Optimisation of Charcoal and Sago (Metroxylon sagu) Bark Ash to Improve Phosphorus Availability in Acidic Soils
Abstract
:1. Introduction
2. Materials and Methods
2.1. Soil Sampling, Preparation, and Selected Physico-Chemical Analyses
2.2. Charcoal and Sago Bark Ash Characterisation
2.3. Incubation Set Up
- T1:
- Soil only
- T2
- Soil + ERP
- T3
- Soil + ERP + 51.4 g charcoal
- T4
- Soil + ERP + 25.7 g sago bark ash
- T5
- Soil + ERP + 51.4 g charcoal + 25.7 g sago bark ash
- T6:
- Soil + ERP + 38.6 g charcoal + 19.3 g sago bark ash
- T7:
- Soil + ERP + 25.7 g charcoal + 19.3 g sago bark ash
- T8:
- Soil + ERP + 12.9 g charcoal + 19.3 g sago bark ash
- T9:
- Soil + ERP + 38.6 g charcoal + 12.9 g sago bark ash
- T10:
- Soil + ERP + 25.7 g charcoal + 12.9 g sago bark ash
- T11:
- Soil + ERP + 12.9 g charcoal + 12.9 g sago bark ash
- T12:
- Soil + ERP + 38.6 g charcoal + 6.4 g sago bark ash
- T13:
- Soil + ERP + 25.7 g charcoal + 6.4 g sago bark ash
- T14:
- Soil + ERP + 12.9 g charcoal + 6.4 g sago bark ash
2.4. Experimental Design and Statistical Analysis
3. Results
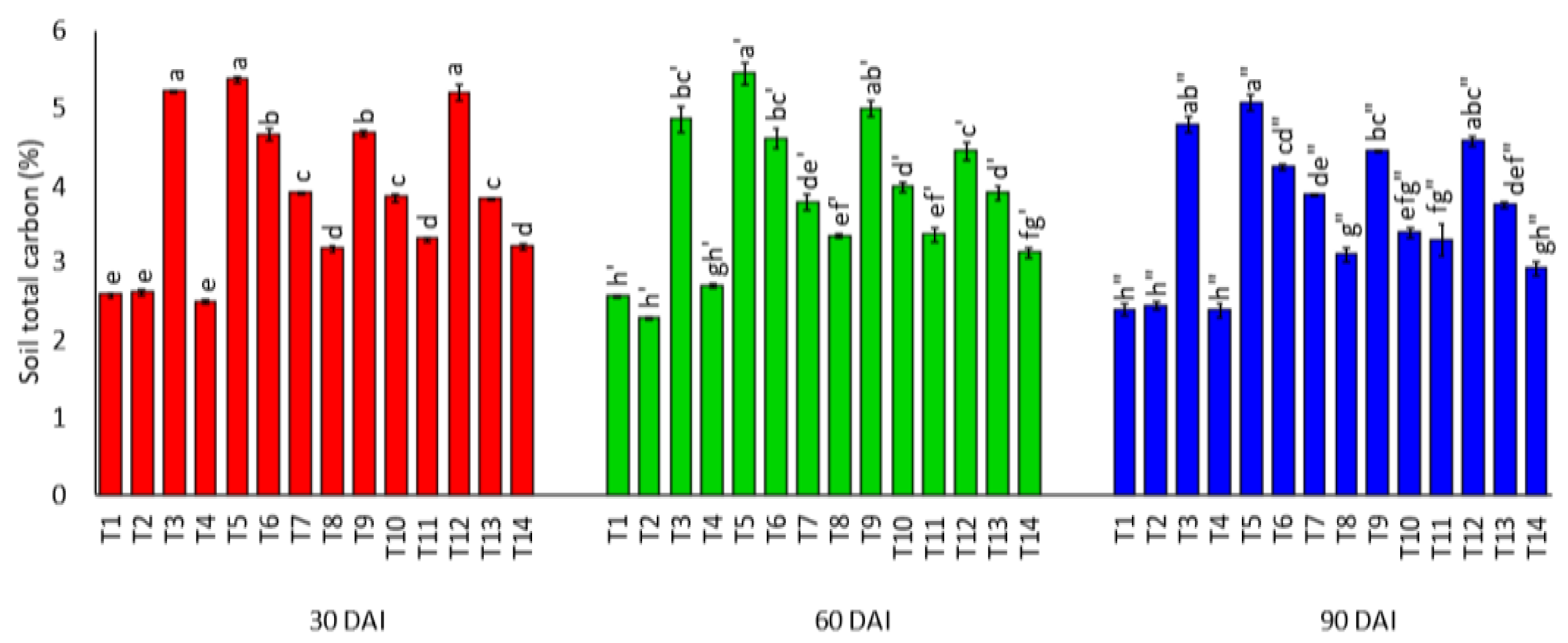
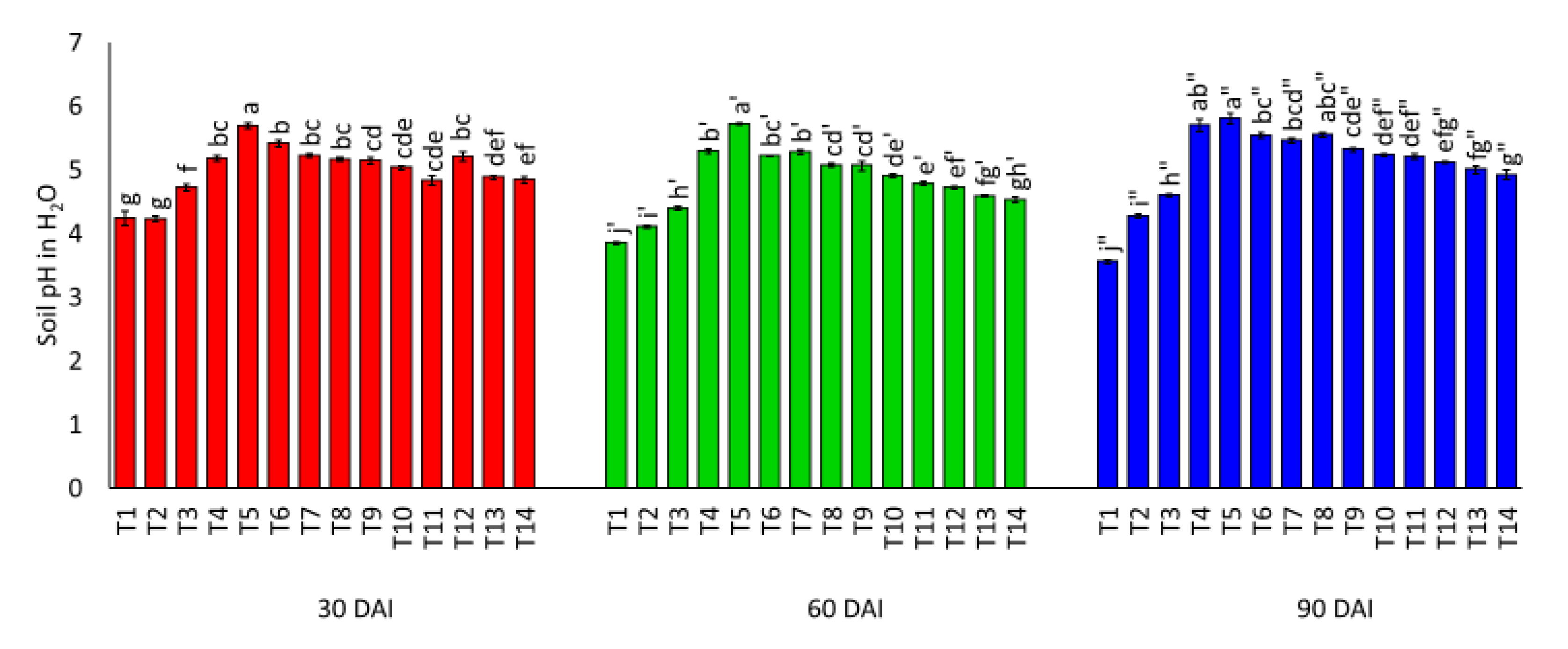
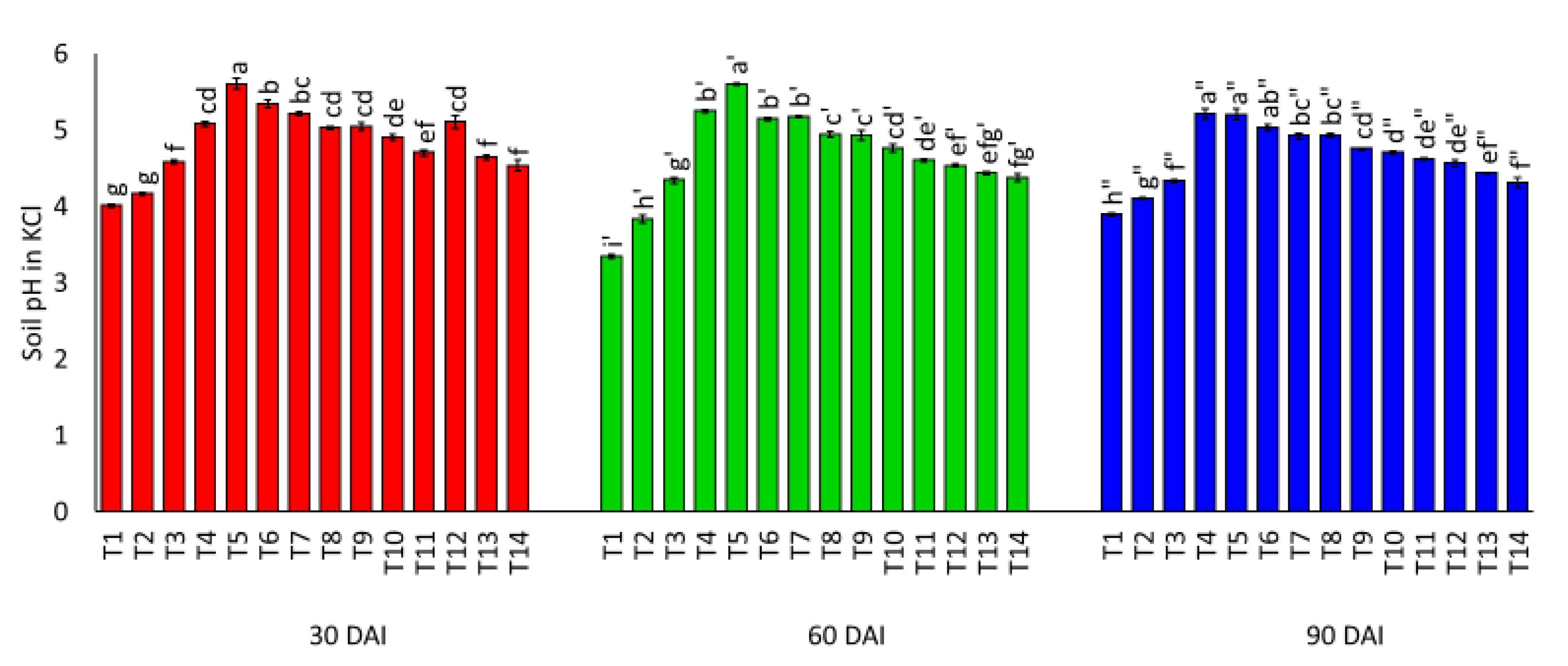
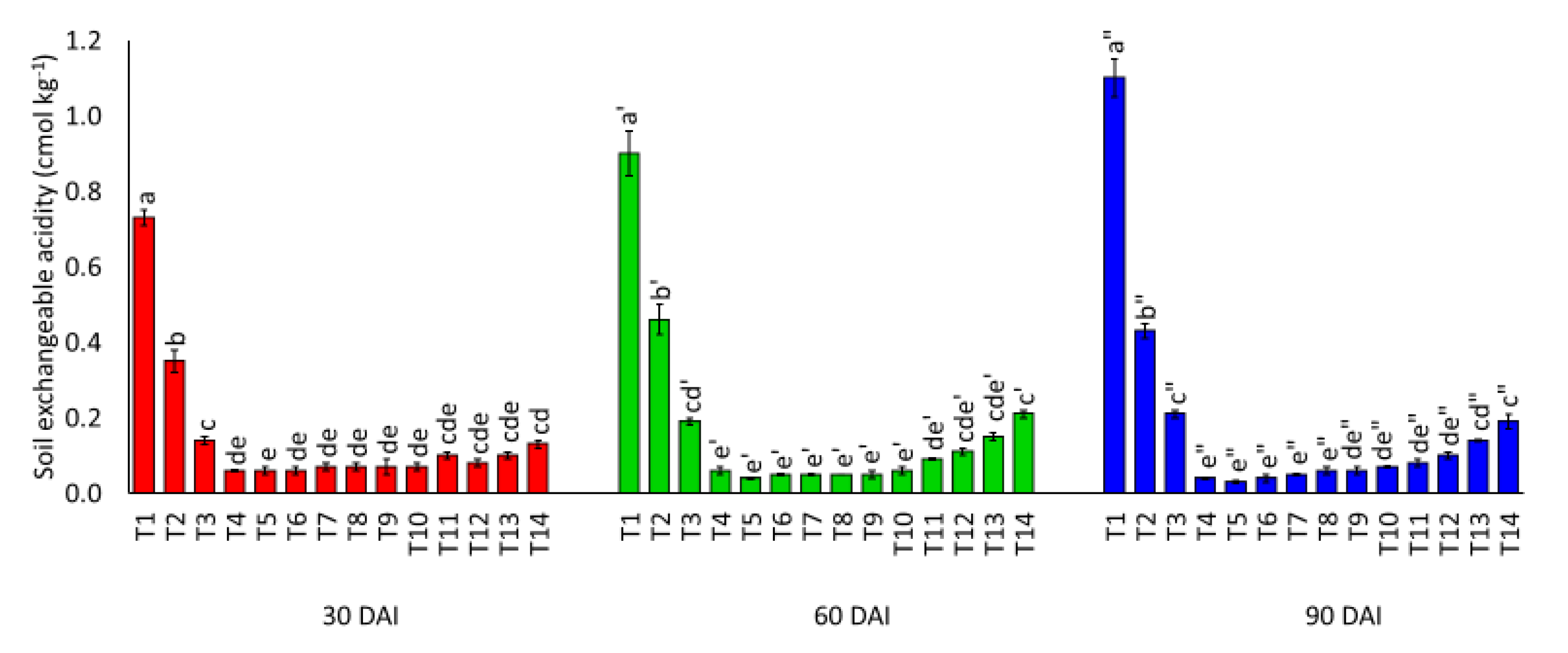
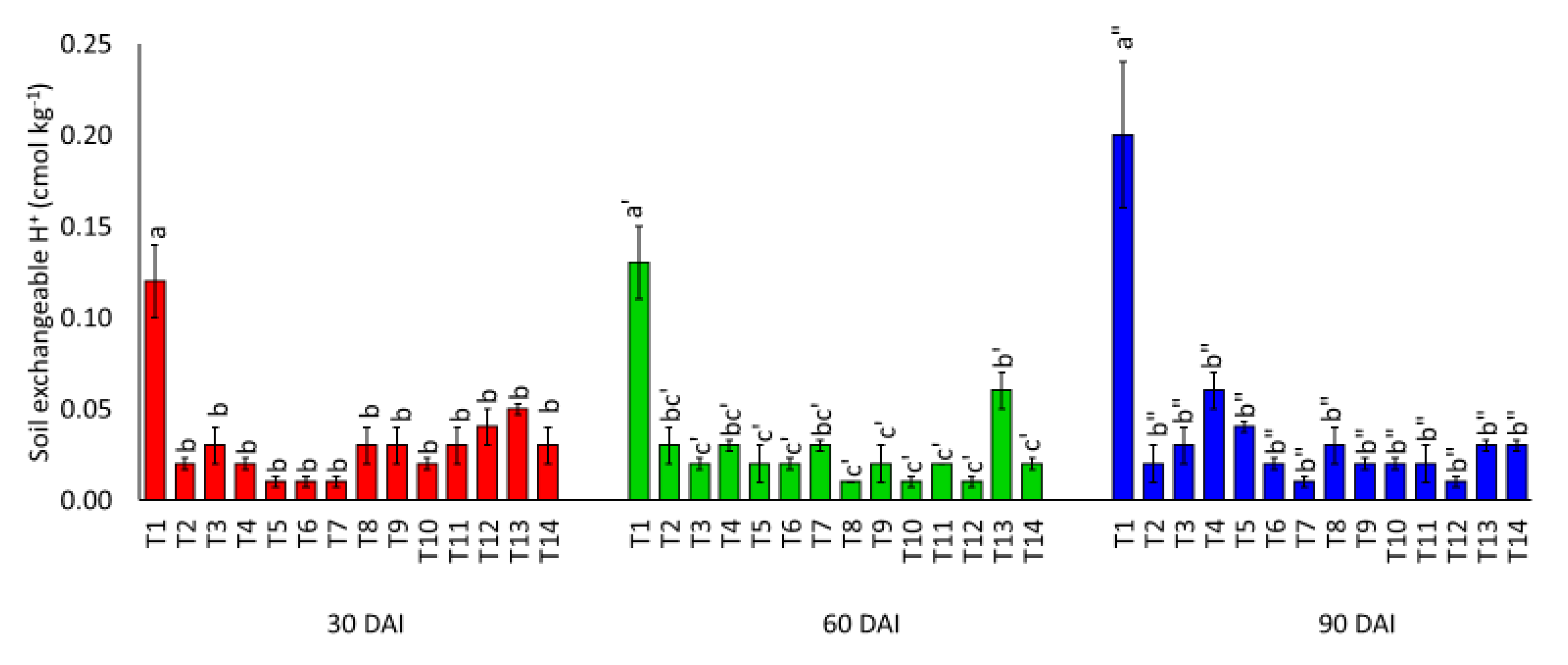
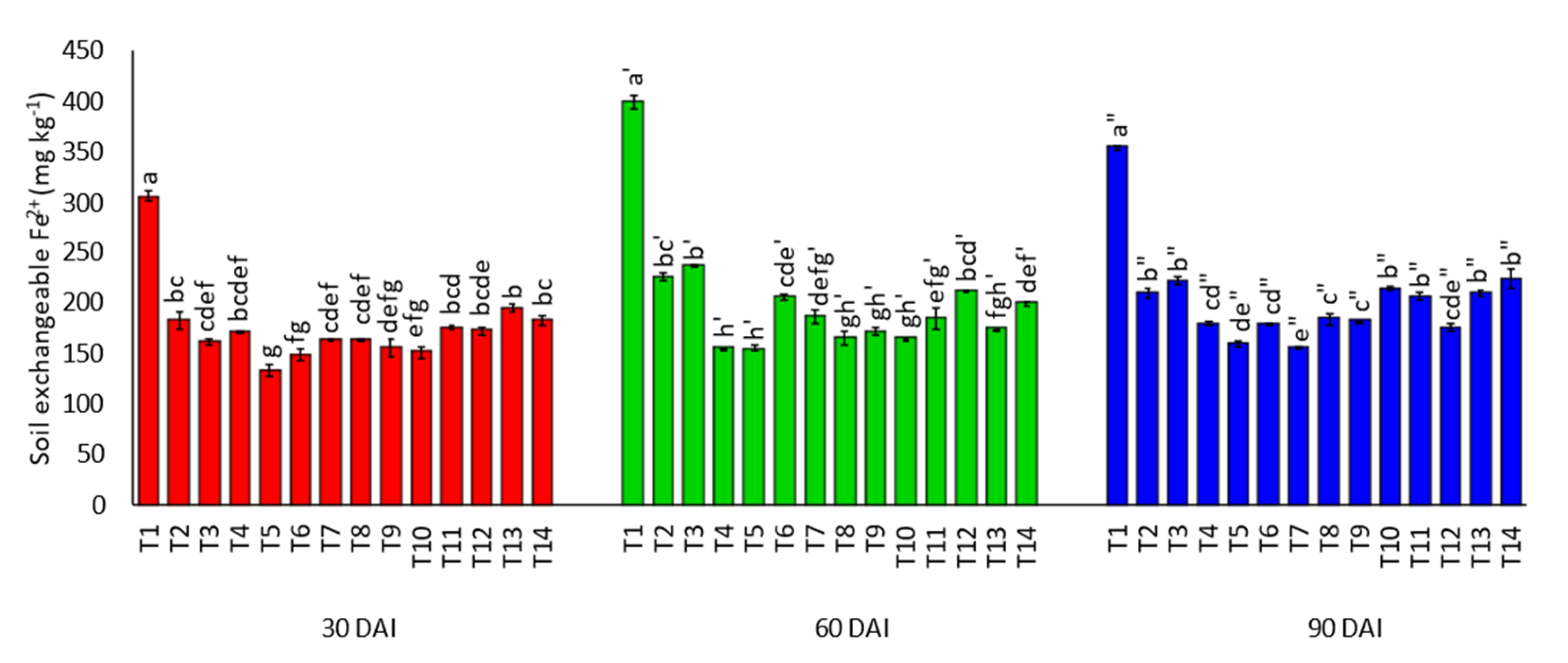
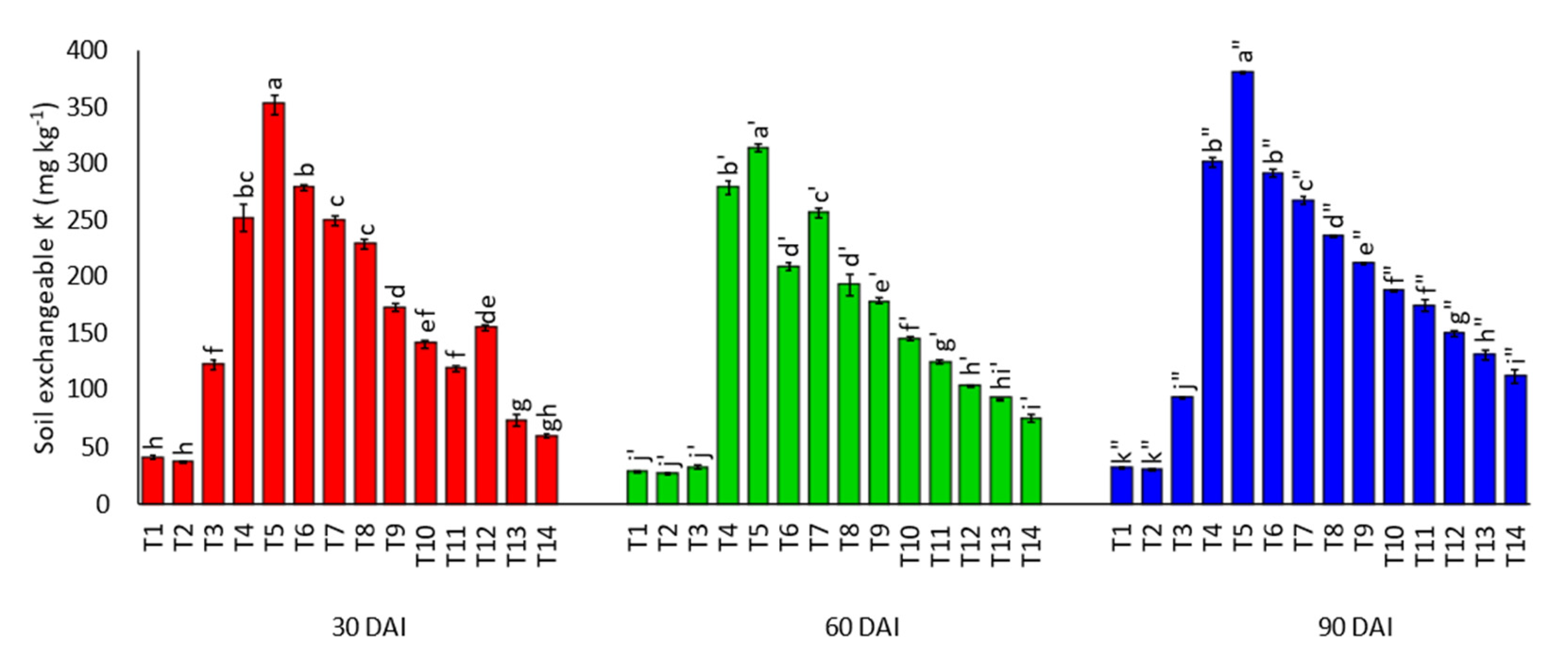
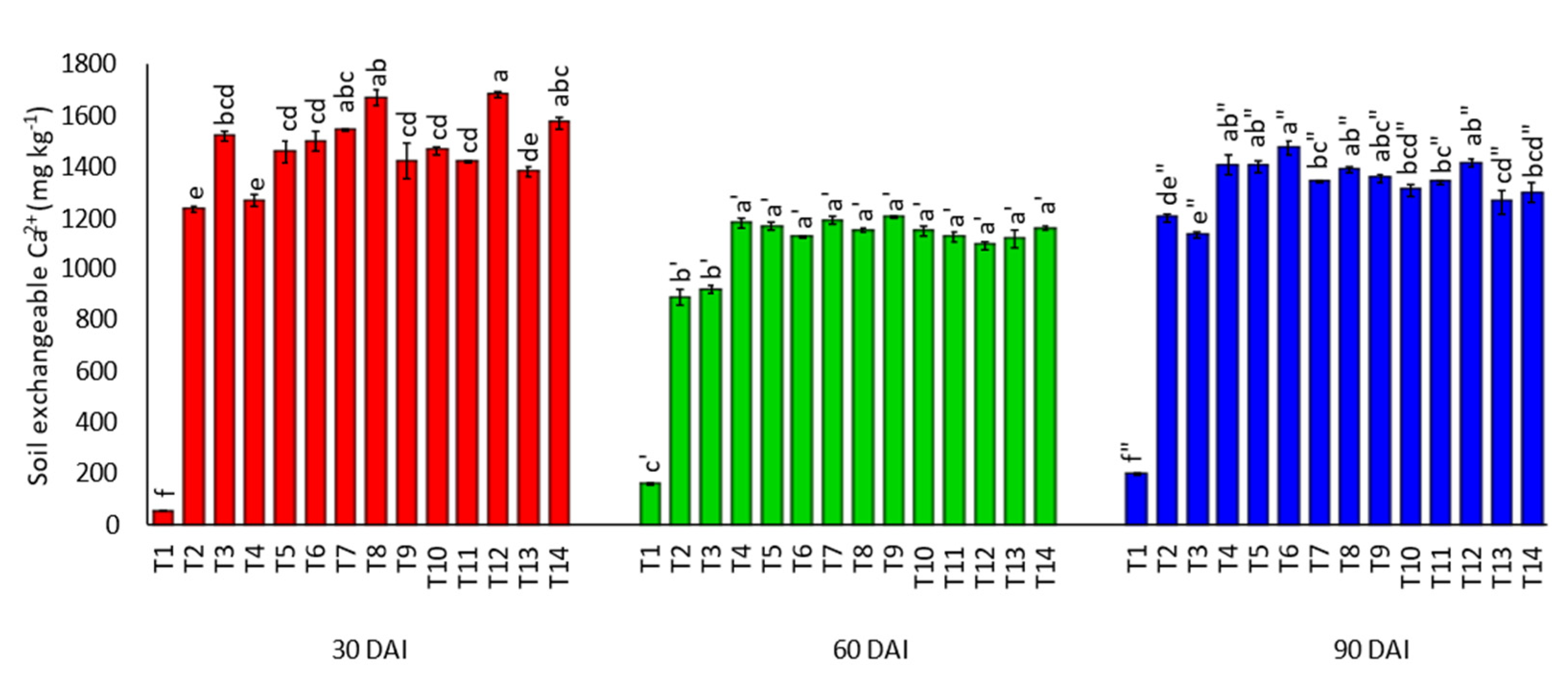
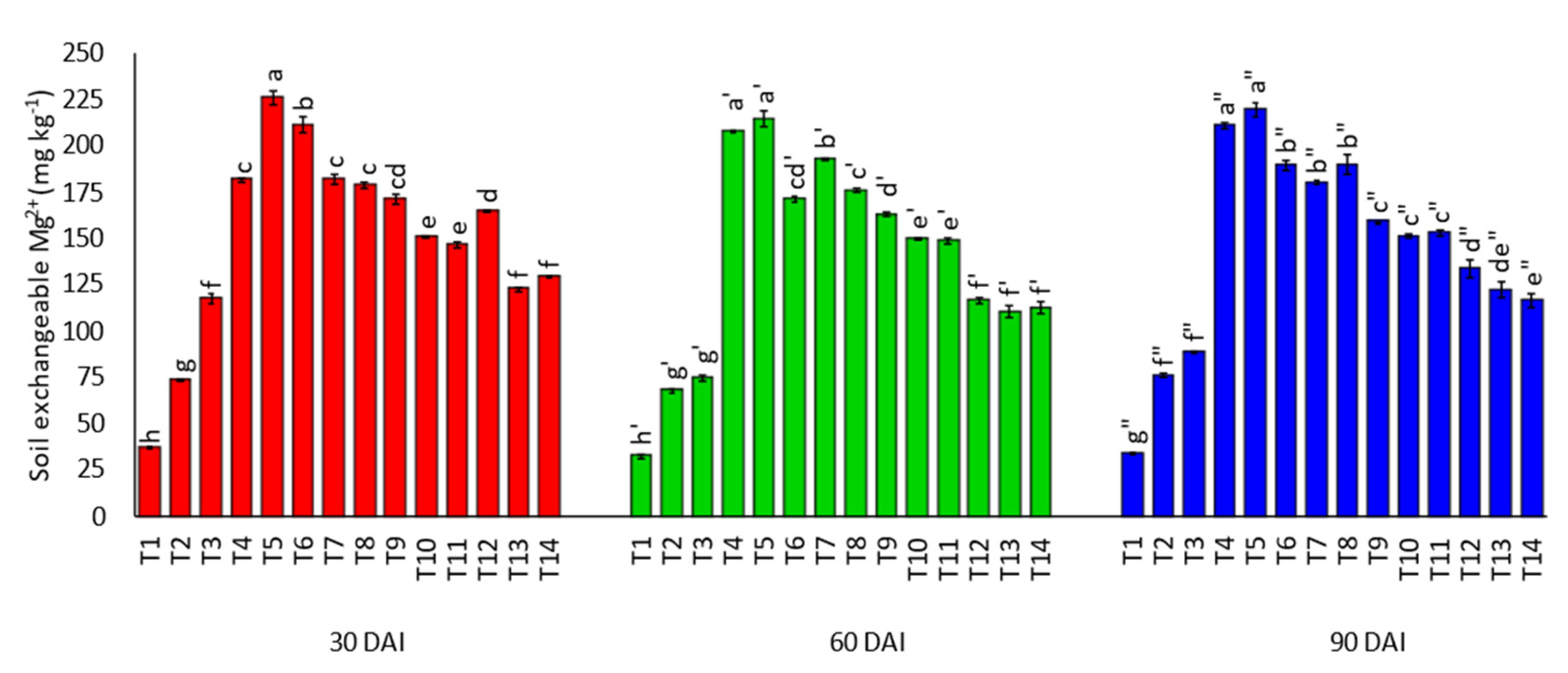
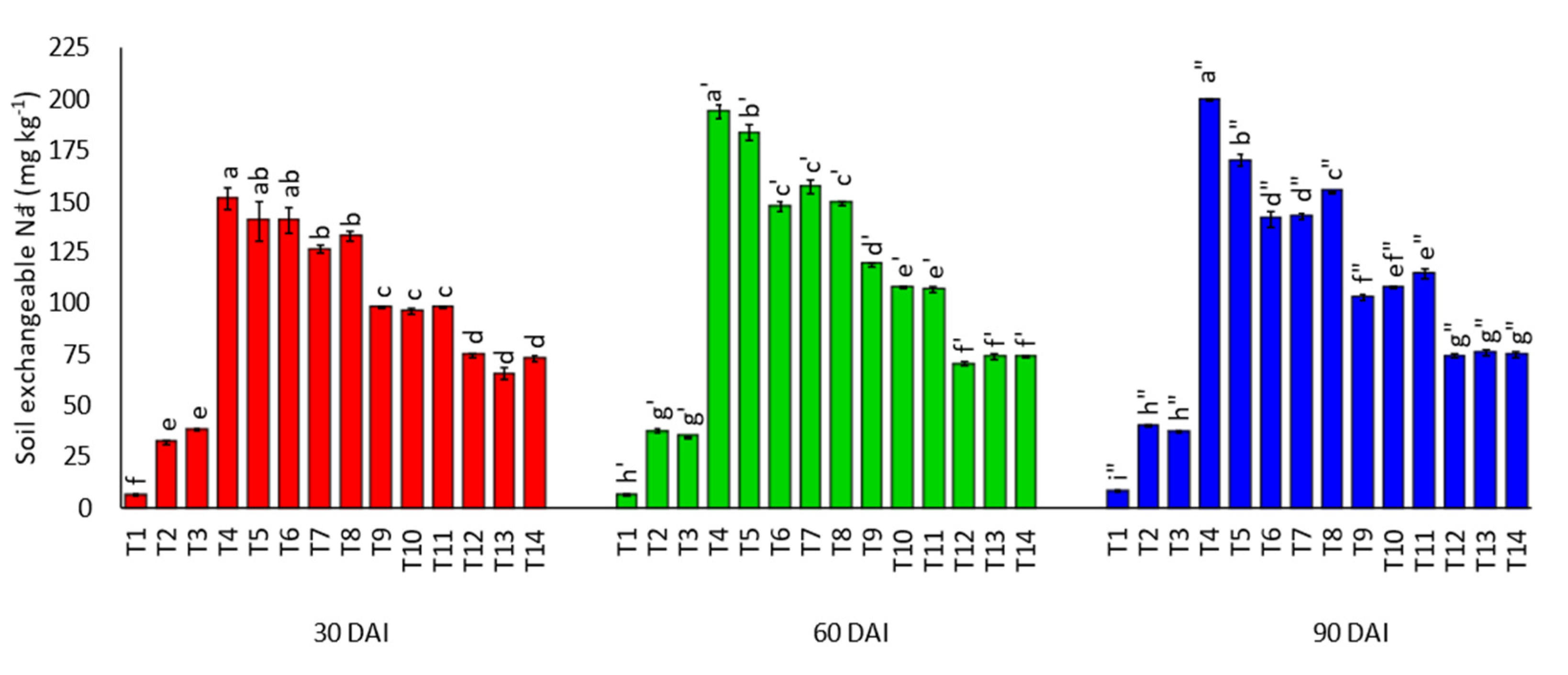
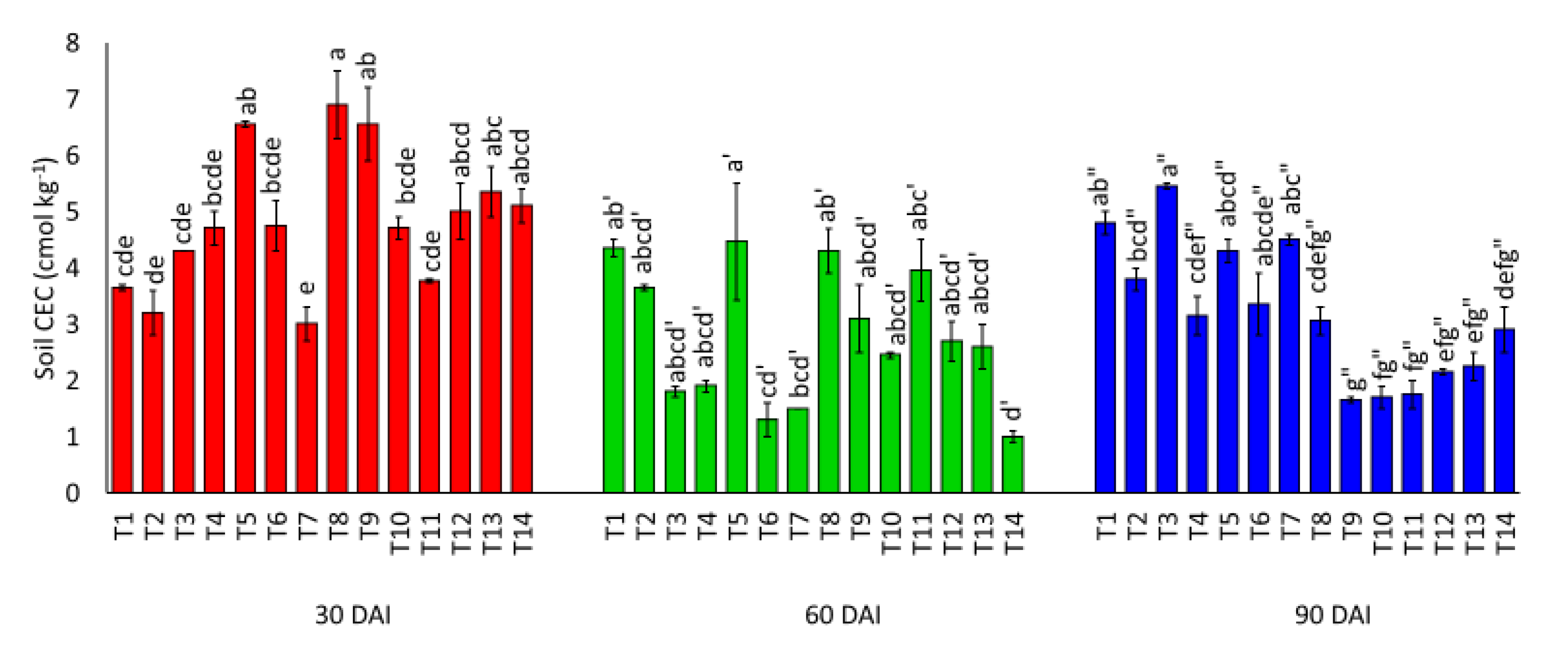
3.1. Effects of Amending Egypt Rock Phosphate with Charcoal and Sago Bark Ash on Selected Soil Chemical Properties
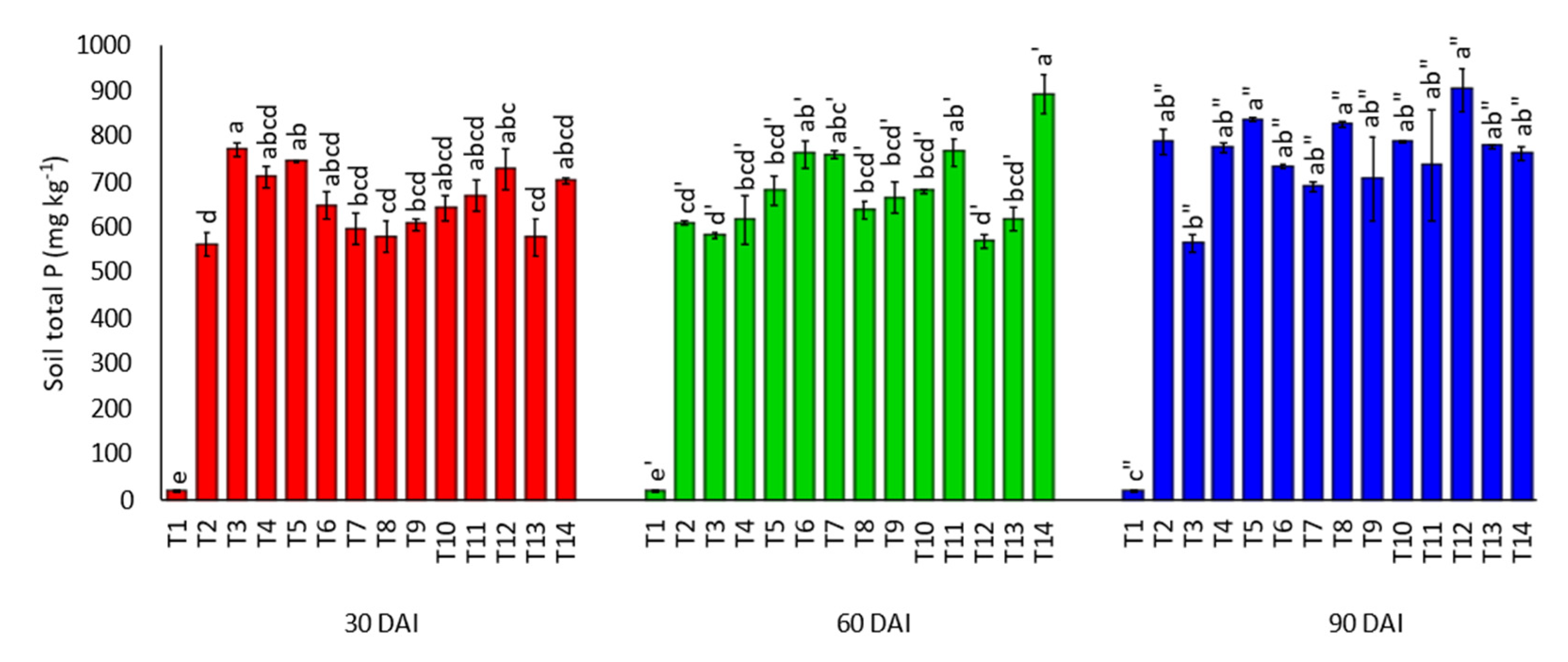
3.2. Effects of Amending Egypt Rock Phosphate with Charcoal and Sago Bark Ash on Soil Total Phosphorus and Mehlich-Phosphorus
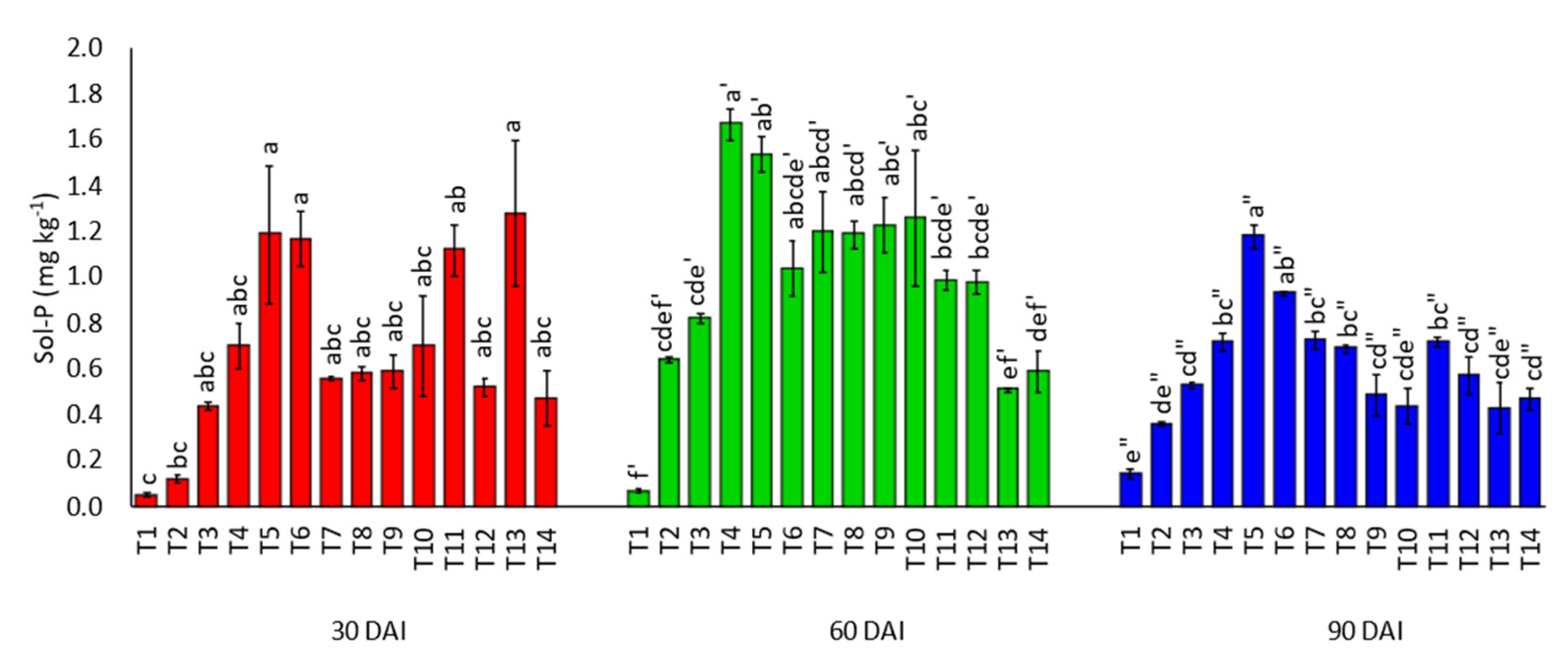
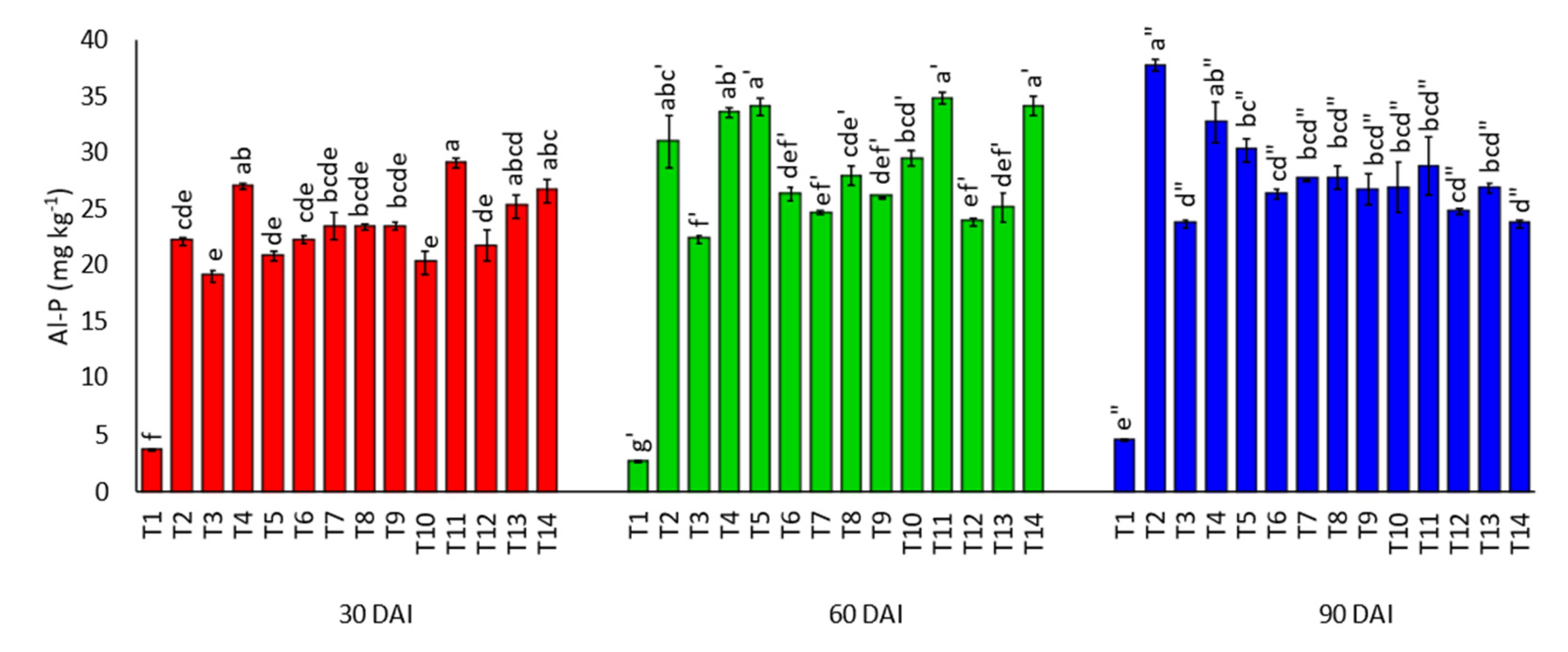
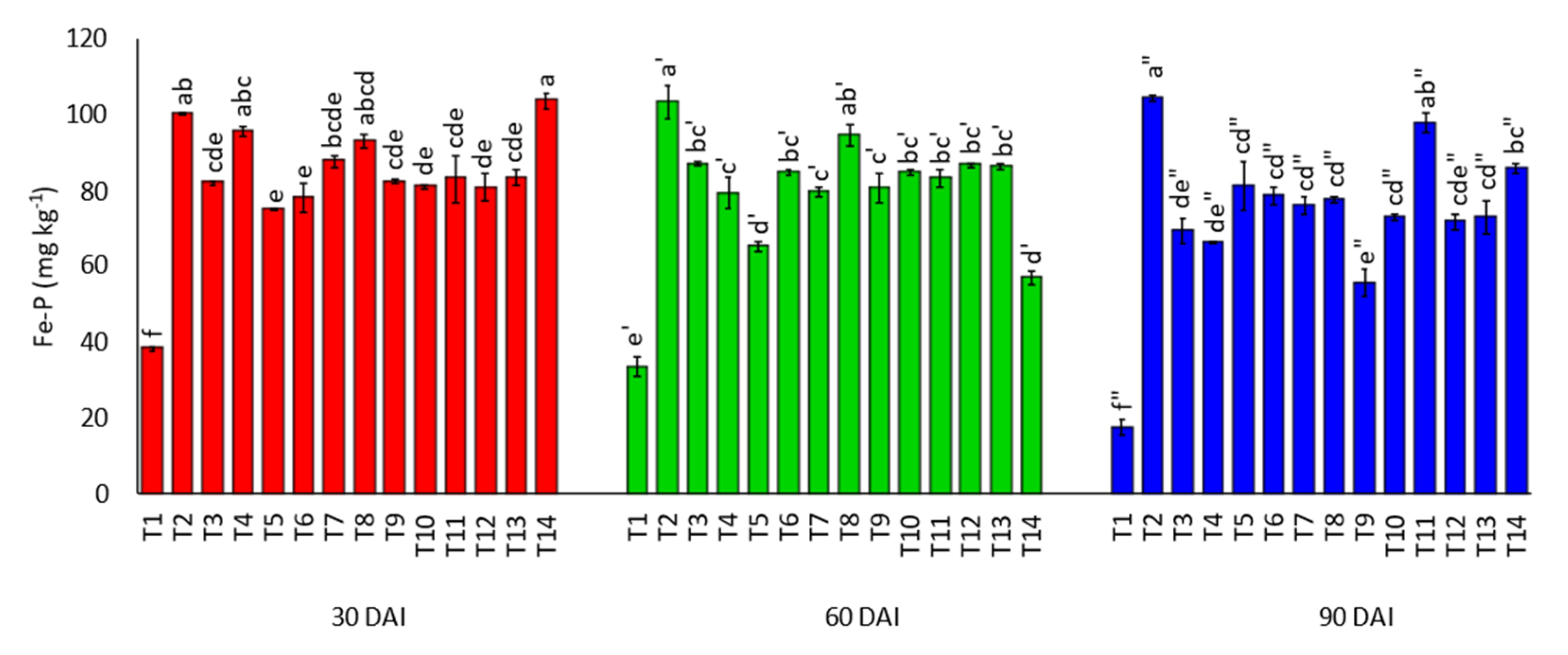
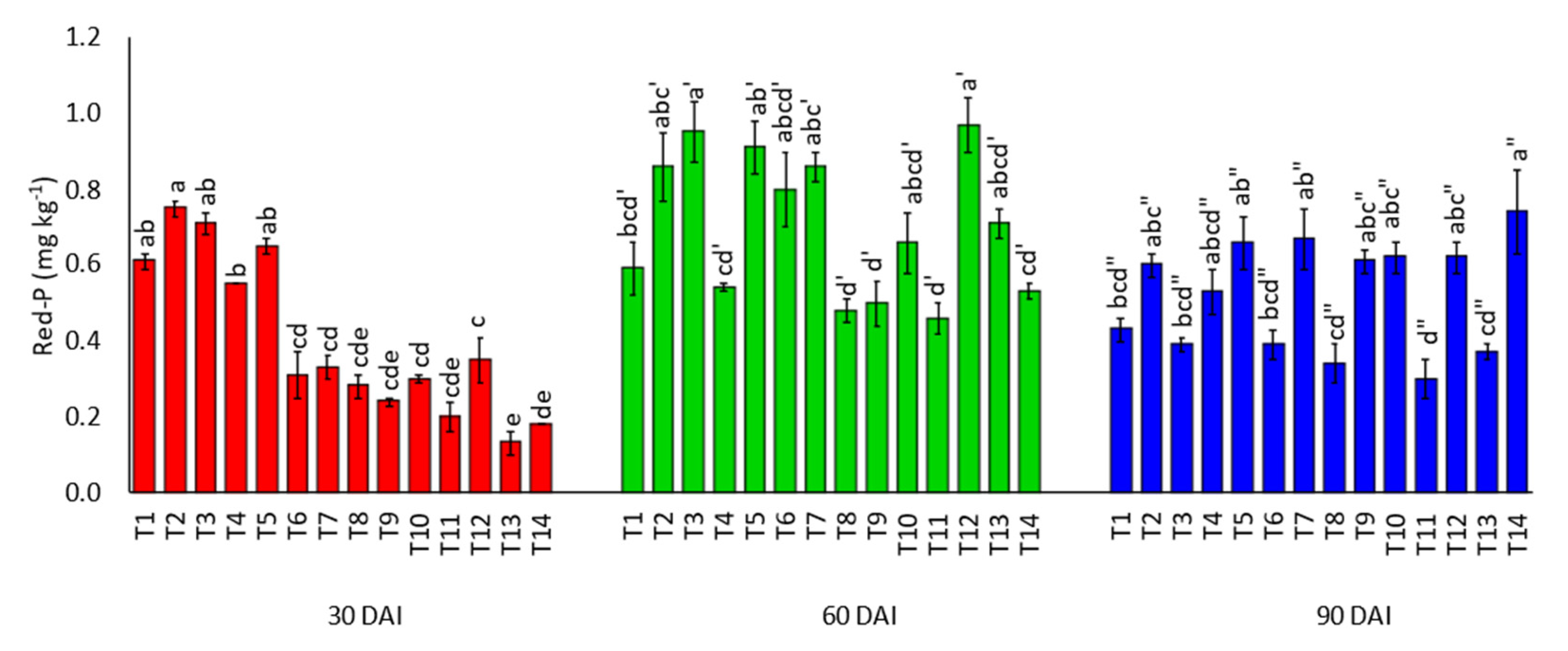
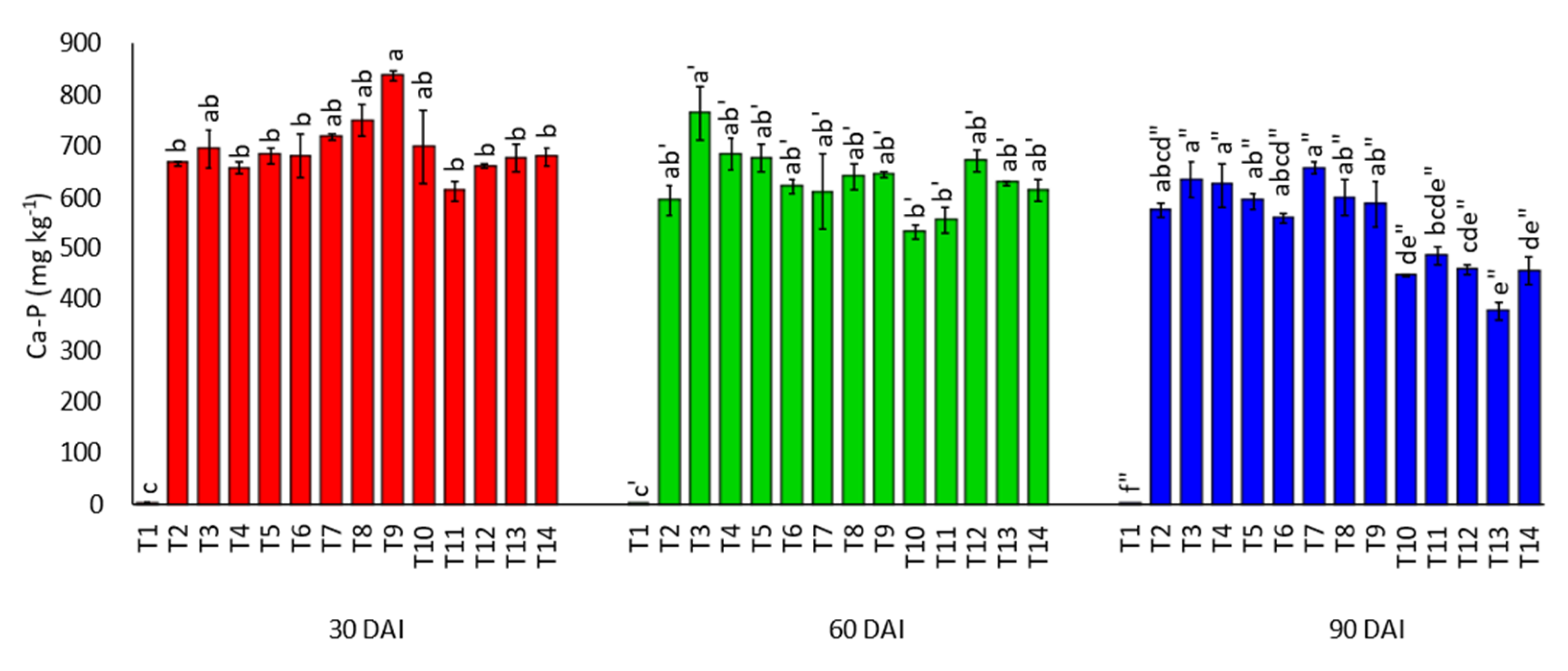
3.3. Effects of Amending Egypt Rock Phosphate with Charcoal and Sago Bark Ash on Soil Inorganic Phosphorus Fractions
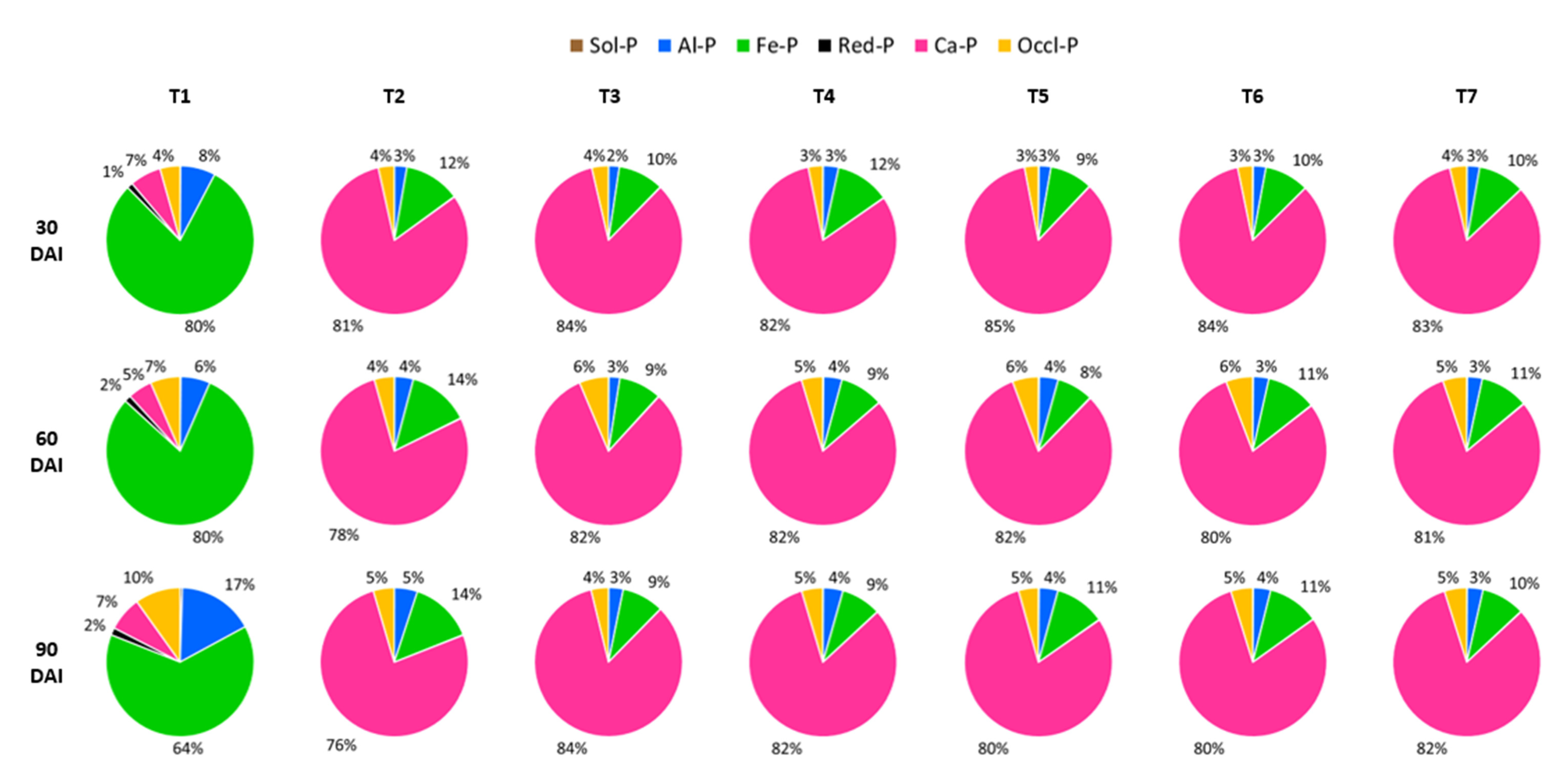
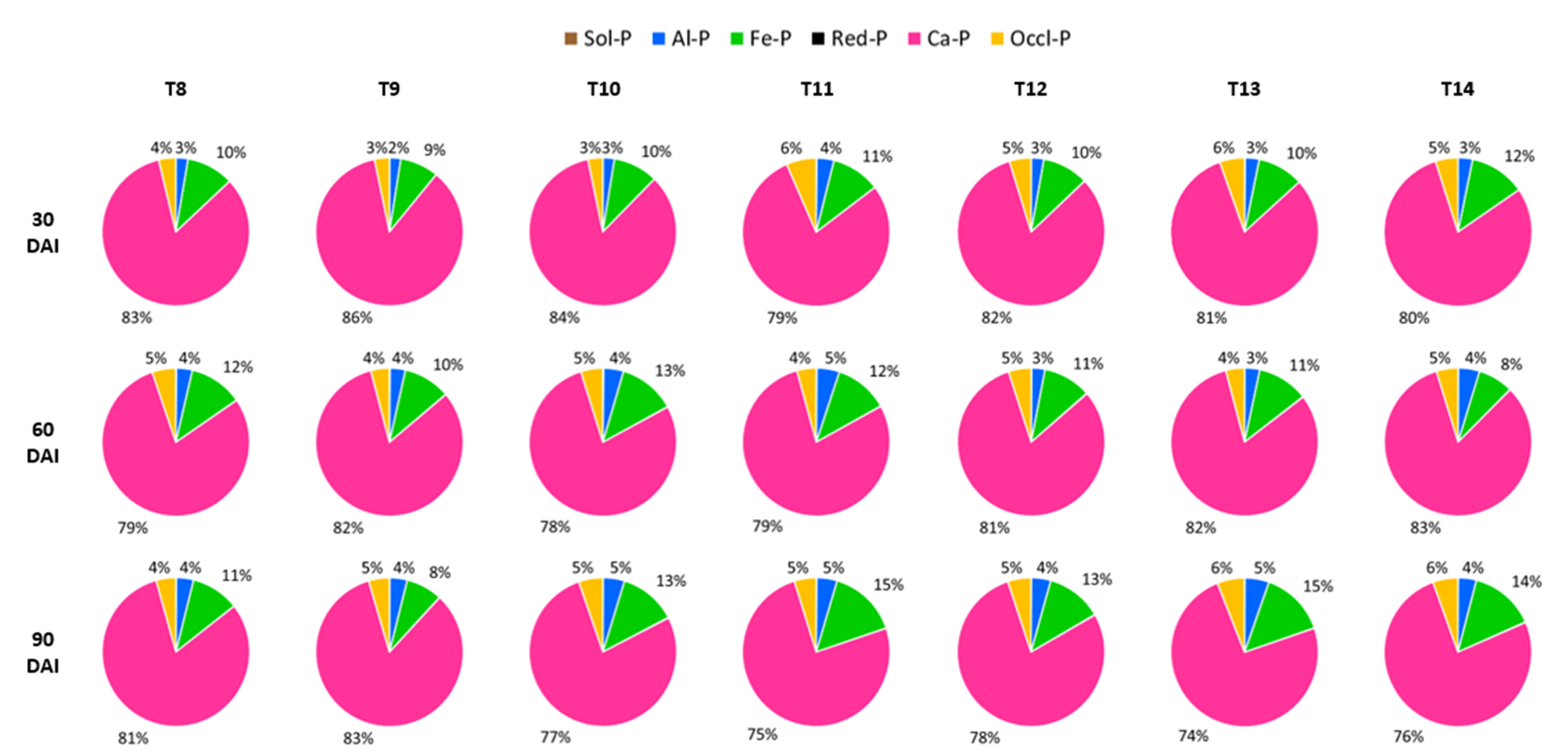
3.4. Percentages of Soil Inorganic Phosphorus Distribution by Treatment after Thirty, Sixty, and Ninety Days of Incubation
4. Discussion
4.1. Selected Soil Chemical Properties at Thirty, Sixty, and Ninety Days of Incubation
4.2. Total Phosphorus and Mehlich-Phosphorus at Thirty, Sixty, and Ninety Days of Incubation
4.3. Soil Inorganic Phosphorus Fractions at Thirty, Sixty, and Ninety Days of Incubation
4.4. Percentages of Soil Inorganic Phosphorus Distribution after Incubation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gichangi, E.M.; Mnkeni, P.N.S.; Muchaonyerwa, P. Phosphate sorption characteristics and external P requirements of selected South African soils. J. Agric. Rural Dev. Trop. Subtrop. 2008, 109, 139–149. [Google Scholar]
- Brady, N.C.; Weil, R.R. The Nature and Properties of Soils, 14th ed.; Pearson Prentice Hall: Upper Saddle River, NJ, USA, 2008; pp. 1–31. [Google Scholar]
- Meyer, J.B.; Frapolli, M.; Keel, C.; Maurhofer, M. Pyrroloquinoline quinone biosynthesis gene pqqC, a novel molecular marker for studying the phylogeny and diversity of phosphate-solubilizing pseudomonads. Appl. Environ. Microbiol. 2011, 77, 7345–7354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adnan, A.; Mavinic, D.S.; Koch, F.A. Pilot-scale study of phosphorus recovery through struvite crystallisation-examining to process feasibility. J. Environ. Eng. Sci. 2003, 2, 315–324. [Google Scholar] [CrossRef]
- Ch’ng, H.Y.; Ahmed, O.H.; Majid, N.M.A. Improving phosphorus availability in an acid soil using organic amendments produced from agroindustrial wastes. Sci. World J. 2014, 2014, 1–6. [Google Scholar] [CrossRef]
- Tsado, P.A.; Osunde, O.A.; Igwe, C.A.; Adeboye, M.K.A.; Lawal, B.A. Phosphorus sorption characteristics of some selected soil of the Nigerian Guinea Savanna. Int. J. Agric. Sci. 2012, 2, 613–618. [Google Scholar]
- Li, J.; Shi, Y.L.; Chen, Z.W. Research on phosphorus in southern red soils of China. Chin. J. Soil Sci. 2011, 42, 763–768. [Google Scholar]
- Chien, S.H.; Prochnow, L.I.; Tu, S.; Snyder, C.S. Agronomic and environmental aspects of phosphate fertilizers varying in source and solubility: An update review. Nutr. Cycl. Agroecosystems 2011, 89, 229–255. [Google Scholar] [CrossRef]
- Wang, Y.L.; Zhang, Y.H.; He, Y.Q. Effect of soil matrix components on phosphate sorption index in red soil. Acta Pedol. Sin. 2012, 49, 552–559. [Google Scholar]
- Troeh, F.R.; Thompson, L.M. Soils and Soil Fertility, 6th ed.; Blackwell: New York, NY, USA, 2005. [Google Scholar]
- Ch’ng, H.Y.; Ahmed, O.H.; Majid, N.M.A. Improving phosphorus availability, nutrient uptake and dry matter production of Zea mays L. on a tropical acid soil using poultry manure biochar and pineapple leaves compost. Exp. Agric. 2015, 52, 447–465. [Google Scholar] [CrossRef]
- Brady, N.C.; Weil, R.R. The Nature and Properties of Soils, 13th ed.; Pearson Education Inc: Upper Saddle River, NJ, USA, 2002. [Google Scholar]
- Hinsinger, P. Bioavailability of soil inorganic P in the rhizosphere as affected by root-induced chemical changes: A review. Plant Soil 2001, 237, 173–195. [Google Scholar] [CrossRef]
- Takeda, M.; Nakamoto, T.; Miyazawa, K.; Murayama, T.; Okada, H. Phosphorus availability and soil biological activity in an Andosol under compost application and winter cover cropping. Appl. Soil Ecol. 2009, 42, 86–95. [Google Scholar] [CrossRef]
- Rahman, Z.A.; Gikonyo, E.; Silek, B.; Goh, K.J.; Soltanghesis, A. Evaluation of phosphate rock sources and rate of application on oil palm yield grown on peat soils of Sarawak, Malaysia. J. Agron. 2014, 13, 12–22. [Google Scholar] [CrossRef] [Green Version]
- Elser, J.; Bennett, E. Phosphorus cycle: A broken biogeochemical cycle. Nature 2011, 478, 29–31. [Google Scholar] [CrossRef] [PubMed]
- Obersteiner, M.; Peñuelas, J.; Ciais, P.; van der Velde, M.; Janssens, I.A. The phosphorus trilemma. Nat. Geosci. 2013, 6, 897–898. [Google Scholar] [CrossRef]
- Roy, E.D.; Richards, P.D.; Martinelli, L.A.; Coletta, L.D.; Lins, S.R.M.; Vazquez, F.F.; Willig, E.; Spera, S.A.; VanWey, L.K.; Porder, S. The phosphorus cost of agricultural intensification in the tropics. Nat. Plants 2016, 2, 16043. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Zhu, Y. Potential pollution and recommended critical levers of phosphorus in paddy soils of the southern Lake Tai area, China. Geoderma 2003, 115, 45–54. [Google Scholar] [CrossRef]
- Ruban, V.; Lopez-Sanchez, J.F.; Pardo, P.; Rauret, G.; Muntau, H.; Quevauviller, P.H. Selection and evaluation of sequential extraction procedures for the determination of phosphorus forms in lake sediments. J. Environ. Monit. 1999, 1, 51–56. [Google Scholar] [CrossRef]
- Sharpley, A.; Wang, X. Managing agricultural phosphorus for water quality: Lessons from the USA and China. J. Environ. Sci. 2014, 26, 1770–1782. [Google Scholar] [CrossRef]
- Vohla, C.; Kõiv, M.; Bavor, H.J.; Chazarenc, F.; Mander, Ü. Filter materials for phosphorus removal from wastewater in treatment wetlands-A review. Ecol. Eng. 2011, 37, 70–89. [Google Scholar] [CrossRef]
- Haynes, R.J.; Mokolobate, M.S. Amelioration of Al toxicity and P deficiency in acid soils by additions of organic residues: A critical review of the phenomenon and the mechanisms involved. Nutr. Cycl. Agroecosystems 2001, 59, 47–63. [Google Scholar] [CrossRef]
- Antoniadis, V.; Hatzis, F.; Bachtsevanidis, D.; Koutroubas, S.D. Phosphorus availability in low-P and acidic soils as affected by liming and P addition. Commun. Soil Sci. Plant Anal. 2015, 46, 1288–1298. [Google Scholar] [CrossRef]
- Parham, J.A.S.P.; Deng, S.; Raun, W.; Johnson, G. Long-term cattle manure application in soil. Biol. Fertil. Soils 2002, 35, 328–337. [Google Scholar] [CrossRef]
- Shamshuddin, J.; Daud, N.W. Classification and management of highly weathered soils in Malaysia for production of plantation crops. Princ. Appl. Assess. Soil Sci. 2011, 2011, 75–86. [Google Scholar] [CrossRef] [Green Version]
- Shamshuddin, J.; Fauziah, C.I.; Anda, M.; Kapok, J.; Shazana, M.A.R.S. Using ground basalt and/or organic fertilizer to enhance productivity of acid soils in Malaysia for crop production. Malays. J. Soil Sci. 2011, 15, 127–146. [Google Scholar]
- Myers, R.J.K.; De Pauw, E. Strategies for management of soil acidity. In Plant-Soil Interaction at Low pH: Principles and Management; Grundon, N.J., Raiment, G.E., Probert, M.E., Eds.; Springer: Dordrecht, The Netherlands, 1995; pp. 729–741. [Google Scholar]
- Shafie, S.M.; Othman, Z.; Hami, N. Potential utilisation of wood residue in Kedah: A preliminary study. J. Technol. Oper. Manag. 2017, 12, 60–69. [Google Scholar] [CrossRef]
- Hoi, W.K.; Razak, M.A.; Puad, E.; Azmi, M.H. Wood as a Potential Source of Energy. In Proceedings of the Conference of Forestry and Forest Products Research 90, Forest Research Institute Malaysia, Kepong, Selangor, Malaysia, 3 October 1990. [Google Scholar]
- Bujang, K. Potentials of bioenergy from the sago industries in Malaysia. In EOLSS-Encyclopedia of Life Support Systems; Doelle, H.W., Rokem, S., Berovic, M., Eds.; EOLSS Publisher: Oxford, UK, 2006; pp. 124–131. [Google Scholar]
- Singhal, R.S.; Kennedy, J.F.; Gopalakrishnan, S.M.; Kaczmarek, A.; Knill, C.J.; Akmar, P.F. Industrial production, processing, and utilization of sago palm-derived products. Carbohydr. Polym. 2008, 72, 1–20. [Google Scholar] [CrossRef]
- Vikineswary, S.; Shim, Y.L.; Thambirajah, J.J.; Blakebrough, N. Possible microbial utilization of sago processing wastes. Resour. Conserv. Recycl. 1994, 11, 289–296. [Google Scholar] [CrossRef]
- Wahi, R.; Abdullah, L.C.; Mobarekeh, M.N.; Ngaini, Z.; Yaw, T.C.S. Utilization of esterified sago bark fibre waste for removal of oil from palm oil mill effluent. J. Environ. Chem. Eng. 2017, 5, 170–177. [Google Scholar] [CrossRef]
- Amin, N.; Sabli, N.M.; Izhar, S.; Yoshida, H. Sago Wastes and Its Applications. Pertanika J. Sci. Technol. 2019, 27, 1841–1862. [Google Scholar]
- Ch’ng, H.Y. Phosphorus Management for Tropical Acid Soils Using Amendments from Agro-Industrial Wastes. Ph.D. Thesis, Universiti Putra Malaysia, Serdang, Selangor, Malaysia, 2015. [Google Scholar]
- Ohno, T.; Amirbahman, A. Phosphorus availability in boreal forest soils: A geochemical and nutrient uptake modeling approach. Geoderma 2010, 155, 46–54. [Google Scholar] [CrossRef]
- Ohno, T.; Fernandez, I.J.; Hiradate, S.; Sherman, J.F. Effects of soil acidification and forest type on water soluble soil organic matter properties. Geoderma 2007, 140, 176–187. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, X.; Zhang, H.; Christie, P.; Li, X.; Horlacher, D.; Liebig, H.P. Evaluation of current fertilizer practice and soil fertility in vegetable production in the Beijing region. Nutr. Cycl. Agroecosystems 2004, 69, 51–58. [Google Scholar] [CrossRef] [Green Version]
- Imbufe, A.U.; Patti, A.F.; Burrow, D.; Surapaneni, A.; Jackson, W.R.; Milner, A.D. Effects of potassium humate on aggregate stability of two soils from Victoria, Australia. Geoderma 2005, 125, 321–330. [Google Scholar] [CrossRef]
- Galinato, S.; Yoder, J.; Granatstein, D. The economic value of biochar in crop production and carbon sequestration. Energy Policy 2011, 39, 6344–6350. [Google Scholar] [CrossRef]
- Lehmann, J. A handful of carbon. Nature 2007, 447, 143–144. [Google Scholar] [CrossRef]
- DeLuca, T.H.; Aplet, G.H. Charcoal and carbon storage in forest soils of the Rocky Mountain West. Front. Ecol. Environ. 2008, 6, 18–24. [Google Scholar] [CrossRef]
- Ogawa, M. Rehabilitation of Pine with Charcoal and Mycorrhiza; Chikushishokan Publishing: Tokyo, Japan, 2007. [Google Scholar]
- Herrmann, S.; Oelmuller, R.; Buscot, F. Manipulation of the onset of ectomycorrhiza formation by indole-2-acetic acid, activated charcoal or relative humidity in the association between oak micocuttings and Piloderma croceum: Influence on plant development and photosynthesis. J. Plant Physiol. 2004, 161, 509–517. [Google Scholar] [CrossRef] [Green Version]
- Topoliantz, S.; Ponge, J.F.; Ballof, S. Manioc peel and charcoal: A potential organic amendment for sustainable soil fertility in the tropics. Biol. Fertil. Soils 2005, 41, 15–21. [Google Scholar] [CrossRef] [Green Version]
- Rutto, K.L.; Mizutani, F. Effect of mycorrhizal inoculation and activated charcoal on growth and nutrition in peach (Prunus perisica Batsch) seedlings treated with peach root-bark extracts. J. Jpn. Soc. Hortic. Sci. 2006, 75, 463–468. [Google Scholar] [CrossRef]
- Warnock, D.D.; Lehmann, J.; Kuypern, T.W.; Rilling, M.C. Mycorrhizal response to charcoal in soil-concepts and mechanisms. Plant Soil 2007, 300, 9–20. [Google Scholar] [CrossRef]
- Grogan, P.J.; Baar, T.; Bruns, D. Below-ground ectomycorrhizal community structure in a recently burned bishop pine forest. J. Ecol. 2000, 88, 1051–1062. [Google Scholar] [CrossRef] [Green Version]
- Glaser, B.; Lehmann, J.; Zesh, W. Ameliorating physical and chemical properties of highly weathered soils in the tropics with charcoal-a review. Biol. Fertil. Soil 2002, 35, 219–230. [Google Scholar] [CrossRef]
- Lehmann, J.; da Silva, J.P., Jr.; Rondon, M.; Cravo, M.D.S.; Greenwood, J.; Nehls, T.; Steiner, C.; Glaser, B. Slash-and-char-a feasible alternative for soil fertility management in the central Amazon. In Proceedings of the 17th World Congress of Soil Science, Bangkok, Thailand, 14 August 2002. [Google Scholar]
- Tryon, E.H. Effect of charcoal on certain physical, chemical, and biological properties of forest soils. Ecol. Monogr. 1948, 18, 81–115. [Google Scholar] [CrossRef]
- Etiegni, L.; Campbell, A.G. Physical and chemical characteristics of wood ash. Bioresour. Technol. 1991, 37, 173–178. [Google Scholar] [CrossRef]
- Ohno, T. Neutralization of soil acidity and release of phosphorus and potassium by wood ash. J. Environ. Qual. 1992, 21, 433–438. [Google Scholar] [CrossRef]
- Demeyer, A.; Nkana, J.V.; Verloo, M. Characteristics of wood ash influence on soil properties and nutrient uptake: An overview. Bioresour. Technol. 2001, 77, 287–295. [Google Scholar] [CrossRef]
- Khanna, P.K.; Raison, R.J.; Falkiner, R.A. Chemical properties of ash derived from Eucalyptus litter and its effects on forest soils. For. Ecol. Manag. 1994, 66, 107–125. [Google Scholar] [CrossRef]
- Ludwig, B.; Rumpf, S.; Mindrup, M.; Meiwes, K.J.; Khanna, P.K. Effects of lime and wood ash on soilsolution chemistry, soil chemistry and nutritional status of a pine stand in northern Germany. Scand. J. For. Res. 2002, 17, 225–237. [Google Scholar] [CrossRef]
- Saarsalmi, A.; Mälkönen, E.; Piirainen, S. Effects of wood ash fertilization on forest soil chemical properties. Silva. Fenn. 2001, 35, 355–368. [Google Scholar] [CrossRef] [Green Version]
- Ferriero, A.; Merino, A.; Diaz, N.; Pineiro, J. Improving the effectiveness of wood-ash fertilization in mixed mountain pastures. Grass Forage Sci. 2011, 66, 337–350. [Google Scholar] [CrossRef]
- Dixon, R.K.; Wisniewski, J. Global forest systems: An uncertain response to atmospheric pollutants and global climate change. Water Air Soil Pollut. 1995, 85, 101–110. [Google Scholar] [CrossRef]
- Bouyoucos, G.J. Hydrometer method improved for making particle size analysis of soils. Agron. J. 1962, 54, 464–465. [Google Scholar] [CrossRef]
- Peech, H.M. Hydrogen-ion Activity. In Methods of Soil Analysis Chemical and Microbiological Properties; Black, C.A., Evans, D.D., White, J.L., Ensminger, L.E., Clark, F.E., Eds.; American Society of Agronomy: Madison, WI, USA, 1965; pp. 914–926. [Google Scholar]
- Chefetz, B.; Hatcher, P.H.; Hadar, Y.; Chen, Y. Chemical and biological characterization of organic matter during composting of municipal solid waste. J. Environ. Qual. 1996, 25, 776–785. [Google Scholar] [CrossRef]
- Bremner, J.M. Total Nitrogen. In Methods of Soil Analysis: Part 2; Black, C.A., Evans, D.D., Ensminger, L.E., White, J.L., Clark, F.F., Dinauer, R.C., Eds.; American Society of Agronomy: Madison, WI, USA, 1965; pp. 1149–1178. [Google Scholar]
- Cottenie, A. Soil testing and plant testing as a basis of fertilizer recommendation. FAO Soils Bull. 1980, 38, 70–73. [Google Scholar]
- Rowell, D.L. Soil Science: Methods and Application; Longman Publishers Ltd.: London, UK, 1994; p. 169. [Google Scholar]
- Bernas, B. New method for decomposition and comprehensive analysis of silicates by atomic absorption spectrometry. Anal. Chem. 1968, 40, 1682–1686. [Google Scholar] [CrossRef]
- Mehlich, A. Determination of P, Ca, Mg, K, Na and NH4. Short Test Methods Used in Soil Testing Division; Department of Agriculture: Raleigh, NC, USA, 1953. [Google Scholar]
- Kuo, S. Phosphorus. In Methods of soil analysis. Part 3: Chemical Methods; Sparks, D.L., Ed.; The American Society of Anesthesiologists, Soil Science Society of America: Madison, WI, USA, 1996; pp. 869–920. [Google Scholar]
- Murphy, J.; Riley, J.P. A modified single solution method for the determination of phosphate in natural waters. Anal. Chim. Acta 1962, 27, 31–36. [Google Scholar] [CrossRef]
- Paramananthan, S. Soil of Malaysia: Their Characteristics and Identification; Academy of Sciences Malaysia: Kuala Lumpur, Malaysia, 2000; Volume 1, pp. 11–125. [Google Scholar]
- Malaysian Agricultural Research and Development Institute (MARDI). Jagung Manis Baru, Masmadu [New Sweet Corn, Masmadu]; Malaysian Agricultural Research and Development: Serdang, Selangor, Malaysia, 1993; pp. 3–5. [Google Scholar]
- Free, H.F.; McGill, C.R.; Rowarth, J.S.; Hedley, M.J. The effect of biochars on maize (Zea mays) germination. N. Z. J. Agric. Res. 2010, 53, 1–4. [Google Scholar] [CrossRef]
- Ndor, E.; Dauda, S.N.; Azagaku, E.D. Response of maize varieties (Zea mays) to biochar amended soil in Lafia, Nigeria. J. Exp. Agric. Int. 2015, 5, 525–531. [Google Scholar] [CrossRef]
- Mandre, M.; Pärn, H.; Ots, K. Short-term effects of wood ash on the soil and the lignin concentration and growth of Pinus sylvestris L. For. Ecol. Manag. 2006, 223, 349–357. [Google Scholar] [CrossRef]
- Ozolincius, R.; Buozyte, R.; Varnagiryte, K.I. Wood ash and nitrogen influence on ground vegetation cover and chemical composition. Biomass Bioenergy 2007, 31, 710–716. [Google Scholar] [CrossRef]
- Perucci, P.; Monaci, E.; Onofri, A.; Vischetti, C.; Casucci, C. Changes in physico-chemical and biochemical parameters of soil following addition of wood ash: A field experiment. Eur. J. Agron. 2008, 28, 155–161. [Google Scholar] [CrossRef]
- Hasbullah, N.A.; Ahmed, O.H.; Ab Majid, N.M. Effects of Amending Phosphatic Fertilizers with Clinoptilolite Zeolite on Phosphorus Availability and Its Fractionation in an Acid Soil. Appl. Sci. 2020, 10, 3162. [Google Scholar] [CrossRef]
- Bidin, A. Phosphorus utilization in rainfed agriculture in Malaysia. In Proceedings of the Second IMPHOS Regional Seminar: Southern Asia, Fertility Association of India, New Delhi, India, 26–30 August 1986; pp. 161–172. [Google Scholar]
- Phounglamcheik, A.; Wang, L.; Romar, H.; Kienzl, N.; Broström, M.; Ramser, K.; Skreiberg, Ø.; Umeki, K. Effects of Pyrolysis conditions and feedstocks on the properties and gasification reactivity of charcoal from woodchips. Energy Fuels 2020, 34, 8353–8365. [Google Scholar] [CrossRef]
- Phonphuak, N.; Thiansem, S. Effects of charcoal on physical and mechanical properties of fired test briquettes. Sci. Asia 2011, 37, 120–124. [Google Scholar] [CrossRef]
- Asap, A.; Haruna, A.O.; Majid, N.M.A.; Ali, M. Amending triple superphosphate with chicken litter biochar improves phosphorus availability. Eurasian J. Soil Sci. 2018, 7, 121–132. [Google Scholar] [CrossRef]
- Ch’ng, H.Y.; Sanusi, S.; Othman, S.B. Effect of Christmas Island rock phosphate and rice straw compost application on soil phosphorus availability and maize (Zea mays L.) growth in a tropical acid soil of Kelantan, Malaysia. Open Agric. 2020, 5, 150–158. [Google Scholar] [CrossRef]
- Saarsalmi, A.; Mälkönen, E.; Kukkola, M. Effect of wood ash fertilization on soil chemical properties and stand nutrient status and growth of some coniferous stands in Finland. Scand. J. For. Res. 2004, 19, 217–233. [Google Scholar] [CrossRef]
- Ohno, T.; Erich, M.S. Effect of wood ash application on soil pH and soil test nutrient levels. Agric. Ecosyst. Environ. 1990, 32, 223–239. [Google Scholar] [CrossRef]
- Meiwes, K.J. Application of lime and wood ash to decrease acidification of forest soils. Water Air Soil Pollut. 1995, 85, 143–152. [Google Scholar] [CrossRef]
- Ch’ng, H.Y.; Haruna, A.O.; Majid, N.M.N.A.; Jalloh, M.B. Improving soil phosphorus availability and yield of Zea mays L. using biochar and compost derived from agro-industrial wastes. Ital. J. Agron. 2019, 14, 34–42. [Google Scholar] [CrossRef] [Green Version]
- Sanusi, S.; Ch’ng, H.Y.; Othman, S. Effects of incubation period and Christmas Island rock phosphate with different rate of rice straw compost on phosphorus availability in acid soil. AIMS Agric. Food 2018, 3, 384–396. [Google Scholar] [CrossRef]
- Noack, S.R.; McLaughlin, M.J.; Smernik, R.J.; McBeath, T.M.; Armstrong, R.D. Phosphorus speciation in mature wheat and canola plants as affected by phosphorus supply. Plant. Soil 2014, 378, 125–137. [Google Scholar] [CrossRef]
- Penn, C.J.; Camberato, J.J. A critical review on soil chemical processes that control how soil pH affects phosphorus availability to plants. Agriculture 2019, 9, 120. [Google Scholar] [CrossRef] [Green Version]
- Erich, M.S.; Ohno, T. Phosphorus availability to corn from wood ash-amended soils. Water Air Soil Pollut. 1992, 64, 475–485. [Google Scholar] [CrossRef]
- Erich, M.S. Agronomic effectiveness of wood ash as a source of phosphorus and potassium. Am. Soc. Agron. Crop. Sci. Soc. Am. Soil Sci. Soc. Am. 1991, 20, 576–581. [Google Scholar] [CrossRef]
- Hongqing, H.; Chunying, T.; Chongfa, C.; Jizheng, H.; Xueyuan, L. Availability and residual effects of phosphate rocks and inorganic P fractionation in a red soil of Central China. Nutr. Cycl. Agroecosystems 2001, 59, 251–258. [Google Scholar] [CrossRef]
- Hasbullah, N.A.; Osumanu, H.A.; Nik, M. Effects of Clinoptilolite zeolite on phosphorus dynamics and yield of Zea mays L. cultivated on an acid soil. PLoS ONE 2018, 13, e0204401. [Google Scholar] [CrossRef]
- Hasbullah, N.A. Use of Clinoptilolite Zeolite to Improve Efficiency of Phosphorus Use in Acid Soils. Ph.D. Thesis, Universiti Putra Malaysia, Serdang, Selangor, Malaysia, 2016. [Google Scholar]



| Property | Value Obtained | Standard Range * | |
|---|---|---|---|
| pH (water) | 4.61 | 4.6–4.9 | |
| pH (KCl) | 3.95 | 3.8–4.0 | |
| EC (µS cm−1) | 35.10 | NA | |
| Bulk density (g cm−1) | 1.25 | NA | |
| Total carbon (%) | 2.16 | 0.57–2.51 | |
| Total N (%) | 0.08 | 0.04–0.17 | |
| Total P (mg kg−1) | 23.65 | NA | |
| Available P (mg kg−1) | 1.13 | NA | |
| CEC | cmol kg−1 | 4.67 | 3.86–8.46 |
| Exchangeable acidity | 1.15 | NA | |
| Exchangeable Al3+ | 1.02 | NA | |
| Exchangeable H+ | 0.13 | NA | |
| Exchangeable K+ | 0.06 | 0.05–0.19 | |
| Exchangeable Ca2+ | 0.02 | 0.01 | |
| Exchangeable Mg2+ | 0.22 | 0.07–0.21 | |
| Exchangeable Na+ | 0.03 | 0.01 | |
| Exchangeable Mn2+ | 0.01 | NA | |
| Exchangeable Fe2+ | 1.09 | NA | |
| Soil texture | Sand (%): 71.9 | Sand (%): 72–76 | |
| Silt (%): 13.5 | Silt (%): 8–9 | ||
| Clay (%): 14.6 | Clay (%): 16–19 | ||
| Sandy loam | Sandy clay loam |
| Inorganic Phosphorus | Percentage (%) |
|---|---|
| Loosely soluble phosphorus (Sol-P) | 0 |
| Aluminium bound phosphorus (Al-P) | 11 |
| Iron bound phosphorus (Fe-P) | 67 |
| Reductant soluble phosphorus (Red-P) | 3 |
| Calcium bound phosphorus (Ca-P) | 7 |
| Occluded phosphorus (Occl-P) | 12 |
| Total | 100 |
| Property | Charcoal | Sago Bark Ash | |
|---|---|---|---|
| pH (water) | 7.74 | 9.99 | |
| pH (KCl) | 7.31 | 9.66 | |
| EC (dS m−1) | 0.27 | 5.75 | |
| Available P (mg kg−1) | 31.25 | 55.83 | |
| Exchangeable K+ | cmol kg−1 | 3.67 | 23.33 |
| Exchangeable Ca2+ | 11.71 | 16.77 | |
| Exchangeable Mg2+ | 3.37 | 3.57 | |
| Exchangeable Na+ | 0.43 | 1.51 | |
| Exchangeable Fe2+ | 0.15 | 0.03 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Johan, P.D.; Ahmed, O.H.; Maru, A.; Omar, L.; Hasbullah, N.A. Optimisation of Charcoal and Sago (Metroxylon sagu) Bark Ash to Improve Phosphorus Availability in Acidic Soils. Agronomy 2021, 11, 1803. https://doi.org/10.3390/agronomy11091803
Johan PD, Ahmed OH, Maru A, Omar L, Hasbullah NA. Optimisation of Charcoal and Sago (Metroxylon sagu) Bark Ash to Improve Phosphorus Availability in Acidic Soils. Agronomy. 2021; 11(9):1803. https://doi.org/10.3390/agronomy11091803
Chicago/Turabian StyleJohan, Prisca Divra, Osumanu Haruna Ahmed, Ali Maru, Latifah Omar, and Nur Aainaa Hasbullah. 2021. "Optimisation of Charcoal and Sago (Metroxylon sagu) Bark Ash to Improve Phosphorus Availability in Acidic Soils" Agronomy 11, no. 9: 1803. https://doi.org/10.3390/agronomy11091803
APA StyleJohan, P. D., Ahmed, O. H., Maru, A., Omar, L., & Hasbullah, N. A. (2021). Optimisation of Charcoal and Sago (Metroxylon sagu) Bark Ash to Improve Phosphorus Availability in Acidic Soils. Agronomy, 11(9), 1803. https://doi.org/10.3390/agronomy11091803





