Abstract
Water scarcity is one of the most limiting environmental factors for crop productivity globally, where biochar application can play a role. To test this, a glasshouse experiment was conducted with aged biochar application with water regimes on arbuscular mycorrhizal fungi colonisation, plant growth, phosphorus nutrition and leaf gas exchange in wheat and subterranean clover. Six treatment combinations (two biochar levels of 0 and 6 t ha−1; and three water regimes of well-watered, water-stressed and watering intermittently) were arranged in factorial completely randomized block design with three replications. The wheat and subterranean clover were grown and harvested 14, 24, 34 and 44 days after sowing. In this study, aged biochar had no significant effect on plant growth for both wheat and clover, regardless of water regimes. Shoot and root dry weights increased in well-watered conditions compared to water-stressed conditions. Root length and colonised root length increased with biochar addition for wheat, mostly in well-watered treatment. Phosphorus uptake increased in biochar treatment, and the effect was higher in well-watered conditions. Leaf photosynthetic rate (PN), stomatal conductance (gs), intercellular CO2 concentration (Ci) and transpiration rate (E) increased with biochar addition. For clover, the effect was higher in water-stressed than well-watered conditions. These results suggest that biochar may enhance water availability to clover plants in water-stressed conditions. However, the positive effect of biochar on plant growth under water-stressed conditions, varied with plant species, needs to be explored further in a range of crop species and biochar sources in addition to the material used in the current investigation.
1. Introduction
Water scarcity is one of the most limiting global environmental factors for crop productivity [1]. With the prevalence of global climate change, most agricultural areas are expected to undergo a high frequency of dry conditions, primarily in the Mediterranean and subtropical rain-fed cropping systems [2,3]. For example, South-Western Australia is projected to experience more than double the drought frequency by 2030 [4]. Furthermore, prolonged water stress may change soil biological processes [5] that can diminish plant growth and productivity by adversely affecting various aspects of plant physiology, especially photosynthetic capacity [6]. Therefore, the inherent benefits of soil amendments to enhance the access to soil water by plants, which could happen through improved arbuscular mycorrhizal (AM) colonisation, must be further explored.
Arbuscular mycorrhizal fungi create symbiotic associations with most terrestrial plant species [7]. Studies have shown that, besides increasing plant phosphorus availability, AM symbiosis can influence the water relations of the plant, thereby affecting plant tissue hydration and physiology [8,9]. It is believed that the AM fungi symbiosis contributes to plant drought tolerance through its effect on plant nutrition, physiology, plant cells and vegetative plant growth [9,10]. Arbuscular mycorrhizal root colonisation improves the soil structure, regulates host plant mineral nutrition and enhances plant growth [11]. Furthermore, AM fungi have been widely reported to invigorate the growth of stressed plants [8]. For instance, under saline conditions, AM fungi, as an essential stress bio-ameliorator, increased the potential of the plant to withstand salinity stress [12]. Asrar and Elhindi [13] recorded stimulation of all growth parameters of all AM fungi colonized marigold (Tagetes erecta) plants. Principally, AM fungi counter plant drought stress by maintaining plant water uptake [14], through osmotic adjustment [15], through trehalose biosynthesis [16], by increasing the level of antioxidants compounds [17] and by expressing drought-related genes in the host plant [18]. More importantly, AM fungi root colonisation improves plant access to water and nutrients by modifying the plant root architecture [12]. Modification of the plant physiological status by enhancing leaf area and nutrient content (e.g., P content) optimises the photosynthesis rate of the host plant [19,20].
Biochar, a charred organic matter, produced by heating solid biomass under limited oxygen conditions (a process called pyrolysis), has received attention due to its potential to enhance soil fertility and water holding capacity [21,22,23]. Biochar has been used, both scientifically and commercially as a soil amendment. For example, biochar has been considered an essential resource in soil carbon sequestration, amendment of agricultural soils, management of waste and in environmental remediation [24,25]. Aside from its positive effect in increasing greenhouse gases sequestration, biochar, when applied to soil, can enhance soil properties and functions [26,27]. Applying biochar to soil in suitable amounts can enhance soil pH, cation exchange capacity (CEC), water and nutrient holding capacities, microbe response and nitrogen uptake [28]. For example, the addition of biochar to acidic soil improved soil microbial activity, pH, electrical conductivity (EC) and CEC [29,30]. Biochar can also increase crop and biomass yield. For example, the application of biochar to soil increased growth and yield of maize [31]. Dong et al. [32] recorded an increase in rice yield and N retention when rice straw biochar was applied in a waterlogged paddy field. Furthermore, biochar is thought to influence leaf gas exchange through its effect on photosynthesis parameters and leaf characteristics [33]. More recently, Abideen et al. [34] reported a significant increase in chlorophyll content, net photosynthesis rate, leaf gas exchange and plant water use efficiency of herbaceous perennial grass (Phragmites karka) and soybeans with biochar soil amendment, respectively.
Plant root colonisation by AM fungi has been demonstrated to improve the capacity of the plants to tolerate several growth-inhibiting factors, thereby enhancing crop growth and yield [35]. The application of biochar can improve AM fungi’s effectiveness and functionality [36] but these responses are varied with crop species and biochar types produced from various feedstocks [37]. It is well known that biochar is resistant to weathering and not easily decomposed [38], and therefore can act as a shelter for AM fungi hyphae and protect them from fungal grazers [39], thereby enhancing the symbiosis between the fungus and host plant. Several other mechanisms by which biochar could influence mycorrhizal colonization have been discussed [28]. The ability of biochar to improve soil physio-chemical properties can increase soil nutrient availability and enhance mycorrhizal root colonization [40,41]. Lehmann et al. [42] suggested that biochar application can directly improve crop productivity due to its nutrient content and release properties and indirectly by improving soil nutrient retention. Biochar can also indirectly affect mycorrhizae through its effects on other microbes [28].
The positive influence of biochar addition on AM fungi root colonisation has been justified [28,39]. However, minimal information has been presented about the effect of biochar on mycorrhizal root colonisation under drought stress conditions. Therefore, the objective of the present study was to examine the influence of biochar, water regimes and their combination on plant growth, mycorrhizal colonisation, P nutrition and leaf gas exchange of wheat and subterranean clover in glasshouse conditions. The underlying hypothesis was that the addition of aged biochar would increase plant growth, P uptake, leaf gas exchange and mycorrhizal colonisation under water-stressed conditions.
2. Materials and Methods
2.1. Soil and Biochar Sources
Sandy clay loam (0–100 cm) was collected from Walkaway, Western Australia (WA) (28° 56’ 21.6” S, 114°50’ 6.3” E) and was amended with 50 kg ha−1 rock minerals fertiliser (N 10%, P 7.5%, K 4.5%, S 1.2%) as basal dressing. The biochar used in this experiment was jarrah wood (Eucalyptus marginata), obtained from an old large open pile of metallurgical charcoal at the site of an iron foundry at Wundowie, Western Australia. This wood biochar was produced as byproduct from jarrah wood burning during iron metal galvanising at around 550–650 °C and had been piled at an open place for a long time (stockpiling with continuous top-up for about 35 years). Both soil and biochar were sieved to 2 mm. The chemical characteristics of the soil and biochar are summarised in Table 1.

Table 1.
Chemical characteristics of the field soil and biochar.
2.2. Experimental Design
The experiment was conducted under glasshouse conditions at the University of Western Australia (31° 58’ 29.4” S, 115° 49’ 4.2” E). The layout was a factorial and randomized complete block design with four treatments and three replications. The treatments comprised three water regimes, two biochar rates, two crops and four harvesting times. The watering strategies were as follows: 80% FC, WW (well-watered); 40% FC, WS (water-stressed); and WI, watered intermittently for periodic water-stressed (1 week well-watered then 1 week water-stressed). The pots were watered every second day by weighing. The two biochar application rates were: B0 (0 t ha−1) and B6 (6 t ha−1). Each pot contained 1 kg soil that had been mixed with 4 g (6 t ha−1) biochar or mixed without biochar [43]. Five seeds of wheat (Triticum aestivum L. var. Brookton) and subterranean clover (Trifolium subterranean L. var. Seaton Park) were respectively sown to each 1.3 L plastic pot [37] and allowed to grow for five days after germination before thinning them to three most vigorous plants that were selected for experiments. Plants were maintained in a glasshouse under ambient light conditions with a photoperiod of 16/8 h and a temperature of 22/17 °C (day/night). Each pot was watered by weighing as assigned watering regimes throughout the growth of the plants. Plants were harvested 14, 24, 34 and 44 days after germination. A total of 144 experimental pots (2 biochar rates × 3 water regimes × 2 crops × 4 harvests × 3 replications) were analysed.
2.3. Plant Growth and P Analyses
Shoots were cut from each plant at each harvest, and roots were washed with tap water to remove soil and organic matter. Shoot and root dry weights (DW) were measured after oven drying the samples at 65 °C for 72 h. The oven-dried shoot samples were then ground and digested in 3:1 HNO3-HClO4 [44], and P concentration in the digested sample was measured using the molybdenum-blue method [45]. Shoot P uptake per pot was calculated by multiplying shoot P concentration by total shoot weight.
2.4. Determination of Total Root Length and AM Fungal Colonisation
After washing the roots with water at each harvest, root subsamples (∼1 g) were taken to determine total root length and AM fungal colonised root length. The root subsamples were cut into pieces of ∼1 cm and cleared in 10% KOH, acidified and stained with Trypan blue (0.05%) in lactoglycerol and de-stained in lactoglycerol [46,47]. Total root length and percent root length colonised by AM fungi was assessed by the gridline intercept method under an optical microscope at 100× magnification [48].
2.5. Leaf Gas Exchange
Leaf gas exchange was measured using a LI-COR 6400 portable photosynthesis system (LI-COR, Lincoln, NE, USA). The measurements performed were photosynthesis (PN), stomatal conductance (gs) and transpiration (E). The measurements were performed on the youngest-fully emerged and the entire leaf before each harvest starting from 2nd harvest.
2.6. Statistical Analysis
Statistical analyses were conducted using XLSTAT. Two-way analysis of variance (ANOVA) was carried out to determine the statistical significance of the treatments (biochar and water regimes) on measured parameters. Parameters analyzed were shoot and root dry weight, AM fungal colonization, total and colonized root length, shoot P concentration and leaf gas exchange. The experiment used four main factors: watering regime, crop, biochar and harvest. However, since typically, two crops have different characteristics and growing crops are different at each growth stage, the two crops were analyzed separately and each harvest was analyzed independently to aid better understanding and interpretation.
3. Results
3.1. Plant Growth and P Uptake
3.1.1. Wheat
The shoot dry weight (DW) of wheat was significantly affected by watering regimes at all four harvest times (Table S1; p < 0.05). On average, the shoot DW of wheat was greatest under well-watered (WW) treatment and least under water-stressed (WS). By 4th harvest, the average shoot DW for the well-watered treatment was 4.99 g plant−1 compared to 3.42 and 4.34 g plant−1 under water-stressed and intermittent-watering (WI) treatments, respectively. However, biochar amendment did not affect shoot DW of wheat at all four harvest times (Table 2).

Table 2.
Shoot and root dry weights, root length, shoot P concentration and P uptake per unit pot of wheat for the well-watered (WW), water-stressed (WS) and water intermittently (WI) treatments at four harvest times with 6 t ha−1 biochar (B6) or without application of biochar (B0).
Root DW of wheat was not significantly affected by biochar application at the 2nd, 3rd and 4th harvests (Table 2). However, it was reduced (by about 15%) when biochar was applied at 1st harvest. The effect of watering regimes on root DW of wheat was significant at all four harvests (Table 2; p < 0.05). Root DW was greatest under well-watered and least under water-stressed conditions.
Besides the 4th harvest, root length was not significantly affected by biochar addition and its interaction with irrigation at 1st, 2nd and 3rd harvests (Table S1 and Table 2). However, root length was significantly affected by water regimes at all harvests. It was greatest under well-watered treatment compared to water-stressed and intermittent watering treatments. At the 4th harvest, the effect of biochar and water regimes on root length was significant (Table S1; p < 0.05). The wheat’s root length significantly increased with biochar application by 17% under well-watered conditions and 37% under intermittent watering but decreased by about 12% under water-stressed conditions.
Phosphorus uptake by wheat was not significantly affected by the addition of biochar at the 1st, 2nd and 4th harvests (Table S1 and Table 2). However, the effect of biochar was significant at the 3rd harvest (p < 0.05). In this case, biochar application increased foliar P uptake by about 13%. The effect of water regimes on foliar P uptake was significant at all harvest times (Table 2; p < 0.05) and was, generally, greatest under well-watered and least under water-stressed treatments. In addition, the combined effect of biochar and water regimes on foliar P uptake was significant at 3rd and 4th harvests (Table S1; p < 0.05). At both harvests, foliar P uptake increased with biochar addition under well-watered treatment but decreased under water-stressed treatment (9 and 16% at 3rd and 4th harvests, respectively).
3.1.2. Subterranean Clover
The shoot DW of subterranean clover was not significantly affected by biochar application until the 3rd harvest (Table S2 and Table 3). However, the interaction between biochar and water regimes significantly affected shoot DW at the 4th harvest (p < 0.05). In this case, biochar application increased shoot DW by 16% under WW and 0.3% under WI, but decreased shoot DW by 7% under WS water regimes significantly affected shoot DW of subterranean clover at 1st, 2nd and 3rd harvests (Table 3; p < 0.05) and was greatest for well-watered and least for water-stressed treatment.

Table 3.
Shoot and root dry weights, root length, shoot P concentration and P uptake per pot of subterranean clover for the well-watered (WW), water-stressed (WS) and water intermittently (WI) treatments at four harvest times with 6 t ha−1 biochar (B6) or without application of biochar (B0).
Root DW of subterranean clover was not significantly affected by the application of biochar and its interactions with water regimes (Table S2). On the contrary, water regimes significantly affected root DW at the 2nd, 3rd and 4th harvests (Table S2 and Table 3; p < 0.05), and the effect was most remarkable for well-watered treatment. On average, root DW for well-watered treatment was highest (0.86–2.57 g plant−1) compared to intermittent watering (0.6–2.3 g plant−1) and water-stressed (0.3–1.4 g plant−1).
Biochar application and its interaction with water regimes had no significant effect on total root length of subterranean clover at the 1st, 2nd and 4th harvests (Table S2 and Table 3). However, biochar substantially impacted root length at the 3rd harvest (p < 0.05). In this case, biochar application, on average, decreased the root length of subterranean clover by about 9%. On the other hand, water regimes significantly affected total root length at all harvest times (p < 0.05). Furthermore, increased total root length was greatest for well-watered treatment and least for water-stressed (Table 3).
Foliar P uptake was not significantly affected by biochar addition at the 1st and 2nd harvest times (Table 3). However, the effect of biochar and water regimes on foliar P uptake of subterranean clover was significant at 3rd and 4th harvests (p < 0.05). At 3rd harvest, biochar application increased foliar P uptake by 33%, under well-watered and 4%, under intermittent watering but decreased P uptake by 40% under water-stressed treatment. At the 4th harvest, foliar P uptake increased by 52% under well-watered treatment but decreased by 17 and 6% under water-stressed and periodic water-stressed treatments, respectively (Table 3).
3.2. AM Fungal Colonisation
3.2.1. Wheat
Biochar application and its interaction with water regimes had no significant effect on root AM fungi colonization of wheat at 1st and 2nd harvests (Table S3). However, biochar application significantly increased AM fungi colonization of wheat by about 19% at 3rd harvest (Figure 1). The effect of water regimes on AM fungi colonization of wheat was significant from 2nd harvest (Table S3; p < 0.05). On average, the percentage root length colonized by AM fungi was greatest under WI treatment and the least under WW treatment (Figure 1). The interaction between biochar and water regimes on AM fungi colonization was significant in the 4th harvest (Table S3). The percent root length colonized by AM fungi increased by 44% under WW, 1% under WS and 76% under WI (Figure 1).
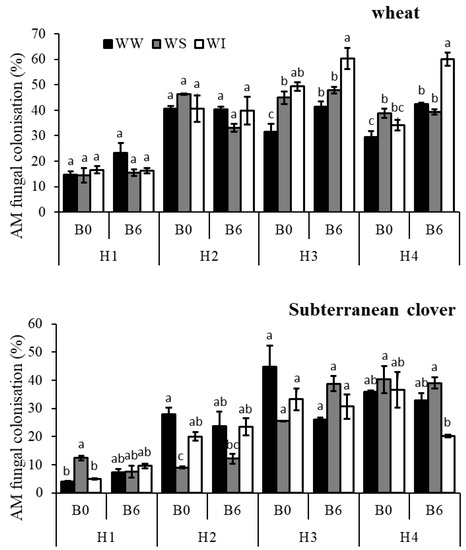
Figure 1.
AM fungi colonisation of wheat and subterranean clover for the; WW, well-watered; WS, water-stressed; WI, water intermittently treatments at four harvest times with 6 t ha−1 biochar (B6) or without biochar application (B0); different letters on top of bars indicate significant difference between treatments at p < 0.05 in each harvest.
3.2.2. Subterranean Clover
The AM fungi root colonisation of subterranean clover responded differently to different water regimes. On average, AM fungi colonization was most significant for WS treatment and least for WW treatment at 1st and 4th harvests (Figure 1). However, the opposite is correct in 2nd and 3rd harvests. The effect of biochar and water regimes on root AM fungi colonization of subterranean clover was significant at 1st and 3rd harvests (Table S3; p < 0.05). At 1st harvest percent root length colonized by AM fungi increased under WW and WI treatments but decreased under WS conditions (Figure 1). In this regard, percent AM fungi colonization increased by about 77% under WW and 92% under WI but decreased by 40% for WS. Biochar addition had no significant effect on AM fungal colonization of subterranean clover at the 2nd harvest (Table S3; p < 0.05).
3.3. Colonised Root Length
3.3.1. Wheat
Biochar application significantly increased AM fungi colonized root length (CRL) of wheat at 1st, 2nd and 4th harvests but caused a significant decrease at the 3rd harvest (Figure 2; p < 0.05). The combined effect of biochar and water regimes significantly affected AM fungi colonized root length of wheat at 1st, 3rd and 4th harvests (Table S3). In this regard, biochar application increased AM fungi colonized root length under WW and WI treatments but had a negative effect under WS treatment (Figure 2). Under WS conditions, biochar application decreased AM fungi colonized root length of wheat by 27, 36,18 and 11% across the four harvests, respectively.
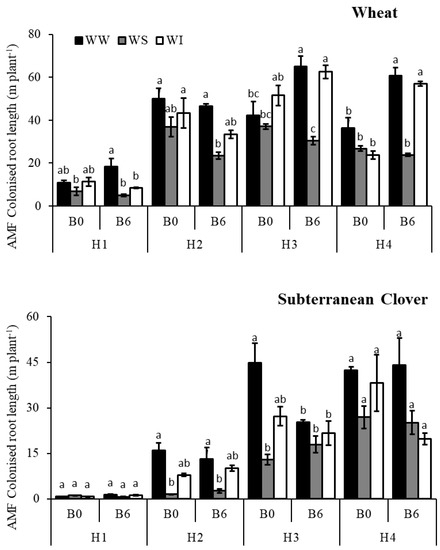
Figure 2.
AM fungi colonised root length of wheat and subterranean clover for the; WW, well-watered; WS, water-stressed; WI, water intermittently at four harvest times with 6 t ha−1 biochar (B6) or without biochar application (B0); different letters on top of bars indicate significant difference between treatments at p < 0.05 in each harvest.
3.3.2. Subterranean Clover
Biochar application significantly decreased AM fungi colonized root length (CRL) of subterranean clover by about 24% at 3rd harvest (Figure 2; p < 0.05). However, there was no significant effect of biochar on AM fungi colonized root length of subterranean clover at 1st, 2nd and 4th harvests (Table S3). On the contrary, the AM fungi colonized root length of subterranean clover significantly increased with water regimes at 2nd, 3rd and 4th harvests (p < 0.05). In this regard, the interaction with biochar was not significant (Table S3). At all these three harvests AM fungi colonized root length of subterranean clover was greatest for WW treatment and least for WS treatment (Figure 2). However, the combined effect of biochar and water regimes on AM fungi colonized root length of clover was significant at the 1st harvest (Table S3). In this case, AM fungi colonized root length increased with biochar application under WW (by 81%) and WI conditions (by 45%) but decreased under WS conditions by 38% (Figure 2).
3.4. Leaf Gas Exchange
3.4.1. Wheat
All leaf gas exchange parameters (photosynthesis, stomatal conductance, internal CO2 concentration and transpiration) of the wheat crop were significantly affected by biochar application and water regimes (Figure 3; p < 0.05).
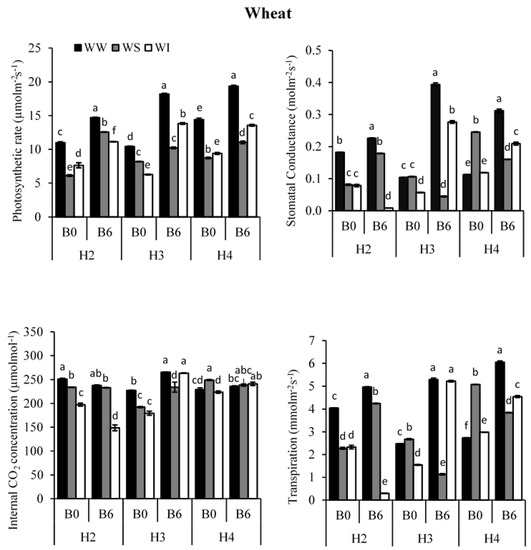
Figure 3.
Effect of biochar and water regimes on photosynthesis (µmolm−2s−1), stomatal conductance (molm−2s−1), internal CO2 concentration (µmolmol−1) and Transpiration (mmolm−2s−1) of wheat for the well-watered (WW); water-stressed (WS) and water intermittently (WI) treatments; at four harvest times; with 6 t ha−1 biochar (B6) or without biochar application (B0); different letters on top of columns indicate a significant difference between treatments at p < 0.05 in each harvest.
The photosynthetic rate (PN) of wheat was significantly affected by biochar and water regimes (Table S4; p < 0.05). Biochar addition significantly increased PN under all water regimes, and it was generally higher for WW treatment than WS and WI treatments (Figure 3). When averaged across biochar levels and all three harvesting times, PN was highest for WW treatment and least under WS condition. However, biochar application at H2 was greatest for WS treatment compared to WW and WI treatments (Figure 3). At H2, Biochar increased photosynthesis by 105% under WS compared to 34 and 46% for WW and WI treatments, respectively.
Leaf stomatal conductance (gs) of wheat was significantly affected by biochar and water regimes (Table S4; p < 0.05). Leaf gs increased with biochar addition for WW and WI treatments (Figure 3; p < 0.05), except at 2nd harvest where the addition of biochar decreased gs by about 89%. Conversely, biochar decreased gs of wheat under WS conditions at 3rd and 4th, except for 2nd harvest where biochar addition increased gs by almost 120% (Figure 3). The differences among WW, WS and WI treatments were most pronounced at 3rd harvest.
Leaf intercellular CO2 concentration (Ci) of wheat was significantly affected by biochar and water regimes (Table S4; p < 0.05). Leaf Ci increased by adding biochar under WW and WI treatments at 3rd and 4th harvests (Figure 3). In this regard, under WW treatment Ci increased by 17% at H3 and by 3% at H4, while under WI it increased by 47% at H3 and 8% at H4 (Figure 3). On the contrary, under WS treatment Ci of wheat decreased with the addition of biochar at H4. In addition, at H2 biochar and water regimes had a negative effect on Ci of wheat.
The transpiration rate (E) of wheat significantly increased with the addition of biochar (Table S4; p < 0.05). Therefore, it was generally more significant for WW treatment than WS and WI treatments (Figure 3). Under WW, the addition of biochar significantly increased E by 23% at H2, 114 at H3 and 122% at H4. Under WI condition, E of wheat increased by 238% at H3 and by 52% at H4 when biochar was added but decreased by 87% at H2 (Figure 3). However, under water-stressed conditions, the addition of biochar decreased E by 58% at H3 and 24% at H4, except for H2 where E increased by about 87%.
3.4.2. Subterranean Clover
All leaf gas exchange parameters (PN, gs, Ci and E) of the subterranean clover crop were significantly affected by the combined effect of biochar and water regimes (Figure 4; p < 0.05).
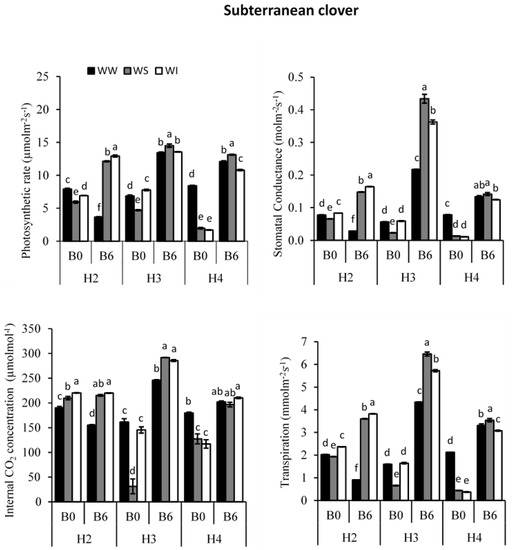
Figure 4.
Effect of biochar and water regime on photosynthesis (µmolm−2s−1), stomatal conductance (molm−2s−1), internal CO2 concentration (µmolmol−1) and Transpiration (mmolm−2s−1) of subterranean clover for the well-watered (WW); water-stressed (WS) and water intermittently (WI) treatments; at four harvest times; with 6 t ha−1 biochar (B6) or without biochar application (B0); different letters on top of columns indicate significant difference between treatments at p < 0.05 in each harvest.
leaf PN of subterranean clover was significantly affected by biochar and water regimes (Table S4; p < 0.05). The PN increased dramatically at all three harvest times with biochar application and under all water regimes, except for well-watered treatment at H2 (Figure 4). It was generally higher for WS treatment compared to WW and WI treatments. The differences among the water regimes were most pronounced at H4 (Figure 4).
Biochar and water regimes significantly affected leaf gs of subterranean clover (Table S4; p < 0.05). The gs increased with biochar application under all watering regimes, except at H2 for WW, where biochar application decreased gs by about 63% (Figure 4). Leaf gs was generally greatest for WS treatment and least for WW treatment. Under WS conditions, biochar application increased gs by 181% at H3 and 98% at H4, compared to WW (29 and 71%) and WI (5 and 103%), respectively. It is worth noting that the differences among WW, WS and WI treatments were most pronounced at H3 in B6.
Biochar and water regimes significantly affected leaf Ci of subterranean clover (Table S4; p < 0.05). Leaf Ci increased by biochar application under all water regimes at 3rd and 4th harvests (Figure 4). At both H3 and H4, the effect of biochar on Ci was the least under WW. At H3, Ci increased by 52% under WW, 833% under WS and 97% under WI, while at H4, it increased by 12% under WW, 54% under WS and 80% under WI (Figure 4). However, at H2, biochar application significantly decreased Ci under WW and WI treatments.
Biochar and water regimes significantly affected leaf E of subterranean clover (Table S4; p < 0.05). The E increased with the addition of biochar (except at H2 for WW). It was generally greater for WS than WW and WI treatments (Figure 4). Under WS conditions, biochar addition increased E by 86% at H2, 883% at H3 and 707% at H4. For WI treatment, biochar application increased E by 61% at H2, 247% at H3 and 727% at H4 (Figure 4). However, for WW treatment, E increased at H3 and H4 (by about 172 and 56%, respectively) but decreased at H2 by 55%.
4. Discussion
The proposed hypothesis was supported in this study as biochar did not significantly affect shoot and root dry weights for both wheat and subterranean clover, regardless of the water regimes. Similar results of biochar having a slightly or no effect on plant growth have been reported [49]. However, positive effects of biochar on plant growth have also been reported [50]. These inconsistent results of the effect of biochar on plant growth have been associated with biochar properties that depends on feedstock properties [51], soil properties [52] and biochar application rate [53]. The insignificant effect of biochar on plant growth in the current study could partly be ascribed to its pH being acidic because of aging. Biochar undergoes slow oxidation in the soil with aging [54]. Aging can change biochar’s chemistry, functionality and microporous structure due to the introduction of surface carboxylic and carbonyl groups [54,55,56]. In a pot study, Lin et al. [56] recorded an increase in aluminium toxicity and acidity when aged biochar was added to acid soil. Similarly, the pH of the biochar used in this study was unusually low compared to other biochars [56,57], which could have negatively affected root growth and development [58]. This could explain the significant positive correlation between shoot DW and root DW for wheat and subterranean clover (r = 0.87 and r = 0.89, respectively). In addition, the biochar application rate used in the present study was relatively low. Previous studies that recorded positive effects used rates greater than used here [33, 59]. In a field study, Xu et al. [33] observed that a biochar rate of at least 20 to 40 t ha−1 was needed for agronomic benefits to be recorded in peanut plants, which may also apply to the plants used in the current study.
The effect of water regimes on AM fungi colonization increased over time and was greater for WW and WI treatments and least for WS treatment for both wheat and subterranean clover. These results agree with Al-Karaki et al. [10] and the general observation that AM fungi levels are lower under water-stressed than well-watered conditions [60,61]. In the present study, the AM fungi colonization under WW conditions was higher than under WS conditions by 39% for wheat and 37% for subterranean clover. These results are similar to Asrar et al. [62], who recorded a significant decrease in AM fungi colonization in snapdragon plants under water-stressed conditions. On the contrary, results have also been reported where water-stressed conditions did not affect the percent of roots colonized by AM fungi.
Biochar and mycorrhizal interaction can increase plant growth by enhancing plant nutrient uptake or indirectly through interacting with beneficial soil biological processes, as reviewed by Warnock et al. [28] and Lehmann et al. [27]. In the present study, we assessed the effect of biochar on AM fungal colonization under well-watered, water-stressed and intermittently watered conditions. The effect ranged from negative, with no impact, to positive. Although the results were inconsistent across the harvesting times, the overall trend showed the lowest mycorrhizal colonization in the water-stressed treatment. These conflicting results of the effect of biochar on AM fungi colonization have been reported in other studies [41,63]. However, Augé [64] reported that, though the effect of biochar on AM fungi colonization in drought conditions has been inconsistent, more increases have been recorded than decreases. It has been suggested that drought usually affects the abundance, composition and activity of AM fungi [65]. Although positive and negative effects of biochar addition on AM fungi root colonization have been observed [36,41], the impact of biochar on the beneficial influence of AM fungi symbioses is inadequately understood [66]. Warnock et al. [28] mainly associated the negative impact of biochar amendments on AM fungi plants with nutrient effects. Lehmann, et al. [27] found positive results of biochar amendment on soil P uptake in AM fungi plants. However, in the current study, biochar amendment decreased P uptake for both wheat and subterranean clover under water-stressed conditions. Similarly Liu et al. [67] found a negative effect of biochar addition on host plant P uptake in potato. So far, it is poorly understood whether the negative impact of biochar on AM fungi colonization is due to its influence on the plants, AM fungi or soil [67]. The effect of biochar on P uptake in clover plants was remarkable, especially in the presence of water in more developed plants, and it is important to highlight as its influence on P uptake under water stress conditions was low in the third harvest at 34 days after germination was significant.
The effect of biochar and water regimes on plant leaf gas exchange has been described in different crops [24,68]. In the present study, leaf gas exchange parameters such as PN, gs, Ci and E significantly increased with biochar addition in wheat and subterranean clover in all water regimes. Similar results were reported by Abideen et al. [34] in Phragmites karka and Miyashita et al. [69] in kidney beans. In the present study, the effect of biochar on PN, gs, Ci and E was more pronounced in WS treatment than WW and WI treatments. Drought decreases gs in several plants [70,71]. To minimize the loss of water and maintain homeostasis, plants tend to close stomata under drought stress conditions [72], reducing leaf transpiration and photosynthetic rates [73]. Biochar addition increased gs and Ci which are leading to increase PN and E. Akhtar et al. [74] attributed the positive effect of biochar addition on plant gas exchange to the capacity of biochar to increase soil water holding capacity (WHC), which could enable plants sustain adequate water status and therefore an increased gs. It is important to note that in the present study, the positive effect of biochar addition on leaf gas exchange did not translate to increased shoot and root DW. This could be due to shorter period the plants could grow in the glasshouse before they were harvested, and the lower application rate used.
5. Conclusions
Although there were no significant effects of biochar application on plant growth for wheat and subterranean clover in this short-term experiment, the results indicated that biochar positively impacted P nutrition, AM colonisation, root length and leaf gas exchange. In addition, biochar application significantly increased the leaf gas exchange parameters suggesting enhanced water availability in the rhizosphere of biochar amended plants. These results support the expectation that biochar addition may hold or help water supply in dry conditions in a field experiment. However, the positive effect of biochar on plant growth under water-stressed conditions, varied with plant species, needs to be explored further in a range of crop species and biochar sources in addition to the material used in the current investigation. A long-term investigation with other biochars application is also needed to compare their effects of biochar aging on plant growth, nutrition and plant gas exchange.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agronomy12102420/s1. Table S1: Shoot and root dry weights, root length, shoot P concentration and P uptake per unit pot of wheat for the well-watered (WW), water-stressed (WS) and water intermittently (WI) treatments at four harvest times with 6t ha-1 biochar (B6) or without application of biochar (B0); Table S2: Shoot and root dry weights, root length, shoot P concentration and P uptake per unit pot of subterranean clover for the well-watered (WW), water-stressed (WS) and water intermittently (WI) treatments at four harvest times with 6 t ha-1 biochar (B6) or without application of biochar (B0); Table S3: ANOVA output for AM fungi colonisation and AM colonised root length (CRL) for wheat and subterranean clover as affected by biochar and water regimes in each harvest; Table S4: ANOVA output for leaf photosynthetic rate (PN), stomatal conductance (gs), intercellular CO2 concentration (Ci) and transpiration rate (E) of wheat and subterranean clover as affected by biochar and water regimes.
Author Contributions
Conceptualization, M.S., P.M. and Z.M.S.; methodology, M.S., P.M. and Z.M.S.; software, Z.M.S.; validation, M.S., P.M. and Z.M.S.; formal analysis, Z.M.S.; investigation, M.S. and P.M.; resources, Z.M.S.; data curation, P.M.; writing—original draft preparation, P.M.; writing—review and editing, M.S., P.M., and Z.M.S.; visualization, P.M.; supervision, Z.M.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Solaiman, Z.M.; Sarcheshmehpour, M.; Abbott, L.K.; Blackwell, P. Effect of biochar on arbuscular mycorrhizal colonisation, growth, P nutrition and leaf gas exchange of wheat and clover influenced by different water regimes. In Proceedings of the 19th World Congress of Soil Science: Soil Solutions for a Changing World, Brisbane, Australia, 1–6 August 2010; Symposium 2.3. 1 The Soil-Root Interface. International Union of Soil Sciences (IUSS), c/o Institut für Bodenforschung: Vienna, Austria, 2010. [Google Scholar]
- Aydinalp, C.; Cresser, M.S. The effects of global climate change on agriculture. Am. Eurasian J. Agric. Environ. Sci. 2008, 3, 672–676. [Google Scholar]
- Turner, N.C.; Molyneux, N.; Yang, S.; Xiong, Y.-C.; Siddique, K. Climate change in south-west Australia and north-west China: Challenges and opportunities for crop production. Crop Pasture Sci. 2011, 62, 445–456. [Google Scholar] [CrossRef]
- Kirono, D.; Kent, D.; Hennessy, K.; Mpelasoka, F. Characteristics of Australian droughts under enhanced greenhouse conditions: Results from 14 global climate models. J. Arid Environ. 2011, 75, 566–575. [Google Scholar] [CrossRef]
- Talmon, Y.; Sternberg, M.; Grünzweig, J.M. Impact of rainfall manipulations and biotic controls on soil respiration in Mediterranean and desert ecosystems along an aridity gradient. Glob. Chang. Biol. 2011, 17, 1108–1118. [Google Scholar] [CrossRef]
- Osakabe, Y.; Osakabe, K.; Shinozaki, K.; Tran, L.-S.P. Response of plants to water stress. Front. Plant Sci. 2014, 5, 86. [Google Scholar] [CrossRef]
- Leigh, J.; Hodge, A.; Fitter, A.H. Arbuscular mycorrhizal fungi can transfer substantial amounts of nitrogen to their host plant from organic material. New Phytol. 2009, 181, 199–207. [Google Scholar] [CrossRef]
- Hashem, A.; Kumar, A.; Al-Dbass, A.M.; Alqarawi, A.A.; Al-Arjani, A.-B.F.; Singh, G.; Farooq, M.; Abd_Allah, E.F. Arbuscular mycorrhizal fungi and biochar improves drought tolerance in chickpea. Saudi J. Biol. Sci. 2019, 26, 614–624. [Google Scholar] [CrossRef]
- Augé, R. Arbuscular mycorrhizae and soil/plant water relations. Can. J. Soil Sci. 2004, 84, 373–381. [Google Scholar] [CrossRef]
- Al-Karaki, G.; McMichael, B.; Zak, J. Field response of wheat to arbuscular mycorrhizal fungi and drought stress. Mycorrhiza 2004, 14, 263–269. [Google Scholar] [CrossRef]
- Kumar, A.; Sharma, S.; Mishra, S.; Dames, J.F. Arbuscular mycorrhizal inoculation improves growth and antioxidative response of Jatropha curcas (L.) under Na2SO4 salt stress. Plant Biosyst. Int. J. Deal. All Asp. Plant Biol. 2013, 149, 260–269. [Google Scholar]
- Hameed, A.; Wu, Q.-S.; Abd-Allah, E.F.; Hashem, A.; Kumar, A.; Lone, H.A.; Ahmad, P. Role of AM Fungi in Alleviating Drought Stress in Plants. In Use of Microbes for the Alleviation of Soil Stresses; Springer: Cham, Switzerland, 2014; pp. 55–75. [Google Scholar]
- Asrar, A.-W.A.; Elhindi, K.M. Alleviation of drought stress of marigold (Tagetes erecta) plants by using arbuscular mycorrhizal fungi. Saudi J. Biol. Sci. 2011, 18, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Symanczik, S.; Lehmann, M.F.; Wiemken, A.; Boller, T.; Courty, P.-E. Effects of two contrasted arbuscular mycorrhizal fungal isolates on nutrient uptake by Sorghum bicolor under drought. Mycorrhiza 2018, 28, 779–785. [Google Scholar] [CrossRef] [PubMed]
- Dar, Z.M.; Masood, A.; Asif, M.; Malik, M.A. Review on Arbuscular Mycorrhizal Fungi: An Approach to Overcome Drought Adversities in Plants. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 1040–1049. [Google Scholar] [CrossRef][Green Version]
- Zacarías, J.J.J.; Altamirano-Hernández, J.; Cabriales, J.J.P. Nitrogenase activity and trehalose content of nodules of drought-stressed common beans infected with effective (Fix+) and ineffective (Fix−) rhizobia. Soil Biol. Biochem. 2004, 36, 1975–1981. [Google Scholar] [CrossRef]
- Ruiz-Lozano, J.M. Arbuscular mycorrhizal symbiosis and alleviation of osmotic stress. New perspectives for molecular studies. Mycorrhiza 2003, 13, 309–317. [Google Scholar] [CrossRef]
- Li, T.; Hu, Y.; Hao, Z.; Li, H.; Wang, Y.; Chen, B. First cloning and characterization of two functional aquaporin genes from an arbuscular mycorrhizal fungus Glomus intraradices. New Phytol. 2013, 197, 617–630. [Google Scholar] [CrossRef] [PubMed]
- Boyer, L.R.; Brain, P.; Xu, X.-M.; Jeffries, P. Inoculation of drought-stressed strawberry with a mixed inoculum of two arbuscular mycorrhizal fungi: Effects on population dynamics of fungal species in roots and consequential plant tolerance to water deficiency. Mycorrhiza 2015, 25, 215–227. [Google Scholar] [CrossRef]
- Zhu, X.Q.; Wang, C.Y.; Chen, H.; Tang, M. Effects of arbuscular mycorrhizal fungi on photosynthesis, carbon content, and calorific value of black locust seedlings. Photosynthetica 2014, 52, 247–252. [Google Scholar] [CrossRef]
- Joseph, S.; Arbestain, M.C.; Lin, Y.; Munroe, P.; Chia, C.H.; Hook, J.; Van Zwieten, L.; Kimber, S.; Cowie, A.; Singh, B.; et al. An investigation into the reactions of biochar in soil. Soil Res. 2010, 48, 501–515. [Google Scholar] [CrossRef]
- Lehmann, J. A handful of carbon. Nature 2007, 447, 143–144. [Google Scholar] [CrossRef]
- Yu, O.-Y.; Raichle, B.; Sink, S. Impact of biochar on the water holding capacity of loamy sand soil. Int. J. Energy Environ. Eng. 2013, 4, 44. [Google Scholar] [CrossRef]
- Afshar, R.K.; Hashemi, M.; DaCosta, M.; Spargo, J.; Sadeghpour, A. Biochar Application and Drought Stress Effects on Physiological Characteristics of Silybum marianum. Commun. Soil Sci. Plant Anal. 2016, 47, 743–752. [Google Scholar] [CrossRef]
- Iijima, M.; Yamane, K.; Izumi, Y.; Daimon, H.; Motonaga, T. Continuous Application of Biochar Inoculated with Root Nodule Bacteria to Subsoil Enhances Yield of Soybean by the Nodulation Control using Crack Fertilization Technique. Plant Prod. Sci. 2015, 18, 197–208. [Google Scholar] [CrossRef]
- Lehmann, J.; Gaunt, J.; Rondon, M. Bio-char Sequestration in Terrestrial Ecosystems—A Review. Mitig. Adapt. Strateg. Glob. Change 2006, 11, 403–427. [Google Scholar] [CrossRef]
- Lehmann, J.; Rillig, M.C.; Thies, J.; Masiello, C.A.; Hockaday, W.C.; Crowley, D. Biochar effects on soil biota—A review. Soil Biol. Biochem. 2011, 43, 1812. [Google Scholar] [CrossRef]
- Warnock, D.D.; Lehmann, J.; Kuyper, T.W.; Rillig, M.C. Mycorrhizal responses to biochar in soil—Concepts and mechanisms. Plant Soil 2007, 300, 9–20. [Google Scholar] [CrossRef]
- Rutigliano, F.; Romano, M.; Marzaioli, R.; Baglivo, I.; Baronti, S.; Miglietta, F.; Castaldi, S. Effect of biochar addition on soil microbial community in a wheat crop. Eur. J. Soil Biol. 2014, 60, 9–15. [Google Scholar] [CrossRef]
- Chintala, R.; Mollinedo, J.; Schumacher, T.E.; Malo, D.D.; Julson, J.L. Effect of biochar on chemical properties of acidic soil. Arch. Agron. Soil Sci. 2014, 60, 393–404. [Google Scholar] [CrossRef]
- Doan, T.T.; Henry-Des-Tureaux, T.; Rumpel, C.; Janeau, J.-L.; Jouquet, P. Impact of compost, vermicompost and biochar on soil fertility, maize yield and soil erosion in Northern Vietnam: A three year mesocosm experiment. Sci. Total Environ. 2015, 514, 147–154. [Google Scholar] [CrossRef]
- Dong, D.; Feng, Q.; McGrouther, K.; Yang, M.; Wang, H.; Wu, W. Effects of biochar amendment on rice growth and nitrogen retention in a waterlogged paddy field. J. Soils Sediments 2015, 15, 153–162. [Google Scholar] [CrossRef]
- Xu, C.-Y.; Hosseini-Bai, S.; Hao, Y.; Rachaputi, R.C.N.; Wang, H.; Xu, Z.; Wallace, H. Effect of biochar amendment on yield and photosynthesis of peanut on two types of soils. Environ. Sci. Pollut. Res. 2015, 22, 6112–6125. [Google Scholar] [CrossRef]
- Abideen, Z.; Koyro, H.; Huchzermeyer, B.; Ansari, R.; Zulfiqar, F.; Gul, B. Ameliorating effects of biochar on photosynthetic efficiency and antioxidant defence of Phragmites karka under drought stress. Plant Biol. 2020, 22, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Javaid, A.; Riaz, T. Mycorrhizal colonization in different varieties of gladiolus and its relation with plant vegetative and reproductive growth. Int. J. Agric. Biol. 2008, 10, 278–282. [Google Scholar]
- Warnock, D.D.; Mummey, D.L.; McBride, B.; Major, J.; Lehmann, J.; Rillig, M.C. Influences of non-herbaceous biochar on arbuscular mycorrhizal fungal abundances in roots and soils: Results from growth-chamber and field experiments. Appl. Soil Ecol. 2010, 46, 450–456. [Google Scholar] [CrossRef]
- Solaiman, Z.M.; Abbott, L.K.; Murphy, D.V. Biochar phosphorus concentration dictates mycorrhizal colonisation, plant growth and soil phosphorus cycling. Sci. Rep. 2019, 9, 5062. [Google Scholar] [CrossRef]
- Verheijen, F.; Jeffery, S.; Bastos, A.C.; Van der Velde, M.; Diafas, I. Biochar application to soils. In A Critical Scientific Review of Effects on Soil Properties, Processes and Functions; Joint Research Centre, Institute for Environment and Sustainability: Ispra, Italy, 2009. [Google Scholar]
- Mau, A.; Utami, S. Effects of biochar amendment and arbuscular mycorrhizal fungi inoculation on availability of soil phosphorus and growth of maize. J. Degrad. Min. Lands Manag. 2014, 1, 69. [Google Scholar]
- Ishii, T.; Kadoya, K. Effects of Charcoal as a Soil Conditioner on Citrus Growth and Vesicular-Arbuscular Mycorrhizal Development. J. Jpn. Soc. Hortic. Sci. 1994, 63, 529–535. [Google Scholar] [CrossRef]
- Yamato, M.; Okimori, Y.; Wibowo, I.F.; Anshori, S.; Ogawa, M. Effects of the application of charred bark of Acacia mangium on the yield of maize, cowpea and peanut, and soil chemical properties in South Sumatra, Indonesia. Soil Sci. Plant Nutr. 2006, 52, 489–495. [Google Scholar] [CrossRef]
- Lehmann, J.; Pereira da Silva, J.; Steiner, C.; Nehls, T.; Zech, W.; Glaser, B. Nutrient availability and leaching in an archaeological Anthrosol and a Ferralsol of the Central Amazon basin: Fertilizer, manure and charcoal amendments. Plant Soil 2003, 249, 343–357. [Google Scholar] [CrossRef]
- Solaiman, Z.; Blackwell, P.; Abbott, L.; Storer, P. Direst and residual effect of biochar application on mycorrhizal root colonisation, growth and nutrition of wheat. Soil Res. 2010, 48, 546–554. [Google Scholar] [CrossRef]
- Jackson, M.L.; Ulrich, A. Analytical methods for use in plant analysis. Coll. Agric. Exp. State Bull. 1959, 766, 35. [Google Scholar]
- Murphy, J.A.; Riley, J.P. A modified single solution method for the determination of phosphate in natural waters. Anal. Chim. Acta 1962, 27, 31–36. [Google Scholar] [CrossRef]
- Abbott, L.; Robson, A. Infectivity and effectiveness of five endomycorrhizal fungi: Competition with indigenous fungi in field soils. Aust. J. Agric. Res. 1981, 32, 621–630. [Google Scholar] [CrossRef]
- Solaiman, Z.M.; Abbott, L.K. Influence of arbuscular mycorrhizal fungi, inoculum level and phosphorus placement on growth and phosphorus uptake of Phyllanthus calycinus under jarrah forest soil. Biol. Fertil. Soils 2008, 44, 815–821. [Google Scholar] [CrossRef]
- Giovannetti, M.; Mosse, B. AN EVALUATION OF TECHNIQUES FOR MEASURING VESICULAR ARBUSCULAR MYCORRHIZAL INFECTION IN ROOTS. New Phytol. 1980, 84, 489–500. [Google Scholar] [CrossRef]
- Prendergast-Miller, M.; Duvall, M.; Sohi, S. Localisation of nitrate in the rhizosphere of biochar-amended soils. Soil Biol. Biochem. 2011, 43, 2243–2246. [Google Scholar] [CrossRef]
- Abbas, T.; Rizwan, M.; Ali, S.; Adrees, M.; Mahmood, A.; Zia-Ur-Rehman, M.; Ibrahim, M.; Arshad, M.; Qayyum, M.F. Biochar application increased the growth and yield and reduced cadmium in drought stressed wheat grown in an aged contaminated soil. Ecotoxicol. Environ. Saf. 2018, 148, 825–833. [Google Scholar] [CrossRef] [PubMed]
- Omondi, M.O.; Xia, X.; Nahayo, A.; Liu, X.; Korai, P.K.; Pan, G. Quantification of biochar effects on soil hydrological properties using meta-analysis of literature data. Geoderma 2016, 274, 28–34. [Google Scholar] [CrossRef]
- Haider, G.; Steffens, D.; Moser, G.; Müller, C.; Kammann, C.I. Biochar reduced nitrate leaching and improved soil moisture content without yield improvements in a four-year field study. Agric. Ecosyst. Environ. 2017, 237, 80–94. [Google Scholar] [CrossRef]
- Baronti, S.; Vaccari, F.; Miglietta, F.; Calzolari, C.; Lugato, E.; Orlandini, S.; Pini, R.; Zulian, C.; Genesio, L. Impact of biochar application on plant water relations in Vitis vinifera (L.). Eur. J. Agron. 2014, 53, 38–44. [Google Scholar] [CrossRef]
- Dong, X.; Li, G.; Lin, Q.; Zhao, X. Quantity and quality changes of biochar aged for 5 years in soil under field conditions. CATENA 2017, 159, 136–143. [Google Scholar] [CrossRef]
- Mia, S.; Dijkstra, F.A.; Singh, B. Aging Induced Changes in Biochar’s Functionality and Adsorption Behavior for Phosphate and Ammonium. Environ. Sci. Technol. 2017, 51, 8359–8367. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Zhang, L.; Riaz, M.; Zhang, M.; Xia, H.; Lv, B.; Jiang, C. “Assessing the potential of biochar and aged biochar to alleviate aluminum toxicity in an acid soil for achieving cabbage productivity”. Ecotoxicol. Environ. Saf. 2018, 161, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Mickan, B.S.; Abbott, L.K.; Stefanova, K.; Solaiman, Z.M. Interactions between biochar and mycorrhizal fungi in a water-stressed agricultural soil. Mycorrhiza 2016, 26, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Bopp, C.; Christl, I.; Schulin, R.; Evangelou, M.W.H. Biochar as possible long-term soil amendment for phytostabilisation of TE-contaminated soils. Environ. Sci. Pollut. Res. 2016, 23, 17449–17458. [Google Scholar] [CrossRef]
- Zhang, Q.; Song, Y.; Wu, Z.; Yan, X.; Gunina, A.; Kuzyakov, Y.; Xiong, Z. Effects of six-year biochar amendment on soil aggregation, crop growth, and nitrogen and phosphorus use efficiencies in a rice-wheat rotation. J. Clean. Prod. 2020, 242, 118435. [Google Scholar] [CrossRef]
- Ryan, M.; Ash, J. Colonisation of wheat in southern New South Wales by vesicular-arbuscular mycorrhizal fungi is significantly reduced by drought. Aust. J. Exp. Agric. 1996, 36, 563–569. [Google Scholar] [CrossRef]
- Al-Karaki, G.N.; Clark, R.B. Growth, mineral acquisition, and water use by mycorrhizal wheat grown under water stress. J. Plant Nutr. 1998, 21, 263–276. [Google Scholar] [CrossRef]
- Asrar, A.A.; Abdel-Fattah, G.M.; Elhindi, K.M. Improving growth, flower yield, and water relations of snapdragon (Antirhinum majus L.) plants grown under well-watered and water-stress conditions using arbuscular mycorrhizal fungi. Photosynthetica 2012, 50, 305–316. [Google Scholar] [CrossRef]
- Wallstedt, A.; Coughlan, A.; Munson, A.D.; Nilsson, M.-C.; Margolis, H.A. Mechanisms of interaction between Kalmia angustifolia cover and Picea mariana seedlings. Can. J. For. Res. 2002, 32, 2022–2031. [Google Scholar] [CrossRef]
- Augé, R.M. Water relations, drought and vesicular-arbuscular mycorrhizal symbiosis. Mycorrhiza 2001, 11, 3–42. [Google Scholar] [CrossRef]
- Gehring, C.A.; Swaty, R.; Deckert, R. Mycorrhizas, drought, and host-plant mortality. In Mycorrhizal Mediation of Soil; Elsevier: Amsterdam, The Netherlands, 2017; pp. 279–298. [Google Scholar]
- LeCroy, C.; Masiello, C.A.; Rudgers, J.A.; Hockaday, W.C.; Silberg, J.J. Nitrogen, biochar, and mycorrhizae: Alteration of the symbiosis and oxidation of the char surface. Soil Biol. Biochem. 2013, 58, 248–254. [Google Scholar] [CrossRef]
- Liu, C.; Liu, F.; Ravnskov, S.; Rubaek, G.H.; Sun, Z.; Andersen, M.N. Impact of Wood Biochar and Its Interactions with Mycorrhizal Fungi, Phosphorus Fertilization and Irrigation Strategies on Potato Growth. J. Agron. Crop Sci. 2017, 203, 131–145. [Google Scholar] [CrossRef]
- Wang, F.; Liu, Y.; Li, X.; Liu, F. Effects of biochar amendment, CO2 elevation and drought on leaf gas exchange, biomass production and water use efficiency in maize. Pak. J. Bot. 2018, 50, 1347–1353. [Google Scholar]
- Miyashita, K.; Tanakamaru, S.; Maitani, T.; Kimura, K. Recovery responses of photosynthesis, transpiration, and stomatal conductance in kidney bean following drought stress. Environ. Exp. Bot. 2005, 53, 205–214. [Google Scholar] [CrossRef]
- Liu, F.; Song, R.; Zhang, X.; Shahnazari, A.; Andersen, M.N.; Plauborg, F.; Jacobsen, S.-E.; Jensen, C.R. Measurement and modelling of ABA signalling in potato (Solanum tuberosum L.) during partial root-zone drying. Environ. Exp. Bot. 2008, 63, 385–391. [Google Scholar] [CrossRef]
- Pazzagli, P.T.; Weiner, J.; Liu, F. Effects of CO2 elevation and irrigation regimes on leaf gas exchange, plant water relations, and water use efficiency of two tomato cultivars. Agric. Water Manag. 2016, 169, 26–33. [Google Scholar] [CrossRef]
- Zoghi, Z.; Hosseini, S.M.; Kouchaksaraei, M.T.; Kooch, Y.; Guidi, L. The effect of biochar amendment on the growth, morphology and physiology of Quercus castaneifolia seedlings under water-deficit stress. Eur. J. For. Res. 2019, 138, 967–979. [Google Scholar] [CrossRef]
- Mannan, M.; Halder, E.; Karim, M.; Ahmed, J. Alleviation of Adverse Effect of Drought Stress on Soybean (Glycine max L.) by Using Poultry Litter Biochar. Bangladesh Agron. J. 2016, 19, 61–69. [Google Scholar] [CrossRef][Green Version]
- Akhtar, S.S.; Li, G.; Andersen, M.N.; Liu, F. Biochar enhances yield and quality of tomato under reduced irrigation. Agric. Water Manag. 2014, 138, 37–44. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).