Reducing the Halotolerance Gap between Sensitive and Resistant Tomato by Spraying Melatonin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Culturing
2.2. Treatment of Experiment
2.3. Experimental Method
2.4. Data Analysis
3. Results
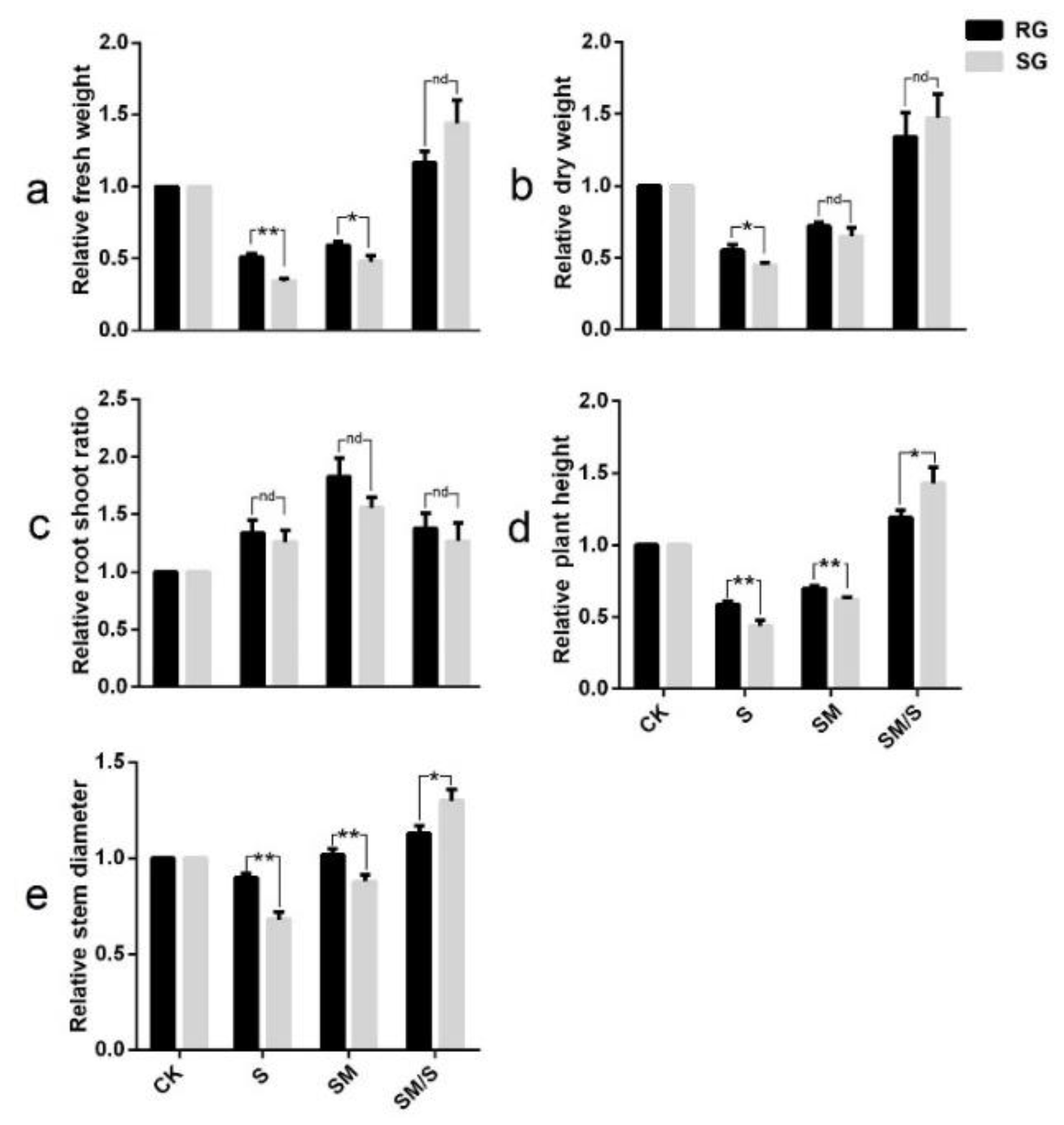
3.1. The Effect of Exogenous Melatonin on the Growth of Tomato Seedlings under Normal Conditions and Salt Stress
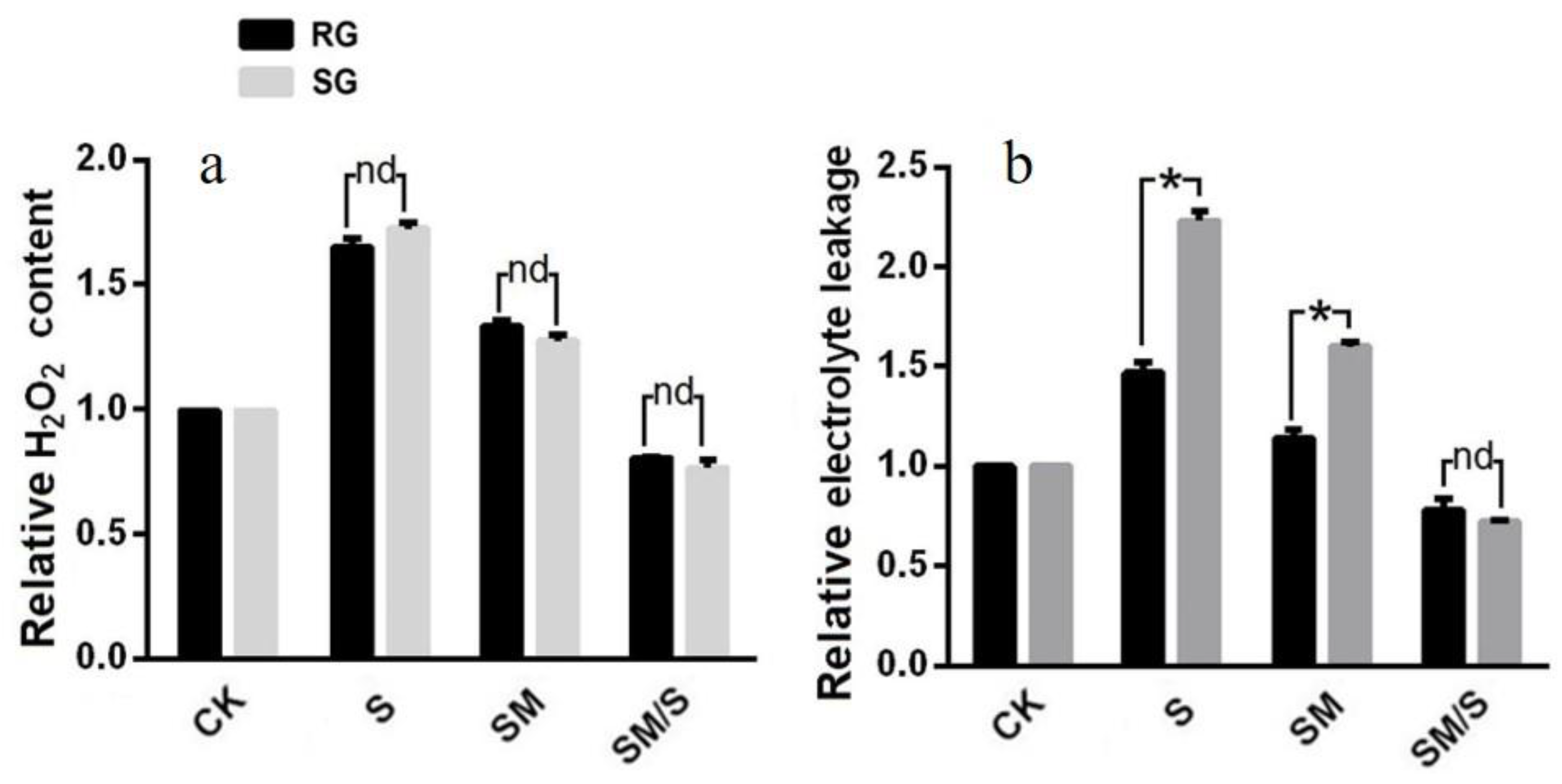
3.2. The Effect of Exogenous Melatonin on the H2O2 Content and Electrolyte Leakage of Tomato Seedlings under Normal Conditions and Salt Stress
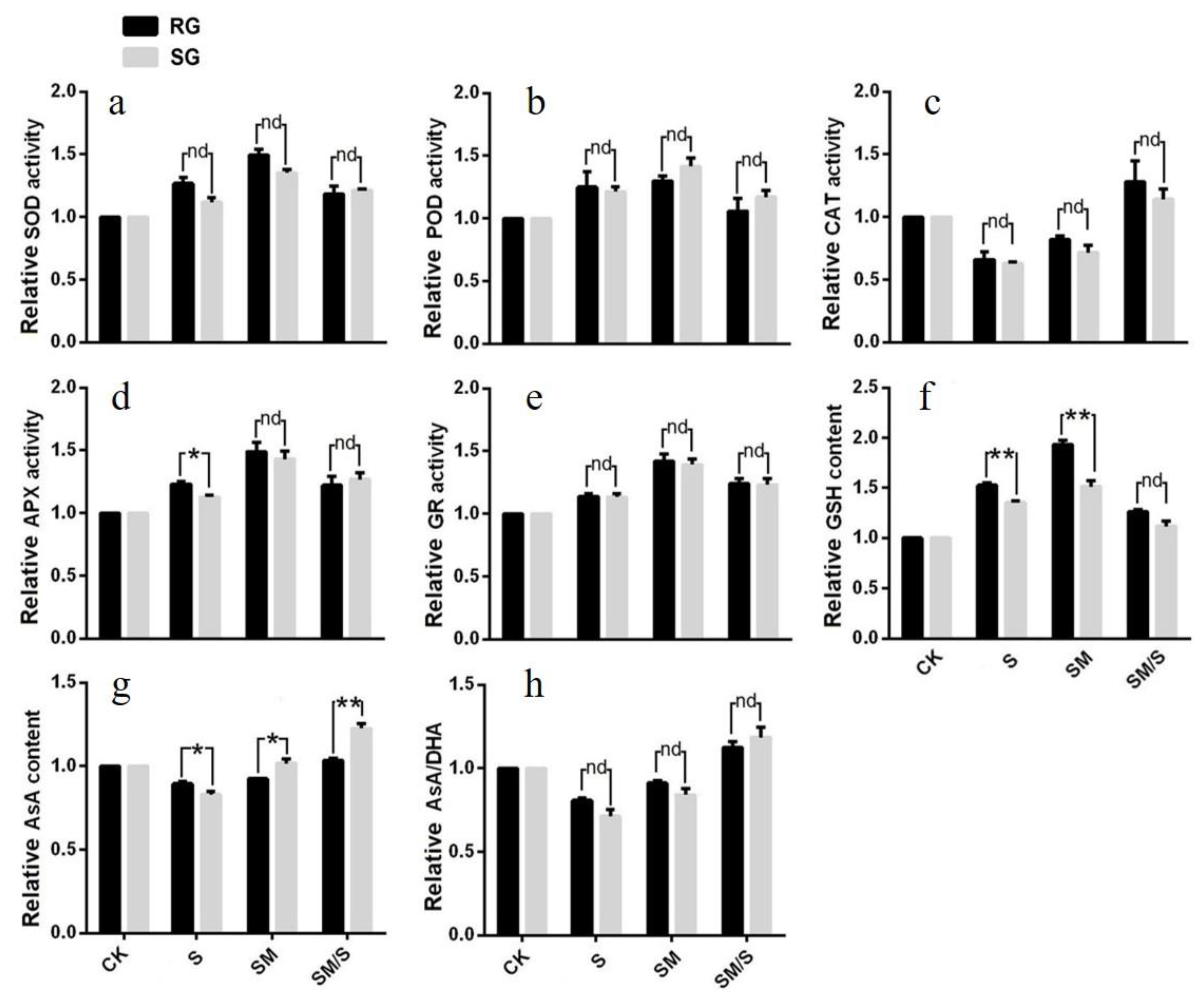
3.3. The Effect of Exogenous Melatonin on the Activity of SOD, POD, CAT, and AsA–GSH Cycles of Tomato Seedlings under Normal Conditions and Salt Stress
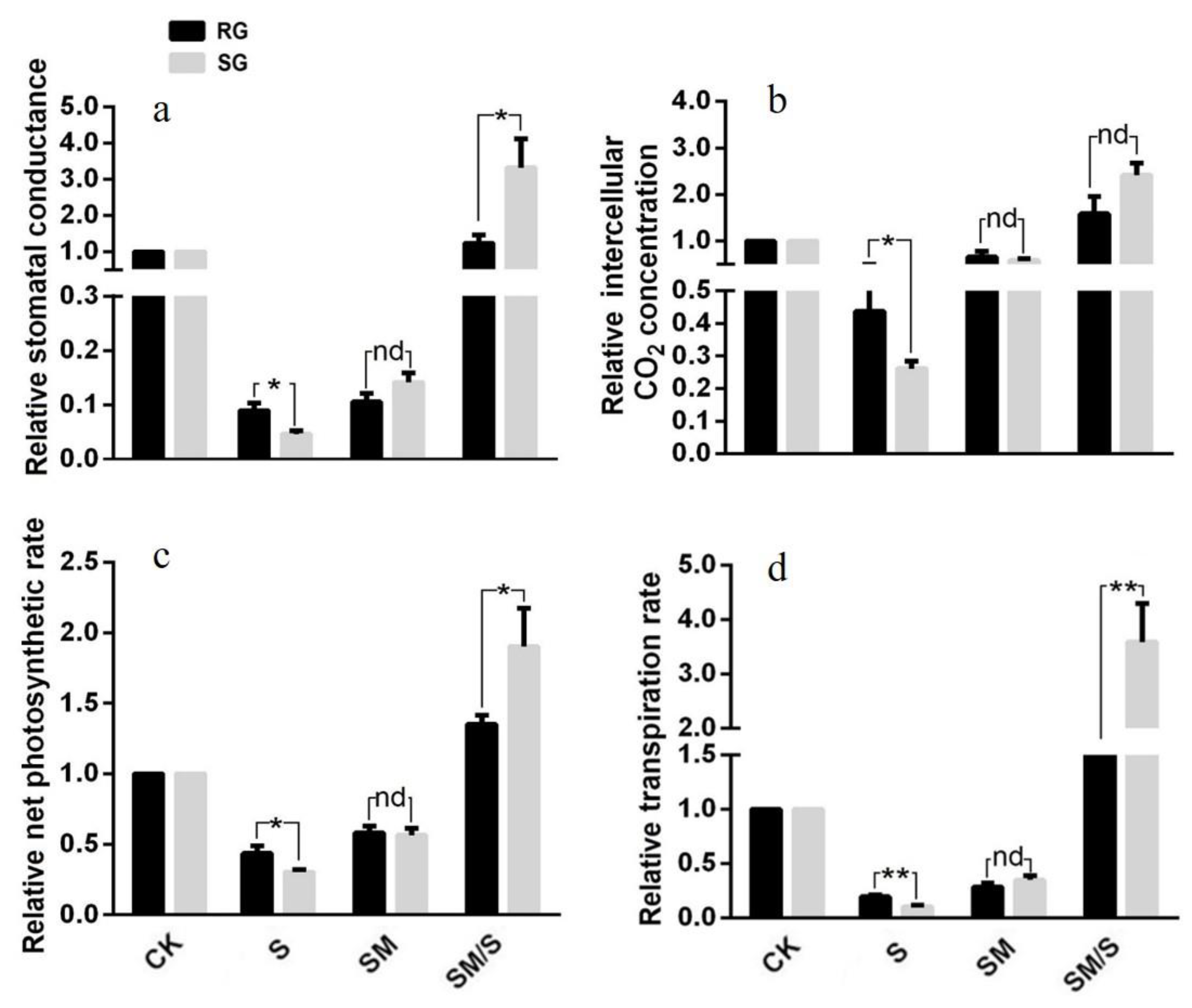
3.4. The Effect of Exogenous Melatonin on the Photosynthetic Parameters of Tomato Seedlings under Normal Conditions and Salt Stress
3.5. The Effect of Exogenous Melatonin on the Ultrastructure of the Chloroplast of Tomato Leaves under Salt Stress
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dubbels, R.; Reiter, R.; Klenke, E.; Goebel, A.; Schnakenberg, E.; Ehlers, C.; Schiwara, H.; Schloot, W. Melatonin in edible plants identified by radioimmunoassay and by high performance liquid chromatography-mass spectrometry. J. Pineal Res. 1995, 18, 28–31. [Google Scholar] [CrossRef] [PubMed]
- Hattori, A.; Migitaka, H.; Iigo, M.; Itoh, M.; Yamamoto, K.; Ohtani-Kaneko, R.; Hara, M.; Suzuki, T.; Reiter, R.J. Identification of melatonin in plants and its effects on plasma melatonin levels and binding to melatonin receptors in vertebrates. Biochem. Mol. Biol. Int. 1995, 35, 627–634. [Google Scholar] [PubMed]
- Beilby, M.J.; Al Khazaaly, S.; Bisson, M.A. Salinity-Induced Noise in Membrane Potential of Characeae Chara australis: Effect of Exogenous Melatonin. J. Membr. Biol. 2014, 248, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Kostopoulou, Z.; Therios, I.; Roumeliotis, E.; Kanellis, A.; Molassiotis, A. Melatonin combined with ascorbic acid provides salt adaptation in Citrus aurantium L. seedlings. Plant. Physiol. Biochem. 2015, 86, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, P.; Wei, Z.; Liang, D.; Liu, C.; Yin, L.; Jia, D.; Fu, M.; Ma, F. The mitigation effects of exogenous melatonin on salinity-induced stress in Malus hupehensis. J. Pineal Res. 2012, 53, 298–306. [Google Scholar] [CrossRef]
- Mukherjee, S.; David, A.; Yadav, S.; Baluška, F.; Bhatla, S.C. Salt stress-induced seedling growth inhibition coincides with differential distribution of serotonin and melatonin in sunflower seedling roots and cotyledons. Physiol. Plant. 2014, 152, 714–728. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.-J.; Zhang, N.; Yang, R.-C.; Wang, L.; Sun, Q.-Q.; Li, D.-B.; Cao, Y.-Y.; Weeda, S.; Zhao, B.; Ren, S.; et al. Melatonin promotes seed germination under high salinity by regulating antioxidant systems, ABA and GA4 interaction in cucumber (Cucumis sativus L.). J. Pineal Res. 2014, 57, 269–279. [Google Scholar] [CrossRef]
- Tan, D.-X.; Hardeland, R.; Manchester, L.C.; Korkmaz, A.; Ma, S.; Rosales-Corral, S.; Reiter, R.J. Functional roles of melatonin in plants, and perspectives in nutritional and agricultural science. J. Exp. Bot. 2011, 63, 577–597. [Google Scholar] [CrossRef]
- Reiter, R.J.; Tan, D.-X.; Zhou, Z.; Cruz, M.H.C.; Fuentes-Broto, L.; Galano, A. Phytomelatonin: Assisting Plants to Survive and Thrive. Molecules 2015, 20, 7396–7437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, H.; Tan, D.-X.; Reiter, R.J.; Ye, T.; Yang, F.; Chan, Z. Melatonin induces class A1 heat-shock factors (HSFA1s) and their possible involvement of thermotolerance in Arabidopsis. J. Pineal Res. 2015, 58, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Arnao, M.B.; Hernández-Ruiz, J. Protective effect of melatonin against chlorophyll degradation during the senescence of barley leaves. J. Pineal Res. 2008, 46, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Byeon, Y.; Park, S.; Kim, Y.S.; Back, K. Microarray analysis of genes differentially expressed in melatonin-rich transgenic rice expressing a sheep serotonin N -acetyltransferase. J. Pineal Res. 2013, 55, 357–363. [Google Scholar] [CrossRef]
- Sarropoulou, V.; Dimassi-Theriou, K.; Therios, I.; Koukourikou-Petridou, M. Melatonin enhances root regeneration, photo-synthetic pigments, biomass, total carbohydrates and proline content in the cherry rootstock phl-c (prunus avium × prunus cerasus). Plant. Physio Bioch. 2012, 61, 162–168. [Google Scholar] [CrossRef]
- Szafrańska, K.; Glińska, S.; Janas, K.M. Ameliorative effect of melatonin on meristematic cells of chilled and re-warmed Vigna radiata roots. Biol. Plant. 2013, 57, 91–96. [Google Scholar] [CrossRef]
- Wang, P.; Yin, L.; Liang, D.; Li, C.; Ma, F.; Yue, Z. Delayed senescence of apple leaves by exogenous melatonin treatment: Toward regulating the ascorbate-glutathione cycle. J. Pineal Res. 2011, 53, 11–20. [Google Scholar] [CrossRef]
- Wang, P.; Sun, X.; Li, C.; Wei, Z.; Liang, D.; Ma, F. Long-term exogenous application of melatonin delays drought-induced leaf senescence in apple. J. Pineal Res. 2012, 54, 292–302. [Google Scholar] [CrossRef]
- Weeda, S.; Zhang, N.; Zhao, X.; Ndip, G.; Guo, Y.; Buck, G.A.; Fu, C.; Ren, S. Arabidopsis Transcriptome Analysis Reveals Key Roles of Melatonin in Plant Defense Systems. PLoS ONE 2014, 9, e93462. [Google Scholar] [CrossRef] [Green Version]
- Liu, N.; Jin, Z.; Wang, S.; Gong, B.; Wen, D.; Wang, X.; Wei, M.; Shi, Q. Sodic alkaline stress mitigation with exogenous melatonin involves reactive oxygen metabolism and ion homeostasis in tomato. Sci. Hortic. 2015, 181, 18–25. [Google Scholar] [CrossRef]
- Turk, H.; Erdal, S.; Genisel, M.; Atici, O.; Demir, Y.; Yanmis, D. The regulatory effect of melatonin on physiological, biochemical and molecular parameters in cold-stressed wheat seedlings. Plant. Growth Regul. 2014, 74, 139–152. [Google Scholar] [CrossRef]
- Wei, W.; Li, Q.; Chu, Y.-N.; Reiter, R.J.; Yu, X.-M.; Zhu, D.-H.; Zhang, W.-K.; Ma, B.; Lin, Q.; Zhang, J.-S.; et al. Melatonin enhances plant growth and abiotic stress tolerance in soybean plants. J. Exp. Bot. 2014, 66, 695–707. [Google Scholar] [CrossRef] [Green Version]
- Arnao, M.B.; Ruiz, J.H. Functions of melatonin in plants: A review. J. Pineal Res. 2015, 59, 133–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meng, J.-F.; Xu, T.-F.; Wang, Z.-Z.; Fang, Y.-L.; Xi, Z.-M.; Zhang, Z.-W. The ameliorative effects of exogenous melatonin on grape cuttings under water-deficient stress: Antioxidant metabolites, leaf anatomy, and chloroplast morphology. J. Pineal Res. 2014, 57, 200–212. [Google Scholar] [CrossRef] [PubMed]
- Yadu, B.; Chandrakar, V.; Meena, R.K.; Poddar, A.N.; Keshavkant, S. Spermidine and Melatonin Attenuate Fluoride Toxicity by Regulating Gene Expression of Antioxidants in Cajanus cajan L. J. Plant. Growth Regul. 2018, 37, 1113–1126. [Google Scholar] [CrossRef]
- Shi, H.; Chen, K.; Wei, Y.; He, C. Fundamental Issues of Melatonin-Mediated Stress Signaling in Plants. Front. Plant. Sci. 2016, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Selvakumar, G.; Kim, K.; Hu, S.; Sa, T. Effect of salinity on plants and the role of arbuscular mycorrhizal fungi and plant growth-promoting rhizobacteria in alleviation of salt stress. In Physiological Mechanisms and Adaptation Strategies in Plants under Changing Environment; Ahmad, P., Wani, M.R., Eds.; Springer: New York, NY, USA, 2013; pp. 115–144. [Google Scholar] [CrossRef]
- Rozema, J.; Flowers, T. Crops for a Salinized World. Science 2008, 322, 1478–1480. [Google Scholar] [CrossRef]
- Zhou, X.; Zhao, H.; Cao, K.; Hu, L.; Du, T.; Baluška, F.; Zou, Z. Beneficial roles of melatonin on redox regulation of photo-synthetic electron transport and synthesis of D1 protein in tomato seedlings under salt stress. Front. Plant. Sci. 2016, 7. [Google Scholar] [CrossRef] [Green Version]
- Alexieva, V.; Sergiev, I.; Mapelli, S.; Karanov, E. The effect of drought and ultraviolet radiation on growth and stress markers in pea and wheat. Plant. Cell Environ. 2001, 24, 1337–1344. [Google Scholar] [CrossRef]
- Bajji, M.; Bertin, P.; Lutts, S.; Kinet, J.M. Evaluation of drought resistance-related traits in durum wheat somaclonal lines se-lected in vitro. Anim Prod. Sci. 2004, 44, 27–35. [Google Scholar] [CrossRef]
- Beyer, W.F., Jr.; Fridovich, I. Assaying for superoxide dismutase activity: Some large consequences of minor changes in conditions. Anal. Biochem. 1987, 161, 559–566. [Google Scholar] [CrossRef]
- Muñoz-Muñoz, J.; García-Molina, F.; García-Ruiz, P.; Arribas, E.; Tudela, J.; García-Cánovas, F.; Rodríguez-López, J. Enzymatic and chemical oxidation of trihydroxylated phenols. Food Chem. 2009, 113, 435–444. [Google Scholar] [CrossRef]
- Aebi, H. Methods in enzymology. Catalase Vitro 1984, 105, 121–126. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Hydrogen Peroxide is Scavenged by Ascorbate-specific Peroxidase in Spinach Chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar] [CrossRef]
- Zhu, H.; Cao, Z.; Zhang, L.; Trush, M.A.; Li, Y. Glutathione and glutathione-linked enzymes in normal human aortic smooth muscle cells: Chemical inducibility and protection against reactive oxygen and nitrogen species-induced injury. Mol. Cell. Biochem. 2007, 301, 47–59. [Google Scholar] [CrossRef]
- Zhang, J.; Kirkham, M.B. Antioxidant responses to drought in sunflower and sorghum seedlings. New Phytol. 1996, 132, 361–373. [Google Scholar] [CrossRef]
- Nagalakshmi, N.; Prasad, M. Responses of glutathione cycle enzymes and glutathione metabolism to copper stress in Scenedesmus bijugatus. Plant. Sci. 2001, 160, 291–299. [Google Scholar] [CrossRef]
- Arnon, D.I. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant. Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Chartzoulakis, K.; Klapaki, G. Response of two greenhouse pepper hybrids to NaCl salinity during different growth stages. Sci. Hortic. 2000, 86, 247–260. [Google Scholar] [CrossRef]
- Cuartero, J.; Bolarin, M.C.; Asins, M.; Moreno, V. Increasing salt tolerance in the tomato. J. Exp. Bot. 2006, 57, 1045–1058. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.-K. Salt and Drought Stress Signal Transduction in Plants. Annu. Rev. Plant. Biol. 2002, 53, 247–273. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.Y.; Liu, J.L.; Wang, W.X.; Sun, Y. Exogenous melatonin improves growth and photosynthetic capacity of cucumber under salinity-induced stress. Photosynthetica 2016, 54, 19–27. [Google Scholar] [CrossRef]
- Chaves, M.M.; Flexas, J.; Pinheiro, C. Photosynthesis under drought and salt stress: Regulation mechanisms from whole plant to cell. Ann. Bot. 2009, 103, 551–560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Von Caemmerer, S.; Farquhar, G.D. Some relationships between the biochemistry of photosynthesis and the gas exchange of leaves. Planta 1981, 153, 376–387. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Tan, D.-X.; Liang, D.; Chang, C.; Jia, D.; Ma, F. Melatonin mediates the regulation of ABA metabolism, free-radical scavenging, and stomatal behaviour in two Malus species under drought stress. J. Exp. Bot. 2014, 66, 669–680. [Google Scholar] [CrossRef] [PubMed] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, R.; Cen, B.; Jiang, F.; Sun, M.; Wen, J.; Cao, X.; Cui, S.; Kong, L.; Zhou, N.; Wu, Z. Reducing the Halotolerance Gap between Sensitive and Resistant Tomato by Spraying Melatonin. Agronomy 2022, 12, 84. https://doi.org/10.3390/agronomy12010084
Zhou R, Cen B, Jiang F, Sun M, Wen J, Cao X, Cui S, Kong L, Zhou N, Wu Z. Reducing the Halotolerance Gap between Sensitive and Resistant Tomato by Spraying Melatonin. Agronomy. 2022; 12(1):84. https://doi.org/10.3390/agronomy12010084
Chicago/Turabian StyleZhou, Rong, Benjian Cen, Fangling Jiang, Mintao Sun, Junqin Wen, Xue Cao, Shouyao Cui, Lingpeng Kong, Niannian Zhou, and Zhen Wu. 2022. "Reducing the Halotolerance Gap between Sensitive and Resistant Tomato by Spraying Melatonin" Agronomy 12, no. 1: 84. https://doi.org/10.3390/agronomy12010084
APA StyleZhou, R., Cen, B., Jiang, F., Sun, M., Wen, J., Cao, X., Cui, S., Kong, L., Zhou, N., & Wu, Z. (2022). Reducing the Halotolerance Gap between Sensitive and Resistant Tomato by Spraying Melatonin. Agronomy, 12(1), 84. https://doi.org/10.3390/agronomy12010084








