply on root activity of drip-irrigated spring wheat. Note: XC 38: Xinchun 38; XC 49: Xinchun 49. Nck: 300 kg·ha−1; N0: 0 kg·ha−1; A1: 240 kg·ha−1; A2: 210 kg·ha−1. V: variety, N: different nitrogen treatments, and Y: year. “**” indicates significant difference at 0.01 levels, ns indicates insignificant difference. Different lowercase letters indicate significant differences between treatments at the 0.05 level.
From the jointing stage to the milk ripening stage, the root NR activity in the 0–60 cm soil layer increased first and then decreased and reached the maximum at the flowering stage (22.61–49.04 μ·g−1FW) (Figure 2). The effect of the nitrogen application rate on NR activity of root was significant, decreased in the order A1 > Nck > A2 > N0 as N application decreased in all stages, and there were significant differences between treatments. At the flowering stage, A1 increased by an average of 16.11% compared with Nck. With the deepening of the soil layer, the NR activity of roots in the 40–60 cm soil layer was the highest at the flowering stage. Under A1 treatment, the root NR activity in the 40–60 cm soil layer increased by 21.32–49.97% and 10.98–16.32% on average compared with 0–20 and 20–40 cm, respectively. At the flowering stage, root NR activity of cv. XC 38 increased by 0.61% on average compared to cv. XC 49 under A1 treatment. The interaction effect of the variety and nitrogen application rate on root NR activity reached a significant level.
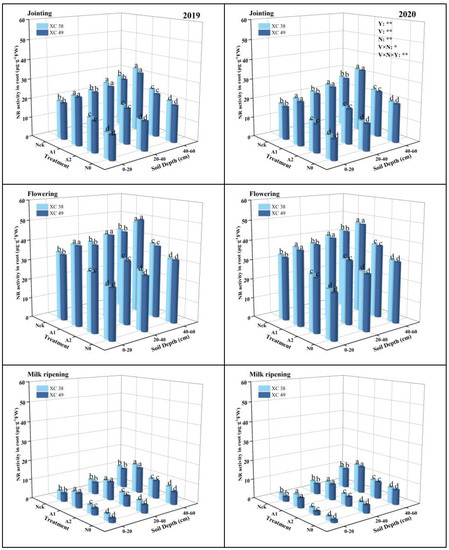
Figure 2.
Effect of nitrogen supply on root NR activity of drip-irrigated spring wheat. Note: XC 38: Xinchun 38; XC 49: Xinchun 49. Nck: 300 kg·ha−1; N0: 0 kg·ha−1; A1: 240 kg·ha−1; A2: 210 kg·ha−1. V: variety, N: different nitrogen treatments, and Y: year. “*” and “**” indicate significant differences at 0.05 and 0.01 levels, ns indicates insignificant differences. Different lowercase letters indicate significant differences between treatments at the 0.05 level.
As shown in Figure 3, the root GS activity of the two wheat varieties had the same change trend under different nitrogen supply conditions, reaching the peak at the flowering stage, and the A1 treatment was significantly higher than other treatments (p < 0.05). Under different nitrogen supply levels, the root GS activity of the two varieties decreased in the order A1 > Nck > A2 > N0. At the flowering stage, the A1 treatment was, on average, 30.73–119.03% higher than the other treatments. The deeper root GS activity was higher than the shallow layer, and the root GS activity in the 40–60 cm soil layer was, on average, 32.34–49.97 and 12.47–16.32% higher than the 0–20 cm and 20–40 cm. From different years, the root GS activity of wheat in 2019 was higher than that in 2020 and significantly reduced from the flowering to the milk ripening period. Differences in the reduction range among treatments decreased by 62.15%, 60.90%, 58.58%, and 57.21%, respectively. The root GS activity of cv. XC 38 was 4.28% higher than that of cv. XC 49 under the A1 treatment at the flowering stage. The nitrogen application and variety intercropping significantly affected root GS activity at all periods.
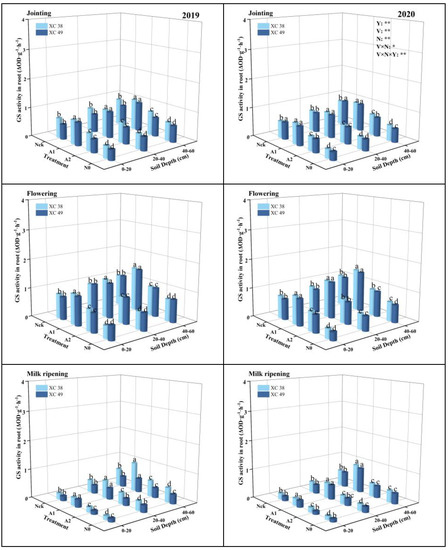
Figure 3.
Effect of nitrogen supply on root GS activity of drip-irrigated spring wheat. Note: XC 38: Xinchun 38; XC 49: Xinchun 49. Nck: 300 kg·ha−1; N0: 0 kg·ha−1; A1: 240 kg·ha−1; A2: 210 kg·ha−1. V: variety, N: different nitrogen treatments, and Y: year. “*” and “**” indicate significant differences at 0.05 and 0.01 levels, ns indicates insignificant differences. Different lowercase letters indicate significant differences between treatments at the 0.05 level.
The response of root GOGAT activity to nitrogen varied in the same way as NR and GS activity (Figure 4). The root GOGAT activity showed a trend of increasing and then decreasing with decreasing N application, reaching its maximum at the A1 treatment, and the GOGAT activity at the A1 treatment was, on average, 36.04–92.23% higher than the rest of the treatments at flowering. In terms of different soil layers, root GOGAT activity was the greatest in the 40–60 cm soil layer under the A1 treatment, indicating that moderate N reduction was conducive to maintaining root physiological activity. The average decrease during the flowering–milk ripening period in 2019 (76.13–77.66%) was lower than that in 2020 (76.86–78.11%). At flowering, root GOGAT activity of cv. XC 38 was, on average, 4.30% higher than cv. XC 49 under A1 treatment. Nitrogen application had a significant effect on root GOGAT activity, but the interaction between cultivar and N application did not have a considerable impact on GOGAT activity.
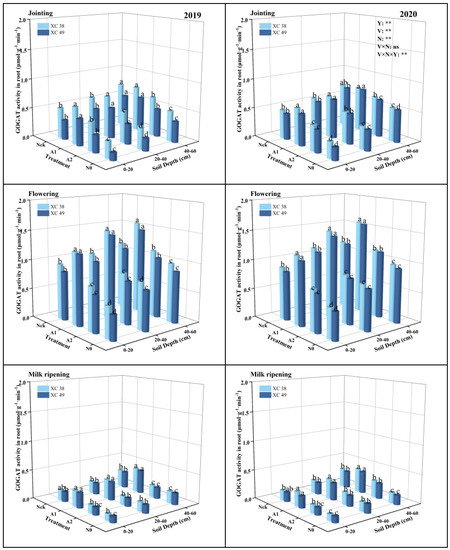
Figure 4.
Effect of nitrogen supply on root GOGAT activity of drip-irrigated spring wheat. Note: XC 38: Xinchun 38; XC 49: Xinchun 49. Nck: 300 kg·ha−1; N0: 0 kg·ha−1; A1: 240 kg·ha−1; A2: 210 kg·ha−1. V: variety, N: different nitrogen treatments, and Y: year. “**” indicates significant differences at 0.01 levels, ns indicates insignificant differences. Different lowercase letters indicate significant differences between treatments at the 0.05 level.
The effect of the nitrogen application rate on root GPT activity is shown in Figure 5. All treatments showed a trend of increasing and then decreasing with the advancement of the growth process, reaching a peak at the flowering stage. The effect of the nitrogen application rate on root GPT activity was significant. GPT activity increased first and then decreased with the decrease in the nitrogen application rate. The performance of each treatment decreased in the order A1 > Nck > A2 > N0. The difference between A1 and the other treatments was significant (p < 0.05): 37.68%, 50.35%, and 100.23% higher than CK, A2, and N0, respectively. At the flowering stage, the GPT activity of deep roots at A1 treatment was 20.35–49.97% and 7.28–16.32% higher than that in the shallow and middle layers, respectively. The GPT activity of cv. XC 38 roots was, on average, 3.98% higher than cv. XC 49 under A1 treatment. The common effect of variety and N application on root GPT activity reached a significant level.
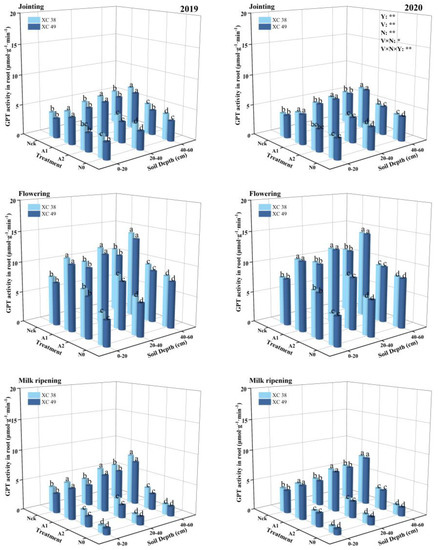
Figure 5.
Effect of nitrogen supply on root GPT activity of drip-irrigated spring wheat. Note: XC 38: Xinchun 38; XC 49: Xinchun 49. Nck: 300 kg·ha−1; N0: 0 kg·ha−1; A1: 240 kg·ha−1; A2: 210 kg·ha−1. V: variety, N: different nitrogen treatments, and Y: year. “*” and “**” indicate significant differences at 0.05 and 0.01 levels, ns indicates insignificant differences. Different lowercase letters indicate significant differences between treatments at the 0.05 level.
As shown in Table 3 and Table 4, the root MDA content of the two varieties increased and reached a maximum at the milk ripening stage. Nitrogen application significantly affected root MDA content, which first decreased and then increased with the decrease in the nitrogen application rate. The root MDA content of the A1 treatment was the lowest, significantly different from Nck. At the milk ripening stage, A1 decreased by 7.26–25.57% compared with other treatments. Among varieties, the root MDA content of cv. XC 38 was 3.50% lower than cv. XC 49 on average. The interaction between the variety and nitrogen application rate had no significant effect on MDA content.

Table 3.
Effect of different nitrogen application rates on root antioxidant enzymes activity and MDA content of drip-irrigated spring wheat in 2019.

Table 4.
Effect of different nitrogen application rates on root antioxidant enzymes activity and MDA content of drip-irrigated spring wheat in 2020.
From the jointing stage to the milk ripening stage, the root SOD activity of different treatments increased first and then decreased and reached the maximum value at the flowering stage, which was 262.08–363.38 U·g−1·min−1 (Table 3 and Table 4). The effect of the nitrogen application rate on root SOD activity was significant: with the gradual decrease in the nitrogen application rate, SOD activity reached the maximum under A1 treatment, which was significantly different from other treatments, and was 8.10%, 13.58%, and 22.16% higher than Nck, A2, and N0, respectively. Under A1 treatment, the root SOD activity in the 40–60 cm soil layer was 3.98–25.42% and 1.80–13.79% higher than that in 0–20 cm and 20–40 cm, respectively. Among the years, the root SOD activity of wheat in 2019 was better than that in 2020, and the decline in root SOD activity during the flowering–milk ripening period in 2019 (61.59–64.90%) was less than that in 2020 (61.90–65.23%). Among the varieties, the root SOD activity of cv. XC 38 increased by an average of 7.25% compared with cv. XC 49.
The response of POD activity to nitrogen application was consistent with that of SOD (Table 3 and Table 4). Nitrogen application had a significant effect on root POD activity, with the A1 treatment performing the best, maintaining root POD activity at around 180 U·g−1·min−1 at flowering, significantly increasing by 5.42% to 27.79% compared with other treatments. Within two years, the root POD activity was higher in 2019 than in 2020, and SOD activity decreased significantly from the flowering to the milk ripening stage. There was a difference in the reduction range among the treatments, which decreased by 81.58%, 80.32%, 79.12%, and 77.96%, respectively. At the flowering stage, root POD activity of cv. XC 38 under A1 treatment was 4.42% higher than that of cv. XC 49. The reciprocal effect of the variety and N application on root POD activity could reach a highly significant level in all periods.
3.3. Dry Matter and Yield
As can be seen from Table 5, the shoot dry matter accumulation of aboveground parts tended to increase as the fertility period progressed, reaching a maximum at the milk ripening stage. The effect of nitrogen application on shoot dry matter accumulation reached a significant level. With shoot dry matter accumulation increasing and then decreasing as the amount of nitrogen fertilizer applied decreased, the A1 treatment was significantly higher than the rest of the treatments, with an average increase of 14.54%, 28.12%, and 79.38% for the A1 treatment compared to the Nck, A2, and N0 treatments at the milk ripening stage, respectively. The effect of variety and N application interactions on spike dry matter accumulation was not significant, while the effect of N application on spike dry matter reached a significant level, with spike dry matter showing a trend of increasing and then decreasing with decreasing N fertilizer application, with the A1 treatment being the highest. The yield was affected by the amount of nitrogen application. With the increase in the nitrogen application, the yield decreased in the order A1 > Nck > A2 > N0, in which A1 was significantly higher than Nck, which was 2.58–42.02% higher than other treatments. Under this treatment, the yield of cv. XC 38 (7321.43–7429.53 kg·ha−1) was higher than that of cv. XC 49 (7116.33–7164.36 kg·ha−1). The interaction of the variety and nitrogen application rate significantly affected yields.

Table 5.
Effect of nitrogen supply on shoot dry matter and spike dry matter of wheat.
With the increase in nitrogen reduction, the spike mass fraction increased first and then decreased (Figure 6), with A1 treatment being the highest, which increased by 6.8% to 47.45% on average compared with other treatments. From 2019 to 2020, the change trend of the spike mass fraction of the two varieties was the same. Among varieties, the spike mass fraction of cv. XC 38 was 0.52–9.02% higher than that of cv. XC 49. In two years, the interaction between nitrogen treatment and variety had a significant effect on the spike mass fraction.
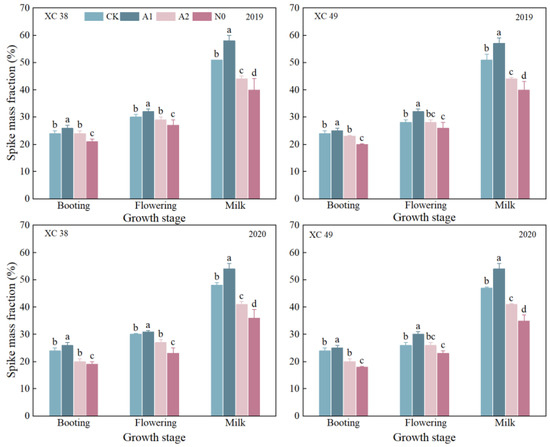
Figure 6.
Effect of nitrogen reduction treatments on the spike mass fraction in drip-irrigated spring wheat. Note: spike mass fraction = spike dry mass/shoot dry mass. XC 38: Xinchun 38; XC 49: Xinchun 49. Nck: 300 kg·ha−1; N0: 0 kg·ha−1; A1: 240 kg·ha−1; A2: 210 kg·ha−1. V: variety, N: different nitrogen treatments. Different lowercase letters indicate significant differences between treatments at the 0.05 level.
The fitting analysis of yields showed that the wheat yield showed a quadratic parabola trend with the increase in nitrogen application (Figure 7). When the amount of nitrogen application was A1, the yield was the highest, and when it exceeded A1, the yield decreased with the increase in nitrogen application. According to the equation, the maximum yield and nitrogen application rate of XC 38 and XC 49 were x = 261 and 287.5 kg·ha−1, respectively, and the corresponding maximum yields were 7311.20 and 7453.78 kg·ha−1.
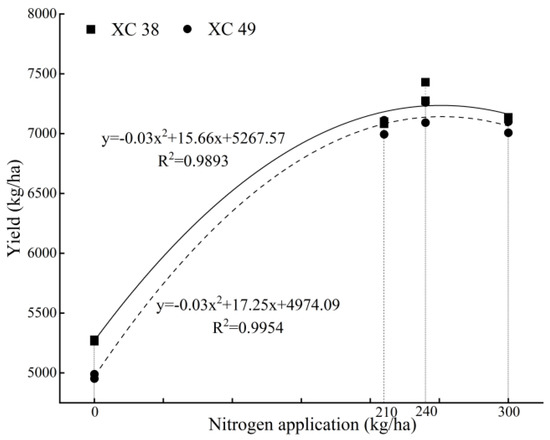
Figure 7.
Nitrogen fertilizer yield effect curve.
3.4. Correlation between Wheat Root Traits
It can be seen from Figure 8 that root morphological traits (RLD, RVD, RMD) were highly significantly positively correlated with root physiological indexes (NR, GS, GOGAT, GPT, SOD, POD) (p < 0.01), while root MDA content was only highly significantly negatively correlated with RVD. The nitrogen metabolism enzymes activities (NR, GS, GOGAT, GPT) were significantly positively correlated with root activity, SOD, POD, and yield, but there was no correlation between root GPT activity and MDA content. The antioxidant enzymes activities (SOD, POD) in roots were significantly correlated with other traits (except MDA content). The yield was significantly positively correlated with root morphological characteristics (RLD, RVD, RMD), nitrogen metabolism enzymes activities (NR, GS, GOGAT, GPT), antioxidant enzymes activities (SOD, POD), and root activity.
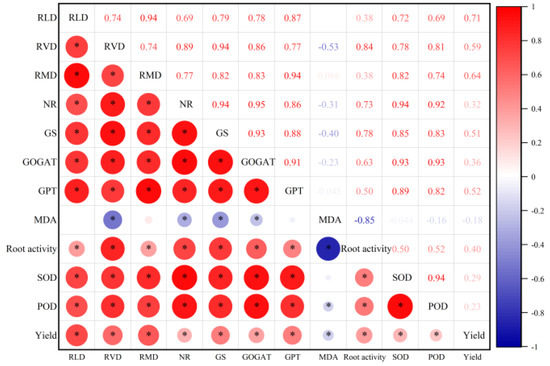
Figure 8.
Interrelationship of root system indicators in drip-irrigated spring wheat. RLD: Root length density; RVD: Root volume density; RMD: Root mass density; NR: Nitrate reductase; GS: Glutamine synthetase; GOGAT: Glutamate synthase; GPT: Glutamic pyruvic transaminase; MDA: Malondialdehyde; SOD: Superoxide dismutase; POD: Peroxide dismutase; “*” indicates significant differences at 0.05 level.
3.5. Regression Analysis of Root Morphology and Physiological Traits
The stepwise regression analysis of wheat root morphological and physiological traits and yields showed that (Table 6) the root activity of the whole soil layer had the most significant impact on yields, with a contribution rate of 65.9%. Among the morphological parameters, RVD, RLD, and RMD at the depth of 0–20 cm contributed 48.6%, 68.2%, and 67.5% to the yield, respectively. Among the physiological parameters, GS and MDA at the depth of 0–20 cm had the most significant effect on the yield, with contribution rates of 41.2% and 73.0%. The root activity, NR, GOGAT, GPT, SOD, and POD activities at the depth of 40–60 cm determined 68.6%, 50.9%, 46.2%, 60.0%, 59.8%, and 47.1% of the yield, respectively.

Table 6.
Stepwise regression between root traits and yields.
4. Discussion
4.1. Effect of Nitrogen Application on the Morphological Characteristics of the Root
The root is the main organ for plants to absorb nutrients, and the root morphological structure is an essential factor affecting the root system’s absorbing of nutrients [33]. Studies have shown that root morphological characteristics (including root length, root volume, density, and other factors) have a positive effect on nitrogen absorption [34,35]. Chu et al.’s [20] research showed that when the amount of nitrogen application was insufficient, crops could improve nitrogen absorption by increasing deep RLD and RVD. When nitrogen was appropriate, RLD and RVD increased significantly, which was conducive to reducing the loss of nitrogen in the soil [36]. Hao et al. [37] reported that an appropriate nitrogen application rate could obtain better root morphological characteristics and increase the proportion of the root system absorbed by nitrogen. In our experiment, we found that with the increase in the nitrogen supply level, RLD, RVD, and RMD all showed a trend of increasing first and then decreasing, reaching their maximum value under A1 treatment, and excessive nitrogen could not continuously improve the morphological indicators. However, Wang et al. [38] showed that wheat roots increased to a certain extent with RVD, and the excess roots were not conducive to the formation of yields, which needs further study. Gan et al. [39] believed that nitrogen application only affected the increase in the root volume in the area closest to nitrogen application. This study also had the same results. The results showed that wheat roots were mainly distributed in the 0–20 cm soil layer, and RLD, RVD, and RMD were significantly higher than those in 20–40 cm and 40–60 cm soil layers. Pang et al. [40] found that wheat varieties with a strong nitrogen absorption and utilization capacity generally have higher root growth and distribution density. In this experiment, the root morphological characteristics of strong gluten wheat cv. XC 38 under different treatments was better than cv. XC 49, indicating that cv. XC 38 had relatively developed roots, which was conducive to crops’ effective capture of nitrogen in the soil. The correlation analysis showed that RLD, RVD, and RMD were significantly positively correlated with the spike dry matter and yield, and A1 treatment could make wheat form good root morphological characteristics to better transport nutrients to aboveground parts and achieve high yields. The results of stepwise regression showed that RVD, RLD, and RMD of the 0–20 cm soil layer determined 48.6%, 68.2%, and 67.5% of the yield, respectively. The above results showed that A1 treatment benefited the improvement of RVD, RLD, and RMD, which was an essential reason for the advancement of root morphological indexes with the appropriate amount of nitrogen.
4.2. Effect of Nitrogen Application on the Physiological Characteristics of the Root
The strength of nitrogen metabolism is particularly important for the improvement of spring wheat yields, and nitrogen can affect the nitrogen metabolism enzyme activities. Therefore, the appropriate amount of nitrogen application can promote plant nitrogen metabolism [41]. It has been shown that the level of nitrogen supply was significantly and positively correlated with the nitrogen metabolism enzyme activities. Within a certain range, NR, GS, and GOGAT activities increase with increasing nitrogen application, thus promoting nitrogen assimilation in the plant [42]. The study showed that the NR, GS, GOGAT, and GPT activities in the root increased significantly with increasing N application from 0 to 240 kg·ha−1, but no significant changes in the activities of these key enzymes of N metabolism were observed with increasing N application to 270 kg·ha−1 [43]. Echarte et al. [44] studied maize of different ages and found that the new varieties were more efficient in using nitrogen than the old ones, indicating that the new varieties were better than the old ones in all physiological indicators under low nitrogen conditions. In this experiment, both varieties reached the maximum nitrogen metabolism enzymes activities at a 240 kg·ha−1 nitrogen supply level, but the root traits of strong wheat cv. XC 38 increased by 8.24–23.58%, 4.11–12.54%, and 3.89–9.37%, respectively, compared to medium gluten wheat cv. XC 49, which showed that cv. XC 38 was a nitrogen-efficient variety. The results of correlation analysis indicated that there were significant correlations between root NR, GS, GOGAT, and GPT activities and antioxidant enzyme activities, root activity, and root morphological characteristics, indicating a powerful synergistic effect between root morphology and physiological indicators. Stepwise regression analysis showed that GS and MDA at 0–20 cm root were more significant determinants of yields. Root activity, NR, GOGAT, GPT, SOD, and POD activities in the 40–60 cm soil layer roots contributed to yields at higher levels. Therefore, applying N at the A1 (240 kg·ha−1) treatment increased the root N-metabolizing enzyme activities and fully harmonized the relationship between N-metabolizing enzyme activity and root morphology to maintain a high physiological activity of the root.
The aging process of organisms is a process of metabolic imbalance and accumulation of reactive oxygen species. The damage of oxygen free radicals directly affects the aging process of plants, and the synergistic effect of protective enzymes such as SOD and pod in plants can remove excess MDA, maintain the metabolic balance of reactive oxygen species, protect membrane structure, and delay aging [45]. Nitrogen is an important component of plant protective enzymes, as the level of nitrogen significantly affects the activity of crop protective enzymes. Appropriate application of nitrogen fertilizer can improve the activity of crops’ protective enzymes, and excessive application can inhibit its activity [46]. This study also concluded that when the nitrogen application rate was in the range of 0–240 kg·ha−1, the root SOD and POD activities increased with the increase in the nitrogen level. When the nitrogen application rate increased to 300 kg·ha−1, the activity of antioxidant enzymes showed a downward trend, indicating that diminishing returns occurred due to an excessive nitrogen supply. An appropriate amount of nitrogen supply can increase the antioxidant enzyme activities and reduce the toxicity of reactive oxygen species, which is conducive to maintaining a higher physiological activity of roots. From the perspective of soil depth, the antioxidant enzyme activities of spring wheat roots increased with the increase in soil depth, which may be because the root tip cells are more active and sensitive to nitrogen changes. Different varieties of the same crop have different physiological responses to nitrogen nutrition levels. Research showed that with the increase in nitrogen, the antioxidant enzyme activities of nitrogen-efficient varieties was higher than that of inefficient nitrogen varieties [47]. In this study, the root SOD and POD activities of strong gluten wheat cv. XC 38 under A1 treatment were 7.25% and 4.42% higher than those of cv. XC 49, respectively, indicating that strong gluten wheat had a higher nitrogen utilization efficiency. Correlation analysis showed that SOD and POD activities were significantly positively correlated with root morphological indexes and nitrogen metabolism enzyme activities and negatively correlated with root MDA content. Stepwise regression analysis showed that the MDA content of roots in the 0–20 cm soil layer contributed the most to the yield, and the SOD and POD activities of roots in 40–60 cm soil layer determined the yield by 59.8% and 47.1%, indicating that the physiological activity of deep roots was the main factor determining whether wheat was high-yield. Therefore, based on the existing amount of nitrogen application in Xinjiang, an appropriate reduction can reduce the damage of high nitrogen to the root system, make the root system maintain high activity, and ensure a strong ability for nutrient absorption and transport.
The strength of root activity reflects the adaptability of plants to adversity and their ability to absorb nutrients. A higher root activity helps to coordinate the contradiction between high yields and premature root failure [48]. For the change in nitrogen, the root system is more sensitive than the aboveground part. High nitrogen and low nitrogen can reduce the root activity of wheat [49]. Under the experimental conditions, the root activity increased first and then decreased with the increase in nitrogen application. The root activity of wheat in the A1 treatment was the best, which was 21.04–79.93% higher than other treatments. The root activity of the two spring wheat varieties under the same nitrogen treatment was also different. The root activity of cv. XC 38 was 3.17% higher than that of cv. XC 49, indicating that nitrogen-efficient varieties had more advantages in production to obtain high yields and quality. Correlation analysis showed that root activity and root morphology (RLD, RVD, RMD) were significantly correlated with the nitrogen metabolism enzymes activities (NR, GS, GOGAT, GPT) and antioxidant enzyme activities (SOD, POD), which showed that A1 treatment could increase the physiological activity of wheat roots, ensure that wheat had vigorous root activity, maintain strong nutrient absorption capacity of roots, and then increase the nutrient absorption of wheat and promote the process of nitrogen metabolism. Stepwise regression analysis showed that root activity in deep roots (40–60 cm) contributed the most to yields, while A1 treatment could significantly improve root activity in deep roots. Therefore, the application of nitrogen fertilizer during the wheat growth period in Northern Xinjiang can be appropriately reduced to improve the root activity of spring wheat.
4.3. Effect of Nitrogen Application on Dry Matter Accumulation, Transport, and Yield of Wheat
Nitrogen is the primary factor to promote wheat growth and yield formation, and nitrogen uptake is the primary factor to improve yield. Some studies have shown that there are three main directions after the application of nitrogen fertilizer: plant absorption, soil residue, and loss of multiple ways such as leaching, denitrification, ammonia volatilization. Our research team showed that the proportion of nitrogen fertilizer absorption, soil residue, and loss in wheat maturity was 29.6%, 27.6% and 42.8%, respectively, but some studies believed that the three proportions were 49%, 30% and 21%, respectively [50]. The research results were inconsistent, which may be related to the impact of many factors such as the nitrogen application amount, yield level, soil fertility status, and variety differences on nitrogen fertilizer utilization in production practice. Therefore, increasing nitrogen uptake of wheat can regulate the accumulation and transportation of assimilates, which is conducive to dry matter accumulation and yield formation [51]. Xu et al. [52] believed that when the nitrogen application rate in North China was 0–240 kg·ha−1, with the increase in the nitrogen application rate, the rice yield increased from 5190 kg·ha−1 to 9370 kg·ha−1, and the continuous increase of nitrogen application had no significant effect on the yields. Zhang et al. [53] believed that when the nitrogen application rate in the Huang Huai Hai wheat area increased from 0 kg·ha−1 to 210 kg·ha−1, the spike mass fraction increased from 45.13% to 46.82%. The nitrogen application rate increased to 360 kg·ha−1, while the spike mass fraction decreased to 44.34%. The results showed that the shoot dry matter, spike dry matter, and yield reached the maximum at A1 treatment (240 kg·ha−1), and the spike mass fraction was the highest at the milk ripening stage. At this time, shoot dry matter and spike dry matter were 14.54–79.38% and 29.04–165.13% higher than other treatments. When the amount of nitrogen application exceeded 240 kg·ha−1, shoot dry matter, spike dry matter, and yield decreased, which may be due to the “luxury” absorption phenomenon caused by excessive nitrogen application, which eventually led to the decline in wheat yields. Compared with the A1 treatment, the shoot dry matter of Nck and A2 significantly decreased, which was caused by the late leaf senescence and the transfer of the growth center to the spike. Too little nitrogen application may lead to insufficient dry matter accumulation. High nitrogen application will lead to the vigorous growth of wheat stems and leaves and reduce the spike mass fraction, which is not conducive to increasing yields. The yields of strong gluten wheat (XC 38) were higher than that of medium gluten wheat (XC 49), indicating that cv. XC 38 was a variety with high nitrogen absorption and utilization efficiency. High nitrogen (Nck) treatment led to the excessive growth of nutrients, resulting in a decline in yield. Proper nitrogen reduction (A1) could effectively improve the morphological and physiological characteristics of roots, improve the spike mass fraction, and promote the increase in yields. It is suggested that the nitrogen application rate of the two varieties in the Xinjiang wheat area should be controlled at about 240 kg·ha−1, which can promote the rational distribution and transfer of dry matter to spikes.
5. Conclusions
The interaction of different nitrogen levels and varieties on the root morphology and physiological shape of wheat improved the root morphological characteristics, promoted root nitrogen metabolism and antioxidant enzyme activities, increased root activity, promoted dry matter translocation to spikes, and contributed to higher yields with the A1 (240 kg·ha−1) treatment. The root morphological characteristics, nitrogen metabolism, and antioxidant enzymes of strong gluten wheat cv. XC 38 were better than those of medium gluten wheat cv. XC 49. Therefore, the use of a strong gluten wheat variety (XC 38) combined with the appropriate regulation of nitrogen application (240 kg·ha−1) coordinated the growth of wheat roots and aboveground parts to promote wheat yield improvement.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agronomy12112618/s1, Figure S1: Daily average temperature and precipitation during the growth period of spring wheat from 2019 to 2020; Figure S2: Schematic diagram of field plantin; Table S1: Physical and chemical properties of soil used in the experiment; Table S2 Nitrogen application rate of spring wheat under drip irrigation in each treatment and each growth period.
Author Contributions
Conceptualization, R.W., G.J. and H.Y.; software and validation, R.W.; formal analysis, H.W., H.Y. and Z.C.; investigation, R.W., H.W., Z.C., F.J., T.Z. and B.X.; data curation, R.W.; writing—original draft preparation, R.W.; writing—review and editing, G.J. and J.L.; supervision, J.L.; project administration, G.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research and APC were funded by projects of the key project of the National Natural Science Foundation of China (Project No. 31760346).
Data Availability Statement
The datasets generated for this study are available upon request to the corresponding author.
Acknowledgments
We are grateful to Guiying Jiang (Shihezi University) for help with paper writing. We would also like to thank the reviewers for helping us improve our original manuscript. This study was supported by projects of the key project of the National Natural Science Foundation of China (Project No. 31760346).
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Ferreira, I.E.P.; Zocchi, S.S.; Baron, D. Reconciling the mitscherlich’s law of diminishing returns with liebig’s law of the minimum. some results on crop modeling. Math. Biosci. 2017, 293, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; He, J.Z.; Zhang, F.S.; Shen, Q.R.; Wu, J.S. Healthy soils for sustainable food production and environmental quality. Front. Agric. Sci. Eng. 2020, 7, 347–355. [Google Scholar] [CrossRef]
- Jin, S.Q.; Zhou, F. Zero growth of chemical fertilizer and pesticide use: China’s objectives, progress and challenges. J. Resour. Ecol. 2018, 9, 50–58. [Google Scholar]
- Qi, W.Z.; Liu, H.H.; Liu, P.; Dong, S.T.; Zhao, B.Q.; So, H.B.; Li, G.; Liu, H.D.; Zhang, J.W.; Zhao, B. Morphological and physiological characteristics of corn (Zea mays L.) roots from cultivars with different yield potentials. Eur. J. Agron. 2012, 38, 54–63. [Google Scholar] [CrossRef]
- Wang, J.T.; Du, G.F.; Tian, J.S.; Jiang, C.D.; Zhang, Y.L.; Zhang, W.F. Mulched drip irrigation increases cotton yield and water use efficiency via improving fine root plasticity. Agric. Water Manag. 2021, 255, 106992. [Google Scholar] [CrossRef]
- Eghball, B.; Maranville, J.W. Root development and nitrogen influx of corn genotypes grown under combined drought and nitrogen stresses. Agron. J. 1993, 85, 147–152. [Google Scholar] [CrossRef]
- Středa, T.; Dostál, V.; Horáková, V.; Chloupek, O. Effective use of water by wheat varieties with different root system sizes in rain-fed experiments in Central Europe. Agric. Water Manag. 2012, 104, 203–209. [Google Scholar] [CrossRef]
- Chloupek, O.; Dostál, V.; Středa, T.; Psota, V.; Dvořáčková, O. Drought tolerance of barley varieties in relation to their root system size. Plant Breed. 2010, 129, 630–636. [Google Scholar] [CrossRef]
- Herrera, J.M.; Noulas, C.; Feil, B.; Stamp, P.; Liedgens, M. Nitrogen and genotype effects on root growth and root survivorship of spring wheat. J. Plant Nutr. Soil Sci. 2013, 176, 561–571. [Google Scholar] [CrossRef]
- Xue, Y.F.; Zhang, W.; Liu, D.Y.; Yue, S.C.; Cui, Z.L.; Chen, X.P.; Zou, C.Q. Effects of nitrogen management on root morphology and zinc translocation from root to shoot of winter wheat in the field. Field Crops Res. 2014, 161, 38–45. [Google Scholar] [CrossRef]
- Wang, F.; Gao, J.; Liu, Y.; Tian, Z.; Muhammad, A.; Zhang, Y.X.; Jiang, D.; Cao, W.X.; Dai, T.B. Higher ammonium transamination capacity can alleviate glutamate inhibition on winter wheat (Triticum aestivum L.) root growth under high ammonium stress. PLoS ONE 2016, 11, e0160997. [Google Scholar] [CrossRef] [PubMed]
- Ahanger, M.A.; Qin, C.; Begum, N.; Qi, M.; Dong, X.X.; El-Esawi, M.; El-Sheikh, M.A.; Alatar, A.A.; Zhang, L.X. Nitrogen availability prevents oxidative effects of salinity on wheat growth and photosynthesis by up-regulating the antioxidants and osmolytes metabolism, and secondary metabolite accumulation. BMC Plant Biol. 2019, 19, 479. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.W.; Wang, H.Z.; Zhai, Z.H.; Sun, M.; Li, Y.J. Effect of water and nitrogen coupling on root morphology and physiology, yield and nutrition utilization for rice. Trans. Chin. Soc. Agric. Eng. 2015, 31, 132–141. (In Chinese) [Google Scholar] [CrossRef]
- Wang, J.Z.; Huang, G.B.; Zhang, C.N.; Yang, Y.J.; Zhao, H.J.; Zhu, X.Y.; Ma, P.F. Influence of nitrogen fertilizer rate on carbon-nitrogen metabolism and nitrogen use efficiency of summer maize under high and medium yield levels. Acta Ecol. Sin. 2009, 29, 2045–2052. (In Chinese) [Google Scholar] [CrossRef]
- Xu, G.W.; Lu, D.K.; Wang, H.Z.; Li, Y.J. Morphological and physiological traits of rice roots and their relationships to yield and nitrogen utilization as influenced by irrigation regime and nitrogen rate. Agric. Water Manag. 2018, 203, 385–394. [Google Scholar] [CrossRef]
- Liao, Z.Q.; Zeng, H.L.; Fan, J.L.; Lai, Z.L.; Zhang, C.; Zhang, F.C.; Wang, H.D.; Cheng, M.H.; Guo, J.J.; Li, Z.J.; et al. Effects of plant density, nitrogen rate and supplemental irrigation on photosynthesis, root growth, seed yield and water-nitrogen use efficiency of soybean under ridge-furrow plastic mulching. Agric. Water Manag. 2022, 268, 107688. [Google Scholar] [CrossRef]
- Ahmad, I.; Kamran, M.; Yang, X.N.; Meng, X.P.; Ali, S.; Ahmad, S.; Zhang, X.D.; Bilegjargal, B.; Ahmad, B.; Liu, T.N.; et al. Effects of applying uniconazole alone or combined with manganese on the photosynthetic efficiency, antioxidant defense system, and yield in wheat in semiarid regions. Agric. Water Manag. 2019, 216, 400–414. [Google Scholar] [CrossRef]
- Ahmad, I.; Zhu, G.L.; Zhou, G.S.; Song, X.D.; Ibrahim, M.E.H.; Salih, E.G.I. Effect of N on growth, antioxidant capacity, and chlorophyll content of sorghum. Agronomy 2022, 12, 501. [Google Scholar] [CrossRef]
- Xu, S.J.; Wang, L.; De, M.Q.G.; Wang, J.B.; Qi, H.Y.; Zhu, G.L. Responses of antioxidant enzyme activities, yield, and oil content to nitrogen fertilizer in dwarf castor hybrids in the Xiliaohe Plain. Soil Fertil. Sci. China 2021, 4, 192–202. (In Chinese) [Google Scholar] [CrossRef]
- Chu, G.; Xu, R.; Chen, S.; Xu, C.M.; Liu, Y.H.; Abliz, B.; Zhang, X.F.; Wang, D.Y. Root morphological-physiological traits for japonica/indicahybrid rice with better yield performance under low N conditions. Food Energy Secur. 2022, 2, 355. [Google Scholar] [CrossRef]
- Zheng, X.J.; Yu, Z.; Zhang, Y.W.; Zhang, Y.L.; Shi, Y. Effect of nitrogen rates on wheat photosynthesis, anatomical parameters and photoassimilate partitioning in North China. Int. J. Plant Prod. 2021, 15, 161–172. [Google Scholar] [CrossRef]
- Si, Z.Y.; Zain, M.; Mehmood, F.; Wang, G.S.; Gao, Y.; Duan, A. Effects of nitrogen application rate and irrigation regime on growth, yield, and water-nitrogen use efficiency of drip-irrigated winter wheat in the north china plain. Agric. Water Manag. 2020, 231, 106002. [Google Scholar] [CrossRef]
- Dunbabin, V.M.; Postma, J.A.; Schnepf, A.; Pagès, L.; Javaux, M.; Wu, L.H.; Leitner, D.; Chen, Y.L.; Rengel, Z.; Diggle, A.J. Modelling root-soil interactions using three-dimensional models of root growth, architecture and function. Plant Soil 2013, 372, 93–124. [Google Scholar] [CrossRef]
- Huo, L.Q.; Guo, Z.J.; Wang, Q.; Cheng, L.; Jia, X.; Wang, P.; Gong, X.Q.; Li, C.Y.; Ma, F.W. Enhanced autophagic activity improved the root growth and nitrogen utilization ability of apple plants under nitrogen starvation. Int. J. Mol. Sci. 2021, 22, 8085. [Google Scholar] [CrossRef] [PubMed]
- Ju, C.X.; Zhu, Y.W.; Liu, T.; Sun, C.M. The effect of nitrogen reduction at different stages on grain yield and nitrogen use efficiency for nitrogen efficient rice varieties. Agronomy 2021, 11, 462. [Google Scholar] [CrossRef]
- Gwenzi, W.; Veneklaas, E.J.; Holmes, K.W.; Bleby, T.M.; Philips, I.R.; Hinz, C. Spatial analysis of fine root distribution on a recently constructed ecosystem in a water-limited environment. Plant Soil 2011, 344, 255–272. [Google Scholar] [CrossRef]
- Mehrabi, F.; Sepaskhah, A.R.; Ahmadi, S.H. Winter wheat root distribution with irrigation, planting methods, and nitrogen application. Nutr. Cycl. Agroecosyst. 2021, 119, 231–245. [Google Scholar] [CrossRef]
- Cruz, C.; Bio, A.F.M.; Domínguez-Valdivia, M.D.; Aparicio-Tejo, P.M.; Lamsfus, C.; Martins-Loução, M.A. How does glutamine synthetase activity determine plant tolerance to ammonium? Planta 2006, 223, 1068–1080. [Google Scholar] [CrossRef]
- Singh, R.P.; Srivastava, H.S. Increase in glutamate synthase (NADH) activity in maize seedlings in response to nitrate and ammonium nitrogen. Physiol. Plant. 1986, 66, 413–416. [Google Scholar] [CrossRef]
- Baars, J.J.P.; Op den Camp, H.J.M.; Hermans, J.M.H.; Mikes, V.; Drift, C.; Van Griensven, L.J.L.D.; Vogels, G.D. Nitrogen assimilating enzymes in the white button mushroom Agaricus bisporus. Microbiology 1994, 140, 1161–1168. [Google Scholar] [CrossRef]
- Guo, Z.J.; Shi, Y.; Yu, Z.W.; Zhang, Y.L. Supplemental irrigation affected flag leaves senescence post-anthesis and grain yield of winter wheat in the Huang-Huai-Hai Plain of China. Field Crops Res. 2015, 180, 100–109. [Google Scholar] [CrossRef]
- Man, J.G.; Shi, Y.; Yu, Z.W.; Zhang, Y.L. Root growth, soil water variation, and grain yield response of winter wheat to supplemental irrigation. Plant Prod. Sci. 2016, 19, 193–205. [Google Scholar] [CrossRef]
- Xin, W.; Liu, H.L.; Zhao, H.W.; Wang, J.G.; Zheng, H.L.; Jia, Y.; Yang, L.M.; Wang, X.P.; Li, J.M.; Li, X.W.; et al. The response of grain yield and root morphological and physiological traits to nitrogen levels in paddy rice. Front. Plant Sci. 2021, 12, 713814. [Google Scholar] [CrossRef] [PubMed]
- Meng, T.Y.; Wei, H.H.; Li, X.Y.; Dai, Q.G.; Huo, Z.Y. A better root morpho-physiology after heading contributing to yield superiority of japonica/indica hybrid rice. Field Crops Res. 2018, 228, 135–146. [Google Scholar] [CrossRef]
- Zhu, K.Y.; Zhou, Q.; Shen, Y.; Yan, J.Q.; Xu, Y.J.; Wang, Z.Q.; Yang, J.C. Agronomic and physiological performance of an indica-japonica rice variety with a high yield and high nitrogen use efficiency. Crop Sci. 2020, 60, 1556–1568. [Google Scholar] [CrossRef]
- Chandna, R.; Hakeem, K.R.; Khan, F.; Ahmad, A.; Iqbal, M. Variability of nitrogen uptake and assimilation among N-efficient and N-inefficient wheat (Triticum aestivum L.) genotypes. J. Plant Interact. 2012, 7, 367–375. [Google Scholar] [CrossRef]
- Hao, Z.; Xue, Y.G.; Wang, Z.Q.; Yang, J.C.; Zhang, J.H. Morphological and physiological traits of roots and their relationships with shoot growth in “super” rice. Field Crops Res. 2009, 113, 31–40. [Google Scholar] [CrossRef]
- Wang, J.T.; Du, G.F.; Tian, J.S.; Zhang, Y.L.; Jiang, C.D.; Zhang, W.F. Effect of irrigation methods on root growth, root-shoot ratio and yield components of cotton by regulating the growth redundancy of root and shoot. Agric. Water Manag. 2020, 234, 106120. [Google Scholar] [CrossRef]
- Gan, Y.T.; Campbell, C.A.; Janzen, H.H.; Lemke, R.L.; Basnyat, P.; McDonald, C.L. Nitrogen accumulation in plant tissues and roots and N mineralization under oilseeds, pulses, and spring wheat. Plant Soil 2010, 332, 451–461. [Google Scholar] [CrossRef]
- Pang, J.Y.; Palta, J.A.; Rebetzke, G.J.; Milroy, S.P. Wheat genotypes with high early vigour accumulate more nitrogen and have higher photosynthetic nitrogen use efficiency during early growth. Funct. Plant Biol. 2013, 41, 215–222. [Google Scholar] [CrossRef]
- Ma, G.; Liu, W.X.; Li, S.S.; Zhang, P.P.; Wang, C.Y.; Lu, H.F.; Wang, L.F.; Xie, Y.X.; Ma, D.Y.; Kang, G.Z. Determining the Optimal N input to improve grain yield and quality in winter wheat with reduced apparent N loss in the North China Plain. Front. Plant Sci. 2019, 10, 181. [Google Scholar] [CrossRef] [PubMed]
- Forde, B.; Lorenzo, H. The nutritional control of root development. Plant Soil 2001, 232, 51–68. [Google Scholar] [CrossRef]
- Ju, C.X.; Liu, T.; Sun, C.M. Panicle nitrogen strategies for nitrogen-efficient rice varieties at a moderate nitrogen application rate in the lower reaches of the Yangtze River, China. Agronomy 2021, 11, 192. [Google Scholar] [CrossRef]
- Echarte, L.; Rothstein, S.; Tollenaar, M. The response of leaf photosynthesis and dry Matter accumulation to nitrogen supply in an older and a newer maize hybrid. Crop Sci. 2008, 48, 656–665. [Google Scholar] [CrossRef]
- Liu, R.X.; Zhou, Z.G.; Guo, W.Q.; Chen, B.L.; Oosterhuis, D.M. Effects of Nfertilization on root development and activity of water-stressed cotton (Gossypium hirsutum L.) plants. Agric. Water Manag. 2008, 95, 1261–1270. [Google Scholar] [CrossRef]
- Iqbal, A.; Dong, Q.; Wang, X.R.; Gui, H.P.; Zhang, H.H.; Zhang, X.L.; Song, M.Z. High nitrogen enhance drought tolerance in cotton through antioxidant enzymatic activities, nitrogen metabolism and osmotic adjustment. Plants 2020, 9, 178. [Google Scholar] [CrossRef]
- Yousuf, P.Y.; Abd_Allah, E.F.; Nauman, M.; Asif, A.; Hashem, A.; Alqarawi, A.A.; Ahmad, A. Responsive proteins in wheat cultivars with contrasting nitrogen efficiencies under the combined stress of high temperature and low nitrogen. Genes 2017, 8, 356. [Google Scholar] [CrossRef] [PubMed]
- Li, G.H.; Fu, P.X.; Cheng, G.G.; Lu, W.P.; Lu, D.L. Delaying application time of slow-release fertilizer increases soil rhizosphere nitrogen content, root activity, and grain yield of spring maize. Crop J. 2022, in press. [CrossRef]
- Chen, R.; Xiong, X.P.; Cheng, W.H. Root characteristics of spring wheat under drip irrigation and their relationship with aboveground biomass and yield. Sci. Rep. 2021, 11, 4913. [Google Scholar] [CrossRef]
- Shi, Z.L.; Jing, Q.; Cai, J.; Jiang, D.; Cao, W.X.; Dai, T.B. The fates of 15N fertilizer in relation to root distributions of winter wheat under different N splits. Eur. J. Agron. 2012, 40, 86–93. [Google Scholar] [CrossRef]
- Duan, J.Z.; Wu, Y.P.; Zhou, Y.; Ren, X.X.; Shao, Y.H.; Feng, W.; Zhu, Y.J.; Wang, Y.H.; Guo, T.C. Grain number responses to pre-anthesis dry matter and nitrogen in improving wheat yield in the Huang-Huai Plain. Sci. Rep. 2018, 8, 7126. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.W.; Song, K.J.; Lu, D.K.; Wang, H.Z.; Chen, M.C. Influence of water management and nitrogen application on rice root and shoot traits. Agron. J. 2019, 111, 2232–2244. [Google Scholar] [CrossRef]
- Zhang, X.T.; Huang, Y.F.; Ma, X.J.; Ye, Y.L. Effects of seeding rate and nitrogen level on dry matter accumulation, translocation and grain yield in two genotypes of winter wheat (Triticum aestivum). Plant Physiol. J. 2017, 53, 1067–1076. (In Chinese) [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).