Identification of the Functional Modules of SlPP2C.D—SlSAUR and Their Roles in Abscisic Acid-Mediated Inhibition of Tomato Hypocotyl Elongation
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
2.2. Root Length and Shoot Length Measurement
2.3. Conserved Domain Analysis
2.4. RNA Extraction and Quantitative RT-PCR (qRT-PCR) Analysis
2.5. Organ-Specific Expression Analysis
2.6. Polygenetic Tree by Maximum-Likelihood Method
2.7. Y2H Assays
3. Results
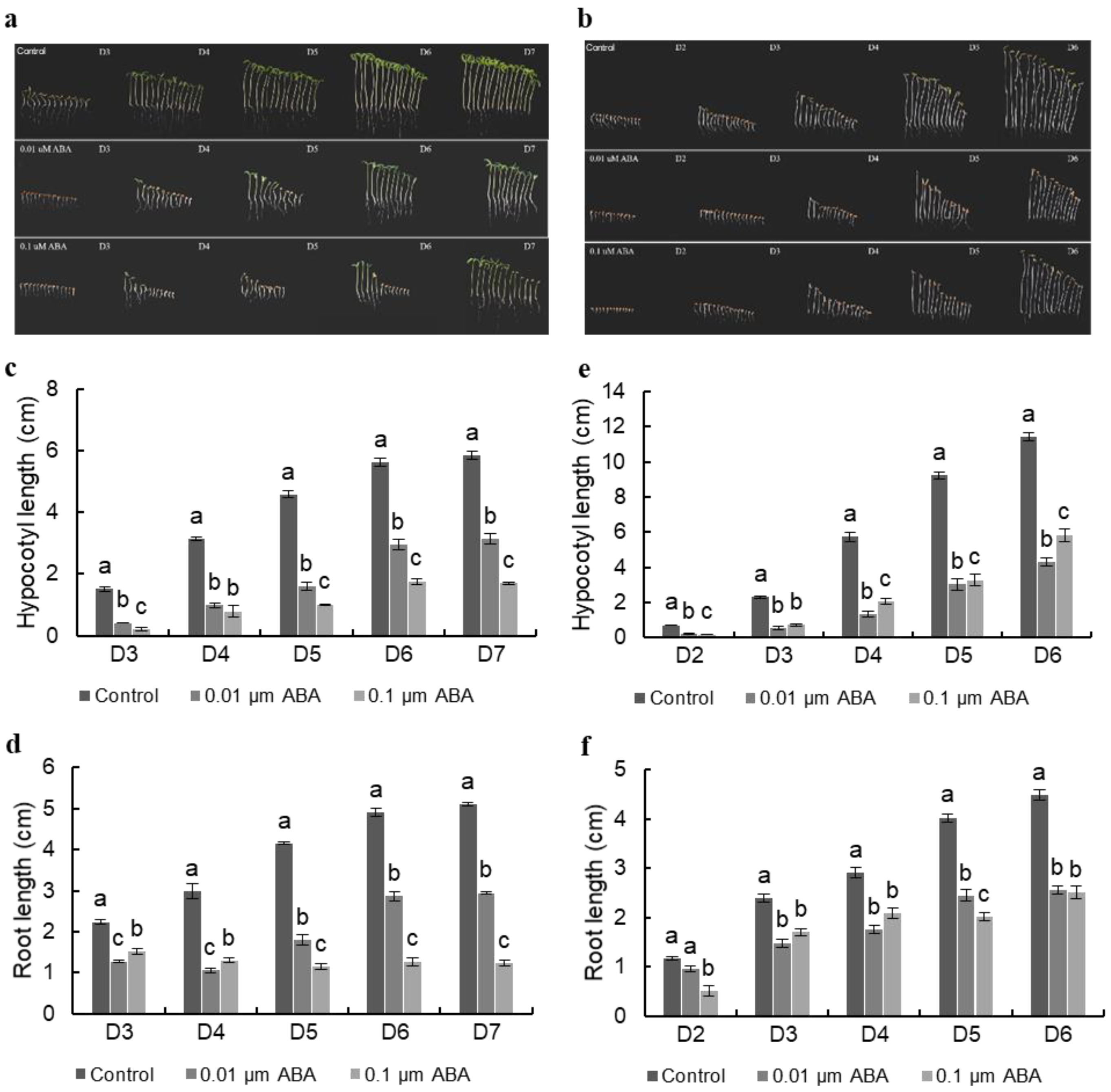
3.1. ABA Suppresses Hypocotyl Elongation in Both Light-Grown and Dark-Grown Seedlings in a Dose-Dependent Manner
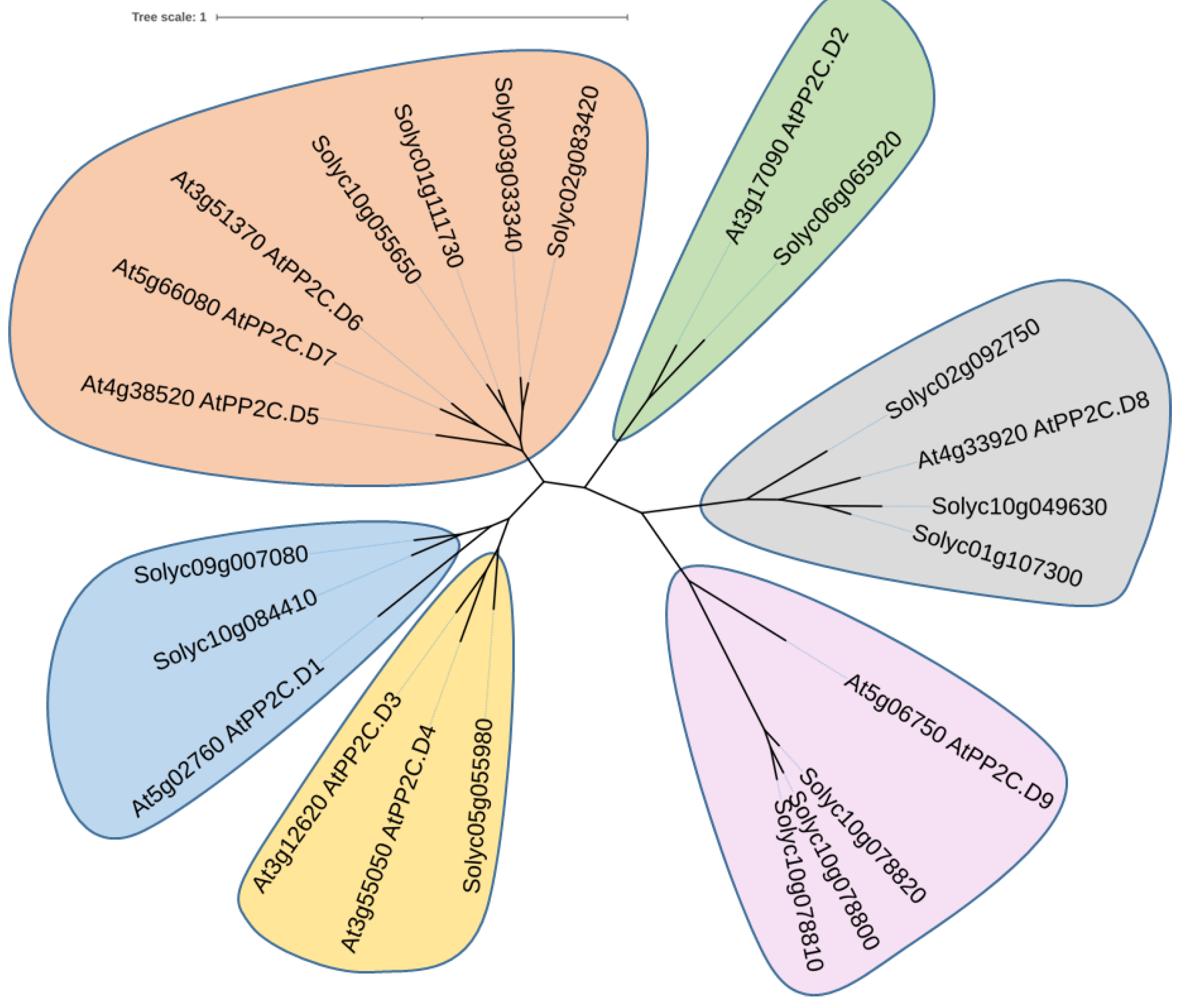
3.2. Characterization of D-Clade PP2C (SlPP2C.D) Proteins in Tomato
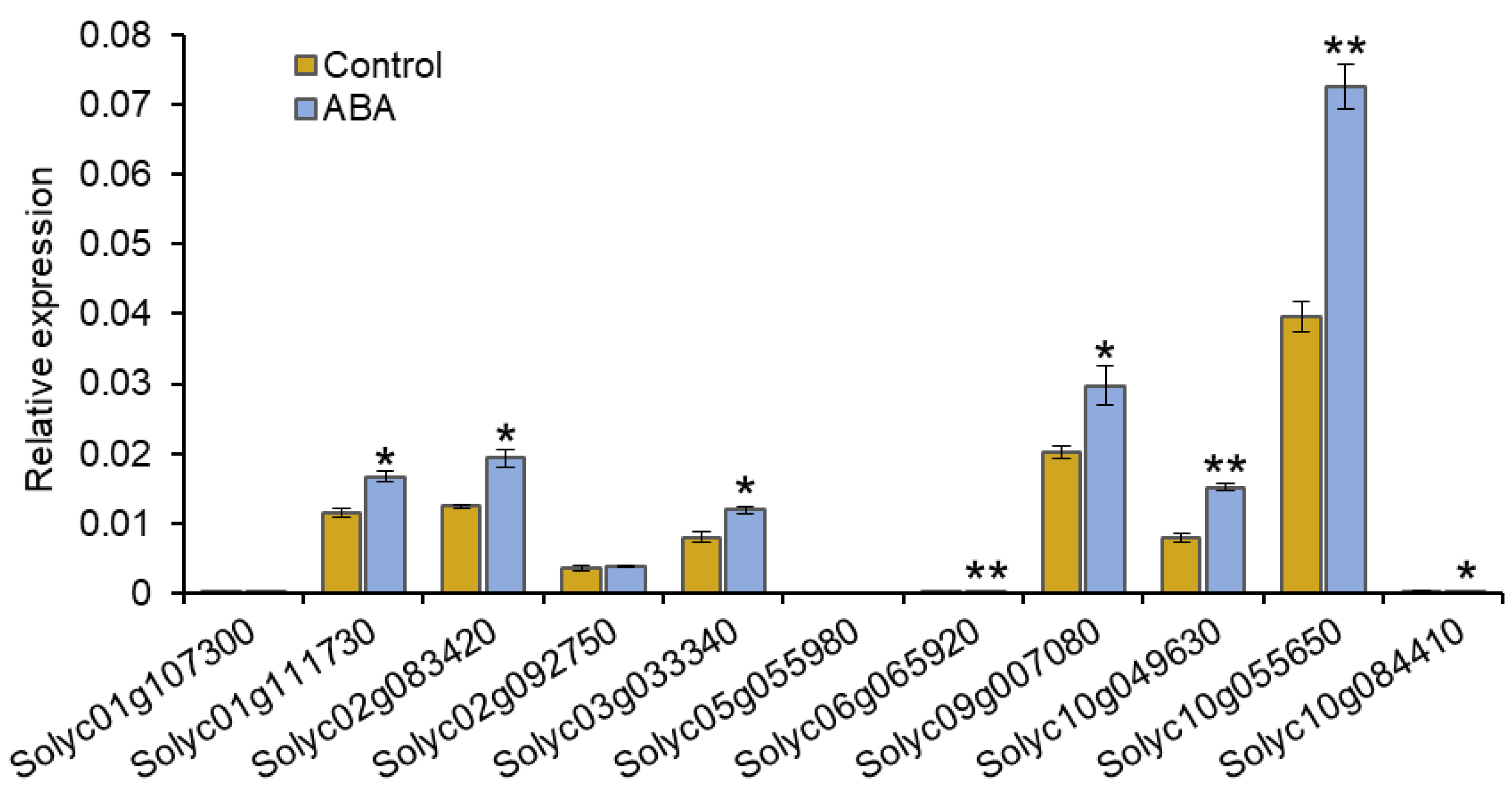
3.3. Expression of SlPP2C.D Genes in Response to ABA
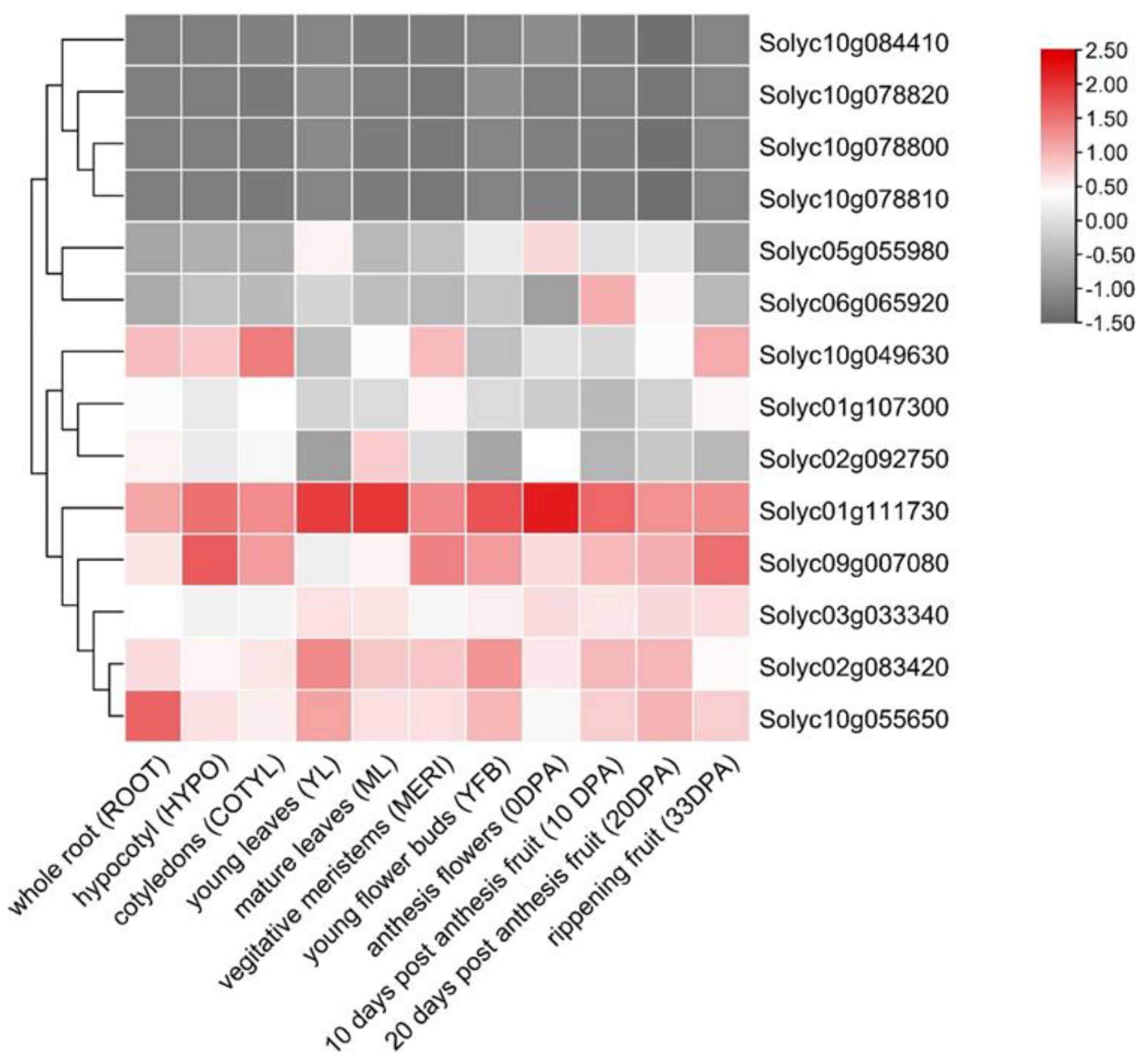
3.4. Organ-Specific Expression of SlPP2C.D Genes
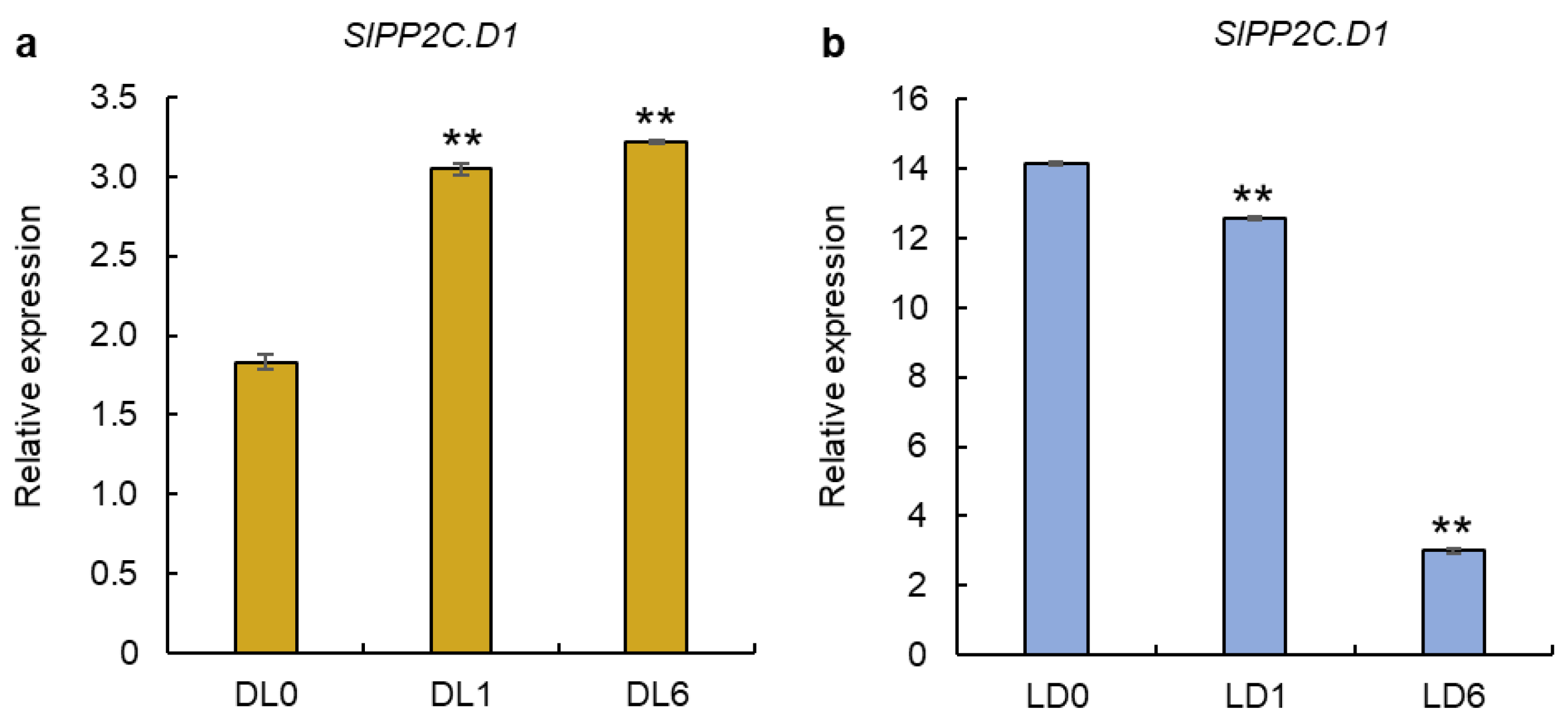
3.5. Expression of SlPP2C.D1 Gene in Light-Grown and Dark-Grown Seedlings
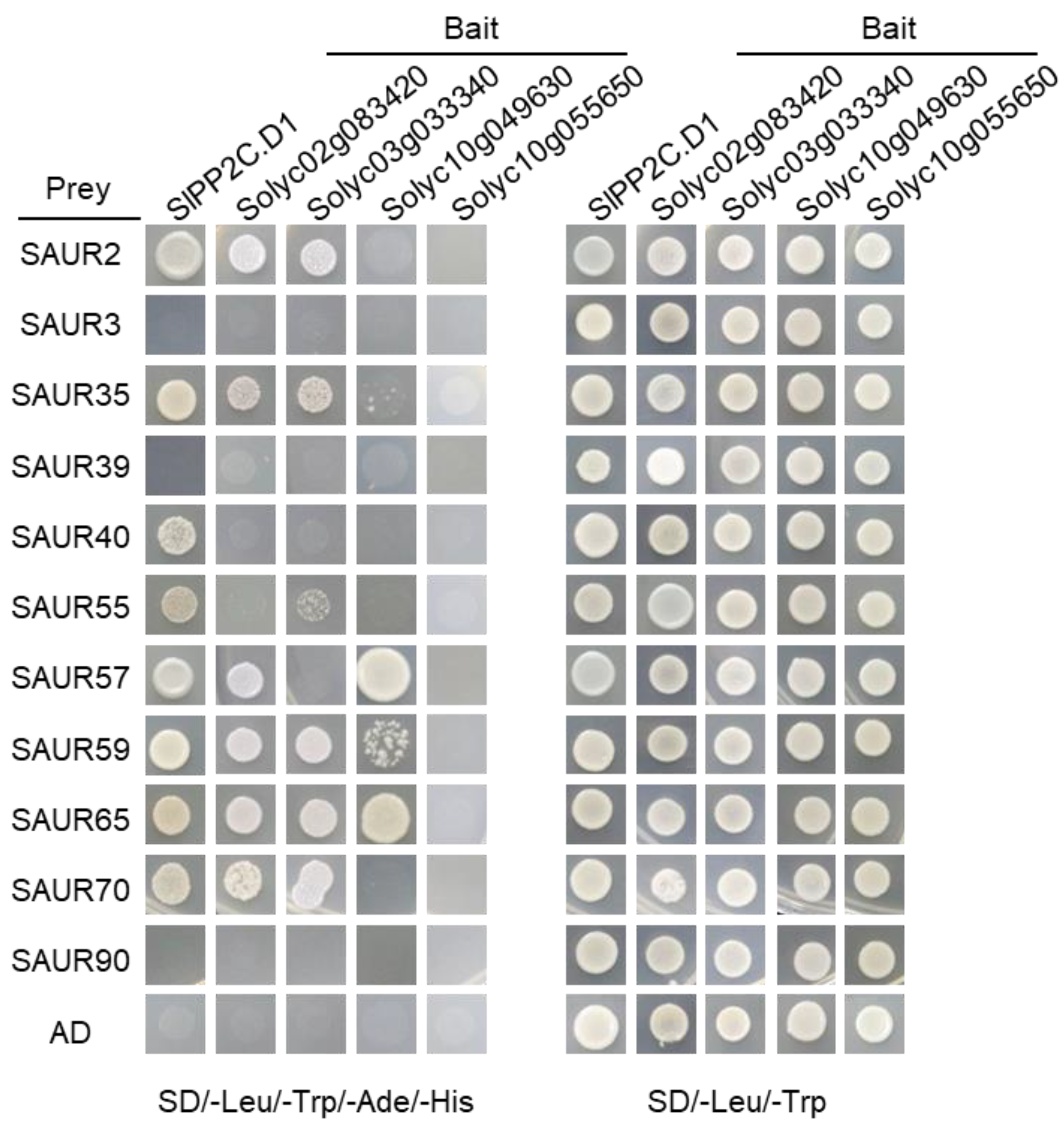
3.6. Characterization of the Interaction between SlPP2C.Ds and SlSAUR Proteins
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gendreau, E.; Traas, J.; Desnos, T.; Grandjean, O.; Caboche, M.; Hofte, H. Cellular basis of hypocotyl growth in Arabidopsis thaliana. Plant Physiol. 1997, 114, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Stamm, P.; Kumar, P.P. The phytohormone signal network regulating elongation growth during shade avoidance. J. Exp. Bot. 2010, 61, 2889–2903. [Google Scholar] [CrossRef] [PubMed]
- Rayle, D.L.; Cleland, R.E. The Acid Growth Theory of auxin-induced cell elongation is alive and well. Plant Physiol. 1992, 99, 1271–1274. [Google Scholar] [CrossRef] [PubMed]
- Rayle, D.L.; Cleland, R. Enhancement of wall loosening and elongation by Acid solutions. Plant Physiol. 1970, 46, 250–253. [Google Scholar] [CrossRef] [PubMed]
- McQueen-Mason, S.; Durachko, D.M.; Cosgrove, D.J. Two endogenous proteins that induce cell wall extension in plants. Plant Cell 1992, 4, 1425–1433. [Google Scholar] [PubMed]
- Nishitani, K.; Vissenberg, K. Roles of the XTH Protein Family in the Expanding Cell. In The Expanding Cell Plant Cell Monographs; Verbelen, J.P., Vissenberg, K., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; Volume 6, pp. 89–116. [Google Scholar]
- Hocq, L.; Pelloux, J.; Lefebvre, V. Connecting Homogalacturonan-Type Pectin Remodeling to Acid Growth. Trends Plant Sci. 2017, 22, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Hager, A. Role of the plasma membrane H+-ATPase in auxin-induced elongation growth: Historical and new aspects. J. Plant Res. 2003, 116, 483–505. [Google Scholar] [CrossRef]
- Katou, K.; Okamoto, H. Symplast as a Functional Unit in Plant Growth. In International Review of Cytology; Jeon, K.W., Friedlander, M., Eds.; Academic Press: Cambridge, MA, USA, 1992; Volume 142, pp. 263–304. [Google Scholar]
- Takahashi, K.; Hayashi, K.; Kinoshita, T. Auxin activates the plasma membrane H+-ATPase by phosphorylation during hypocotyl elongation in Arabidopsis. Plant Physiol. 2012, 159, 632–641. [Google Scholar] [CrossRef]
- Fuglsang, A.T.; Visconti, S.; Drumm, K.; Jahn, T.; Stensballe, A.; Mattei, B.; Jensen, O.N.; Aducci, P.; Palmgren, M.G. Binding of 14-3-3 protein to the plasma membrane H+-ATPase AHA2 involves the three C-terminal residues Tyr(946)-Thr-Val and requires phosphorylation of Thr(947). J. Biol. Chem. 1999, 274, 36774–36780. [Google Scholar] [CrossRef]
- Svennelid, F.; Olsson, A.; Piotrowski, M.; Rosenquist, M.; Ottman, C.; Larsson, C.; Oecking, C.; Sommarin, M. Phosphorylation of Thr-948 at the C terminus of the plasma membrane H+-ATPase creates a binding site for the regulatory 14-3-3 protein. Plant Cell 1999, 11, 2379–2391. [Google Scholar]
- Falhof, J.; Pedersen, J.T.; Fuglsang, A.T.; Palmgren, M. Plasma Membrane H+-ATPase Regulation in the Center of Plant Physiology. Mol. Plant 2016, 9, 323–337. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, T.; Shimazaki, K. Blue light activates the plasma membrane H+-ATPase by phosphorylation of the C-terminus in stomatal guard cells. EMBO J. 1999, 18, 5548–5558. [Google Scholar] [CrossRef] [PubMed]
- Okumura, M.; Takahashi, K.; Inoue, S.; Kinoshita, T. Evolutionary appearance of the plasma membrane H +-ATPase containing a penultimate threonine in the bryophyte. Plant Signal. Behav. 2012, 7, 979–982. [Google Scholar] [CrossRef]
- Okumura, M.; Inoue, S.; Takahashi, K.; Ishizaki, K.; Kohchi, T.; Kinoshita, T. Characterization of the plasma membrane H+-ATPase in the liverwort Marchantia polymorpha. Plant Physiol. 2012, 159, 826–834. [Google Scholar] [CrossRef] [PubMed]
- Nuhse, T.S.; Bottrill, A.R.; Jones, A.M.; Peck, S.C. Quantitative phosphoproteomic analysis of plasma membrane proteins reveals regulatory mechanisms of plant innate immune responses. Plant J. 2007, 51, 931–940. [Google Scholar] [CrossRef]
- Cleland, R.E. Fusicoccin-induced growth and hydrogen ion excretion of Avena coleoptiles: Relation to auxin responses. Planta 1976, 128, 201–206. [Google Scholar] [CrossRef]
- Marre, E. Fusicoccin: A Tool in Plant Physiology. Annu. Rev. Plant Physiol. 1979, 30, 273–288. [Google Scholar] [CrossRef]
- Chen, Y.; Hoehenwarter, W.; Weckwerth, W. Comparative analysis of phytohormone-responsive phosphoproteins in Arabidopsis thaliana using TiO2-phosphopeptide enrichment and mass accuracy precursor alignment. Plant J. 2010, 63, 1–17. [Google Scholar] [CrossRef]
- Hayashi, Y.; Takahashi, K.; Inoue, S.; Kinoshita, T. Abscisic acid suppresses hypocotyl elongation by dephosphorylating plasma membrane H+-ATPase in Arabidopsis thaliana. Plant Cell Physiol. 2014, 55, 845–853. [Google Scholar] [CrossRef]
- Duby, G.; Poreba, W.; Piotrowiak, D.; Bobik, K.; Derua, R.; Waelkens, E.; Boutry, M. Activation of plant plasma membrane H+-ATPase by 14-3-3 proteins is negatively controlled by two phosphorylation sites within the H+-ATPase C-terminal region. J. Biol. Chem. 2009, 284, 4213–4221. [Google Scholar] [CrossRef]
- Fuglsang, A.T.; Guo, Y.; Cuin, T.A.; Qiu, Q.; Song, C.; Kristiansen, K.A.; Bych, K.; Schulz, A.; Shabala, S.; Schumaker, K.S.; et al. Arabidopsis protein kinase PKS5 inhibits the plasma membrane H+ -ATPase by preventing interaction with 14-3-3 protein. Plant Cell 2007, 19, 1617–1634. [Google Scholar] [CrossRef] [PubMed]
- Fuglsang, A.T.; Borch, J.; Bych, K.; Jahn, T.P.; Roepstorff, P.; Palmgren, M.G. The binding site for regulatory 14-3-3 protein in plant plasma membrane H+-ATPase: Involvement of a region promoting phosphorylation-independent interaction in addition to the phosphorylation-dependent C-terminal end. J. Biol. Chem. 2003, 278, 42266–42272. [Google Scholar] [CrossRef] [PubMed]
- Rudashevskaya, E.L.; Ye, J.; Jensen, O.N.; Fuglsang, A.T.; Palmgren, M.G. Phosphosite mapping of P-type plasma membrane H+-ATPase in homologous and heterologous environments. J. Biol. Chem. 2012, 287, 4904–4913. [Google Scholar] [CrossRef] [PubMed]
- Haruta, M.; Sabat, G.; Stecker, K.; Minkoff, B.B.; Sussman, M.R. A peptide hormone and its receptor protein kinase regulate plant cell expansion. Science 2014, 343, 408–411. [Google Scholar] [CrossRef]
- Fuglsang, A.T.; Kristensen, A.; Cuin, T.A.; Schulze, W.X.; Persson, J.; Thuesen, K.H.; Ytting, C.K.; Oehlenschlaeger, C.B.; Mahmood, K.; Sondergaard, T.E.; et al. Receptor kinase-mediated control of primary active proton pumping at the plasma membrane. Plant J. 2014, 80, 951–964. [Google Scholar] [CrossRef]
- Lin, W.; Zhou, X.; Tang, W.; Takahashi, K.; Pan, X.; Dai, J.; Ren, H.; Zhu, X.; Pan, S.; Zheng, H.; et al. TMK-based cell-surface auxin signalling activates cell-wall acidification. Nature 2021, 599, 278–282. [Google Scholar] [CrossRef]
- Pei, D.; Hua, D.; Deng, J.; Wang, Z.; Song, C.; Wang, Y.; Wang, Y.; Qi, J.; Kollist, H.; Yang, S.; et al. Phosphorylation of the plasma membrane H+-ATPase AHA2 by BAK1 is required for ABA-induced stomatal closure in Arabidopsis. Plant Cell 2022, 34, 2708–2729. [Google Scholar] [CrossRef]
- Kerk, D.; Templeton, G.; Moorhead, G.B. Evolutionary radiation pattern of novel protein phosphatases revealed by analysis of protein data from the completely sequenced genomes of humans, green algae, and higher plants. Plant Physiol. 2008, 146, 351–367. [Google Scholar] [CrossRef]
- Cohen, P. The structure and regulation of protein phosphatases. Annu. Rev. Biochem. 1989, 58, 453–508. [Google Scholar]
- Cohen, P.T. Novel protein serine.threonine phosphatases: Variety is the spice of life. Trends Biochem. Sci. 1997, 22, 245–251. [Google Scholar] [CrossRef]
- Spartz, A.K.; Ren, H.; Park, M.Y.; Grandt, K.N.; Lee, S.H.; Murphy, A.S.; Sussman, M.R.; Overvoorde, P.J.; Gray, W.M. SAUR Inhibition of PP2C-D Phosphatases Activates Plasma Membrane H+-ATPases to Promote Cell Expansion in Arabidopsis. Plant Cell 2014, 26, 2129–2142. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Gray, W.M. SAUR Proteins as Effectors of Hormonal and Environmental Signals in Plant Growth. Mol. Plant 2015, 8, 1153–1164. [Google Scholar] [CrossRef]
- Chen, K.; Li, G.J.; Bressan, R.A.; Song, C.P.; Zhu, J.K.; Zhao, Y. Abscisic acid dynamics, signaling, and functions in plants. J. Integr. Plant Biol. 2020, 62, 25–54. [Google Scholar] [CrossRef] [PubMed]
- Brookbank, B.P.; Patel, J.; Gazzarrini, S.; Nambara, E. Role of Basal ABA in Plant Growth and Development. Genes 2021, 12, 1936. [Google Scholar] [CrossRef] [PubMed]
- Wakabayashi, K.; Sakurai, N.; Kuraishi, S. Role of the outer tissue in abscisic acid-mediated growth suppression of etiolated squash hypocotyl segments. Physiol. Plant. 1989, 75, 151–156. [Google Scholar] [CrossRef]
- Miao, R.; Yuan, W.; Wang, Y.; Garcia-Maquilon, I.; Dang, X.L.; Li, Y.; Zhang, J.H.; Zhu, Y.Y.; Rodriguez, P.L.; Xu, W.F. Low ABA concentration promotes root growth and hydrotropism through relief of ABA INSENSITIVE 1-mediated inhibition of plasma membrane H+-ATPase 2. Sci. Adv. 2021, 7, eabd4113. [Google Scholar] [CrossRef] [PubMed]
- Merlot, S.; Gosti, F.; Guerrier, D.; Vavasseur, A.; Giraudat, J. The ABI1 and ABI2 protein phosphatases 2C act in a negative feedback regulatory loop of the abscisic acid signalling pathway. Plant J. 2001, 25, 295–303. [Google Scholar] [CrossRef]
- Qiu, J.; Ni, L.; Xia, X.; Chen, S.; Zhang, Y.; Lang, M.; Li, M.; Liu, B.; Pan, Y.; Li, J.; et al. Genome-Wide Analysis of the Protein Phosphatase 2C Genes in Tomato. Genes 2022, 13, 604. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Ren, H.; Park, M.Y.; Spartz, A.K.; Wong, J.H.; Gray, W.M. A subset of plasma membrane-localized PP2C.D phosphatases negatively regulate SAUR-mediated cell expansion in Arabidopsis. PLoS Genet. 2018, 14, e1007455. [Google Scholar] [CrossRef] [PubMed]
- Lorrai, R.; Boccaccini, A.; Ruta, V.; Possenti, M.; Costantino, P.; Vittorioso, P. Abscisic acid inhibits hypocotyl elongation acting on gibberellins, DELLA proteins and auxin. AoB Plants 2018, 10, ply061. [Google Scholar] [PubMed]
- Humplik, J.F.; Bergougnoux, V.; Jandova, M.; Simura, J.; Pencik, A.; Tomanec, O.; Rolcik, J.; Novak, O.; Fellner, M. Endogenous abscisic acid promotes hypocotyl growth and affects endoreduplication during dark-induced growth in tomato (Solanum lycopersicum L.). PLoS ONE 2015, 10, e0117793. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.D.; Wang, J.; Guo, X.N.; Shang, B.S.; Yan, H.R.; Zhang, X.; Zhao, X. A high concentration of abscisic acid inhibits hypocotyl phototropism in Gossypium arboreum by reducing accumulation and asymmetric distribution of auxin. J. Exp. Bot. 2021, 72, 6365–6381. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Hettenhausen, C.; Sun, G.; Zhuang, H.; Li, J.H.; Wu, J. The parasitic plant Cuscuta australis is highly insensitive to abscisic acid-induced suppression of hypocotyl elongation and seed germination. PLoS ONE 2015, 10, e0135197. [Google Scholar] [CrossRef]
- Hayashi, Y.; Nakamura, S.; Takemiya, A.; Takahashi, Y.; Shimazaki, K.; Kinoshita, T. Biochemical characterization of in vitro phosphorylation and dephosphorylation of the plasma membrane H+-ATPase. Plant Cell Physiol. 2010, 51, 1186–1196. [Google Scholar] [CrossRef]
- Humplik, J.F.; Bergougnoux, V.; Van Volkenburgh, E. To Stimulate or Inhibit? That Is the Question for the Function of Abscisic Acid. Trends Plant Sci. 2017, 22, 830–841. [Google Scholar] [CrossRef]
- Cramer, G.R.; Quarrie, S.A. Abscisic acid is correlated with the leaf growth inhibition of four genotypes of maize differing in their response to salinity. Funct. Plant Biol. 2002, 29, 111–115. [Google Scholar] [CrossRef]
- Zhang, J.; Davies, W.J. Does ABA in the Xylem Control the Rate of Leaf Growth in Soil-Dried Maize and Sunflower Plants? J. Exp. Bot. 1990, 41, 1125–1132. [Google Scholar] [CrossRef]
- Sondergaard, T.E.; Schulz, A.; Palmgren, M.G. Energization of transport processes in plants. roles of the plasma membrane H+-ATPase. Plant Physiol. 2004, 136, 2475–2482. [Google Scholar] [CrossRef]
- Hayashi, M.; Inoue, S.; Takahashi, K.; Kinoshita, T. Immunohistochemical detection of blue light-induced phosphorylation of the plasma membrane H+-ATPase in stomatal guard cells. Plant Cell Physiol. 2011, 52, 1238–1248. [Google Scholar] [CrossRef] [PubMed]
- Witthoft, J.; Caesar, K.; Elgass, K.; Huppenberger, P.; Kilian, J.; Schleifenbaum, F.; Oecking, C.; Harter, K. The activation of the Arabidopsis P-ATPase 1 by the brassinosteroid receptor BRI1 is independent of threonine 948 phosphorylation. Plant Signal. Behav. 2011, 6, 1063–1066. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Du, M.; Bou Daher, F.; Liu, Y.; Steward, A.; Tillmann, M.; Zhang, X.; Wong, J.H.; Ren, H.; Cohen, J.D.; Li, C.; et al. Biphasic control of cell expansion by auxin coordinates etiolated seedling development. Sci. Adv. 2022, 8, eabj1570. [Google Scholar] [CrossRef] [PubMed]
- Sun, N.; Wang, J.; Gao, Z.; Dong, J.; He, H.; Terzaghi, W.; Wei, N.; Deng, X.W.; Chen, H. Arabidopsis SAURs are critical for differential light regulation of the development of various organs. Proc. Natl. Acad. Sci. USA 2016, 113, 6071–6076. [Google Scholar] [CrossRef]
- Wang, J.; Sun, N.; Zhang, F.; Yu, R.; Chen, H.; Deng, X.W.; Wei, N. SAUR17 and SAUR50 Differentially Regulate PP2C-D1 during Apical Hook Development and Cotyledon Opening in Arabidopsis. Plant Cell 2020, 32, 3792–3811. [Google Scholar] [CrossRef]
- Kodaira, K.S.; Qin, F.; Tran, L.S.; Maruyama, K.; Kidokoro, S.; Fujita, Y.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Arabidopsis Cys2/His2 zinc-finger proteins AZF1 and AZF2 negatively regulate abscisic acid-repressive and auxin-inducible genes under abiotic stress conditions. Plant Physiol. 2011, 157, 742–756. [Google Scholar] [CrossRef]
- Chae, K.; Isaacs, C.G.; Reeves, P.H.; Maloney, G.S.; Muday, G.K.; Nagpal, P.; Reed, J.W. Arabidopsis SMALL AUXIN UP RNA63 promotes hypocotyl and stamen filament elongation. Plant J. 2012, 71, 684–697. [Google Scholar] [CrossRef]
- He, Y.; Liu, Y.; Li, M.; Lamin-Samu, A.T.; Yang, D.; Yu, X.; Izhar, M.; Jan, I.; Ali, M.; Lu, G. The Arabidopsis SMALL AUXIN UP RNA32 Protein Regulates ABA-Mediated Responses to Drought Stress. Front. Plant Sci. 2021, 12, 625493. [Google Scholar] [CrossRef]
- Wu, J.; Liu, S.; He, Y.; Guan, X.; Zhu, X.; Cheng, L.; Wang, J.; Lu, G. Genome-wide analysis of SAUR gene family in Solanaceae species. Gene 2012, 509, 38–50. [Google Scholar] [CrossRef]
- Qiu, T.; Qi, M.; Ding, X.; Zheng, Y.; Zhou, T.; Chen, Y.; Han, N.; Zhu, M.; Bian, H.; Wang, J. The SAUR41 subfamily of SMALL AUXIN UP RNA genes is abscisic acid inducible to modulate cell expansion and salt tolerance in Arabidopsis thaliana seedlings. Ann. Bot. 2020, 125, 805–819. [Google Scholar] [CrossRef]
| Query | PL 1 (aa 2) | From | To | E-Value | Accession | Short Name |
|---|---|---|---|---|---|---|
| Solyc01g107300 | 376 | 42 | 337 | 4.67 × 10−62 | cd00143 | PP2Cc 3 |
| Solyc01g111730 | 388 | 49 | 356 | 2.06 × 10−64 | cd00143 | PP2Cc |
| Solyc02g083420 | 390 | 48 | 355 | 3.33 × 10−64 | cd00143 | PP2Cc |
| Solyc02g092750 | 366 | 32 | 336 | 4.59 × 10−58 | cd00143 | PP2Cc |
| Solyc03g033340 | 397 | 48 | 355 | 9.29 × 10−64 | cd00143 | PP2Cc |
| Solyc05g055980 | 384 | 53 | 360 | 2.43 × 10−68 | cd00143 | PP2Cc |
| Solyc06g065920 | 374 | 51 | 351 | 2.28 × 10−55 | cd00143 | PP2Cc |
| Solyc09g007080 | 378 | 43 | 350 | 1.25 × 10−72 | cd00143 | PP2Cc |
| Solyc10g049630 | 380 | 34 | 335 | 1.20 × 10−59 | cd00143 | PP2Cc |
| Solyc10g055650 | 339 | 1 | 306 | 1.44 × 10−64 | cd00143 | PP2Cc |
| Solyc10g078800 | 1312 | 51 | 342 | 3.46 × 10−55 | cd00143 | PP2Cc |
| Solyc10g078800 | 551 | 844 | 1.28 × 10−45 | cd00143 | PP2Cc | |
| Solyc10g078800 | 974 | 1266 | 1.36 × 10−54 | cd00143 | PP2Cc | |
| Solyc10g078810 | 438 | 63 | 339 | 2.40 × 10−53 | cd00143 | PP2Cc |
| Solyc10g078820 | 460 | 63 | 341 | 1.23 × 10−57 | cd00143 | PP2Cc |
| Solyc10g084410 | 376 | 42 | 349 | 1.38 × 10−72 | cd00143 | PP2Cc |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, X.; Fei, S.; Wang, S.; He, Y.; Zhu, Z.; Liu, Y. Identification of the Functional Modules of SlPP2C.D—SlSAUR and Their Roles in Abscisic Acid-Mediated Inhibition of Tomato Hypocotyl Elongation. Agronomy 2022, 12, 2542. https://doi.org/10.3390/agronomy12102542
Zheng X, Fei S, Wang S, He Y, Zhu Z, Liu Y. Identification of the Functional Modules of SlPP2C.D—SlSAUR and Their Roles in Abscisic Acid-Mediated Inhibition of Tomato Hypocotyl Elongation. Agronomy. 2022; 12(10):2542. https://doi.org/10.3390/agronomy12102542
Chicago/Turabian StyleZheng, Xiaolin, Shihong Fei, Shajun Wang, Yong He, Zhujun Zhu, and Yuanyuan Liu. 2022. "Identification of the Functional Modules of SlPP2C.D—SlSAUR and Their Roles in Abscisic Acid-Mediated Inhibition of Tomato Hypocotyl Elongation" Agronomy 12, no. 10: 2542. https://doi.org/10.3390/agronomy12102542
APA StyleZheng, X., Fei, S., Wang, S., He, Y., Zhu, Z., & Liu, Y. (2022). Identification of the Functional Modules of SlPP2C.D—SlSAUR and Their Roles in Abscisic Acid-Mediated Inhibition of Tomato Hypocotyl Elongation. Agronomy, 12(10), 2542. https://doi.org/10.3390/agronomy12102542







