PotatoMASH—A Low Cost, Genome-Scanning Marker System for Use in Potato Genomics and Genetics Applications
Abstract
1. Introduction
2. Materials and Methods
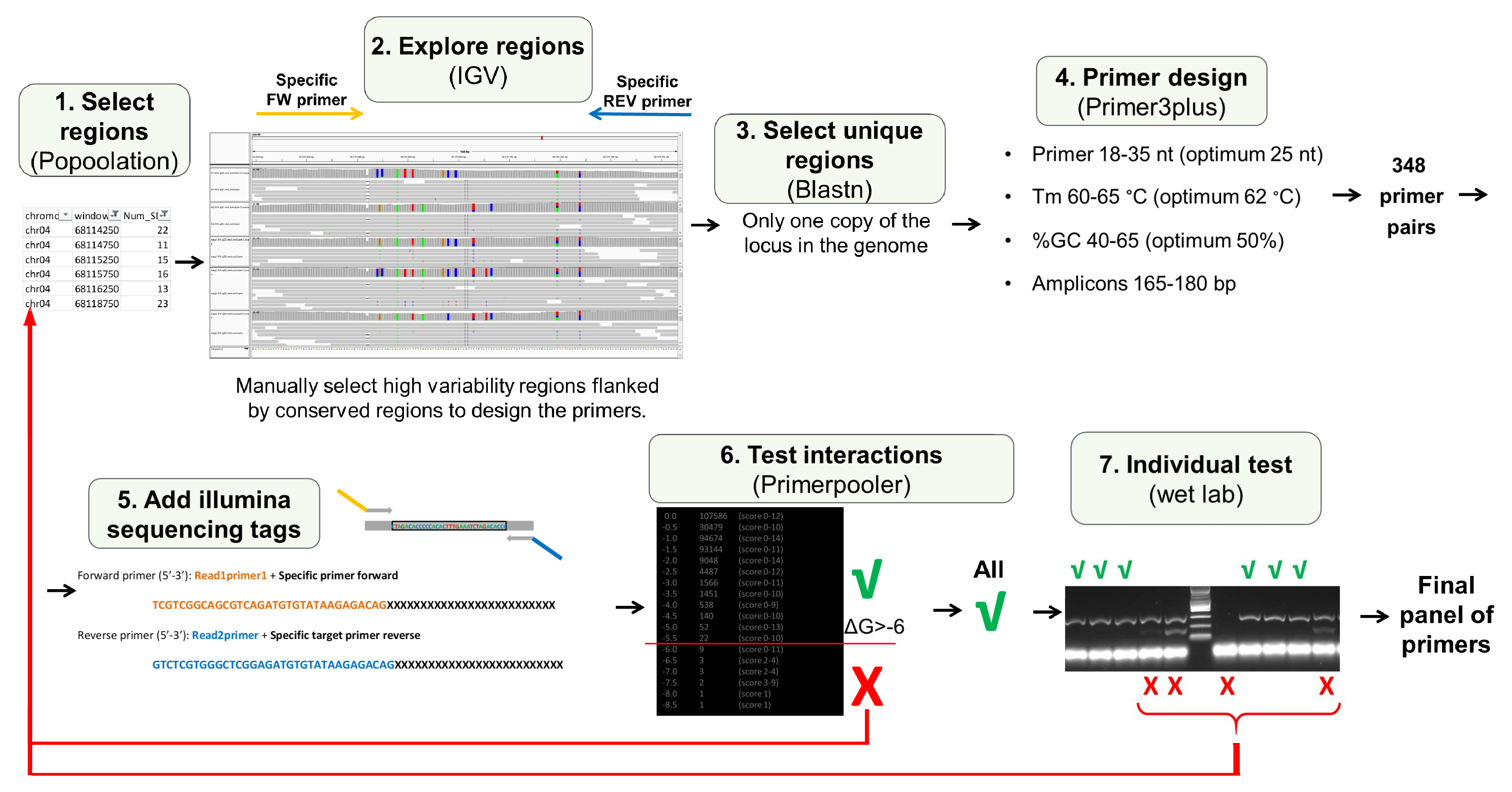
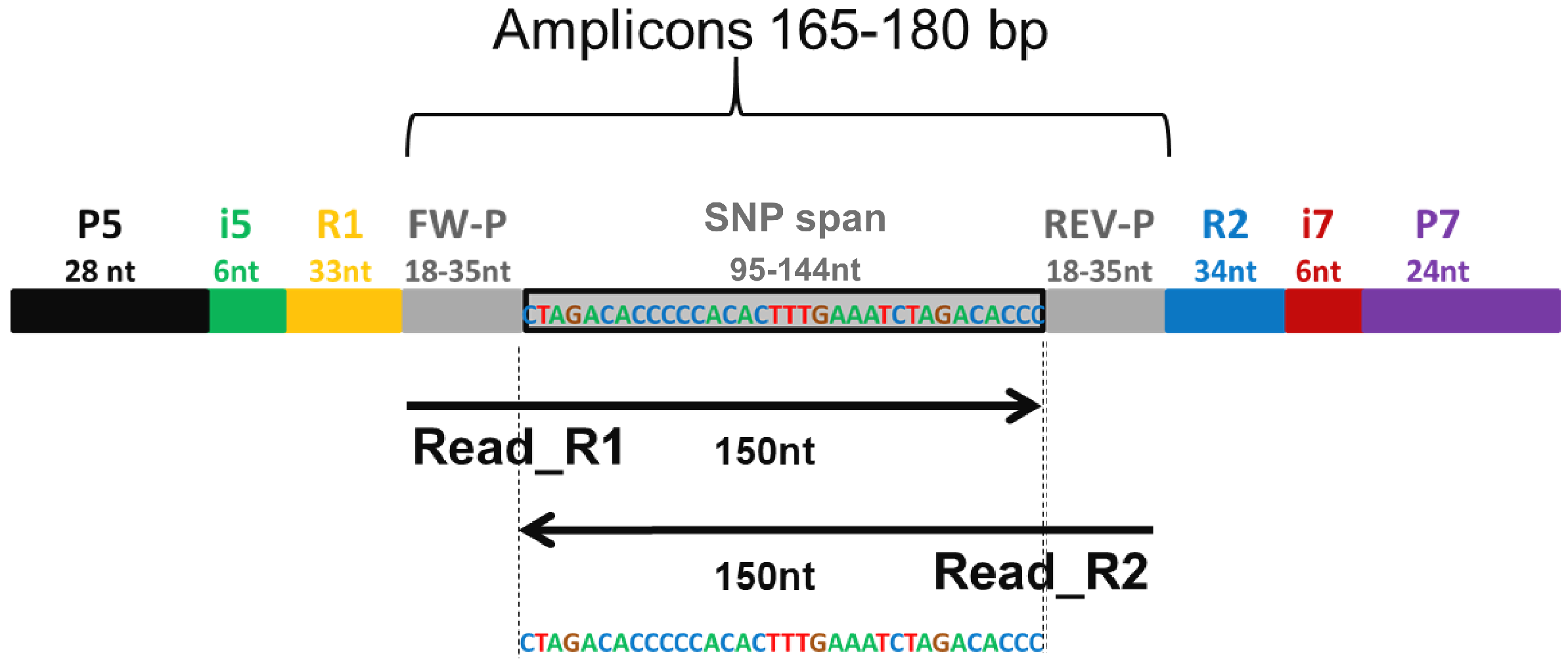
2.1. PotatoMASH Primers Panel Design
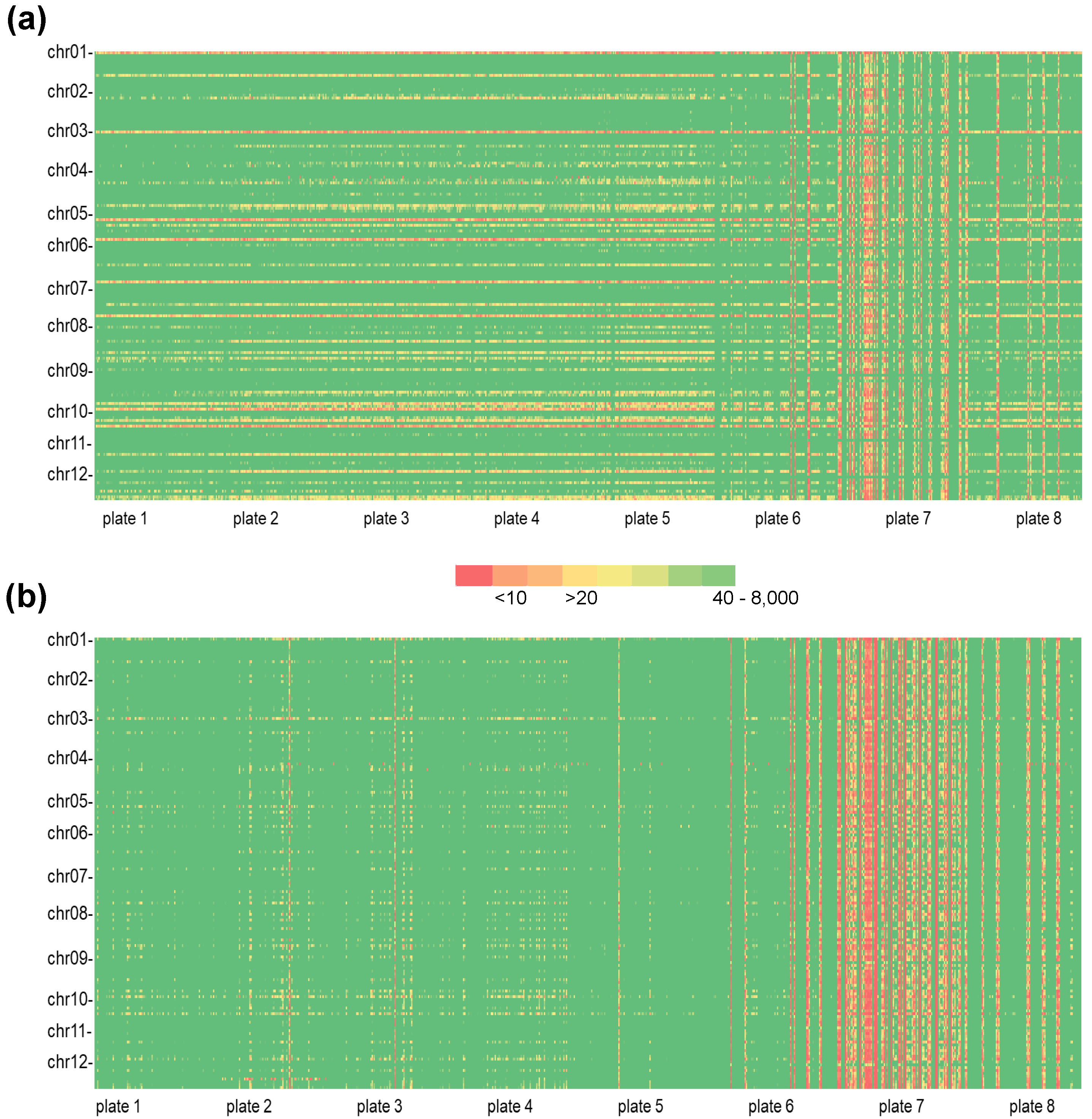
2.2. Genotyping Panel
2.3. PotatoMASH Library Construction
2.4. Multiallelic Haplotype Analysis
2.5. Haplotype-Based GWAS to Identify QTL Associated with Fry Colour
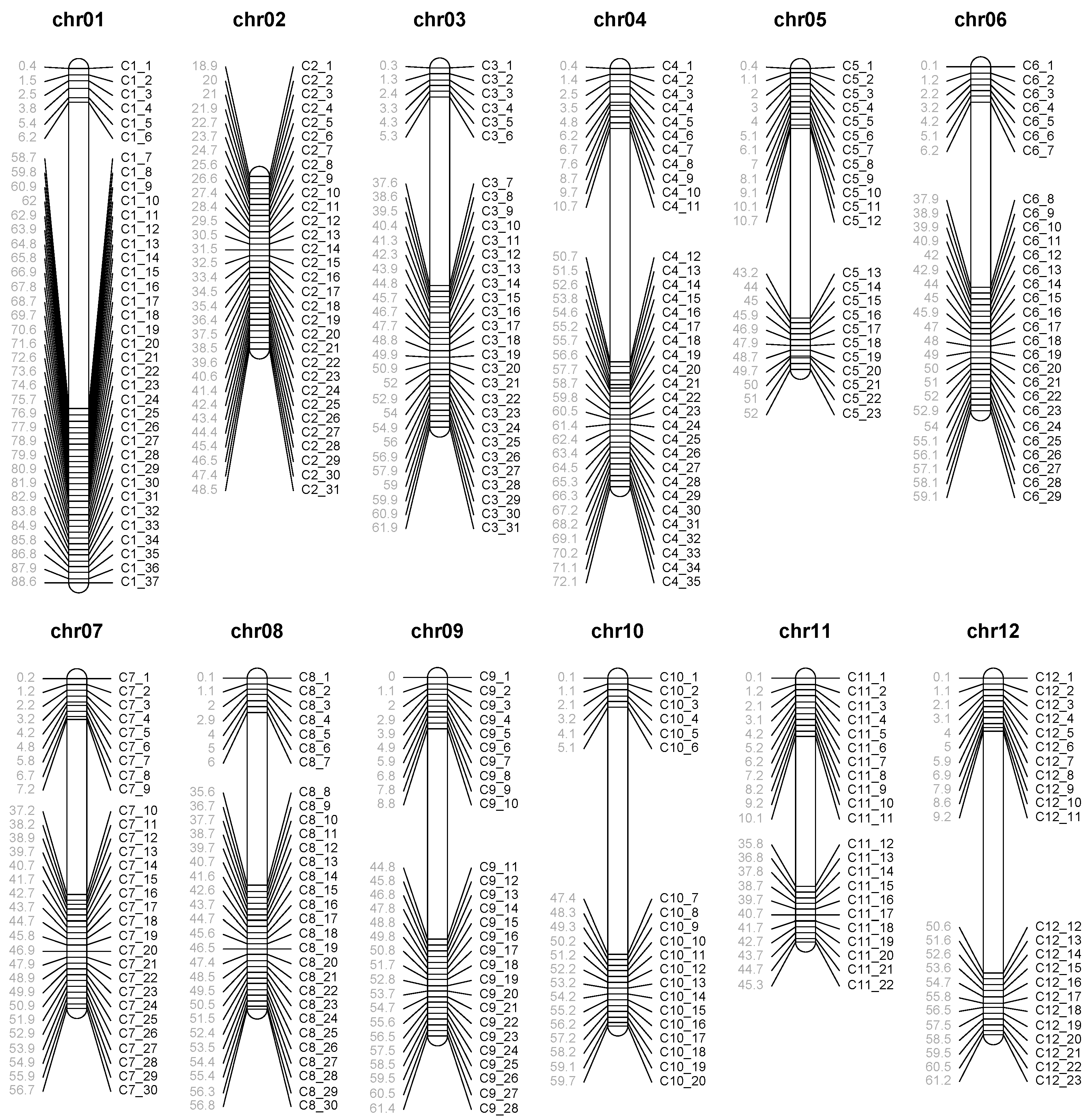
2.6. Mapping Population Genotyping and Linkage Map Construction
3. Results
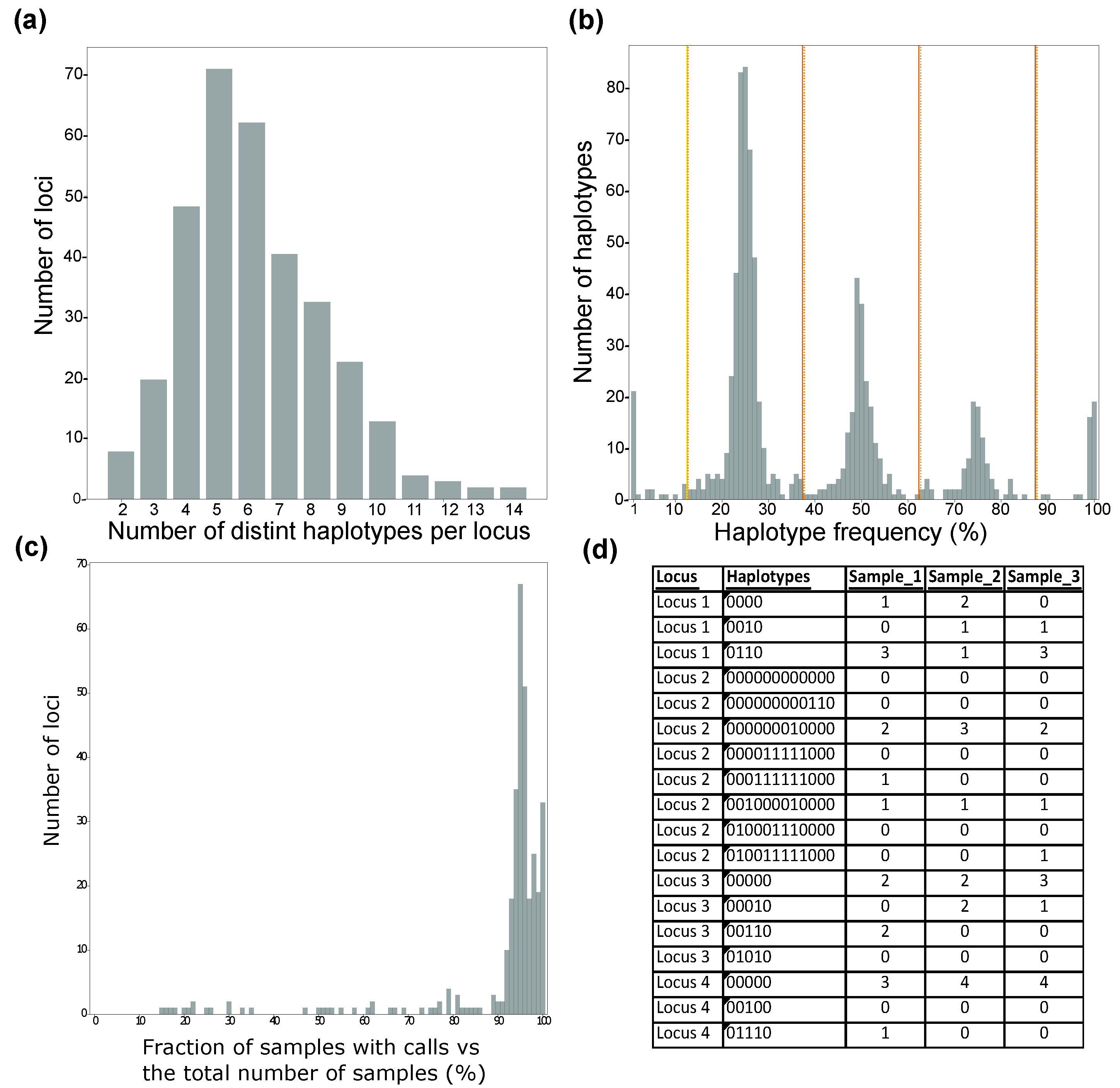
3.1. Potato Multi-Allele Scanning Haplotags (PotatoMASH) as a Genotyping System
3.2. Demonstrating the Expandability of the PotatoMASH Platform Using Targeted R-Locus Markers
3.3. Haplotype-Based GWAS to Identify QTL Associated with Fry Colour
3.4. Linkage Map Construction Using PotatoMASH Haplotypes
4. Discussion
4.1. Coverage and Allelic Diversity
4.2. Performance of PotatoMASH across the Core Loci
4.3. Cost Considerations
4.4. Utility of PotatoMASH in Discovery Genetics and Breeding Applications
4.5. Potential Applications for PotatoMASH in Potato Breeding
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| PotatoMASH Step and Supplies | Provider/Code | Pack Price (EUR) | Pack Units | Preps | Sample Cost (EUR) |
|---|---|---|---|---|---|
| DNA extraction: 705 samples by CTAB method + 60 samples by sigma Kit | 0.247 | ||||
| CTAB Lysis buffer | Applichem A4150 | 85.6 | 1 L | 1000 | |
| GenElute™ Plant Genomic DNA Miniprep Kit | Sigma G2N10-70KT | 150 | 70 | 70 | |
| 2 mL tubes for CTAB method | Greiner 623201CI | 11.43 | 1000 | 500 | 0.023 |
| Isopropanol for CTAB method | Fisher Chem. P749015 | 5.3 | 1 L | 2000 | 0.003 |
| Ethanol for CTAB method | Sigma 24105-M | 8.4 | 2.5 L | 3000 | 0.003 |
| 1.5 mL tubes for CTAB method | Sarstedt 72.690.001 | 35 | 5000 | 5000 | 0.007 |
| 1 mL tips | Fisherbrand 11548442 | 8.92 | 1000 | 916 | 0.010 |
| 200 L tips | Sarstedt 70.760.002 | 40 | 10,000 | 10,000 | 0.004 |
| 96-well PCR plate | Thermo Sci. 10425733 | 58.27 | 25 | 2400 | 0.024 |
| plate adhesive lid | Greiner 676001 | 14.3 | 100 | 9600 | 0.001 |
| Template DNA normalization: | |||||
| Quant-iT™ PicoGreen® dsDNA Assay Kit | Thermofisher P7589 | 510 | 2000 | 1916 | 0.266 |
| Nunc™ F96 MicroWell™ Black Plates | Thermofisher 236105 | 112 | 50 | 4400 | 0.025 |
| 10 L tips for quantitation | Greiner 771290 | 9 | 1000 | 1000 | 0.009 |
| 200 L tips for quantitation | Sarstedt 70.760.002 | 40 | 10,000 | 10,000 | 0.004 |
| 10 L filter tips for normalization | Sarstedt 70.1130.210 | 70 | 1920 | 1920 | 0.036 |
| 96-well PCR plate | Thermo Sci. 10425733 | 58.27 | 25 | 2400 | 0.024 |
| plate adhesive lid | Greiner 676001 | 14.3 | 100 | 9600 | 0.001 |
| PotatoMASH PCR1: | |||||
| QIAGEN Multiplex PCR Plus Kit (100) | Qiagen 6152 | 185 | 2.55 mL | 700 | 0.264 |
| PotatoMASH Primers (n = 347, 750 uM each) | IDT | 5119 | 20 L | 85,714 | 0.060 |
| 96-well PCR plate | Sarstedt 72.1978.202 | 69.75 | 25 | 2400 | 0.029 |
| 10 L filter tips | Sarstedt 70.1130.210 | 70 | 1920 | 1920 | 0.036 |
| Adhesive aluminium foil plate lid | Sarstedt 95.1995 | 50.5 | 100 | 9600 | 0.005 |
| PotatoMASH PCR2: | |||||
| 100 L filter tips to dilute PCR1 | Sarstedt 70.760.212 | 70.8 | 1920 | 1,920 | 0.037 |
| QIAGEN Multiplex PCR Plus Kit (100) | Qiagen 6152 | 185 | 2.55 mL | 490 | 0.378 |
| i5 and i7 Primers (n = 96 + 8) at 100 M | IDT | 667 | 300 L | 2875 | 0.232 |
| 96-well PCR plate | Sarstedt 72.1978.202 | 69.75 | 25 | 2400 | 0.029 |
| 10 L filter tips | Sarstedt 70.1130.210 | 70 | 1920 | 960 | 0.073 |
| Adhesive aluminium foil plate lid | Sarstedt 95.1995 | 50.5 | 100 | 9600 | 0.005 |
| Library normalization, Pooling wells, and Concentration-purification: | |||||
| SequalPrep™ Normalization Plate Kit | Invitrogen A1051001 | 1050 | 10 | 960 | 1.094 |
| 10 L tips for binding step | Greiner 771290 | 9 | 1000 | 500 | 0.018 |
| 200 L tips for washing step | Sarstedt 70.760.002 | 40 | 10,000 | 10,000 | 0.004 |
| 200 L tips for elution step | Sarstedt 70.760.002 | 40 | 10,000 | 10,000 | 0.004 |
| plate adhesive lid | Greiner 676001 | 14.3 | 100 | 9600 | 0.001 |
| Size selection, Purification, Quantification, and Pooling plates: | |||||
| Buffer PB | Qiagen 19066 | 87 | 500 mL | 6400 | 0.014 |
| 15 mL tubes | Sarstedt 62.554.002 | 55 | 500 | 48,000 | 0.001 |
| QIAquick PCR purification Kit | Qiagen 28704 | 104.14 | 50 | 4800 | 0.022 |
| 1 mL tips | Fisherbrand 11548442 | 8.92 | 1000 | 6857 | 0.001 |
| Wizard SV Gel and PCR Clean-Up System | Promega A9281 | 94 | 50 | 4800 | 0.020 |
| AMPure XP magnetic beads | Beckman C. A63881 | 1326 | 60 mL | 57,600 | 0.023 |
| 100 L filter tips | Sarstedt 70.760.212 | 70.8 | 1920 | 30,720 | 0.002 |
| Qubit™ dsDNA BR Assay Kit | Thermofisher Q32853 | 275 | 500 | 48,000 | 0.006 |
| Qubit™ assay tubes | Thermofisher Q32856 | 70 | 500 | 48,000 | 0.001 |
| Library quality assessment and Sequencing: | |||||
| One Lane Illumina HiseqX 50% PhiX, paired-end 2 × 150 nt reads. | Novogene (UK) | 1307 | 1 lane | 765 | 1.708 |
| Minor inherent expenses: | 0.125 | ||||
| Other supplies which individual cost per sample is too low such as gloves, RNAse (macherey 740505, 0.000016 EUR/sample), ddHO, one 10 mL pipette to dispense PB buffer, Agarose, TBE buffer, GelRed dye, 100 bp DNA ladder, one scalpel to cut gel slices, 200 uL tips and ethanol to wash the ampure beads, 10 uL tips for Qubit quantitation to pool the final library, and library shipment to UK with coolers. | |||||
| Total cost per sample: | 4.882 | ||||
| Without library normalization: | 3.759 |
References
- Hamilton, J.P.; Hansey, C.N.; Whitty, B.R.; Stoffel, K.; Massa, A.N.; Van Deynze, A.; De Jong, W.S.; Douches, D.S.; Buell, C.R. Single nucleotide polymorphism discovery in elite North American potato germplasm. BMC Genom. 2011, 12, 302. [Google Scholar] [CrossRef] [PubMed]
- Vos, P.G.; Uitdewilligen, J.G.; Voorrips, R.E.; Visser, R.G.; van Eck, H.J. Development and analysis of a 20 K SNP array for potato (Solanum tuberosum): An insight into the breeding history. Theor. Appl. Genet. 2015, 128, 2387–2401. [Google Scholar] [CrossRef] [PubMed]
- Sverrisdóttir, E.; Byrne, S.; Sundmark, E.H.R.; Johnsen, H.Ø.; Kirk, H.G.; Asp, T.; Janss, L.; Nielsen, K.L. Genomic prediction of starch content and chipping quality in tetraploid potato using genotyping-by-sequencing. Theor. Appl. Genet. 2017, 130, 2091–2108. [Google Scholar] [CrossRef]
- Byrne, S.; Meade, F.; Mesiti, F.; Griffin, D.; Kennedy, C.; Milbourne, D. Genome-wide association and genomic prediction for fry color in potato. Agronomy 2020, 10, 90. [Google Scholar] [CrossRef]
- Wickland, D.P.; Battu, G.; Hudson, K.A.; Diers, B.W.; Hudson, M.E. A comparison of genotyping-by-sequencing analysis methods on low-coverage crop datasets shows advantages of a new workflow, GB-eaSy. BMC Bioinform. 2017, 18, 586. [Google Scholar] [CrossRef] [PubMed]
- Uitdewilligen, J.G.; Wolters, A.M.A.; D’hoop, B.B.; Borm, T.J.; Visser, R.G.; Van Eck, H.J. A next-generation sequencing method for genotyping-by-sequencing of highly heterozygous autotetraploid potato. PLoS ONE 2013, 8, e62355. [Google Scholar] [CrossRef]
- Jupe, F.; Witek, K.; Verweij, W.; Śliwka, J.; Pritchard, L.; Etherington, G.J.; Maclean, D.; Cock, P.J.; Leggett, R.M.; Bryan, G.J.; et al. Resistance gene enrichment sequencing (R en S eq) enables reannotation of the NB-LRR gene family from sequenced plant genomes and rapid mapping of resistance loci in segregating populations. Plant J. 2013, 76, 530–544. [Google Scholar] [CrossRef]
- Campbell, N.R.; Harmon, S.A.; Narum, S.R. Genotyping-in-Thousands by sequencing (GT-seq): A cost effective SNP genotyping method based on custom amplicon sequencing. Mol. Ecol. Resour. 2015, 15, 855–867. [Google Scholar] [CrossRef]
- Vos, P.G.; Paulo, M.J.; Voorrips, R.E.; Visser, R.G.; van Eck, H.J.; van Eeuwijk, F.A. Evaluation of LD decay and various LD-decay estimators in simulated and SNP-array data of tetraploid potato. Theor. Appl. Genet. 2017, 130, 123–135. [Google Scholar] [CrossRef]
- Sharma, S.K.; MacKenzie, K.; McLean, K.; Dale, F.; Daniels, S.; Bryan, G.J. Linkage disequilibrium and evaluation of genome-wide association mapping models in tetraploid potato. G3 Genes Genomes Genet. 2018, 8, 3185–3202. [Google Scholar] [CrossRef]
- Consortium, P.G.S. Genome sequence and analysis of the tuber crop potato. Nature 2011, 475, 189. [Google Scholar]
- Wolters, A.M.A.; Uitdewilligen, J.G.; Kloosterman, B.A.; Hutten, R.C.; Visser, R.G.; van Eck, H.J. Identification of alleles of carotenoid pathway genes important for zeaxanthin accumulation in potato tubers. Plant Mol. Biol. 2010, 73, 659–671. [Google Scholar] [CrossRef] [PubMed]
- Uitdewilligen, J. Discovery and Genotyping of Existing and Induced DNA Sequence Variation in Potato; Wageningen University and Research: Wageningen, The Netherlands, 2012. [Google Scholar]
- Uitdewilligen, J.; Wolters, A.; van Eck, H.; Visser, R. Allelic variation for alpha-Glucan Water Dikinase is associated with starch phosphate content in tetraploid potato. Plant Mol. Biol. 2022, 108, 1–12. [Google Scholar] [CrossRef]
- Tinker, N.A.; Bekele, W.A.; Hattori, J. Haplotag: Software for haplotype-based genotyping-by-sequencing analysis. G3 Genes Genomes Genet. 2016, 6, 857–863. [Google Scholar] [CrossRef] [PubMed]
- Baral, K.; Coulman, B.; Biligetu, B.; Fu, Y.B. Advancing crested wheatgrass [Agropyron cristatum (L.) Gaertn.] breeding through genotyping-by-sequencing and genomic selection. PLoS ONE 2020, 15, e0239609. [Google Scholar] [CrossRef]
- Canales, F.J.; Montilla-Bascón, G.; Bekele, W.; Howarth, C.; Langdon, T.; Rispail, N.; Tinker, N.A.; Prats, E. Population genomics of Mediterranean oat (A. sativa) reveals high genetic diversity and three loci for heading date. Theor. Appl. Genet. 2021, 134, 2063–2077. [Google Scholar] [CrossRef]
- Schaumont, D.; Veeckman, E.; Van der Jeugt, F.; Haegeman, A.; van Glabeke, S.; Bawin, Y.; Lukasiewicz, J.; Blugeon, S.; Barre, P.; de la O Leyva-Perez, M.; et al. Stack Mapping Anchor Points (SMAP): A versatile suite of tools for read-backed haplotyping. bioRxiv 2022. [Google Scholar] [CrossRef]
- Tang, X.; de Boer, J.M.; van Eck, H.J.; Bachem, C.; Visser, R.G.; de Jong, H. Assignment of genetic linkage maps to diploid Solanum tuberosum pachytene chromosomes by BAC-FISH technology. Chromosome Res. 2009, 17, 899–915. [Google Scholar] [CrossRef]
- Sharma, S.K.; Bolser, D.; de Boer, J.; Sønderkær, M.; Amoros, W.; Carboni, M.F.; D’Ambrosio, J.M.; de la Cruz, G.; Di Genova, A.; Douches, D.S.; et al. Construction of reference chromosome-scale pseudomolecules for potato: Integrating the potato genome with genetic and physical maps. G3 Genes Genomes Genet. 2013, 3, 2031–2047. [Google Scholar] [CrossRef]
- Meade, F.; Byrne, S.; Griffin, D.; Kennedy, C.; Mesiti, F.; Milbourne, D. Rapid development of KASP markers for disease resistance genes using pooled whole-genome resequencing. Potato Res. 2020, 63, 57–73. [Google Scholar] [CrossRef]
- Hardigan, M.A.; Crisovan, E.; Hamilton, J.P.; Kim, J.; Laimbeer, P.; Leisner, C.P.; Manrique-Carpintero, N.C.; Newton, L.; Pham, G.M.; Vaillancourt, B.; et al. Genome reduction uncovers a large dispensable genome and adaptive role for copy number variation in asexually propagated Solanum tuberosum. Plant Cell 2016, 28, 388–405. [Google Scholar] [CrossRef]
- Li, H. Aligning sequence reads, clone sequences and assembly contigs with BWA-MEM. arXiv 2013, arXiv:1303.3997. [Google Scholar]
- Kofler, R.; Orozco-terWengel, P.; De Maio, N.; Pandey, R.V.; Nolte, V.; Futschik, A.; Kosiol, C.; Schlötterer, C. PoPoolation: A toolbox for population genetic analysis of next generation sequencing data from pooled individuals. PLoS ONE 2011, 6, e15925. [Google Scholar] [CrossRef] [PubMed]
- Robinson, J.T.; Thorvaldsdóttir, H.; Winckler, W.; Guttman, M.; Lander, E.S.; Getz, G.; Mesirov, J.P. Integrative genomics viewer. Nat. Biotechnol. 2011, 29, 24–26. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Untergasser, A.; Cutcutache, I.; Koressaar, T.; Ye, J.; Faircloth, B.C.; Remm, M.; Rozen, S.G. Primer3—New capabilities and interfaces. Nucleic Acids Res. 2012, 40, e115. [Google Scholar] [CrossRef]
- He, C.; Holme, J.; Anthony, J. SNP genotyping: The KASP assay. In Crop Breeding; Humana Press: New York, NY, USA, 2014; pp. 75–86. [Google Scholar]
- Yuan, J.; Bizimungu, B.; De Koeyer, D.; Rosyara, U.; Wen, Z.; Lagüe, M. Genome-wide association study of resistance to potato common scab. Potato Res. 2020, 63, 253–266. [Google Scholar] [CrossRef]
- Brown, S.S.; Chen, Y.W.; Wang, M.; Clipson, A.; Ochoa, E.; Du, M.Q. PrimerPooler: Automated primer pooling to prepare library for targeted sequencing. Biol. Methods Protoc. 2017, 2, bpx006. [Google Scholar] [CrossRef]
- Magoč, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef]
- Gordon, A.; Hannon, G. Fastx-Toolkit. FASTQ/A Short-Reads Pre-Processing Tools. 2010. Available online: http://hannonlab.cshl.edu/fastx_toolkit (accessed on 26 August 2022).
- Li, H. A statistical framework for SNP calling, mutation discovery, association mapping and population genetical parameter estimation from sequencing data. Bioinformatics 2011, 27, 2987–2993. [Google Scholar] [CrossRef]
- Rosyara, U.R.; De Jong, W.S.; Douches, D.S.; Endelman, J.B. Software for genome-wide association studies in autopolyploids and its application to potato. Plant Genome 2016, 9, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Tang, D.; Huang, W.; Yang, Z.; Zhang, Y.; Hamilton, J.P.; Visser, R.G.; Bachem, C.W.; Buell, C.R.; Zhang, Z.; et al. Haplotype-resolved genome analyses of a heterozygous diploid potato. Nat. Genet. 2020, 52, 1018–1023. [Google Scholar] [CrossRef]
- Meade, F.; Hutten, R.; Wagener, S.; Prigge, V.; Dalton, E.; Kirk, H.G.; Griffin, D.; Milbourne, D. Detection of novel QTLs for late blight resistance derived from the wild potato species Solanum microdontum and Solanum pampasense. Genes 2020, 11, 732. [Google Scholar] [CrossRef]
- Bourke, P.M.; van Geest, G.; Voorrips, R.E.; Jansen, J.; Kranenburg, T.; Shahin, A.; Visser, R.G.; Arens, P.; Smulders, M.J.; Maliepaard, C. polymapR—Linkage analysis and genetic map construction from F1 populations of outcrossing polyploids. Bioinformatics 2018, 34, 3496–3502. [Google Scholar] [CrossRef] [PubMed]
- Preedy, K.; Hackett, C. A rapid marker ordering approach for high-density genetic linkage maps in experimental autotetraploid populations using multidimensional scaling. Theor. Appl. Genet. 2016, 129, 2117–2132. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Amadeu, R.R.; Munoz, P.R.; Endelman, J.B. Haplotype Reconstruction in Connected Tetraploid F1 Populations. Genetics 2021, 219, iyab106. [Google Scholar] [CrossRef] [PubMed]
- Rouppe Van der Voort, J.; Van der Vossen, E.; Bakker, E.; Overmars, H.; Van Zandvoort, P.; Hutten, R.; Klein Lankhorst, R.; Bakker, J. Two additive QTLs conferring broad-spectrum resistance in potato to Globodera pallida are localized on resistance gene clusters. Theor. Appl. Genet. 2000, 101, 1122–1130. [Google Scholar] [CrossRef]
- van Eck, H.J.; Vos, P.G.; Valkonen, J.P.; Uitdewilligen, J.G.; Lensing, H.; de Vetten, N.; Visser, R.G. Graphical genotyping as a method to map Ny (o, n) sto and Gpa5 using a reference panel of tetraploid potato cultivars. Theor. Appl. Genet. 2017, 130, 515–528. [Google Scholar] [CrossRef]
- Prodhomme, C.; Vos, P.G.; Paulo, M.J.; Tammes, J.E.; Visser, R.G.; Vossen, J.H.; van Eck, H.J. Distribution of P1 (D1) wart disease resistance in potato germplasm and GWAS identification of haplotype-specific SNP markers. Theor. Appl. Genet. 2020, 133, 1859–1871. [Google Scholar] [CrossRef]
- Grech-Baran, M.; Witek, K.; Szajko, K.; Witek, A.I.; Morgiewicz, K.; Wasilewicz-Flis, I.; Jakuczun, H.; Marczewski, W.; Jones, J.D.; Hennig, J. Extreme resistance to Potato virus Y in potato carrying the Rysto gene is mediated by a TIR-NLR immune receptor. Plant Biotechnol. J. 2020, 18, 655–667. [Google Scholar] [CrossRef]
- Tang, D.; Jia, Y.; Zhang, J.; Li, H.; Cheng, L.; Wang, P.; Bao, Z.; Liu, Z.; Feng, S.; Zhu, X.; et al. Genome evolution and diversity of wild and cultivated potatoes. Nature 2022, 606, 535–541. [Google Scholar] [CrossRef] [PubMed]
- Willemsen, J. The Identification of Allelic Variation in Potato. Ph.D. Thesis, Wageningen University and Research, Wageningen, The Netherlands, 2018. [Google Scholar]
- Bao, Z.; Li, C.; Li, G.; Wang, P.; Peng, Z.; Cheng, L.; Li, H.; Zhang, Z.; Li, Y.; Huang, W.; et al. Genome architecture and tetrasomic inheritance of autotetraploid potato. Mol. Plant 2022, 15, 1211–1226. [Google Scholar] [CrossRef] [PubMed]
- Selga, C.; Koc, A.; Chawade, A.; Ortiz, R. A bioinformatics pipeline to identify a subset of SNPs for genomics-assisted potato breeding. Plants 2021, 10, 30. [Google Scholar] [CrossRef] [PubMed]
- Sallam, A.H.; Conley, E.; Prakapenka, D.; Da, Y.; Anderson, J.A. Improving prediction accuracy using multi-allelic haplotype prediction and training population optimization in wheat. G3 Genes Genomes Genet. 2020, 10, 2265–2273. [Google Scholar] [CrossRef]
- Batista, L.G.; Mello, V.H.; Souza, A.P.; Margarido, G.R. Genomic prediction with allele dosage information in highly polyploid species. Theor. Appl. Genet. 2021, 135, 723–739. [Google Scholar] [CrossRef]
- Slater, A.T.; Cogan, N.O.; Hayes, B.J.; Schultz, L.; Dale, M.F.B.; Bryan, G.J.; Forster, J.W. Improving breeding efficiency in potato using molecular and quantitative genetics. Theor. Appl. Genet. 2014, 127, 2279–2292. [Google Scholar] [CrossRef]


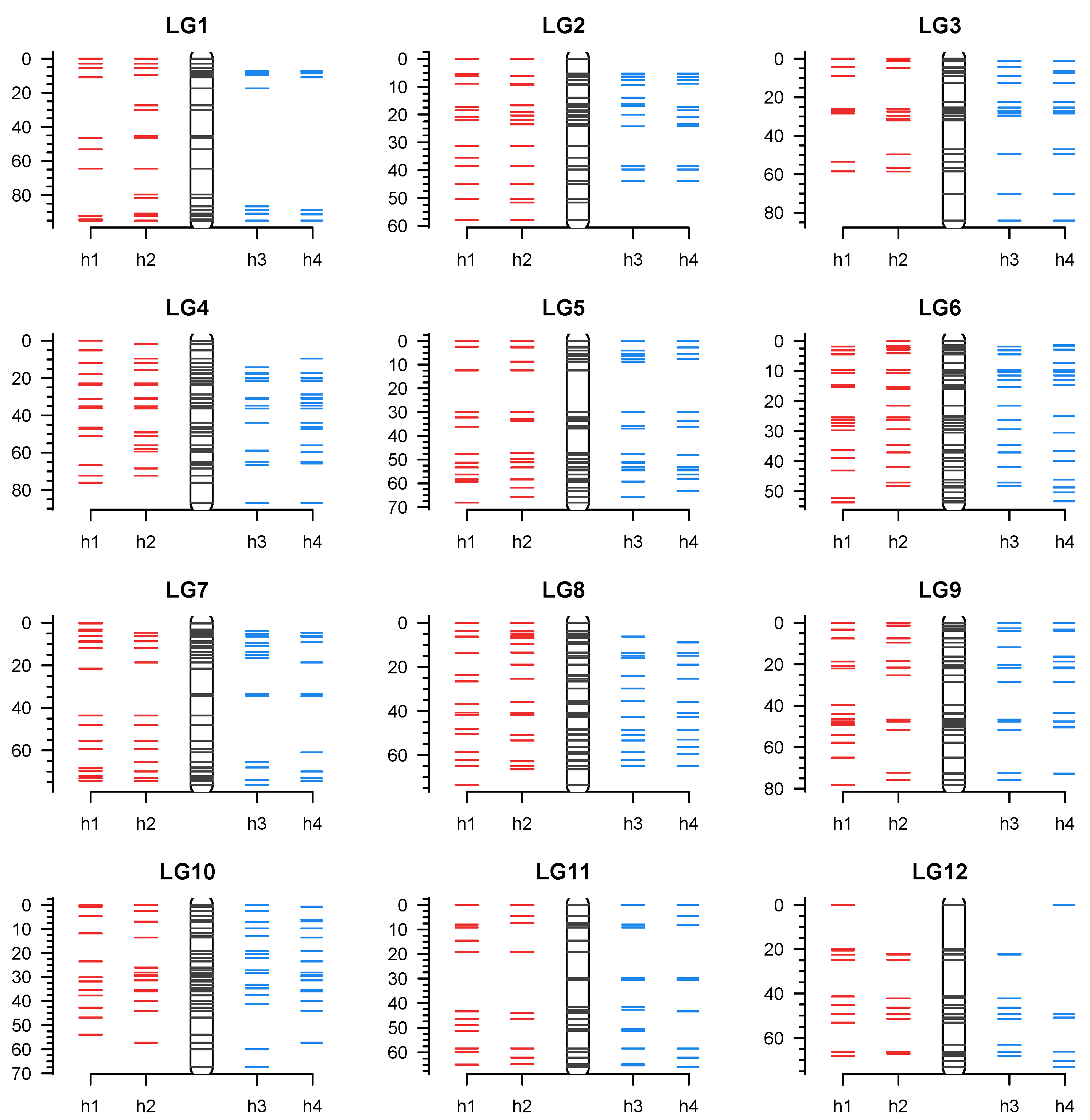
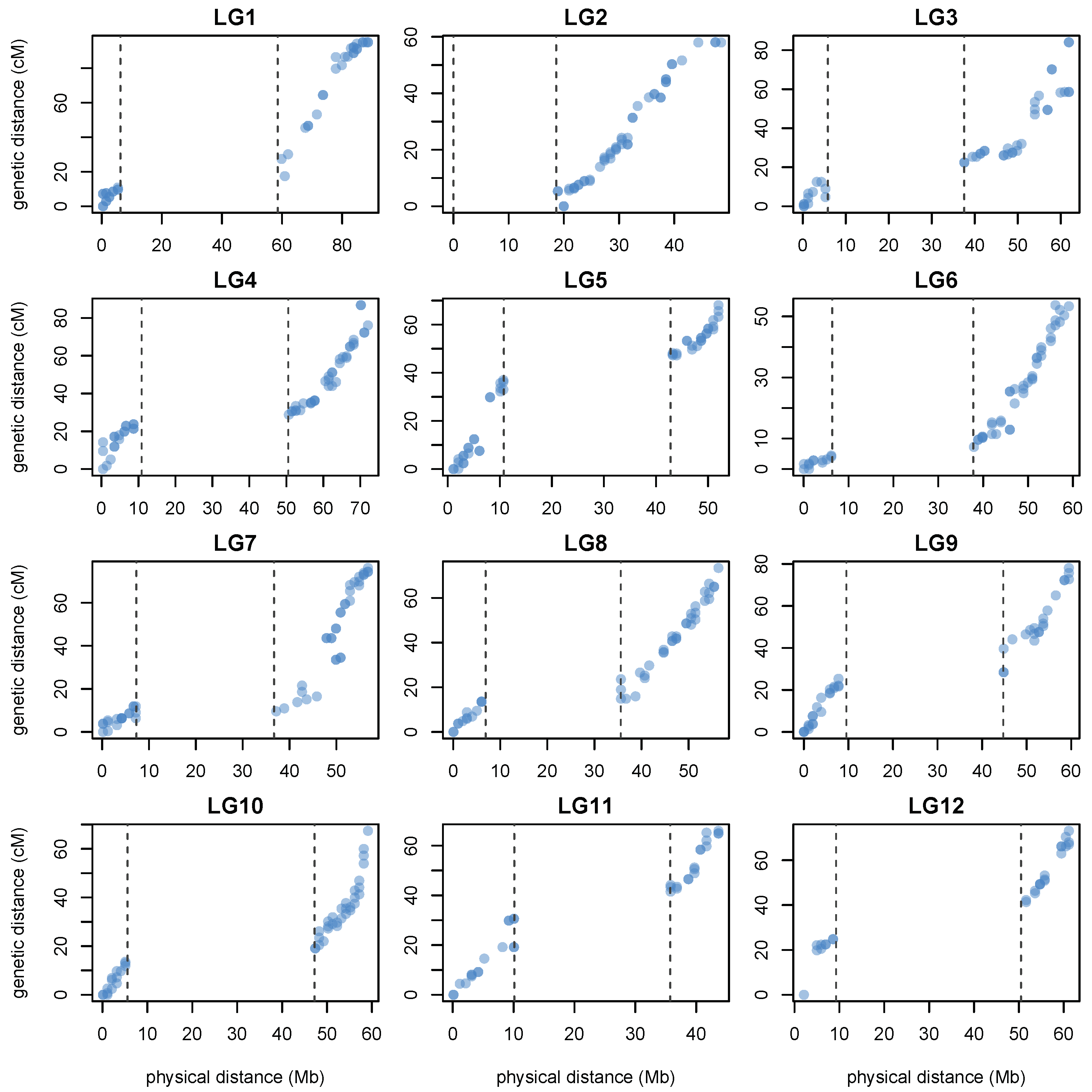
| Chr/arm | Start | End | Length | Core | Diagnostic | Total |
|---|---|---|---|---|---|---|
| (Mb) | Loci | Loci | Loci | |||
| chr1/1 | 1 | 6,236,423 | 6.2 | 6 | ||
| chr1/2 | 58,566,960 | 88,663,952 | 30.1 | 31 | ||
| chr2 | 18,620,376 | 48,614,681 | 30.0 | 31 | 2 | |
| chr3/1 | 1 | 5,853,851 | 5.9 | 6 | ||
| chr3/2 | 37,557,548 | 62290286 | 24.7 | 25 | ||
| chr4/1 | 1 | 10,893,487 | 10.9 | 11 | 2 | |
| chr4/2 | 50,527,797 | 72,208,621 | 21.7 | 24 | ||
| chr5/1 | 1 | 10,773,566 | 10.8 | 12 | 1 | |
| chr5/2 | 42,795,302 | 52,070,158 | 9.3 | 11 | 1 | |
| chr6/1 | 1 | 6,372,027 | 6.4 | 7 | 1 | |
| chr6/2 | 37,792,178 | 59,532,096 | 21.7 | 22 | ||
| chr7/1 | 1 | 7,298,544 | 7.3 | 9 | ||
| chr7/2 | 36,698,521 | 56,760,843 | 20.1 | 21 | ||
| chr8/1 | 1 | 6,899,227 | 6.9 | 7 | ||
| chr8/2 | 35,611,618 | 56,938,457 | 21.3 | 23 | ||
| chr9/1 | 1 | 9,549,714 | 9.5 | 10 | ||
| chr9/2 | 44,754,712 | 61,540,751 | 16.8 | 18 | ||
| chr10/1 | 1 | 5,591,854 | 5.6 | 6 | ||
| chr10/2 | 47,231,005 | 59,756,223 | 12.5 | 14 | ||
| chr11/1 | 1 | 10,117,653 | 10.1 | 11 | 2 | |
| chr11/2 | 35,737,669 | 45,475,667 | 9.7 | 11 | ||
| chr12/1 | 1 | 9,273,808 | 9.3 | 11 | ||
| chr12/2 | 50,482,591 | 61,165,649 | 10.7 | 12 | 1 | |
| Total | 318 Mb | 339 | 10 | 347 |
| Ref. | Resistance to: | Name | SNP Position | Locus | Haplotype | Concord. |
|---|---|---|---|---|---|---|
| [29] | S. scabies | c2_17867 | chr02:36548178 [T/C] | C2_B2 | 011110 | 99.9% |
| [29] | S. scabies | c2_17864 | chr02:36550070 [T/C] | C2_B3 | 0011110 | 99.9% |
| TPBP | G. pallida (Pa2/3) | Gpa4 | chr04:4782401 [A/G] | C4_5 | 001110 | 99.9% |
| [21] | P. infestans | R2 | chr04:6191864 [A/T] chr04:6191873,76,77 [TGATT/CGAAA] | C4_6 | Not detected | NA |
| [40] | G. pallida | Gpa5 | chr05:5485534 [T/A] | C5_B9 | 0110101010111100101 | 96.5% |
| [21] | G.rostochiensis(P1/4) | H1 | chr05:49238169 [T/A] | C5_B10 | 000000110 | 100% |
| TPBP | P. infestans | Rpi-blb2 | chr06:775752 [G/A] | C6_B1 | Not detected | NA |
| [41] | PVY | Ny(o,n)sto | chr11:284162 [T/C] 68 [T/C] | C11_B1 | 000101010010 | 98.95% |
| [42] | S. endobioticum | Sen1 | chr11:3928601 [A/G] | C11_B3 | 001100 | 100% |
| [43] | PVY | Ry-sfto | chr12:59957417 [G/A] | C12_B6 | Not detected | NA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leyva-Pérez, M.d.l.O.; Vexler, L.; Byrne, S.; Clot, C.R.; Meade, F.; Griffin, D.; Ruttink, T.; Kang, J.; Milbourne, D. PotatoMASH—A Low Cost, Genome-Scanning Marker System for Use in Potato Genomics and Genetics Applications. Agronomy 2022, 12, 2461. https://doi.org/10.3390/agronomy12102461
Leyva-Pérez MdlO, Vexler L, Byrne S, Clot CR, Meade F, Griffin D, Ruttink T, Kang J, Milbourne D. PotatoMASH—A Low Cost, Genome-Scanning Marker System for Use in Potato Genomics and Genetics Applications. Agronomy. 2022; 12(10):2461. https://doi.org/10.3390/agronomy12102461
Chicago/Turabian StyleLeyva-Pérez, Maria de la O., Lea Vexler, Stephen Byrne, Corentin R. Clot, Fergus Meade, Denis Griffin, Tom Ruttink, Jie Kang, and Dan Milbourne. 2022. "PotatoMASH—A Low Cost, Genome-Scanning Marker System for Use in Potato Genomics and Genetics Applications" Agronomy 12, no. 10: 2461. https://doi.org/10.3390/agronomy12102461
APA StyleLeyva-Pérez, M. d. l. O., Vexler, L., Byrne, S., Clot, C. R., Meade, F., Griffin, D., Ruttink, T., Kang, J., & Milbourne, D. (2022). PotatoMASH—A Low Cost, Genome-Scanning Marker System for Use in Potato Genomics and Genetics Applications. Agronomy, 12(10), 2461. https://doi.org/10.3390/agronomy12102461







