Sole and Combined Application of Phosphorus and Glucose and Its Influence on Greenhouse Gas Emissions and Microbial Biomass in Paddy Soils
Abstract
1. Introduction
2. Materials and Methods
2.1. Soil Sampling, Site and Characteristics
2.2. Incubation and Treatment Details
2.3. N2O and CH4 Emission Sampling Method
2.4. Chemical Analysis
2.5. Statistical Analysis
3. Results
3.1. Effect of Phosphorus and Glucose Addition on N2O and CH4 Emissions
3.2. Effects of Phosphorus and Glucose Addition on Soil Chemical Properties
3.3. Alterations in Soil Microbial Biomass after Phosphorus and Glucose Addition
3.4. Relative Contribution of Different Soil Properties and Soil Microbial Biomass to CH4 and N2O Emissions
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cook, E. (Ed.) Agriculture, Forestry and Fishery Statistics—2015 Edition; Publications Office of the European Union: Luxembourg, 2016. [Google Scholar]
- Myhre, G.; Shindell, D.; Bréon, F.M.; Collins, W.; Fuglestvedt, J.; Huang, J.; Koch, D.; Lamarque, J.F.; Lee, D.; Mendoza, B.; et al. Anthropogenic and natural radiative forcing. In Climate Change 2013. The Physical Science Basis; Stocker, T.F., Qin, D., Plattner, G.-K., Tignor, M., Allen, S.K., Boschung, J., Nauels, A., Xia, Y., Bex, V., Midgley, P.M., Eds.; Cambridge University Press: Cambridge, UK, 2013; p. 714. [Google Scholar]
- Yang, B.; Xiong, Z.; Wang, J.; Xu, X.; Huang, Q.; Shen, Q. Mitigating net global warming potential and greenhouse gas intensities by substituting chemical nitrogen fertilizers with organic fertilization strategies in rice—Wheat annual rotation systems in China: A 3-year field experiment. Ecol. Eng. 2015, 81, 289–297. [Google Scholar] [CrossRef]
- Butterbach-Bahl, K.; Diaz-Pines, E.; Dannenmann, M. Soil traces gas emissions and climate change. In Global Environmental Change; Freedman, B., Ed.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 325–334. [Google Scholar]
- Ravishankara, A.R.; Daniel, J.S.; Portmann, R.W. Nitrous oxide (N2O): The dominant ozone-depleting substance emitted in the 21st century. Science 2009, 326, 123–125. [Google Scholar] [CrossRef] [PubMed]
- Food and Agricultural Organization of the United Nations. OECD-FAO Agricultural Outlook 2011–2030; Food and Agricultural Organization of the United Nations: Rome, Italy, 2010. [Google Scholar]
- Bridgham, S.D.; Cadillo-Quiroz, H.; Keller, J.K.; Zhuang, Q. Methane emissions from wetlands: Biogeochemical, microbial, and modelling perspectives from local to global scales. Glob. Change Biol. 2013, 19, 13–25. [Google Scholar] [CrossRef]
- Liu, R.; Hayden, H.L.; Suter, H.; Hu, H.; Lam, S.K.; He, J.-Z.; Mele, P.M.; Chen, D. The effect of temperature and moisture on the source of N2O and contributions from ammonia oxidizers in an agricultural soil. Biol. Fertil. Soils 2017, 53, 141–152. [Google Scholar] [CrossRef]
- Dalal, R.C.; Wang, W.; Robertson, G.; Parton, W.J. Nitrous oxide emission from Australian agricultural lands and mitigation options: A review. Soil Res. 2003, 41, 165–195. [Google Scholar] [CrossRef]
- Conrad, R. Microbial ecology of methanogens and methanotrophs. Adv. Agron. 2007, 96, 1–63. [Google Scholar]
- Mori, T.; Ohta, S.; Ishizuka, S.; Konda, R.; Wicaksono, A.; Heriyanto, J.; Hardjono, A. Effects of phosphorus addition on N2O and NO emissions from soils of an Acacia mangium plantation. Soil Sci. Plant Nutr. 2010, 56, 782–788. [Google Scholar] [CrossRef]
- Mori, T.; Ohta, S.; Ishizuka, S.; Konda, R.; Wicaksono, A.; Heriyanto, J.; Hardjono, A. Effects of phosphorus addition with and without ammonium, nitrate, or glucose on N2O and NO emissions from soil sampled under Acacia mangium plantation and incubated at 100% of the water-filled pore space. Biol. Fertil. Soils 2013, 49, 13–21. [Google Scholar] [CrossRef]
- Mehnaz, K.R.; Keitel, C.; Dijkstra, F.A. Effects of carbon and phosphorus addition on microbial respiration, N2O emission, and gross nitrogen mineralization in a phosphorus-limited grassland soil. Biol. Fertil. Soils 2018, 54, 481–493. [Google Scholar] [CrossRef]
- Mehnaz, K.R.; Dijkstra, F.A. Denitrification and associated N2O emissions are limited by phosphorus availability in a grassland soil. Geoderma 2016, 284, 34–41. [Google Scholar] [CrossRef]
- Yvon-Durocher, G.; Allen, A.P.; Bastviken, D.; Conrad, R.; Gudasz, C.; St-Pierre, A.; Thanh-Duc, N.; del Giorgio, P. Methane fluxes show consistent temperature dependence across microbial to ecosystem scales. Nature 2014, 507, 488–491. [Google Scholar] [CrossRef] [PubMed]
- Bodelier, P.E. Bypassing the methane cycle. Nature 2015, 523, 534–535. [Google Scholar] [CrossRef] [PubMed]
- Conrad, R.; Chan, O.C.; Claus, P.; Casper, P. Characterization of methanogenic archaea and stable isotope fractionation duringmethane production in the profundal sediment of an oligotrophic lake (Lake Stechlin, Germany). Limnol. Oceanogr. 2007, 52, 13–93. [Google Scholar] [CrossRef]
- Krüger, M.; Frenzel, P.; Kemnitz, D.; Conrad, R.; Krüger, M. Activity, structure and dynamics of the methanogenic archaeal community in a flooded Italian rice field. FEMS Microbiol. Ecol. 2005, 51, 323–331. [Google Scholar] [CrossRef]
- Ma, K.; Conrad, R.; Lu, Y. Responses of methanogen mcrA genes and their transcripts to an alternate dry/wet cycle of paddy field soil. Appl. Environ. Microbiol. 2012, 78, 445–454. [Google Scholar] [CrossRef]
- Garland, G.; Bünemann, E.K.; Oberson, A.; Frossard, E.; Snapp, S.; Chikowo, R.; Six, J. Phosphorus cycling within soil aggregate fractions of a highly weathered tropical soil: A conceptual model. Soil Biol. Biochem. 2018, 116, 91–98. [Google Scholar] [CrossRef]
- Medvedeff, C.A.; Inglett, K.S.; Inglett, P.W. Evaluation of direct and indirect phosphorus limitation of methanogenic pathways in a calcareous subtropical wetland soil. Soil Biol. Biochem. 2014, 69, 343–345. [Google Scholar] [CrossRef]
- Conrad, R.; Klose, M.; Claus, P. Phosphate inhibits acetotrophic methanogenesis on rice roots. Appl. Environ. Microbiol. 2000, 66, 828–831. [Google Scholar] [CrossRef]
- Lund, M.; Christensen, T.R.; Mastepanov, M.; Lindroth, A.; Str€om, L. Effects of N and P fertilization on the greenhouse gas exchange in two northern peat lands with contrasting N deposition rates. Biogeosciences 2009, 6, 2135–2144. [Google Scholar] [CrossRef]
- Zhang, T.; Zhu, W.; Mo, J.; Liu, L.; Dong, S. Increased phosphorus availability mitigates the inhibition of nitrogen deposition on CH4 uptake in an old-growth tropical forest, southern China. Biogeosciences 2011, 8, 2805–2813. [Google Scholar] [CrossRef]
- Cleveland, C.C.; Townsend, A.R.; Schmidt, S.K. Phosphorus limitation of microbial processes in moist tropical forests: Evidence from short-term laboratory incubations and field studies. Ecosystems 2002, 5, 680–691. [Google Scholar] [CrossRef]
- Ilstedt, U.; Singh, S. Nitrogen and phosphorus limitations of microbial respiration in a tropical phosphorus-fixing acrisol (ultisol) compared with organic compost. Soil Biol. Biochem. 2005, 37, 1407–1410. [Google Scholar] [CrossRef]
- Cleveland, C.C.; Liptzin, D. C:N:P stoichiometry in soil: Is there a Bred field ratio for the microbial biomass? Biogeochemistry 2007, 85, 235–252. [Google Scholar] [CrossRef]
- Turner, B.L.; Wright, S.J. The response of microbial biomass and hydrolytic enzymes to a decade of nitrogen, phosphorus, and potassium addition in a lowland tropical rain forest. Biogeochem 2014, 117, 115–130. [Google Scholar] [CrossRef]
- Mori, T.; Ohta, S.; Ishizuka, S.; Konda, R.; Wicaksono, A.; Heriyanto, J. Phosphorus application reduces N2O emissions from tropical leguminous plantation soil when phosphorus uptake is occurring. Biol. Fertil. Soils 2014, 50, 45–51. [Google Scholar] [CrossRef]
- Mori, T.; Yokoyama, D.; Kitayama, K. Contrasting effects of exogenous phosphorus application on N2O emissions from two tropical forest soils with contrasting phosphorus availability. SpringerPlus 2016, 5, 12–37. [Google Scholar] [CrossRef]
- Mori, T.; Wachrinrat, C.; Staporn, D.; Meunpong, P.; Suebsai, W.; Matsubara, K.; Boonsri, K.; Lumban, W.; Kuawong, M.; Phukdee, T.; et al. Effects of phosphorus addition on nitrogen cycle and fluxes of N2O and CH4 in tropical tree plantation soils in Thailand. Agric. Nat. Resour. 2017, 51, 91–95. [Google Scholar] [CrossRef]
- Olsen, S.R. Estimation of Available Phosphorus in Soils by Extraction with Sodium Bicarbonate; US Government Printing Office: Washington, DC, USA, 1954. [Google Scholar]
- Brookes, P.; Landman, A.; Pruden, G.; Jenkinson, D. Chloroform fumigation and the release of soil nitrogen: A rapid direct extraction method to measure microbial biomass nitrogen in soil. Soil Biol. Biochem. 1985, 17, 837–842. [Google Scholar] [CrossRef]
- Brookes, P.C.; Powlson, D.S.; Jenkinson, D.S. Measurement of microbial biomass phosphorus in soil. Soil Biol. Biochem. 1982, 14, 319–329. [Google Scholar] [CrossRef]
- Beck, T.; Joergensen, R.; Kandeler, E.; Makeschin, F.; Nuss, E.; Oberholzer, H.; Scheu, S. An inter-laboratory comparison of ten different ways of measuring soil microbial biomass C. Soil Biol. Biochem. 1997, 29, 1023–1032. [Google Scholar] [CrossRef]
- Davidson, E.A. The contribution of manure and fertilizer nitrogen to atmospheric nitrous oxide since 1860. Nat. Geosci. 2009, 2, 659–662. [Google Scholar] [CrossRef]
- Morley, N.; Baggs, E.M. Carbon and oxygen controls on N2O and N2 production during nitrate reduction. Soil Biol. Biochem. 2010, 42, 1864–1871. [Google Scholar] [CrossRef]
- Le Mer, J.; Roger, P. Production, oxidation, emission and consumption of methane by soils: A review. Eur. J. Soil Biol. 2001, 37, 25–50. [Google Scholar] [CrossRef]
- Kim, G.W.; Gwon, H.S.; Jeong, S.T.; Hwang, H.Y.; Kim, P.J. Different responses of nitrogen fertilization on methane emission in rice plant included and excluded soils during cropping season. Agric. Ecosyst. Environ. 2016, 230, 162–168. [Google Scholar] [CrossRef]
- Signor, D.; Cerri, C.E.P. Nitrous oxide emissions in agricultural soils: A review. Pesqui. Agropecuária Trop. 2013, 43, 322–338. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.Y.; Su, S.L.; Li, C.S. Quantifying methane emissions from rice paddies in Northeast China by integrating remote sensing mapping with a biogeochemical model. Biogeosciences 2011, 8, 1225–1235. [Google Scholar] [CrossRef]
- Kim, S.Y.; Veraart, A.J.; Meima-Franke, M.; Bodelier, P.L.E. Combined effects of carbon, nitrogen and phosphorus on CH4 production and denitrification in wetland sediments. Geoderma 2015, 259–260, 354–361. [Google Scholar] [CrossRef]
- Han, X.G.; Sun, X.; Wang, C.; Wu, M.X.; Dong, D.; Zhong, T.; Thies, J.E.; Wu, W.X. Mitigating methane emission from paddy soil with rice-straw biochar amendment under projected climate change. Sci. Rep. 2016, 6, 24731. [Google Scholar] [CrossRef]
- Bååth, E.; Anderson, T.-H. Comparison of soil fungal/bacterial ratios in a pH gradient using physiological and PLFA-based techniques. Soil Biol. Biochem. 2003, 35, 955–963. [Google Scholar] [CrossRef]
- Krüger, M.; Frenzel, P.; Conrad, R. Microbial processes influencing methane emission from rice fields. Glob. Chang. Biol. 2001, 7, 49–63. [Google Scholar] [CrossRef]
- Hue, N.V.; Adams, F. Effect of phosphorus level on nitrification rates in three low-phosphorus ultisols. Soil Sci. 1984, 137, 324–331. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, Y.-R.; Zhang, C.-J.; Zhang, L.-M.; Han, L.-L.; Shen, J.-P.; He, J.-Z. Responses of soil nitrous oxide production and abundances and composition of associated microbial communities to nitrogen and water amendment. Biol. Fertil. Soils 2017, 53, 601–611. [Google Scholar] [CrossRef]
- Wrage, N.; Velthof, G.L.; van Beusichem, M.L.; Oenema, O. Role of nitrifier denitrification in the production of nitrous oxide. Soil Biol. Biochem. 2001, 33, 1723–1732. [Google Scholar] [CrossRef]
- Song, C.; Yang, G.; Liu, D.; Mao, R. Phosphorus availability as a primary constraint on methane emission from a freshwater wetland. Atmos. Environ. 2012, 59, 202–206. [Google Scholar] [CrossRef]
- Krause, S.M.B.; Le Roux, X.; Niklaus, P.A.; Van Bodegom, P.M.; Lennon, J.T.; Bertilsson, S.; Grossart, H.-P.; Philippot, L.; Bodelier, P.L.E. Trait-based approaches for understanding microbial biodiversity and ecosystem functioning. Front. Microbiol. 2014, 5, 251. [Google Scholar] [CrossRef]
- Konieczna, A.; Roman, K.; Borek, K.; Grzegorzewska, E. GHG and NH3 emissions vs. energy efficiency of maize production technology: Evidence from Polish farms; A further study. Energies 2021, 14, 5574. [Google Scholar] [CrossRef]


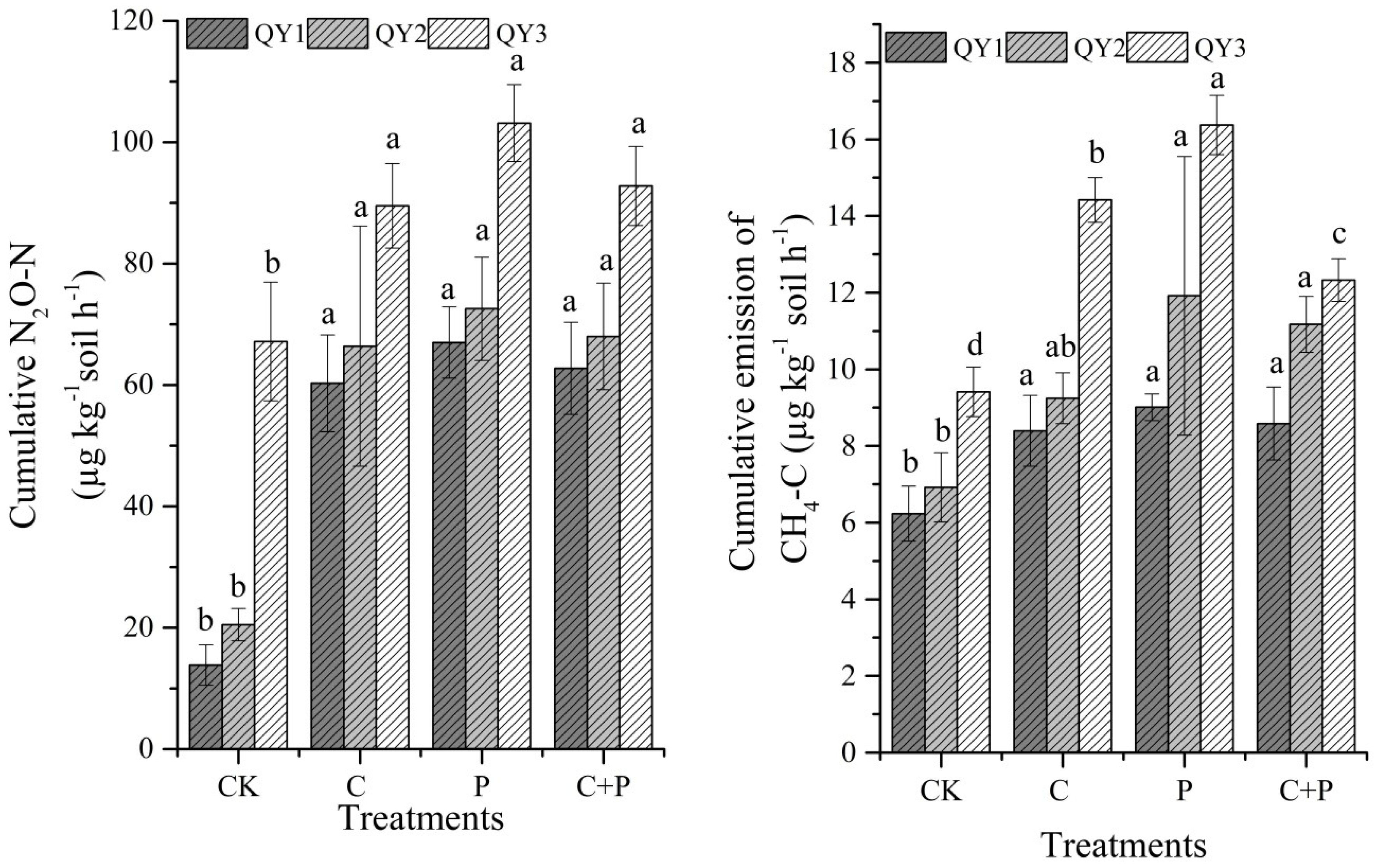

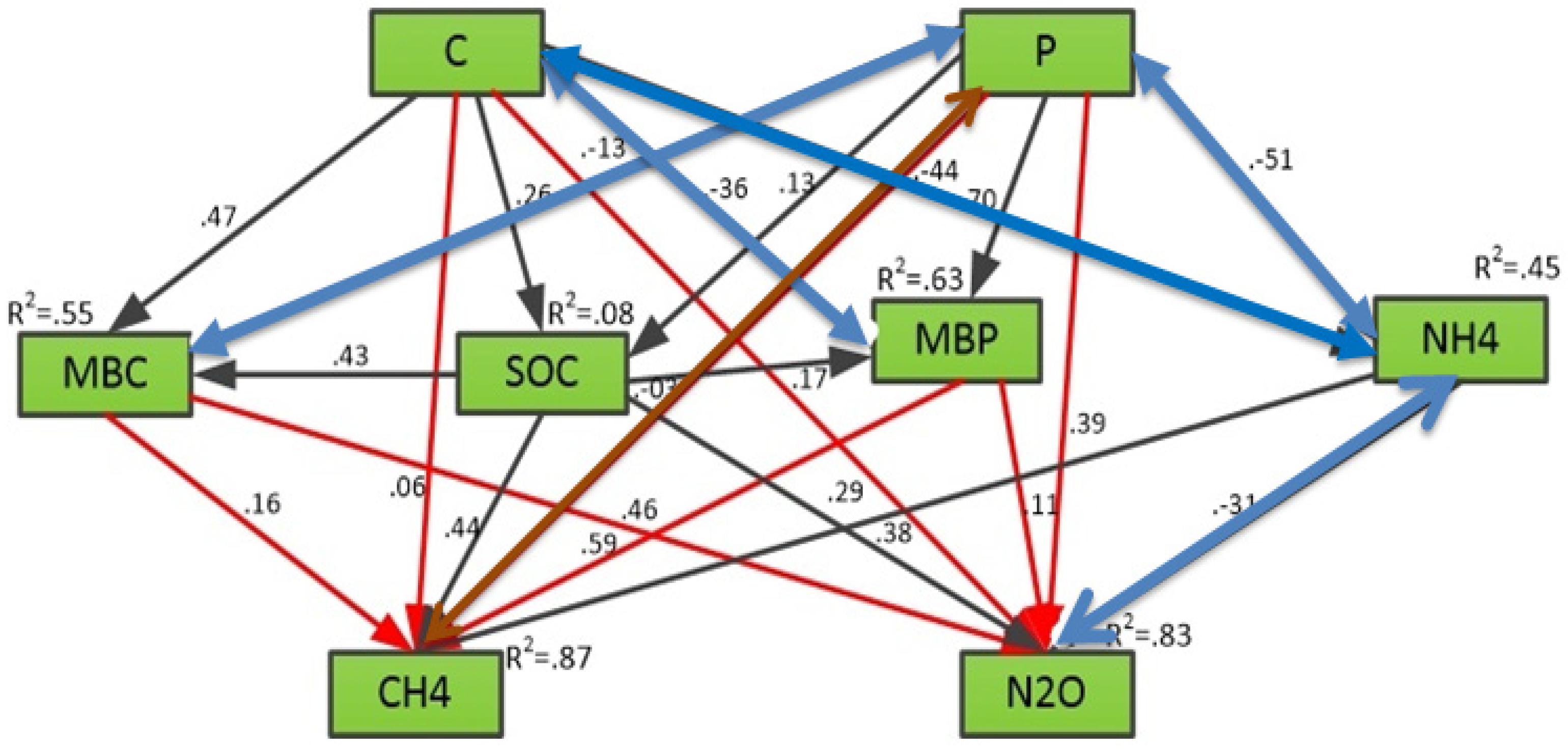
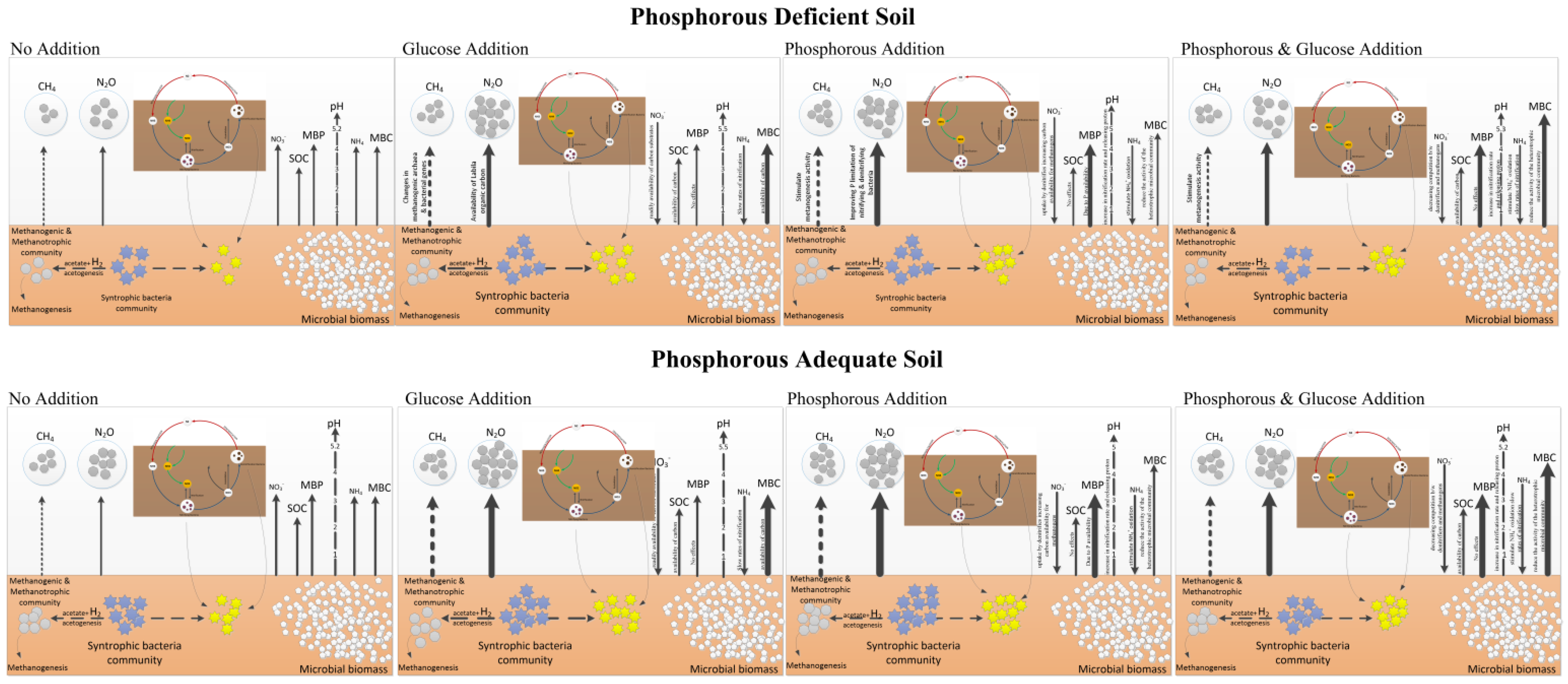
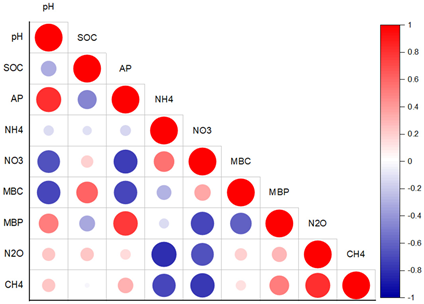
| Fields | pH | SOC (gkg−1) | AP (mgPkg−1) | TP (g·kg−1) | TN (g·kg−1) | AN (mgNkg−1) |
|---|---|---|---|---|---|---|
| P-deficient soil (QY1) | 5.54 ± 0.13 | 18.1 ± 0.91 | 9.03 ± 0.67 | 1.47 ± 0.05 | 1.9 ± 0.09 | 189.6 ± 14.7 |
| P-adequate soil (QY2) | 5.59 ± 0.06 | 20.8 ± 1.2 | 39.3 ± 1.1 | 1.98 ± 0.16 | 2.1 ± 0.05 | 243.1 ± 57.2 |
| P-adequate soil (QY3) | 5.66 ± 0.03 | 28.9 ± 0.58 | 56.8 ± 7.9 | 2.17 ± 0.13 | 2.8 ± 0.03 | 275.8 ± 43.2 |
| Phosphorus-Deficient Soil (QY1) | ||||||||
| Treatments | pH | SOC | AP | NH4 | NO3 | MBC | MBP | |
| Glu addition | p addition | gkg−1 | mgPkg−1 | mgNkg−1 | mgNkg−1 | mgCkg−1 | mgPkg−1 | |
| Without | Without | 5.25 ± 0.08 b | 16.4 ± 1.18 ab | 9.6 ± 1.1 bc | 1.20 ± 0.5 a | 8.09 ± 0.88 a | 144.6 ± 3.9 bc | 14.2 ± 1.3 b |
| With | Without | 5.51 ± 0.15 a | 18.2 ± 1.36 a | 7.9 ± 1.3 c | 0.38 ± 0.04 b | 7.16 ± 0.97 ab | 168.5 ± 5.04 a | 13.1 ± 0.78 b |
| Without | With | 5.06 ± 0.06 c | 16.1 ± 0.96 b | 12.7 ± 2.8 a | 0.34 ± 0.04 b | 4.75 ± 0.96 c | 139.6 ± 5.7 c | 20.7 ± 3.0 a |
| With | With | 5.33 ± 0.59 b | 17.6 ± 0.99 ab | 10.1 ± 3.3 b | 0.49 ± 0.09 b | 6.05 ± 0.46 bc | 150.4 ± 3.2 b | 16.1 ± 1.7 ab |
| ANOVA p values | ||||||||
| Glu | 0.023 | 0.05 | 0.089 | ns | ns | 0.0003 | ns | |
| P | 0.016 | ns | 0.066 | ns | ns | 0.002 | 0.013 | |
| P × C | ns | ns | ns | 0.02 | 0.031 | 0.032 | ns | |
| Phosphorus-Adequate Soil (QY2) | ||||||||
| Treatments | pH | SOC | AP | NH4 | NO3 | MBC | MBP | |
| Glu addition | p addition | gkg−1 | mgPkg−1 | mgNkg−1 | mgNkg−1 | mgCkg−1 | mgPkg−1 | |
| Without | Without | 5.2 ± 0.2 a | 20.6 ± 2.3 a | 41.6 ± 5.3 bc | 1.76 ± 0.22 a | 9.48 ± 1.5 a | 141.1 ± 13.7 b | 14.0 ± 0.6 b |
| With | Without | 5.56 ± 0.2 a | 24.4 ± 4.0 a | 40.4 ± 7.2 c | 0.419 ± 0.13 b | 7.18 ± 0.64 b | 160.8 ± 7.4 a | 14.4 ± 1.1 b |
| Without | With | 5.12 ± 0.40 a | 19.7 ± 1.3 a | 43.8 ± 9.7 a | 0.298 ± 0.08 b | 5.97 ± 1.2 b | 143.3 ± 3.6 b | 24.7 ± 3.4 a |
| With | With | 5.30 ± 0.1 a | 22.4 ± 2.4 a | 43.0 ± 5.5 ab | 0.455 ± 0.12 b | 6.76 ± 0.25 b | 152.8 ± 5.7 ab | 17.8 ± 1.7 b |
| ANOVA p values | ||||||||
| Glu | ns | ns | ns | ns | ns | 0.0125 | ns | |
| P | ns | ns | 0.007 | ns | ns | ns | 0.049 | |
| P × C | ns | ns | ns | 0.005 | 0.035 | ns | ns | |
| Phosphorus-Adequate Soil (QY3) | ||||||||
| Treatments | pH | SOC | AP | NH4 | NO3 | MBC | MBP | |
| Glu addition | p addition | gkg−1 | mgPkg−1 | mgNkg−1 | mgNkg−1 | mgCkg−1 | mgPkg−1 | |
| Without | Without | 5.08 ± 0.06 b | 25.6 ± 1.3 a | 52.1 ± 11.4 ab | 1.70 ± 0.52 a | 10.4 ± 1.8 a | 169.1 ± 3.5 c | 18.42 ± 1.1 b |
| With | Without | 5.52 ± 0.25 a | 28.4 ± 1.5 a | 51.0 ± 15.8 b | 0.47 ± 0.06 b | 7.85 ± 0.33 b | 182.8 ± 3.4 a | 16.8 ± 0.97 b |
| Without | With | 5.06 ± 0.20 ab | 24.1 ± 2.3 a | 56.6 ± 8.4 a | 0.40 ± 0.02 b | 6.74 ± 0.45 b | 162.3 ± 7.6 bc | 30.3 ± 3.4 a |
| With | With | 5.21 ± 0.09 b | 27.1 ± 2.2 a | 53.3 ± 5.3 ab | 0.613 ± 0.25 b | 8.46 ± 1.2 b | 171.7 ± 6.2 ab | 21.04 ± 1.1 b |
| ANOVA p values | ||||||||
| Glu | ns | ns | ns | ns | ns | ns | ns | |
| P | ns | ns | ns | ns | ns | ns | 0.05 | |
| P × C | ns | ns | ns | 0.002 | 0.010 | ns | 0.005 | |
| Phosphorus-deficient soil (QY1) |
 |
| Phosphorous-adequate soil (QY2) |
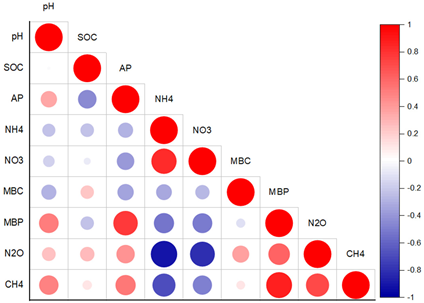 |
| Phosphorus-adequate soil (QY3) |
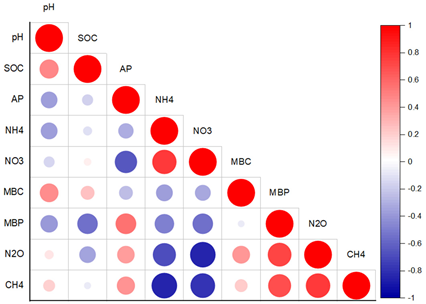 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shah, A.; Huang, J.; Khan, M.N.; Han, T.; Ali, S.; Daba, N.A.; Du, J.; Li, D.; Zhang, L.; Fahad, S.; et al. Sole and Combined Application of Phosphorus and Glucose and Its Influence on Greenhouse Gas Emissions and Microbial Biomass in Paddy Soils. Agronomy 2022, 12, 2368. https://doi.org/10.3390/agronomy12102368
Shah A, Huang J, Khan MN, Han T, Ali S, Daba NA, Du J, Li D, Zhang L, Fahad S, et al. Sole and Combined Application of Phosphorus and Glucose and Its Influence on Greenhouse Gas Emissions and Microbial Biomass in Paddy Soils. Agronomy. 2022; 12(10):2368. https://doi.org/10.3390/agronomy12102368
Chicago/Turabian StyleShah, Asad, Jing Huang, Muhammad Numan Khan, Tianfu Han, Sehrish Ali, Nano Alemu Daba, Jiangxue Du, Dongchu Li, Lu Zhang, Shah Fahad, and et al. 2022. "Sole and Combined Application of Phosphorus and Glucose and Its Influence on Greenhouse Gas Emissions and Microbial Biomass in Paddy Soils" Agronomy 12, no. 10: 2368. https://doi.org/10.3390/agronomy12102368
APA StyleShah, A., Huang, J., Khan, M. N., Han, T., Ali, S., Daba, N. A., Du, J., Li, D., Zhang, L., Fahad, S., Liu, S., Liu, L., Gao, J., Xu, Y., He, Z., & Zhang, H. (2022). Sole and Combined Application of Phosphorus and Glucose and Its Influence on Greenhouse Gas Emissions and Microbial Biomass in Paddy Soils. Agronomy, 12(10), 2368. https://doi.org/10.3390/agronomy12102368






