Abstract
The agricultural productivity of farmland in Northeast China’s Liaohe Plain is restricted by the salinity and sodicity of the soils, which have additionally low organic matter content. In order to improve saline–sodic soils, organic amendments are frequently applied. Our objective was to clarify how different organic amendments affect the diversity and composition of soil microbes, as well as how these factors are related to crop yield. In 2020–2021, we conducted an experiment with different organic amendments. The treatments included the application of crop residue incorporation (SR), lignite humic acid (LHA; 6 ton/ha), or cow manure (FM; 30 ton/ha), and a control (CK). The results show that, compared with CK, the content of SOM in soil treated with organic amendments increased by 5.3–7.4 g/kg; the available potassium (AK) of the LHA treatment was significantly higher than that of the FM and SR treatments by 32.17 and 42.79 mg/kg, respectively; and the available phosphorus (AP) of the LHA treatment was significantly higher than that of the SR treatment by 7.19 mg/kg. The pH and EC1:5 values of the LHA treatment were significantly lower than those of CK by 1.36 units and 0.2 mS/cm, respectively. The application of organic amendments and changes in environmental conditions also significantly affected community structure and increased soil microbial richness and diversity. SR treatment increased the abundance of Acidobacteria. Further FAPROTAX (Functional Annotation of Prokaryotic Taxa) analysis showed that organic amendments can increase the abundance of microbes involved in the carbon and nitrogen cycle processes, such as aerobic_ammonia_oxidation, aerobic_chemoheterotrophy, nitrification, etc., which increases the kernel number per row and increases crop yield. LHA can increase the microbial abundance of the nitrogen cycle and reduce soil carbon mineralization, while also increasing soil nutrients and crop yield. This study provides a comprehensive understanding of the application of organic amendments in saline–sodic cultivated land.
1. Introduction
In agricultural production, salinity and sodicity are important factors limiting crop yield. Soil salinity poses a significant risk to soil productivity in cultivated land due to its negative effects on microbial activity and soil physicochemical properties []. On the one hand, soil salinity affects the intracellular concentration of various ions in plant cells, which, in turn, affects plant metabolic activities, which are toxic to subterranean microorganisms and are associated with carbon mineralization and nutrient cycling []. On the other hand, high salinity and pH in the soil can cause osmotic and ionic stress in plants, which can lead to nutrient imbalance and may reduce the photosynthetic capacity of plants, which, in turn, reduces growth and leads to plant death [,]. This is especially true in the Northeast Liaohe Plain, where large tracts of saline–sodic soil are cultivated as backup farmland resources.
The application of organic materials is considered an effective strategy to improve the quality of saline–sodic soils [,]. Reclamation of saline–sodic soil with organic amendments can increase soil nutrient content, improve soil structure, and increase soil macroaggregates [,]. Crop residue is the most commonly used organic material to improve and enhance soil quality, Zhang, et al. [] showed that the combined application of residue pellets and desulfurized gypsum improved the alkalinity, nutrient content, and aggregate stability of soil, thereby improving soil structure. Zhang, et al. [] showed that continuous use of farmyard manure can significantly reduce exchangeable sodium ions, reduce soil salinity, increase soil nutrients and organic matter, and improve plant growth. However, long-term or repeated field applications of conventional organic inputs can be problematic due to the high turnover of these conventional organic materials []. For example, their overuse may increase the risk of nitrogen leaching and phosphorus runoff leading to eutrophication, or phosphorus leaching in soils with low phosphorus retention []. Conventional organic materials (e.g., compost, crop residue, etc.), especially manure, are also sources of greenhouse gas emissions []. In addition to farmyard manure, lignite humic acids are widely used to improve saline soils due to their low pH, abundant organic functional groups, favorable soil aggregate formation, and enhanced crop root uptake []. The results of Manasa et al. [] showed that lignite humic acid can reduce the pH value, decrease soil Na+ content, increase soil K+ content, and improve soil microbial and enzymatic activities.
In addition, the use of organic amendments can also improve agricultural soil health by altering the soil microbiome []. For example, microorganisms metabolize and decompose organic matter, promoting the accumulation and utilization of nutrients []; microorganisms also contribute to the formation and size of aggregates via the production of mucus and polysaccharides []. The application of organic amendments increases microbial diversity in saline–alkali soils and enriches microbial communities towards microbial species responsible for soil aggregate stability (e.g., Ascomycetes) []. The microbial community is related not only to the organic matter content, but also to the type of organic amendments [], and significant differences were found in the relative strength of functional groups in different organic amendments []. Organic matter that is rich in aromatic hydrocarbons and stable carbon structures is less easily decomposed, affecting the carbon and nitrogen cycles in which microorganisms drive the metabolic transformation of organic matter [,]. Furthermore, different organic matter amendments may also introduce different microorganisms into the soil, especially the application of farmyard manure []. Therefore, the different decomposability of organic amendments may result in different improvement effects and different microbial response mechanisms []. However, whether the application of different organic amendments can improve the diversity, composition, and structure of soil microbial communities in slightly saline–alkali soils is unclear.
This study investigated the effects of different organic material additions (cow dung, residue, and lignite humic acid) on the properties and bacterial communities of saline–sodic soil and spring maize (Zea mays L.) yield. We hypothesized that (i) organic amendments can improve the properties of saline–sodic soil, increase soil nutrients, and improve crop yield; (ii) different amendments have different effects on soil microbial diversity, which are jointly affected by soil properties and organic amendments; and (iii) microorganisms of carbon and nitrogen cycling in saline–sodic soil treated with different organic amendments may affect crop yield.
2. Materials and Methods
2.1. Experimental Site
The experiment was carried out on salinized farmland (43°49′18″ N, 122°09′24″ E) in Sanjiazi Village, Huatugula Township, Horqin Zuoyizhongqi, Inner Mongolia, which is located in the eastern part of the West Liaohe Plain and belongs to the typical inland saline–sodic cultivated soil. The soil texture is sandy loam. The climate of the test area is a mid-temperate continental monsoon climate, with four distinct seasons and large daily and annual temperature differences; the highest temperature throughout the year is 35.8 °C, the lowest temperature is −25.1 °C, the annual average temperature is 7 °C, and the frost-free period is 202 days. The annual precipitation is 342–392 mm.
2.2. Experimental Design
The experiment consisted of 4 treatments with 5 replicate plots grown in a completely randomized plot design. A total of 20 field plots were used, and each plot was 30 m long, 6 m wide, and 180 m2 in area. The 4 treatments were: a control treatment (CK); lignite humic acid treatment (LHA) at 6 ton/ha (according to the manufacturer’s recommended usage); maize residue incorporation treatment (SR) with the full amount returned to the soil; and cow dung treatment (FM) at 30 ton/ha (according to the general application rate of local farmers). In April 2020 and April 2021, the organic materials were evenly distributed on the soil surface and 20 cm rotary tillage was performed. The details of the organic materials used are shown in Table 1.

Table 1.
Chief characteristics of the amendments used in the study.
The experiment ran from late April 2020 to late October 2021, that is, the sunflower (Helianthus annuus)–spring maize rotation, and the sunflowers were sown on 19 June 2020. The variety was Zhengbo No. 3, the row spacing was 60 cm × 110 cm, and the planting density was 15,150 plants/ha. Maize was planted in wide and narrow rows; the variety was Dika 159, the average row spacing was 60 cm, the plant spacing was 20 cm, and the planting density was 83,340 plants/ha. During the experiment, each plot received the same irrigation and field management practices. Shallow burial drip irrigation was used, and the other field management measures were the same.
2.3. Soil Sampling and Laboratory Analysis
The initial conditions for basic soil properties were determined from samples taken from the top 0–20 cm of the topsoil in late April 2020 (before the start of the experiment) (Table 2). Before establishing field plots, the experimental field was leveled using a rotary tiller to ensure uniform soil conditions. Soil sampling was carried out in late October 2021 during the spring maize harvest season, and the samples obtained at this time were taken as the final state of soil properties. In each plot, 1 soil bulk density (BD) sample [] was taken, and 5 soil samples were collected from the top 0–20 cm surface layer and mixed to form a representative sample. Therefore, a total of 20 mixed topsoil samples were collected in late October 2021, together with 20 soil bulk density samples. The obtained soil samples were stored at 4 °C until they were brought to the laboratory for immediate processing. The soil sample at this time was subdivided into two subsamples: the first subsample was air-dried, pulverized, and passed through a 1 mm sieve for the determination of soil salinity expressed as EC1:5 [], pH1:5, soil organic matter (SOM), total nitrogen (TN), and available phosphorus (AP) []; the second subsample was sieved with a 2 mm mesh for downstream processing, including microbial genomic DNA extraction, amplification, and pyrosequencing.

Table 2.
Physicochemical characteristics of saline–sodic soil.
2.4. Bulk Soil DNA Extraction, PCR Amplification, and Sequencing
According to the manufacturer’s instructions, the total DNA of soil microorganisms was extracted using the OMEGA Soil DNA Kit (D5625 01) (Omega Bio-Tek, Norcross, GA, USA), and the molecular size was determined via 0.8% agarose gel electrophoresis. A luminometer was used to quantify DNA. In this experiment, the highly variable V3V4 region of the bacterial 16S rRNA gene with a length of about 468 bp was selected for sequencing. PCR amplification selects bacterial 16S rDNAV3V4 region-specific primers, 338F (5′ barcode+ACTCCTACGGGAGGCAGCA 3′) and 806R (GGACTACHVGGGTWTCTAAT 3′). After PCR, the products were further purified and pooled in equimolar concentrations to use the sequencing program on the platform. For eligible libraries, we performed 2 × 250 bp paired-end sequencing on an Illumina NovaSeq machine using the NovaSeq 6000 SP Reagent Kit (500 cycles). Microbiome bioinformatics analysis was performed using QIIME 2 2019.4 with slight modifications according to the official tutorials (https://docs.qiime2.org/2022.8/ (accessed on 31 August 2022)). Taxonomy was assigned to ASVs using the classify-Sckit Learn naïve Bayes taxonomy classifier in the feature-classifier plugin [] against the Silva_132 99% OTU reference sequences.
2.5. Statistical Analysis
We compared the yield, soil chemistry, and microbial properties between treatments via one-way ANOVA and least significant difference (LSD) tests using R (v.4.1.3), analyzed their correlations via Pearson correlation analysis, and plotted heatmaps using the R ggcor package (v.0.9.8.1). To identify variations in soil bacterial communities under various treatments, principal coordinate analysis (PCoA) was carried out using the R “vegan” package (v2.5-7). The link between environmental conditions and bacterial community structure was investigated using Mantel tests and redundancy analysis (RDA). We used the R “microeco” package (v.0.11.0) for FAPROTAX analysis (v.1.2.4) [], with nonparametric tests and linear discriminant analysis for biomarker discovery []; based on the results of the previous step, we used the top 200 rich taxa and 50 differential features in the tree to draw a cladogram.
3. Results
3.1. Physical and Chemical Properties
Compared with that in CK, the content of SOM in organic modifier treatments increased by 62–87% (Table 3). The contents of AP, AK, and TN in organically modified treatments were significantly higher than those in CK; the AK of LHA was significantly higher than that of FM and SR by 29% and 43%, respectively, while the AP of LHA was significantly higher than that of SR by 30%. The BD of the organic amendment treatments decreased by 4–7%, and the pH and EC1:5 values of the soil surface were affected differently by the addition of amendments. The pH and EC values of LHA were significantly lower than those of CK, by 1.36 units and 0.2 mS/cm, respectively. Correlation analysis showed that nutrient content was significantly positively correlated with organic matter content and significantly negatively correlated with pH, EC1:5, and BD (Figure 1).

Table 3.
Effects of different organic amendment materials on soil physical and chemical properties.
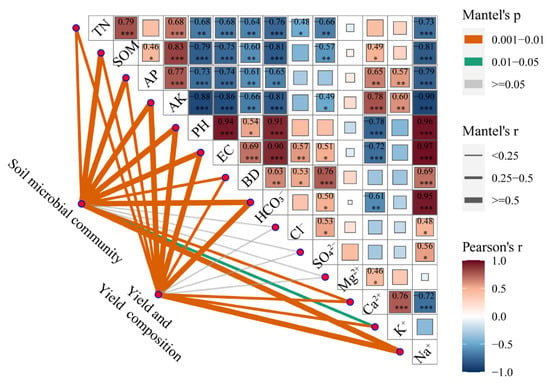
Figure 1.
Mantel analysis of the soil microbial structure, grain yield, and yield components in response to environmental factors. The labels indicate significant levels tested by Pearson correlation (* p < 0.05, ** p < 0.01, *** p < 0.001).
3.2. Yield and Yield Components
Organic amendment materials had a significant impact on yield and yield components (Table 4). The grain yields of LHA, SR, and FM were significantly higher than that of CK, by 34%, 21%, and 31%, respectively. Among the yield components, kernel numbers per row for LHA, SR, and FM were significantly higher than that for CK, by 15%, 12%, and 14%, respectively. The 1000-grain weights for LHA, SR, and FM were significantly higher than that for CK, by 6%, 2%, and 6%, respectively. LHA, SR, and FM ear numbers were significantly higher than that for CK, by 6%, 5%, and 7%, respectively. There was no significant difference in the number of spike rows. Nutrient content and pH, EC, BD, Na+, K+, Ca2+, and HCO3- concentrations significantly affected yield and yield components (Figure 1).

Table 4.
Effects of different organic amendment materials on soil physical and chemical properties.
3.3. Microbial Composition: Basic Composition and Specific Species
Illumina sequencing of 16S rRNA gene amplicons and sparseness of the same sequencing depth were used to further investigate changes in bacterial community composition due to the application of different organic materials. A total of 1,380,994 high-quality bacterial sequences were obtained. At the OTU level, there were 622 OTUs shared across all samples, accounting for 38% of overall abundance, with a lesser unique OTU of 4.8 for lignite humic acid amendment (Figure S1). Meanwhile, the main bacterial phyla in this study were Proteobacteria (29.2%), Actinobacteria (25.8%), Acidobacteria (15.8%), Chloroflexi (13.2%), Firmicutes (5.3%), and Bacteroidetes (3.4%) in all soil samples (Figure S2).
Variations in the dominant bacterial phyla between treatments were evident. The proportion of Acidobacteria in the SR treatment was significantly increased to 22%, while the corresponding proportion in FM and CK was significantly decreased to 13% and 11%, respectively; however, the proportion of Firmicutes in the SR treatment was significantly decreased to 3% (Figure 2a, Table S1). At the genus level, organic material treatments significantly increased the abundances of Subgroup_6, Skermanella, Micrococcaceae RB41, Rubrobacter, and JG30-KF-CM45 (Figure 2b). Furthermore, linear discriminant analysis indicated that the number of genus biomarkers varied between treatments (Figure 3), with RB41 in SR; Skermanella, Rubrobacter, Blastococcus, and JG30−KF−CM45 in FM; MB−A2−108 and MND1 in LHA; and Propionibacteriaceae and AKYG1722 in the CK treatment.
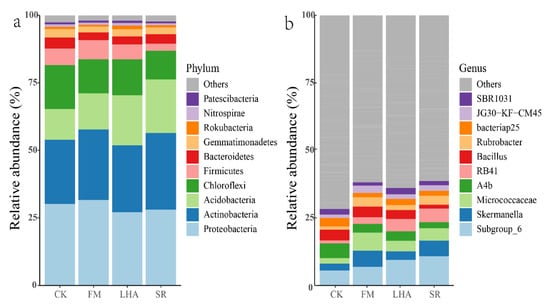
Figure 2.
The relative abundances of the top 10 dominant phyla (a) and the top 10 dominant genera (b) in bulk soil.
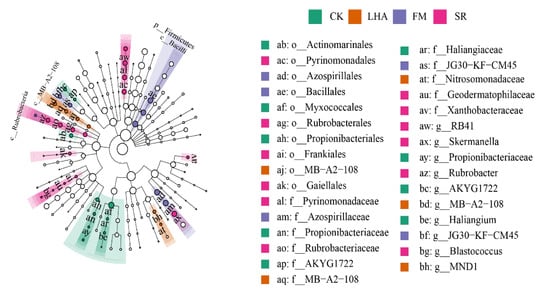
Figure 3.
Linear discriminant analysis effect size (LEfSe) analysis to identify differences in abundant taxa between different treatment samples.
3.4. Soil Bacterial Diversity and Its Influencing Factors
To investigate whether soil microbes are involved in plant growth and affect changes in soil properties after organic amendment application, soil bacterial communities were analyzed by high-throughput sequencing of 16S rRNA gene amplicons. Among the alpha diversity indexes, the Shannon index, Chao1, ACE diversity, and the richness index in organic amendment treatments were significantly higher than those in CK (p < 0.05) (Figure 4). Furthermore, the LHA, FM, and SR treatments exhibited higher degrees of variation in their rarefaction curves than did the CK treatment (Figure S3).
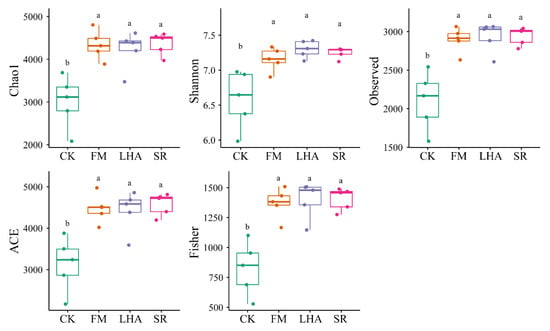
Figure 4.
Bacterial alpha diversity in the soils in the different treatments. Different letters represent significant difference (p < 0.05) by LSD test.
PCoA was used to analyze the bacterial community structure in soil samples based on the bacterial OTU taxonomy. According to the PCoA results, the substrate distance between bacterial communities was significantly affected by organic amendments. The PCoA1 and PCoA2 principal components accounted for 25% and 11% of species variation, respectively (Figure 5a). The distribution of OTUs in the samples was affected by organic amendment type. The bacterial communities in the samples were divided into four groups, namely, CK, FM, LHA, and SR (p < 0.05) (Table S2).
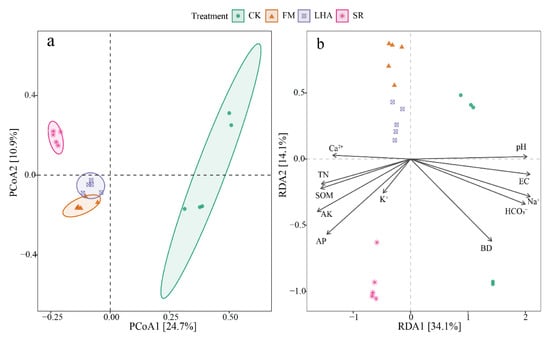
Figure 5.
Principal coordination analysis (PCoA) (a) of the bacterial community structure and redundancy analysis (RDA) (b) of the relationship between environmental factors and bacterial community based on Bray–Curtis dissimilarity.
The correlation analysis of soil environmental factors and microbial community structure showed that the soil TN, SOM, AP, AK, pH, EC, BD, SAR, HCO3−, Ca2+, K+, and Na+ concentrations had a significant effect on bacterial community structure (p < 0.05) (Figure 1). RDA further revealed the relationship between the soil bacterial community structure and environmental variables. The results of RDA analysis showed that the first and second ranking axes explained 27.7% and 11.8% of the microbial community structure variation, respectively, and physicochemical factors had different effects on soil microbial community structure (Figure 5b). With significant effects, pH, EC, BD, and HCO3− were positively correlated with the microbial community structure of the control soil, while TN, SOM, AP, AK, Ca2+, and K+ were positively correlated with the soil microbial community structure in the organic amendment treatments.
3.5. Relationship between Functional Characteristics of Soil Bacteria and Yield
Elemental cycling analysis (FAPROTAX) was used to analyze the different functional characteristics of the bacterial communities in this study. The FAPROTAX annotations indicated that three main functional groups (average relative abundance above 0.5%) were involved in carbon cycling, namely, the aerobic_chemoheterotrophy, chemoheterotrophy, and anaerobic_chemoheterotrophy groups. The abundance of the aerobic_chemoheterotrophy and chemoheterotrophy groups in FM and SR was significantly higher than that in the CK treatment, and the abundance of the aerobic_chemoheterotrophy and chemoheterotrophy groups in FM was significantly higher than that in the LHA treatment (Figure 6a). The addition of organic matter had no significant effect on the abundance of the anaerobic_chemoheterotrophy group. In addition, the main functional groups involved in nitrogen cycling included the aerobic_ammonia_oxidation, aerobic_nitrite_oxidation, nitrate_reduction, nitrification, and nitrogen_fixation groups. Compared with CK, LHA and SR significantly increased the relative abundance of the aerobic_ammonia_oxidation and nitrification groups in soil, and the relative abundance of the nitrate_reduction and nitrogen_fixation groups in the FM treatment was significantly higher than that in other treatments. Correlation analysis of the yield and yield components with the abundance of carbon and nitrogen cycle groups showed that the yield and kernel number per row were significantly positively correlated with the relative abundance of the acrobic_chemoheterotrophy, nitrification, and aerobic_ammonia_oxidation groups (Figure 6b). In addition, the bacterial abundance of the chemoheterotrophy group was positively correlated with the kernel number per row.
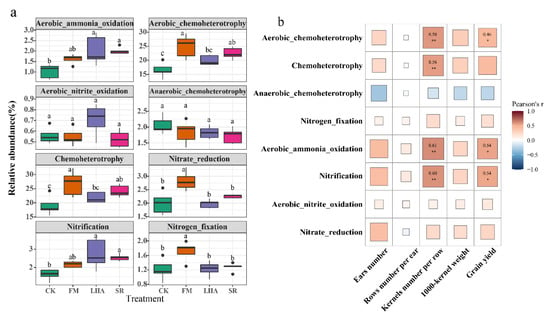
Figure 6.
Functional prediction of bacterial groups in the different treatments using FAPROTAX. Different letters indicate significant differences between the different treatments at the p < 0.05 level (a); Pearson correlation analysis of the functionally predicted abundance of bacterial communities with yield and yield components (b). The labels indicate significant levels tested by Pearson correlation (* p < 0.05, ** p < 0.01).
4. Discussion
Soil salinity and alkalinity are land degradation processes that severely alter soil quality and, thus, plant productivity. Amendment with organic matter can increase resistance to soil degradation []. The sequestration of farmland SOC mainly depends on the dynamic balance between the input of exogenous organic materials, such as organic fertilizers and field biological residues, and the loss of soil organic matter in the form of mineralization and leaching [,]. The input of exogenous organic matter is the SOC content, and the properties of soil organic matter also profoundly affect the stability of soil organic matter [,]. The application of LHA, FM, and SR treatments significantly increased soil SOC content when compared to CK, according to the findings of our two-year short-term organic improvement trial. Further, the total carbon input of the FM treatment was 6.06 Cton/ha higher than those of the other treatments. However, the results showed that there was no significant difference in the increase in organic matter, which may be due to the fact that FM provided a large amount of active substances to the soil, which was beneficial to the mineralization of microorganisms, and most of them were decomposed []. This was also reflected in the increase in carbon-cycle-related microorganisms (aerobic chemical heterotrophy, chemical heterotrophy) in the FM treatment (Figure 6a). LHA delivered 2.26 Cton/ha. LHA is rich in aliphatic compounds and has a slower rate of microbial decomposition; therefore, the soil organic matter carbon content was high []. The application of organic materials can reduce soil bulk density and increase soil porosity [], which was also confirmed in this study. The soil bulk density of treated soils was lower than that of the control soil. Soil structure improvement increases the hydraulic conductivity of saturated topsoil [], which reduces EC and accelerates Na+ leaching, resulting in lower pH [,]. This explains the lower EC level in organic-material-modified soil than in CK soil. Compared with other organic materials, lignite humic acid decreased soil pH more rapidly and increased AK and AP, which was due to the higher content of available nutrients in lignite humic acid. Seedling emergence is extremely sensitive to salinity, which may be the main reason for the decline in yield in unreclaimed saline fields []. In the present study, the number of ears in the control was significantly lower than those in amendment treatments, because the soil conditions in the root zone were improved by the organic material before sowing, which was beneficial to the emergence and growth of maize []. At the same time, the nutrients produced by the decomposition of organic matter enhance crop growth, which, in turn, increases crop yield, especially in nutrient-deficient saline–sodic soils [].
Compared with those under CK, the diversity and richness under organic material amendment treatments significantly increased. This is due to the increased supply of soil organic carbon in cow dung, residue, and humic acid, which is the main source of energy and material for microorganisms []. However, different organic matter components may have different effects on soil microbes. Composted cow dung can provide more unstable components of organic matter []. Residue is rich in cellulose polysaccharides and lignin derivatives [], while lignite humic acid is rich in aliphatic compounds []. This leads to differences in the selective utilization and metabolism of organic matter by microorganisms [], resulting in microbial structural diversity and differences in microbial survival in modified soils—a key factor affecting microbial survival in amended soils. As further evidence that this also affected the relative abundance of bacteria at the phylum and genus levels, Acidobacteria were significantly increased in the SR and LHA treatments, which may have increased the conversion efficiency of carbon and nitrogen in the soil [], while organic amendments increased the abundance of Skermanella, Micrococcaceae, Rubrobacter, and other genera to improve the crop growth environment, which was beneficial to the growth of maize []. For example, Skermanella and Micrococcaceae can inhibit the growth of phytopathogens and enhance the salt tolerance of plants [,]. Micrococcaceae promote plant growth through phosphate solubilization, biological control activity, auxin production, ACC deaminase activity, and siderophore production [].
Organic amendments are able to alter microbial community structure, which significantly increases microbial diversity (Shannon, richness, and phylogenetic diversity) and varies with the organic amendment type, microbiota, and soil properties [,]. This study also showed that physicochemical factors have a significant effect on soil microbial community structure. pH, EC, and BD are the main shaping factors of microbial community structure in unimproved saline–sodic soils. After reclamation, organic matter nutrients and Ca2+ have significant effects on the soil microbial community structure in soils reclaimed with organic matter. These results suggest that organic amendments changed the soil environment and, thus, the soil community structure [,]. The types of applied amendments are closely related to the microbial community structure and function. Compared with CK, organic amendments increased the relative abundance of microorganisms related to soil carbon and nitrogen cycles and promoted the decomposition of carbon and nitrogen, which was related to the dynamics of microbial communities [] or the increase in microbial activity []. Lignite humic acid could reduce the carbon-related microbial abundance compared to other treatments, which was consistent with []; for nitrogen and carbon cycling, lignite humic acid amendment can increase almost all gene families involved in nitrogen cycling, except nirA and hao, and reduce the gene abundance of the carbohydrate metabolism module. In addition to increasing the bacterial abundance of saline–sodic soils, organic materials can improve soil aggregate structure [], improve soil properties, and increase crop yields. Shu, et al. [] showed that the impact of microbial function on crop yield cannot be ignored. On the one hand, organic materials can directly increase soil nutrients and directly promote plant growth; on the other hand, organic amendments may lead to higher relative abundance and diversity of carbon–nitrogen cycling microorganisms []. This can improve the nitrogen supply capacity of the soil, promote the kernel number per row at the filling stage, and thus increase crop yield [].
5. Conclusions
Our study showed that the application of organic amendments in saline–sodic soil with low organic matter content improved the soil properties; changed the microbial composition, structure, and function; and improved crop yield. Specifically, compared with the control, reclamation with organic amendments increased the organic matter content and nutrient content of saline–sodic land; reduced the soil bulk density, surface soil EC1:5, and pH; and then promoted crop growth and increased crop yield. Organic amendments significantly affected the soil microbial community structure and increased soil microbial richness and functional changes. For example, crop residue incorporation increased the abundance of Acidobacteria. Further analysis by FAPROTAX showed that lignite amendments increased the microbial abundance of nitrogen cycle groups, decreased the microbial abundance of carbon cycle groups, increased soil nutrients, decreased soil carbon mineralization, and increased crop yield. In summary, LHA can not only enhance the physicochemical properties of saline–sodic soil, but also reduce the abundance of carbon-cycle-related microorganisms and increase the soil carbon sequestration potential.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agronomy12102294/s1, Figure S1: Soil microbe Venn diagram; Figure S2: Pie chart of mean abundance at different phylum levels across all treatments; Figure S3: Bacterial diversity sparse curve; Table S1: Relative abundance of dominant bacteria in soil samples with different treatments; Table S2: Results of Adonis test to analyze the effects of different treatments on soil bacterial community compositions.
Author Contributions
Conceptualization: Z.W.; Data Curation: L.G.; Formal Analysis: L.G.; Investigation: Z.N., J.Z. and S.Z.; Project Admtainistration: F.A.; Visualization: L.G. and L.Z.; Writing—Original Draft Preparation: L.G. and T.T.; Funding Acquisition: Z.W. and F.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Strategic Priority Research Program of the CAS [XDA28010403], the National Natural Science Foundation of China [Nos. 41971066], the Key Laboratory Foundation of Mollisols Agroecology [2020ZKHT-03], the Science–Technology Development Initiative of Jilin Province [20200402005NC].
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
There are no conflicts of interest related to this research.
References
- Haj-Amor, Z.; Araya, T.; Kim, D.-G.; Bouri, S.; Lee, J.; Ghiloufi, W.; Yang, Y.; Kang, H.; Jhariya, M.K.; Banerjee, A.; et al. Soil salinity and its associated effects on soil microorganisms, greenhouse gas emissions, crop yield, biodiversity and desertification: A review. Sci. Total Environ. 2022, 843, 156946. [Google Scholar] [CrossRef] [PubMed]
- Horn, D.J.V.; Okie, J.G.; Buelow, H.N.; Gooseff, M.N.; Barrett, J.E.; Takacs-Vesbach, C.D.; Kostka, J.E. Soil Microbial Responses to Increased Moisture and Organic Resources along a Salinity Gradient in a Polar Desert. Appl. Environ. Microbiol. 2014, 80, 3034–3043. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Liu, Y.; Ferreira, J.F.S.; Wang, M.; Na, J.; Huang, J.; Liang, Z. Long-term combined effects of tillage and rice cultivation with phosphogypsum or farmyard manure on the concentration of salts, minerals, and heavy metals of saline-sodic paddy fields in Northeast China. Soil Tillage Res. 2022, 215, 105222. [Google Scholar] [CrossRef]
- Zhu, H.; Yang, J.; Yao, R.; Wang, X.; Xie, W.; Zhu, W.; Liu, X.; Cao, Y.; Tao, J. Interactive effects of soil amendments (biochar and gypsum) and salinity on ammonia volatilization in coastal saline soil. CATENA 2020, 190, 104527. [Google Scholar] [CrossRef]
- Kumar, C.; Ramawat, N.; Verma, A.K. Organic fertigation system in saline-sodic soils: A new paradigm for the restoration of soil health. Agron. J. 2022, 114, 317–330. [Google Scholar] [CrossRef]
- Wu, Y.; Li, Y.; Zheng, C.; Zhang, Y.; Sun, Z. Organic amendment application influence soil organism abundance in saline alkali soil. Eur. J. Soil Biol. 2013, 54, 32–40. [Google Scholar] [CrossRef]
- Meng, Q.; Ma, X.; Zhang, J.; Yu, Z. The long-term effects of cattle manure application to agricultural soils as a natural-based solution to combat salinization. CATENA 2019, 175, 193–202. [Google Scholar] [CrossRef]
- Medina Litardo, R.C.; García Bendezú, S.J.; Carrillo Zenteno, M.D.; Pérez-Almeida, I.B.; Parismoreno, L.L.; Lombeida García, E.D. Effect of mineral and organic amendments on rice growth and yield in saline soils. J. Saudi Soc. Agric. Sci. 2022, 21, 29–37. [Google Scholar] [CrossRef]
- Zhang, W.; Zhao, Y.; Wang, S.; Li, Y.; Liu, J.; Zhuo, Y.; Zhang, W. Combined Application of Flue Gas Desulfurization Gypsum and Straw Pellets to Ameliorate Sodicity, Nutrient Content, and Aggregate Stability of Sodic Soil. J. Soil Sci. Plant Nutr. 2021, 21, 1806–1816. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, H.; Liu, X.; Chen, Y.; Lu, Y.; Shen, M.; Dang, K.; Zhao, Y.; Dong, Y.; Li, Q.; et al. Organic fertilizer enhances rice growth in severe saline–alkali soil by increasing soil bacterial diversity. Soil Use Manag. 2022, 38, 964–977. [Google Scholar] [CrossRef]
- Gregorich, E.G.; Janzen, H.; Ellert, B.H.; Helgason, B.L.; Qian, B.; Zebarth, B.J.; Angers, D.A.; Beyaert, R.P.; Drury, C.F.; Duguid, S.D.; et al. Litter decay controlled by temperature, not soil properties, affecting future soil carbon. Glob. Chang. Biol. 2017, 23, 1725–1734. [Google Scholar] [CrossRef] [PubMed]
- Horta, C.; Roboredo, M.; Carneiro, J.P.; Duarte, A.C.; Torrent, J.; Sharpley, A. Organic amendments as a source of phosphorus: Agronomic and environmental impact of different animal manures applied to an acid soil. Arch. Agron. Soil Sci. 2018, 64, 257–271. [Google Scholar] [CrossRef]
- Wood, J.D.; VanderZaag, A.C.; Wagner-Riddle, C.; Smith, E.L.; Gordon, R.J. Gas emissions from liquid dairy manure: Complete versus partial storage emptying. Nutr. Cycl. Agroecosystems 2014, 99, 95–105. [Google Scholar] [CrossRef]
- Chen, Z.; Li, Y.; Zhang, X.; Xiong, Y.; Huang, Q.; Jin, S.; Shijun, S.; Daocai, C.; Huang, G. Effects of lignite bioorganic product on sunflower growth, water and nitrogen productivity in saline-sodic farmlands at Northwest China. Agric. Water Manag. 2022, 271, 107806. [Google Scholar] [CrossRef]
- Manasa, M.R.K.; Katukuri, N.R.; Darveekaran Nair, S.S.; Haojie, Y.; Yang, Z.; Guo, R.b. Role of biochar and organic substrates in enhancing the functional characteristics and microbial community in a saline soil. J. Environ. Manag. 2020, 269, 110737. [Google Scholar] [CrossRef] [PubMed]
- Jacoby, R.; Peukert, M.; Succurro, A.; Koprivova, A.; Kopriva, S. The role of soil microorganisms in plant mineral nutrition—current knowledge and future directions. Front. Plant Sci. 2017, 8, 1617. [Google Scholar] [CrossRef] [PubMed]
- Sahin, U.; Eroğlu, S.; Sahin, F. Microbial application with gypsum increases the saturated hydraulic conductivity of saline–sodic soils. Appl. Soil Ecol. 2011, 48, 247–250. [Google Scholar] [CrossRef]
- Tian, S.; Zhu, B.; Yin, R.; Wang, M.; Jiang, Y.; Zhang, C.; Li, D.; Chen, X.; Kardol, P.; Liu, M. Organic fertilization promotes crop productivity through changes in soil aggregation. Soil Biol. Biochem. 2022, 165, 108533. [Google Scholar] [CrossRef]
- Chang, C.; Tian, L.; Tian, Z.; McLaughlin, N.; Tian, C. Change of soil microorganism communities under saline-sodic land degradation on the Songnen Plain in northeast China#. J. Plant Nutr. Soil Sci. 2022, 185, 297–307. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, S.; Ma, R.; Chen, M.; Wei, W.; Ding, X. Carbon sequestration under different organic amendments in saline-alkaline soils. CATENA 2021, 196, 104882. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, H.; Lv, J. Evaluation of the applicability of organic amendments from microbially driven carbon and nitrogen transformations. Sci. Total Environ. 2022, 817, 153020. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Zhang, X.-X.; Guo, X.; Wang, D.; Chu, H. Bacterial diversity in soils subjected to long-term chemical fertilization can be more stably maintained with the addition of livestock manure than wheat straw. Soil Biol. Biochem. 2015, 88, 9–18. [Google Scholar] [CrossRef]
- Yang, Y.; Li, G.; Min, K.; Liu, T.; Li, C.; Xu, J.; Hu, F.; Li, H. The potential role of fertilizer-derived exogenous bacteria on soil bacterial community assemblage and network formation. Chemosphere 2022, 287, 132338. [Google Scholar] [CrossRef] [PubMed]
- Chahal, S.S.; Choudhary, O.P.; Mavi, M.S. Organic amendments decomposability influences microbial activity in saline soils. Arch. Agron. Soil Sci. 2017, 63, 1875–1888. [Google Scholar] [CrossRef]
- Blake, G.R.; Hartge, K.H. Bulk Density. In Methods of Soil Analysis; Soil Science Society of America: Madison, WI, USA, 1986; pp. 363–375. [Google Scholar]
- Chi, C.-M.; Wang, Z.-C. Characterizing Salt-Affected Soils of Songnen Plain Using Saturated Paste and 1:5 Soil-to-Water Extraction Methods. Arid. Land Res. Manag. 2010, 24, 1–11. [Google Scholar] [CrossRef]
- Rayment, G.E.; Lyons, D.J. Soil Chemical Methods: Australasia; CSIRO Publishing: Clayton, Australia, 2011; Volume 3. [Google Scholar]
- Bokulich, N.A.; Kaehler, B.D.; Rideout, J.R.; Dillon, M.; Bolyen, E.; Knight, R.; Huttley, G.A.; Caporaso, J.G. Optimizing taxonomic classification of marker-gene amplicon sequences with QIIME 2’s q2-feature-classifier plugin. Microbiome 2018, 6, 90. [Google Scholar] [CrossRef]
- Liu, C.; Cui, Y.; Li, X.; Yao, M. microeco: An R package for data mining in microbial community ecology. FEMS Microbiol. Ecol. 2020, 97, fiaa255. [Google Scholar] [CrossRef]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef]
- Leogrande, R.; Vitti, C. Use of organic amendments to reclaim saline and sodic soils: A review. Arid. Land Res. Manag. 2019, 33, 1–21. [Google Scholar] [CrossRef]
- Jingkuan, W.; Yingde, X.U.; Fan, D.; Xiaodan, G.A.O.; Shuangyi, L.I.; Liangjie, S.U.N.; Tingting, A.N.; Jiubo, P.E.I.; Ming, L.I.; Yang, W.; et al. Process of Plant Residue Transforming into Soil Organic Matter and Mechanism of its Stabilization: A Review. Acta Pedol. Sin. 2019, 56, 528–540. [Google Scholar]
- De Mastro, F.; Cocozza, C.; Traversa, A.; Savy, D.; Abdelrahman, H.M.; Brunetti, G. Influence of crop rotation, tillage and fertilization on chemical and spectroscopic characteristics of humic acids. PLoS ONE 2019, 14, e0219099. [Google Scholar] [CrossRef] [PubMed]
- Abdelrahman, H.M.; Olk, D.C.; Dinnes, D.; Ventrella, D.; Miano, T.; Cocozza, C. Occurrence and abundance of carbohydrates and amino compounds in sequentially extracted labile soil organic matter fractions. J. Soils Sediments 2016, 16, 2375–2384. [Google Scholar] [CrossRef]
- Abdelrahman, H.; Cocozza, C.; Olk, D.C.; Ventrella, D.; Montemurro, F.; Miano, T. Changes in Labile Fractions of Soil Organic Matter During the Conversion to Organic Farming. J. Soil Sci. Plant Nutr. 2020, 20, 1019–1028. [Google Scholar] [CrossRef]
- Morvan, T.; Nicolardot, B. Role of organic fractions on C decomposition and N mineralization of animal wastes in soil. Biol. Fertil. Soils 2009, 45, 477–486. [Google Scholar] [CrossRef]
- Piccolo, A.; Spaccini, R.; Nieder, R.; Richter, J. Sequestration of a Biologically Labile Organic Carbon in Soils by Humified Organic Matter. Clim. Chang. 2004, 67, 329–343. [Google Scholar] [CrossRef]
- Gonçalo Filho, F.; da Silva Dias, N.; Suddarth, S.R.P.; Ferreira, J.F.S.; Anderson, R.G.; dos Santos Fernandes, C.; de Lira, R.B.; Neto, M.F.; Cosme, C.R. Reclaiming Tropical Saline-Sodic Soils with Gypsum and Cow Manure. Water 2020, 12, 57. [Google Scholar] [CrossRef]
- Song, X.; Sun, R.; Chen, W.; & Wang, M. Effects of surface straw mulching and buried straw layer on soil water content and salinity dynamics in saline soils. Can. J. Soil Sci. 2020, 100, 58–68. [Google Scholar] [CrossRef]
- Nan, J.; Chen, X.; Chen, C.; Lashari, M.S.; Deng, J.; Du, Z. Impact of flue gas desulfurization gypsum and lignite humic acid application on soil organic matter and physical properties of a saline-sodic farmland soil in Eastern China. J. Soils Sediments 2016, 16, 2175–2185. [Google Scholar] [CrossRef]
- Chaganti, V.N.; Crohn, D.M. Evaluating the relative contribution of physiochemical and biological factors in ameliorating a saline–sodic soil amended with composts and biochar and leached with reclaimed water. Geoderma 2015, 259, 45–55. [Google Scholar] [CrossRef]
- Maas, E.; Hoffman, G.; Chaba, G.; Poss, J.; Shannon, M. Salt sensitivity of corn at various growth stages. Irrig. Sci. 1983, 4, 45–57. [Google Scholar] [CrossRef]
- Fouladidorhani, M.; Shayannejad, M.; Shariatmadari, H.; Mosaddeghi, M.R.; Arthur, E. Biochar, manure, and super absorbent increased wheat yields and salt redistribution in a saline-sodic soil. Agron. J. 2020, 112, 5193–5205. [Google Scholar] [CrossRef]
- Srivastava, P.K.; Gupta, M.; Shikha; Singh, N.; Tewari, S.K. Amelioration of Sodic Soil for Wheat Cultivation Using Bioaugmented Organic Soil Amendment. Land Degrad. Dev. 2016, 27, 1245–1254. [Google Scholar] [CrossRef]
- Molina-Herrera, S.; Romanyà, J. Synergistic and antagonistic interactions among organic amendments of contrasted stability, nutrient availability and soil organic matter in the regulation of C mineralisation. Eur. J. Soil Biol. 2015, 70, 118–125. [Google Scholar] [CrossRef]
- Wu, Y.; Li, Y.; Zhang, Y.; Bi, Y.; Sun, Z. Responses of Saline Soil Properties and Cotton Growth to Different Organic Amendments. Pedosphere 2018, 28, 521–529. [Google Scholar] [CrossRef]
- Chen, X.; Wu, J.; Opoku-Kwanowaa, Y. Effects of Returning Granular Corn Straw on Soil Humus Composition and Humic Acid Structure Characteristics in Saline-Alkali Soil. Sustainability 2020, 12, 1005. [Google Scholar] [CrossRef]
- Raguraj, S.; Kasim, S.; Jaafar, N.M.; Nazli, M.H.; Amali, R.K.A. A comparative study of tea waste derived humic-like substances with lignite-derived humic substances on chemical composition, spectroscopic properties and biological activity. Environ. Sci. Pollut. Res. 2022, 29, 60631–60640. [Google Scholar] [CrossRef]
- Li, L.; Xu, M.; Eyakub Ali, M.; Zhang, W.; Duan, Y.; Li, D. Factors affecting soil microbial biomass and functional diversity with the application of organic amendments in three contrasting cropland soils during a field experiment. PLoS ONE 2018, 13, e0203812. [Google Scholar] [CrossRef]
- Kielak, A.M.; Barreto, C.C.; Kowalchuk, G.A.; Van Veen, J.A.; Kuramae, E.E. The ecology of Acidobacteria: Moving beyond genes and genomes. Front. Microbiol. 2016, 7, 744. [Google Scholar] [CrossRef]
- Liu, M.; Wang, C.; Wang, F.; Xie, Y. Vermicompost and humic fertilizer improve coastal saline soil by regulating soil aggregates and the bacterial community. Arch. Agron. Soil Sci. 2019, 65, 281–293. [Google Scholar] [CrossRef]
- Panneerselvam, P.; Kumar, U.; Sahu, S.; Mohapatra, S.D.; Dangar, T.K.; Parameswaran, C.; Jahan, A.; Senapati, A.; Govindharaj, G.P.P. Larvicidal potential of Skermanella sp. against rice leaf folder (Cnaphalocrosis medinalis Guenee) and pink stem borer (Sesamia inferens Walker). J. Invertebr. Pathol. 2018, 157, 74–79. [Google Scholar] [CrossRef]
- Velázquez-Becerra, C.; Macías-Rodríguez, L.I.; López-Bucio, J.; Flores-Cortez, I.; Santoyo, G.; Hernández-Soberano, C.; Valencia-Cantero, E. The rhizobacterium Arthrobacter agilis produces dimethylhexadecylamine, a compound that inhibits growth of phytopathogenic fungi in vitro. Protoplasma 2013, 250, 1251–1262. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Fan, M.; Wang, E.; Chen, W.; Wei, G. Interactions of plant growth-promoting rhizobacteria and soil factors in two leguminous plants. Appl. Microbiol. Biotechnol. 2017, 101, 8485–8497. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Liu, C.; Song, C.; Wang, X.; Ma, X.; Gao, J.; Gao, S.; Wang, L. Linking soil organic carbon mineralization with soil microbial and substrate properties under warming in permafrost peatlands of Northeastern China. Catena 2021, 203, 105348. [Google Scholar] [CrossRef]
- Condron, L.; Stark, C.; O’Callaghan, M.; Clinton, P.; Huang, Z. The Role of Microbial Communities in the Formation and Decomposition of Soil Organic Matter. In Soil Microbiology and Sustainable Crop Production; Dixon, G.R., Tilston, E.L., Eds.; Springer: Dordrecht, The Netherlands, 2010; pp. 81–118. [Google Scholar]
- Yang, Y.; Liu, H.; Dai, Y.; Tian, H.; Zhou, W.; Lv, J. Soil organic carbon transformation and dynamics of microorganisms under different organic amendments. Sci. Total Environ. 2021, 750, 141719. [Google Scholar] [CrossRef]
- Zhao, S.; Qiu, S.; Xu, X.; Ciampitti, I.A.; Zhang, S.; He, P. Change in straw decomposition rate and soil microbial community composition after straw addition in different long-term fertilization soils. Appl. Soil Ecol. 2019, 138, 123–133. [Google Scholar] [CrossRef]
- Farrell, M.; Kuhn, T.K.; Macdonald, L.M.; Maddern, T.M.; Murphy, D.V.; Hall, P.A.; Singh, B.P.; Baumann, K.; Krull, E.S.; Baldock, J.A. Microbial utilisation of biochar-derived carbon. Sci. Total Environ. 2013, 465, 288–297. [Google Scholar] [CrossRef]
- Chen, Z.; Huang, G.; Li, Y.; Zhang, X.; Xiong, Y.; Huang, Q.; Jin, S. Effects of the lignite bioorganic fertilizer on greenhouse gas emissions and pathways of nitrogen and carbon cycling in saline-sodic farmlands at Northwest China. J. Clean. Prod. 2022, 334, 130080. [Google Scholar] [CrossRef]
- Luo, S.; Wang, S.; Tian, L.; Shi, S.; Xu, S.; Yang, F.; Li, X.; Wang, Z.; Tian, C. Aggregate-related changes in soil microbial communities under different ameliorant applications in saline-sodic soils. Geoderma 2018, 329, 108–117. [Google Scholar] [CrossRef]
- Shu, X.; He, J.; Zhou, Z.; Xia, L.; Hu, Y.; Zhang, Y.; Zhang, Y.; Luo, Y.; Chu, H.; Liu, W.; et al. Organic amendments enhance soil microbial diversity, microbial functionality and crop yields: A meta-analysis. Sci. Total Environ. 2022, 829, 154627. [Google Scholar] [CrossRef]
- Harindintwali, J.D.; Zhou, J.; Muhoza, B.; Wang, F.; Herzberger, A.; Yu, X. Integrated eco-strategies towards sustainable carbon and nitrogen cycling in agriculture. J. Environ. Manag. 2021, 293, 112856. [Google Scholar] [CrossRef]
- Fan, K.; Delgado-Baquerizo, M.; Guo, X.; Wang, D.; Zhu, Y.-g.; Chu, H. Biodiversity of key-stone phylotypes determines crop production in a 4-decade fertilization experiment. ISME J. 2021, 15, 550–561. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).